Abstract
Background
Risk factors for poor asthma outcomes may have considerable influence on the control level and medical care of asthmatic patients. Our objective was to conduct a study that provides data on the level of symptom control and the frequency of specific risk factors for poor asthma outcomes on a large patient cohort.
Methods
A cross-sectional, non-interventional real-life study was conducted among asthmatic patients treated by respiratory specialists in Hungary. Asthma control and risk factor assessment were done according to Global Initiative for Asthma guideline (Box 2–2). In the data analysis, phase descriptive statistics, graphical outputs, and Fisher’s exact tests were used.
Results
Of 12743 patients enrolled by 187 specialists, asthma was well controlled in 36.0%, partially controlled in 29.29%, and uncontrolled in 34.71% of the cases. The most common comorbidities were rhinitis/sinusitis (66.84%), cardiovascular diseases (43.81%), and gastroesophageal reflux disease (20.11%). The following risk factors had the strongest relationship with uncontrolled disease: incorrect inhaler technique causing side effects (odds ratio, OR 4.86, 3.51–6.8), previous severe exacerbation (OR 4.79, 4.02–5.72), high short-acting beta agonist (SABA) use (OR 4.46, 4.03–4.93), incorrect inhaler technique associated with an exacerbation (OR 3.91, 3.06–5.03), and persistently low forced expiratory volume in 1 s (FEV1, OR 3.14, 2.8–3.52). The most frequent risk factors were smoking (OR 1.47, 1.36–1.59) and obesity (OR 1.34, 1.24–1.45). Furthermore, high loss of control was associated with an initial low FEV1 (OR 2.21, 2.01–2.44), frequent oral corticosteroid (OCS) use (OR 1.83, 1.64–2.05), poor adherence to treatment (OR 2.51, 2.21–2.86), and allergen exposure (OR 1.63, 1.47–1.81).
Conclusions
This study indicated that the presence of risk factors for poor asthma outcomes listed by the Global Initiative for Asthma document significantly influenced actual control level in a real-world large patient cohort, with high SABA use, previous severe exacerbation, incorrect inhaler technique, persistently low FEV1, and poor adherence to treatment having the highest impact.
Keywords: asthma, risk factors, poor outcomes, exacerbation, reliever use, comorbidity
Background
Asthma is a chronic pulmonary disease with considerable economic burden.1 In 2015, asthma was the most prevalent chronic respiratory disease, affecting 358 million people, meaning twice the number of cases compared to chronic obstructive pulmonary disease (COPD).2
The prevalence of asthma in Hungary was found to be 7.6%, which corresponds to the European average.3 International strategies set forth by the Global Initiative for Asthma (GINA) document, as well as the local national asthma guideline, help in effective clinical management and propose therapeutic decisions to be made based on the level of asthma control. The aim of asthma treatment is to achieve a controlled condition and to maintain it on the long term.4 Despite the presence of established treatment guidelines and high accessibility to inhaled therapies, asthma morbidity is significant, and many asthma patients still experience persistent symptoms, poor disease control, and exacerbations.5,6 A recent European study of 8000 asthmatic patients treated in general practice showed that 45% of them were uncontrolled and 44% required at least 1 course of oral corticosteroids in the last year.7 Poor asthma control is associated with negative outcomes, including impaired health-related quality of life (HR-QoL), great use of health care resources, work, and activity impairment, resulting in substantial direct and indirect costs.4,8 At the same time, negative outcomes of asthma are associated with risk factors some of which are modifiable.6
The GINA 2014 document was the first to formally describe asthma evaluation beyond control assessment.4 Achieving and maintaining a controlled condition is still an important aim of asthma treatment; however, decreasing the risks of negative outcomes caused by asthma is also a top priority. Minimizing the risk of poor asthma outcomes, namely future exacerbations, development of fixed airflow limitation, and side effects, are also aims of asthma management. Consequently, the therapeutic strategy is not merely determined by symptom control, but also identifying the specific risk factors of poor asthma outcomes. Poor asthma symptom control itself increases the risk of exacerbations.9 However, up till now, the frequency of specific risk factors for poor asthma outcomes determined by the GINA document, together with their relationship to disease control have not yet been evaluated. There is no full-scale GINA determined risk factor assessment in a large asthmatic population, either in a national or international cohort, and consequently, no reliable data are available in respect to how much the presence of a specific risk factor influences current control.
Our study was designed to examine not only the current asthma control but also the importance and impact of certain risk factors determined by the GINA document and their relationship to uncontrolled status. Our objective was to conduct a wide-ranging, representative real-life study in asthma, which would provide data both on symptomatic control level and the frequency of risk factors associated with poor asthma outcomes in a specialist treated patient population.
Methods
Selection of the patients
This was a non-interventional cross-sectional study under real-life circumstances. Inclusion of the patients and data recording was performed on a single occasion. For detailed data collection purposes, a doctor and a patient questionnaire were developed. In order to eliminate seasonal effects, patient recruitment was carried out throughout an entire year (from 11–05-2015 to 19–05-2016). To obtain a non-biased patient enrolment, every health institution could include a maximum of 15 patients on 5 pre-determined consecutive workdays per month. Enrolment was conducted randomly with the inclusion of consecutive asthma patients who wished to participate. Given that in Hungary, pulmonologist specialists have the exclusive responsibility to diagnose and treat asthma patients, the examinations and data collection were done solely by respiratory specialists. The enrolment of patients took place in dispensaries, outpatient clinics specializing in pulmonology, and in outpatient departments of hospitals in all regions of Hungary. Table 1 contains the inclusion and exclusion criteria of the study.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| - Adult asthmatic patients - Asthma diagnosis for >6 months - Maintenance therapy unchanged in the last month - Out-patient - No hospitalisation in the last month - No significant, untreated chronic disease |
- Lack of consent by patient - Inability to complete patient related questionnaires - Permanent need for maintenance systemic corticosteroid treatment - Acute exacerbations at time of inclusion in study - Active tuberculosis - Malignant disease in a palliative treatment phase |
The designing and the implementation of the study were carried out observing good clinical practice (GCP guidelines) and the Declaration of Helsinki. Patients were included in the study on a voluntary basis after providing them with information and after signing a written contract, without any remuneration.
Recorded data
A comprehensive data collection form was used to record patient demographic characteristics, major medical history, smoking habits, comorbidities, risk factors, current control state, medications, and all relevant physical assessments. Laboratory tests were not performed. Asthma control, treatment steps, and risk factor assessment were done according to GINA 2014 (Box 2–2 and Box 3–5.). The treatment steps were derived from actual prescribed maintenance therapy. Comorbidities, allergen history, hospitalization, previous intubation, low initial forced expiratory volume in 1 s (FEV1) at the time of diagnosis, and the time of asthma diagnosis were collected by reviewing clinical records of enrolled patients. BMI was calculated based on the patient’s measured height and weight at the time of examination. Poor adherence was defined by the physician based on the patient’s data. In addition, data from the patient survey were also documented. If spirometry was performed on the medical visit of the patient, FEV1, forced vital capacity (FVC), and FEV1/FVC were recorded.
Statistical analysis
Data collection and database management were conducted by AdWare Research Ltd. (Balatonfüred, Hungary), and the statistical analysis by Adatrendező Ltd. (Budapest, Hungary). In the data analysis phase, descriptive statistics, graphical outputs, and Fisher’s exact tests were used. Odds ratios were provided with 95% confidence intervals (CI). For statistical analysis, we used the open source Python 2.7.12 on a MAC operating system (Anaconda Inc., Austin, TX) and R for Windows 3.4.2 (R Core Team 2017., R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
Results
During the 1-year period of inclusion, an average of 69 patients were included per investigational sites. This involved 187 centers, representing 35% of the pulmonologists currently working in outpatient medical clinics in Hungary. Table 2 contains the demographic data and main clinical characteristics of the included 12743 patients.
Table 2.
Main demographic data, clinical characteristics and lung function parameters of patients
| Study population | |||
|---|---|---|---|
| N | % | ||
| Number of patients | 12743 | 100 | |
| Number of cases in regions of Hungary | East | 5149 | 40.4 |
| West | 3984 | 31.3 | |
| Central | 3610 | 28.3 | |
| Examined patients according to seasonality | April–September | 6923 | 54.3 |
| October–March | 5820 | 45.7 | |
| Years since the diagnosis of asthma | 0–1 years | 310 | 2.4 |
| 2–5 years | 3280 | 25.7 | |
| 6–10 years | 3295 | 25.9 | |
| 11–20 years | 3930 | 30.8 | |
| >20 years | 1913 | 15.0 | |
| No data | 15 | 0.1 | |
| GINA based treatment categories | STEP 1 | 274 | 2.15 |
| STEP 2 | 990 | 7.77 | |
| STEP 3 | 4759 | 37.35 | |
| STEP 4 | 6390 | 50.14 | |
| STEP 5 | 330 | 2.59 | |
| Gender | Male | 4059 | 31.9 |
| Female | 8684 | 68.1 | |
| Smoking habit | Smoker | 1669 | 13.1 |
| Former smoker | 2584 | 20.3 | |
| Never smoked | 8476 | 66.5 | |
| No data | 14 | 0.1 | |
| Age distribution | 18–30 | 1261 | 9.9 |
| 31–45 | 2466 | 19.4 | |
| 46–65 | 5687 | 44.6 | |
| >65 | 3329 | 26.1 | |
| Body mass index distribution | <18.5 | 228 | 1.8 |
| 18.5–24.9 | 3453 | 27.1 | |
| 25–29.9 | 4487 | 35.2 | |
| 30–34.9 | 3009 | 23.6 | |
| ≥35 | 1566 | 12.3 | |
| FEV1 (forced expiratory volume in 1 s, % predicted) distribution | >80% | 7527 | 59.1 |
| 60–80% | 3399 | 26.7 | |
| <60% | 1461 | 11.5 | |
| No data | 356 | 2.8 | |
Abbreviation: GINA, global initiative for asthma.
Concerning age distribution, 9.9% of the patients were 18–30 years, 19.4% were 31–45 years, 44.6% were 46–65 years old, and 26.1% were older than 65 years. Patients diagnosed with asthma for more than 1 year represented 97.4% of the cohort. Men represented 31.9%. With respect to seasonality, 54.3% of the patients were examined from April to September, while 45.7% were examined from October to March. Patient inclusion was also in line with population densities in the geographical regions. The majority of patients received maintenance therapy on GINA step 2, 3, and 4.
Patients who had never smoked represented 66.5% of the cohort, 20.3% had smoked previously, yet quit, and 13.1% were smokers at the time of the examination. The average body mass index (BMI) was 28.46±5.7 kg/m2. Mean forced expiratory flow in 1 s (FEV1) value was 84.29% (2.34 L), mean forced vital capacity (FVC) 94.18% (3.13 L), and mean FEV1/FVC 83.74%. Regarding the FEV1, 38.2% of the patients had values lower than 80% predicted, and 11.5% of the patients had values lower than 60%.
Table 3 summarizes the medically diagnosed comorbidities of the whole patient population.
Table 3.
Comorbidities of study participants (data are presented as numbers and percentages)
| Comorbidities | N | % |
|---|---|---|
| Cardiovascular disease | 5583 | 43.81 |
| Hypertension | 5226 | 41.01 |
| Cardiac insufficiency | 1250 | 9.81 |
| Acute myocardial infarction | 210 | 1.65 |
| Atrial fibrillation (chronic) | 160 | 1.26 |
| Other cardiac arrhythmia | 731 | 5.70 |
| Other cardiac history | 215 | 1.69 |
| Rhinitis and/or Sinusitis | 8517 | 66.84 |
| Gastroesophageal reflux disease (GERD) | 2563 | 20.11 |
| Diabetes | 1129 | 8.86 |
| Impaired glucose tolerance (IGT) | 1058 | 8.30 |
| Concomitant COPD | 1002 | 7.86 |
| Osteoporosis | 1081 | 8.48 |
| Prostate hyperplasia | 317 | 2.49 |
| Glaucoma | 227 | 1.78 |
| Cerebrovascular events | 311 | 2.44 |
| Other comorbidities | 1320 | 10.36 |
The most commonly recorded comorbidities (66.84% of all patients) were rhinitis and/or sinusitis. Cardiovascular disease was the second, affecting 43.81% of all patients. Gastroesophageal reflux disease (GERD) was diagnosed in 20.11% and COPD in 7.86% of the patients.
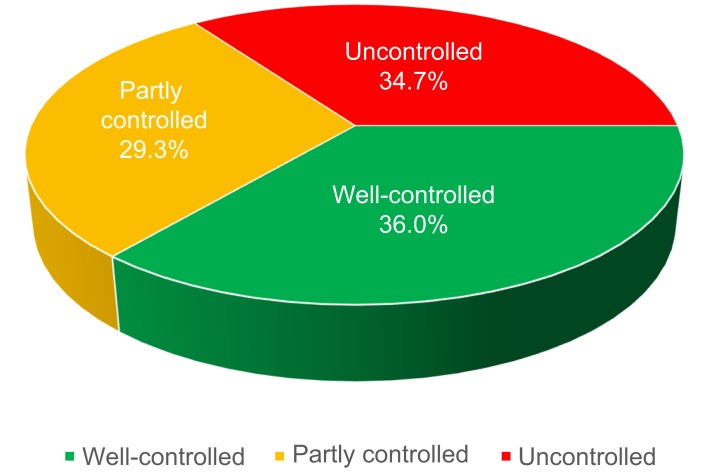
Measuring asthma control was mandatory in all cases, and the results are summarized in Figure 1. Well-controlled asthma was found in 36.0% of the patients; however, in 29.29% of the patients, it was partially controlled, and in 34.71% of the cases, asthma was uncontrolled.
Figure 1.
Proportion of patients with different levels of asthma control according to GINA guideline.
The risk factors of poor asthma outcomes determined by the GINA document (exacerbation, fixed airway obstruction, and side effects of medications) were also recorded. Table 4 and Figures S1–S3 contain control level of patients together with the specific risk factors.
Table 4.
Frequency of risk factors, control levels of affected patients, and relationship between the risk factor and uncontrolled status (Fisher’s exact test odds ratio)
| Risk factors | Number of patients affected by the risk factor | Frequency of risk factor | Ratio of uncontrolled patients | Number of uncontrolled patients | Number of partly controlled patients | Number of well- controlled patients | Odds ratios (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Risk factors for exacerbation | |||||||||
| 1 | Excessive SABA use (>1 pack/month) | 2045 | 16.05% | 64.55% | 1,320 | 460 | 265 | 4.46 (4.03-4.93) | p<0.001 |
| 2 | Inadequate ICS (not prescribed ICS, poor adherence, no proper inhaler technique) | 2250 | 17.66% | 44.36% | 998 | 679 | 573 | 1.64 (1.50-1.81) | p<0.001 |
| 2a | Poor adherence | 1076 | 8.44% | 55.11% | 593 | 332 | 151 | 2.51 (2.21-2.86) | p<0.001 |
| 2b | Incorrect inhaler technique in connection with exacerbation | 304 | 2.39% | 66.78% | 203 | 69 | 32 | 3.91 (3.06-5.03) | p<0.001 |
| 3 | Low FEV1<60% predicted (actual) | 1461 | 11.47% | 59.14% | 864 | 327 | 270 | 3.14 (2.80-3.52) | p<0.001 |
| 4 | Exposures (smoking, allergen exposure if sensitized) | 10,210 | 80.12% | 35.57% | 3632 | 2991 | 3587 | 1.22 (1.11-1.35) | p<0.001 |
| 5 | Comorbidities (Overweight, rhinosinusitis, confirmed food allergy) | 10,193 | 79.99% | 35.34% | 3602 | 3007 | 3584 | 1.15 (1.05-1.27) | p=0.002 |
| 5a | Obesity (above BMI 30) | 4575 | 35.90% | 39.04% | 1786 | 1353 | 1436 | 1.34 (1.24-1.45) | p<0.001 |
| 5b | Allergen exposure if sensitized (allergen history administered) | 8517 | 66.84% | 34.79% | 2963 | 2462 | 3092 | 1.01 (0.94-1.09) | p=0.767 |
| 5b1 | Allergen exposure if sensitized (in the patient’s allergic season) | 1786 | 14.02% | 44.62% | 797 | 561 | 428 | 1.63 (1.47-1.81) | p<0.001 |
| 6 | Pregnancy | 43 | 0.34% | 25.58% | 11 | 13 | 19 | 0.65 (0.29-1.32) | p=0.261 |
| 7 | Ever intubated or in intensive care unit for asthma | 83 | 0.65% | 56.63% | 47 | 15 | 21 | 2.47 (1.56-3.93) | p<0.001 |
| 8 | ≥1 severe exacerbation in last 12 months | 651 | 5.11% | 70.05% | 456 | 115 | 80 | 4.79 (4.02-5.72) | p<0.001 |
| Risk factors for developing fixed airflow limitation | |||||||||
| 9 | Lack of ICS treatment | 1331 | 10.44% | 35.24% | 469 | 418 | 444 | 1.03 (0.91-1.16) | p=0.67 |
| 10 | Exposures (Tobacco smoke, noxious chemicals, occupational exposures) | 4736 | 37.17% | 41.22% | 1952 | 1423 | 1361 | 1.58 (1.46-1.70) | p<0.001 |
| 10a | Tobacco smoke (history of active smoking) | 4253 | 33.38% | 40.58% | 1726 | 1292 | 1235 | 1.47 (1.36-1.59) | p<0.001 |
| 10a1 | Smoking (currently active smokers) | 1669 | 13.10% | 42.30% | 706 | 512 | 451 | 1.58 (1.42-1.76) | p<0.001 |
| 10b | Noxious chemicals or occupational exposures | 793 | 6.22% | 48.68% | 386 | 227 | 180 | 1.86 (1.60-2.15) | p<0.001 |
| 11 | Low initial FEV1 (<60% predicted) | 1999 | 15.69% | 50.73% | 1,014 | 508 | 477 | 2.21 (2.01-2.44) | p<0.001 |
| Risk factors for medication side effects | |||||||||
| 12 | Systemic side effect | 1550 | 12.16% | 47.23% | 732 | 369 | 449 | 1.82 (1.63-2.03) | p<0.001 |
| 12a | Frequent OCS | 1513 | 11.87% | 47.46% | 718 | 353 | 442 | 1.83 (1.64-2.05) | p<0.001 |
| 12b | P450 inhibitor | 52 | 0.41% | 42.31% | 22 | 17 | 13 | 1.38 (0.76-2.48) | p=0.247 |
| 13 | No proper inhaler technique which caused side effect | 190 | 1.49% | 71.58% | 136 | 33 | 21 | 4.86 (3.51-6.80) | p<0.001 |
Abbreviations: ICS, inhaled corticosteroid; BMI, body mass index; OCS, oral corticosteroid; SABA, short-acting beta agonist; FEV1, forced expiratory volume in 1 s.
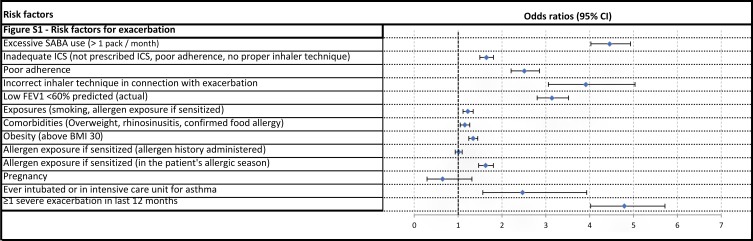
Figure S1.
Risk factors for exacerbation.
Figure S3.
Risk factors for medication side effects.
Among the risk factors named by the GINA and detected in our study, improper inhaler technique which caused side effects showed the strongest relation to an uncontrolled state (OR 4.86, CI 3.51–6.8). As high as 71.58% of the affected patients were uncontrolled. The second highest OR of 4.79 was observed for patients who had at least 1 severe exacerbation in the last 12 months; 70.05% were uncontrolled. Patients with high short-acting beta agonist (SABA) use were also predisposed to loss of asthma control, with 64.55% were uncontrolled (OR 4.46, CI 4.03–4.93). The fourth highest OR (3.91, CI 3.06–5.03) was found in those with incorrect inhaler technique associated with an exacerbation.
Poor adherence to ICS showed a strong relation to uncontrolled disease in our study as 55.11% were uncontrolled, resulting in an OR of 2.51 (CI 2.21–2.86). Low FEV1 at diagnosis had an OR of 2.21 (CI 2.01–2.44) for loss of asthma control. As an initially low FEV1 value predisposed an uncontrolled state, an actual low FEV1 was a stronger risk factor for uncontrolled disease (OR 3.14, CI 2.8–3.52).
Inhaled noxious chemicals or occupational exposures excluding smoking affected 48.68%, who were poorly controlled (OR 1.86, 1.6–2.15). Smoking affected 4253 patients, and poor control was present in 40.58% of them (OR 1.47, 1.36–1.59). The most common risk factor was the presence of chronic rhinosinusitis. A history of allergies was found in 8517 patients, with similar disease control as the whole cohort; however, out of 1786 patients who had an allergic condition at the time of examination, 44.62% were uncontrolled and demonstrated an OR of 1.63 (1.47–1.81) for loss of asthma control. In 1513 patients, systemic corticosteroid treatment was necessary, and poor asthma control was observed in 47.46% of them. Frequent OCS related to systemic side effects resulted in a high uncontrolled level (OR 1.83, 1.64–2.05). In 4575 cases, the BMI was >30 kg/m2, and 39.04% of these patients were poorly controlled (OR 1.34, CI 1.24–1.45).
Discussion
In Hungary, similarly to many countries of Eastern Europe, the diagnosis and treatment of asthmatic patients is the responsibility of the pulmonologists. A whole range of therapeutic options suggested by the GINA guidelines is available. Nonetheless, based on the current study, which was the largest study examining asthma control in Eastern Europe, 36% of the patients were well controlled, 29.29% were partially controlled, and 34.71% were uncontrolled.
Regarding the rate of controlled asthma, our investigation demonstrated nearly equivalent result to a recent specialist-based cross-sectional study of adult asthma in Japan. Adachi et al found, despite receiving treatment from an allergy and/or respiratory specialist, only 35.1% of the patients had controlled asthma.10 In Turkey, Gemicioglu et al observed the same rate of controlled patients. The percentage of patients with total control in the elderly and young groups were 33.9% and 37.1% at first visit.11 These results draw attention to the need for improving asthma management. There is growing evidence that environmental pollution aggravates asthma. One limitation is that this factor has not been studied.12
Uncontrolled disease may be related to the presence of risk factors documented by the GINA guidelines. This current study was the first large-scale, specialist-evaluated cohort which aimed to determine the frequency of specific risk factors identified by the GINA document. Our aim was to validate the known risk factors of non-control in a large asthma cohort in real life. Based on the data collected by pulmonologists during patient visits, GINA defined risk factors for poor asthma outcomes proved to be related to uncontrolled disease; the strength of this relationship varied depending on the risk factor. In everyday clinical practice, it is important to know that the frequencies and the relationships to poor control of listed risk factors vary over a wide range.
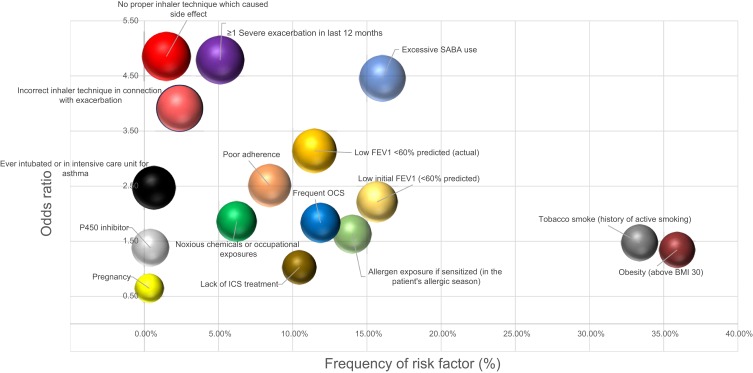
The frequency of specific risk factors and the odds ratio of its relationship with control loss are visualized in Figure 2. The risk factors that appear more frequently than average and those which strongly linked to a poor outcome should be prioritized and monitored continuously.
Figure 2.
The frequency of specific risk factors and the odds ratio of its relationship with uncontrolled status.
Note: Size of bubbles represent the ratio of uncontrolled patients.
Abbreviations: BMI, body mass index; ICS, ICS, inhaled corticosteroid; OCS, oral corticosteroid; FEV1, forced expiratory volume in 1s; SABA, short-acting beta agonist.
In our study, besides excessive SABA use, yearly exacerbating disease pattern, improper inhaler technique, and low FEV1 (<60% predicted) were the most strongly related to suboptimal disease control. It was not surprising that high SABA use had an especially high OR. Of the patients who used more than 1 pack of salbutamol a month, 64.55% were uncontrolled at the time of the survey, and in total only 13% of them were well controlled. This result clearly shows that there is an overuse of salbutamol that is related to uncontrolled disease.
Similarly to high SABA use, the chance of an uncontrolled status (eg, exacerbations) amongst patients who had at least 1 severe exacerbation per year was exceedingly high. This confirms the results of the TENOR Study Group that severe asthma exacerbations are a strong independent factor predicting future exacerbations.13 Although patients in acute exacerbation were excluded from our study, 5.11% of the patients had severe exacerbation within the last year, and 70.05% of them were poorly controlled at the time of the study.
Incorrect inhaler technique was recorded in two aspects listed in the GINA guideline. In the present study, incorrect inhaler technique showed a strong relationship to uncontrolled status. This is in line with a recent study, which showed that incorrect inhaler technique seems to be frequent in real-life settings. Melani et al found that 12–43.5% of the patients make at least 1 critical error in inhalation technique, and hospitalization or emergency department visit due to these errors occurred in 30% of the patients.14 The study showed that different failures of device use may lead to different levels of impairment to successful therapy. Incorrect inhaler technique in real-life setting may be an important risk factor to loss of control (named as poor asthma outcome in our study) and also exacerbations, because poor inhaler technique may cause low drug deposition resulting in deterioration of the effect of the drug. On the other hand, improper inhaler technique may worsen drug adherence. Thus, educational programs, which are inexpensive and effective, may help in preventing the development of loss of asthma control.15,16
Data recording of incorrect inhaler technique on the other hand represents a limitation of our study, as it was the doctors’ task to determine inhalation technique, and also whether the patient had an exacerbation or side effect due to incorrect inhaler technique. GINA underlines the fact that low FEV1 is known to be a strong independent predictor of future exacerbations. Our results are in concordance with these findings, both when low FEV1 was measured at the time of diagnosis or with maintenance therapy. However, our results raise a hypothesis that patients are at higher risk of poor outcomes if their low FEV1 exists despite the use of maintenance therapy. Although we experienced low FEV1 in only 11.47% of our patients, it may be considered as a very strong predictor of uncontrolled status with an OR of 3.14. Interestingly, low initial FEV1 values also showed a significant relationship to loss of asthma control, with an OR of 2.21. Our results were consistent with those of Osborne and co-workers, who found that patients with low FEV1 at any time of their life are at a significantly higher risk of exacerbations, which underscores the importance of spirometry in asthma care.17
Notably in our study, the incidence of frequent OCS users was high, and despite the effective systemic effect of this medication, their control was significantly lower than average. In the CHAS study, Gonzalez et al observed a high level of uncontrolled asthma (63.9%) which was strongly associated with oral corticosteroid treatment (OR=6.55).18
In our investigation, the incidence of poor adherence to treatment was lower than in other specific adherence-focused studies.19,20 Our method had limitations in recognizing all non-adherent patients, which may be the consequence of deficiencies in the collection of adherence data by specialists in everyday clinical practice. However, our results still confirmed the well-established evidence of poor adherent patients having a high probability of an uncontrolled status.21
Three risk factors were identified in our study affecting a high number of patients but having a weaker relationship with loss of asthma control. The smoker group represented 33.38% of all the patients and was related to suboptimal control with an OR of 1.47. Smoking is a frequent factor behind suboptimal asthma control, with the risk of evolving COPD. Among smokers, 13.1% of the patients were currently active smokers and their OR was 1.58 (CI 1.42–1.76). Consequently, active smoking may be considered as a frequent factor behind suboptimal asthma control. Furthermore, for ex-smokers, the chance of poor outcome remains higher for a long time after quitting.
Many studies support an association between obesity and asthma prevalence.22–28 It has also been proved that patients with obesity are more likely to have uncontrolled asthma compared to eutrophic patients.29 Additionally, an association between obesity and increased asthma severity in adults has been demonstrated.30 The National Asthma Survey, one of the largest asthma surveys in the USA, showed that obesity is associated with several measures of asthma severity and control, including symptoms, missed workdays, medication use, and GINA severity classification.31 In our study, the risk of an uncontrolled status was also higher due to obesity.
A history of rhinosinusitis or food allergy was especially prevalent amongst Hungarian asthmatic patients; however, it showed no strong relationship with poor asthma outcomes. Inhaled allergens cause problems and symptoms at a specific time of the year. Therefore, we separately analyzed the patient group who had seasonal allergies at the time of data registration. Out of 1786 patients who were examined in their sensitized allergen period, 44.62% had bad asthma control, which was higher compared to the rest of the patients and correlated significantly with a higher chance for bad asthma outcomes. A limitation of our study is that bronchiectasis was not actively screened, which could impact on control status of moderate to severe asthma patients.32 Finally, we identified infrequent risk factors as well which were less likely to worsen asthma control. Patients who had been pregnant in the last 12 months represented 0.34% of the cohort, but this condition had hardly any effect on current asthma control. At the time of enrolment, 43 patients were pregnant; however, due to the smaller number of participants, it was not possible to reliably determine what effect pregnancy had on current asthma state. The rate of the side effects that stem from the co-administration of a P450 inhibitor compared to the examined risk factors is still an important result despite its lower incidence. The risk of uncontrolled asthma was not associated with a lack of ICS. The reason for this may be that pulmonologists underestimate the lack of ICS resulting from non-adherence. On the other hand, there could be a patient population who used maintenance therapy as needed without losing control of their asthma. This hypothesis was supported by Papi et al, who found that patients with mild persistent asthma who have infrequent symptoms may not require regular treatment with inhaled corticosteroids.33
Conclusion
The results of this large real-world study, conducted for the first time in Eastern Europe by respiratory specialists, may contribute to uncovering the most important causes of poor asthma control in everyday clinical practice, together with determining the impact of different risk factors in leading to poor asthma outcomes, thus gaining a better understanding of the disease. We found that the risk factors listed by the GINA document significantly influence the control level of asthmatic patients. High SABA use, exacerbation history, incorrect inhaler technique, persistently low FEV1, and poor adherence to treatment are of outstanding significance in influencing asthma control and leading to poor outcomes. In order to further improve disease control, substantial attention might be paid to recognizing risk factors for poor asthma outcomes.
Acknowledgments
The authors thank to Abonyi-Tóth Zsolt (Data Processor Ltd.) for statistical analysis and to Proof-Reading-Service.com Ltd. for language check. The study was funded by Chiesi Hungary Ltd.
Abbreviation list
OR, odds ratio; CI, confidence intervals; COPD, chronic obstructive pulmonary disease; GINA, Global Initiative for Asthma; HR-QoL, health-related quality of life; eCRF, electronic case report form; GCP, good clinical practice; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; BMI, body mass index; GERD, gastroesophageal reflux disease; IGT, Impaired glucose tolerance; SABA, short-acting beta agonist; ICS, inhaled corticosteroid, ICU, intensive care unit.
Ethics approval and consent to participate
All procedures were performed in accordance with the ethical standards of the National Scientific and Research Ethics Committee of Hungary (ad.7864-2/2015/EKU [ad.48/2015]) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
GT contributed to conception and design of the study, to literature search, and writing the manuscript. AH participated in literature search and made contributions to the acquisition and interpretation of data, and revised the manuscript. VM contributed to the literature search and revised the manuscript. LT and ZS contributed to conception and design of the study, to the acquisition and interpretation of data, and revised the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
VM, LT, and ZS had consultant arrangements with AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, GSK, Novartis, Orion, TEVA, and Takeda. GT and AH are employees of Chiesi Hungary Ltd. VM reports personal fees from GSK, AstraZeneca, Berlin Chemie, Chiesi, Boehringer Ingelheim, Novartis, Orion, and TEVA, including non-financial support from Takeda, outside the submitted work. The authors report no other conflicts of interest in this work.
Supplementary materials
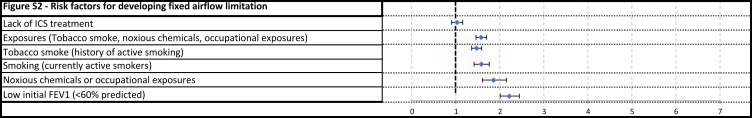
Figure S2.
Risk factors for developing fixed airflow limitation.
References
- 1.World Health Organisation. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach; 2007. Available from: http://www.who.int/respiratory/publications/global_surveillance/en/. Accessed October16, 2018.
- 2.Soriano JB, Abajobir AA, Abate KH, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi: 10.1016/S2213-2600(17)30234-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.To T, Stanojevic S, Moores G, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012;12:204. doi: 10.1186/1471-2458-12-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Initiative for Asthma. Global strategy for asthma management and prevention; 2018. Available from:https://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention. Accessed 16October 2018.
- 5.Beasly R, Semprini A, Mitchell EA. Risk factors for asthma: is prevention possible? Lancet. 2015;386(9998):1075–1085. doi: 10.1016/S0140-6736(15)00156-7 [DOI] [PubMed] [Google Scholar]
- 6.Bosnic-Anticevich S, Kritikos V, Carter V, et al. Lack of asthma and rhinitis control in general practitioner-managed patients prescribed fixed-dose combination therapy in Australia. Journal of Asthma. 2018;55(6):684–694. doi: 10.1080/02770903.2017.1353611 [DOI] [PubMed] [Google Scholar]
- 7.Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24:14009. doi: 10.1038/npjpcrm.2014.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Berge M, Ten Hacken NHT, Kerstjens HAM, Postma DS. Management of asthma with ICS and LABAs: different treatment strategies. Clin Med Ther. 2009;1:77–93. [Google Scholar]
- 9.Schatz M, Zeiger RS, Yang SJ, et al. The relationship of asthma impairment determined by psychometric tools to future asthma exacerbations. Chest. 2012;141(1):66–72. doi: 10.1378/chest.11-0574 [DOI] [PubMed] [Google Scholar]
- 10.Adachi M, Hozawa S, Nishikawa M, Yoshida A, Jinnai T, Tamura G. Asthma control and quality of life in a real-life setting: a cross-sectional study of adult asthma patients in Japan (ACQUIRE-2). Journal of Asthma. 2018;1–10. doi: 10.1080/02770903.2018.1514628 [DOI] [PubMed] [Google Scholar]
- 11.Gemicioglu B, Bayram H, Cimrin A, et al. Asthma control and adherence in newly diagnosed young and elderly adult patients with asthma in Turkey. Journal of Asthma. 2019;56(5):553–561. doi: 10.1080/02770903.2018.1471707 [DOI] [PubMed] [Google Scholar]
- 12.Muñoz X, Barreiro E, Bustamante V, Lopez-Campos JL, González-Barcala FJ, Cruz MJ. Diesel exhausts particles: their role in increasing the incidence of asthma. Reviewing the evidence of a causal link. Sci Total Environ. 2019;652:1129–1138. ISSN 0048-9697. doi: 10.1016/j.scitotenv.2018.10.188 [DOI] [PubMed] [Google Scholar]
- 13.Miller MK, Lee JH, Miller DP, Wenzel SE; TENOR Study Group. Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med. 2007;101(3):481–489. doi: 10.1016/j.rmed.2006.07.005 [DOI] [PubMed] [Google Scholar]
- 14.Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;150(6):930–938. doi: 10.1016/j.rmed.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 15.González-Barcala F-J, García-Couceiro N, Facal D. Educación en asma. Arch Bronconeumol. 2016;52:543–544. doi: 10.1016/j.arbres.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 16.Gelzer AD, Gao W, Keleti D, et al. Multifaceted interventions improve medication adherence and reduce acute hospitalization rates in medicaid patients prescribed asthma controllers. Journal of Asthma. 2019;56(2):190–199. doi: 10.1080/02770903.2018.1439954 [DOI] [PubMed] [Google Scholar]
- 17.Osborne ML, Pedula KL, O’Hollaren M, et al. Assessing future need for acute care in adult asthmatics: the Profile of Asthma Risk Study: a prospective health maintenance organization-based study. Chest. 2007;132(4):1151–1161. doi: 10.1378/chest.05-3084 [DOI] [PubMed] [Google Scholar]
- 18.Barcala FJG, de la Fuente-Cid R, Álvarez-Gil R, Tafalla M, Nuevo J, Caamaño-Isorna F. Factors associated with asthma control in primary care patients: the CHAS Study. Arch Bronconeumol. 2010:46(7):358–363. ISSN 0300-2896. doi: 10.1016/j.arbres.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Ulrik CS, Backer V, Søes-Petersen U, Lange P, Harving H, Plaschke PP. The patient’s perspective: adherence or non-adherence to asthma controller therapy? J Asthma. 2006;43:701–704. doi: 10.1080/02770900600925569 [DOI] [PubMed] [Google Scholar]
- 20.Bender BG, Rand C. Medication non-adherence and asthma treatment cost. Curr Opin Allergy Clin Immunol. 2004;4:191–195. doi: 10.1097/00130832-200406000-00009 [DOI] [PubMed] [Google Scholar]
- 21.Papi A, Ryan D, Soriano JB, et al. Relationship of inhaled corticosteroid adherence to asthma exacerbations in patients with moderate-to-severe asthma. J Allergy Clin Immunol Pract. 2018. doi: 10.1016/j.jaip.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Dales R, Krewski D, Breithaupt K. Increased effects of smoking and obesity on asthma among female Canadians: the National Population Health Survey, 1994–1995. Am J Epidemiol. 1999;150(3):255–262. doi: 10.1093/oxfordjournals.aje.a009996 [DOI] [PubMed] [Google Scholar]
- 23.Huovinen E, Kaprio J, Koskenvuo M. Factors associated to lifestyle and risk of adult onset asthma. Respir Med. 2003;97(3):273–280. doi: 10.1053/rmed.2003.1419 [DOI] [PubMed] [Google Scholar]
- 24.Luder E, Ehrlich RI, Lou WY, Melnik TA, Kattan M. Body mass index and the risk of asthma in adults. Respir Med. 2004;98(1):29–37. [DOI] [PubMed] [Google Scholar]
- 25.Nystad W, Meyer HE, Nafstad P, Tverdal A, Engeland A. Body mass index in relation to adult asthma among 135,000 Norwegian men and women. Am J Epidemiol. 2004;160(10):969–976. doi: 10.1093/aje/kwh212 [DOI] [PubMed] [Google Scholar]
- 26.Shaheen SO, Sterne JA, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax. 1999;54(5):396–402. doi: 10.1136/thx.54.5.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanley AH, Demissie K, Rhoads GG. Asthma development with obesity exposure: observations from the cohort of the National Health and Nutrition Evaluation Survey Epidemiologic Follow-up Study (NHEFS). J Asthma. 2005;42(2):97–99. doi: 10.1081/JAS-51338 [DOI] [PubMed] [Google Scholar]
- 28.Young SY, Gunzenhauser JD, Malone KE, McTiernan A. Body mass index and asthma in the military population of the northwestern United States. Arch Intern Med. 2001;161(13):1605–1611. doi: 10.1001/archinte.161.13.1605 [DOI] [PubMed] [Google Scholar]
- 29.Neffen H, Chahuàn M, Hernández DD, et al. Key factors associated with uncontrolled asthma – the Asthma Control in Latin America Study. Journal of Asthma. 2019:1–10. doi: 10.1080/02770903.2018.1553050. [DOI] [PubMed] [Google Scholar]
- 30.Akerman MJ, Calacanis CM, Madsen MK. Relationship between asthma severity and obesity. J Asthma. 2004;41(5):521–526. [DOI] [PubMed] [Google Scholar]
- 31.Taylor B, Mannino D, Brown C, Crocker D, Twum-Baah N, Holguin F. Body mass index and asthma severity in the National Asthma Survey. Thorax. 2008;63(1):14–20. doi: 10.1136/thx.2007.082784 [DOI] [PubMed] [Google Scholar]
- 32.Padilla-Galo A, Olveira C, Fernández de Rota-Garcia L, et al. Factors associated with bronchiectasis in patients with uncontrolled asthma; the NOPES score: a study in 398 patients. Respir Res. 2018;19:43. doi: 10.1186/s12931-018-0746-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papi A, Canonica GW, Maestrelli P, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med. 2007;356(20):2040–2052. doi: 10.1056/NEJMc063190 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.