Abstract
Background
Fluconazole is lifesaving for treatment and prevention of cryptococcosis; however, optimal dosing is unknown. Initial fluconazole doses of 100 mg to 2000 mg/day have been used. Prevalence of fluconazole nonsusceptible Cryptococcus is increasing over time, risking the efficacy of long-established standard dosing. Based on current minimum inhibitory concentration (MIC) distribution, we modeled fluconazole concentrations and area under the curve (AUC) relative to MIC to propose a rational fluconazole dosing strategy.
Method
We conducted a systematic review using the MEDLINE database for reports of fluconazole MIC distribution against clinical Cryptococcus isolates. Then, we utilized fluconazole concentrations from 92 Ugandans who received fluconazole 800mg/day coupled with fluconazole’s known pharmacokinetics to predict plasma fluconazole concentrations for doses ranging from 100 mg to 2000 mg via linear regression. The fluconazole AUC above MIC ratio were calculated using Monte Carlo simulation and using the MIC distribution elucidated during the systemic review.
Results
We summarized 21 studies with 11 049 clinical Cryptococcus isolates. Minimum inihibitory concentrations were normally distributed with a geometric mean of 3.4 µg/mL, median (MIC50) of 4 µg/mL, and 90th percentile (MIC90) of 16 µg/mL. The median MIC50 trended upwards from 4 µg/mL in 2000–2012 to 8 µg/mL in 2014–2018. Predicted subtherapeutic fluconazole concentrations (below MIC) would occur in 40% with 100 mg, 21% with 200 mg, and 9% with 400 mg. The AUC:MIC ratio >100 would occur in 53% for 400 mg, 74% for 800 mg, 83% for 1200 mg, and 88% for 1600 mg.
Conclusions
Currently recommended fluconazole doses may be inadequate for cryptococcosis. Further clinical studies are needed for rational fluconazole dose selection.
Keywords: cryptococcal meningitis, Cryptococcus, fluconazole, fungal drug resistance, systematic review
The systematic review demonstrates the uptrend in the minimum inhibitory concentration of fluconazole against Cryptococcus species. Based on the fluconazole pharmacokinetic data, the historically recommended fluconazole dose for cryptococcal meningitis treatment may not be adequate enough to reach an optimal therapeutic goal.
INTRODUCTION
Cryptococcus is the leading cause of meningitis in persons with AIDS [1]. Cryptococcosis accounts for approximately 15% of AIDS-related deaths worldwide [2]. Globally, deaths from cryptococcal meningitis are still unacceptably high despite the expanded rollout of antiretroviral therapy (ART) and increased availability of effective antifungal agents [2]. Cryptococcal meningitis can be prevented by cryptococcal antigen (CrAg) screenings of at-risk populations with advanced AIDS and then providing preemptive fluconazole therapy [3]; however, the optimal fluconazole dose is not known. Varying fluconazole doses between 100mg/day and 2000mg/day have been used for treatment or preemptive therapy [3].
One of the crucial factors influencing fluconazole dosing is the in vitro minimum inhibitory concentration (MIC) of fluconazole against Cryptococcus. The MIC ≤8 µg/mL is considered fully susceptible to fluconazole; above this threshold, treatment outcomes could be less optimal [4, 5]. Fluconazole therapeutic drug monitoring is not routinely used in clinical practice due to relatively predictable pharmacokinetics and infrequent resistance of initial Cryptococcus isolates [6–8]. However, a recent systematic review of 4995 clinical isolates in 29 studies from 1988 to 2017 revealed that fluconazole nonsusceptible Cryptococcus neoformans and Cryptococcus gattii isolates ranged from 0%–50% with a mean prevalence of 12%, and an increasing trend over time [9]. The concentration-time area under the curve (AUC) relative to the MIC is a central pharmacodynamic principle associated with fluconazole’s mycologic activity. Based on pharmacokinetic and pharmacodynamic modeling in animals, an AUC:MIC ratio ≥389 is the mean stasis endpoint desired for maximal fluconazole activity against Cryptococcus in mice [10–12]. The correlation between the MIC distribution, steady-state fluconazole concentrations in humans, and exposure in an HIV-infected population have not been well studied. Our study aimed to characterize the MIC distribution using a systematic review of published Cryptococcus MICs, simulate steady-state fluconazole exposures in humans, and propose a rational fluconazole dose for different phases of cryptococcal meningitis treatment and preemptive therapy in asymptomatic CrAg-positive persons.
METHODS
We determined the MIC distribution of Cryptococcus from a systematic review of the literature, and distribution of fluconazole plasma concentrations from a Uganda HIV-infected cryptococcal cohort to then model the probability of achieving therapeutic fluconazole concentrations for the population as well as for specific MICs.
MIC Distribution
We performed a systematic literature search from the MEDLINE database using key words from the Medical Subject Heading (MeSH) database. Search terms were [“fluconazole” or “antifungal agents”] and [“Cryptococcus” or “Cryptococcus gattii” or “Cryptococcus neoformans” or “cryptococcosis”] and [“microbial sensitivity test” or “fungal drug resistance”]. The search strategy identified studies reporting the MIC distribution of fluconazole against clinical Cryptococcus isolates from January 1, 2000, to May 31, 2018. The inclusion criteria included experimental studies, observational studies, and case series describing fluconazole MIC distribution in clinical isolates. Environmental isolates or animal studies were excluded. Data extracted from the selected studies were year, country, the number of isolates, and the pattern of overall MIC distribution.
Plasma Fluconazole Concentration
Plasma fluconazole concentrations were determined as part of the Adjunctive Sertraline for the Treatment of HIV-associated Cryptococcal Meningitis (ASTRO-CM) trial (Clinical Trials registration number NCT01802385) [13]. We used fluconazole plasma concentrations obtained at steady state in 92 HIV-infected Ugandan adults with cryptococcal meningitis who received 800mg of oral fluconazole daily. One to three fluconazole concentrations were measured per participant during the second week of induction of meningitis therapy and averaged within participant. The details of the protocol, including fluconazole measurement method, were described in a previous study [14, 15].
Fluconazole Concentration and AUC Above the MIC Prediction Model
Fluconazole is well known to have linear pharmacokinetics [11, 16–18]. For this reason, we used a linear regression model for predicting the plasma concentrations following oral fluconazole at 100 mg, 200 mg, 400 mg, 1200 mg, 1600 mg, and 2000 mg/day from the measured concentrations following oral fluconazole 800 mg daily given as directly observed therapy while hospitalized [15, 19]. We performed a Monte Carlo simulation with 1 million replicates based on the normal distribution of steady-state fluconazole concentrations from the different doses. We restricted the modeled distribution to ±2 standard deviations (SD), thereby truncating at <2.5% or >97.5% distributions. This truncation eliminated nonsensical, negative modeled fluconazole values. We modeled the MIC distribution with 1 million replicates using a normal distribution of a log2 geometric mean MIC ± SD and then back transforming into the geometric MIC. The proportion of fluconazole concentration relative to MIC then was calculated from the 1 million replicates. Fluconazole’s long terminal half-life leads to little variability across the dosing interval [20], so the AUC:MIC ratio also was calculated from the simulated patients using fluconazole concentration multiplied by 24 hours and divided by MIC. Analyses were performed using Excel 2016 (Microsoft, Redmond, WA).
We summarized the proportions of simulated patients achieving plasma exposures above the MIC (relevant as a target for secondary prophylaxis). We additionally summarized proportions achieving a >100 AUC:MIC ratio. This ratio is less than the optimal AUC:MIC ratio of 389 for induction therapy determined in a murine model using ~7 log10Cryptococcus colony forming units/g tissue [10]. This lower target (>100 AUC:MIC) is an approximation consistent with clinical success with consolidation therapy of 400mg/day in the setting of historically lower MICs ≤2 µg/mL and lower fungal burdens at the time of consolidation therapy.
RESULTS
Cryptococcus Susceptibility to Fluconazole
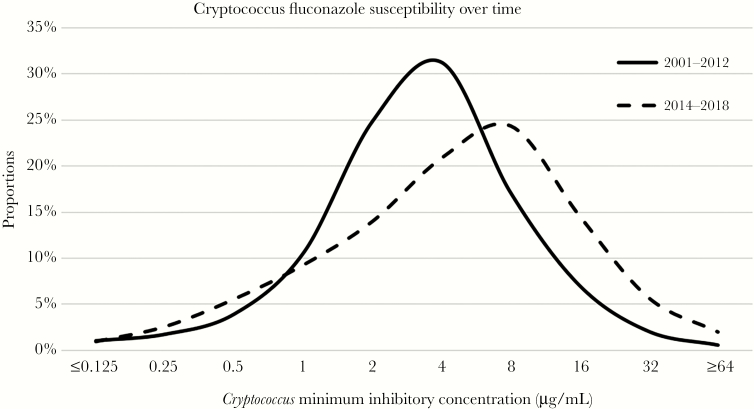
A total of 681 studies were identified and screened for Cryptococcus susceptibility. Only 96 studies met the inclusion criteria, 75 of these studies were excluded due to the absence of presenting MIC distribution (Supplementary Figure 1). The final quantitative synthesis included 11 049 clinical Cryptococcus isolates reported from 21 studies (Table 1) [6, 7, 21–37]. Overall, 68% of the clinical isolates came from 2 large global studies [7, 37] that were collected from multiple geographic sites. All studies used broth microdilution methods for MIC determination. The MICs were normally distributed on a log2 scale (Supplementary Figure 2) with a geometric mean of 3.4 µg/mL and a geometric standard deviation of ±1.53 log2 µg/mL. The median MIC50 was 4 µg/mL. A total of 1255 clinical isolates (11.3%) had MIC >8 µg/mL with 90th percentile (MIC90) being 16 µg/mL. When the MIC distribution was divided into 2 groups by date of publication, 13 studies (n = 9507) published in 2000–2012 and 8 studies (n = 1542) published in 2014–2018, the median MIC50 trended upwards from 4 µg/mL to 8 µg/mL (Figure 1).
Table 1.
Distribution of Cryptococcus Clinical Isolates Minimum Inhibitory Concentrations
| First Author | Year | Population | N | Cryptococcus Minimum Inhibitory Concentrations, µg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ≥64 | ||||
| Cogliati [21] | 2018 | Italy | 295 | 3 | 17 | 35 | 46 | 67 | 88 | 35 | 4 | 0 | 0 |
| Kassi [22] | 2018 | Ivory coast | 50 | 9 | 14 | 13 | 8 | 0 | 1 | 5 | 0 | 0 | 0 |
| Worasilchai [23] | 2017 | Thailand | 74 | 0 | 0 | 5 | 17 | 35 | 17 | 0 | 0 | 0 | 0 |
| Gago [24] | 2016 | Spain | 28 | 0 | 0 | 0 | 0 | 0 | 2 | 8 | 6 | 4 | 8 |
| Cordoba [25] | 2016 | Argentina | 702 | 2 | 7 | 8 | 19 | 43 | 116 | 256 | 172 | 63 | 16 |
| Smith [26] | 2015 | Uganda | 198 | 1 | 2 | 8 | 20 | 20 | 39 | 49 | 37 | 17 | 5 |
| Van Wyk [27] | 2014 | South Africa | 155 | 0 | 0 | 9 | 19 | 42 | 53 | 23 | 6 | 1 | 2 |
| Morales [28] | 2014 | Brazil | 40 | 0 | 0 | 8 | 14 | 9 | 6 | 0 | 0 | 3 | 0 |
| Espinel-Ingroff [7] | 2012 | Global | 5733 | 97 | 149 | 319 | 705 | 1629 | 1868 | 668 | 206 | 67 | 25 |
| Matos [29] | 2012 | Brazil | 60 | 0 | 0 | 0 | 1 | 2 | 12 | 28 | 9 | 2 | 6 |
| Lockhart [7] | 2012 | USA | 298 | 0 | 0 | 9 | 17 | 41 | 71 | 100 | 40 | 20 | 0 |
| Govender [30] | 2011 | South Africa | 487 | 0 | 1 | 27 | 92 | 196 | 138 | 30 | 3 | 0 | 0 |
| Pfaller [31] | 2011 | Global | 285 | 0 | 3 | 2 | 24 | 62 | 140 | 45 | 8 | 1 | 0 |
| Mdodo [32] | 2011 | Kenya | 66 | 0 | 2 | 1 | 5 | 16 | 32 | 8 | 2 | 0 | 0 |
| Illnait-Zaragozí [7] | 2010 | Cuba | 19 | 0 | 2 | 2 | 4 | 4 | 5 | 2 | 0 | 0 | 0 |
| Iqbal [33] | 2009 | USA | 43 | 0 | 0 | 1 | 0 | 4 | 16 | 10 | 10 | 2 | 0 |
| Fusco-Almeida [34] | 2007 | Brazil | 83 | 0 | 0 | 0 | 2 | 0 | 12 | 42 | 20 | 7 | 0 |
| Bii [35] | 2007 | Kenya | 80 | 0 | 0 | 0 | 1 | 3 | 3 | 12 | 46 | 6 | 9 |
| Serena [36] | 2005 | Spain | 20 | 0 | 0 | 0 | 1 | 1 | 1 | 4 | 2 | 7 | 4 |
| Pfaller [37] | 2005 | Global | 1811 | 0 | 7 | 11 | 72 | 327 | 598 | 489 | 235 | 72 | 0 |
| Brandt [6] | 2001 | USA | 522 | 0 | 0 | 0 | 77 | 77 | 77 | 189 | 85 | 9 | 8 |
| Total (Cumulative %) | 11 049 |
112
1.0% |
204
2.9% |
458
7.0% |
1144
17.4% |
2578
40.7% |
3295
70.5% |
2003
88.6% |
891
96.7% |
281
99.2% |
83
100% |
||
Figure 1.
Fluconazole Minimum Inhibitory Concentration Distribution for Cryptococcus from Years 2001–2012 and 2014–2018
Minimum inhibitory concentration distribution of 11 049 clinical isolates published from January 1, 2000, to May 31, 2018, were normally distributed with a geometric mean of 3.4 µg/mL, median (MIC50) of 4 µg/mL, and 90th percentile (MIC90) of 16 µg/mL. When divided into 2 groups from the publication years of 2000–2012 (13 studies, n = 9507) and years of 2014–2018 (8 studies, n = 1542), the median MIC50 was up trending from 4 µg/mL to 8 µg/mL.
Modeled Fluconazole Concentrations
The mean plasma fluconazole concentration at steady state in 92 patients who received 800mg of oral fluconazole from the previous study as described above was 42.6 (±21.3 SD) µg/mL (median, 41.2; interquartile range, 29.8 to 52.7 µg/mL). The predicted mean plasma fluconazole concentrations (µg/mL) are reported in Table 2.
Table 2.
Distribution of Plasma Fluconazole Concentrations and Proportion Achieving Therapeutic Concentrations
| Fluconazole Dose | 100 mg | 200 mg | 400 mg | 800 mg | 1200 mg | 1600 mg | 2000 mg |
|---|---|---|---|---|---|---|---|
| Mean plasma concentration (±SD), µg/mL | 5.3 ± 2.7 | 10.7 ± 5.3 | 21.3 ± 10.7 | 42.6 ± 21.3 | 63.9 ± 31.9 | 85.2 ± 42.6 | 106 ± 53 |
| % below MIC | 39.8% | 20.7% | 8.8% | 3.3% | 1.7% | 1.1% | 0.8% |
| Mean AUC24 (±SD) mg*h/L | 127.8 ± 63.9 | 255.6 ± 127.8 | 511.2 ± 255.6 | 1022.4 ± 511.2 | 1533.6 ± 766.8 | 2044.8 ± 1022.4 | 2556 ± 1278 |
| % AUC:MIC > 100 | 13.4% | 30.6% | 53.2% | 74.2% | 83.3% | 88.3% | 91.2% |
| % AUC:MIC > 389 optimal | 1.5% | 6.1% | 17.2% | 36.3% | 49.7% | 59.2% | 66.1% |
Abbreviations: AUC, area under the curve; MIC, minimum inhibitory concentration; SD, standard deviation.
Projected Fluconazole Concentrations Above MICs
The Monte Carlo simulation with 1 000 000 simulated patients based on the normal distribution pattern of MICs and measured fluconazole concentrations demonstrated the projected fluconazole concentration above MICs. Only 60% of those receiving oral fluconazole at 100 mg daily were projected to reach a therapeutic concentration in blood above the Cryptococcus MIC, and the remaining 40% would be expected to have subtherapeutic concentrations. For oral fluconazole at 200 mg, the percentage of predicted fluconazole concentration above MICs was 79%, 400 mg was 91%, 800 mg was 97%, 1200 mg was 98%, and 1600 mg was 99% based on the total distribution of Cryptococcus isolates (Table 2).
Projected AUC:MIC Therapeutic Ratios
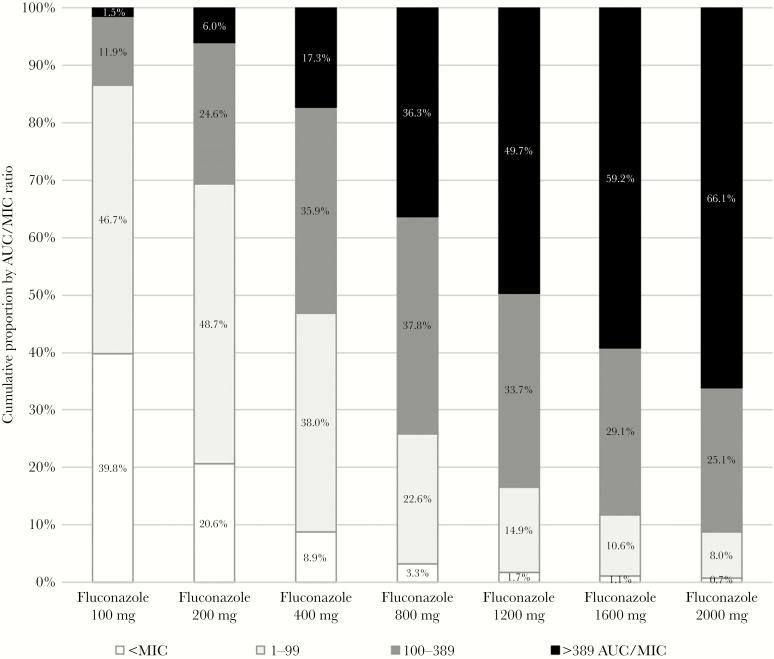
The projected AUC:MIC ratio was calculated from simulated patients, using steady state concentrations. Therapeutic concentrations with AUC:MIC ratio of >100 were present in 13% of fluconazole 100 mg, 31% of fluconazole 200 mg, 53% of fluconazole 400 mg, 74% of fluconazole 800 mg, 83% of fluconazole 1200 mg, 88% of fluconazole 1600 mg, and 91% of fluconazole 2000 mg (Table 2). Regarding induction therapy, the percent of AUC:MIC ratio above 389 for fluconazole 400 mg was 17%, 800 mg was 36%, 1200 mg was 50%, 1600 mg was 59%, and 2000 mg was 66% (Figure 2).
Figure 2.
Proportion Achieving Target Fluconazole AUC:MIC Ratio
The percent of AUC:MIC ratio presented by category for various doses of fluconazole. The desired optimal target dose for induction therapy would be a >389 AUC:MIC ratio, which is directly proportional to the fluconazole dose. The percent of <MIC reflects the percent of subtherapeutic fluconazole levels below the MIC, which decrease with higher fluconazole doses. At present, ~21% of persons receiving 200 mg/day are projected to not achieve fluconazole plasma levels above MIC based on the current MIC distribution of Cryptococcus isolates.
The Projected Dose of Fluconazole Based on Given MIC
Based on the given MIC, the percentage of fluconazole doses reaching therapeutic exposures above MIC can be projected (Table 3). For MICs ≤4 µg/mL, standard secondary prophylaxis at 200 mg/day will achieve levels above the MIC in >90% of persons. Yet as the MIC rises to 8 µg/mL, only 70% achieve therapeutic exposures, and only 14% of persons receiving 200 mg are projected to achieve therapeutic exposures in plasma above the MIC when it is 16 µg/mL. Among Cryptococcus isolates with higher MICs, higher fluconazole doses would be necessary to achieve therapeutic concentrations. Overall, >90% would achieve therapeutic exposures with 400 mg at MIC of 8 µg/mL, >90% with 800mg at 16 µg/mL, and >85% with 1200 mg at 32 µg/mL, and >70% with 1600 mg at 64 µg/mL (Supplementary Table 1).
Table 3.
Possible Rational Fluconazole Dosing Based on Cryptococcus Minimum Inhibitory Concentration
| Cryptococcus MIC | Fluconazole Therapy | |||
|---|---|---|---|---|
| Consolidation/Preemptive Therapy | Projected at Target of >100 AUC:MIC | Secondary Prophylaxis | Prophylaxis Above MIC | |
| Current Recommendations [3, 42] | 400–800 mg | 53–74% | 200 mg | 79% |
| Customized Recommendations | ||||
| Unknown MIC | 800–1200 mg | 74–83% | 400 mg | 91% |
| ≤2 µg/mL | 400 mg | >91% | 200 mg | 97% |
| 4 µg/mL | 800 mg | 91% | 200 mg | 92% |
| 8 µg/mLa | 1200 mg | 85% | 400 mg | 92% |
| 16 µg/mL | 1600 mg | 68% | 800 mg | 92% |
| 32 µg/mL | 2000 mg | 30%b | 1200 mg | 86% |
| 64 µg/mL | 2000 mg | 0%b | 1600 mg | 70% |
Abbreviations: MIC, minimum inhibitory concentration.
aThere are no official breakpoints for Cryptococcus, but the 2017 Clinical and Laboratory Standards Institute Performance Standards for Antifungal Susceptibility Testing of Yeasts guidelines for Candida albicans cite MICs ≥8 µg/L as considered resistant [39]. Some microbiology laboratories may provide Candida susceptibility breakpoints for context, but physicians should be aware that such thresholds are not clinically validated for Cryptococcus. These suggestions would be relevant for settings where alternative therapies are unavailable or cost-prohibitive.
bFor isolates with MIC ≥32 µg/mL, alternative therapy should be considered as probability of achieving therapeutic fluconazole levels is low.
Refer to Supplementary Table 1 for % above MIC and Supplementary Table 2 for >100 AUC:MIC targets.
For consolidation therapy or preemptive therapy, higher AUC:MIC ratios may be necessary but less than necessary for induction therapy. The traditional 400mg/day dose would achieve >100 AUC:MIC ratio in >90% of persons with a MIC of 2 µg/mL and 68% of persons with a MIC of 4 µg/mL, but only 11% of persons would achieve >100 AUC:MIC ratio with a MIC of 8 µg/mL. As Cryptococcus MICs double, similar proportions achieve >100 AUC:MIC ratio target as fluconazole doses double (Supplementary Table 2). Thus, at 800 mg/day of fluconazole therapy, >90% of persons with MIC of ≤4 µg/mL and ~68% of persons with MIC of 8 µg/mL would achieve >100 AUC:MIC target.
DISCUSSION
In our systematic review, we found that the susceptibility to fluconazole was normally distributed with a geometric mean of 3.4 µg/mL, MIC50 of 4 µg/mL, and MIC90 of 16 µg/mL. Cryptococcus MIC to fluconazole appears to be increasing over time when comparing reported isolates from the year 2000–2012 versus 2014–2018. Ongoing surveillance of antifungal susceptibilities is needed. Based on steady-state fluconazole concentrations from people with advanced HIV disease in Uganda, we found that currently recommended doses are far below optimal AUC targets for induction therapy, projected in ~50% of persons receiving 1200 mg/day and 36% of persons receiving 800 mg/day. Although with induction amphotericin therapy, “optimal” consolidation therapy may not be essential with induction amphotericin therapy [26]. Subtherapeutic concentrations below the MIC would be achieved in 40% of persons receiving 100 mg/day, 21% receiving 200 mg/day, and 9% receiving 400 mg/day. This implies among those receiving the 400 mg/day consolidation dosing, 9% would achieve levels below the MIC and 21% below the MIC when receiving secondary prophylaxis with 200 mg/day. Whether low plasma levels of fluconazole are associated with mortality is an area of further exploration.
Witt et al studied the treatment of AIDS-associated cryptococcal meningitis and found that the MIC of Cryptococcus is an essential factor in determining the treatment outcome [38]. Nevertheless, the breakpoint for Cryptococcus species was not well established given the Clinical and Laboratory Standards Institute Performance Standards for Antifungal Susceptibility Testing of Yeasts was developed for Candida species [39]. The resistance for Candida albicans was defined by MIC ≥8 µg/mL. Previous studies have shown that the MIC breakpoint >8 µg/mL for Cryptococcus species was related to poor outcome and considered as resistant [4, 5]. In our systematic review, 30% of clinical isolates had MIC >4 µg/mL and 11% had MIC >8 µg/mL. The recent systematic review showed that the resistance rates are varied, ranging from 0%–50% with a mean resistance of 10.6% (95% confidence interval [CI], 5.5–15.6) for incident isolates and 24.1% (95% CI, −3.1–51.2) for relapse isolates [9]. Historically, clinical outcomes have not been associated with different MIC breakpoints when using amphotericin [5, 26]; however, fluconazole monotherapy for induction meningitis therapy is associated with 70% mortality in routine care [40, 41]. Thus, comparing outcomes versus MIC during induction therapy likely may not be the correct metric in choosing consolidation therapy doses as the success with consolidation therapy is generally high.
The current treatment dose of fluconazole for cryptococcal infection depends on the phase of treatment and HIV infection status. In the 2010 guidelines from the Infectious Disease Society of American [42] and the 2011 guidelines from World Health Organization (WHO) [43], the recommended fluconazole dose was 400 mg/day for consolidation phase and 200 mg/day for maintenance phase in HIV-infected patients with cryptococcal infection. The 2018 WHO cryptococcal disease guidelines recommend 800 mg/day during consolidation therapy [3]. Higher doses up to 1600 mg or 2000 mg/day may be preferred if an alternative therapy to amphotericin B and flucytosine is necessary in the induction phase. With asymptomatic cryptococcal antigenemia, the 2010 IDSA recommendation is fluconazole 400 mg/day [42], whereas WHO recommends 800 mg/day [3]. Previous studies [17, 44, 45] have shown that high dose fluconazole up to 2000 mg/day is generally well tolerated. In an analysis of patients with coccidioidomycosis receiving high dose fluconazole, the cumulative incidence of the adverse effects was approximately 20% through 8 weeks and continued to increase with the longer durations of therapy [46]. Common adverse effects observed with a higher dose of fluconazole include dry skin (17%), alopecia (16%), fatigue (11%), nausea (10%), hepatic transaminitis (6%), cheilitis (5%), and isolated alkaline phosphatase elevation (2%) [45, 46]. Some degree of nausea is common with fluconazole doses at or above 800 mg/day and generally can be decreased by splitting the dose twice daily. Another potential concern would be increasing drug to drug interactions, historically a concern with nevirapine, yet there are few data on dose dependency of drug to drug interactions.
The IDSA guidelines also recommended against in vitro susceptibility testing for two main reasons. The first reason is that the MIC breakpoint has not been validated well, and the second reason is the low resistance rate. Although historically accurate, triazole fungicide use in agriculture has increased exponentially worldwide, including in Sub-Saharan Africa. Tebuconazole, a commonly used agricultural triazole fungicide, readily induces cross-resistance to fluconazole in Cryptococcus [47], and it is possible that agricultural azole use may be why Cryptococcus MICs appear to be increasing per our systematic review. Our study reports 11% of isolates to have MICs >8 µg/mL, suggesting current dose recommendations are not adequate for all instances of Cryptococcus infection. The predominant focus on induction therapy mostly has ignored dosing considerations for preemptive therapy for asymptomatic CrAg-positive persons, consolidation phase of meningitis therapy, and secondary prophylaxis.
Some recommended fluconazole monotherapy doses for asymptomatic cryptococcal antigenemia potentially are subtherapeutic, based on our modeling. Among persons with cryptococcal meningitis, mortality continues to occur during consolidation therapy [48, 49], and relapse occurs in approximately 5% of cryptococcal patients [14, 50]. Although this morbidity and mortality may be viewed as expected, using subtherapeutic antifungal regimens may be adversely contributing in addition to the complexities of HIV care. Those with nonsterile Cerebrospinal fluid (CSF) at 2 weeks historically have been at clear risk of excess 10-week mortality and paradoxical immune reconstitution inflammatory syndrome [49, 51, 52]. Since 2010 in Uganda, we have used an enhanced consolidation therapy of 800 mg/day through 6 weeks and ART initiation or switch [48], and others have used 800 mg/day through 10 weeks [53]. The duration of this enhanced dosing still may be too short and secondary prophylaxis dosing too low depending on the efficacy of the induction regimen used.
Although immune reconstitution with ART may obscure overt culture-positive relapse, the failure to eradicate Cryptococcus may contribute to paradoxical immune reconstitution inflammatory syndrome (IRIS) [52], including persons having higher serum CrAg titers at the time of starting ART (at 4 weeks) being at higher risk [54]. In Uganda, as we have increased our fluconazole consolidation therapy, our incidence of paradoxical IRIS decreased from 45% with 400 mg/day to <15% with 800 mg/day [14, 48, 54].
Based on the given Cryptococcus MIC, our model predicted rational fluconazole doses for consolidation therapy and preemptive therapy based on the proportion of persons with >100 AUC:MIC. Given the current population MIC distribution, the recommended consolidation dose of 800 mg will yield ~74% of persons with >100 AUC:MIC ratio. Yet, in dropping to 400 mg, only ~53% would achieve >100 AUC:MIC ratio. The original rationale for decreasing preemptive therapy for cryptococcal antigenemia to 400 mg after 2 weeks was the potential drug to drug interaction with nevirapine. Nevirapine now is used rarely, and fluconazole 800 mg/day or 1200 mg/day may be a more effective choice with uptrending of MICs. We found that the appropriate dose for MIC ≥8 µg/mL likely may be higher than 800 mg, and local susceptibility patterns may inform national guidelines. Similarly, in secondary prophylaxis, a customized fluconazole dose likely should be considered for MIC of 8 µg/mL (400 mg), 16 µg/mL (800 mg), 32 µg/mL (1200 mg), and 64 µg/mL (1600 mg), or alternative therapy used; however, in low and middle income countries where there is no alternative therapy [55], recommending use of itraconazole or voriconazole is not particularly helpful. We provide some considerations of rational fluconazole dosing for consolidation therapy and secondary prophylaxis in the absence of alternative therapies (Table 3).
Our study is a large systematic review that compiled the MIC distribution from more than 10 000 clinical isolates over the past 2 decades and predicted the customized fluconazole dose for the population-based MIC target, and our study has some limitations. First, the fluconazole concentrations were obtained from 1 clinical study that included 92 HIV-infected Ugandans that used the linear kinetic model for predicting the exposure from different doses. Our steady plasma concentrations were within 10%–20% of observations in US, Vietnamese, and other Ugandan patients [12, 17]. Second, the timing of the fluconazole level draw was variable, which either could under- or overestimate the AUC calculation, but multiple samples were averaged. Third, fungal susceptibility testing is fraught with challenges, including reproducibility as well as inoculum effects where in vivo susceptibility may differ from standardized in vitro susceptibility testing. Finally, the exact target AUC above MIC ratio for consolidation therapy is unknown, and we have made an arbitrary approximation. Human data and excellent modeling has been conducted for fluconazole induction therapy [12], yet consolidation therapy recommendations has been derived mostly from expert opinion. Based on historical experience, the doses of fluconazole used during consolidation have been less than that used for induction fluconazole monotherapy. Even now, data are limited in the selection of consolidation therapy doses with a lack of clinical studies demonstrating strong associations between suboptimal fluconazole AUC:MIC ratio and treatment failure [56]. This is an area of further research, requiring adequate sample sizes.
In conclusion, we found that MIC distribution of Cryptococcus species to fluconazole was normally distributed and there was high incident (11%) of fluconazole-“resistant” Cryptococcus. Moreover, the standard fluconazole doses developed in the 1990s may no longer be sufficient based on increasing fluconazole MIC. Consolidation therapy and preemptive therapy doses should likely be routinely increased to at least 800 mg/day. Using low dose fluconazole at 100 mg/day for preemptive therapy in asymptomatic CrAg-positive persons does not make rational sense, based on our current understanding of fluconazole pharmacokinetics. Clinical studies using pharmacokinetics of fluconazole and MIC distribution linked to outcomes are needed to justify the rational fluconazole dose selection. Testing Cryptococcus susceptibility may allow a rational selection of fluconazole dose, especially in resource-limited settings, where alternative antifungal options are limited.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the support from the National Institute of Neurologic Diseases and Stroke (R01NS086312) and Fogarty International Institute (K01TW010268) and the National Institute of Allergy and Infectious Diseases (U01AI125003, K23AI138851, K08AI134262).
Potential conflicts of interest. There are no conflicts of interest by any of the authors. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Durski KN, Kuntz KM, Yasukawa K, et al. Cost-effective diagnostic checklists for meningitis in resource-limited settings. J Acquir Immune Defic Syndr 2013; 63:e101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. http://www.who.int/hiv/pub/guidelines/cryptococcal-disease/en/. Published March 2018. Accessed April 1, 2018. [PubMed] [Google Scholar]
- 4. Cheong JW, McCormack J. Fluconazole resistance in cryptococcal disease: emerging or intrinsic? Med Mycol 2013; 51:261–9. [DOI] [PubMed] [Google Scholar]
- 5. Aller AI, Martin-Mazuelos E, Lozano F, et al. Correlation of fluconazole MICs with clinical outcome in cryptococcal infection. Antimicrob Agents Chemother 2000; 44:1544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brandt ME, Pfaller MA, Hajjeh RA, et al. Trends in antifungal drug susceptibility of Cryptococcus neoformans isolates in the United States: 1992 to 1994 and 1996 to 1998 . Antimicrob Agents Chemother 2001; 45: 3065–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Espinel-Ingroff A, Aller AI, Canton E, et al. Cryptococcus neoformans-Cryptococcus gattii species complex: an international study of wild-type susceptibility endpoint distributions and epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole. Antimicrob Agents Chemother 2012; 56:5898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casadevall A, Spitzer ED, Webb D, Rinaldi MG. Susceptibilities of serial Cryptococcus neoformans isolates from patients with recurrent cryptococcal meningitis to amphotericin B and fluconazole. Antimicrob Agents Chemother 1993; 37:1383–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bongomin F, Oladele RO, Gago S, et al. A systematic review of fluconazole resistance in clinical isolates of Cryptococcus species. Mycoses 2018; 61:290–7. [DOI] [PubMed] [Google Scholar]
- 10. Sudan A, Livermore J, Howard SJ, et al. Pharmacokinetics and pharmacodynamics of fluconazole for cryptococcal meningoencephalitis: implications for antifungal therapy and in vitro susceptibility breakpoints. Antimicrob Agents Chemother 2013; 57:2793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alves IA, Staudt KJ, Carreño FO, et al. Population pharmacokinetic modeling to describe the total plasma and free brain levels of fluconazole in healthy and Cryptococcus neoformans infected rats: how does the infection impact the drug’s levels on biophase? Pharm Res 2018; 35:132. [DOI] [PubMed] [Google Scholar]
- 12. Stott KE, Beardsley J, Kolamunnage-Dona R, et al. Population pharmacokinetics and cerebrospinal fluid penetration of fluconazole in adults with cryptococcal meningitis. Antimicrob Agents Chemother 2018; 62:e00885-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rhein J, Hullsiek KH, Tugume L, et al. Adjunctive sertraline in HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect Dis 2019; 19:843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rhein J, Morawski BM, Hullsiek KH, et al. ; ASTRO-CM Study Team Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect Dis 2016; 16:809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rhein J, Nielsen K, Boulware DR, Meya DB. Sertraline for HIV-associated cryptococcal meningitis - Authors’ reply. Lancet Infect Dis 2016; 16:1111–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Humphrey MJ, Jevons S, Tarbit MH. Pharmacokinetic evaluation of UK-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob Agents Chemother 1985; 28:648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anaissie EJ, Kontoyiannis DP, Huls C, et al. Safety, plasma concentrations, and efficacy of high-dose fluconazole in invasive mold infections. J Infect Dis 1995; 172:599–602. [DOI] [PubMed] [Google Scholar]
- 18. Shiba K, Saito A, Miyahara T. Pharmacokinetic evaluation of fluconazole in healthy volunteers. Jpn J Antibiot 1989; 42:17–30. [PubMed] [Google Scholar]
- 19. Rajasingham R, Meya DB, Boulware DR. Are fluconazole or sertraline dose adjustments necessary with concomitant rifampin? HIV Med 2018; 19:e64–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Debruyne D, Ryckelynck JP. Clinical pharmacokinetics of fluconazole. Clin Pharmacokinet 1993; 24:10–27. [DOI] [PubMed] [Google Scholar]
- 21. Cogliati M, Prigitano A, Esposto MC, et al. Epidemiological trends of cryptococcosis in Italy: molecular typing and susceptibility pattern of Cryptococcus neoformans isolates collected during a 20-year period. Med Mycol 2018; 56:963–71. [DOI] [PubMed] [Google Scholar]
- 22. Kassi FK, Bellet V, Drakulovski P, et al. Comparative typing analyses of clinical and environmental strains of the Cryptococcus neoformans/Cryptococcus gattii species complex from Ivory Coast. J Med Microbiol 2018; 67:87–96. [DOI] [PubMed] [Google Scholar]
- 23. Worasilchai N, Tangwattanachuleeporn M, Meesilpavikkai K, et al. Diversity and antifungal drug susceptibility of Cryptococcus isolates in Thailand. Med Mycol 2017; 55:680–5. [DOI] [PubMed] [Google Scholar]
- 24. Gago S, Serrano C, Alastruey-Izquierdo A, et al. Molecular identification, antifungal resistance and virulence of Cryptococcus neoformans and Cryptococcus deneoformans isolated in Seville, Spain. Mycoses 2017; 60:40–50. [DOI] [PubMed] [Google Scholar]
- 25. Córdoba S, Isla MG, Szusz W, et al. Susceptibility profile and epidemiological cut-off values of Cryptococcus neoformans species complex from Argentina. Mycoses 2016; 59:351–6. [DOI] [PubMed] [Google Scholar]
- 26. Smith KD, Achan B, Hullsiek KH, et al. ; ASTRO-CM/COAT Team Increased antifungal drug resistance in clinical isolates of Cryptococcus neoformans in Uganda. Antimicrob Agents Chemother 2015; 59:7197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Wyk M, Govender NP, Mitchell TG, Litvintseva AP; GERMS-SA Multilocus sequence typing of serially collected isolates of Cryptococcus from HIV-infected patients in South Africa. J Clin Microbiol 2014; 52:1921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morales BP, Junior IN, Trilles L, et al. Determination of the minimum inhibitory concentration of Cryptococcus neoformans and Cryptococcus gattii against fluconazole by flow cytometry. Med Mycol 2014; 52:90–8. [DOI] [PubMed] [Google Scholar]
- 29. Saag MS, Cloud GA, Graybill JR, et al. National Institute of Allergy and Infectious Diseases Mycoses Study Group. A comparison of itraconazole versus fluconazole as maintenance therapy for AIDS-associated cryptococcal meningitis. Clin Infect Dis 1999; 28:291–6. [DOI] [PubMed] [Google Scholar]
- 30. Govender NP, Patel J, van Wyk M, Chiller TM, Lockhart SR. Trends in antifungal drug susceptibility of Cryptococcus neoformans isolates obtained through population-based surveillance in South Africa in 2002–2003 and 2007–2008. Antimicrob Agents Chemother 2011; 55:2606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pfaller MA, Castanheira M, Diekema DJ, et al. Wild-type MIC distributions and epidemiologic cutoff values for fluconazole, posaconazole, and voriconazole when testing Cryptococcus neoformans as determined by the CLSI broth microdilution method. Diagn Microbiol Infect Dis 2011; 71:252–9. [DOI] [PubMed] [Google Scholar]
- 32. Mdodo R, Moser SA, Jaoko W, et al. Antifungal susceptibilities of Cryptococcus neoformans cerebrospinal fluid isolates from AIDS patients in Kenya. Mycoses 2011; 54:e438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iqbal N, DeBess EE, Wohrle R, et al. ; Cryptococcus gattii Public Health Working Group Correlation of genotype and in vitro susceptibilities of Cryptococcus gattii strains from the Pacific Northwest of the United States. J Clin Microbiol 2010; 48:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsumoto MT, Fusco-Almeida AM, Baeza LC, et al. Genotyping, serotyping and determination of mating-type of Cryptococcus neoformans clinical isolates from São Paulo State, Brazil. Rev Inst Med Trop Sao Paulo 2007; 49:41–7. [DOI] [PubMed] [Google Scholar]
- 35. Bii CC, Makimura K, Abe S, et al. Antifungal drug susceptibility of Cryptococcus neoformans from clinical sources in Nairobi, Kenya. Mycoses 2007; 50:25–30. [DOI] [PubMed] [Google Scholar]
- 36. Serena C, Fernández-Torres B, Pastor FJ, et al. In vitro interactions of micafungin with other antifungal drugs against clinical isolates of four species of Cryptococcus. Antimicrob Agents Chemother 2005; 49:2994–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pfaller MA, Messer SA, Boyken L, et al. Global trends in the antifungal susceptibility of Cryptococcus neoformans (1990 to 2004). J Clin Microbiol 2005; 43:2163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Witt MD, Lewis RJ, Larsen RA, et al. Identification of patients with acute AIDS-associated cryptococcal meningitis who can be effectively treated with fluconazole: the role of antifungal susceptibility testing. Clin Infect Dis 1996; 22:322–8. [DOI] [PubMed] [Google Scholar]
- 39. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antifungal Susceptibility Testing of Yeasts. 1st ed. CLSI supplement M60. Wayne, PA: CLSI; 2017. [Google Scholar]
- 40. Rajasingham R, Rolfes MA, Birkenkamp KE, et al. Cryptococcal meningitis treatment strategies in resource-limited settings: a cost-effectiveness analysis. PLoS Med 2012; 9:e1001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beyene T, Zewde AG, Balcha A, et al. Inadequacy of high-dose fluconazole monotherapy among cerebrospinal fluid cryptococcal antigen (CrAg)-positive human immunodeficiency virus-infected persons in an Ethiopian CrAg screening program. Clin Infect Dis 2017; 65:2126–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010; 50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. World Health Organization. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. www.who.int/hiv/pub/cryptococcal_disease2011. Published December 2011. Accessed January 1, 2017. [PubMed] [Google Scholar]
- 44. Zhao HZ, Wang RY, Wang X, et al. High dose fluconazole in salvage therapy for HIV-uninfected cryptococcal meningitis. BMC Infect Dis 2018; 18:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Milefchik E, Leal MA, Haubrich R, et al. Fluconazole alone or combined with flucytosine for the treatment of AIDS-associated cryptococcal meningitis. Med Mycol 2008; 46:393–5. [DOI] [PubMed] [Google Scholar]
- 46. Davis MR, Nguyen MH, Donnelley MA, Thompson Iii GR. Tolerability of long-term fluconazole therapy. J Antimicrob Chemother 2019; 74:768–71. [DOI] [PubMed] [Google Scholar]
- 47. Bastos RW, Carneiro HCS, Oliveira LVN, et al. Environmental triazole induces cross-resistance to clinical drugs and affects morphophysiology and virulence of Cryptococcus gattii and C. neoformans. Antimicrob Agents Chemother 2018; 62:e01179-01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rolfes MA, Rhein J, Schutz C, et al. Cerebrospinal fluid culture positivity and clinical outcomes after amphotericin-based induction therapy for cryptococcal meningitis. Open Forum Infect Dis 2015; 2:ofv157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jarvis JN, Bicanic T, Loyse A, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated Cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 2014; 58:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boulware DR, Meya DB, Muzoora C, et al. ; COAT Trial Team Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van der Horst CM, Saag MS, Cloud GA, et al. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N Engl J Med 1997; 337:15–21. [DOI] [PubMed] [Google Scholar]
- 52. Chang CC, Dorasamy AA, Gosnell BI, et al. Clinical and mycological predictors of cryptococcosis-associated Immune reconstitution inflammatory syndrome (C-IRIS). AIDS 2013; 27:2089–2099. [DOI] [PubMed] [Google Scholar]
- 53. Molloy SF, Kanyama C, Heyderman RS, et al. ; ACTA Trial Study Team Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med 2018; 378:1004–17. [DOI] [PubMed] [Google Scholar]
- 54. Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLOS Med 2010; 7:e1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kneale M, Bartholomew JS, Davies E, Denning DW. Global access to antifungal therapy and its variable cost. J Antimicrob Chemother 2016; 71:3599–606. [DOI] [PubMed] [Google Scholar]
- 56. Manosuthi W, Chetchotisakd P, Nolen TL, et al. ; BAMSG 3-01 Study Team Monitoring and impact of fluconazole serum and cerebrospinal fluid concentration in HIV-associated cryptococcal meningitis-infected patients. HIV Med 2010; 11:276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.