Abstract
Purpose
To explore if bexarotene (BEX) synergistically enhances docetaxel (DTX) cytotoxicity in castration-resistant prostate cancer cell lines.
Materials and methods
MTT assay was used to measure the cytotoxic effect of DTX and BEX on castration-resistant prostate cancer (CRPC) cell proliferation and the combination index (CI) values calculated to analyze the interaction between DTX and BEX. Flow cytometry and Western blot analysis identified the underlying mechanism for the synergistic effect of BEX and DTX.
Results
When mitotic slippage happens, BEX can synergistically strengthen the anti-proliferation of DTX in a way of significantly down-regulating cyclinB1 and CDK1 expression, and then arresting cells in G2 phase.
Conclusion
Results from this study showed that BEX-induced G2 arrest and DTX-induced mitotic arrest probably contributed to the synergistic effect of BEX and DTX.
Keywords: docetaxel, bexarotene, prostate cancer, combination therapy, cell cycle arrest
Introduction
Prostate cancer (PCa) is the most prevalent cancer in males and has become the third major cause of cancer-related death in men in the United States, with an estimated 161,360 new cases, and 16,730 deaths in 2017.1 Androgen deprivation therapy (ADT) is the cornerstone for treating metastatic prostate cancer. Nevertheless, a substantial proportion of patients who initially responded to ADT would inevitably develop castration-resistant prostate cancer (CRPC),2 and warrant the development of other CRPC treatment approaches. In 2004, docetaxel (DTX) was approved by the US Food and Drug Administration as a first-line treatment for CRPC. However, dose-related DTX toxicities always limit its clinical use, and most CRPC patients will ultimately develop resistance to chemotherapy. Combination therapy of DTX with other agents has demonstrated its potential to reduce dose-related toxicities and maintain or even enhance efficacy.3
Retinoid X receptors (RXRs), a type of intracellular receptors of retinoids, serve as transcriptional factors via homodimerization or heterodimerization with multiple nuclear receptors, including retinoic acid receptors and peroxisome proliferator-activated receptors. RXRs thus have a significant role in regulating tumor cell proliferation, differentiation, and apoptosis.4 Bexarotene (BEX) is a synthetic retinoid analog that binds specifically and activates RXRs and approved for treating cutaneous T-cell lymphoma (CTCL). Hypertriglyceridemia, hypercholesterolemia, and hypothyroidism, significant side-effects of BEX are manageable and reversible with corresponding therapy. Considering the nonoverlapping adverse-effect profile of BEX with most cytotoxic agents, it is used frequently in combination with most chemotherapy drugs. A phase I trial of 15 patients identified a combination of 25 mg/m2/week DTX and 400 mg/m2/day BEX as the maximum dose in several solid tumors. Three of 10 non-small-cell lung carcinoma (NSCLC) patients and one angiosarcoma patient showed stable disease.5 Another two randomized phase III trials showed that BEX was well tolerated in advanced NSCLC patients when added to the traditional chemotherapy (cisplatin/vinorelbine or carboplatin/paclitaxel). Although BEX, in combination with chemotherapy, failed to prolong survival in advanced NSCLC, a subgroup of patients who suffered severe hypertriglyceridemia in the BEX treatment group showed prolonged survival with BEX.6–8 Previously, Yen et al (2006) showed that low-dose BEX (1 µM) exposure reduced the spontaneous mutation rate and thus effectively delayed the development of resistance to chemotherapy drugs (paclitaxel, doxorubicin, and cisplatin) in PC3 cells. Moreover, the addition of BEX desensitized the resistant cells to the cytotoxic agents.9–11 However, the potential role of BEX and DTX combination regime in treating CRPC is not thoroughly addressed.
This study aimed to explore the ability of BEX to enhance DTX therapeutic effects in CRPC. We showed synergistic cytotoxicity of BEX and DTX in PC3 and DU145 CRPC cell lines. Following prolonged DTX-induced mitosis arrest, some prostate cancer cells “slip” out of mitosis and this event can be related to drug resistance.12,13 We revealed that when mitotic slippage happens, BEX can synergistically strengthen the anti-proliferation of DTX in a way of significantly down-regulating cyclinB1 and CDK1 expression, subsequently arresting cells in G2 phase.
Materials and methods
Cell lines and reagents
The human PCa androgen-independent cell lines PC3 and DU145 were from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences. The cells were cultured in RPMI-1640 media supplemented with 10% fetal bovine serum (Cellmax, Peking, China) and penicillin-streptomycin. The cells were incubated at 37 °C and 5% CO2 atmosphere. DTX was obtained from Jiangsu Hengrui Medicine Company (Jiangsu, China) and stored at 4 °C in the dark. BEX was from the Ligand Pharmaceuticals Inc. (San Diego, CA, USA.) and stored at −20 °C.
MTT assay
Cells (6×103/well) were seeded in 96-well plates and allowed to adhere overnight. Varying concentrations of DTX, BEX, and combination of drugs added to cells. After 24 and 48 h of incubation, one-tenth volume of MTT (5 mg/mL, dissolved in PBS buffer) was added to each well, and the plate incubated for another 2 h. The medium was removed, and 150 μL of dimethyl sulfoxide (DMSO) added to dissolve the formazan crystals, and absorbance measured at 550 nm in a microplate reader (Bio-Tek, VT, USA). The IC50 was defined as half-maximum inhibitory concentration, and all results calculated as percentages of controls.
Cell cycle
Analysis of cell cycle profile was by using the Cell Cycle Staining Kit (Multi Science, Hangzhou, China) following the manufacturer’s instructions. Cells were seeded in six-well plates and treated with DTX (5 nΜ or 10 nΜ) and BEX (20 and 40 μM) alone or with a combination of drugs for 24 or 48 h. Cells were harvested, washed with PBS, and resuspended in 500 μL DNA staining solution containing 5 μL permeabilization solution. Cells were incubated in the dark for 30 min, and cell cycle analysis performed using FACSCalibur (BD, NJ, USA).
Apoptosis
Cell apoptosis was analyzed using the Cell Apoptosis Kit (Multi Science, Hangzhou, China) following the manufacturer’s instructions. Cells were seeded in six-well plates and treated with DTX (5 nΜ or 10 nΜ) and BEX (20 and 40 μM) alone or with a combination of drugs for 24 and 48 h. Cells were then harvested, washed with PBS, and resuspended in 500 μL binding buffer with 5 μL Annexin V-FITC and 10 μL PI. Cells were incubated in the dark for 5 min, and cell apoptosis analysis was performed using FACSCalibur (BD, NJ, USA).
Western blot
Cells after treatment were washed with cold PBS and lysed on ice for 10 min in cell lysis buffer (Beyotime, Shanghai, China) containing PMSF (FDbio-tech, Hangzhou, China) and protease inhibitor cocktail (Biotool, Huston, USA). Lysates collected and centrifuged at 12,000× g for 20 min. Protein concentrations quantified by BCA Protein Assay Kit (Thermo Fisher, MA, USA), and 20 µg total protein separated on SDS/PAGE gels and subsequently transferred to polyvinylidene difluoride membranes. The membranes were blocked in 5% nonfat milk at room temperature for 1 h. Afterward, the membranes were incubated overnight at 4 °C with primary antibodies for anti-cyclinD1, anti-cyclinE2, anti-cyclinB1, anti-CDK1, anti–phospho-histone H3 (Cell Signal Technology, MA, USA), and GAPDH (Abcam, MA, USA), and followed by incubation with appropriate horseradish peroxidase-conjugated secondary antibodies (anti-mouse IgG, HRP-linked antibody and anti-rabbit IgG, and HRP-linked antibody) (Cell Signal Technology, MA, USA) for 2 h at room temperature. The hybridization signals detected using an Enhanced Chemiluminescence Detection kit (Fdbio-tech, Hangzhou, China).
Statistical analyses
The results presented as the mean ± SD of at least three independent experiments. Student’s t-test and compared the means. p-values, <0.05 considered statistically significant.
Results
Effect of BEX and DTX alone or in combination on cell viability
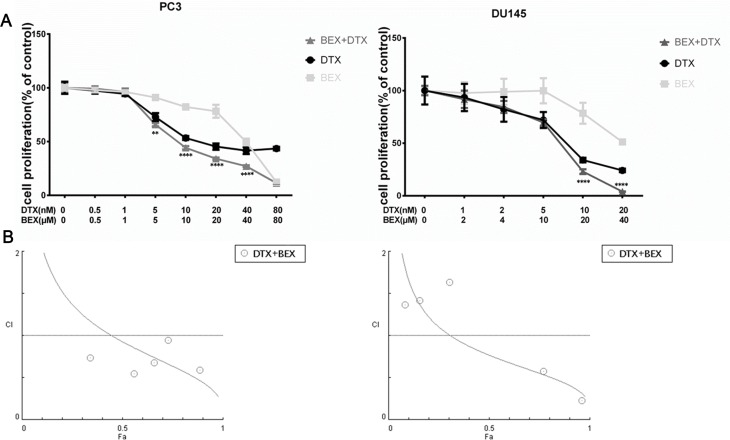
MTT assay was used to investigate the effects of DTX and BEX on PC3 and DU145 cell proliferation. We used sequential DTX and BEX doses singly or simultaneously to evaluate their antitumor efficacy and explore whether they can act synergistically. As shown in Figure 1A, DTX inhibited cells in a dose-dependent manner, and the IC50 of DTX in PC3 and DU145 were 22.83±3.49 nM and 6.65±1.45 nM, respectively. BEX monotherapy of cells showed an antiproliferative effect at a high dose, and the IC50s were 40.62±0.45 µM (PC3) and 50.20±4.10 µM (DU145). The antiproliferative effects of DTX and BEX combination were evaluated. In DU145 cells, a significant reduction in cell viability was observed after adding either 20 or 40 µM BEX to 5 nM and 10 nM DTX; in PC3 cells, only 40 µM BEX enhanced the effect of DTX (Figure 1B). Based on the above IC50s, we selected a DTX concentration of 10 nM and 5 nM; BEX concentration of 20 and 40 µM for PC3 and DU145 cells respectively for further analysis. To characterize the interaction between DTX and BEX, we analyzed the combination index (CI) values using Chou and Talalay method, based on the median-effect principle and widely accepted for characterizing drug interactions.14,15 We treated PC3 and DU145 cells with BEX and DTX alone or in combination at a ratio of 1:1000 (PC3) or 1:2000 (DU145) (Figure 2A) and calculated the CI values at different growth inhibition (Fa) levels using CompuSyn software. CI values of >1 indicate antagonism, =1 indicate additivity, and <1 show synergy. Figure 2B shows that in PC3 cells, CI<1 seen when Fa>45%; in DU145 cells, CI<1 observed when Fa>35%. For anticancer agents, synergism at a high Fa level is more relevant to therapy than synergy at a low Fa level. Conclusively, DTX, and BEX exhibited a synergistic effect in inhibiting PC3 and DU145 cell viability.
Figure 1.
Effects of BEX and DTX alone or in combination on cell viability. (A). Dose-response curves of DTX and BEX against PC3 and DU145 cells. (B). DTX and BEX exhibited a synergistic effect on the inhibition of PC3 and DU145 cell proliferation. The data represent the mean ± SD of at least three independent experiments (*p<0.01, **p<0.01, ***p<0.005, ****p<0.0001, Student’s t-test).
Figure 2.
CI plot for combinations of DTX and BEX in PC3 and DU145 cells. (A). PC3 and DU145 cells were treated with a sequential dose of BEX and DTX alone or in combination at a ratio of 1:1000 (PC3) or 1:2000 (DU145) for 48 h. Circles, BEX; square, DTX; triangle, BEX+DTX. (B). CI values at different levels of growth-inhibitory effect (Fa). CI =1, >1, and <1 indicate an additive, antagonistic, and synergistic effects, respectively; circles represent BEX: DTX combinations tested. The data represent the mean ± SD of at least three independent experiments. (**p<0.01, ****p<0.0001, Student’s t-test).
Effect of BEX on DTX-induced cell cycle arrest and cell apoptosis
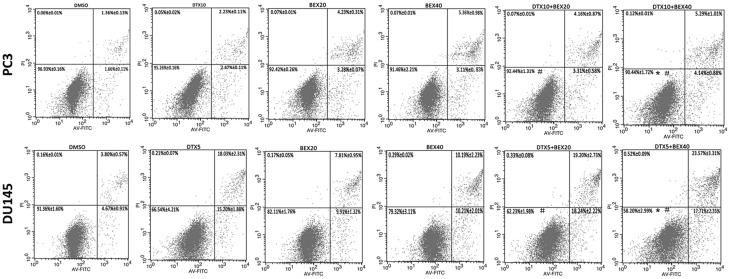
The therapeutic activity of DTX is due to a two-step mechanism: 1) stabilizing microtubules and triggering mitotic cell cycle arrest, and 2) inducing cell apoptosis.16 Similarly, previous studies showed that in CTCL cells, BEX exerted its antineoplastic effect by promoting cell cycle arrest (both G1/S arrest and G2/M arrest) and cell apoptosis.17–19 Given that, we hypothesized that BEX could enhance DTX-induced cell cycle arrest or cell apoptosis in CRPC cell lines. First, we used the Cell Cycle Staining Kit to assess DTX and BEX effects on the distribution of cell cycle phase in PC3 and DU145 cells. After 24 h treatment, DTX dramatically arrested CRPC cells in G2/M phase while BEX (40 µM) caused G1 arrest. Compared to DTX monotherapy, although combination treatment reduced DTX-induced G2/M arrest, it arrested more cells in G1 phase, the effect attributed to an additive effect of the different mechanisms of DTX and BEX (Figure 3A and C). After 48 h of treatment, BEX alone slightly arrested cells in G1 phase and DTX monotherapy arrested cells in G2/M phase in the two cell lines (Figure 3B and D). However, because of mitotic slippage, DTX-induced G2/M arrest was reduced significantly in PC3 cells, consistent with the previous study,20 and was prominent in DU145 cells, but also showed a slight decrease (Figure 3B). When compared with DTX monotherapy, concurrent treatment with BEX (20 µM and 40 µM) and DTX resulted in a more significant G2/M arrest in DU145 cells, indicating BEX had the potential to enhance the DTX-induced G2/M arrest. However, in PC3 cells, only 40 µM BEX triggered a statistically significant increase in G2/M arrest, consistent with the MTT assay (Figure 3B). Later, the apoptotic effect of DTX + BEX detected using Annexin V-FITC/APC staining. As shown in Figure 4, BEX monotherapy caused mild apoptosis in PC3 while in DU145, the induced apoptosis was stronger. Although, there were significant differences between DTX alone, and DTX +40 µM BEX, the increase in apoptotic cells is minimal. It appears that BEX failed to increase the pro-apoptotic effect of DTX. Together, the results mentioned above showed the synergistic antiproliferative effect of DTX and BEX likely mediated by an enhancement of G2/M arrest.
Figure 3.
Effects of BEX and DTX on cell cycle phase distribution. (A,B). PC3 were exposed to BEX (20 and 40 µM) and 10 nM DTX alone or in combination for 24h (A) or 48 h (B). (C,D). DU145 were exposed to BEX (20 and 40 µM) and 5 nM DTX alone or in combination for 24h (C) or 48 h (D). PC3 and DU145 cells were exposed to BEX (20 and 40 µM) and 10 nM (PC3) or 5 nM (DU145) DTX alone or in combination for 48 h. Cell cycle phase distribution determined by flow cytometry. The data represent the mean ± SD of at least three independent experiments (*p<0.05, **p<0.01, ***p<0.0005, Student’s t-test, #vs the DTX5 or DTX10 group.).
Figure 4.
Effects of BEX and DTX on cell apoptosis. PC3 and DU145 cells were exposed to BEX (20 and 40 µM) and 10 nM (PC3) or 5 nM (DU145) DTX alone or in combination for 48 h. Cell apoptosis determined by flow cytometry. The data represent the mean ± SD of at least three independent experiments. *p<0.05, Student's t-test, # vs. the DTX5 or DTX10 group.
Effect of BEX and DTX alone or in combination on the expression of cyclins and CDK1
Cyclin and cyclin-dependent kinases (CDKs) are a group of crucial regulators in cell cycle progression. Abnormal expression profile of these regulatory proteins will disrupt the transition between G1, S, and G2/M phases.21 We examined the expression of several cell-cycle-related proteins, such as cyclin D1, cyclin E2, CDK1, cyclin B1, and phospho-histone H3 (p-H3), using Western blot analysis to evaluate the synergistic effect of DTX and BEX. Figure 5 shows the similar expression pattern of these proteins in two cell lines. After 24 h of BEX treatment, cyclin D1, and cyclin E2 decreased while cyclin B1, CDK1, and p-H3 remained similar, consistent with BEX-induced G1 arrest (Figure 5A). Twenty-four hours after DTX treatment, cyclin B1 and p-H3 (markers of mitosis) increased markedly (Figure 5B), confirming cell cycle analysis results as well as results from previous studies showing that antimitotic compounds lead to mitotic arrest.22 After 48 h, BEX not only decreased the expression of cyclin D1 and cyclin E2 but repressed cyclin B1 and CDK1 expression, which was also obtained by N. Nieto-Rementerı´a.17 Cyclin B1 is a central regulator of progression from G2 to mitosis. It associates with CDK1 to form a complex that is essential for G2/M transition.23 Reduced levels of cyclin B leave the CDK1 inactive, which may contribute to cell cycle arrest in G2.24 However, according to results from the cell cycle analysis, it appeared that BEX monotherapy failed to arrest CRPC cells in G2 phase (Figure 3B). Forty-eight hours after DTX monotherapy, the expression of cyclin B1 and p-H3 decreased to the expression level in the control group in PC3 cells but remained overexpressed in DU145 cells (Figure 5B). It suggested that PC3 were reaching mitosis and subsequently undergoing mitotic slippage, which might potentially confer acquired resistance following DTX treatment,25,26 while slippage in DU145 cells was not distinct. This result was in absolute concordance with the cell cycle analysis results (Figure 3B and D). Interestingly, combined therapy led to a substantial downregulation in the expression of cyclinB1 and CDK1. The downregulation of cyclin B1 probably resulted from BEX, but could also contribute to mitotic slippage, because cyclin B1 falls below a threshold level that is required to maintain the mitotic state during mitotic slippage.26 Since the decrease in mitotic slippage resulted in downregulation of both cyclin B1 and p-H3 levels,20 BEX monotherapy, and combined therapy did not affect the expression of p-H3 (Figure 5B), indicating that the enhanced downregulation of cyclin B1 was probably not due to the mitotic slippage. Therefore, we considered that it was BEX that led to the suppression of cyclin B1 and CDK1 in combination therapy, and subsequent G2 phase arrest in cells. DTX also led to the downregulation of cyclinD1 in either PC3 or DU145 cells after 24 or 48 h of DTX treatment. Cyclin D1 is involved in G1/S transition,21 but the results of cell cycle analysis showed no DTX-induced G1/S arrest, suggesting an insignificant role for cyclinD1 in the combination treatment. The above data showed that although mitotic slippage hinders DTX cytotoxicity by promoting cells to prematurely exit mitotic arrest, BEX-induced repression of cyclin B1 and CDK1 would probably synergistically enhance the anti-proliferative activity of DTX by arresting cells in the G2 phase.
Figure 5.
Effects of BEX and DTX on cell cycle regulation of cyclins and CDK1. PC3 and DU145 cells were exposed to BEX (20 and 40 µM) and 10 nM (PC3) or 5 nM (DU145) DTX alone or in combination for 24h (A) or 48 h (B). The expression of cyclinD1, cyclinE2, cyclinB1, and CDK1 determined by Western blot analysis.
Discussion
DTX-based chemotherapy is a widely used treatment regimen for CRPC. However, long-term DTX administration results in toxicity represented by myelosuppression and drug resistance and eventually contributes to undesired clinical outcomes.27 Therefore, combination therapy has become a popular approach against DTX cytotoxicity and drug resistance.28–30 BEX has nonoverlapping adverse effect profile and previously shown to synergistically enhance the growth inhibition activity of DTX in breast cancer and NSCLC. BEX effectively prevents and overcomes DTX resistance in several tumors.9–11 In the current study, we showed the synergy between DTX and BEX via targeting the cell cycle regulators, thereby inhibiting proliferation in androgen-independent PCa cell lines, which in turn suppress the progression of CRPC.
The CI values were calculated in PC3 and DU145 cells to examine the interaction between DTX and BEX. When growth inhibition reached up to 50%, CI values of <1 were detected suggesting that DTX and BEX exhibited a synergistic effect on inhibiting PC3 and DU145 cells proliferation. Considering both DTX and BEX had the potential to induce cell-cycle arrest and cell apoptosis, we hypothesized that the underlying mechanism of synergism is due to the enhancement of cell-cycle arrest or cell apoptosis. Our data indicated that BEX-induced G2 arrest and DTX-induced mitotic arrest contributed to the synergistic therapeutic effects of BEX and DTX.
Cell cycle progression is positively governed by cyclins (D, E, A, and B)/CDKs (CDK4, CDK6, CDK2, and CDK1) complex, which mediate DNA replication, cell division, and cell proliferation. G1, S, G2/M are three checkpoints controlling the cell cycle progression in cancer cells.21 As mentioned above, previous studies showed that both DTX and BEX induce cell cycle arrest in cancer cells. One study using three CTCL cell lines proposed that BEX exerted its antineoplastic activity via induction of apoptosis rather than cell cycle arrest.19 However, Rementeria et al considered the cell cycle as the main target of BEX. It was documented earlier that BEX activated the p53/p73 pathway by phosphorylating ataxia telangiectasia-mutated protein, and also modulated several downstream target genes, like cyclinB1, CDK1, and Bax, ultimately leading to G1/S and G2/M arrest in malignant cells.17 In addition, BEX repressed cyclin D1 and cyclin D3 expression in NSCLC.31,32 Consistent with the literature, our study confirmed the inhibitory effect of BEX on the expression of cyclinD1, cyclinB1, and CDK1, suggesting that BEX repressed cyclin D1 and cyclin E2 after 24 h, but observed inhibition of cyclin B1 and CDK1 expression only after 48 h. The above findings explain the underlying mechanism of BEX arrest of CRPC cells in G1/S over 24 h and its role in enhancing the DTX effect by arresting cells in the G2 phase over 48 h. Beyond that, our data showed that BEX could also significantly inhibit the expression of cyclinE2 in CRPC cell lines, which partly gave rise to the G1 arrest induced by BEX monotherapy. Taxanes (paclitaxel, docetaxel) bind to tubulin and promote tubulin polymerization, which interferes with the function of the mitotic spindle resulting in mitotic arrest.33 However, following long-term mitotic arrest, cells exit mitosis by slippage into a tetraploid G1 state, from which they either die, arrest in G1, or initiate a new round of the cell cycle, and this event could be related to paclitaxel resistance.12,34 It was described that PC3 cells reached mitosis arrest after 24 h of paclitaxel treatment, but most cells would exit from mitosis by slippage, also observed in our study.20 As seen from the results of cell cycle analysis and Western blot, DU145 cells maintained a more efficient mitotic arrest and underwent less mitotic slippage, suggesting they are more sensitive to DTX. When compared to DTX monotherapy, combination therapy with DTX and BEX caused a more pronounced reduction in cyclinB1 and CDK1, while the expression of p-H3 was unchanged. The upregulation in cyclin B and p-H3, two markers of DTX-induced mitotic arrest and mitotic slippage, showed a decrease in their expression. As a result, we inferred that the reduction in cyclin B1 and CDK1 levels was caused by BEX, leading to G2 arrest. As explained above, the synergistic effect of DTX and BEX was likely due to the combination of DTX-induced mitotic arrest and BEX-induced G2 arrest. Of note, in PC3 cells, 20 µM BEX failed to impose a synergistic arrest on G2/M phase when combined with 10 nM DTX, explained by the fact that CDK1 increased slightly with 10 nM DTX and 20 µM BEX combination compared with 10 nM DTX monotherapy.
Cell apoptosis was unrelated to the synergistic effect between DTX and BEX, and DTX exerts its proapoptotic effect by inducing Bcl-2 phosphorylation or downregulation of Bcl-xL, thereby subsequently promoting apoptosis.16 Previous studies showed that BEX upregulated the expression of Bax and induced a redistribution of Bax from the cytosol to the mitochondrial.17 Theoretically, as both mechanisms reinforce the function of Bax, the combination therapy may have resulted in an enhancement of cell apoptosis. However, practically, BEX failed to enhance the proapoptotic effect of DTX.
One of the limitations of this study is that we have not conducted in vivo experiments to validate the efficacy of BEX and DTX combination therapy in CRPC. Further, in vivo studies are warranted to elucidate the synergistic effects of BEX and DTX. In summary, our study provides a theoretical basis for BEX improving the therapeutic outcomes of DTX in CRPC via enhancing G2/M arrest. A combination of DTX and BEX has the potential to be a new approach to battle CRPC in the future, and further studies are warranted.
Conclusion
The present study identified the ability of BEX to enhance the therapeutic effects of DTX in CRPC by synergistic inhibition of cyclinB1 and CDK1 expression levels. Based on these results, combination therapy with DTX and BEX may become a new therapeutic strategy to battle CRPC in the future. However, additional animal and patient studies are required to validate this strategy.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers: 81773789, 81672520LI, 81870484, 81702504, 81702508); Zhejiang Provincial Natural Science Foundation of China (grant numbers: LY17H160020, LY17H050003); Zhejiang Science and Technology Project (grant number: 2017C33058) and Zhejiang Medical and Health Plan Project (grant numbers: 2019ZD007, 2019KY413).
Author contributions
Danyang Shen offered constructive ideas and came up with the research hypothesis. Danyang Shen, Huan Wang, and Qiming Zheng formulated the methodology and plan. Danyang Shen and Liqun Xia organized and supervised the project and were also involved in manuscript writing; they were responsible for the study as a whole. Li Q Xia and Gong H Li provided personnel support, environmental, tools, financial support, and instruments vital for the project. Danyang Shen and Qiming Zheng procured the biological materials and reagents. Danyang Shen, Sheng Cheng, Liwei Xu, and Mingchao Wang executed the experiments and also handled data management and reporting. Huan Wang and Qiming Zheng were responsible for data interpretation and presentation of the results. Taking responsibility in this necessary function have been provided by Sheng Cheng and Liwei Xu. Dangyang Shen was responsible for writing the manuscript. Li Q Xia, Gong H Li, and Mingchao Wang reviewed the article before submission for spelling, grammar, and intellectual content. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Ali ZS, Mengesha T, Allaqaband S, Jahangir A. Androgen deprivation therapy for prostate cancer and incident atrial fibrillation. J Am Coll Cardiol. 2018;71(11):378. doi: 10.1016/S0735-1097(18)30919-7 [DOI] [Google Scholar]
- 3.McKeage K. Docetaxel A review of its use for the first-line treatment of advanced castration-resistant prostate cancer. Drugs. 2012;72(11):1559–1577. doi: 10.2165/11209660-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 4.Minucci S, Leid M, Toyama R, et al. Retinoid X receptor (RXR) within the RXR-retinoic acid receptor heterodimer binds its ligand and enhances retinoid-dependent gene expression. Mol Cell Biol. 1997;17(2):644–655. doi: 10.1128/mcb.17.2.644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wildi JD, Baggstrom MQ, Suresh R, Read W, Fracasso PM, Govindan R. A phase I study of docetaxel and bexarotene. Chemotherapy. 2008;54(2):125–130. doi: 10.1159/000119706 [DOI] [PubMed] [Google Scholar]
- 6.Tyagi P. Bexarotene in combination with chemotherapy fails to prolong survival in patients with advanced non-small-cell lung cancer: results from the SPIRIT I and II trials. Clin Lung Cancer. 2005;7(1):17–19. doi: 10.1016/S1525-7304(11)70385-0 [DOI] [PubMed] [Google Scholar]
- 7.Blumenschein GR, Khuri FR, von Pawel J, et al. Phase III trial comparing carboplatin, paclitaxel, and bexarotene with carboplatin and paclitaxel in chemotherapy-naive patients with advanced or metastatic non-small-cell lung cancer: SPIRIT II. J Clin Oncol. 2008;26(11):1879–1885. doi: 10.1200/JCO.2007.12.2689 [DOI] [PubMed] [Google Scholar]
- 8.Ramlau R, Zatloukal P, Jassem J, et al. Randomized phase III trial comparing bexarotene (L106949)/cisplatin/vinorelbine with cisplatin/vinorelbine in chemotherapy-naive patients with advanced or metastatic non-small-cell lung cancer: SPIRIT I. J Clin Oncol. 2008;26(11):1886–1892. doi: 10.1200/JCO.2007.12.2614 [DOI] [PubMed] [Google Scholar]
- 9.Yen WC, Lamph WW. A selective retinoid X receptor agonist bexarotene (LGD1069, Targretin) prevents and overcomes multidrug resistance in advanced prostate cancer. Prostate. 2006;66(3):305–316. doi: 10.1002/pros.20347 [DOI] [PubMed] [Google Scholar]
- 10.Yen WC, Lamph WW. The selective retinoid X receptor agonist bexarotene (LGD1069, Targretin) prevents and overcomes multidrug resistance in advanced breast carcinoma. Mol Cancer Ther. 2005;4(5):824–834. doi: 10.1158/1535-7163.MCT-05-0018 [DOI] [PubMed] [Google Scholar]
- 11.Yen WC, Corpuz MR, Prudente RY, et al. A selective retinoid X receptor agonist bexarotene (Targretin) prevents and overcomes acquired paclitaxel (Taxol) resistance in human non-small cell lung cancer. Clin Cancer Res. 2004;10(24):8656–8664. doi: 10.1158/1078-0432.CCR-04-0979 [DOI] [PubMed] [Google Scholar]
- 12.Weaver BA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: the mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8(1):7–12. doi: 10.1016/j.ccr.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 13.Sudo T, Nitta M, Saya H, Ueno NT. Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res. 2004;64(7):2502–2508. [DOI] [PubMed] [Google Scholar]
- 14.Chou TC, Talalay P. Quantitative-analysis of dose-effect relationships - the combined effects of multiple-drugs or enzyme-inhibitors. Adv Enzyme Regul. 1984;22:27–55. [DOI] [PubMed] [Google Scholar]
- 15.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–681. doi: 10.1124/pr.58.3.10 [DOI] [PubMed] [Google Scholar]
- 16.Pienta KJ. Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer. Semin Oncol. 2001;28(4 Suppl 15):3–7. [DOI] [PubMed] [Google Scholar]
- 17.Nieto-Rementeria N, Perez-Yarza G, Boyano MD, et al. Bexarotene activates the p53/p73 pathway in human cutaneous T-cell lymphoma. Br J Dermatol. 2009;160(3):519–526. doi: 10.1111/j.1365-2133.2008.08931.x [DOI] [PubMed] [Google Scholar]
- 18.Zhang CL, Hazarika P, Ni X, Weidner DA, Duvic M. Induction of apoptosis by bexarotene in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. Clin Cancer Res. 2002;8(5):1234–1240. [PubMed] [Google Scholar]
- 19.Budgin JB, Richardson SK, Newton SB, et al. Biological effects of bexarotene in cutaneous T-cell lymphoma. Arch Dermatol. 2005;141(3):315–321. doi: 10.1001/archderm.141.3.315 [DOI] [PubMed] [Google Scholar]
- 20.Castilla C, Flores ML, Medina R, et al. Prostate cancer cell response to paclitaxel is affected by abnormally expressed securin PTTG1. Mol Cancer Ther. 2014;13(10):2372–2383. doi: 10.1158/1535-7163.MCT-13-0405 [DOI] [PubMed] [Google Scholar]
- 21.Shapiro GI, Harper JW. Anticancer drug targets: cell cycle and checkpoint control. J Clin Invest. 1999;104(12):1645–1653. doi: 10.1172/JCI9054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8(5):379–393. doi: 10.1038/nrm2163 [DOI] [PubMed] [Google Scholar]
- 23.Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller JL. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990;60(3):487–494. doi: 10.1016/0092-8674(90)90599-a [DOI] [PubMed] [Google Scholar]
- 24.Krause K, Wasner M, Reinhard W, et al. The tumour suppressor protein p53 can repress transcription of cyclin B. Nucleic Acids Res. 2000;28(22):4410–4418. doi: 10.1093/nar/28.22.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng B, Crasta K. Consequences of mitotic slippage for antimicrotubule drug therapy. Endocr Relat Cancer. 2017;24(9):T97z–T106. doi: 10.1530/ERC-17-0147 [DOI] [PubMed] [Google Scholar]
- 26.Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14(2):111–122. doi: 10.1016/j.ccr.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 27.Baker J, Ajani J, Scotte F, et al. Docetaxel-related side effects and their management. Eur J Oncol Nurs. 2009;13(1):49–59. doi: 10.1016/j.ejon.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 28.Alshaker H, Wang Q, Kawano Y, et al. Everolimus (RAD001) sensitizes prostate cancer cells to docetaxel by down-regulation of HIF-1 alpha and sphingosine kinase 1. Oncotarget. 2016;7(49):80943–80956. doi: 10.18632/oncotarget.13115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroon J, Puhr M, Buijs JT, et al. Glucocorticoid receptor antagonism reverts docetaxel resistance in human prostate cancer. Endocr Relat Cancer. 2016;23(1):35–45. doi: 10.1530/ERC-15-0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim R, Chiorean EG, Amin M, et al. Phase 2 study of combination SPI-1620 with docetaxel as second-line advanced biliary tract cancer treatment. Brit J Cancer. 2017;117(2):189–194. doi: 10.1038/bjc.2017.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng Q, Sekula D, Guo YL, et al. UBE1L causes lung cancer growth suppression by targeting cyclin D1. Mol Cancer Ther. 2008;7(12):3780–3788. doi: 10.1158/1535-7163.MCT-08-0753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dragnev KH, Petty WJ, Shah SJ, et al. A proof-of-principle clinical trial of bexarotene in patients with non-small cell lung cancer. Clin Cancer Res. 2007;13(6):1794–1800. doi: 10.1158/1078-0432.CCR-06-1836 [DOI] [PubMed] [Google Scholar]
- 33.Bhalla KN. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22(56):9075–9086. doi: 10.1038/sj.onc.1207233 [DOI] [PubMed] [Google Scholar]
- 34.Huang HC, Shi J, Orth JD, Mitchison TJ. Evidence that mitotic exit is a better cancer therapeutic target than spindle assembly. Cancer Cell. 2009;16(4):347–358. doi: 10.1016/j.ccr.2009.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]