Abstract
Background and Purpose
DAWN and DEFUSE 3 established thrombectomy for patients with emergent large vessel occlusions (ELVO) presenting 6–24 hours after symptom onset. Given the greater inclusivity of DEFUSE 3 we evaluated the effect of thrombectomy in DEFUSE 3 patients who would have been excluded from DAWN.
Methods
Eligibility criteria of the DAWN trial were applied to DEFUSE 3 patient data to identify DEFUSE 3 patients not meeting DAWN criteria (DEFUSE 3 Non-DAWN). Reasons for DAWN exclusion in DEFUSE 3 were infarct core too large (CTL), NIHSS 6–9 and mRS 2. Subgroups were compared to the DEFUSE 3 Non-Dawn and entire DEFUSE 3 cohorts.
Results
There were 71 DEFUSE 3 Non-DAWN patients; 31 patients with NIHSS 6–9, 33 with CTL, and 13 with pre-morbid mRS 2 (some patients met multiple criteria). For CTL patients, median 24hour infarct volume was 119ml (IQR 74.6–180) versus 31.5ml (IQR 17.6–64.3) for Core Not Too Large (CNTL) patients, p= <0.001. Complications and functional outcomes were similar between the groups. Thrombectomy in CTL patients compared to the remaining DEFUSE 3 Non-Dawn patients conveyed benefit for functional outcome (OR 20.9, CI 1.3–337.8). Comparing the NIHSS 6–9 group with the NIHSS ≥10 patients, mRS 0–2 outcomes were achieved in 74% versus 22% (p=<0.001), with mortality in 6% versus 23% (p=0.024) respectively. For patients with NIHSS 6–9 compared to the remaining DEFUSE 3 Non-Dawn patients thrombectomy trended towards a better chance of functional outcome (OR 1.86, CI 0.36–9.529)
Conclusions
Patients with pretreatment core infarct volumes <70ml but too large for inclusion by DAWN criteria demonstrate benefit from endovascular therapy. More permissive pretreatment core thresholds in core-clinical mismatch selection paradigms may be appropriate. In contrast to data supporting a beneficial treatment effect across the full range of NIHSS scores in the entire DEFUSE 3 population, only a trend towards benefit of thrombectomy in patients with NIHSS 6–9 was found in this small subgroup.
Keywords: Thrombectomy, large core, late window
Introduction
Mechanical thrombectomy for emergent large vessel occlusion stroke (ELVO) is well established as standard of care for patients presenting within 6 hours of last known well (LKW) time.1 The treatment benefit is such that stroke systems of care are being designed to support the rapid mobilization of resources sufficient to treat these emergent large vessel occlusion patients (ELVO).2 Recently, two extended time window trials, Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN)3 and the Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3)4 trial were published. These trials proved that patients with documented ELVO and limited infarcted brain tissue identified on advanced imaging who undergo endovascular therapy in treatment windows later than 6 hours have better functional outcomes than patients treated with standard medical therapy alone. The powerful treatment effect was maintained for appropriately selected populations up to 16 hours (in DEFUSE 3)4 and 24 hours (in DAWN)3 from last known well time (LKW).
In response to this new data the American Heart Association/American Stroke Association (AHA/ASA) Acute Stroke Guidelines for 2018 recommend thrombectomy up to 24hrs under trial inclusion criteria, a position embraced by NeuroInterventional societies.5, 6 The DAWN trial used a selection paradigm that assigned a pre-treatment core infarct threshold (maximum of 50ml) based on patient age and presenting National Institutes of Health Stroke Scale (NIHSS). DEFUSE 3 was more inclusive, taking patients broadly with pre-treatment core infarct volumes up to 70ml who had substantial penumbral volumes. Benefit of thrombectomy was sustained for the DEFUSE 3 patients who were not candidates for inclusion in DAWN.4
A deeper understanding of treatment effect in DEFUSE 3 patients who would have been excluded from the DAWN trial is important, because this group of patients is heterogenous and includes patients with larger core volumes as well as lower presenting NIHSS scores. We hypothesized that the favorable treatment effect is maintained across all subgroups of DEFUSE 3 patients who would have been excluded from DAWN, but the magnitude of treatment signal might differ between subgroups. Differences in treatment effect, if identified, may help guide treatment decisions or inform the study design for future clinical trials in ELVO patients.
Methods
DEFUSE 3 was a randomized, open treatment, blinded endpoint trial to compare endovascular therapy plus medical management versus medical management alone in patients with acute ischemic stroke. Details of the study have been previously published.4 The authors declare that all supporting data are available within the article. The study was funded by the National Institutes of Health (NIH) through StrokeNet, a network of over 300 American hospitals with a central institutional review board that provided ethics approval for the study. Patients were enrolled at 38 US centers if they met both clinical and imaging eligibility requirements and could undergo initiation of endovascular treatment between 6 and 16 hours from LKW (including symptoms on waking). All patients or their legal representatives provided written informed consent before randomization.
Patient Eligibility
Eligible patients had an NIHSS of ≥6, pre-stroke mRS 0–2, initial infarct volume (ischemic core) <70ml, a ratio of volume of ischemic tissue (penumbra) to infarction of ≥1.8 and an absolute volume of potentially reversible ischemia of ≥15ml. Estimates of the volume of the ischemic core and penumbral regions were calculated with automated image processing software, called RAPID (iSchemaView, Menlo Park, California), using CT or MRI perfusion imaging. Upon meeting eligibility criteria, patients were randomly assigned in a 1:1 ratio to endovascular therapy plus medical management vs. medical management alone using a web-based dynamic randomization system.
Standard medical therapy based on current AHA/ASA Guidelines was administered to patients in both arms of the study, including tPA for eligible patients presenting with 4.5 hours of onset who were subsequently randomized in the DEFUSE 3 time-window. For endovascular patients, groin puncture had to occur within 90 minutes of the qualifying imaging, and any FDA-approved thrombectomy device could be used for thrombectomy, at the discretion of the neuro-interventionalist.
By comparison, eligibility for ELVO patients after 6 hours from LKW in the DAWN trial required an NIHSS of ≥10, pre-stroke mRS 0 or 1, and evidence of a clinical deficit in excess of the volume of infarcted tissue (core-clinical mismatch). Established core infarct was defined on MR-DWI or automated CT perfusion maps using the same automated software as DEFUSE 3, and patients categorized into 3 groups using the following parameters: 0–20ml core infarct and NIHSS ≥10 and age ≥80 years old, 0–30ml core infarct and NIHSS ≥10 and age <80 years old, 31–50ml core infarct and NIHSS ≥20 and age <80 years old. The trial utilized the Trevo device (Stryker Neurovascular) exclusively. Trial details are also previously published.3 Both trials were IRB approved and HIPAA compliant.
Outcomes
The primary outcome measure in DEFUSE 3 was modified Rankin Score (mRS) 7 at 90 days. A score of 0–2 points (functional independence) served as the secondary efficacy measure. For the current study, the secondary outcome measure (mRS 0–2 at 90 days) was used as the primary endpoint, because the small sample size resulted in violation of the proportional odds assumption for the ordinal analysis. A variety of additional outcome measures (angiographic, radiologic and safety) were assessed.
Subgroup Determination
The eligibility criteria of the DAWN trial were applied to the DEFUSE 3 patient data and DEFUSE 3 patients not meeting DAWN criteria (DEFUSE 3 non-DAWN) were identified. Three reasons led to DAWN exclusion criteria that would still have allowed inclusion to be met for DEFUSE 3 patients: infarct core too large (based on DAWN NIHSS and age criteria), NIHSS too low (presentation scores of 6–9), or baseline mRS of 2 at presentation. Only 13 DEFUSE 3 patients had a pre-stroke mRS of 2, which was inadequate to address whether these patients had a favorable response to thrombectomy; therefore, these patients were categorized by their NIHSS scores, based on the fact they would have not proceeded to imaging evaluation under the DAWN trial exclusion process. Three had NIHSS 6–9 on presentation and therefore these 3 were included in the analysis of patients with NIHSS too low for DAWN. Analysis was therefore performed for Core Too Large (CTL) versus Core Not Too Large (CNTL) patients, and for NIHSS 6–9 versus NIHSS ≥10 patients. These individual subgroups were compared to the remainder of the DAWN-ineligible DEFUSE 3 population, as well as the entire DEFUSE 3 population. The primary outcome analyzed was dichotomized functional neurological outcome (mRS 0–2), with relationships between clinical, imaging and procedural outcomes assessed.
Statistical Analysis
For the description of the general characteristics of the study population, and comparison to the DEFUSE 3 population as a whole, percentages are reported for categorical variables, means and standard deviations for parametric variables, and medians and interquartile ranges (IQRs) for nonparametric variables. The Pearson χ2 test, Student’s t-test, and Wilcoxon rank-sum tests, as appropriate, were used to compare categorical and continuous variables between patient groups, respectively. Adjusted logistic models of the mRS 0–2 outcome were developed for the patients from DEFUSE 3 that would have been ineligible for DAWN based on low baseline NIHSS (6–9) or core volumes that were too large (patients aged ≥80 years with core volume >20ml, patients aged <80 years with baseline NIHSS <20 and core volume >30ml, and all patients with core volumes >50ml). In 3 cases, patients had both baseline NIHSS scores less than 10 and a core too large for DAWN eligibility. These patients were classified as ineligible based on their NIHSS scores because that criterion alone would have excluded them from DAWN imaging evaluation.
Data already supports the benefit of thrombectomy for the DEFUSE 3 Non-Dawn population as whole.4 Our goal of evaluating the effect of thrombectomy within that population, and whether one subgroup within that population benefits more than another, was restricted to comparing subgroups within the DEFUSE 3 Non-DAWN population alone. Results of the logistic models were reported as raw and adjusted risk-ratios. The adjusted risk-ratios were evaluated with 95% confidence intervals. Distribution of the mRS among the DEFUSE 3 Non-DAWN patient subgroups was presented in horizontal bar graphs.
Results
DEFUSE 3 patients were recruited from May 2016 to May 2017, when the trial was terminated early for efficacy by the DSMB; 182 patients were randomized. Applying the eligibility criteria of the DAWN trial to the DEFUSE 3 data, direct population overlap was 62%. For the 71 enrolled DEFUSE 3 non-DAWN eligible patients the reasons for ineligibility were as follows: 31 patients with NIHSS too low (presentation scores of 6–9), 33 unique patients with infarct core too large (based on DAWN NIHSS and age criteria, with 8 patients overlapping more than a single DAWN core volume category), and 13 patients with baseline mRS of 2 at presentation (some patients met more than one criteria).
CTL Patients
Baseline Characteristics
Table 1 details characteristics and outcomes for the patients with CTL compared to the entire DEFUSE 3 CNTL patients. The CTL population contained 42% of patients older than 79 years, compared to 21% in the CNTL group (p=0.016). No significant difference in method of stroke detection was present; approximately 50% of both groups were diagnosed with wake-up strokes. Core size was determined by CTP in 70% of patients in the CTL group, compared to 74% of patients in the CNTL group. Median ASPECTS for the CTL patients was 7, compared to 8 for the CNTL patients (p= 0.02), with no differences noted for level of vascular occlusion. Consistent with the difference in ASPECTS, ischemic core volume was 45ml (IQR 37.6–60.4) compared to 7.3ml (IQR 0–13.9) for the CNTL patients. Significant difference was also present for the measured volume of the perfusion lesion. For the CTL patients, median volume was 145.8ml (IQR 127.3–184.7), and for the CNTL median perfusion volume was 98.7ml (IQR 67.5–144.4). Similar proportions of patients in each group were randomized for intervention (55% of the CTL and 50% of the CNTL cohort).
Table 1:
Characteristics of DEFUSE 3 Non-Dawn Core-Too-Large (CTL) versus all DEFUSE 3 Core Not Too Large (CNTL) Patients
| Baseline | CTL (N=33) | DEFUSE 3 CNTL (N=149) | p-value |
|---|---|---|---|
| Age- median (IQR) Age ≥80yrs- n(%) |
72 (58–83) 14 (42) |
70 (60–78) 32 (21) |
0.512 0.016 |
| Male- n(%) | 19 (58) | 71 (48) | 0.302 |
| NIHSS- Median (IQR) | 18 (14–21) | 15 (11–20) | 0.174 |
| Wake-up Stroke- n(%) Witnessed onset- n(%) Unwitnessed onset- n(%) |
17 (52) 9 (27) 7 (21) |
74 (50) 57 (38) 18 (12) |
0.278 |
| tPA treatment- n(%) | 6 (18) | 12 (8) | 0.103 |
| Randomized to intervention- n(%) | 18 (55) | 74 (50) | 0.612 |
| Imaging | |||
| Imaging with MRI/MRP- n (%) | 10 (30) | 39 (26) | 0.6285 |
| ASPECTS on baseline CT- median (IQR) | 7 (7–9) | 8 (7–9) | 0.020 |
| ICA Occlusion- n(%) | 14 (42) | 54 (36) | 0.507 |
| Good collaterals on CTA- n(%) | 10 (45) | 87 (81) | <0.001 |
| Ischemic core volume, ml- median(IQR) Perfusion lesion volume, ml- median (IQR) |
45.2 (37.6–60.4) 145.8 (127.3–184.7) |
7.3 (0–13.9) 98.7 (67.5–144.4) |
<0.001 <0.001 |
| Time and Process (in HH:MM) | |||
| Onset to imaging- median (IQR) | 9:08 (7:02–11:09) | 10:24 (8:23–12:17) | 0.030 |
| Imaging to puncture- median (IQR) | 0:54 (0:36–1:34) | 0:59 (0:41–1:20) | 0.957 |
| Puncture to reperfusion- median (IQR) | 0:36 (0:27–0:55) | 0:40 (0:27–1:00) | 0.635 |
| Onset to reperfusion- median (IQR) | 11:44 (10:14–13:05) | 12:19 (9:44–13:42) | 0.601 |
| Outcomes | |||
| Functional independence at 90 days- n(%) | 8 (24) | 48 (32) | 0.369 |
| mTICI 2b/3- n(% of endovascular patients) | 16 (89) | 54 (73) | 0.222 |
| Infarct volume at 24hrs- median (IQR) | 119.0 (74.6–180.0) | 31.5 (17.6–64.3) | <0.001 |
| Infarct growth- median (IQR) | 83.9 (39.4–135.8) | 23.8 (11.7–51.1) | <0.001 |
| Reperfusion >90% at 24hrs- n(%) | 13 (50) | 58 (50) | 1.0 |
| Complete recanalization on MRA/CTA- n(%) | 13 (50) | 66 (49) | 0.945 |
| Reperfusion >90% at 24hrs and/or complete recanalization on MRA/CTA – n(%) | 13 (48) | 67 (49) | 0.970 |
| Safety | |||
| Death at 90 days- n(%) | 6 (18) | 30 (20) | 0.799 |
| Symptomatic intracranial hemorrhage- n(%) | 3 (9) | 7 (5) | 0.391 |
| Parenchymal hematoma type 2- n(%) | 2 (6) | 9 (6) | 1.0 |
| Early neurological deterioration- n(%) | 3 (9) | 17 (11) | 0.774 |
Notes: ASPECTS: N=115 & 27; Perfusion lesion volume: N=147 & 33; Good collaterals (modified Tan method, CTA only): N= 22 & 108; Imaging to puncture- median: N=74 & 18; Puncture to reperfusion: N=64 & 16; Onset to reperfusion: N=64 & 16; Infarct volume at 24hrs: N=147 & 32; Infarct growth: N=147 & 32; mTICI 2b/3: N=74 & 18; Reperfusion >90% at 24hrs: N=116 & 26; Complete recanalization on MRA/CTA: N=134 &26; Reperfusion >90% at 24hrs and/or complete recanalization on MRA/CTA: N=138 & 27
Process and Procedural Variables
Time from onset to reperfusion (11 hours 44 minutes for the CTL and 12 hours 19 minutes for CNTL group) and other process metrics were similar between the 2 groups. Target reperfusion was also similar, achieved in 89% of the CTL group compared to 73% of the CNTL group.
Outcomes
No differences in functional outcome were seen between the 2 groups (mRS of 0–2 in 24% of the CTL versus 32% of the CNTL group) despite significant differences in infarct growth between enrollment and 24-hour scans. The CTL group experienced infarct growth of 83.9ml (IQR 39.4–135.8) compared to 23.8ml (IQR space 11.7–51.1) in the CNTL group. For CTL patients, median infarct volume at 24 hours was 119ml (IQR 74.6–180), compared to only 31.5ml (IQR 17.6–64.3) for the CNTL patients. Despite these differences in infarct volumes, rates of death and symptomatic intracranial hemorrhage were equivalent between the 2 groups.
Figure 1 shows the mRS distributions for the patients treated with endovascular versus best medical management for the DEFUSE 3 Non-DAWN patients divided as CTL versus CNTL. For patients with CTL, after adjustment for prognostic factors of age and NIHSS, the OR for functional outcome was 20.9 (CI 1.3–337.8).
Figure 1:
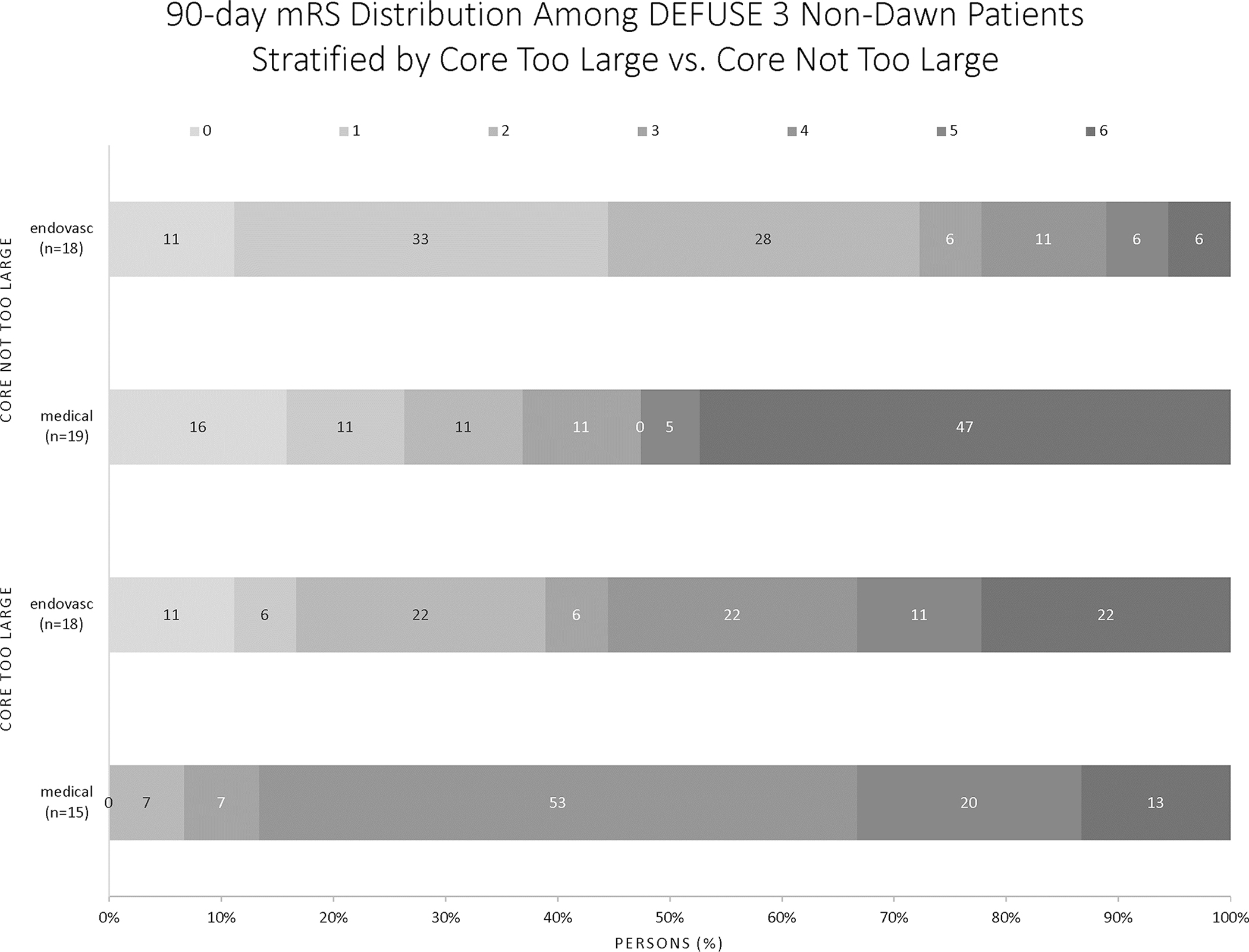
Functional Outcomes for CTL versus CNTL patients in the DEFUSE 3 Non-DAWN population
NIHSS 6–9 Patients
Baseline Characteristics
Table 2 details characteristics and outcomes for the patients with NIHSS 6–9 compared to the entire DEFUSE 3 NIHSS ≥10 patients. Median NIHSS was 8 (IQR 7–9) for this population, compared to 18 (IQR 14–21) for the NIHSS ≥10 cohort. No differences in age, sex, randomization to endovascular therapy or tPA exposure were present. Thirty five percent of the NIHSS 6–9 group was imaged with MRI, compared to 25% of the patients with NIHSS ≥10. Wake up strokes accounted for almost 40% of the NIHSS 6–9 population. Nineteen patients (61%) of the NIHSS 6–9 group and 73 patients (48%) of the NIHSS ≥10 groups were randomized to intervention (p=0.189)
Table 2:
Characteristics of DEFUSE 3 Non-Dawn NIHSS 6–9 versus all DEFUSE 3 NIHSS ≥10 Patients
| Baseline | NIHSS 6–9 (N=31) | DEFUSE 3 NIHSS ≥10 (N=151) | p-value |
|---|---|---|---|
| Age- median (IQR) Age ≥80yrs- n(%) |
63 (57–73) 5 (15) |
72 (60–80) 41 (27) |
0.048 0.198 |
| Male- n(%) | 13 (42) | 79 (52) | 0.292 |
| NIHSS- Median (IQR) | 8 (7–9) | 18 (14–21) | <0.001 |
| Wake-up Stroke- n(%) | 12 (39) | 79 (52) | 0.382 |
| Witnessed onset- n(%) | 14 (45) | 52 (34) | |
| Unwitnessed onset- n(%) | 5 (16) | 20 (13) | |
| tPA treatment- n(%) | 5 (16) | 13 (9) | 0.318 |
| Randomized to intervention- n(%) | 19 (61) | 73 (48) | 0.189 |
| Imaging | |||
| Imaging with MRI/MRP- n (%) | 11 (35) | 38 (25) | 0.238 |
| ASPECTS on baseline CT- median (IQR) | 9 (8–10) | 8 (7–9) | 0.003 |
| ICA Occlusion- n(%) | 9 (29) | 59 (39) | 0.293 |
| Good collaterals on CTA- n(%) | 14 (67) | 83 (76) | 0.361 |
| Ischemic core volume, ml- median(IQR) | 5.0 (0–17.4) | 10.1 (2.7–26.3) | 0.043 |
| Perfusion lesion volume, ml- median (IQR) | 100.1 (66.5–136.4) | 116.9 (77.6–158.8) | 0.057 |
| Time and Process (in HH:MM) | |||
| Onset to imaging- median (IQR) | 10:28 (8:29–12:41) | 10:15 (7:59–11:44) | 0.536 |
| Imaging to puncture- median (IQR) | 1:01 (0:51–1:30) | 0:56 (0:39–1:26) | 0.481 |
| Puncture to reperfusion- median (IQR) | 0:31 (0:25–1:00) | 0:40 (0:28–0:59) | 0.279 |
| Onset to reperfusion- median (IQR) | 12:21 (11:22–15:29) | 12:08 (9:32–13:22) | 0.285 |
| Outcomes | |||
| Functional independence at 90 days- n(%) | 23 (74) | 33 (22) | <0.001 |
| mTICI 2b/3- n(% of endovascular patients) | 13 (68) | 57 (78) | 0.547 |
| Infarct volume at 24hrs- median (IQR) | 33.3 (9.5–64.8) | 39.8 (24.2–110.4) | 0.026 |
| Infarct growth- median (IQR) | 23.2 (6.7–43.1) | 29.1 (13.2–88.1) | 0.117 |
| Reperfusion >90% at 24hrs- n(%) | 14 (52) | 57 (50) | 0.831 |
| Complete recanalization on MRA/CTA- n(%) | 13 (48) | 66 (50) | 0.889 |
| Reperfusion >90% at 24hrs and/or complete recanalization on MRA/CTA – n(%) | 14 (50) | 66 (48) | 0.860 |
| Safety | |||
| Death at 90 days- n(%) | 2 (6) | 34 (23) | 0.024 |
| Symptomatic intracranial hemorrhage- n(%) | 0 (0) | 10 (7) | 0.216 |
| Parenchymal hematoma type 2- n(%) | 0 (0) | 11 (7) | 0.216 |
| Early neurological deterioration- n(%) | 4 (13) | 16 (11) | 0.753 |
Notes: ASPECTS: N=121 & 21; Perfusion lesion volume: N=149 & 31; Good collaterals (modified Tan method, CTA only): N= 21 & 109; Imaging to puncture: N=73 & 19; Puncture to reperfusion: N=66 & 14; Onset to reperfusion: N=66 & 14; Infarct volume at 24hrs: N=148 & 31; Infarct growth: N=148 & 31; mTICI 2b/3: N=73 & 19; Reperfusion >90% at 24hrs: N=115 & 27; Complete recanalization on MRA/CTA: N=133 & 27; Reperfusion >90% at 24hrs and/or complete recanalization on MRA/CTA: N=137 & 28
Process and Procedural Variables
No differences were present between the two groups for process timing, with 12 hours and 21 minutes median time to reperfusion for the NIHSS 6–9 group, compared to 12 hours and 8 minutes for the NIHSS ≥10 patients. Reperfusion rates were similar between the groups, 68% versus 78% respectively (p=0.547), with similar maintained reperfusion at 24hrs.
Outcomes
Ninety-day functional independence was more likely in the low NIHSS group, with 23 patients (74%) in the NIHSS 6–9 group achieving mRS 0–2 compared to 33 patients (22%) of the NIHSS ≥10 group (p= <0.001). The overlapping odds ratios for NIHSS 6–9 vs. ≥10 showed no interaction with treatment effect. Six percent of the NIHSS 6–9 group died, compared to 23% of the NIHSS ≥10 group (p=0.024). Figure 2 shows the mRS distributions for the DEFUSE 3 Non-DAWN patients treated with endovascular versus best medical management for the NIHSS 6–9 and the NIHSS ≥10 groups. For patients with NIHSS 6–9 thrombectomy conveyed a better chance of functional outcome, with an OR of 1.86 (CI 0.36–9.529), however results were not statistically significant in this small subgroup.
Figure 2:
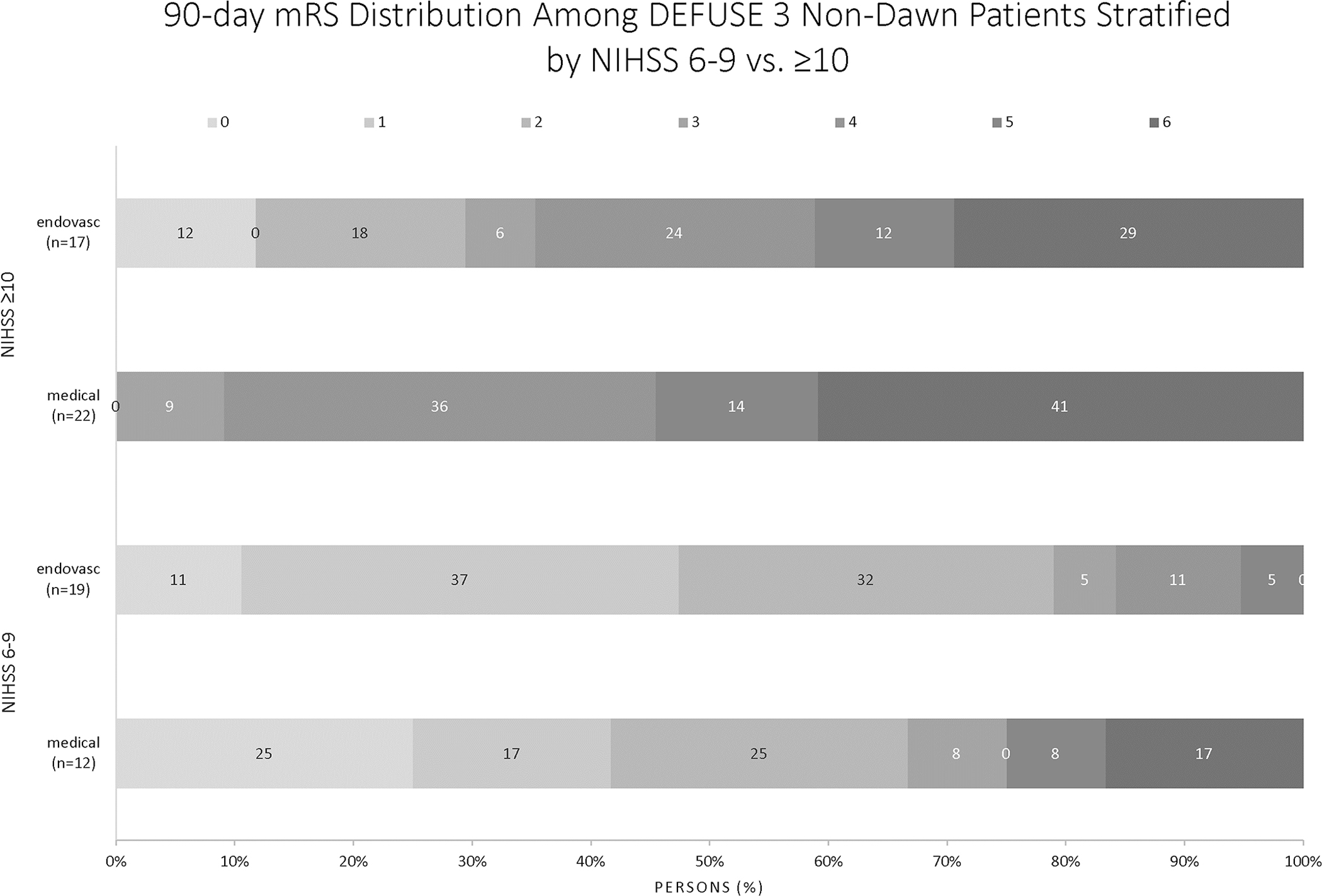
Functional Outcomes for NIHSS 6–9 versus NIHSS ≥10 patients in the DEFUSE 3 Non-DAWN population
Discussion
Our findings demonstrate that for patients treated with mechanical thrombectomy between 6 and 16 hours from LKW, the benefit of thrombectomy appears sustained for DEFUSE 3 patients meeting DAWN exclusion criteria. Despite the small number of patients in the “core too large” subgroup, significant treatment benefit was detected. For those with low NIHSS, there was a favorable trend in this small subgroup. The comparison group in this study was restricted to patients with NIHSS ≥10 who did not meet DAWN criteria, rather than all DEFUSE 3 patients, as detailed in Methods. When the entire DEFUSE 3 population was evaluated, patients with NIHSS as low as 6 were shown to have a significant benefit utilizing the ordinal analysis of the mRS, the primary endpoint of the study.8
It is important to view these results in the context of current stroke care. With irrefutable proof of benefit now established for mechanical thrombectomy, further improvements in treatment delivery and patient outcomes involve three major areas of focus. First, refinement of our identification and triage of ELVO. Second, improved thrombectomy techniques to achieve greater rates of rapid recanalization, and the evaluation of the role of neuroprotection. Third, expansion of our pool of potential patients by identifying a favorable treatment effect in populations currently excluded from thrombectomy care.9 The recent publication of DAWN and DEFUSE 3 adds powerfully to this last goal, demonstrating treatment benefit extending into later treatment windows. As we aim to expand candidacy further still, understanding treatment effect within this late window population is paramount.
Larger pretreatment core infarct increases the likelihood of a poor functional outcome. 10, 11 These observations have understandably led to a therapeutic nihilism towards patients with larger established core infarct, and proved foundational for selection criteria in certain early window trials12–14 as well as DAWN3 and DEFUSE 3.4 Post-treatment infarct volumes have been predictors of 90 day mRS in post-hoc exploratory analyses of the SWIFT PRIME15 and ESCAPE16 trials. The present analysis of CTL patients demonstrated benefit from thrombectomy for a dichotomized outcome of functional independence. The CTL treatment result is noteworthy also for the fact that over 40% of the group were at least 80 years old. These findings suggest a more permissive pretreatment core threshold for core-clinical mismatch selection paradigms may be appropriate.
Not all large core patients are likely to have similar benefit. The threshold pretreatment volume for preserved benefit in patients with larger established core infarct is uncertain. There is promising, emerging data for treatment effect in patients with large established core infarcts. A recent subgroup analysis of MR CLEAN suggested that patients with ASPECTS 5–7 benefitted from thrombectomy, 17 with limited benefit observed for patients with ASPECTS 0–4. Of 53 patients in the THRACE trial with pretreatment core infarct greater than 70ml a total of 12 achieved functional outcome, with outcome determined in part by level of vascular occlusion.18 Therefore, while the frequency of good outcomes is lower in patients that present with large infarcts, there remains a population that likely benefits. Our CTL patients had less robust collaterals and a median infarct volume of 115ml when imaged 24 hours after randomization, and benefit of thrombectomy was demonstrated, despite infarct growth being significantly greater than in the CNTL group. Given similar levels of vascular occlusion between the two groups this likely signifies a relative collateral inadequacy in the CTL patients, and therefore faster infarct progression. As the limits of pretreatment core infarct are tested further it also is likely that our expectations for outcome will need to evolve for patients with core infarct greater than 70ml. In DEFUSE 3 as well as the present analysis, a dichotomized outcome based on functional independence was utilized, but for large core patients this could miss improvement in severe disability or death, and other, more discriminatory metrics are likely to be required to understand the impact of treatment in this population.
Core infarct volume alone is unlikely the sole determinant of treatment response for patients with larger established infarcts at presentation. Patients with large amounts of viable penumbra despite the size of their core infarct may benefit from thrombectomy.19, 20 In this study, patients with core infarcts too large for inclusion in DAWN also demonstrated significant penumbral tissue. DEFUSE 3 inclusions specifically required a mismatch ratio of 1.8 or greater, indicating penumbral tissue almost double the volume of core, and for these patients benefit of thrombectomy is strongly maintained. For patients with large pretreatment core infarcts and limited penumbral tissue volume the impact of recanalization is uncertain.
The potential for increased complications is a concern in late window reperfusion for patients with larger core infarcts.19 We found no evidence of harm in this population, with comparable mortality and symptomatic hemorrhage rates to patients with smaller, DAWN-eligible core infarct sizes. No clear signal for harm has emerged from studies in earlier time windows enrolling patients with lower ASPECTS.21, 22 It is possible that the risk of hemorrhage and reperfusion injury is less than historically feared. Further, late window patients are rarely tPA eligible and are therefore not at risk for tPA-related hemorrhage.23
Similar to patients with large pretreatment core infarct, patients with low NIHSS also represent an area of potential expansion for thrombectomy inclusion.9 Patients in early time windows with NIHSS 6–9 benefit from thrombectomy. This study demonstrates a trend towards this at later timepoints too, though that does not reach statistical significance for the mRS 0–2 outcome. When evaluating the full mRS scale in an adjusted model using the complete DEFUSE 3 database there was benefit on the ordinal shift analysis for patients with a baseline NIHSS as low as 6, with evidence that more severe levels of disability at 90 days were reduced by thrombectomy among patients with mild NIHSS scores at baseline.8 Disability can result from neurological worsening as collaterals fail. Benefit from thrombectomy in patients with lower NIHSS is not as intuitive as for patients with high NIHSS; patients with maintained neurological examinations may have comparatively more adequate collaterals, maintaining not only viability but even function of mildly ischemic tissue. Penumbral sustenance into late windows further selects for those patients with adequate collaterals.
The present study is subject to several limitations, including those typical of subgroup analyses. DEFUSE 3 was terminated early when results of DAWN were presented, and therefore equipoise for further randomization was lost. This limits the sample size for the present analyses. The small sample size limits statistical power, and these data should thus be considered as hypothesis-generating, needing confirmation from larger randomized trials. Further, we cannot comment on treatment in patients after 16 hours. However, these findings provide new insights into the treatment effect in DEFUSE 3 non-DAWN patients and inform future efforts to expand thrombectomy candidacy.
Conclusions
Patients with pretreatment core infarct volumes <70ml but too large for inclusion by DAWN criteria demonstrate benefit from endovascular therapy. This suggests a more permissive pretreatment core threshold for core-clinical mismatch selection paradigms may be appropriate. Enrollment of larger pretreatment core volumes represents an important area of future study. While treatment effect was maintained across all NIHSS thresholds in the DEFUSE 3 study for the primary endpoint, in this subgroup only a trend towards benefit of thrombectomy was seen in late window patients with less severe clinical deficit on presentation.
Acknowledgments
Specific Funding:
DEFUSE 3 was supported by grants from the NIH.
Footnotes
Disclosures:
Leslie-Mazwi, Mlynash, Lansberg: None
Hirsch-Consultant/Advisory Board – Medtronic, Cerenova
Hamilton: Consultant/Advisory Board- Penumbra, Medtronic; Research support- Stanford
Patel: Honoraria- Penumbra, Microvention
Schwamm: Consultant/Advisory board/DSMB-lifeIMAGE, Penumbra, Medtronic, Viz.ai; Research Support- Genentech
Marks: Honoraria-Medtronic, ThrombX Medical
Albers: Ownership-iSchemaView, Consultant/Advisory Board-iSchemaView, Medtronic
References
- 1.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–1731 [DOI] [PubMed] [Google Scholar]
- 2.Leslie-Mazwi T, Chandra RV, Baxter BW, Arthur AS, Hussain MS, Singh IP, et al. Elvo: An operational definition. Journal of neurointerventional surgery 2018;10:507–509 [DOI] [PubMed] [Google Scholar]
- 3.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21 [DOI] [PubMed] [Google Scholar]
- 4.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2018;49:e46–e110 [DOI] [PubMed] [Google Scholar]
- 6.Leslie-Mazwi T, Chandra RV, Fraser JF, Hoh B, Baxter BW, Albuquerque FC, et al. Aha/asa 2018 ais guidelines: Impact and opportunity for endovascular stroke care. Journal of neurointerventional surgery 2018;10:813–817 [DOI] [PubMed] [Google Scholar]
- 7.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke; a journal of cerebral circulation 1988;19:604–607 [DOI] [PubMed] [Google Scholar]
- 8.Albers GWMM, Lansberg M; on behalf of the DEFUSE 3 Investigators. Key subgroup analyses from the defuse 3 study European Stroke Journal 2018;3:204 [Google Scholar]
- 9.Jovin TG, Albers GW, Liebeskind DS, Consortium SI. Stroke treatment academic industry roundtable: The next generation of endovascular trials. Stroke; a journal of cerebral circulation 2016;47:2656–2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo AJ, Verduzco LA, Schaefer PW, Hirsch JA, Rabinov JD, Gonzalez RG. Mri-based selection for intra-arterial stroke therapy: Value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke; a journal of cerebral circulation 2009;40:2046–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo AJ, Chaudhry ZA, Nogueira RG, Lev MH, Schaefer PW, Schwamm LH, et al. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke; a journal of cerebral circulation 2012;43:1323–1330 [DOI] [PubMed] [Google Scholar]
- 12.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-pa vs. T-pa alone in stroke. N Engl J Med 2015;372:2285–2295 [DOI] [PubMed] [Google Scholar]
- 13.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. The New England journal of medicine 2015;372:1009–1018 [DOI] [PubMed] [Google Scholar]
- 14.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. The New England journal of medicine 2015;372:1019–1030 [DOI] [PubMed] [Google Scholar]
- 15.Albers GW, Goyal M, Jahan R, Bonafe A, Diener HC, Levy EI, et al. Relationships between imaging assessments and outcomes in solitaire with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke. Stroke 2015;46:2786–2794 [DOI] [PubMed] [Google Scholar]
- 16.Al-Ajlan FS, Goyal M, Demchuk AM, Minhas P, Sabiq F, Assis Z, et al. Intra-arterial therapy and post-treatment infarct volumes: Insights from the escape randomized controlled trial. Stroke; a journal of cerebral circulation 2016;47:777–781 [DOI] [PubMed] [Google Scholar]
- 17.Yoo AJ, Berkhemer OA, Fransen PSS, van den Berg LA, Beumer D, Lingsma HF, et al. Effect of baseline alberta stroke program early ct score on safety and efficacy of intra-arterial treatment: A subgroup analysis of a randomised phase 3 trial (mr clean). Lancet Neurol 2016;15:685–694 [DOI] [PubMed] [Google Scholar]
- 18.Gautheron V, Xie Y, Tisserand M, Raoult H, Soize S, Naggara O, et al. Outcome after reperfusion therapies in patients with large baseline diffusion-weighted imaging stroke lesions: A thrace trial (mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke) subgroup analysis. Stroke; a journal of cerebral circulation 2018;49:750–753 [DOI] [PubMed] [Google Scholar]
- 19.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (defuse) study. Annals of neurology 2006;60:508–517 [DOI] [PubMed] [Google Scholar]
- 20.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. Mri profile and response to endovascular reperfusion after stroke (defuse 2): A prospective cohort study. Lancet Neurol 2012;11:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. The New England journal of medicine 2015;372:11–20 [DOI] [PubMed] [Google Scholar]
- 22.Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (thrace): A randomised controlled trial. Lancet Neurol 2016;15:1138–1147 [DOI] [PubMed] [Google Scholar]
- 23.Docagne F, Parcq J, Lijnen R, Ali C, Vivien D. Understanding the functions of endogenous and exogenous tissue-type plasminogen activator during stroke. Stroke; a journal of cerebral circulation 2015;46:314–320 [DOI] [PubMed] [Google Scholar]


