Abstract
The performance of three filtering facepiece respirators (two models of N99 and one N95) challenged with an inert aerosol (NaCl) and three virus aerosols (enterobacteriophages MS2 and T4 and Bacillus subtilis phage)—all with significant ultrafine components—was examined using a manikin-based protocol with respirators sealed on manikins. Three inhalation flow rates, 30, 85, and 150 l min−1, were tested. The filter penetration and the quality factor were determined. Between-respirator and within-respirator comparisons of penetration values were performed. At the most penetrating particle size (MPPS), >3% of MS2 virions penetrated through filters of both N99 models at an inhalation flow rate of 85 l min−1. Inhalation airflow had a significant effect upon particle penetration through the tested respirator filters. The filter quality factor was found suitable for making relative performance comparisons. The MPPS for challenge aerosols was <0.1 μm in electrical mobility diameter for all tested respirators. Mean particle penetration (by count) was significantly increased when the size fraction of <0.1 μm was included as compared to particles >0.1 μm. The filtration performance of the N95 respirator approached that of the two models of N99 over the range of particle sizes tested (∼0.02 to 0.5 μm). Filter penetration of the tested biological aerosols did not exceed that of inert NaCl aerosol. The results suggest that inert NaCl aerosols may generally be appropriate for modeling filter penetration of similarly sized virions.
Keywords: filter, penetration, respirator, ultrafine, virus
INTRODUCTION
Filtering facepiece respirators (FFRs) are protective devices used in numerous workplaces to reduce airborne particulate exposures. The US Bureau of Labor Statistics in partnership with the National Institute for Occupational Safety and Health (NIOSH) estimated in 2001 that over 200 000 private establishments in the US—totaling ∼1.9 million workers—had utilized disposable particulate FFRs in the 12 months prior to being surveyed (NIOSH, 2003).
Certification of respirator filtration under 42 CFR 84.181, non-powered air-purifying particulate filter efficiency level determination, is the portion of the regulations most salient to this paper (DHHS, 1995). Certification protocols test the filtration capability of respirators utilizing one of two polydisperse challenge aerosols: NaCl (for use against solid aerosols) or dioctylphthalate (DOP, for use against oil-based liquid aerosols). The challenge aerosols are intended to possess a mass median aerodynamic diameter of ∼0.3 μm, which is the approximate most penetrating particle size (MPPS) for filters as predicted by classic mechanical filtration theory (Hinds, 1999; Lee and Mukund, 2001). Certification conditions are supposed to represent the ‘worst case’ or ‘very severe’ scenario in testing filtration, i.e. a certified air-purifying FFR is intended to filter workplace aerosols as effectively (or more effectively) as it does when tested with the challenge aerosols under the NIOSH testing protocol.
Some limitations of the existing protocol for testing filters with electret properties challenged with ultrafine particles (<0.1 μm) have been discussed in our recent paper (Eninger et al., 2008). Previous studies, reviewed in the above-cited paper have shown that for many electret filter materials, including those used to manufacture N-type respirator filters (N95 and N99), an uncharged or Boltzmann-charged (charge neutral) aerosol has the MPPS ≤ 0.1 μm in physical diameter. The shift in the MPPS from ∼0.3 to <0.1 μm has been attributed to the electret properties of the respirator filter (Lathrache and Fissan, 1986; Lathrache et al., 1986), specifically to the polarization force affecting an electrically neutral particle and consequently changing the function of penetration versus particle size (Martin and Moyer, 2000; Balazy et al., 2006a).
The conventional protocol utilizes two aerosol photometers—one before and one after the filter—to measure the particle penetration (DHHS, 1995; TSI, 2005, 2006). Photometer output signals are approximately proportional to aerosol mass and used to calculate filter penetration, P, as:
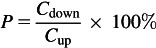 |
(1) |
where Cdown is the challenge aerosol concentration downstream of the respirator filter, and Cup is the aerosol concentration upstream. However, photometry does not effectively detect the ultrafine aerosol fraction; it generally poorly detects the contribution of particles below ∼0.2 μm (Gebhart, 2001; Eninger et al., 2008). As a challenge aerosol, NaCl has a significant fraction within the ultrafine size range: ∼68% of particles by count are <0.1 μm (while the DOP challenge has ∼10% of particles <0.1 μm by count). However, the amount of light scatter available for photometer detection contributed by the ultrafine fraction of both challenge aerosols is negligible, making the test conditions not fully adequate for the filter performance evaluation at MPPS <0.1 μm. Thus, the existing NIOSH certification protocol has a limitation in providing respirator users and occupational hygiene/health professionals with information on the ability of a respirator to filter ultrafine aerosols (Eninger et al., 2008).
At the same time, the need in controlling ultrafine particle exposures has increased in recent years. Although the ultrafine component of occupational aerosols rarely contributes in a major way to exposure in mass terms, it can pose a significant exposure in terms of particle count or surface area (Donaldson et al., 2001). Welding fume, diesel exhaust and some biological airborne particles are examples of aerosols containing a considerable ultrafine fraction (Vincent and Clement, 2000). An expanding source of ultrafine occupational exposures is the employment of engineered nanoparticles (Roco and Bainbridge, 2001; Maynard and Kuempel, 2005). Potential health effects of nanoparticle exposure are of an increasing interest (HSE, 2004; NIOSH, 2004, 2005a,b; Oberdörster et al., 2007). Biological aerosols such as airborne viruses and fungal fragments often belong to the ultrafine fraction (Reponen et al., 2001; Cho et al., 2005). Both severe acute respiratory syndrome (SARS) and highly pathogenic influenza are caused by virions that can be <0.1 μm. Recent work by Morawska (2006) demonstrated that bioaerosol droplets can quickly dry in air to submicrometer and even ultrafine sizes and remain airborne for prolonged periods, thus representing a risk for infection.
Despite the need, there are limited data that can be utilized by health and safety practitioners for guidance in selecting respiratory protective devices for use with ultrafine aerosols, including airborne viruses. While some data on the filter performance of N95 respirators against nanoscale particles and MS2 virions have been recently published by this research group (Balazy et al., 2006a,b), no similar performance information is available for N99 respirators (which are increasingly used in occupational environments, including healthcare settings). The purpose of this investigation was 2-fold: (i) to evaluate size-fractioned filter penetration of N99 FFRs against inert and biological ultrafine aerosols at a wide range of inhalation flow rates—from 30 to 150 l min−1 and (ii) to compare respirator filter penetration values within- and between-filter classes, model and challenge aerosol type (inert and biological). Thus, it is intended to serve as a follow-up of our previous work (Balazy et al., 2006a,b) that examined N95 respirators at 30 and 85 l min−1. The data collected in the present study provide respirator users with additional information for comparing filtration of N99 and N95 FFRs against ultrafine particles, including virions.
METHODS
Study design
The initial filter penetration through two N99 FFRs and one N95 FFR (selected for comparison) was evaluated at three flow rates (30, 85 and 150 l min−1) against two types of challenge aerosol: inert and biological. The selected inert aerosol, NaCl of ∼20 to 500 nm in particle size, was utilized in testing all three respirators; the biological aerosols included MS2 bacteriophage virus (used to test all three respirators), Bacillus subtilis bacteriophage virus (N95 respirators) and enterobacteriophage virus type T4 (N95 respirators). Since most occupational exposures to ultrafine particles are low in mass terms and most FFRs are intended to be disposable respirators, the majority of their use (particularly in healthcare settings) will be in conditions with little or no particle loading. Therefore, this study examined only initial respirator filter performance and did not address filter loading. Additionally, this study did not evaluate the respirator face-seal leakage.
Test system
The test system presented in Fig. 1 has been described in our earlier publications (Balazy et al., 2006a,b). Challenge aerosol penetration through the respirators was evaluated using a manikin-based protocol. The respirator was sealed to a manikin face, leak tested and placed inside of a 0.096 m3 test chamber. The challenge aerosol concentrations were measured upstream and downstream of the respirator facepiece. The aerosols were generated with a 6-jet Collison nebulizer (BGI Inc., Waltham, MA, USA), diluted and dried with clean air, charge equilibrated to a Boltzmann charge distribution using a Kr85 sealed source (Model 3054, TSI Inc., Minneapolis, MN, USA) and fed to the top of the test chamber. Constant inhalation flow was drawn through the probed manikin while size-fractioned particle counts from 20 to 500 nm in diameter were recorded outside and inside of the respirator facepiece using a Wide-range Particle Spectrometer (WPS, Model 1000 XP, MSP Corp., Shoreview, MN, USA) connected to the data acquisition system.
Fig. 1.
Filter penetration test system. Diagram adapted from Balazy et al. (2006a,b).
Respirator selection and test conditions
The two models of N99 and one model of N95 FFRs selected for this study are commonly used in industry and healthcare settings, based on the recommendations from the University of Cincinnati Occupational Pulmonary Services (Director, Roy McKay) that performs respirator fit testing and training for numerous industries in the US. The N95 respirator was of the same make and model as tested in our previous studies (Balazy et al., 2006a,b). This model demonstrated relatively higher filtration of ultrafine particles when compared to other N95 models evaluated in our laboratory. Different manufacturers supplied the two N99 respirators (N99-A and N99-B).
The constant airflows (Q) of 30, 85 and 150 l min−1 were selected to represent different inhalation regimes. The first represents inhalation during low/moderate-intensity work. The second corresponds to a hard workload and is used by NIOSH for respirator filtration certification. The flow rate of 150 l min−1 was intended to represent an instantaneous peak inspiratory flow (PIF) during moderate to strenuous work (Harber et al., 1984; Lafortuna et al., 1984; Cassidy et al., 2003). Consensus is not found in the literature for a representative occupational ventilation rate for PIF. However, the range of PIFs for the 95th percentile minute volume for occupational tasks is estimated to range between 182 and 295 l min−1 (Caretti et al., 2004). Therefore, the choice of 150 l min−1 may underestimate a worst case PIF. Studying respirator filtration at higher inhalation flow rates is salient, at least, for two reasons. First, the rate established by the NIOSH protocol (85 l min−1) may be exceeded during more strenuous occupational tasks. Second, modern FFR media relies upon electret properties for much of the overall filtration efficiency (Martin and Moyer, 2000; Caretti et al., 2004). For ultrafine particles, the primary filter capture mechanisms are diffusion and electrostatic interaction, which are both strongly dependent upon respirator face velocity. This suggests the lowest collection efficiency (highest penetration) at the highest inhalation flow rate (Lathrache and Fissan, 1986; Lathrache et al., 1986; Lee and Mukund, 2001).
Temperature and relative humidity were monitored during the tests using a DeltaTrak Thermo-Hygrometer (Model 13306, DeltaTRAK, Inc., Pleasanton, CA, USA). Relative humidity was maintained between 40 and 45% while temperature ranged from 23 to 26°C.
Selection and preparation of viruses
Three viruses were selected for use in filtration testing: enterobacteriophage types MS2 and T4 and bacteriophage B. subtilis SP01. These were chosen for their small particle sizes, low pathogenicity and ease of preparation and use. We intended to perform the tests with (i) the smallest virions as well as (ii) larger ones—of similar dimensions to those of the SARS coronavirus [∼80 nm diameter (Goldsmith et al., 2004)] and influenza A virus subtype H5N1 [∼80 to 100 nm diameter (Madigan and Martinko, 2006a)]. MS2 has about the smallest size among viruses. T4 and B. subtilis bacteriophage are larger and close to the SARS coronavirus and H5N1 by their volumetric equivalent sizes. It is acknowledged, however, that the latter two simulants are considerably different from the targets in terms of virion shape and aspect ratio, which may influence their filtration properties (Willeke et al., 1996; Flagan, 2001; Rengasamy et al., 2004). This is addressed further in the discussion.
MS2 is an icosahedral RNA bacteriophage which infects the male Escherichia coli bacteria (Valegård et al., 1990). An icosahedron is a symmetric polyhedron with 20 triangular faces (Fig. 2); its shape is close to spherical (Madigan and Martinko, 2006a). A single MS2 virion has a physical diameter of ∼28 nm (Valegård et al., 1990; Madigan and Martinko, 2006b). T4 bacteriophage—which also infects many E. coli bacterial strains—is a double-stranded DNA bacteriophage with asymmetric icosahedral head, helical tail, endplate and tail fibers as shown in Fig. 2. A mature T4 virion is non-spherical. It is ∼225 nm along its longest axis including the head (∼85 × 100 nm), the tail (∼25 × 100 nm) and the endplate (∼50 × 25 nm) (Leiman et al., 2003). Bacillus subtilis bacteriophage SP01 is also a double-stranded DNA bacteriophage with a structure similar to that of the T4 bacteriophage except with a roughly symmetrical icosahedral head (Hemphill and Whitely, 1975). A mature B. subtilis bacteriophage SP01 is typically 237 nm along its longest axis with a head and tail that measure 87 × 90 and 20 × 147 nm, respectively (Hemphill and Whitely, 1975).
Fig. 2.
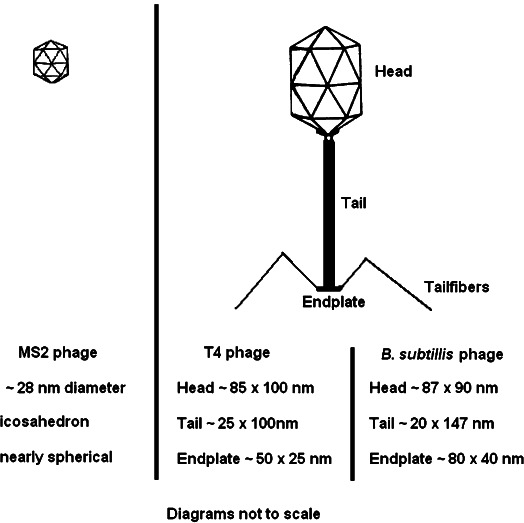
Shape and dimensions of the bacteriophages used in this study.
MS2 (ATCC 15597-B1) and B. subtilis bacteriophage (ATCC 27370-B1) suspensions were prepared using lysis of host bacterial solutions—E. coli (ATCC 15597) and B. subtilis (ATCC 27370), respectively. This was followed by centrifugation to remove bacterial cells, their debris and particles from the medium then filtration with 0.4 μm sterile Millipore filter (Millipore Corp., Billerica, MA, USA). T4 bacteriophage suspensions were prepared from freeze-dried phage vial (ATCC 35060-B4) by adding 9 ml of Luria–Bertani broth followed by serial dilution. Suspensions of each phage for aerosol experiments were diluted to titre of 108–109 plaque-forming units per ml as determined by a modified plaque assay (ISO, 2000). ASTM reagent water purity type I ultrafiltered water was used for all suspensions (ASTM, 2006).
Filter penetration and quality factor
Particle concentrations were measured size selectively outside and inside the respirator filter when the inhalation flow was applied. The data were recorded in 24 size channels of the WPS’ differential mobility analyzer ranging from 0.021 to 0.449 μm in particle electrical mobility diameter. Size-fractioned penetration was calculated using equation (1). Another metric of filter performance determined in this study was the filter quality factor, qf, which incorporates airflow resistance (characterized by the pressure drop, Δp, in mmH2O) and the particle penetration (P, %) (Hinds, 1999).
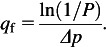 |
(2) |
An ideal respirator filter is characterized by low penetration and low pressure drop. Pressure drop across the filter media was measured at each inhalation flow rate using a magnehelic pressure gage (Dwyer Instruments, Inc., Michigan City, IN, USA).
Data analysis
The tests were replicated three times for each of the tested respirators and challenge aerosols. The mean, peak and standard deviation of the size-fractioned particle penetration were calculated for each combination of respirator, airflow rate and challenge aerosol. The pressure drop measured for a given respirator and airflow was applied to the corresponding size-fractioned penetration value to obtain the filter quality factor. Mean penetration (±1 SD) and filter quality factor were then plotted against electrical mobility particle diameter.
Between-respirator comparisons of the aerosol penetration were performed for two challenge aerosols: NaCl and MS2. The particle penetration through filters of all three respirator models was compared first using NaCl data and then using MS2 data. Within-respirator comparisons of penetration values for NaCl versus MS2 were also performed for all three tested respirator models. This database allowed us to compare the filter penetration of inert NaCl particles and airborne virions of the same particle sizes. Lastly, a within-respirator comparison with respect to penetration of NaCl, B. subtilis bacteriophage and T4 bacteriophage was performed for the N95 respirator. This also allowed comparing the filtration efficiency of inert particles to that of two biological aerosols. Overall, six comparative analyses were performed, as summarized below.
Between-respirator comparisons:within-respirator comparisons:
(1) NaCl challenge aerosol: compare penetration through N99-A, N99-B and N95 filters;
(2) MS2 challenge aerosol: compare penetration through N99-A, N99-B and N95 filters;
(3) Model N99-A: compare penetration of NaCl and MS2;
(4) Model N99-B: compare penetration of NaCl and MS2;
(5) Model N95: compare penetration of NaCl and MS2 and
(6) Model N95: compare penetration of NaCl to that of phage B. subtilis and phage T4.
Comparisons 1 and 2 were performed using analysis of variance (ANOVA). Comparisons 3–5 were run using Student's t-test. Both ANOVA and Student's t-test with Bonferroni adjustment were utilized for Comparison 6. All tests were performed using Excel (Microsoft Corp., Redmond, WA, USA) at a significance level of 0.05.
RESULTS AND DISCUSSION
Aerosol penetration and filter quality factor
Aerosol penetration, pressure drop and quality factor for each test aerosol and inhalation flow rate are summarized in Table 1. For NaCl, the following specific particle sizes and ranges were selected for this summary table:
Table 1.
Summary of aerosol penetration (P), pressure drop and quality factor (qf) for three respirators
| Aerosol: NaCI | ||||||||||
| Respirator | Q (l min−1) | Pressure drop (mmH2O) | P 0.1μm (%) | q f 0.1 μm (1 per mmH2O) | P 0.3 μm (%) | q f 0.3 μm (1 per mmH2O) | P 0.02–0.5 μm (%) | q f 0.02–0.5 μm (1 per mmH2O) | P 0.1–0.5 μm (%) | q f 0.1–0.5 μm (1 per mmH2O) |
| N99 Model A | 30 | 3.90 ± 0.20 | 0.66 ± 0.04 | 1.29 ± 0.06 | 0.35 ± 0.03 | 1.45 ± 0.10 | 0.75 ± 0.04 | 1.29 ± 0.06 | 0.43 ± 0.03 | 1.41 ± 0.08 |
| 85 | 10.67 ± 0.58 | 2.92 ± 0.46 | 0.33 ± 0.03 | 0.99 ± 0.24 | 0.44 ± 0.05 | 3.20 ± 0.46 | 0.34 ± 0.03 | 1.60 ± 0.30 | 0.40 ± 0.04 | |
| 150 | 24.33 ± 2.08 | 5.14 ± 0.58 | 0.12 ± 0.01 | 2.07 ± 0.26 | 0.16 ± 0.01 | 5.93 ± 0.61 | 0.12 ± 0.01 | 3.13 ± 0.38 | 0.15 ± 0.01 | |
| N99 Model B | 30 | 4.53 ± 0.15 | 0.74 ± 0.10 | 1.08 ± 0.06 | 0.44 ± 0.22 | 1.21 ±0.09 | 0.56 ± 0.11 | 1.20 ± 0.05 | 0.56 ± 0.18 | 1.17 ± 0.07 |
| 85 | 13.00 ± 1.00 | 2.78 ± 0.34 | 0.28 ± 0.02 | 1.27 ± 0.50 | 0.34 ± 0.05 | 2.36 ± 0.20 | 0.31 ± 0.02 | 1.65 ± 0.14 | 0.32 ± 0.02 | |
| 150 | 24.67 ± 1.15 | 4.87 ± 0.94 | 0.12 ± 0.01 | 3.07 ± 1.96 | 0.15 ± 0.02 | 4.23 ± 1.27 | 0.13 ± 0.01 | 3.60 ± 1.53 | 0.14 ± 0.01 | |
| N95 | 30 | 2.70 ± 0.10 | 0.83 ± 0.20 | 1.78 ± 0.10 | 0.48 ± 0.14 | 1.99 ± 015 | 0.87 ± 0.21 | 1.80 ± 0.10 | 0.66 ± 0.21 | 1.89 ± 0.14 |
| 85 | 7.57 ± 0.75 | 2.60 ± 0.51 | 0.49 ± 0.02 | 1.34 ± 0.44 | 0.58 ± 0.02 | 2.85 ± 0.44 | 0.49 ± 0.03 | 1.74 ± 0.41 | 0.54 ± 0.03 | |
| 150 | 15.83 ± 2.02 | 4.65 ± 0.48 | 0.20 ± 0.03 | 2.84 ± 0.38 | 0.23 ± 0.04 | 5.16 ± 0.35 | 0.19 ± 0.03 | 3.42 ± 0.46 | 0.26 ± 0.03 | |
| Aerosol: MS2 | aerosol: Bacillus subtilis phage | aerosol: T4 phage | ||||||||
| Respirator | Q (l min−1) | P 0.02–0.09 μm (%) | Respirator | Q (l min−1) | P 0.1 μm (%) | Respirator | Q (l min−1) | P 0.1 μm (%) | ||
| N99 Model A | 30 | 1.03 ± 0.55 | N95 | 30 | 0.58 ± 0.22 | N95 | 30 | 0.23 ± 0.01 | ||
| 85 | 3.43 ± 0.86 | 85 | 1.90 ± 0.19 | 85 | 0.95 ± 0.11 | |||||
| 150 | 5.45 ± 0.35 | 150 | 3.81 ± 0.60 | 150 | 2.18 ± 0.37 | |||||
| N99 Model B | 30 | 0.96 ± 0.12 | ||||||||
| 85 | 3.28 ± 0.20 | |||||||||
| 150 | 5.70 ± 0.61 | |||||||||
| N95 | 30* | 1.69 ± 0.38 | ||||||||
| 85* | 3.45 ± 0.48 | |||||||||
| 150 | 5.64 ± 1.94 | |||||||||
MS2 data for the N95 respirator are taken from Balazy et al., 2006b for 30 and 84 l min−1.
(i) 0.1 μm representing the approximate mobility sizes of phage B. subtilis and phage T4;
(ii) 0.3 μm representing the presently accepted MPPS;
(iii) 0.02–0.5 μm (integrated mean) representing overall penetration over the entire measured range of NaCl particle sizes and
(iv) 0.1–0.5 μm (integrated mean) representing the particle sizes which primarily contribute to filter efficiency determination using the NIOSH certification protocol.
For viruses, the following particle sizes were designated:
(i) 0.02–0.09 μm to represent the nominal virion size of MS2 and to include aggregates; the rationale for the selection of this particle size range is discussed in greater detail in Balazy et al. (2006b) (note that, resulting from slightly different WPS settings, the upper limit was modified—from 0.08 μm in Balazy et al. to 0.09 μm in this study) and
(ii) 0.1 μm to represent the approximate mobility sizes of phage B. subtilis and phage T4. A single WPS channel with a midpoint of 0.1 μm (range 0.094–0.11 μm) was used for the larger virions because a steep drop in the challenge aerosol particle size distribution beyond 0.1 μm suggested that aggregates, if present, did not considerably contribute to the total particle count.
NaCl challenge aerosol.
Particle penetration increased with increasing airflow for all three respirators (Fig. 3) with the overall mean penetration at 150 l min−1 exceeding that at 30 l min−1 by an average factor of 7.9 (N99 Model A), 7.6 (N99 Model B) and 5.9 (N95). For all three respirators and inhalation airflows, the MPPS was <0.1 μm. Peak penetrations for N99 Model A were 10.2, 5.9 and 1.3%, respectively, for the high, medium and low flow rates; mean penetration at 85 l min−1 was 3.2% (for all particle sizes from 0.02 to 0.5 μm) and 1.6% (calculated specifically for particles from 0.1 to 0.5 μm). For N99 Model B, peak penetrations were 6.6, 4.3 and 1.0% at Q = 150, 85 and 30 l min−1, respectively. The mean penetration at 85 l min−1 was 2.4% for particles 0.02–0.5 μm and 1.7% for 0.1–0.5 μm. The N95 respirator filter, peak penetrations were 8.1, 4.8 and 1.4% at each respective inhalation flow rate. At Q = 85 l min−1, mean penetrations 2.9% (integrated 0.02–0.5 μm) and 1.7% (integrated >0.1–0.5 μm). Mean penetration was significantly higher for all three respirators when taking into account ultrafine sizes as compared to those >0.1 μm. The N95 data are consistent with previous observations using N95 FFRs (Balazy et al., 2006a). It is apparent from Fig. 3 that penetration was quite similar between respirators even though respirator classes differed (N95 versus N99).
Fig. 3.
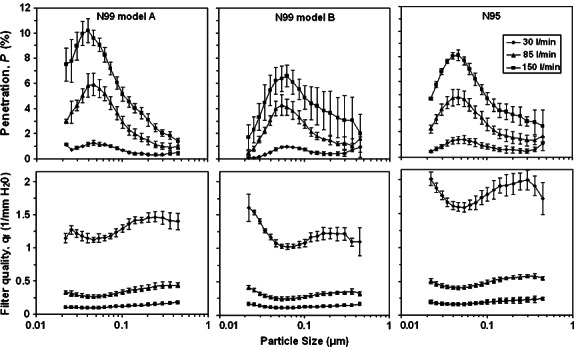
Aerosol penetration and filter quality factor of three respirators as a function of the particle size and inhalation flow rate for NaCl challenge aerosol.
Table 1 shows the pressure drop values across the filter for each respirator and airflow. The N95 FFR demonstrated the lowest resistance at each airflow while N99 Model B possessed the highest. The pressure drop values are consistent with those reported previously for N95 FFRs and N99 filter cartridges by Martin and Moyer (2000). Although Δp differed, particle penetrations appear similar. This can be explained by the charge densities carried by the filter material. Use of electret filters (with charged fibers) allows for increased filter efficiency without increased breathing resistance. The tested N95 filter likely possesses a higher charge density and lower packing density than the N99 respirators.
The size-fractioned filter quality factor (qf) is also shown in Fig. 3 as a function of the NaCl particle size and inhalation flow rate. It is not as dependent on the particle size as filter penetration. While qf is similar between respirators operating at 85 and 150 l min−1, the N95 demonstrates higher quality factor at 30 l min−1 due to its lower pressure drop: 2.7 ± 0.10 mmH2O as compared to 3.9 ± 0.20 and 4.5 ± 0.15 mmH2O measured for N99-A and –B, respectively. The qf value determined for a specific particle sizes of 0.1 and 0.3 μm were similar to the mean value obtained for the size range of 0.02 to 0.5 μm. Filter quality factor was significantly lower for all particle sizes (integrated mean, 0.02–0.5 μm) than for particles calculated specifically for >0.1 μm.
The utility of filter quality factor in assessing the respirator filter performance is not presently established. One reason is that respirator performance also depends upon face-seal leakage, which is not accounted for in filtration studies. Whether face-seal leakage and filter resistance are related in FFRs has not been thoroughly investigated. Although wearer comfort is expected to increase with increasing qf for specific filtration efficiency, this has not been quantitatively studied, and physiologically meaningful differences of filter quality factor have not been assessed. Quality factor has been used previously as a tool for comparing respirators. Han (2000) ranked respirator performance using qf at inhalation flow rates from 10 to 85 l min−1 and utilized a plot of flow rate versus qf to compare FFR. Also, Chen et al. (1992) utilized qf to compare performance of filtering facepieces and respirator cartridges.
MS2 phage challenge aerosol.
Table 1 and Fig. 4 present the mean penetration values for MS2 virus with a designated particle size range of 0.02–0.09 μm. Strongly populated by single virions as well as virus aggregates, this size range accounted for ∼82% of the upstream particle count. Airflow had a strong effect: mean penetration at 150 l min−1 exceeded that at 30 l min−1 by a factor of 5.3 (N99-A), 5.9 (N99-B) and 3.3 (N95). Similar to the trend observed with the NaCl aerosol in this study [and the conclusion made by Balazy et al. (2006a,b) for N95 FFR], at Q = 85 l min−1, the MPPS was < 0.1 μm for all three respirators; peak penetrations were 4.3% (N99-A), 4.6% (N99-B) and 4.3% (N95, data from Balazy et al., 2006b), while mean penetrations were 3.4, 3.3 and 3.5%, respectively. Figure 4 demonstrates relatively high variability in the penetration of the N95 respirator at Q = 150 l min−1, but—again—the trend is consistent with previous observations at 30 and 85 l min−1 (Balazy et al., 2006b).
Fig. 4.
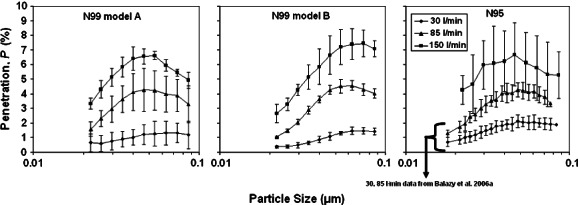
Aerosol penetration through three respirators as a function of the particle size and inhalation flow rate for MS2 bacteriophage challenge aerosol.
Bacillus subtilis and T4 phage challenge aerosols.
Table 1 and Fig. 5 present the data for N95 respirator filter challenged with the B. subtilis phage and T4 phage viruses. The effect of airflow on penetration is readily apparent with the overall mean penetration at 150 l min−1 exceeding that at 30 l min−1 by an average factor of 6.6 (B. subtilis phage) and 9.5 (T4 phage). At 85 l min−1, peak penetrations were 3.4% for the B. subtilis phage aerosol and 2.6% for the T4 phage aerosol occurred at 0.04 μm, which is smaller than the mobility sizes of single virions of B. subtilis and T4 phages estimated based on their physical dimensions. This is attributed to the presence of remnant solutes, biological fragments and impurities associated with preparation and freeze drying. Penetration at the single virion mobility diameter, calculated specifically at 0.1 μm and Q = 85 l min−1 were 1.9% (B. subtilis phage) and 0.95% (T4 phage). Low particle counts for the T4 challenge aerosol resulted in large standard deviations in penetration measurements beyond ∼0.12 μm.
Fig. 5.
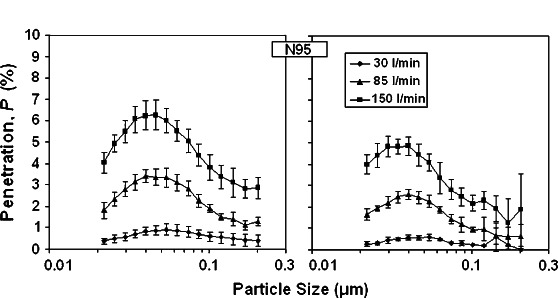
Aerosol penetration through N95 respirator as a function of the particle size and inhalation flow rate for two challenge viruses: Bacillus subtilis bacteriophage (left) and T4 bacteriophage (right).
Between and within-respirator comparisons
The penetration of NaCl aerosol in two particle size ranges was compared between respirators as shown in Fig. 6. Although we expected differences in filtration between respirator classes (N99 was expected to be more efficient in collecting particles than N95), no significant differences in mean penetration were observed for the range of 0.02–0.5 μm (Fig. 6a) or 0.1–0.5 μm (Fig. 6b). However, due to the small sample size, we fall short of concluding that the performance of N99 FFRs is generally no better than that of N95 FFR for the particles up to 0.5 μm. It seems more reasonable to state that filtration of a ‘better’ N95 FFR may approach the performance of some N99 FFR models over the particle sizes observed here when measured by count.
Fig. 6.
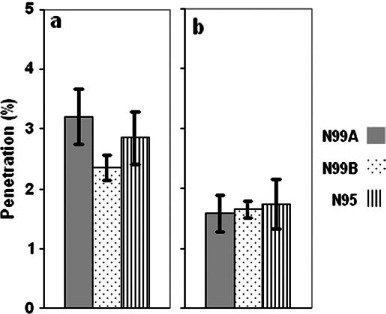
Between-respirator comparison: mean penetration of NaCl [integrated for the size range of 0.02–0.5 μm (a) and 0.1–0.5 μm (b)] at 85 l min−1.
Mean penetration was also compared by particle size range and differed significantly; when analysis was limited to particles of >0.1 μm, mean penetration for all three respirators was significantly lower (P = 0.01) than for particles ranging from 0.02 to 0.5 μm. The greatest contribution to penetration occurred at <0.1 μm for all three respirators. Utilizing a protocol that can also measure the ultrafine component of the test aerosol may result in discovering significantly higher filter penetration (by particle count) than it is anticipated. These observations do not mean that the tested respirators fail to comply with their respective NIOSH certification criteria because the NIOSH certification protocol uses a different method to measure aerosol concentrations to calculate filter penetration (DHHS, 1995).
While no differences were observed between respirators when comparing mean penetration of MS2 aerosol in the designated particle range of 0.02–0.09 μm (see Fig. 7), we also compared the penetration of NaCl to (i) the MS2-containing aerosol for each respirator over the integrated size range of 0.02–0.09 μm (Fig. 8) and (ii) the two larger phages B. subtilis and T4 at their estimated mobility diameter of 0.1 μm (see Fig. 9). The Figures show comparisons for Q = 85 l min−1. These served as direct comparisons of inert particle penetration to that of aerosols containing biological particles over the same mobility diameters. Two differences were observed. At 150 l min−1, there was a significant difference in penetration between MS2 (5.4%) and NaCl (8.5%) for N99 Model A over the integrated size range of 0.02–0.09 μm (P = 0.01, not shown in figure). Also, we found a significant difference between NaCl and T4 phage at 85 l min−1 (P = 0.005) where T4 phage penetration was 0.95% compared to 2.6% for NaCl (Fig. 9) for the N95 FFR. Overall, no biological aerosol penetration exceeded that of inert aerosols.
Fig. 7.
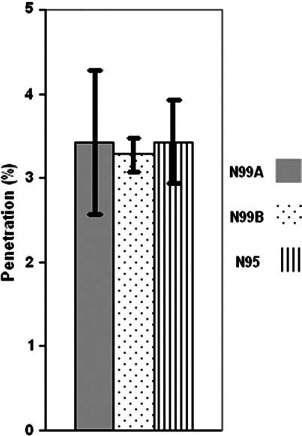
Between-respirator comparison: mean penetration of MS2 (integrated for the size range of 0.02–0.09 μm) at 85 l min−1.
Fig. 8.
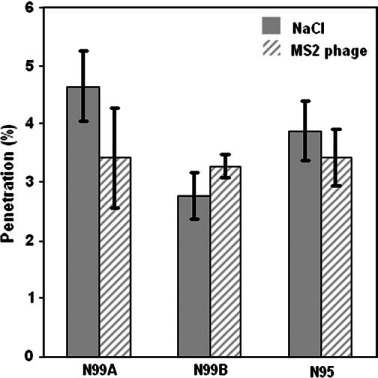
Within-respirator comparison: mean penetration of NaCl and MS2 (integrated for the size range of 0.02–0.09 μm) at 85 l min−1.
Fig. 9.
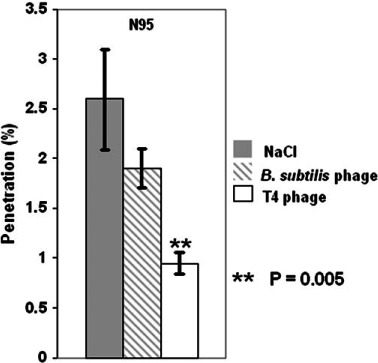
Within-respirator comparison for N95 at 85 l min−1: mean penetration of NaCl compared to Bacillus subtilis phage and T4 phage at 0.1 μm.
Several properties of airborne virus particles may have influenced filtration in this study and could have contributed to the observed—although inconsistent—differences between the inert and biological aerosols. Particle parameters that effect diffusion, the electrostatic collection mechanism or particle adhesion to the filter fibers are believed to be relevant. Particle shape may affect virus particle filtration since it can influence its polarization and formation of dipole charges in an electrical field (Flagan, 2001). Also, shape can influence particle drag by altering terminal velocity toward an influencing fiber, changing the probability of capture (Flagan, 2001). Dynamic shape factors that aid in describing behavior of airborne virus particles have not been investigated. Lastly, shape may also influence filtration through particle rebound. Boskovic et al. (2005, 2007) recently observed differences in filtration efficiency between spheres and perfect cubes of the same electrical mobility diameter up to 0.3 μm. Greater penetration of cubes was ascribed to differences in rebound probability during tumbling at the fiber surface. It is not presently known how the shape of virus aerosol particle may affect its rebound during filtration.
Electrical properties of virions may also influence filtration. With a neutralized aerosol, the virus particle permittivity or dielectric constant is of interest. This represents the ability of a particle to polarize when in an electric field. The degree of polarization will be proportional to the force of attraction between the particle and the influencing fiber (the polarization force). It has been shown theoretically and experimentally that particles with high-dielectric constant are captured by an electret filter with greater efficiency than those with low-dielectric constant (Oh et al., 2002; Yang and Lee, 2005; Wei et al., 2006). The dielectric constant of NaCl is ∼6. While the dielectric constant of the tested virions is not known, similar size virions have been estimated to have dielectric constants of >55 (Aristides et al., 2007; Lepizco-Encinas and Rito-Palomares, 2007).
CONCLUSIONS AND FUTURE WORK
The penetration of four challenge aerosols through three N-type FFRs at three inhalation flow rates was determined. Challenges remain in aerosolizing viruses with the intention of creating a monodisperse aerosol consisting of single virions. As seen in this and other studies, remnant solutes, biological fragments and the possibility of aggregate formation can significantly contribute to the resulting particle size distributions.
Inhalation airflow had a significant impact upon particle penetration. The primary mechanisms of ultrafine particle capture—diffusion and electret charge interaction—are heavily influenced by the filter face velocity. Since the selected 150 l min−1 flow may underestimate the 95th percentile PIF during occupational tasks, additional study seems feasible in this area to better define a very severe or worst case condition. Also, further study of respirator penetration during cyclic breathing with high PIFs is needed.
The pressure drop across the filters was determined and the filter quality factor calculated providing information on relative performance of the respirator filters. However, the salience of this information without reference to performance during respirator wear is limited. Investigation of whether filter quality factor is predictive of actual workplace protection would determine whether it is a meaningful metric of FFR performance.
The MPPS was <0.1 μm for all aerosol challenges. This has been demonstrated previously for electret-type filter materials using physiologically relevant airflows. As a corollary, we also observed that overall respirator penetration increases significantly—when measured by count—if the ultrafine fraction of the test aerosol is properly detected and included in the integration. This finding is important because the NIOSH filter certification protocol assumes an MPPS of 0.3 μm (by mass) and cannot adequately measure aerosol particles <0.1 μm due to limitations of photometry.
We observed that a better performing N95 FFR can approach the filtration performance of some N99 FFRs over the tested particle size range. However, this should be considered with caution and not generalized because the presented results were obtained for a single model of N95 compared to two specific models of N99.
Overall, viral penetration through the tested FFRs did not exceed that of inert NaCl aerosol. We observed a difference between inert and bioaerosol filtration where NaCl penetration exceeded that of MS2 (for N99 Model A at 150 l min−1) and that of T4 phage (for N95 FFR at 85 l min−1) which may be attributed to a number of causes. The results suggest that inert aerosols may generally be appropriate for modeling filter penetration of similarly size viruses.
FUNDING
National Institute for Occupational Safety and Health Education and Research Center Pilot Project Research Training Program (T42/OH008432-02) through the University of Cincinnati Education and Research Center (Cincinnati, OH, USA). Koken Ltd (Tokyo, Japan) to T.H. Finnish Work Environment Fund to H.H.-T.
Acknowledgments
The authors would like to express special gratitude to Roy McKay of the University of Cincinnati for helping to select respirators for this study.
References
- American Society for Testing and Materials (ASTM) Committee D19.02 on general specifications, technical resources, and statistical methods. 2006. ASTM D1193–06. Standard Specification for Reagent Water. West Conshohocken, PA. [Google Scholar]
- Aristides D, Tercero-Espinoza LA, Zhang B, et al. Using non-uniform electric fields to accelerate the transport of viruses to surfaces from media of physiologal ionic strength. Langmuir. 2007;23:3840–8. doi: 10.1021/la061486l. [DOI] [PubMed] [Google Scholar]
- Balazy A, Toivola M, Reponen T, et al. Manikin-based performance evaluation of N95 filtering-facepiece respirators challenged with nanoparticles. Ann Occup Hyg. 2006;50:259–69. doi: 10.1093/annhyg/mei058. [DOI] [PubMed] [Google Scholar]
- Balazy A, Toivola M, Adhikari A, et al. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am J Infect Control. 2006;34:51–7. doi: 10.1016/j.ajic.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Boskovic L, Altman IS, Agranovski IE, et al. Influence of particle shape on filtration processes. Aerosol Sci Technol. 2005;39:1184–90. [Google Scholar]
- Boskovic L, Agranovski IE, Braddock RD. Filtration of nanosized particles with different shape on oil coated fibres. J Aerosol Sci. 2007;38:1220–29. [Google Scholar]
- Caretti DM, Gardner PD, Coyne KM. Workplace breathing rates: defining anticipated values and ranges for respirator certification testing. 2004. Report ECBC-TR-316, Edgewood Chemical Biological Center, US Army Research. Aberdeen Proving Ground, MD: Development and Engineering Command. [Google Scholar]
- Cassidy PE, Anderson NJ, Janssen LL, et al. Mean and maximum peak inspiratory flow values and durations in normal adults exercising at light, moderate, and heavy workloads. 2003 Proceedings, AIHCE. [Google Scholar]
- Chen CC, Lehtimäki M, Willeke K. Aerosol penetration through filtering facepieces and respirator cartridges. AIHAJ. 1992;53:566–74. doi: 10.1080/15298669291360166. [DOI] [PubMed] [Google Scholar]
- Cho S-H, Seo S-C, Schmechel D, et al. Aerodynamic characteristics and respiratory deposition of fungal fragments. Atmos Environ. 2005;39:5454–65. [Google Scholar]
- DHHS. 42 CFR 84 Respiratory protective devices; Final rules and notice. 1995. Federal Register 60:110. Public Health Service. Morgantown, WV: Department of Health and Human Services (DHHS) [DOI] [PubMed] [Google Scholar]
- Donaldson K, Stone V, Renwick L, et al. Ultrafine particles. Occup Environ Med. 2001;58:211–6. doi: 10.1136/oem.58.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eninger RM, Takeshi H, Reponen T, et al. What does respirator certification tell us about filtration of ultrafine particles? J Occup Environ Hyg. 2008;5:286–95. doi: 10.1080/15459620801960153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagan RC. Electrical techniques. In: Baron PA, Willeke K, editors. Aerosol measurement: principles, techniques and applications. New York: Wiley-Interscience; 2001. pp. 537–68. [Google Scholar]
- Gebhart J. Optical direct reading techniques: light intensity systems. In: Baron PA, Willeke K, editors. Aerosol measurement. Principles, techniques and applications. New York: Wiley-Interscience; 2001. pp. 419–54. [Google Scholar]
- Goldsmith CS, Tatti KM, Ksiazek TG, et al. Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis. 2004;10:320–26. doi: 10.3201/eid1002.030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D-H. Performance of respirator filters using quality factor in Korea. Ind Health. 2000;38:380–4. doi: 10.2486/indhealth.38.380. [DOI] [PubMed] [Google Scholar]
- Harber P, Tamimie J, Emory J, et al. Effects of exercise using industrial respirators. AIHAJ. 1984;45:603–9. doi: 10.1080/15298668491400331. [DOI] [PubMed] [Google Scholar]
- Hemphill HE, Whiteley HR. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975;39:257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds WC. Aerosol technology: properties, behavior, and measurement of airborne particles. 2nd edn. New York: John Wiley & Sons, Inc; 1999. Filtration; pp. 182–205. [Google Scholar]
- HSE. 2004. Health effects of particles produced for nanotechnologies. UK Health and Safety Executive. [Google Scholar]
- International Organization for Standardization (ISO) Water quality—detection and enumeration of bacteriophages—part 2: enumeration of somatic coliphages. Geneva, Switzerland, ISO 10705-2:2000: 2000. [Google Scholar]
- Lafortuna CL, Minetti AE, Mognoni A. Inspiratory flow pattern in humans. J Appl Physiol. 1984;57:1111–9. doi: 10.1152/jappl.1984.57.4.1111. [DOI] [PubMed] [Google Scholar]
- Lathrache R, Fissan HJ. Enhancement of particle deposition in filters due to electrostatic effects. Oostende: Proc. 4th World Filtration Congr. 1986;7:55–63. [Google Scholar]
- Lathrache R, Fissan HJ, Neumann S. Deposition of submicron particles on electrically charged fibers. J Aerosol Sci. 1986;17:446–9. [Google Scholar]
- Lee KW, Mukund R. Filter collection. In: Baron PA, Willeke K, editors. Aerosol measurement. principles, techniques and applications. New York: Wiley-Interscience; 2001. pp. 197–229. [Google Scholar]
- Leiman PG, Kanamaru S, Mesyanzhinov VV, et al. Structure and morphogenesis of bacteriophage T4. Cell Mol Life Sci. 2003;60:2356–70. doi: 10.1007/s00018-003-3072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepizco-Encinas BH, Rito-Palomares M. Dielectrophoresis for the manipulation of nanobioparticles. Electrophoresis. 2007;28:4521–38. doi: 10.1002/elps.200700303. [DOI] [PubMed] [Google Scholar]
- Madigan MT, Martinko JM. Essentials of virology. 11th edn. Upper Saddle River, NJ: Pearson Prentice Hall; 2006. Brock biology of microorganisms; pp. 230–54. [Google Scholar]
- Madigan MT, Martinko JM. Viral diversity. 11th ed. Upper Saddle River, NJ: Pearson Prentice Hall; 2006. Brock biology of microorganisms; pp. 503–30. [Google Scholar]
- Martin SB, Moyer ES. Electrostatic respirator filter media: filter efficiency and most penetrating particle size effects. Appl Occup Environ Hyg. 2000;15:609–17. doi: 10.1080/10473220050075617. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Kuempel ED. Airborne nanostructured particles and occupational health. J Nanoparticle Res. 2005;7:587–614. [Google Scholar]
- Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air. 2006;16:335–47. doi: 10.1111/j.1600-0668.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) and the Department of Labor. Bureau of labor statistics, Morgantown, WV: Respirator usage in private sector firms, 2001. 2003. [Google Scholar]
- National Institute for Occupational Safety and Health. Nanotechnology & workplace safety and health. 2004 [Google Scholar]
- National Institute for Occupational Safety and Health. Current Intelligence Bulletin: evaluation of health hazard and recommendations for occupational exposure to titanium dioxide. Draft. 2005;100:2–3. [Google Scholar]
- National Institute for Occupational Safety and Health. Approaches to safe nanotechnology: an Information exchange with NIOSH. Cincinnati, OH. 2005 [Google Scholar]
- Oberdörster G, Stone V, Donaldson K. Toxicology of nanoparticles: a historical perspective. Nanotoxicology. 2007;1:2–25. [Google Scholar]
- Oh Y-H, Jeon K-J, Jung A-I, Jung Y-W. A simulation study on the collection of submicron particles in a unipolar charged fiber. Aerosol Sci Tech. 2002;36:573–82. [Google Scholar]
- Rengasamy A, Zhuang Z, BerryAnn R. Respiratory protection against bioaerosols: literature review and research needs. Am J Inf Control. 2004;32:345–54. doi: 10.1016/j.ajic.2004.04.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reponen T, Willeke K, Grinshpun SA, et al. Biological particle sampling. In: Baron PA, Willeke K, editors. Aerosol measurement. principles, techniques and applications. New York: Wiley-Interscience; 2001. pp. 751–77. [Google Scholar]
- Roco MC, Bainbridge W. Societal implications of nanoscience and nanotechnology. Arlington, VA: National Science Foundation; 2001. [Google Scholar]
- TSI, Inc. Operation and Service Manual. Revision A. St Paul, Shoreview, MN: 2005. Model 8587A laser photometer. [Google Scholar]
- TSI, Inc. Operation and service manual. Revision H. St Paul, Shoreview, MN: 2006. CertiTest® model 8127/8130 automated filter tester. [Google Scholar]
- Valegård K, Liljas L, Fridborg K, et al. The three-dimensional structure of the bacterial virus MS2. Nature. 1990;345:36–41. doi: 10.1038/345036a0. [DOI] [PubMed] [Google Scholar]
- Vincent JH, Clement CF. Ultrafine particles in workplace atmospheres. Phil. Trans. R. Soc. Lond. A. 2000;358:2673–82. [Google Scholar]
- Wei J, Chun-Shun C, Cheong-Ki C, et al. The aerosol penetration through an electret fibrous filter. Chinese Physics. 2006;15:1864–70. [Google Scholar]
- Willeke K, Qian Y, Donnelly J, et al. Penetration of airborne microorganisms through a surgical mask and a dust/mist respirator. AIHAJ. 1996;57:348–55. doi: 10.1080/15428119691014882. [DOI] [PubMed] [Google Scholar]
- Yang S, Lee WM. Filtration characteristics of a fibrous filter pretreated with anionic surfactants for monodisperse solid aerosols. Aerosol Sci. 2005;36:419–37. [Google Scholar]


