Abstract
Phrenic long-term facilitation (pLTF) is a form of serotonin-dependent respiratory motor plasticity induced by moderate acute intermittent hypoxia (AIH), but not by moderate acute sustained hypoxia (ASH) of similar cumulative duration. Thus, moderate AIH-induced pLTF is sensitive to the pattern of hypoxia. On the other hand, pLTF induced by severe AIH protocols is neither pattern sensitive nor serotonin dependent (it converts to an adenosine-dependent mechanism). Although moderate AIH also induces hypoglossal LTF (hLTF), no data are available concerning its sensitivity/insensitivity to the pattern of hypoxia. Since hLTF following moderate hypoxia is serotonin-dependent, we hypothesized that hLTF is pattern-sensitive, similar to serotonin-dependent pLTF. Integrated hypoglossal nerve activity was recorded in urethane-anesthetized, vagotomized, paralyzed, and ventilated rats exposed to isocapnic AIH (3, 5 min episodes of 11% O2) or ASH (a single 25 min episode of 11% O2). Similar to previous studies of pLTF, hypoglossal motor output was elevated for more than 1 h following AIH (50 ± 20%, P < 0.01), but not ASH (−6 ± 9%, P > 0.05). Frequency LTF was not observed following either hypoxic exposure. Thus, in agreement with our hypothesis, hypoglossal LTF following moderate AIH is pattern-sensitive, similar to phrenic LTF.
Keywords: plasticity, hypoxia, pattern, control of breathing
Introduction
Patterned stimulation is often more effective than continuous stimulation at inducing plasticity in the central nervous system. For example, stimulus presentations spaced over time are more effective at inducing long term memory formation versus an equal duration continuous presentation (Ebbinghaus, 1913; Beck et al., 2000; Sutton et al., 2002; Cepeda et al., 2006). This phenomenon, known as the “spacing effect,” is expressed widely throughout the animal kingdom, and has been extensively studied in several animal models and humans because of its potential utility in education (Kerfoot, 2010), psychology (Goverover et al., 2009b), advertising (Janiszewski et al., 2003; Appleton‐Knapp et al., 2005) and physical rehabilitation (Goverover et al., 2009a). Consistent with the spacing effect in learning and memory, patterned stimulation (intermittent vs. continuous) is also more effective at eliciting synaptic plasticity in the nervous system. For example, intermediate-term memory formation and synaptic facilitation in Aplysia (Mauelshagen et al., 1998; Sutton et al., 2002), and hippocampal long-term potentiation in rodents (Kauer, 1999; Nguyen et al., 2000; Scharf et al., 2002) exhibit pattern sensitivity, with intermittent more effective than continuous stimulus presentations. Despite its importance to neuroplasticity, mechanisms giving rise to pattern sensitivity are not well known.
Some forms of hypoxia-induced respiratory motor plasticity exhibit clear pattern sensitivity (Baker & Mitchell, 2000; Wilkerson et al., 2008). For example, serotonin-dependent phrenic long-term facilitation (pLTF) can be elicited by moderate acute intermittent (AIH), but not moderate acute sustained hypoxia (ASH) of similar cumulative duration (Mitchell et al., 2001; Baker & Mitchell, 2000). Both severe intermittent and sustained hypoxia also elicit pLTF, although this form of pLTF is mediated by a distinct, adenosine-dependent mechanism (Nichols et al., 2012; Devinney et al., 2016). While episodic spinal serotonin receptor activation is required to elicit phrenic motor facilitation in rats, a single, larger serotonin injection fails to elicit the response (MacFarlane & Mitchell, 2009a). Further, whereas intermittent but not sustained serotonin receptor activation elicits phrenic long-term facilitation in neonatal brainstem-spinal cord preparations, similar pattern sensitivity is not observed in inspiratory intercostal activity (Lovett-Barr et al., 2006). Thus, all forms of respiratory motor plasticity do not exhibit similar pattern sensitivity to the inducing stimulus.
Another form of hypoxia-induced respiratory motor plasticity is hypoglossal long-term facilitation (hLTF; Bach and Mitchell, 1996). Similar to pLTF, hLTF is expressed as a progressive increase in hypoglossal motor output induced by moderate AIH (Bach & Mitchell, 1996; Baker-Herman and Strey, 2011). Hypoglossal LTF might stabilize the upper airways, maintaining upper airway patency during sleep (Fuller, 2005; Mahamed and Mitchell, 2007; Baker-Herman and Strey, 2011). Thus, greater understanding of distinctions between the mechanisms giving rise to pLTF versus hLTF could lead to new therapeutic approaches that minimize certain forms of obstructive sleep apnea (Baker-Herman and Strey, 2011). However, little is known concerning similarities and differences in the mechanisms of pLTF and hLTF, and there are no reports concerning pattern-sensitivity of hLTF with moderate hypoxia. Here, we demonstrate that hLTF exhibits pattern sensitivity, similar to moderate AIH-induced pLTF.
Materials and Methods
Experiments were performed on 3–5 month old male Sprague-Dawley rats (colony PO4, Charles River Inc., Wilmington, MA). Rats were individually housed in a controlled environment (12h light/dark cycle), with food and water ad libitum. The University of Wisconsin, School of Veterinary Medicine Animal Care and Use Committee approved all protocols.
Surgical Preparation and Nerve Isolation.
Rats were initially anesthetized in a closed chamber containing isoflurane followed by isoflurane administration through a nose cone (3.0 – 3.5% in 50% O2, balance N2). The trachea was cannulated to enable pump-ventilation (tidal volume, 2 – 2.5 mL; FIO2 = 0.50; Rodent Respirator model 682, Harvard Apparatus, South Natick, MA). A bilateral vagotomy was performed at the mid-cervical level to prevent entrainment of respiratory motor output with the ventilator. Catheters were placed into the tail vein for fluid administration (1:11 by volume NaHCO3:lactated Ringer’s; 2.5 mL/hr) and the femoral artery for blood pressure measurement and to draw blood samples for blood gas analysis. Body temperature was maintained at 37.5 ± 1.0 °C using a rectal probe and custom-designed heated table. The left hypoglossal nerve was isolated using a dorsal approach, cut distally, desheathed and placed on a bipolar silver electrode. Rats were slowly converted to urethane anesthesia (1.6 g/kg, i.v.) and then paralyzed with pancuronium bromide to prevent spontaneous breathing movements (2.5 mg/kg, i.v., supplemented as necessary). End-tidal CO2 was measured throughout the experiment using a flow-through capnograph (Capnogard, Model 1265, Novametrix; Wallingford, CT) with sufficient response time to measure expiratory gases in rats.
Experimental Protocols.
The CO2 apneic threshold was determined by decreasing CO2 levels and/or increasing the ventilator rate until nerve activity ceased. Inspired CO2 levels were then increased; the end-tidal CO2 at which phrenic activity resumed was taken as the recruitment threshold (Mahamed & Mitchell, 2007). End-tidal CO2 levels were then set 1–2 mmHg above the recruitment threshold. A stable hypoglossal neurogram was established and an initial blood sample was taken to establish baseline PaO2, PaCO2, pH, and base excess values (0.3 ml in 0.5 ml heparinized glass syringe; ABL-500, Radiometer, Copenhagen, Denmark; unused blood was returned to the animal). Rats were given 3, 5-min episodes of hypoxia (i.e., AIH; FIO2 = 0.11 ± 0.1, PaO2 = 39 ± 1 mmHg), separated by 5 min of baseline conditions (FIO2 = 0.5, PaO2 > 250 mm Hg), or a single, cumulative 25-min hypoxic episode (i.e., ASH; FIO2 = 0.11 ± 0.1, PaO2 = 38 ± 1 mmHg). Hypoglossal activity was monitored 60 min post-hypoxia to determine LTF magnitude. Arterial blood samples were drawn and analyzed during the final minute of the first hypoxic episode, and 15, 30 and 60 min after the final hypoxic episode. Additional rats that did not receive hypoxia (time controls) were used to verify the stability of nerve output over a similar time period in this preparation. Throughout the protocol, isocapnic conditions (± 1 mmHg from baseline PaCO2) were maintained by adjusting ventilator frequency and/or inspired CO2.
Electrophysiological methods.
Hypoglossal nerve activity was amplified (x 10,000), band pass filtered (100 Hz to 10 kHz; Model 1700, A-M Systems, Inc., Carlsborg, WA), and integrated (time constant = 50 ms, Model MA-821RSP, CWE Inc., Ardmore, PA). Integrated signals were digitized and processed with commercially available software (WINDAQ software, DATAQ Instruments, Akron, OH). Peak integrated hypoglossal burst amplitude, burst frequency, and mean arterial blood pressure were calculated over a 60 second period just prior to the first hypoxic episode (baseline), at the end of the first hypoxic episode or the equivalent time point during sustained hypoxia (short-term hypoxic response), and 30 and 60 min post-hypoxia. Data were included in the analysis only if isocapnic conditions were successfully maintained. Amplitude data are expressed as the change in hypoglossal burst amplitude, expressed as a percent change from baseline values. Frequency data are reported as a change from baseline in bursts per minute (delta burst frequency). Data were compared using a one-way ANOVA or two-way ANOVA with repeated measures design as applicable (Fisher LSD post-hoc test; SigmaStat 2.03, SPSS Inc., Chicago, IL).
Results
Physiological variables
No significant differences were observed in the CO2 recruitment threshold for rats treated with (IH: 41 ± 1 mmHg, n=9; SH: 40 ± 1 mmHg, n=14) or without hypoxia (42 ± 1 mmHg, n=14; p > 0.05). Under baseline conditions, mean arterial blood pressure was not different between treatment groups (IH: 112 ± 5 mmHg, SH: 110 ± 4 mmHg; Table 1; p > 0.05), or time controls that did not receive hypoxia: (112 ± 5 mmHg; Table 1, p > 0.05). Similar to other studies from our laboratory, mean arterial blood pressure significantly decreased during the hypoxic stimulus versus baseline (IH: 62 ± 4 mmHg, SH: 65 ± 7 mmHg; p < 0.05), but not in time controls (112 ± 5 mmHg; Table 1, p > 0.05). We did not observe significant changes in mean arterial blood pressure at 30 min post-hypoxia in any group; however, small but significant decreases were found at the 60 min time point in the AIH (105 ± 4 mmHg), ASH (100 ± 3 mmHg), and time control (105 ± 5 mmHg) (Table 1, p < 0.05). No significant treatment effects were found between groups at any time for mean arterial blood pressure (p > 0.05). PaCO2 was successfully maintained within 1 mmHg of the baseline value throughout experimental protocols. Thus, changes in hypoglossal burst amplitude or frequency were not caused by changes in arterial PCO2 from baseline values.
Table 1. Temporal changes in mean arterial blood pressure (MABP), PaCO2, and PaO2 in rats.
Relative to baseline, mean arterial blood pressure (MABP), arterial partial pressure of CO2 (PaCO2), and arterial partial pressure of O2 (PaO2) were not significantly different over time in rats that did not receive hypoxia (p > 0.05). As expected PaO2 significantly decreased during hypoxia exposure (P < 0.05), but returned to baseline values following exposure. Rat groups treated with intermittent or sustained hypoxia showed similar significant decreases in MABP during hypoxia (p < 0.05) and small but significant decreases in MABP 60 min post-hypoxia compared to baseline (p < 0.05). Overall, there was not a significant treatment effect on MABP (p > 0.05).
| Treatment | N | Baseline | 1st HX | 60 min post |
|---|---|---|---|---|
| 112 ± 5 (MABP) | 112 ± 5 | 105 ± 5 | ||
| No hypoxia | 14 | 47.7 ± 1.2 (PaCO2) | 47.7 ± 1.2 | 47.6 ± 1.2 |
| 267.2 ± 4.0 (PaO2) | 265.9 ± 4.7 | 252.2 ± 8.7 | ||
| 112 ± 5 | 68 ± 4* | 105 ± 4* | ||
| Intermittent hypoxia | 9 | 45.2 ± 1.2 | 44.0 ± 1.7 | 45.9 ± 1.1 |
| 275.7 ± 6.5 | 38.9 ± 0.8* | 262.0 ± 8.1 | ||
| 110 ± 4 | 65 ± 7* | 100 ± 3* | ||
| Sustained hypoxia | 14 | 44.4 ± 1.8 | 43.9 ± 0.8 | 44.0 ± 0.9 |
| 257.0 ± 4.9 | 37.9 ± 1.2* | 245.4 ± 8.0 | ||
Significantly different from baseline within treatment group
Hypoglossal long-term facilitation: AIH versus ASH
Representative integrated hypoglossal neurograms before, during and 60 min following AIH or ASH are presented in Figure 1. During hypoxic exposures, hypoglossal burst amplitude and frequency significantly increased in rats exposed to AIH (203 ± 31% baseline and 16 ± 3 bursts/min, respectively) and ASH hypoxia (236 ± 34% baseline and 8 ± 2 bursts/min, respectively) (Figure 2). There were no significant differences in hypoglossal burst amplitude or frequency during AIH versus ASH (Figure 2, p > 0.05 for both). Time controls showed no significant difference in hypoglossal burst amplitude or delta burst frequency at equivalent times (0 ± 3% baseline and −3 ± 2 bursts/min, respectively; Figure 2; p > 0.05).
Figure 1. Intermittent, but not sustained, hypoxia, elicits long-term facilitation (LTF) of hypoglossal (XII) motor output.
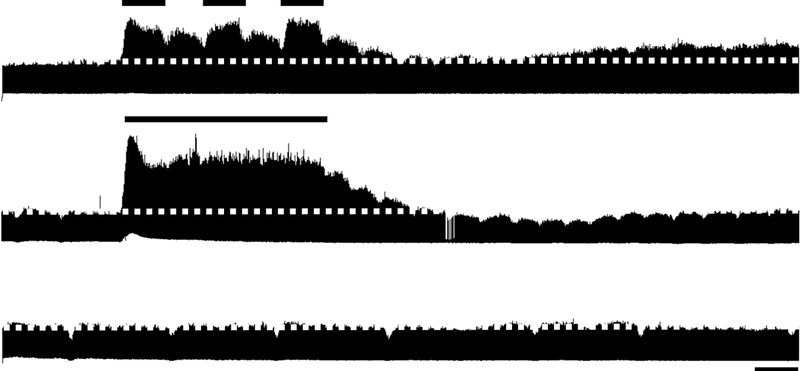
Representative integrated hypoglossal neurograms before, during, and 60 min following intermittent (top) or sustained (middle) hypoxia or no hypoxia (time control, bottom). Relative to baseline (indicated by dotted line), hypoglossal burst amplitude was increased 60 min following intermittent, but not sustained hypoxia. When hypoxia is not presented, hypoglossal motor output remains stable over time (time control). Short bar = 5 min; long bar = 25 min.
Figure 2. Hypoglossal (XII) motor output is similar during intermittent or sustained hypoxia.
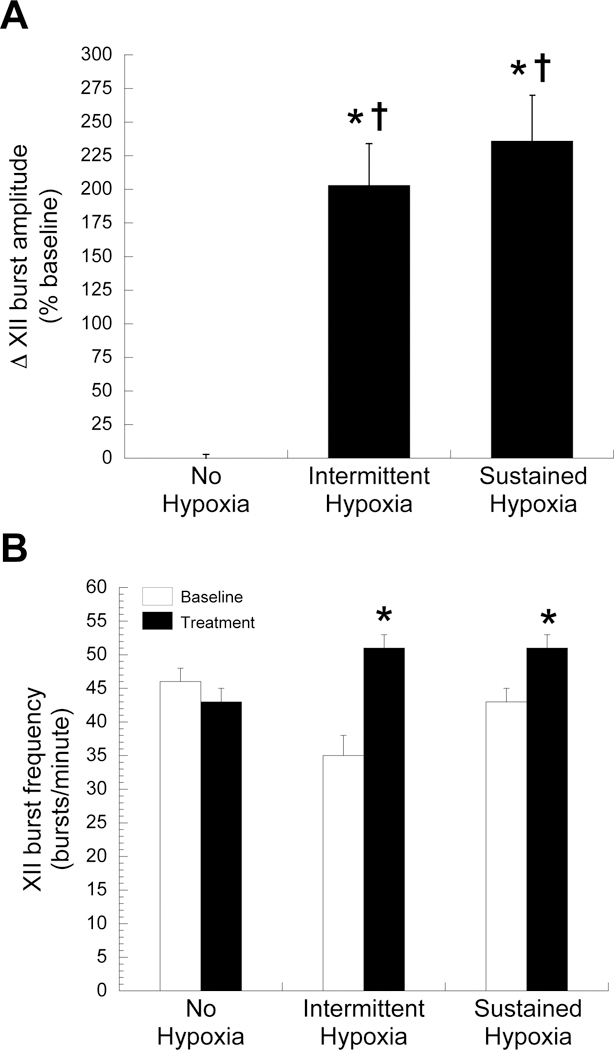
Short-term response of hypoglossal burst amplitude (A) and frequency (B) during intermittent or sustained hypoxia or without hypoxia. Data are presented as mean ± SEM. * Significantly increased from baseline (p < 0.05)
Group data for hypoglossal burst amplitude 60 minutes post-hypoxia are presented in Figure 3. Hypoglossal burst amplitude was significantly increased from baseline 60 min (50 ± 20% baseline, p < 0.01) post-AIH, indicative of hypoglossal LTF (Figure 3A). In contrast, hypoglossal LTF was not observed following ASH (−6 ± 9% baseline, p > 0.05, Figure 3A). Hypoglossal burst amplitude was stable in time control rats; there were no significant changes in hypoglossal burst amplitude versus baseline at 60 minutes in this group (13 ± 9 %baseline, p > 0.05).
Figure 3. Long-term facilitation (LTF) of hypoglossal (XII) burst amplitude, but not burst frequency, is observed following intermittent, but not sustained, hypoxia.

XII burst amplitude (A) but not delta burst frequency (B) is enhanced 60 min following intermittent, but not sustained hypoxia. Data are presented as mean ± SEM. *Significantly increased from baseline (p < 0.05).
Frequency long-term facilitation
Whereas hypoglossal burst frequency increased during hypoxia (Figure 2B; p < 0.05), no significant changes were observed in hypoglossal delta burst frequency 60 min post-AIH (4 ± 3 bursts/min) or post-ASH (1 ± 2 bursts/min; both p > 0.05; Figure 3B). Time control rats did not show significant changes in hypoglossal burst frequency at an equivalent time (2 ± 1 bursts/min; p > 0.05, Figure 3B). Since there were no significant differences between groups 60 minutes post-treatment (p > 0.50), there was no evidence for frequency LTF in these experiments.
Discussion
Surprisingly, despite numerous investigations concerning phrenic LTF and pLTF pattern-sensitivity (Baker & Mitchell, 2000; Wilkerson et al., 2008; Dale-Nagle et al., 2010; Devinney et al., 2013; Devinney et al., 2015; Devinney et al., 2016), there have been no previous reports concerning the existence of pattern sensitivity in hypoglossal LTF (Baker-Herman and Strey, 2011). Using AIH and ASH protocols similar to those previously used to demonstrate pLTF pattern-sensitivity, we report that moderate AIH elicits hLTF, yet moderate ASH does not. Thus, although serotonin-dependent facilitation of intercostal motor activity lacks pattern-sensitivity in neonatal rats (Lovett-Barr et al., 2006), and hLTF differs from pLTF in certain key characteristics (Baker-Herman and Strey, 2011), the hypoglossal and phrenic motor pools exhibit similar sensitivity to the pattern of moderate hypoxia in adult rats.
We do not yet have a full understanding of mechanisms giving rise to pattern-sensitivity of hypoxia-induced respiratory motor plasticity in any motor pool. We do not even know if hLTF pattern-sensitivity occurs via the same mechanisms as pLTF pattern-sensitivity, nor if hLTF is less pattern-sensitive when severe hypoxia is applied (Devinney et al., 2016). A full understanding of mechanisms giving rise to hLTF and hLTF pattern-sensitivity will be essential if we are to fully appreciate the biological and clinical significance of this form of hypoxia-induced plasticity in motor pools innervating the upper airways.
Potential mechanisms of pattern sensitivity in hLTF
Hypoglossal and phrenic LTF exhibit many similarities and differences (Baker-Herman and Strey, 2011). Prominent differences include their responses to sex, sex hormones and ageing (Zabka et al., 2001a, 2001b, 2003, 2005, 2006; Behan et al., 2002, 2003), strain and sub-strain (Fuller et al., 2000; Mitchell et al., 2001; Fuller et al., 2001a; Baker-Herman et al., 2010), and the relative influence of alpha-adrenergic receptors in their underlying mechanisms (Neverova et al., 2007; Huxtable et al., 2014). On the other hand, both hLTF and pLTF require 5-HT2 receptor activation (Fuller et al., 2001b) and reactive oxygen species formation (MacFarlane & Mitchell, 2008a; MacFarlane et al. 2008b), suggesting fundamental similarities in their cellular/synaptic mechanisms.
Although there are no previous studies concerning mechanisms of hLTF sensitivity to the pattern of hypoxia in vivo, one study in medullary slices from juvenile rats showed that episodic, but not continuous, serotonin receptor activation elicits hLTF and increases AMPA-mediated inspiratory currents in hypoglossal motor neurons (Bocchiaro & Feldman, 2004). Similarly, hLTF is elicited by episodic alpha-adrenergic receptor activation in brainstem slice preparations (Neverova et al., 2007). These studies provide at least suggestive evidence that hypoxia-induced hLTF may also be pattern sensitive in adult rats. In contrast, phrenic and intercostal motor outputs differ in their pattern sensitivity to serotonin receptor activation in vitro, suggesting that different motor pools may vary in the extent of pattern-sensitivity to the inducing stimulus (Lovett-Barr et al., 2006).
In previous studies of pLTF pattern sensitivity, we demonstrated that inhibition of protein phosphatases with okadaic acid revealed pLTF following moderate ASH (Wilkerson et al., 2008). Since AIH-induced pLTF requires reactive oxygen species formation (MacFarlane et al., 2008a, 2008b, 2009a, 2009b), and intrathecal okadaic acid restores pLTF in rats with diminished reactive oxygen species formation (MacFarlane et al., 2008b), we suggested that pLTF pattern sensitivity arises through suppression of serine/threonine protein phosphatases that normally constrain pLTF expression via increased reactive species formation that is characteristic of AIH and not ASH (MacFarlane et al., 2008b; Wilkerson et al., 2007; Wilkerson et al., 2008). The relevant reactive oxygen species formation most arises from increased NADPH oxidase activity elicited by episodic serotonin spinal receptor activation (MacFarlane et al., 2009a). Since this form phrenic motor facilitation requires episodic serotonin receptor activation, and is not activated by a single serotonin injection of the same cumulative dose, we suggested that pLTF pattern sensitivity arises downstream from spinal serotonin receptor activation (MacFarlane and Mitchell, 2009a). The protein tyrosine phosphatase corkscrew regulates pattern sensitivity in Drosophila long-term memory induction (Pagani et al., 2009), suggesting that phosphatases play a role in multiple forms of pattern-sensitive neuroplasticity. The requirement for serotonin-dependent reactive oxygen species formation, and suppression of okadaic acid sensitive protein phosphatases in hLTF has not been investigated.
Additional insights concerning mechanisms of pLTF pattern sensitivity were inspired by the realization that multiple competing cellular mechanisms are capable of giving rise to long-lasting phrenic motor facilitation (Dale-Nagle et al., 2010; Devinney et al., 2013). The “Q pathway” to phrenic motor facilitation is initiated by Gq protein coupled metabotropic receptors such as serotonin type 2 receptors, and gives rise to pLTF following moderate AIH (Dale-Nagle et al., 2010). In contrast, the “S pathway” to phrenic motor facilitation is initiated by Gs protein coupled metabotropic receptors such as adenosine 2A receptors (Dale-Nagle et al., 2010). These pathways interact via cross talk inhibition, suggesting that there is a point where they are equal and opposing, cancelling the expression of phrenic motor facilitation (Devinney et al., 2013). Whereas the serotonin-dependent Q pathway predominates with moderate AIH, we proposed that serotonin and adenosine receptor activation are more balanced with ASH, effectively cancelling pLTF expression (Devinney et al., 2016). Selective inhibition of spinal serotonin or adenosine 2A receptors disrupts this balance, revealing pLTF following moderate ASH (Devinney et al., 2016). Similar studies exploring hLTF pattern-sensitivity have not been done.
Significance of pattern sensitivity in respiratory neuroplasticity
The importance of patterned stimulation in evoking plasticity is described in many different models of plasticity, yet the mechanism remains unknown. For example, intermediate-term memory formation and synaptic facilitation in the sea slug Aplysia (Mauelshagen et al., 1998; Sutton et al., 2002), habituation in the crab (Freudenthal et al., 1998), olfactory memory formation in fruit flies (Isabel et al., 2004), and hippocampal long-term potentiation in rodents (Kauer, 1999; Nguyen et al., 2000; Scharf et al., 2002) all exhibit apparent pattern sensitivity to the induction protocol. However, there are certain instances of pattern-insensitive plasticity. In the whole brainstem-spinal cord preparation, long term facilitation of thoracic intercostal motor output is elicited by a continuous infusion of 5-HT (Lovett-Barr et al., 2006). Another example of pattern insensitive respiratory plasticity is ventilatory acclimatization to chronic hypoxia, such as occurs during exposure to high altitude (Dwinell & Powell, 1999). Thus, by describing pattern sensitivity in respiratory neuroplasticity, we may yield insights as to why pattern sensitivity exists in some motor pools, and mechanisms giving rise to that pattern sensitivity. Understanding pattern-sensitive aspects of respiratory neuroplasiticity will also guide its optimal utilization in strategies aiming to increase respiratory motor output through induction of plasticity.
Significance of hLTF
The physiological significance of any form of respiratory plasticity is not completely understood (Mitchell et al., 2001; Mahamed and Mitchell, 2007; Fields and Mitchell, 2015). However, hLTF represents one potential mechanism to increase upper airway tone, thereby preserving upper airway patency and possibly minimizing obstructive apneas due to upper airway collapse in normal individuals or those with sleep-disordered breathing (Mitchell et al., 2001; Fuller, 2005; Mitchell, 2007; Mahamed and Mitchell, 2007; Baker-Herman and Strey, 2011). Furthermore hLTF might represent mechanisms enabling long-term adaptations of hypoglossal motor output during normal ageing (Zabka et al., 2005), or central nervous system injury or disease (Mitchell et al., 2001; Mitchell, 2007). Understanding mechanisms of hypoglossal motor facilitation will aid attempts to utilize hLTF to increase upper airway tone for therapeutic advantage. Elucidating mechanisms of hLTF pattern sensitivity will increase basic understanding of pattern sensitivity in neuroplasticity, and could guide strategies that utilize plasticity to treat disease and/or injury.
In humans, upper airway collapse increases from evening to morning, independent of sleep stage (Mateika et al., 2015, Sforza et al., 1998, Charbonneau et al., 1994). Further, intermittent hypoxia in humans elicits a distinct form of respiratory plasticity known as progressive augmentation (Powell et al., 1998), which increases the number of flow limiting breathing events (Yokhana et al., 2012, Mateika and Syed, 2013; Mateika and Narwani, 2009). Consequently, LTF of upper airway muscle activity may help offset otherwise detrimental forms of respiratory plasticity (El-Chami et al., 2017, Mateika and Komnenov, 2017).
Highlights:
Long-term facilitation of hypoglossal motor output induced by moderate hypoxia is pattern-sensitive
Intermittent, but not sustained, moderate hypoxia, elicits hypoglossal long-term facilitation
Long-term facilitation of hypoglossal motor output results from enhanced hypoglossal burst amplitude versus frequency
Acknowledgements:
This work was supported by the National Institutes of Health (Grants HL080209 and HL111598, GSM).
Support provided by NIH Grants HL080209 and HL111598 (GSM).
Footnotes
Conflicts of Interest: The authors declare no competing financial interests.
References
- Appleton‐Knapp SL, Bjork RA, Wickens TD, 2005. Examining the Spacing Effect in Advertising: Encoding Variability, Retrieval Processes, and Their Interaction. The Journal of Consumer Research 32, 266–276. [Google Scholar]
- Bach KB, Mitchell GS, 1996. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104, 251–260. [DOI] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS, 2000. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J. Physiol. (Lond.) 529 Pt 1, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Bavis RW, Dahlberg JM, Mitchell AZ, Wilkerson JE, Golder FJ, Macfarlane PM, Watters JJ, Behan M, Mitchell GS, 2010. Differential expression of respiratory long-term facilitation among inbred rat strains. Respir Physiol Neurobiol 170, 260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Strey KA, 2011. Similarities and differences in mechanisms of phrenic and hypoglossal motor facilitation. Respir Physiol Neurobiol 179, 48–56. doi: 10.1016/j.resp.2011.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CD, Schroeder B, Davis RL, 2000. Learning performance of normal and mutant Drosophila after repeated conditioning trials with discrete stimuli. J. Neurosci 20, 2944–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Mitchell GS, 2002. Age and gender effects on serotonin-dependent plasticity in respiratory motor control. Respir Physiol Neurobiol 131, 65–77. [DOI] [PubMed] [Google Scholar]
- Behan M, Zabka AG, Thomas CF, Mitchell GS, 2003. Sex steroid hormones and the neural control of breathing. Respir Physiol Neurobiol 136, 249–63. [DOI] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL, 2004. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc. Natl. Acad. Sci. U.S.A 101, 4292–4295. doi: 10.1073/pnas.0305712101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda NJ, Pashler H, Vul E, Wixted JT, Rohrer D, 2006. Distributed practice in verbal recall tasks: A review and quantitative synthesis. Psychol Bull 132, 354–380. doi: 10.1037/0033-2909.132.3.354 [DOI] [PubMed] [Google Scholar]
- Charbonneau M, Marin JM, Olha A, Kimoff RJ, Levy RD, Cosio MG. Changes in obstructive sleep apnea characteristics through the night. Chest. 1994. December;106(6):1695–701. [DOI] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS, 2010. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol. 2010;669:225–30. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Nichols NL, Mitchell GS, 2016. Sustained hypoxia elicits competing spinal mechanisms of phrenic motor facilitation. J Neurosci. 36(30):7877–85. doi: 10.1523/JNEUROSCI.4122-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Fields DP, Huxtable AG, Peterson TJ, Dale EA, Mitchell GS, 2015. Phrenic long-term facilitation requires PKCθ activity within phrenic motor neurons. J Neurosci. 35(21):8107–17. doi: 10.1523/JNEUROSCI.5086-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS, 2013. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann N Y Acad Sci. 1279:143–53. doi: 10.1111/nyas.12085.10.1152/japplphysiol.00204.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwinell MR, Powell FL, 1999. Chronic hypoxia enhances the phrenic nerve response to arterial chemoreceptor stimulation in anesthetized rats. J. Appl. Physiol 87, 817–823. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus H, 1913. Memory: a contribution to experimental psychology. New York City, Teachers College, Columbia University. [Google Scholar]
- El-Chami M, Sudan S, Lin HS, Mateika JH. Exposure to intermittent hypoxia and sustained hypercapnia reduces therapeutic CPAP in participants with obstructive sleep apnea. J Appl Physiol (1985). 2017. July 6:jap.00204.2017. doi: 10.1152/japplphysiol.00204.2017. [DOI] [PubMed] [Google Scholar]
- Fields DP, Mitchell GS 2015. Spinal metaplasticity in respiratory motor control. Front Neural Circuits. 11;9:2, 143–6. doi: 10.3389/fncir.2015.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenthal R, Locatelli F, Hermitte G, Maldonado H, Lafourcade C, Delorenzi A, Romano A, 1998. Kappa-B like DNA-binding activity is enhanced after spaced training that induces long-term memory in the crab Chasmagnathus. Neurosci. Lett. 242, 143–146. [DOI] [PubMed] [Google Scholar]
- Fuller DD, 2005. Episodic hypoxia induces long-term facilitation of neural drive to tongue protrudor and retractor muscles. J. Appl. Physiol 98, 1761–1767. doi: 10.1152/japplphysiol.01142.2004 [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS 2000. Long term facilitation of phrenic motor output. Respir Physiol 121, 135–146. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS, 2001a. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol. Genomics 4, 175–181. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS, 2001b. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J. Appl. Physiol 90, 2001–2006; discussion 2000. [DOI] [PubMed] [Google Scholar]
- Goverover Y, Arango-Lasprilla JC, Hillary FG, Chiaravalloti N, DeLuca J, 2009a. Application of the Spacing Effect to Improve Learning and Memory for Functional Tasks in Traumatic Brain Injury: A Pilot Study. The American Journal of Occupational Therapy 63, 543–548. doi: 10.5014/ajot.63.5.543 [DOI] [PubMed] [Google Scholar]
- Goverover Y, Hillary FG, Chiaravalloti N, Arango-Lasprilla JC, DeLuca J, 2009b. A functional application of the spacing effect to improve learning and memory in persons with multiple sclerosis. J Clin Exp Neuropsychol 31, 513–522. doi: 10.1080/13803390802287042 [DOI] [PubMed] [Google Scholar]
- Huxtable AG, MacFarlane PM, Vinit S, Nichols NL, Dale EA, Mitchell GS, 2014. Adrenergic α₁ receptor activation is sufficient, but not necessary for phrenic long-term facilitation. J Appl Physiol (1985). 116(11):1345–52. doi: 10.1152/japplphysiol.00904.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabel G, Pascual A, Preat T, 2004. Exclusive consolidated memory phases in Drosophila. Science 304, 1024–1027. doi: 10.1126/science.1094932 [DOI] [PubMed] [Google Scholar]
- Janiszewski C, Noel H, Sawyer AG, 2003. A Meta-analysis of the Spacing Effect in Verbal Learning: Implications for Research on Advertising Repetition and Consumer Memory. Journal of Consumer Research: An Interdisciplinary Quarterly, Journal of Consumer Research: An Interdisciplinary Quarterly 30, 138–49. [Google Scholar]
- Kauer JA, 1999. Blockade of hippocampal long-term potentiation by sustained tetanic stimulation near the recording site. J. Neurophysiol 81, 940–944. [DOI] [PubMed] [Google Scholar]
- Kerfoot BP, 2010. Adaptive spaced education improves learning efficiency: a randomized controlled trial. J. Urol. 183, 678–681. doi: 10.1016/j.juro.2009.10.005 [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Mitchell GS, Satriotomo I, Johnson SM, 2006. Serotonin-induced in vitro long-term facilitation exhibits differential pattern sensitivity in cervical and thoracic inspiratory motor output. Neuroscience 142, 885–892. doi: 10.1016/j.neuroscience.2006.06.036 [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS, 2009a. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J. Physiol. (Lond.) 587, 5469–5481. doi: 10.1113/jphysiol.2009.176982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Satriotomo I, Windelborn JA, Mitchell GS, 2009b. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J. Physiol. (Lond.) 587, 1931–1942. doi: 10.1113/jphysiol.2008.165597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS, 2008a. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neuroscience 152, 189–197. doi: 10.1016/j.neuroscience.2007.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Wilkerson JE, Lovett-Barr MR, Mitchell GS, 2008b. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol 164, 263–71. doi: 10.1016/j.resp.2008.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS, 2007. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp. Physiol 92, 27–37. doi: 10.1113/expphysiol.2006.033720 [DOI] [PubMed] [Google Scholar]
- Mateika JH, El-Chami M, Shaheen D, Ivers B. Intermittent hypoxia: a low-risk research tool with therapeutic value in humans. J Appl Physiol (1985). 2015. March 1;118(5):520–32. doi: 10.1152/japplphysiol.00564.2014. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Komnenov D. Intermittent hypoxia initiated plasticity in humans: A multipronged therapeutic approach to treat sleep apnea and overlapping co-morbidities. Exp Neurol. 2017. January;287(Pt 2):113–129. doi: 10.1016/j.expneurol.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Narwani G. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Exp Physiol. 2009. March;94(3):279–96. doi: 10.1113/expphysiol.2008.045153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateika JH, Syed Z. Intermittent hypoxia, respiratory plasticity and sleep apnea in humans: present knowledge and future investigations. Respir Physiol Neurobiol. 2013. September 15;188(3):289–300. doi: 10.1016/j.resp.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauelshagen J, Sherff CM, Carew TJ, 1998. Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica. Learn. Mem 5, 246–256. [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB, 2001. Invited review: Intermittent hypoxia and respiratory plasticity. J. Appl. Physiol 90, 2466–2475. [DOI] [PubMed] [Google Scholar]
- Neverova NV, Saywell SA, Nashold LJ, Mitchell GS, Feldman JL 2007. Episodic stimulation of alpha1-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J Neurosci. 27(16):4435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Dale EA, Mitchell GS, 2012. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol (1985). 112(10):1678–88. doi: 10.1152/japplphysiol.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Duffy SN, Young JZ, 2000. Differential maintenance and frequency-dependent tuning of LTP at hippocampal synapses of specific strains of inbred mice. J. Neurophysiol 84, 2484–2493. [DOI] [PubMed] [Google Scholar]
- Pagani MR, Oishi K, Gelb BD, Zhong Y, 2009. The phosphatase SHP2 regulates the spacing effect for long-term memory induction. Cell 139, 186–198. doi: 10.1016/j.cell.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998. May;112(2):123–34. [DOI] [PubMed] [Google Scholar]
- Scharf MT, Woo NH, Lattal KM, Young JZ, Nguyen PV, Abel T, 2002. Protein synthesis is required for the enhancement of long-term potentiation and long-term memory by spaced training. J. Neurophysiol 87, 2770–2777. [DOI] [PubMed] [Google Scholar]
- Sforza E, Krieger J, Petiau C. Nocturnal evolution of respiratory effort in obstructive sleep apnoea syndrome: influence on arousal threshold. Eur Respir J. 1998. December;12(6):1257–63. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ide J, Masters SE, Carew TJ, 2002. Interaction between Amount and Pattern of Training in the Induction of Intermediate- and Long-Term Memory for Sensitization in Aplysia. Learning & Memory 9, 29–40. doi: 10.1101/lm.44802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JER, Macfarlane PM, Hoffman MS, Mitchell GS, 2007. Respiratory plasticity following intermittent hypoxia: roles of protein phosphatases and reactive oxygen species. Biochem. Soc. Trans 35, 1269–1272. doi: 10.1042/BST0351269 [DOI] [PubMed] [Google Scholar]
- Wilkerson JER, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS, 2008. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J. Neurosci 28, 2949–2958. doi: 10.1523/JNEUROSCI.5539-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokhana SS, Gerst DG 3rd, Lee DS, Badr MS, Qureshi T, Mateika JH. Impact of repeated daily exposure to intermittent hypoxia and mild sustained hypercapnia on apnea severity. J Appl Physiol (1985). 2012. February;112(3):367–77. doi: 10.1152/japplphysiol.00702.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Behan M, 2006. Conversion from testosterone to oestradiol is required to modulate respiratory long-term facilitation in male rats. J Physiol. 576(Pt 3):903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Behan M, 2005. Ageing and gonadectomy have similar effects on hypoglossal long-term facilitation in male Fischer rats. J Physiol. 563(Pt 2):557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Olson EB Jr, Behan M, 2003. Selected contribution: chronic intermittent hypoxia enhances respiratory long-term facilitation in geriatric female rats. J Appl Physiol (1985). 95(6):2614–23; discussion 2604. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS, 2001a. Selected contribution: Time-dependent hypoxic respiratory responses in female rats are influenced by age and by the estrus cycle. J Appl Physiol (1985). 91(6):2831–8. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Behan M, Mitchell GS, 2001b. Long term facilitation of respiratory motor output decreases with age in male rats. J Physiol. 531(Pt 2):509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


