Abstract
In this study, we report a complete (clinical, radiological, and virological) sustained (1 year) response after nivolumab salvage therapy in a progressive multifocal leukoencephalopathy patient. Analyses of the cells infiltrate in a pretreatment brain biopsy suggest that parenchymal programmed cell death-L1+ macrophages could be the T-cells partnership in immune exhaustion and virus escape.
Keywords: immune checkpoint inhibitor, JC virus, nivolumab, PD-1, progressive multifocal leukoencephalopathy
PD-1 and its ligand are pivotal in the T-cell-exhaustion pathway involved in viral infections. In this paper, we report a complete response after nivolumab salvage therapy in a progressive multifocal leukoencephalopathy patient. Analyses of brain biopsy suggest that PD-L1+ macrophages could be the T-cells partnership in immune exhaustion and virus escape.
Progressive multifocal leukoencephalopathy (PML) is a rare opportunistic demyelinating viral disease caused by human polyomavirus JC-virus (JCV) infection [1]. Although asymptomatic primary JCV infection occurs in up to 80% of the general population, PML results from secondary JCV reactivation in patients with defective cellular immunity [2]. The main well characterized immunodeficiencies responsible for PML include secondary forms of human immunodeficiency virus (HIV), hematologic and solid tissue cancers, transplantation, and immunosuppressant use [2, 3]. Progressive multifocal leukoencephalopathy among patients without apparent immune defects remains infrequent [4, 5]. Progressive multifocal leukoencephalopathy prognosis is usually poor and frequently fatal, with 3-month mortality exceeding 20%–50% [6]. Although no effective, specific, antiviral drug is currently available, very recent strategies aiming to increase cellular immunity have opened possibilities for disease control, eg, recombinant interleukin-7 or specific T-cell adoptive therapy [7, 8].
Nivolumab, a fully human immunoglobulin (Ig)G4 anti-programmed cell death-1 (PD-1) immune checkpoint inhibitor antibody, selectively blocks the interaction of the PD-1 receptor with its ligand (PD-L1) by disrupting the negative signal that downregulates T-cell activation and proliferation. Programmed cell death-1 pathway blockade has obtained improvements of overall and progression-free survival rates for increasing numbers of different cancers [9, 10].
T-lymphocyte exhaustion, a form of T-cell dysfunction, commonly occurs in chronic viral infections, including in PML. Indeed, during chronic infections, viruses often use counter immune system subterfuge to hide, escape, or protect themselves from T-cell cytotoxicity [11]. We first hypothesized that T-cell exhaustion, via PD-1 engagement, could participate in JCV sheltering and replication, and, secondarily, that targeted blockade of the PD-1 pathway might achieve control of the pathogenic PML process [12].
In this study, we report a PML patient’s exceptional, favorable outcome with nivolumab salvage therapy. Although his precise primary immunodeficiency remains unknown, we concomitantly immunophenotyped PD-1/PD-L1 on a diagnostic brain biopsy, to explore their hypothetical pivotal pathophysiologic roles and evaluate the effectiveness and safety of therapeutic PD-1 targeting in PML.
CASE REPORT
A 53-year-old man with no known or familial medical history, other than his silicosis, was referred 1 month after the onset of progressive aphasia and weakness, then right-side hemiplegia. Cerebral magnetic resonance imaging (MRI) revealed T2-weighted oval-shaped, hyperintense, nonenhancing, parietal white matter signals. The first cerebrospinal fluid (CSF) analysis revealed normal cytology and negative JCV load. Progressive multifocal leukoencephalopathy was subsequently confirmed on a diagnostic brain biopsy containing large glial cells with intranuclear viral inclusions (Figure 1A), corresponding to anti-JCV positivity on in situ hybridization. The white matter biopsy showed a dense diffuse inflammatory infiltrate, consisting predominantly of foamy macrophages (Figure 1B), with perivascular localization (Figure 1C). Mirtazipine and mefloquine were started. Clinical and MRI signs, reassessed 3 months later, showed clear PML progression, and the JCV-deoxyribonucleic acid load in a second CSF sample was 3.3 log10 copies (Figure 1D).
Figure 1.
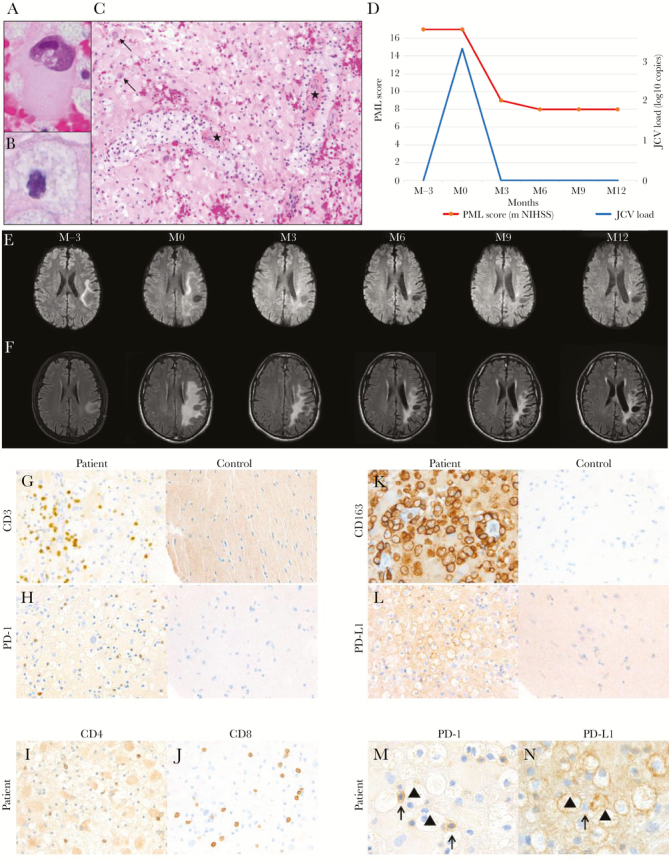
Histology of a nivolumab-treated progressive multifocal leukoencephalopathy (PML) patient’s brain biopsy, and clinical and cerebrospinal fluid (CSF) JC virus (JCV)-load outcomes. (A) shows a large glial cell with intranuclear viral inclusions (hematoxylin-eosin [HES] stain, ×100 magnification). (B) shows a foamy macrophage (HES stain, ×100 magnification). (C) shows brain parenchymal infiltration by numerous foamy macrophages (with thick perivascular cuffs, ★) and reactive astrocytes with enlarged, hyperchromatic, and bizarrely shaped nuclei and large cytoplasm (arrows) (HES stain, ×40 magnification). (D) shows the regular improvement of the patient’s neurologic status, assessed with the modified NIH Stroke Scale (m NIHSS) adapted to PML, after starting nivolumab at month 0 (M0) (red curve). Repeated CSF examinations documented complete JCV-deoxyribonucleic acid load (blue curve) clearance as of M3 after M12. The Nivolumab-Treated Patient’s Sequential Cerebral Magnetic Resonance Imaging (MRI), from Month (M) –3 to M 12, of Progressive Multifocal Leukoencephalopathy (PML). Axial diffusion-weighted images of the left temporoparietal hyperintensity (E) delineating the demyelination front, with demyelination disappearance as of M6 after the first nivolumab infusion (M0). Axial T2-weighted fluid-attenuated inversion-recovery (T2-FLAIR) images of the left temporoparietal hyperintensity (Panel F) from M0 to M12 showing regression of the white-matter lesion and some atrophic changes. Immunohistochemical-labeling of the Progressive Multifocal Leukoencephalopathy Patient’s Brain Biopsy before Nivolumab. Anti-CD3–antibody (G) and anti-PD-1–antibody (H) labeling T cells of the patient and healthy control (40× magnification). Anti-CD4–antibody (I) and anti- CD8–antibody (J) labeling T cells of the patient (40× magnification). Anti-CD163–antibody (K) and anti-PD-L1–antibody labeling (L), of the patient and healthy control, respectively showing diffusely infiltrating macrophages, predominantly in perivascular locations and labeled membranes (40× magnification). Anti-PD-1–antibody immunolabeling of a T cell adjacent to macrophages (▲) (M, (100× magnification). Anti-PD-L1–antibody immunolabeling of macrophages (▲) in contact with T cells (arrows) (N, 100× magnification).
After various repeated explorations, we were unable to identify any primary or secondary immune deficiency, including HIV infection, hematologic and solid tissue cancers, or autoimmune diseases. Despite the patient’s absence of recurrent or unusual infectious diseases, primary immunodeficiency screening was undertaken but revealed no profound T-, B-, or natural killer-cell lymphopenia (616 CD8+ cells/μL, 113 CD19+ cells/μL, 271 CD3–CD16+56+ cells/μL), except a moderate CD4 T cells lymphopenia 365 CD4+ cells/μL (normal range, 530–1300 cells/μL), approximately 25% of CD3+ T cells. Gammaglobins were within the range (IgG, 10 g/L; IgA, 2.78 g/L; IgM, 0.69 g/L), including IgG subclasses. In vitro lymphocyte proliferation assay with phytohemagglutinin was normal. Antidiphteric and antitetanic antibodies were detected in the serum of the patient. Moreover, next-generation sequencing analysis was used, but it did not identify pathogenic defects. Rather than a pathogenic homozygous, only a heterozygous dedicator of cytokinesis 8 protein (DOCK8)-gene mutation (c.4460C>T) was found. Finally, as expected in light of our patient’s clinical context, immunophenotyping showed normal DOCK8 expression, which definitely excluded a related disease.
Without a specific therapeutic strategy to obtain immune recovery in this setting, and in agreement with the patient and his family, compassionate nivolumab salvage therapy was started. See Methods in Supplementary Material for more detail.
RESULTS
Disease Outcome Under Nivolumab
One month after the first nivolumab infusion, neurologic evaluation showed attenuated then stabilized aphasia and regression of the initial complete motor deficit. At month 6, the patient could walk with a cane. Scores obtained with the objective modified NIH Stroke Scale adapted to PML improved regularly: 17, 9, 8, 8, and 8, respectively, at baseline and months 3, 6, 9, and 12 (Figure 1D) [13].
Parallel biologic and imaging assessments of nivolumab treatment showed concordant responses during follow-up. Indeed, repeated CSF analyses showed complete and persistent JCV-load clearance as of month 3 until month 12 (Figure 1D). Likewise, month-3 cerebral MRI findings revealed a reduction of the sizes of the demyelination front (Figure 1E) and white matter lesion (Figure 1F). The demyelination front had disappeared by month 6. From month 9 onward, the only remaining MRI signs were sequelae that paralleled the stable and clearly diminished neurologic deficit.
Adverse Events
No feature of immune reconstitution inflammatory syndrome (IRIS) or any other immune-related adverse events occurred.
Pretreatment Brain Programmed Cell Death (PD)-1/PD-L1 Expression
Histologic examination of the diagnostic brain biopsy showed a mild, diffuse, inflammatory CD3+ T-cell infiltration of the parenchyma (Figure 1G). Most of those infiltrating T-cells had PD-1high expression, suggesting an elevated level of local T-lymphocyte exhaustion (Figure 1H). The T cells corresponded to both CD4+ and CD8+ cells (Figure 1I and J).
Then, to identify a potential partner of PD-1+ T cells, cells involved in immune-cell exhaustion were investigated. As commonly described in PML compared with healthy control brain samples, pronounced gliosis with microglial activation were found in our patient’s biopsy. Reflecting their macrophagic/monocyte lineage, microglial cells are identified by their strong CD163 expression (Figure 1K). Also of note, in this section, most glial cells coexpressed PD-L1 (Figure 1L), which supports their participation in T-cell exhaustion. Finally, PD-1+ T cells were colocalized with PD-L1-expressing macrophages, thereby also indicting that the latter are potential participants in T-cell exhaustion in PML pathogenesis (Figure 1M and N).
DISCUSSION
We described a previously unreported clinical remission of PML under salvage therapy with nivolumab, a specific blocker of the T-cell PD-1/PDL-1 immune-checkpoint, associated with persistent complete JCV clearance from CSF and MRI-assessed brain healing, without any observed adverse event. These concomitant observations implicate overexpression of the PD-1/PD-L1 pathway in JCV pathogenicity, likely contributing to virus escape from host T-cell neutralization. They also shed new light on the local cooperation of some of the central nervous system’s own cells and probably their specific cerebral homing precursors, respectively, glial cells and macrophages, in local exhaustion of the host T-cell defense system in PML pathogenesis.
The T-cell compartment is crucial to controlling chronic infectious diseases. Recovery of anti-JCV CD4+ and CD8+ T-cell responses corresponds to PML patients’ survival [14]. CD8+ T-cell exhaustion, first studied in chronic lymphocytic choriomeningitis virus infection of mice, led to the loss of effector cytokines (eg, interleukin-2, tumor necrosis factor-α, and interferon-γ) and the loss of ex vivo cytotoxicity, both of which are essential to controlling virus replication [15]. T-cell exhaustion was recently highlighted in PML. Programmed cell death-1 is overexpressed on PML patients’ total circulating CD4+ and CD8+ T cells compared with healthy controls. Moreover, PML patients’ had more frequent JCV-specific, PD-1+CD8+ cytotoxic T cells than total CD8+ T lymphocytes, and in vitro PD-1 receptor blockade enhanced the JCV-specific, T-cell immune response in a subgroup of PML patients. At physiologic steady state, as opposed to an inflammatory context, PD-1 is expressed on many cell types and binds to 2 ligands, including PD-L1, which is broadly expressed by hematopoietic and parenchymal cells.
Our patient’s T-cell dysfunction/exhaustion is emphasized by the strong PD-1 expression on many T cells. However, brain-histology findings also suggest related unfavorable and decreased local T-cell homing, reflected by their clear paucity, perhaps attributable to macrophage killing, despite the major JCV-inflicted destruction seen in PML. Our results spotlight glial cells as the potential principal partner contributing to host-defense exhaustion.
To date, antiviral treatment of PML has not been effective, and the only disease-control strategies applied were based on increasing the host’s cellular immunity. In addition to our use of nivolumab, 2 other approaches to improve and/or increase T-cell function/number against JCV were tried, with some interesting results. Interleukin-7, a key cytokine essential for T-cell proliferation, has emerged in Europe to treat PML in the setting of profound CD4 and/or CD8 lymphopenia [7]. However, our patient’s incompletely characterized immune defect did not include profound lymphopenia. In a recent study, Muftuoglu et al [8] reported their findings on 3 immunocompromised PML patients treated with partially human leukocyte antigen-matched, third- party-produced, cryopreserved, BK virus-specific T cells. Two of them experienced clinical sign and PML imaging-feature regression, and their CSF JCV loads were completely cleared [8]. Although such adoptive T-cell immunotherapies are not yet available for routine clinical practice, they highlight, in addition to the potential efficacy of blocking the immune checkpoint pathway, promising perspectives for PML treatment. Combinations of some of these complementary strategies for optimal boosting of host defenses warrant being assessed for refractory PML. Very recently, Cortese et al [16] reported their findings on 8 adults with PML, each with a different underlying predisposing condition, treated with pembrolizumab. Five patients had clinical improvement or stabilization of PML accompanied by a reduction in the JC viral load in the CSF and an increase in in vitro CD4+ and CD8+ anti-JCV activity [16]. The occurrence of IRIS is frequent during treatment with nivolumab and has been reported in the 2 previous cases of PML treated with nivolumab [17, 18]. Our patient did not present IRIS, and probably IRIS is not mandatory for remisson.
CONCLUSIONS
Any conclusions could be drawn from this case report, especially because the precise type of inflammatory syndrome remains unknown, but targeting an immune checkpoint, eg, PD-1, to boost host defenses against JCV seems to be a promising therapeutic option inducing sustained response for PML, but this hypothesis requires prospective studies.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Figure 1. The nivolumab-treated patient’s sequential cerebral magnetic resonance imaging (MRI), from month (M) 3 to M12, of progressive multifocal leukoencephalopathy (PML). Axial diffusion-weighted images of the left temporoparietal hyperintensity (E) delineating the demyelination front, with demyelination disappearance as of M6 after the first nivolumab infusion (M0). Axial T2-weighted fluid-attenuated inversion-recovery (T2-FLAIR) images of the left temporoparietal hyperintensity (F) from M0 to M12 showing regression of the white-matter lesion and some atrophic changes.
Supplementary Figure 2. Immunohistochemical labeling of the progressive multifocal leukoencephalopathy (PML) patient’s brain biopsy before nivolumab. Anti-CD3 antibody (G) and anti-programmed cell death (PD)-1 antibody (H) labeling T cells of the patient and healthy control (×40 magnification). Anti-CD4 antibody (I) and anti-CD8 antibody (J) labeling T cells of the patient (×40 magnification). Anti-CD163 antibody (K) and anti-PD-L1 antibody labeling (L) of the patient and healthy control, respectively, showing diffusely infiltrating macrophages, predominantly in perivascular locations and labeled membranes (×40 magnification). Anti-PD-1 antibody immunolabeling of a T cell adjacent to macrophages (▲) ([M] ×100 magnification). Anti-PD-L1 antibody immunolabeling of macrophages (▲) in contact with T cells (arrows) ([N] ×100 magnification).
Acknowledgments
We thank the patient and his family for their willingness to allow their experience to be shared. We also thank Dr. Guillaume Martin-Blondel for helpful advice concerning the manuscript. In addition, we thank Dr. Capucine Picard for next-generation sequencing analyses. Moreover, we thank the following medical students for their support in managing this patient: Cédric Costa, Anaïs Lecanuet, Martin Gerard, Paul Castan, Laurent D’Arco, Ségolène Fuentes, Marie Cuchet, Charles Maurille, Grégoire Monseau, Eve Calvar, Antoine Hankard, and Claire Delmas. Finally, we thank Janet Jacobson for editorial assistance.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Astrom KE, Mancall EL, Richardson EP. Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin’s disease. Brain J Neurol 1958; 81:93–111. [DOI] [PubMed] [Google Scholar]
- 2. Ferenczy MW, Marshall LJ, Nelson CD, et al. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev 2012; 25:471–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhai S, Brew BJ. Progressive multifocal leukoencephalopathy. Handb Clin Neurol 2018; 152:123–37. [DOI] [PubMed] [Google Scholar]
- 4. Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol 2010; 9:425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zucker BE, Stacpoole SRL. Progressive multifocal leukoencephalopathy in the absence of immunosuppression. J Neurovirol 2018; 24:119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brew BJ, Davies NW, Cinque P, et al. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol 2010; 6:667–79. [DOI] [PubMed] [Google Scholar]
- 7. Alstadhaug KB, Croughs T, Henriksen S, et al. Treatment of progressive multifocal leukoencephalopathy with interleukin 7. JAMA Neurol 2014; 71:1030–5. [DOI] [PubMed] [Google Scholar]
- 8. Muftuoglu M, Olson A, Marin D, et al. Allogeneic BK virus-specific T cells for progressive multifocal leukoencephalopathy. N Engl J Med 2018; 379:1443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015; 372:320–30. [DOI] [PubMed] [Google Scholar]
- 10. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol 2018; 18:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol 2010; 22:223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989; 20:864–70. [DOI] [PubMed] [Google Scholar]
- 14. Gheuens S, Bord E, Kesari S, et al. Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol 2011; 85:7256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 1998; 188:2205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cortese I, Muranski P, Enose-Akahata Y, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med 2019; 380:1597–605. [DOI] [PubMed] [Google Scholar]
- 17. Hoang E, Bartlett NL, Goyal MS, et al. Progressive multifocal leukoencephalopathy treated with nivolumab. J Neurovirol 2019; 25:284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walter O, Treiner E, Bonneville F, et al. Treatment of progressive multifocal leukoencephalopathy with nivolumab. N Engl J Med 2019; 380:1674–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


