Abstract
Spirometry is the current gold standard for diagnosing and monitoring the progression of Chronic Obstructive Pulmonary Disease (COPD). However, many current and former smokers who do not meet established spirometric criteria for the diagnosis of this disease have symptoms and clinical courses similar to those with diagnosed COPD. Large longitudinal observational studies following individuals at risk of developing COPD offer us additional insight into spirometric patterns of disease development and progression. Analysis of forced expiratory maneuver changes over time may allow us to better understand early changes predictive of progressive disease. This review discusses the theoretical ability of spirometry to capture fine pathophysiologic changes in early airway disease, highlights the shortcomings of current diagnostic criteria, and reviews existing evidence for spirometric measures which may be used to better detect early airflow impairment.
Keywords: early COPD, spirometry, obstruction
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide1. Unfortunately, COPD is also often recognized late in the clinical course. Because adequate and timely pharmacologic management and lifestyle modification can impact the disease progression2–6, early identification of COPD is a top priority in global efforts to control this disease.
Spirometry is non-invasive, inexpensive, widely available, and easily reproducible; it remains the gold standard for diagnosis and monitoring of COPD. A wide range of spirometric parameters are routinely reported, but clinical use of measures other than the forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and the ratio of these two measures has been limited. While COPD is defined by a post-bronchodilator ratio of FEV1/FVC <0.70, pathophysiologic changes in the airways and lung parenchyma that characterize COPD start well before this criterion is met7,8. This review discusses the theoretical ability of spirometry to capture fine pathophysiologic changes in early airway disease, highlights the shortcomings of current diagnostic criteria, and discusses existing evidence for selected spirometric indices reflecting early airflow impairment in individuals at risk of COPD.
2. Airway disease preceding COPD
Important pathophysiologic changes occur in the lung prior to the development of a FEV1 to FVC ratio below the threshold of normal (i.e. less than 0.70 or the lower limit of normal)9,10. Early airways changes preceding COPD are localized in small airways11,12 with the development of emphysema in some patients13,14. Small airways, the term which anatomically corresponds to terminal or respiratory bronchioles with a luminal diameter of 2mm or smaller, represent the key anatomic point in the development of COPD. Inflammation in small airways is a determinant of the progression and severity of disease15 and mucus plugging or narrowing and obliteration of the small airways lead to large increase in resistance subsequently leading to development of hyperinflation and emphysema16. Not easily captured by FEV1 and FVC, these smoking-induced changes in small airways are pathologically visible well before conventional spirometric measures change8, and can also be directly measured by catheterization of post-mortem lungs17,18, impulse of forced oscillometry techniques19 or via imaging20.
It is known that tobacco smoke influences epigenetic reprogramming, remodeling, and hyperplasia of airway basal cells21, and compromises regeneration of small airway epithelium22,23. Forty-two percent of current and former smokers with normal spirometry have evidence of emphysema or airway thickening on chest computed tomography (CT) scans24. Low diffusing capacity of the lung for carbon monoxide (DLCO) in smokers with normal FEV1/FVC ratio is not uncommon, and these individuals are at significant risk of developing COPD25,26. Some smokers without COPD, i.e. with “so-called” preserved spirometry, have significant respiratory symptoms, activity limitation, exacerbations and evidence of airway disease in a similar fashion to those who have COPD and similar symptoms27. Initial airway disease extends across a spectrum of spirometric results, including values above an abnormal FEV1/FVC.
Celli and Augustí28, as part of an effort to update COPD taxonomy, drew a parallel with medical concepts of pre-diabetes or pre-hypertension to propose a more general definition for “pre-COPD” as respiratory symptoms with emphysema on CT. The concept of “pre-COPD” syndrome is not new though. Preserved ratio impaired spirometry (PRISm) refers to normal FEV1/FVC ratio but decreased FEV129. Previously widely used GOLD Stage 030, refers to individuals with respiratory symptoms (i.e. cough and sputum production) in the absence of abnormal FEV1/FVC ratio. While individuals with PRISM have higher risk of developing COPD over time31, airway abnormalities present in these clinical scenarios may or may not evolve into COPD over time. Martinez et al32 recognized the need for clear, objective criteria to distinguish these early airway changes from “early” COPD. They proposed that an ever-smoking individual (≥10 pack-years), aged <50 years with either reduced FEV1/FVC below the lower limit of normal (LLN), airway abnormality and/or emphysema on CT, or FEV1 decline ≥60 mL per year may be considered to suffer early COPD. Several other attempts to unify subjective and objective measures in order to more clearly define the early COPD patient33,34 were made, yet no consensus definition exists.
The syndrome encompassing these early airway abnormalities may not be clearly defined, but it is evident that it should be distinguished from “mild” COPD, where the formal diagnosis of COPD is established based on FEV1/FVC34,35 or “early” COPD where manifestations of COPD are present at younger age33. In the search for metrics capable of distinguishing early airway disease from simply mild COPD, a measure of early airflow impairment should:
be present in individuals with FEV1/FVC >0.70 or >LLN and,
serve as an objective correlate to subjective respiratory symptoms and/or,
correlate with other objective features of COPD such as emphysema on computed tomography, reduced DLCO or air trapping and/or,
predict future outcomes, such as accelerated decline in pulmonary function, hospitalization for acute respiratory symptoms consistent with an exacerbation, or mortality
Detailed analysis of lung function decline over time may allow for better understanding of the concept of early disease and help distinguish whether such a measure is predictive of progression to COPD36 or is associated with a separate smoking-related condition, which does not necessary progress to spirometrically defined COPD37.
3. Spirometry in diagnosing COPD
Spirometry is a safe, reproducible, and practical test that is widely used as an objective measure of lung function. While standard spirometric analysis offers a number of parameters, most often used are FEV1 and FVC, and their ratio is considered necessary for the diagnosis and staging of COPD. Pulmonary function tests are generally highly repeatable38 and FEV1 and FVC are more reproducible than expiratory flow measurements39. Nonetheless, some limitations related to the use of FEV1 and FVC in diagnosing COPD need to be pointed out. FEV1 and FVC are variable on a diurnal basis40, and considerable between-test variability has been observed in relation to patient age, sex, smoking status, region, COPD severity41 and even spirometer device selection42. The challenge posed by measurement variability even in the research setting is compounded by variability in the disease itself. There is a growing recognition of various pathologic and clinical phenotypes of COPD43–45, for which different spirometric indices may be relevant. The sensitivity of FEV1 to capture early smoking-related airway disease is limited. It best captures flow-based changes in early portions of forced exhalation, and much less so in later stages of expiration, which is exactly where small airway disease changes could be captured. The FEV1/FVC ratio has limitations for detection of early airflow obstruction. Compared to slow expiratory vital capacity (SVC) or forced inspiratory vital capacity (FIVC), FVC drops to a greater extent in early airflow obstruction due to dynamic air trapping46. This relative decrease in FVC with airway obstruction blunts the sensitivity of FEV1/FVC ratio. Prior international spirometry standardization statements have treated the FEV1/VC denominator differently, including the following: ECCS/ERS 199347 – FIVC or “relaxed expiratory” VC should be used; ATS/ERS 200548 – largest VC should be used (and the ATS 2017 spirometry reporting recommendations49 note measurement of SVC is “a useful adjunct in patients with suspected airflow obstruction”); and GOLD 201635 – only utilizes FVC but mentions ATS/ERS statements are “increasingly suggesting” use of SVC.
There is disagreement between different guidelines and literature50 as to the use of a fixed FEV1/FVC ratio or the LLN for diagnosis of airflow obstruction. It is well recognized that FEV1/FVC declines with age and as such, a fixed ratio leads to high rates of COPD diagnosis in elderly and under-diagnosis of COPD among younger individuals51–53. It is not clear to what degree the increased diagnosis of COPD in the elderly represents overdiagnosis of normal age-related changes versus unrecognized disease. Individuals with FEV1/FVC <0.70 but above LLN have been shown to have more emphysema, air-trapping on CT54,55, lower FEF25-75% and to use more respiratory medication55, as well as to have more hospitalizations and higher mortality,56 than patients with normal lung function by both parameters56. These findings suggest using LLN rather than fixed ratio may fail to identify cases of early airflow compromise, especially in an elderly population. However, in retrospective analysis of the NHANES-III database, Hansen et al57 found an unacceptably high proportion of misdiagnosis with a fixed ratio. Approximately half of abnormal young adults were identified as normal and a fifth of normal older adults were identified as abnormal when compared to LLN57. ATS/ERS 2005 guidelines48 recommend use of LLN for interpretation of FEV1/FVC. GOLD 2016 guidelines35 utilize a fixed ratio, but note that “many experts recommend use of [LLN]” and “FEV1/FVC ratio may need to be lowered to 0.65” as the threshold for abnormality among individuals over 70 years old. Using FEV1/FVC of 0.70 as a threshold for COPD diagnosis is also problematic as significant variability is seen on repeated testing58, suggesting that repeated spirometric assessments may be required. Additionally, it should be noted that there is lack of standardization of pre- versus post-bronchodilator (BD) measurements of FEV1 and FVC. GOLD guidelines recommend post-bronchodilator measurements, but it is not clear that this is necessary or superior to pre-bronchodilator measurements. In the Lung Health Study59 pre- and post-BD measurements predicted mortality equally well. In the more recent COPDGene cohort60, both pre and post-BD predicted certain cardinal features of COPD including symptoms and exercise tolerance. However, post-BD was a better predictor of long-term mortality in COPDGene60 and a prospective study61. There is insufficient data to define which should be used when examining early airflow obstruction preceding COPD, but bronchodilator administration must be accounted for when comparing spirometric indices.
Finally, one conceptual remark relates to the clinical requirement to dichotomize whether the disease is “present” or “absent”, where strict spirometric criteria are needed. However, from a pathophysiologic standpoint, the development of airflow obstruction occurs over many years and the point where these changes are considered a “disease” is arbitrary (Figure 1). Taken together, these arguments suggest that clinically relevant dysfunction may exist despite normal current diagnostic criteria, and additional parameters able to objectively evaluate subtle airway abnormalities could be useful in interpretation of borderline FEV1/FVC.
Figure 1. Select pathologic and clinical changes leading to development of early airflow impairment.
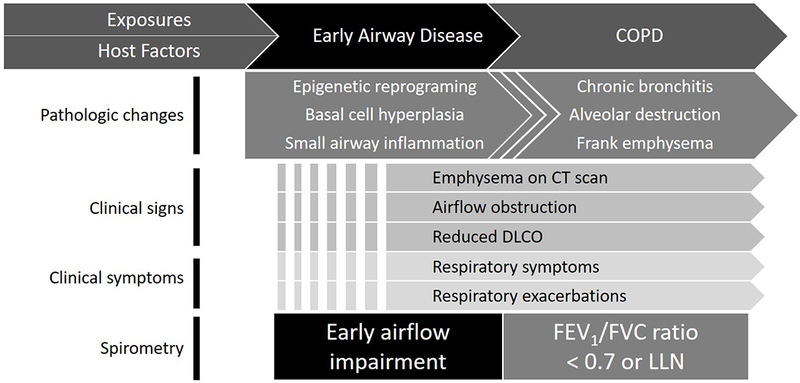
Summary of features underlying early airway disease which may be detected as early airflow impairment. Broken lines indicate variability in onset of described features. COPD = chronic obstructive lung disease; CT = computerized tomography; DLCO = diffusing capacity of lungs for carbon monoxide; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; LLN = lower limit of normal.
4. Spirometric indices of early airflow impairment beyond FEV1 and FVC
Spirometry offers abundant information about the function of the respiratory system, and it extends beyond measures such as FEV1 and FVC. Modern spirometers are built with sensitive real-time flow sensors which directly measure the flow of inhaled or exhaled air and obtain volumes by electronic or numerical integration. They can immediately display the real-time graphical spirogram and calculate reference values including the lower limit of normal. Analysis of spirometric data in the era of digital technology and machine learning, combined with a focus on recognition of early pathologic changes, offers the ability to explore and better understand other, less frequently used spirometric measures than FEV1 and FVC and develop novel parameters which could be used for detection of small airways disease and early airflow impairment. These indices are divided into five categories based on the mathematical approach used to analyze spirometric data. A summary of previously reported parameters and their categorization follows in Table 1.
Table 1. Spirometric indices of airflow impairment.
All suggested cutoffs are for airflow obstruction unless otherwise noted. FEV = forced expiratory volume, subscript denotes time in seconds; FVC = forced expiratory vital capacity; FIVC = forced inspiratory vital capacity; SVC = slow vital capacity; IC = inspiratory capacity; TLC = total lung capacity; FEF = forced expiratory flow, subscript denotes percentage of FVC; PEF = peak expiratory flow; LLN = lower limit of normal; -- = cutoff value is not well defined or not applicable.
| Category | Index | Suggested cutoff | Potential clinical applicability |
|---|---|---|---|
| Lung capacity indices | SVC – FVC | -- | Marker of air trapping; predicts exercise tolerance |
| FIVC – FVC | -- | Marker of air trapping | |
| FVC/SVC | -- | Indicator of small airway disease | |
| FEV1/SVC | < 0.7 or LLN | Obstruction in young individuals | |
| IC | -- | Indicates hyperinflation; predicts respiratory mortality | |
| Time-fractioned lung volume indices | FEV6 | LLN | More reproducible and less difficult to perform than FVC; predictor of lung function decline |
| FEV1/FEV6 | < 0.73 or LLN | In normal FEV1/FVC, associated with air-trapping, diffusion abnormalities, and respiratory exacerbations; identifies smokers | |
| FEV3/FEV6 and FEV3/FVC | LLN | In normal FEV1/FVC, associated with hyperinflation, air trapping, diffusion abnormalities; identifies smokers | |
| FEV0.5 or FEV0.75/FVC | LLN | Obstruction in infants and children | |
| Flow-based indices | FEF25-75 | < 65% predicted or LLN | Lower in some smokers normal FEV1/FVC; correlates with air trapping on CT |
| FEF75-85 | LLN | Distinguishes smokers from nonsmokers | |
| FEF50 (MEF50) or FEF75 | < 60% predicted | Reduced in GOLD zero patients | |
| FEF50/0.5FVC | -- | Correlates with FEV1/FVC | |
| FEF200-1200 | -- | Substitute for PEF | |
| PEF | Males < 350 L/min Females < 250 L/min |
Simple screening for undiagnosed COPD | |
| PIFR | < 60L/min | Predicts COPD-related hospital readmissions | |
| FEF50/FIF50 | -- | Evaluates upper airway obstruction; correlated with emphysema by CT | |
| Curvilinearity Measures | |||
| Classic geometric indices | Global concavity index | Males > 38.4 units Females > 26.3 units |
Based on FEF50, quantifies end-expiratory spirogram concavity |
| Peripheral concavity index | Males > 61.2 units Females > 63.1 units |
Based on FEF75, quantifies end-expiratory spirogram concavity | |
| Angle β | < 180° (concavity) | Lower in patients with dyspnea and wheezing than controls; improves in response to bronchodilators | |
| Slope ratio (SR) | > 1 (concavity) > 2.5 |
Indicates heterogenous lung emptying, obstruction | |
| Flow ratio at 75% FVC (FR75) | < 0 (concavity) | More negative in smokers than non-smokers | |
| Coefficient of maximal mid-expiratory flow (β-MMEF) | > 0.4 | Correlates with risk of hospitalization | |
| Curvature index (kmax) | -- | Exponentially associated with FEV1 | |
| Flow decay | Upper limit of normal (0.802 L−1) | Correlates with other measures of obstruction; not sensitive to artifactually low FVC | |
| Area under the curve in 3 seconds / Area of triangle 3 seconds (AUC3/AT3) | LLN | Surrogate for FEV1/FVC when 6 second expiratory effort not met (particularly young patients with obstruction) | |
| Area under the flow volume curve (AUFVC) | -- | Detects air trapping and hyperinflation; correlates with 6-minute walk | |
| Novel computational indices | Angle of collapse (AC) | < 131° ≤ 137° |
< 131° correlates significantly with emphysema extent; ≤137°asthma-COPD overlap syndrome |
| Volume dependence of slope ratio | SR decreases through exhalation in early COPD; SR increases through exhalation in elderly | Distinguish spirogram concavity caused by mild COPD from concavity due to physiologic changes with age | |
| Transfer function model of flow decline | -- | Correlates with traditional measures of obstruction well; offers additional inputs for machine learning algorithms | |
| Parameter D | -- | Identifies individuals with mild disease or unrecognized disease who have CT findings of structural lung disease | |
| Deep learning algorithms and other machine learning approaches | -- | May detect subtle patterns that distinguish disease from normal variation; may synthesize various indices to improve predictive power for relevant outcomes | |
4.1. Lung capacity indices
A number of lung capacity maneuvers are obtained during PFTs. An advantage of measuring lung capacities is predictability of normal ranges based on genetic sex, age, weight, height and race/ethnicity of the subject62. Spirometry allows for measurement of vital capacity (VC) - a sum of tidal volume (VT), inspiratory and expiratory reserve volumes (IRV and ERV) - and inspiratory capacity (IC), a sum of VT and IRV (Figure 2). VC can be measured while doing a slow (SIVC) or forceful (FIVC) inspiratory maneuver starting from residual volume (RV) up to the level of total lung capacity (TLC), or a slow (SEVC, commonly referred to as SVC) or forceful (FEVC, commonly referred to as FVC) expiration starting from TLC down to the level of RV63. Since airways resistance and effort differ between inspiration and expiration, VC varies in these maneuvers. The differences between the four types of VC are minimal in those with no obstruction. In patients with obstruction, FIVC is usually the largest and FVC the smallest of measured capacities, the latter being most frequently and most significantly affected in COPD63.
Figure 2. Difference between vital capacities.
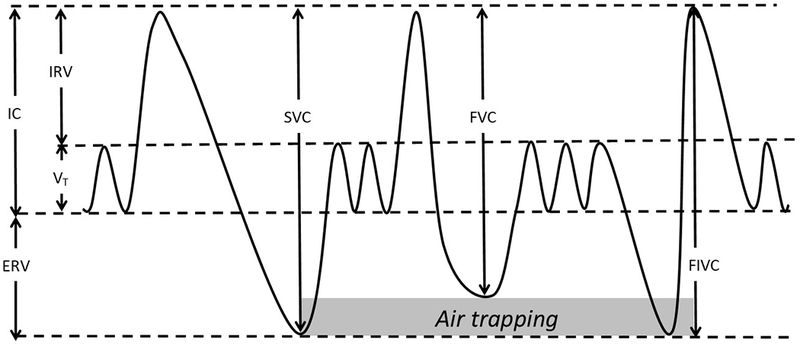
Theoretical time-volume curve for patient with obstruction demonstrating the difference between slow vital capacity (SVC) or forced inspiratory vital capacity (FIVC) and forced vital capacity (FVC) due to dynamic air trapping. Other volumes of note, IC = inspiratory capacity; ERV = expiratory reserve volume; IRV = inspiratory reserve volume; VT = tidal volume.
Although forced maneuvers are effort-dependent, they provide more information on flow-resistive characteristics than tidal maneuvers64. The high pressure generated in forced expiration pre-disposes to air trapping due to dynamic airway compression, leading to a fall in FVC in obstructed lungs. This effect is not as dramatic with a slow expiratory maneuver (SVC) or an inspiratory maneuver. The difference between SVC and FVC (Figure 2) has been described as a marker of air trapping, an early step in the development of obstruction63,65. This difference is also an independent predictor of diminished exercise tolerance and peak oxygen uptake in COPD patients66. However, interpretation of this index is complicated by the observation that body mass index (BMI) has a large impact on baseline vital capacities. In individuals with low BMI, FVC is larger than SVC, whereas FVC is smaller than SVC in individuals with high BMI67. A related index, FVC to SVC ratio (FVC/SVC) may give insight to changes in small airways - an important early step in COPD development. FVC/SVC decreased from baseline in lung transplant patients who develop bronchiolitis obliterans syndrome, a primarily small airways obstructive disease68. Compared to FVC, the stability of SVC may increase the sensitivity of spirometry to detect mild airflow obstruction, regardless of the defining criterion of obstruction (FEV1/FVC <0.70 or <LLN)63,69. Since the discrepancy between FVC and SVC increases with age, a decrement below a fixed FEV1/SVC ratio may better indicate obstruction in young individuals than in the elderly, where specificity may be reduced70. The obvious limiting factor for wider use of this metric is the lack of validation studies that would refer to clinical benefits of this more sensitive metric of diagnosing obstruction. In addition, lack of accepted LLN values for SVC makes interpretation more difficult given the significant impact of age or body habitus. Since expiratory time is usually longer than inspiratory time, and any leak caused by the patient or spirometer can affect expiration more than inspiration, and thus lead to lower FVC than FIVC. While assessing the difference between FIVC and FVC may help in detection of technically inadequate forced expiratory maneuvers, reduced FVC in comparison to FIVC can also be the consequence of the initiation of inhalation before the exhalation is complete in the FVC maneuver, which can be a sign of gas trapping (Figure 2) and can happen in individuals with severe airway obstruction63,65,66,71.
The relationship between FIVC, SVC and FVC remains to be studied in mildly obstructed patients, but the ability to detect air trapping and potentially small airway changes may be useful in identifying early steps in COPD pathophysiology.
Inspiratory capacity (IC) may be helpful in assessing severity, prognosis and response to treatment of airway obstruction. Worsening obstruction and alteration in the elastic properties of the lungs of patients with COPD are associated with the development of progressive lung hyperinflation and decline in the resting IC72. Since bronchodilator (BD) administration can reduce lung hyperinflation in the absence of significant improvement in FEV1 in advanced emphysema, improvement in IC can indirectly reflect the effect of a BD on hyperinflation reduction. Reduced IC in COPD as a consequence of increased functional residual capacity correlates with decreased exercise tolerance73, increased dyspnea74, and all-cause and respiratory mortality75. Compared to FEV1, IC better correlated with symptom severity during acute COPD exacerbation76. IC/TLC ratio <25% has been shown to be a predictor of exacerbations and death in patients with emphysematous COPD77.
4.2. Time-fractioned lung volume indices
Time-based lung volume fractions have the benefit of reproducilibility, simplicity of calculation, and familiarity. The most widely used metric is FEV1. There are several alternatives to FEV1 which provide information about different components of the forced expiratory maneuver (Figure 3).
Figure 3. Time-fractioned lung volumes.
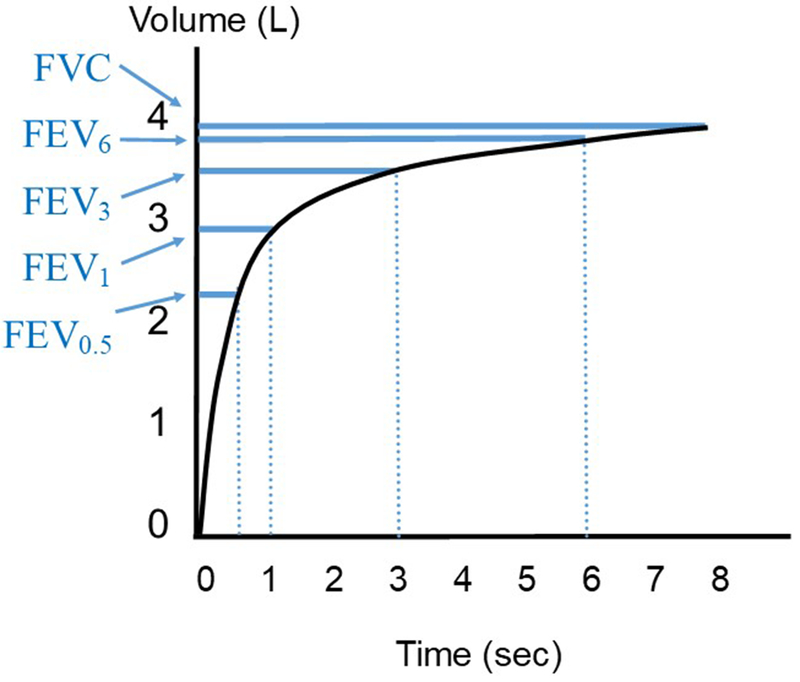
Plot of maximal expiratory volume-time curve, in a theoretical patient with mild obstruction, with commonly obtained time-fractioned lung volumes illustrated in blue. L = liters; sec = seconds; FEV = forced expiratory volume, subscript denotes time in seconds since initiation of force expiratory maneuver; FVC = forced vital capacity.
The forced expiratory volume in six seconds (FEV6) has been used as a potential alternative to FVC. Measuring FEV6 instead of FVC reduces duration of exhalation to six seconds, allowing for standardization of expiratory maneuver and limiting the effect of conscious effort to prolong the maneuver. FEV6 is more reproducible and less difficult to perform than FVC78 and performs well with office-based, hand-held devices79. In patient with COPD, an FEV1/FEV6 ratio in the lowest quartile (<74% predicted) and second lowest quartile (74-84% predicted) was shown to be an independent predictor of mortality and hospitalizations, and low FEV6 may predict future lung function decline80. A meta-analysis of eleven studies showed reduced FEV1/FEV6 ratio to be a sensitive and specific measure of airflow obstruction81. In the NHANES-III cohort, a LLN cutoff for FEV1/FEV6 outperformed FEV1/FVC in identifying smokers78. The most direct evidence of benefit in predicting early airway disease comes from work by Bhatt et al82 using the COPDGene cohort. Patients with FEV1/FEV6 <0.73 but FEV1/FVC above 0.70 or LLN had greater air trapping and airway wall thickness, poorer functional capacity, and a greater number of respiratory exacerbations at follow-up in comparison to those with reduced FEV1/FVC in isolation82. Similar results have been demonstrated in other large cohorts52,83. FEV1/FEV6 was found to be less sensitive than FEV1/FVC to detect obstruction, but those with isolated reduction in FEV1/FEV6 had greater physiologic abnormalities in spirometry, diffusing capacity, and metrics of air trapping83. Based on these data, while FEV1/FEV6 may not be a replacement for FEV1/FVC, inclusion may facilitate the detection of more individuals near conventional diagnostic cutoffs with important features of early airway disease.
In comparison to FEV1, extending the measurement of expired volume to the first three seconds of forced exhalation has the advantage of offering additional insight into air flow through small airways. The forced expiratory volume in three seconds (FEV3) has shown value for detecting early obstruction. Morris et al84,85 in a single center study of over 13,000 patients, demonstrated that an isolated reduction in FEV3/FVC, with normal FEV1/FVC, was associated with greater degrees of hyperinflation (higher RV and TLC), air trapping (RV/TLC ratio), and loss of diffusing capacity of the lung for carbon monoxide (DLCO) compared to those with normal FEV3/FVC and FEV1/FVC. While it has been argued that FEV3/FVC may simply be an overly sensitive measure of mild obstruction of any etiology that lacks specificity86, Hansen et al78,87 established mean and 95% confidence limits for the LLN values for FEV3 and demonstrated that FEV3/FVC and FEV3/FEV6 identified significantly more smokers in the NHANES-III dataset than FEV1/FVC or FEV1/FEV6 respectively78.
FEV0.5/FVC and FEV0.75/FVC are used in measuring obstruction in infants and children with wheezing88. However, despite inclusion in spirometry reference values in the past89,90, these measures have been rarely used in adults.
4.3. Flow-based indices
Instantaneous and mean flows may be derived from various points on the flow volume curve to capture flow dynamics at different portions of the forced expiratory maneuver (Figure 4). Flow-based indices may serve as a more direct measure of small airways than time-based indices as the former may be measured over only the later, effort-independent, portion of the flow-volume curve.
Figure 4. Flow-based indices.
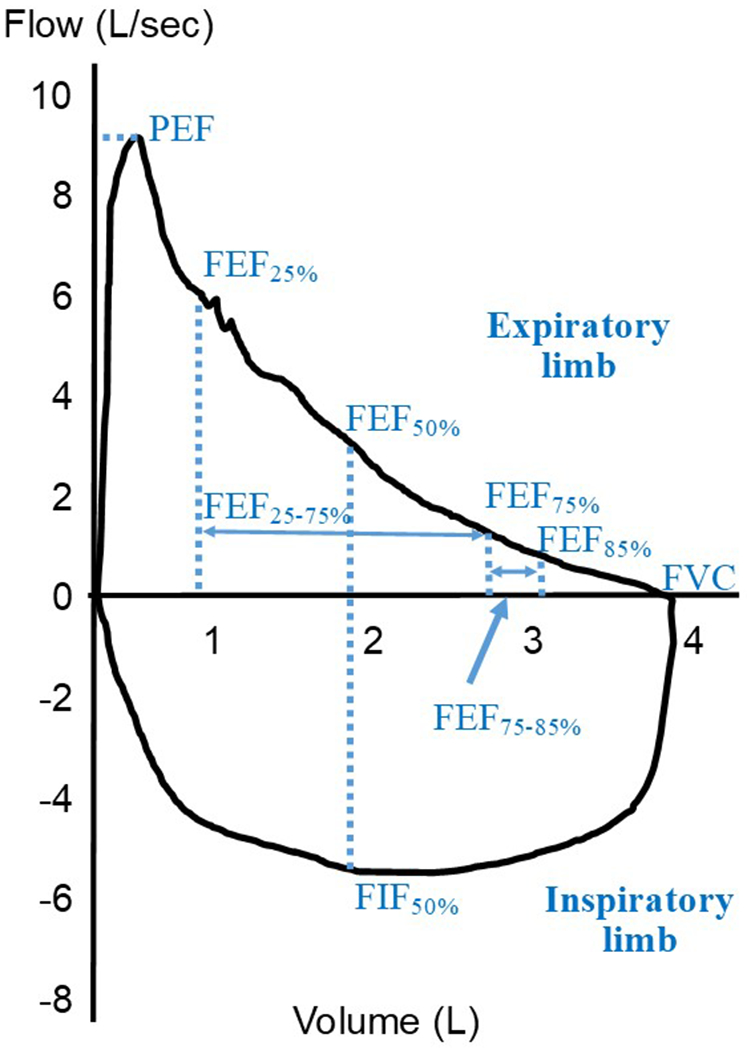
Flow-volume loop of a maximal inspiratory and expiratory maneuver from a theoretical patient with mild obstruction. The location where commonly obtained instantaneous and averaged flows are obtained are demonstrated by dotted lines and solid arrows, respectively. L = liters; sec = seconds; PEF = peak expiratory flow; FEF = forced expiratory flow, subscript denotes percentage of FVC; FIF = forced inspiratory flow; FVC = forced expiratory vital capacity.
The mean forced expiratory flow between 25% and 75% of the forced vital capacity (FEF25-75) is the most studied and most widely reported forced expiratory flow measure. There is a wealth of information linking FEF25-75 to small airway disease in a variety of conditions. It is reduced in early bronchial impairment in allergic rhinitis91, is a marker for early diagnosis of bronchiolitis obliterans92,93 and correlates with eosinophilic inflammation94. In regard to COPD, FEF25-75 is lower in current and former smokers with no evidence of airflow obstruction as conventionally defined in comparison to healthy individuals95. It also correlates with second hand smoke exposure in adolescents96 and with air trapping seen on chest CT imaging97. Nonetheless, the clinical utility of FEF25-75 has been limited, primarily by the wide range of normal values and within-subject variability. Different cut-off values have been proposed to be considered abnormal, the most commonly used cut off being < 65% of predicted flow98. But percentile LLN varies greatly among different patient populations; for example, the 5th percentile LLN is <35% predicted for those over 80 years old99. In NHANES III healthy controls100, height, weight, gender and ethnicity all account for a relatively small portion of variability in FEF25-75, making standardization of this metric challenging. The problems with interpretation of forced expiratory flows are even greater in patients with airway disease. Expiratory flow in small airways is, in part, dependent on the interplay of inward force of high pleural pressure and outward force of elastic recoil – which is dependent on lung volume. It is not practical to measure flows as a percentage of total lung volume, so percentage of FVC is used as a surrogate. FVC occurs at different total lung volumes depending on individual patient characteristics, introducing variability that contributes to large reference intervals for predicted values99,101. Furthermore, as obstruction develops, RV typically increases and FVC occurs at higher lung volumes, which limits even comparison of forced expiratory flow measures in any individual subject as his/her disease progresses. Abston et al102 attempted to address the effect of body mass on lung volumes and flow by describing FEF25-75/FVC and found association with several outcomes including exacerbations and mortality. A systematic study of PFT results from 22,676 consecutive patients at multiple tertiary centers called into question whether maximal mid-expiratory flows add meaningful information to FEV1/FVC, as there was very little discordance between FEF25-75 and FEV/FVC in properly performed spirometry101. When discordant results have been found, Detels et al103 showed that FEV1/FVC identified a greater percentage of smokers as abnormal when the FEF25-75 was normal than vice versa. Similarly, in the NHANES-III database discordant results with FEF25-75 <5th percentile LLN and normal FEV1/FVC often miscategorized never smokers as abnormal and smokers as normal87.
Mean forced expiratory flow at 75%-85% of FVC (FEF75-85), which falls further into the effort-independent portion of the flow volume curve, does distinguish smokers from nonsmokers104. Predicted normal values were found to correlate with height, age, and smoking history105. However, FEF75-85 is highly sensitive to the FVC volume and expiratory time106. FEF50-75, FEF75-85 and FEF85-95 do not predict mortality as accurately as FEV1107.
The instantaneous maximum expiratory flow at 50% of FVC (FEF50 or MEF50) is the flow where half of forced vital capacity (FVC) remains to be exhaled and, unsurprisingly, it strongly correlated with FEF25-75108. It is considered reduced when < 60 % of predicted and may be used as a surrogate of early small airways disease (defined by an abnormally low mid-expiratory flow in the presence of normal FEV1)109. Significant decreases in forced expiratory flow at both 75% (FEF75%) and 50% (FEF50%) of the forced vital capacity were detected in GOLD stage 0 COPD patients compared with those in nonsmokers110. A related metric FEF50/0.5FVC correlates with FEV1/FVC ratio111; however whether any additional information is gained remains unclear. Forced expiratory flows are all sensitive to variability in FVC, and their utility for diagnosis of early airflow obstruction remains undefined.
The peak expiratory flow (PEF) is commonly obtained and is generally used as a dynamic measure for monitoring severity of airflow obstruction during an exacerbation. A protocol using PEF and a simple symptom based score identified patients with COPD in the primary care setting112, and handheld peak flow meters seem to perform better than screening questionnaires alone at identifying undiagnosed COPD113. This may reflect late diagnosis of COPD in practice rather than detection of early disease. Variability among PEF values in healthy subjects is high, particularly females100, which may limit utility for detection of mild deficits. The FEF200-1200 is based on the average expiratory flow rate between 0.2 and 1.2 liters of the FVC114. It has been considered a substitute for PEF, nevertheless it becomes progressively less accurate as the vital capacity becomes smaller106.
Inspiratory flow features have also been reported to correlate with the status and progression of COPD even at early stages. Studies have shown that peak inspiratory flow rate (PIFR) may be reduced among females and advancing age, without a clear correlation between FEV1 and PIFR115. PIFR can be reduced during COPD exacerbations. Reduced PIFR is associated with worse COPD-related symptom burden, increased odds of COPD-related hospital readmissions116, and improved responsiveness to nebulized therapy117.
Another ratio, FEF50/FIF50, is based on maximal flows in inspiration and expiration during the flow-volume–loop maneuver. The flow during the middle of inspiration, measured at 50% of the FVC (FIF50% or MIF50%), is usually greater than the maximal expiratory flow at 50% of FVC (FEF50% or MEF50%). A FEF50/FIF50 ratio is, therefore, usually less than 1. In lesions associated with variable extrathoracic airflow obstruction, the ratio is increased (usually greater than 1), while in lesions associated with variable intrathoracic obstruction, the ratio is diminished (0.2 or less).64 FEF50/FIF50 has been correlated with presence of emphysema on CT118, although its clinical usefulness in detecting early airflow limitation has not been shown.
4.3.1. Measures of maximal expiratory flow volume curvilinearity (MEFVC)
In addition to known spirometric indices, several attempts have been made to model different aspects of a maximal expiratory flow-volume curve (MEFVC). A common hypothesis behind these approaches is that parameters obtained from modelling MEFVC may capture early pathophysiologic changes associated with COPD, as the shape of the MEFVC becomes abnormal before numerically derived spirometric measurements119. These modelling approaches could be broadly divided into two main categories. Classic geometric indices quantify the degree of concavity (section 4). Novel computational indices, shape analyses (section 5), model distinct elements of the MEFVC shape.
4.4. Classic geometric indices
The concavity of the flow volume curve is often utilized by experienced clinicians to provide a gestalt of a patient’s obstructive pattern, although objective criteria for analysis are lacking. The degree of curvature has been of interest since the 1980s, with the development of indices such as angle-β by Kapp et al120. Classic geometric indices are relatively simple calculations based on discrete points on the flow volume curve or on first to second order equations which approximate the curve in order to quantify the degree of concavity. Recently, the number of such approaches has expanded, facilitated by the ease of computerized calculation.
The angle-β measures concavity by quantifying the angle between the slopes of the first and second halves of the expiratory limb of the flow volume curve (Figure 5A). Angle-β has been shown to be lower in patients with asthma, bronchitis, dyspnea, and wheezing than controls117 and improves in response to bronchodilators. However, this measure is highly sensitive to attained FVC; if FVC is artifactually low due to incomplete exhalation, the mid-point will move to a lower volume which on an obstructed curve is closer to the initial steep, exponential decline in flow and thus may dramatically change the angle. In the 1990s O’Donnell et al121 proposed a parameter called flow-ratio at 75% FVC (FR75). FR75 was calculated as the deviation of FEF75 from a straight line joining FEF50 and RV and expressed as a percentage of FEF75. A FR75>0 indicates a convexity of the MEFVC with respect to the volume axis (Figure 5B), while an FR75<0 indicates concavity (Figure 5C), and the magnitude reflects the degree of curvature. O’Donnell et al showed that FR75 was significantly more negative in smokers than in non-smokers and could be used as a sensitive index for early obstructive ventilatory impairment.
Figure 5. Selected curve analyses of maximal expiratory flow volume curve.
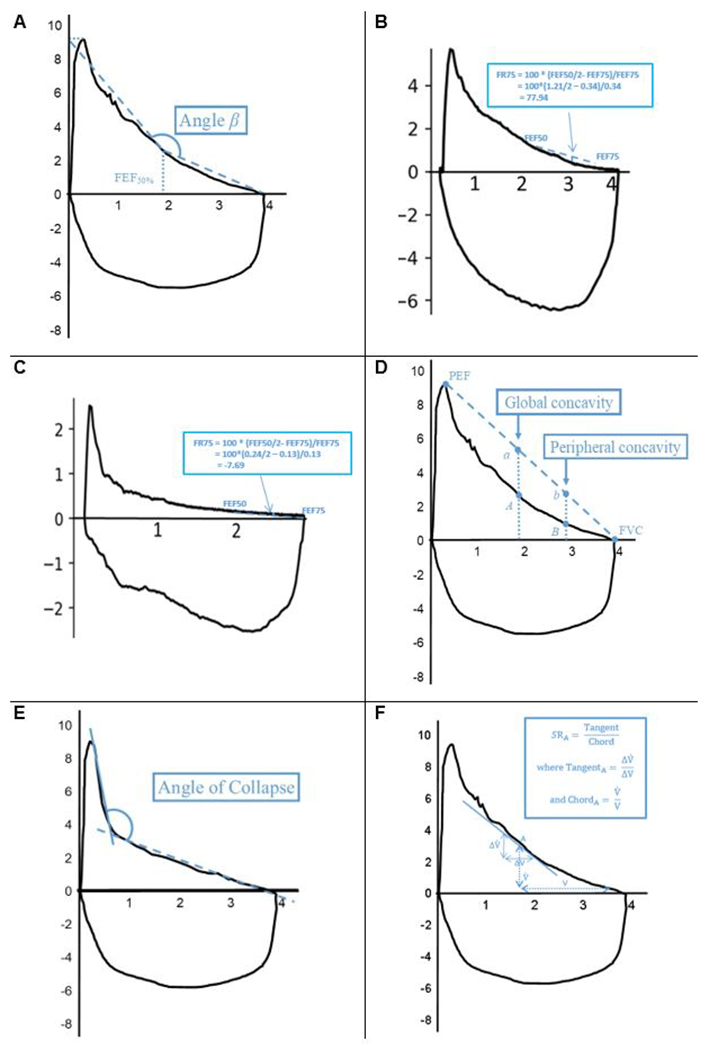
Flow-volume loop of a maximal inspiratory and expiratory maneuver from a theoretical patient with mild obstruction. X-axis = volume in liters, y-axis = flow in liters/second. A. Angle- β. FEF50% = forced expiratory flow at 50% of forced vital capacity (FVC) B. FR75, positive value. FR75 = flow-ratio at 75% FVC. C. FR75, negative value. D. Global and peripheral concavity index. Global concavity index = 100* (reference FEF50% (point a)—measured FEF50% (point A)/ (reference FEF50%); peripheral concavity index = (reference FEF75% (point b)—measured FEF75% (point B)/ (reference FEF75%). PEF = peak expiratory flow. E. Angle of collapse. Angle between two optimal regression lines (solid and dashed lines) of the descending limb of the expiratory curve. F. Slope ratio. Example instantaneous slope ratio calculation at point A (SRA). V = volume remaining to be expired and = flow at this point, chord (ChordA) is defined as the ratio of to V. If an instantaneous change in volume and flow at this point are denoted by , the tangent (TangentA) is defined as the ratio of to ΔV. SRA is calculated as the ratio of TangentA to ChordA.
More recently, Johns et al122 have put forward two related indices of concavity. They argue that the global concavity index is based on FEF50% and quantifies concavity that usually involves the entire descending limb, and the peripheral concavity index is based on FEF75%, which quantifies concavity present near the terminal portion of the curve (Figure 5D). The authors found strong correlation with other measures of forced expiratory flow, greater detection of abnormality than standard indices, and some discordance between global and peripheral indices which may distinguish between different phenotypes of obstruction. More examples of mathematical equations to model the flow volume curve and quantify the concavity of MEFVC include slope ratio123 curvature index (kmax)124, flow-decay125 and β-MMEF126; the last was also associated with increased risk of subsequent hospitalization.
Researchers have also studied the clinical significance of the area under MEFVC (AUFVC) and its other derivatives. AUFVC has been shown to be more sensitive to bronchoconstriction and bronchodilation when compared to FEV1 and other traditional parameters127. It is a good alternative for measuring lung function in pre-school children, especially when FEV1 cannot be obtained due to short expiratory times128. Lee et al129 calculated several ratios involving AUFVC that correlated well with six-minute walking distance in COPD patients. Das et al130 proposed a parameter called AreaFE% where they express AUFVC as a percentage of a healthy reference AUFVC, estimated using predicted values of PEF, FEF25, FEF50, FEF75 and FVC. They concluded that AreaFE% is superior to traditional parameters in detecting the presence of air-trapping (RV/TLC > upper limit of normal) and severe hyperinflation (RV/TLC > 60% and IC/TLC < 25%) in COPD patients. The area under the curve in the first 3 seconds relative to the FVC has been shown to be an adequate substitute for FEV1/FVC in suboptimal spirometry131.
4.5. Novel computational indices
The curvilinearity of the MEFVC has held a major interest among researchers. Intuitively, in view of the variety of underlying pathophysiologic changes which converge to obstruction, there is more information to be gained by analyzing the unique shape of individual flow volume curves rather than simply the degree of concavity. As with classic geometric indices, the availability of computerized analysis, and now machine learning, is rapidly expanding the number of indices developed and the power to validate such indices.
The presence of a particular expiratory flow volume curve shape - the spirographic “kink” - due to pressure-dependent airway collapse in emphysema has long been a known concept132, but Topalovic et al133 provided a mathematical model to quantify it termed the angle of collapse (AC). They did so by calculating the angle between two best fitting regression lines that approximate the flow after PEF (Figure 5E). They showed that an AC below 131 degrees could be considered as a specific cut-off for predicting the presence of emphysema on CT scans in heavy smokers. Wang et al134 further demonstrated that AC correlated significantly with emphysema extent quantified by percentage of low-attenuation areas less than −950 Hounsfield units (%LAA-950) in CT. They also concluded that AC ≤137 degrees could be used as a surrogate criterion for diagnosing asthma-COPD overlap.
Dynamical models describe time-dependent changes of volume or airflow during a spirometric maneuver. In mathematics a dynamical system is one which evolves over time according to a fixed rule. In spirometry the fixed rule may reflect intrinsic characteristics of the lung – i.e. elasticity, airway diameter and branching – which determine the characteristics of flow at a particular volume. One of the earliest works can be traced to the 1970s with Webster et al135 in the early phases of spirometry development, who calculated instantaneous time constants as the ratio of remaining expiratory volume to maximal flow. Mead et al136 developed a similar index called slope ratios (SR) in the late 1970s. SR is the ratio of instantaneous tangent slope (prior to point of interest) to corresponding chord slope (after point of interest) on MEFVC curves (Figure 5F). The plot of SRs against fractional expiratory volumes are sensitive to the shape of the MEFVC. Although Mead concluded that SR plots showed systematic changes with age and they were noticeably different in the abnormal curves of smokers, he speculated that they were not likely to detect early disease. Recently, Dominelli et al123 showed that mild COPD patients had a significantly larger mean SR than healthy individuals. They further concluded that the shape of the instantaneous SR and lung volume plot could, in fact, differentiate age-related changes in non-smokers (where SR was elevated and gradually increased during exhalation) from mild COPD in smokers (where SR was initially more elevated and gradually decreased throughout exhalation).
More complex dynamical models are now being developed through computerized modelling. Topalovic et al137 proposed a transfer function model to describe flow in time after PEF and explained the baseline differences of model parameters such as poles and steady state gain between COPD and non-COPD. In a subsequent work, he applied machine learning to these model parameters as input to detect the presence of small airway disease in a cohort of discordant subjects (FEV1/FVC between LLN and 0.70)138. Recently, Bhatt et al139 derived a metric for airflow-obstruction called parameter D by describing the volume as an exponential function of time. They revealed that parameter D could identify additional subjects, who would be considered normal by traditional criteria, with mild disease or abnormal lung function with greater likelihood of structural lung disease.
The application of machine learning (ML) on spirometry data in detecting early obstruction may hold a promising future. ML has already been successfully applied to data from pulmonary function tests, CT, forced oscillation tests, sounds from lung auscultation and exhaled breath for diagnosing obstructive lung diseases140. The advantage of ML lies in the fact that it can learn complex yet subtle patterns which may distinguish early pathophysiologic changes from the effects of normal aging or smoking. We believe that there will be two different paths in the development of such algorithms. One path will involve extracting parameters through mathematical modelling of flow-volume data and feeding them as an input into a ML model, which then outputs a probability measure of a clinically relevant outcome138. The other path will involve a direct application of ML algorithms to flow-volume data, which in-turn will detect patterns that may associate with early COPD development. While the former approach could work in datasets with very limited samples, we believe the latter approach may require larger datasets as these models would be much larger in terms of computational complexity. However, it is still very early to comment on their comparative advantages.
4.6. Indices outside of the maximal flow volume curve
While not the focus of this review, it should be noted that several indices derived from routine spirometry other than the maximal expiratory or inspiratory flow volume curves have been studied. For example, Williams et al141 analyzed the centroids of flow-time and flow-volume waveforms obtained from tidal breathing in spirometry. They concluded that breathing rate is faster and time to reach PEF is shorter in COPD patients with the centroids left-shifted with increasing asymmetry with airflow obstruction. In one of the only studies involving frequency domain analysis, Anogeianaki et al142 studied the power spectrum characteristics of forced expiratory airflow. They showed that airflow resonances are sub-audible (<20 Hz) and that COPD patients have different power spectral characteristics than healthy individuals below 3.66 Hz. Combined with traditional indices, these approaches may increase the power of spirometry as a single test to distinguish unique patterns of obstruction.
5. Future of spirometry for detecting early obstruction and predicting COPD development
While it has been widely available for decades, the clinical use of spirometry remains primarily limited to FEV1 and FVC analysis. Advances in understanding of the biologic mechanisms underlying early airway abnormalities in smokers hold promise for development of early interventions, highlighting the clinical imperative to identify early disease. In this context, spirometry may be an ideal diagnostic tool as it is widely performed and remains a crucial test in diagnosing and managing COPD. As we broaden our knowledge about early disease through large observational COPD cohorts, in an era of digitalized spirometry and increasingly ubiquitous complex analytic tools, we are offered the possibility to better understand and utilize spirometry. This review highlights simple measures of early airflow compromise such as FEV1/FEV6 or FEV3/FEV6. We also acknowledge the growing interest in measures of curvilinearity, which can provide more granular assessment of lung function. Machine learning holds promise for curve analysis which may detect subtle patterns that distinguish early pathophysiologic changes from the expected changes of aging and may allow synthesis of a variety of measures to form better predictive models for relevant outcomes.
Many of the investigations into alternative indices have been single center and retrospective. There is a need for organization within the field of spirometry to prioritize and expand investigation into promising metrics to drive clinical practice. We hope that classification schema for spirometric indices of early airway disease proposed in this review may provide a framework for further investigation and comparison between various indices of early airflow impairment. It is of crucial importance that investigational efforts in this field continue, in line with the premise that spirometry goes far beyond FEV1/FVC.
HIGHLIGHTS:
Clinically relevant airway abnormalities may precede formal diagnosis of COPD by FEV1/FVC ratio.
Evidence for spirometric indices of early airflow impairment preceding COPD is summarized.
This review offers a classification scheme of existing indices based on mathematical approach.
Digital analysis of spirometry and machine learning provide new avenues to characterize early disease.
Significance:
Spirometry is well-validated in diagnosis of COPD, nevertheless the evidence suggests that early airway abnormalities often start before the formal spirometric diagnosis of COPD. While alternative approaches to identify these subjects (symptom-based, imaging techniques) have been investigated, the full potential of spirometry to identify early disease has not been completely exploited. Multiple spirometric indices - some previously investigated and some being novel - may deserve more systematic evaluation in the era of spirometry digitalization and availability of data from large observational longitudinal cohorts. In this review, we summarize published evidence about alternative spirometric indices of airflow obstruction and propose their systematic categorization which could be utilized in future studies focused on early airway disease.
References:
- 1.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. [DOI] [PubMed] [Google Scholar]
- 2.Csikesz NG, Gartman EJ. New developments in the assessment of COPD: early diagnosis is key. Int J Chron Obstruct Pulmon Dis. 2014;9:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Decramer M, Cooper CB. Treatment of COPD: the sooner the better? Thorax. 2010;65(9):837–841. [DOI] [PubMed] [Google Scholar]
- 4.Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374(9696):1171–1178. [DOI] [PubMed] [Google Scholar]
- 5.Jenkins CR, Jones PW, Calverley PM, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res. 2009;10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of Smoking Intervention and the Use of an Inhaled Anticholinergic Bronchodilator on the Rate of Decline of FEV1: The Lung Health Study. JAMA. 1994;272(19):1497–1505. [PubMed] [Google Scholar]
- 7.Macklem PT. Therapeutic implications of the pathophysiology of COPD. Eur Respir J. 2010;35(3):676–680. [DOI] [PubMed] [Google Scholar]
- 8.Cosio M, Ghezzo H, Hogg JC, et al. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med. 1978;298(23):1277–1281. [DOI] [PubMed] [Google Scholar]
- 9.Tantucci C, Modina D. Lung function decline in COPD. International journal of chronic obstructive pulmonary disease. 2012;7:95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loeckx M, Rodrigues F, Demeyer H, et al. Decline in function in preclinical COPD patients: a 6 years follow up study. European Respiratory Journal. 2017;50(suppl 61):OA3404. [Google Scholar]
- 11.Reid L. Measurement of the bronchial mucous gland layer: a diagnostic yardstick in chronic bronchitis. Thorax. 1960;15:132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thurlbeck WM, Angus GE. A DISTRIBUTION CURVE FOR CHRONIC BRONCHITIS. Thorax. 1964;19:436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunnill MS. The classification and quantification of emphysema. Proceedings of the Royal Society of Medicine. 1969;62(10):1024–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koo HK, Vasilescu DM, Booth S, et al. Small airways disease in mild and moderate chronic obstructive pulmonary disease: a cross-sectional study. Lancet Respir Med. 2018;6(8):591–602. [DOI] [PubMed] [Google Scholar]
- 15.Hogg JC, Chu F, Utokaparch S, et al. The Nature of Small-Airway Obstruction in Chronic Obstructive Pulmonary Disease. New England Journal of Medicine. 2004;350(26):2645–2653. [DOI] [PubMed] [Google Scholar]
- 16.Singh D. Small Airway Disease in Patients with Chronic Obstructive Pulmonary Disease. Tuberculosis and respiratory diseases. 2017;80(4):317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macklem PT, Mead J. Resistance of central and peripheral airways measured by a retrograde catheter. Journal of Applied Physiology. 1967;22(3):395–401. [DOI] [PubMed] [Google Scholar]
- 18.Van Brabandt H, Cauberghs M, Verbeken E, Moerman P, Lauweryns JM, Van de Woestijne KP. Partitioning of pulmonary impedance in excised human and canine lungs. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(6):1733–1742. [DOI] [PubMed] [Google Scholar]
- 19.Oppenheimer BW, Goldring RM, Berger KI. Distal airway function assessed by oscillometry at varying respiratory rate: comparison with dynamic compliance. Copd. 2009;6(3):162–170. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder JD, McKenzie AS, Zach JA, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol. 2013;201(3):W460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crystal RG. Airway basal cells. The “smoking gun” of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190(12):1355–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaykhiev R, Crystal RG. Early events in the pathogenesis of chronic obstructive pulmonary disease. Smoking-induced reprogramming of airway epithelial basal progenitor cells. Ann Am Thorac Soc. 2014;11 Suppl 5:S252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staudt MR, Buro-Auriemma LJ, Walters MS, et al. Airway Basal stem/progenitor cells have diminished capacity to regenerate airway epithelium in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190(8):955–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and Radiologic Disease in Smokers With Normal Spirometry. JAMA Intern Med. 2015;175(9):1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Salcedo P, Divo M, Casanova C, et al. Disease progression in young patients with COPD: rethinking the Fletcher and Peto model. Eur Respir J. 2014;44(2):324–331. [DOI] [PubMed] [Google Scholar]
- 26.Harvey BG, Strulovici-Barel Y, Kaner RJ, et al. Risk of COPD with obstruction in active smokers with normal spirometry and reduced diffusion capacity. Eur Respir J. 2015;46(6):1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodruff PG, Barr RG, Bleecker E, et al. Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function. N Engl J Med. 2016;374(19):1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celli BR, Agustí A. COPD: time to improve its taxonomy? ERJ Open Research. 2018;4(1):00132–02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan ES, Fortis S, Regan EA, et al. Longitudinal Phenotypes and Mortality in Preserved Ratio Impaired Spirometry in the COPDGene Study. Am J Respir Crit Care Med. 2018;198(11):1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. American Journal of Respiratory and Critical Care Medicine. 2001;163(5):1256–1276. [DOI] [PubMed] [Google Scholar]
- 31.Park HJ, Byun MK, Rhee CK, Kim K, Kim HJ, Yoo KH. Significant predictors of medically diagnosed chronic obstructive pulmonary disease in patients with preserved ratio impaired spirometry: a 3-year cohort study. Respir Res. 2018;19(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez FJ, Han MK, Allinson JP, et al. At the Root: Defining and Halting Progression of Early Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2018;197(12):1540–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rennard SI, Drummond MB. Early chronic obstructive pulmonary disease: definition, assessment, and prevention. Lancet (London, England). 2015;385(9979):1778–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siafakas N, Bizymi N, Mathioudakis A, Corlateanu A. EARLY versus MILD Chronic Obstructive Pulmonary Disease (COPD). Respiratory medicine. 2018;140:127–131. [DOI] [PubMed] [Google Scholar]
- 35.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. Available from: https://goldcopd.org/. [DOI] [PubMed]
- 36.Rennard SI. The Promise of Observational Studies (ECLIPSE, SPIROMICS, and COPDGene) in Achieving the Goal of Personalized Treatment of Chronic Obstructive Pulmonary Disease. Semin Respir Crit Care Med. 2015;36(4):478–490. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Roisin R, Han MK, Vestbo J, Wedzicha JA, Woodruff PG, Martinez FJ. Chronic Respiratory Symptoms with Normal Spirometry. A Reliable Clinical Entity? Am J Respir Crit Care Med. 2017;195(1):17–22. [DOI] [PubMed] [Google Scholar]
- 38.Anderson WH, Ha JW, Couper DJ, et al. Variability in objective and subjective measures affects baseline values in studies of patients with COPD. PLoS One. 2017;12(9):e0184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cochrane GM, Prieto F, Clark TJ. Intrasubject variability of maximal expiratory flow volume curve. Thorax. 1977;32(2):171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medarov BI, Pavlov VA, Rossoff L. Diurnal variations in human pulmonary function. Int J Clin Exp Med. 2008;1(3):267–273. [PMC free article] [PubMed] [Google Scholar]
- 41.Enright PL, Beck KC, Sherrill DL. Repeatability of spirometry in 18,000 adult patients. Am J Respir Crit Care Med. 2004;169(2):235–238. [DOI] [PubMed] [Google Scholar]
- 42.Kunzli N, Ackermann-Liebrich U, Keller R, Perruchoud AP, Schindler C. Variability of FVC and FEV1 due to technician, team, device and subject in an eight centre study: three quality control studies in SAPALDIA. Swiss Study on Air Pollution and Lung Disease in Adults. Eur Respir J. 1995;8(3):371–376. [DOI] [PubMed] [Google Scholar]
- 43.Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate End-points (ECLIPSE). Eur Respir J. 2008;31(4):869–873. [DOI] [PubMed] [Google Scholar]
- 44.Agusti A, Calverley PMA, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respiratory research. 2010;11(1):122–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vestbo J, Agusti A, Wouters EF, et al. Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team. Am J Respir Crit Care Med. 2014;189(9):1022–1030. [DOI] [PubMed] [Google Scholar]
- 46.Lutfi MF. The physiological basis and clinical significance of lung volume measurements. Multidisciplinary respiratory medicine. 2017;12:3–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault J-C. Lung volumes and forced ventilatory flows. European Respiratory Journal. 1993;6(Suppl 16):5–40. [DOI] [PubMed] [Google Scholar]
- 48.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. European Respiratory Journal. 2005;26(5):948–968. [DOI] [PubMed] [Google Scholar]
- 49.Culver BH, Graham BL, Coates AL, et al. Recommendations for a Standardized Pulmonary Function Report. An Official American Thoracic Society Technical Statement. Am J Respir Crit Care Med. 2017;196(11):1463–1472. [DOI] [PubMed] [Google Scholar]
- 50.Rennard SI, Vestbo J, Agusti A. What is chronic obstructive pulmonary disease anyway?: Continua, categories, cut points, and moving beyond spirometry. Am J Respir Crit Care Med. 2013;187(10):1036–1037. [DOI] [PubMed] [Google Scholar]
- 51.Swanney MP, Ruppel G, Enright PL, et al. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63(12):1046–1051. [DOI] [PubMed] [Google Scholar]
- 52.Vollmer WM, Gislason T, Burney P, et al. Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J. 2009;34(3):588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mannino DM, Sonia Buist A, Vollmer WM. Chronic obstructive pulmonary disease in the older adult: what defines abnormal lung function? Thorax. 2007;62(3):237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhatt SP, Sieren JC, Dransfield MT, et al. Comparison of spirometric thresholds in diagnosing smoking-related airflow obstruction. Thorax. 2014;69(5):409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pirozzi CS, Gu T, Quibrera P, et al. Heterogeneous Burden of Emphysema and Functional Small Airway Abnormalities in Smokers with FEV<sub>1</sub>/FVC Ratio Above Lower Limit of Normal but Below 0.7 In: D28. RESPIRATORY DISEASE DIAGNOSIS: PULMONARY FUNCTION TESTING AND IMAGING. American Thoracic Society; 2018:A6397–A6397. [Google Scholar]
- 56.Mohamed Hoesein FA, Zanen P, Lammers JW. Lower limit of normal or FEV1/FVC < 0.70 in diagnosing COPD: an evidence-based review. Respiratory medicine. 2011;105(6):907–915. [DOI] [PubMed] [Google Scholar]
- 57.Hansen JE, Sun XG, Wasserman K. Spirometric criteria for airway obstruction: Use percentage of FEV1/FVC ratio below the fifth percentile, not < 70%. Chest. 2007;131(2):349–355. [DOI] [PubMed] [Google Scholar]
- 58.Aaron SD, Tan WC, Bourbeau J, et al. Diagnostic Instability and Reversals of Chronic Obstructive Pulmonary Disease Diagnosis in Individuals with Mild to Moderate Airflow Obstruction. Am J Respir Crit Care Med. 2017;196(3):306–314. [DOI] [PubMed] [Google Scholar]
- 59.Mannino DM, Diaz-Guzman E, Buist S. Pre- and post-bronchodilator lung function as predictors of mortality in the Lung Health Study. Respir Res. 2011;12:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fortis S, Eberlein M, Georgopoulos D, Comellas AP. Predictive value of prebronchodilator and postbronchodilator spirometry for COPD features and outcomes. BMJ Open Respir Res. 2017;4(1):e000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen CZ, Ou CY, Wang WL, et al. Using post-bronchodilator FEV(1) is better than pre-bronchodilator FEV(1) in evaluation of COPD severity. Copd. 2012;9(3):276–280. [DOI] [PubMed] [Google Scholar]
- 62.Degens P, Merget R. Reference values for spirometry of the European Coal and Steel Community: time for change. European Respiratory Journal. 2008;31(3):687–688. [DOI] [PubMed] [Google Scholar]
- 63.Chhabra SK. Forced vital capacity, slow vital capacity, or inspiratory vital capacity: which is the best measure of vital capacity? J Asthma. 1998;35(4):361–365. [DOI] [PubMed] [Google Scholar]
- 64.Miller RD, Hyatt RE. Obstructing lesions of the larynx and trachea: clinical and physiologic characteristics. Mayo Clin Proc. 1969;44(3):145–161. [PubMed] [Google Scholar]
- 65.Brusasco V, Pellegrino R, Rodarte JR. Vital capacities in acute and chronic airway obstruction: dependence on flow and volume histories. Eur Respir J. 1997;10(6):1316–1320. [DOI] [PubMed] [Google Scholar]
- 66.Yuan W, He X, Xu QF, Wang HY, Casaburi R. Increased difference between slow and forced vital capacity is associated with reduced exercise tolerance in COPD patients. BMC Pulm Med. 2014;14:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fortis S, Corazalla EO, Wang Q, Kim HJ. The difference between slow and forced vital capacity increases with increasing body mass index: a paradoxical difference in low and normal body mass indices. Respir Care. 2015;60(1):113–118. [DOI] [PubMed] [Google Scholar]
- 68.Cohen J, Postma DS, Vink-Klooster K, et al. FVC to slow inspiratory vital capacity ratio: a potential marker for small airways obstruction. Chest. 2007;132(4):1198–1203. [DOI] [PubMed] [Google Scholar]
- 69.Mathieu Saint-Pierre JL, Berton Danilo, Zapotichny Angie, Faubert Denis, Crozier-Wells Lori, Tang Julianna, Muir Cathy, Forkert Lutz, O’Donnell Denis, Serafini Jose Alberto Neder. Usefulness of FEV1/SVC to uncover airflow obstruction in subjects with preserved FEV1/FVC. European Respiratory Journal 2016. 48: PA2229; DOI: 101183/13993003congress-2016PA2229. 2016. [Google Scholar]
- 70.Marsh S, Aldington S, Williams M, et al. Complete reference ranges for pulmonary function tests from a single New Zealand population. N Z Med J. 2006;119(1244):U2281. [PubMed] [Google Scholar]
- 71.Engel T, Heinig JH, Madsen F, Nikander K. Peak inspiratory flow and inspiratory vital capacity of patients with asthma measured with and without a new dry-powder inhaler device (Turbuhaler). Eur Respir J. 1990;3(9):1037–1041. [PubMed] [Google Scholar]
- 72.O’Donnell DE, Elbehairy AF, Webb KA, Neder JA, Canadian Respiratory Research N. The Link between Reduced Inspiratory Capacity and Exercise Intolerance in Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. 2017;14(Supplement_1):S30–S39. [DOI] [PubMed] [Google Scholar]
- 73.Diaz O, Villafranca C, Ghezzo H, et al. Role of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitation at rest. Eur Respir J. 2000;16(2):269–275. [DOI] [PubMed] [Google Scholar]
- 74.Marin JM, Carrizo SJ, Gascon M, Sanchez A, Gallego B, Celli BR. Inspiratory capacity, dynamic hyperinflation, breathlessness, and exercise performance during the 6-minute-walk test in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(6):1395–1399. [DOI] [PubMed] [Google Scholar]
- 75.Tantucci C, Donati P, Nicosia F, et al. Inspiratory capacity predicts mortality in patients with chronic obstructive pulmonary disease. Respiratory medicine. 2008;102(4):613–619. [DOI] [PubMed] [Google Scholar]
- 76.Yetkin O, Gunen H. Inspiratory capacity and forced expiratory volume in the first second in exacerbation of chronic obstructive pulmonary disease. Clin Respir J. 2008;2(1):36–40. [DOI] [PubMed] [Google Scholar]
- 77.Zaman M, Mahmood S, Altayeh A. Low inspiratory capacity to total lung capacity ratio is a risk factor for chronic obstructive pulmonary disease exacerbation. Am J Med Sci. 2010;339(5):411–414. [DOI] [PubMed] [Google Scholar]
- 78.Hansen JE, Porszasz J, Casaburi R, Stringer WW. Re-Defining Lower Limit of Normal for FEV1/FEV6, FEV1/FVC, FEV3/FEV6 and FEV3/FVC to Improve Detection of Airway Obstruction. Chronic Obstr Pulm Dis. 2015;2(2):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Frith P, Crockett A, Beilby J, et al. Simplified COPD screening: validation of the PiKo-6(R) in primary care. Prim Care Respir J. 2011;20(2):190–198, 192 p following 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prats E, Tejero E, Pardo P, et al. Prognostic Value of the Six-Second Spirometry in Patients with Chronic Obstructive Pulmonary Disease: A Cohort Study. PLOS ONE. 2015;10(10):e0140855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jing JY, Huang TC, Cui W, Xu F, Shen HH. Should FEV1/FEV6 replace FEV1/FVC ratio to detect airway obstruction? A metaanalysis. Chest. 2009;135(4):991–998. [DOI] [PubMed] [Google Scholar]
- 82.Bhatt SP, Kim YI, Wells JM, et al. FEV(1)/FEV(6) to diagnose airflow obstruction. Comparisons with computed tomography and morbidity indices. Annals of the American Thoracic Society. 2014;11(3):335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morris ZQ, Huda N, Burke RR. The diagnostic importance of a reduced FEV1/FEV6. Copd. 2012;9(1):22–28. [DOI] [PubMed] [Google Scholar]
- 84.Morris ZQ, Coz A, Starosta D. An isolated reduction of the FEV3/FVC ratio is an indicator of mild lung injury. Chest. 2013;144(4):1117–1123. [DOI] [PubMed] [Google Scholar]
- 85.Morris ZQ. In Reply: Isolated Reduction of the FEV3/FVC Ratio as an Indicator of Mild Airflow Obstruction. Chest. 2014;145(3):662–663. [DOI] [PubMed] [Google Scholar]
- 86.Madan K, Hadda V, Khilnani GC, Guleria R. Isolated reduction of the FEV3/FVC ratio as an indicator of mild airflow obstruction. Chest. 2014;145(3):662. [DOI] [PubMed] [Google Scholar]
- 87.Hansen JE, Sun XG, Wasserman K. Discriminating measures and normal values for expiratory obstruction. Chest. 2006;129(2):369–377. [DOI] [PubMed] [Google Scholar]
- 88.Neve V, Hulo S, Edme JL, et al. Utility of measuring FEV0.75/FVC ratio in preschoolers with uncontrolled wheezing disorder. Eur Respir J. 2016;48(2):420–427. [DOI] [PubMed] [Google Scholar]
- 89.Kory RC, Callahan R, Boren HG, Syner JC. The veterans administration-army cooperative study of pulmonary function: I. Clinical spirometry in normal men. The American Journal of Medicine. 1961;30(2):243–258. [DOI] [PubMed] [Google Scholar]
- 90.Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. The American review of respiratory disease. 1976;113(5):587–600. [DOI] [PubMed] [Google Scholar]
- 91.Ciprandi G, Cirillo I, Klersy C, et al. Role of FEF25-75 as an early marker of bronchial impairment in patients with seasonal allergic rhinitis. Am J Rhinol. 2006;20(6):641–647. [DOI] [PubMed] [Google Scholar]
- 92.Patterson GM, Wilson S, Whang JL, et al. Physiologic definitions of obliterative bronchiolitis in heart-lung and double lung transplantation: a comparison of the forced expiratory flow between 25% and 75% of the forced vital capacity and forced expiratory volume in one second. J Heart Lung Transplant. 1996;15(2):175–181. [PubMed] [Google Scholar]
- 93.Sritippayawan S, Keens TG, Horn MV, Starnes VA, Woo MS. What are the best pulmonary function test parameters for early detection of post-lung transplant bronchiolitis obliterans syndrome in children? Pediatr Transplant. 2003;7(3):200–203. [DOI] [PubMed] [Google Scholar]
- 94.Malerba M, Radaeli A, Olivini A, et al. Association of FEF25-75% Impairment with Bronchial Hyperresponsiveness and Airway Inflammation in Subjects with Asthma-Like Symptoms. Respiration. 2016;91(3):206–214. [DOI] [PubMed] [Google Scholar]
- 95.Kornmann O, Beeh KM, Beier J, et al. Newly diagnosed chronic obstructive pulmonary disease. Clinical features and distribution of the novel stages of the Global Initiative for Obstructive Lung Disease. Respiration. 2003;70(1):67–75. [DOI] [PubMed] [Google Scholar]
- 96.Bird Y, Staines-Orozco H. Pulmonary effects of active smoking and secondhand smoke exposure among adolescent students in Juarez, Mexico. Int J Chron Obstruct Pulmon Dis. 2016;11:1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee SM, Seo JB, Lee SM, Kim N, Oh SY, Oh YM. Optimal threshold of subtraction method for quantification of air-trapping on coregistered CT in COPD patients. Eur Radiol. 2016;26(7):2184–2192. [DOI] [PubMed] [Google Scholar]
- 98.Ciprandi G, Capasso M, Tosca M, et al. A forced expiratory flow at 25-75% value <65% of predicted should be considered abnormal: a real-world, cross-sectional study. Allergy Asthma Proc. 2012;33(1):e5–8. [DOI] [PubMed] [Google Scholar]
- 99.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. [DOI] [PubMed] [Google Scholar]
- 101.Quanjer PH, Weiner DJ, Pretto JJ, Brazzale DJ, Boros PW. Measurement of FEF25-75% and FEF75% does not contribute to clinical decision making. Eur Respir J. 2014;43(4):1051–1058. [DOI] [PubMed] [Google Scholar]
- 102.Abston E, Comellas A, Reed RM, et al. Higher BMI is associated with higher expiratory airflow normalised for lung volume (FEF25-75/FVC) in COPD. BMJ Open Respir Res. 2017;4(1):e000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Detels R, Tashkin DP, Simmons MS, et al. The UCLA population studies of chronic obstructive respiratory disease. 5. Agreement and disagreement of tests in identifying abnormal lung function. Chest. 1982;82(5):630–638. [DOI] [PubMed] [Google Scholar]
- 104.Sorbello A, Giudice JC, Komansky H, Gordon R, Kaufman JL. Forced end-expiratory flow (FEF75-85) measurement: use in diagnosis of small airways dysfunction from routine spirometric tracings. J Am Osteopath Assoc. 1981;80(11):731–732. [PubMed] [Google Scholar]
- 105.Morris JF, Koski A, Breese JD. Normal values and evaluation of forced end-expiratory flow. Am Rev Respir Dis. 1975;111(6):755–762. [DOI] [PubMed] [Google Scholar]
- 106.Johnson R. FVC measurements that are mostly gone but not completely forgotten. https://wwwpftforumcom/blog.
- 107.Thomason MJ, Strachan DP. Which spirometric indices best predict subsequent death from chronic obstructive pulmonary disease? Thorax. 2000;55(9):785–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lutfi MF. Patterns of changes and diagnostic values of FEF50%, FEF25%-75% and FEF50%/FEF25%-75% ratio in patients with varying control of bronchial asthma. International journal of health sciences. 2016;10(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guder G, Brenner S, Stork S, et al. Diagnostic and prognostic utility of mid-expiratory flow rate in older community-dwelling persons with respiratory symptoms, but without chronic obstructive pulmonary disease. BMC Pulm Med. 2015;15:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gong SG, Yang WL, Liu JM, Liu WZ, Zheng W. Change in pulmonary function in chronic obstructive pulmonary disease stage 0 patients. Int J Clin Exp Med. 2015;8(11):21400–21406. [PMC free article] [PubMed] [Google Scholar]
- 111.Rodrigues MT, Fiterman-Molinari D, Barreto SS, Fiterman J. The role of the FEF50%/0.5FVC ratio in the diagnosis of obstructive lung diseases. J Bras Pneumol. 2010;36(1):44–50. [DOI] [PubMed] [Google Scholar]
- 112.Martinez FJ, Mannino D, Leidy NK, et al. A New Approach for Identifying Patients with Undiagnosed Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2017;195(6):748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haroon S, Jordan R, Takwoingi Y, Adab P. Diagnostic accuracy of screening tests for COPD: a systematic review and meta-analysis. BMJ open. 2015;5(10):e008133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sharma MM, Nanda PK. FEF200-1200, FEF25-75% and FEF75-85% in non-smokers of either sex and in male smokers residing at an altitude of 2150 M above MSL in Himachal Pradesh. Indian journal of physiology and pharmacology. 1986;30(4):329–333. [PubMed] [Google Scholar]
- 115.Ghosh S, Ohar JA, Drummond MB. Peak Inspiratory Flow Rate in Chronic Obstructive Pulmonary Disease: Implications for Dry Powder Inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30(6):381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Loh CH, Peters SP, Lovings TM, Ohar JA. Suboptimal Inspiratory Flow Rates Are Associated with Chronic Obstructive Pulmonary Disease and All-Cause Readmissions. Annals of the American Thoracic Society. 2017;14(8):1305–1311. [DOI] [PubMed] [Google Scholar]
- 117.Van de Moortele T, Goerke U, Wendt CH, Coletti F. Airway morphology and inspiratory flow features in the early stages of Chronic Obstructive Pulmonary Disease. Clin Biomech (Bristol, Avon). 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cerveri I, Dore R, Corsico A, et al. Assessment of Emphysema in COPD: A Functional and Radiologic Study. Chest. 2004;125(5):1714–1718. [DOI] [PubMed] [Google Scholar]
- 119.Miller RD, Hyatt RE. Evaluation of obstructing lesions of the trachea and larynx by flow-volume loops. Am Rev Respir Dis. 1973;108(3):475–481. [DOI] [PubMed] [Google Scholar]
- 120.Kapp MC, Schachter EN, Beck GJ, Maunder LR, Witek TJ Jr. The shape of the maximum expiratory flow volume curve. Chest. 1988;94(4):799–806. [DOI] [PubMed] [Google Scholar]
- 121.O’Donnell CR, Rose RM. The Flow-Ratio Index: An Approach for Measuring the Influence of Age and Cigarette Smoking on Maximum Expiratory Flow-Volume Curve Configuration. Chest. 1990;98(3):643–646. [DOI] [PubMed] [Google Scholar]
- 122.Johns DP, Walters JA, Walters EH. Diagnosis and early detection of COPD using spirometry. J Thorac Dis. 2014;6(11):1557–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dominelli PB, Foster GE, Guenette JA, et al. Quantifying the shape of the maximal expiratory flow-volume curve in mild COPD. Respir Physiol Neurobiol. 2015;219:30–35. [DOI] [PubMed] [Google Scholar]
- 124.Zheng CJ, Adams AB, McGrail MP, Marini JJ, Greaves IA. A proposed curvilinearity index for quantifying airflow obstruction. Respiratory care. 2006;51(1):40–45. [PubMed] [Google Scholar]
- 125.Oh A, Morris TA, Yoshii IT, Morris TA. Flow Decay: A Novel Spirometric Index to Quantify Dynamic Airway Resistance. Respiratory care. 2017;62(7):928–935. [DOI] [PubMed] [Google Scholar]
- 126.Weiner DJ, Forno E, Sullivan L, Weiner GA, Kurland G. Subjective and Objective Assessments of Flow-Volume Curve Configuration in Children and Young Adults. Annals of the American Thoracic Society. 2016;13(7):1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zapletal A, Hladikova M, Chalupova J, Svobodova T, Vavrova V. Area under the maximum expiratory flow-volume curve--a sensitive parameter in the evaluation of airway patency. Respiration. 2008;75(1):40–47. [DOI] [PubMed] [Google Scholar]
- 128.Stein D, Stein K, Ingrisch S. [Aex - the area under the expiratory flow-volume loop]. Pneumologie (Stuttgart, Germany). 2015;69(4):199–206. [DOI] [PubMed] [Google Scholar]
- 129.Lee J, Lee CT, Lee JH, et al. Graphic analysis of flow-volume curves: a pilot study. BMC Pulm Med. 2016;16:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Das N, Topalovic M, Janssens W. Artificial intelligence in diagnosis of obstructive lung disease: current status and future potential. Curr Opin Pulm Med. 2018;24(2):117–123. [DOI] [PubMed] [Google Scholar]
- 131.Li H, Liu C, Zhang Y, Xiao W. The Concave Shape of the Forced Expiratory Flow-Volume Curve in 3 Seconds Is a Practical Surrogate of FEV1/FVC for the Diagnosis of Airway Limitation in Inadequate Spirometry. Respiratory care. 2017;62(3):363–369. [DOI] [PubMed] [Google Scholar]
- 132.Saltzman HP, Ciulla EM, Kuperman AS. The spirographic “kink”. A sign of emphysema. Chest. 1976;69(1):51–55. [DOI] [PubMed] [Google Scholar]
- 133.Topalovic M, Exadaktylos V, Peeters A, et al. Computer quantification of airway collapse on forced expiration to predict the presence of emphysema. Respir Res. 2013;14:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang W, Xie M, Dou S, Cui L, Xiao W. Computer quantification of “angle of collapse” on maximum expiratory flow volume curve for diagnosing asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis. 2016;11:3015–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Webster PM, Zamel N, Bryan AC, Kruger K. Volume Dependence of Instantaneous Time Constants Derived from the Maximal Expiratory Flow-Volume Curve. American Review of Respiratory Disease. 1977;115(5):805–810. [DOI] [PubMed] [Google Scholar]
- 136.Mead J. Analysis of the configuration of maximum expiratory flow-volume curves. J Appl Physiol Respir Environ Exerc Physiol. 1978;44(2):156–165. [DOI] [PubMed] [Google Scholar]
- 137.Topalovic M, Exadaktylos V, Decramer M, Troosters T, Berckmans D, Janssens W. Modelling the dynamics of expiratory airflow to describe chronic obstructive pulmonary disease. Medical & biological engineering & computing. 2014;52(12):997–1006. [DOI] [PubMed] [Google Scholar]
- 138.Topalovic M, Exadaktylos V, Decramer M, Berckmans D, Troosters T, Janssens W. Using dynamics of forced expiration to identify COPD where conventional criteria for the FEV(1) /FVC ratio do not match. Respirology (Carlton, Vic). 2015;20(6):925–931. [DOI] [PubMed] [Google Scholar]
- 139.Bhatt SP, Bhakta NR, Wilson CG, et al. New Spirometry Indices for Detecting Mild Airflow Obstruction. Scientific reports. 2018;8(1):17484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Das N, Topalovic M, Janssens W. Artificial intelligence in diagnosis of obstructive lung disease: current status and future potential. Current opinion in pulmonary medicine. 2018;24(2):117–123. [DOI] [PubMed] [Google Scholar]
- 141.Williams EM, Powell T, Eriksen M, Neill P, Colasanti R. A pilot study quantifying the shape of tidal breathing waveforms using centroids in health and COPD. Journal of clinical monitoring and computing. 2014;28(1):67–74. [DOI] [PubMed] [Google Scholar]
- 142.Anogeianaki A, Negrev N, Ilonidis G. Contributions of signal analysis to the interpretation of spirometry. Hippokratia. 2007;11(4):187–195. [PMC free article] [PubMed] [Google Scholar]


