Abstract
Background:
Cigarette smoking is known to increase the risk of AF, and a recent cross-sectional analysis suggested that parental smoking may be an AF risk factor.
Objectives:
To assess if parental smoking predicts offspring atrial fibrillation (AF) in the Framingham Heart Study.
Methods:
We analyzed Framingham Offspring cohort participants with parents in the Original cohort with known smoking status during the Offspring’s childhood. Framingham participants were evaluated every 2-8 years and under routine surveillance for incident AF. We assessed AF incidence among Offspring participants exposed to parental smoking through age 18 years and performed a mediation analysis to determine the extent to which Offspring smoking might explain observed associations.
Results:
Of 2,816 Offspring cohort participants with at least one parent in the Original cohort, 82% were exposed to parental smoking. For every pack/day increase in parental smoking, there was an 18% increase in Offspring AF incidence (adjusted hazard ratio [HR], 1.18; 95%CI, 1.00-1.39; p = 0.04). Additionally, parental smoking was a risk factor for Offspring smoking (adjusted odds ratio [OR], 1.34; 95% CI, 1.17-1.54; p<0.001). Offspring smoking mediated 17% (95%CI 1.5-103.3%) of the relationship between parental smoking and Offspring AF.
Conclusions:
Childhood secondhand smoke exposure predicted increased risk for adulthood AF after adjustment for AF risk factors. Some of this relationship may be mediated by a greater propensity among Offspring of smoking parents to smoke themselves. These findings highlight potential new pathways for AF risk that begin during childhood, offering new evidence to motivate smoking avoidance and cessation.
Keywords: Atrial fibrillation, smoking, secondhand smoke
CONDENSED ABSTRACT:
Secondhand smoke exposure and atrial fibrillation (AF) risk remain poorly studied. Of 2,816 Framingham Heart Study Offspring cohort participants with at least one parent in the Original cohort, 82% were exposed to parental smoking during their early childhood and development. For every pack/day increase in parental smoking, there was an 18% increase in Offspring AF incidence (adjusted hazard ratio [HR], 1.18; 95% CI, 1.00-1.39; p=0.04). Childhood secondhand smoke exposure predicted increased risk for adulthood AF, highlighting a potential new mechanistic pathway for AF that begins during childhood.
Introduction
Atrial fibrillation (AF) is increasing in prevalence and expected to affect 16 million Americans by 2050 (1). Cigarette smoking remains one of the most important modifiable cardiovascular risk factors, with a 2017 adult smoking prevalence of 14% in the United States (2). Cigarette smoking has been associated with incident AF in several large epidemiologic studies, with estimates that up to 7% of all AF can be attributed to smoking (3–5).
It is well-established that smoking cessation is difficult, but some evidence suggests that recognizing harmful effects on others may help motivate smoking avoidance (6). Whereas little has been published on the effects of secondhand smoke exposure on AF, we previously showed that exposure to cigarette smoke while in utero or as a child may increase AF risk later in life (7). If true, these findings could incentivize smokers to quit and potential smokers to avoid smoking. The findings might also point to new mechanistic pathways related to AF pathogenesis. However, that study relied on self-reported cigarette smoke exposure, and recall bias may have played a role. We therefore sought to leverage the multi-generational Framingham Heart Study to test the hypothesis that parental smoking predicts offspring AF.
Methods
Patient Population
We analyzed all Framingham Offspring cohort participants with at least one parent in the Original cohort with a known smoking status at any point until their Offspring reached the age of 18 years. The Framingham Heart and Offspring Studies, as well as their design and methods, have been described in prior publications (8,9). In brief, ambulatory men and women participants were recruited into the Original cohort in 1948 and followed at specific intervals for the development of cardiovascular disease. The Framingham Heart Study was then expanded to include the Offspring cohort in 1971 for the children of those in the Original cohort and their spouses, with continued longitudinal follow-up for incident cardiovascular disease. Each cohort was evaluated by a Framingham Heart Study physician approximately every 2 to 4 (Original) and 4 to 8 (Offspring) years, and were under routine surveillance during inter-exam periods for cardiovascular outcomes through review of outside medical records and physician visits adjudicated by Framingham investigators. The most recent examination period for both the Original and Offspring cohorts concluded in 2014. At each visit, a physician obtained a medical history and administered a physical exam. All participants underwent serial standardized questionnaires regarding cigarette-smoking status. To examine a relationship between secondhand smoke exposure in early development and adulthood AF risk, we centered on smoking ascertainment during study examinations for the Original cohort up until the time their respective Offspring cohort participants reached 18 years of age.
Cardiovascular Disease and Risk Factor Definitions
The following cardiovascular risk factors at Exam 1 in the Offspring cohort were utilized as baseline covariates in the study analysis: hypertension, diabetes mellitus, body mass index, coronary heart disease, congestive heart failure, alcohol consumption, and family history of AF. Framingham Heart Study participants prescribed antihypertensive therapy as well as those with either a systolic blood pressure ≥ 140 mmHg or a diastolic blood pressure ≥ 90 mmHg were considered to have hypertension. Body mass index was calculated as weight in kilograms divided by the square of the participant’s height in meters at baseline examination. Either a fasting plasma glucose ≥ 126 mg/dL or treatment with diabetes mellitus medications were used for diabetes mellitus status. The criteria for coronary heart disease included the presence of 2 of the 3 of the following findings: symptoms indicative of ischemia, changes in biomarkers of myocardial necrosis, and serial changes in ECGs suggestive of infarction. An old myocardial infarction was considered to be present when the ECG showed a pathologic Q wave or loss of precordial R waves. Finally, myocardial infarction could be diagnosed based on autopsy. Congestive heart failure was diagnosed based on a minimum of 2 major heart failure or 1 major and 2 minor heart failure criteria (10). Alcohol consumption was defined as any baseline consumption of at least one weekly alcoholic beverage. All Framingham Heart Study participants provided written informed consent, and the Framingham Heart Study protocols were approved by the Boston University Medical Campus Institutional Review Board. This current study utilizing the Framingham Heart Study datasets was approved by the University of California, San Francisco Institutional Review Board.
Smoking Definitions
Smoking was defined in both the Original and Offspring cohorts as participants reporting smoking ≥ 1 cigarette daily during the year prior to their study examination. For those participants who reported smoking, the number of mean packs of cigarettes smoked per day was calculated based on daily number of cigarettes (1 pack representing 20 cigarettes, ½ pack representing 10 cigarettes, and ¼ pack representing 5 cigarettes). Parental smoke exposure for the Offspring cohort was assessed and defined as the presence of parental smoking (either parent) of > 0 mean packs per day at any point in an examination period when his or her Offspring participant was between 0 and 18 years of age. To account for a possible dose-response smoking relationship from variable exposure between individual parents, parental smoke exposure was also defined as the summation of number of cigarettes smoked daily by both the mother and father. Baseline Offspring smoking status was defined as smoking of > 0 mean packs per day at Offspring Exam 1.
Atrial Fibrillation Definitions
Participants in the Original and Offspring cohorts were considered to have AF if an ECG at a Framingham Heart Study examination ever demonstrated AF or atrial flutter. Additionally, Framingham Heart Study investigators routinely reviewed participants’ outside medical records, interim hospitalizations, outside ECGs, and Holter monitor results for a diagnosis of AF. Family history of AF was defined by the diagnosis of AF in either parent at any point during a Framingham follow up examination.
Statistical Analysis
Continuous and ordinal variables are expressed as means and standard deviations or median and interquartile ranges (IQR), respectively. Bivariate comparisons were conducted using logistic models with robust standard errors in order to account for family clustering, and Fisher’s exact test was utilized when such a comparison included at least one cell with no observations. Normally distributed continuous variables were compared using the Student’s t-tests, and variables with either a skewed distribution or that were ordinal were compared using the Wilcoxon rank sum test. The χ2 test was used to compare categorical variables and Fisher’s exact test for categories with < 5 observations. Cox proportional hazard models were used to assess the association of parental smoking from the Original cohort with incident AF among Offspring cohort participants. Given the natural clustering of AF outcomes within families, the models used robust standard errors to account for intrafamily AF clustering. The models were adjusted for established AF risk factors including age, sex, body mass index, coronary heart disease, hypertension, diabetes mellitus, and a family history of AF. Baseline congestive heart failure was not included as a covariate as it was only present in 1 participant in the Offspring cohort.
A priori, it was hypothesized that Offspring cigarette smoking may mediate some of the risk for incident AF as Offspring of smokers may be more likely to smoke themselves. Unadjusted and multivariable adjusted logistic regression models were first used to measure associations between parental smoking as a predictor of Offspring smoking. Cox proportional hazards models were then used to examine the relationship between Offspring smoking as a predictor of Offspring AF incidence. The proportion of AF risk explained by parental smoking that was mediated by Offspring smoking was calculated based on the percent difference in multivariable adjusted beta coefficients for parental smoking with and without Offspring smoking included in the model. A 95% CI was then estimated using bootstrap resampling with 500 replications with robust standard errors accounting for intrafamily AF clustering.
All analyses were performed using STATA version 15 (STATA Corp., College Station, TX) and a two-tailed p-value of <0.05 was considered statistically significant.
Results
Out of 5,124 Offspring cohort participants, 2,816 (55%) had available parental smoking ascertainment during the respective Offspring childhood period up until age 18 years. Secondhand smoke exposure at some point during childhood was experienced by 82% of these Offspring participants. Baseline characteristics of the studied population are listed in Table 1. Those exposed to smoking tended to be older, have hypertension, were more likely to smoke themselves, and more often had a family history of AF. The median number of parental cigarettes smoked daily in the exposed group was 0.5 packs, or 10 cigarettes, per day (interquartile range 0.07 – 1 pack per day).
Table 1.
Baseline Offspring Characteristics, by the Presence or Absence of Parental Smoking (up until age 18)
| Characteristic | Parental Smoking (n = 2316) | No Parental Smoking (n = 500) | p-value |
|---|---|---|---|
| Age, mean, years | 32.9 ± 10.0 | 29.5 ± 7.1 | <0.001 |
| Male Sex | 1100 (48%) | 263 (53%) | 0.03 |
| Body Mass Index, mean, kg/m2 | 24.7 ± 4.2 | 24.6 ± 4.4 | 0.58 |
| Smoking History | 1100 (48%) | 185 (37%) | <0.001 |
| Coronary Heart Disease | 10 (<1%) | 0 (0%) | 0.23 |
| Congestive Heart Failure | 1 (<1%) | 0 (0%) | 1.00 |
| Hypertension | 394 (17%) | 54 (11%) | 0.001 |
| Diabetes Mellitus | 26 (1%) | 1 (<1%) | 0.09 |
| Alcohol Consumption | 1973 (85%) | 397 (79%) | 0.004 |
| Parental AF | 940 (41%) | 176 (35%) | 0.15 |
| Maternal & Paternal Smoking | 797 (34%) | 0 (0%) | |
| Maternal Smoking | 1256 (54%) | 0 (0%) | |
| Paternal Smoking | 1857 (80%) | 0 (0%) |
Included participants are those with parental smoking ascertainment between ages 0 and 18.
AF, atrial fibrillation
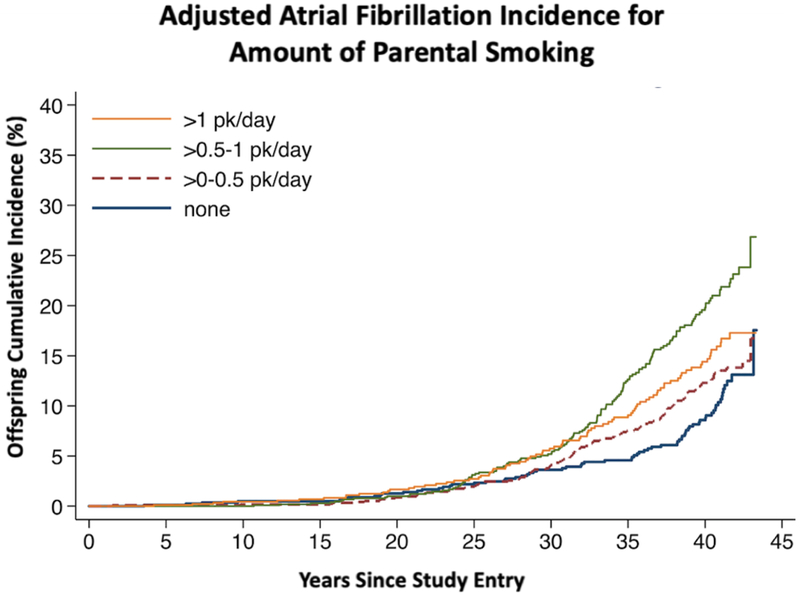
Among the Offspring cohort with available smoking ascertainment, 404 (14.3%) developed AF over a median follow-up of 40.5 years (interquartile range 33.3 – 41.9 years), with an overall incidence rate of 4.02 per 1000 person-years. Those exposed to more parental smoking demonstrated a higher adjusted incidence of AF (Central Illustration). After multivariable adjustment, Offspring participants experienced an 18% increase in AF incidence for every pack per day increase in parental smoking (Table 2).
Central Illustration. Adjusted Atrial Fibrillation Incidence for Amount of Parental Smoking.
Cumulative incidence of Offspring atrial fibrillation by number of cigarettes smoked per day by their parents. Adjusted for Offspring age, sex, race, body mass index, coronary heart disease, diabetes, hypertension, alcohol consumption, and accounting for family clustering of atrial fibrillation.
Table 2.
Parental Smoking (mean packs/day up until Offspring age 18) as a Predictor of Incident Atrial Fibrillation
| Covariate | Unadjusted Models | Multivariable Adjusted Models | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| No Parental Smoking | 1.00 | (Referent) | 1.00 | (Referent) | ||
| Parental Smoking | 1.35 | 1.16-1.58 | <0.001 | 1.18 | 1.00-1.39 | 0.04 |
| No Paternal Smoking | 1.00 | (Referent) | 1.00 | (Referent) | ||
| Paternal Smoking | 1.67 | 1.38-2.02 | <0.001 | 1.24 | 1.01-1.51 | 0.04 |
| No Maternal Smoking | 1.00 | (Referent) | 1.00 | (Referent) | ||
| Maternal Smoking | 1.49 | 1.07-2.07 | 0.02 | 1.16 | 0.83-1.61 | 0.38 |
Models were adjusted for Offspring age, sex, body mass index, coronary heart disease, diabetes, hypertension, alcohol consumption, and accounted for family clustering of atrial fibrillation.
After adjusting for Offspring age, sex, body mass index, coronary heart disease, diabetes, hypertension, alcohol consumption, and family history of AF, parental smoking was associated with a significantly increased odds of Offspring smoking (adjusted odds ratio 1.34, 95% CI 1.17-1.54, p<0.001). Also after adjusting for the same covariates, Offspring smoking was associated with a 32% increase in AF incidence (adjusted hazard ratio 1.32, 95% CI 1.11-1.56, p=0.002). In assessing the proportion of AF risk explained by the indirect pathway of Offspring of smoking parents smoking themselves, adjusting for Offspring smoking in the multivariable model attenuated the relationship between parental smoking and Offspring AF, statistically explaining, or mediating, 17% (95% CI 1.5 to 103.3%) of the relationship between parental smoking and Offspring AF (Table 3.).
Table 3.
Mediation of the Relationship between Parental Smoking (mean packs/day up until Offspring age 18) and Offspring Incident Atrial Fibrillation by Offspring Smoking
| Covariate | Multivariable Adjusted Models Adjusted for Offspring Smoking | Proportion of Associated AF Risk Explained by Offspring Smoking | |||
|---|---|---|---|---|---|
| HR | 95% CI | p-value | % | 95% CI (bias corrected) | |
| Parental Smoking | 1.15 | 0.98-1.35 | 0.10 | 16.57 | 1.49 – 103.26 |
Models were adjusted for Offspring age, sex, body mass index, coronary heart disease, diabetes, hypertension, alcohol consumption, and accounted for family clustering of atrial fibrillation.
Discussion
Primary Findings
Parental smoking was associated with a higher risk of Offspring AF after adjustment for conventional AF risk factors and accounting for AF clustering within families. At least some of that relationship may be mediated by a greater propensity among Offspring of smoking parents to smoke themselves. These findings highlight potential new mechanistic pathways for AF risk that begin during childhood and an association between secondhand smoke exposure and AF risk. These observations may also provide new information pertinent to smoking cessation and avoidance, highlighting the harms that may be associated not only “to others” but to close and the most vulnerable members of the family.
Despite the published health hazards of smoking and public awareness campaigns to reduce smoking, 14% of adult Americans continue to smoke, and each day, 2000 people under the age of 18 smoke their first cigarette. Therefore, smoking remains the primary cause of preventable death, with the number of attributable annual deaths expected to increase from 6 million to 8 million by 2030 (11). In the United States, secondhand smoke exposure is responsible for at least 41,000 deaths annually (11). While there have been numerous published reports of the deleterious effects of smoking on other cardiovascular conditions such as coronary heart disease, the risk of AF secondary to cigarette smoking is less well-defined (12). This is especially pertinent given that recent successes in smoking cessation programs have demonstrated a decreased incidence of myocardial infarction and a reduction of all-cause mortality in those with established coronary heart disease (13,14). With the rising prevalence of AF, even after accounting for the aging population, it is imperative to address modifiable risk factors such as cigarette smoking to reduce the global burden of AF (15). However, most studies to date have focused on primary smoke exposure and not secondhand smoke exposure and AF risk, with a recent meta-analysis demonstrating that active smoking was associated with a 23% higher AF risk across multiple longitudinal studies (5). It is hypothesized that smoking alters plasma catecholamine concentrations and ion channel conduction, increases oxidative stress, and triggers atrial remodeling by disrupting fibroblasts, potentially all contributing to increased AF risk (16–18). We hypothesize that these mechanisms should be no less relevant during passive smoke inhalation, and that this may be even more critical during early human growth and development in the childhood and adolescent years. In addition to these direct mechanisms for AF risk, secondhand smoke is a previously established risk factor for coronary heart disease and diabetes mellitus, each themselves important risk factors for AF development and thus, may indirectly affect AF risk (19–21).
Our study highlights the relationship between smoking and AF risk, with a magnitude very similar to previous studies (3,4). Given this, we recognize that the relationship between parental smoking on Offspring AF may be related to direct (rather than secondhand) smoking itself. Indeed, we found that Offspring of smoking parents were themselves substantially more likely to smoke. As specified a priori, adjustment for Offspring smoking demonstrated that at least some of the observed relationships were mediated by Offspring smoking, suggesting that this learned behavior (smoking parents begetting smoking Offspring) may also explain how parental smoking might predispose to Offspring AF. While the point estimate suggested that only 17% of these relationships were explained by Offspring smoking, the wide 95% confidence intervals make it difficult to determine how much of the enhanced Offspring AF risk may be due to detrimental secondhand smoke effects during development versus the direct smoking that is more likely to occur if one’s parents smoke.
Despite public health efforts in reducing primary smoking and secondhand smoke exposure, 8.4% of women report smoking at some point during pregnancy, and the exposure to secondhand smoke during pregnancy is likely even higher (22). Within a family, smoking status is linked among individual members. Consistent with our findings in the current study, previous investigations have shown that a smoking parent increases the likelihood of his or her partner smoking and a child’s chance of smoking later in life (23,24). Given these relationships, smoking cessation by one parent may lead to cessation by the other and a decreased smoking incidence for their children, thus impacting the overall cardiovascular health and AF burden within the family.
Study Strengths and Limitations
Our study has several strengths including a moderately large sample size, a unique longitudinal, community-based cohort spanning across generations, and long-term and robust follow-up for our primary outcome of interest. In addition to AF, the Framingham Heart Study is renowned for its rigorous ascertainment of relevant covariates that were used in multivariable adjustment. We recognize several limitations to our analysis. Parental smoking status was not available in nearly 45% of potentially exposed Offspring participants. This is attributed to incomplete smoking ascertainment in the early Framingham Heart Study examinations (Exams 1 and 2) due to lack of awareness of the harmful effects of smoking in the 1950’s. Additionally, there may be variations in parental smoke exposure among children of separated, divorced, single parents or other smoking family members that may also live in the household. To minimize this variability, we utilized the total sum of parental smoke exposure between the mother and father. While we cannot exclude the possibility that these limitations introduced some bias, we suspect this primarily decreases our power to detect significant associations and would not meaningfully influence our measures of association in either direction—specifically, our analysis demonstrated a graded response regarding amount of parental smoking and Offspring AF, which at least should maintain internal validity even in a selected sample. We also recognize that AF may be clinically silent and not routinely detected on follow-up examinations. However, as mentioned, the Framingham Heart Study is well-established in its thorough assessment of cardiovascular outcomes, and AF events were ascertained from outside records in addition to routine examinations; again this limitation would be expected to reduce power rather than result in spurious false positive results. Importantly, the demographic makeup of the Framingham Heart Study is a predominantly White cohort in one geographic area, which may limit the generalizability to the remainder of the population. Finally, as this was an observational study, we cannot prove causal effects and recognize that both unmeasured and residual confounding may yet be important factors in explaining our observations.
Conclusions
As the world experiences an increasing burden of AF, it is imperative to identify AF risk factors beyond traditional cardiovascular comorbidities and ideally to highlight lifestyle factors that can be readily modified (25). Smoking remains the most important modifiable cause of death and morbidity in the world, and new information regarding the harms of secondhand smoke may prove useful to motivate smoking cessation and avoidance (11). These data show that parental smoking influences the risk of AF among Offspring, demonstrating chronic deleterious effects that are relevant decades after the initial exposure. These observations reveal an association between an early life exposure and AF which may suggest new mechanistic pathways for AF risk deserving of further exploration. Whether related to acute smoke inhalation during development and/or learned behaviors that influence risk later in life, these observations may provide new evidence to motivate current smokers to quit and potential smokers to avoid smoking altogether.
Clinical Perspectives.
Competency in Medical Knowledge:
Smoking is a risk factor for atrial fibrillation, and secondhand exposure during childhood is associated with a higher risk of developing AF later in life. This may be because the offspring of smoking parents are more likely to smoke themselves, but exposure to smoke during childhood may also have a long-term detrimental effect.
Translational Outlook:
Future studies should investigate the biological mechanisms responsible for atrial substrate modification resulting from smoke exposure in utero and during childhood development.
Acknowledgments
Financial Support: This research was supported by funds from the Tobacco Related-Disease Research Program Office of the University of California (Award #27IR-0027 to GMM), the American Heart Association Clinical Scientist Training Program (Award #17CPOST33660315 to CAG, GMM sponsor), the NIH National Research Service Award Postdoctoral Fellowship (Award #1F32HL140809-01 to CAG, GMM sponsor), and contract #HHSN268201500001I from the NHLBI to the Framingham Heart Study. Dr. Benjamin is supported by R01HL128914; 2R01 HL092577; 2U54HL120163, American Heart Association, 18SFRN34110082.
Relationship with Industry: Dr. Gregory Marcus has received research funding from Jawbone Health, Eight, and Medtronic, and is a consultant for and holds equity in InCarda. The remaining authors have no relationships with industry to report.
ABBREVIATIONS:
- AF
atrial fibrillation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation. 2019;139:e56–e66. [DOI] [PubMed] [Google Scholar]
- 2.Wang TW, Asman K, Gentzke AS, et al. Tobacco Product Use Among Adults—United States, 2017. MMWR Morb Mortal Wkly Rep. 2018;67(44):1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heeringa J, Kors JA, Hofman A, van Rooij FJA, Witteman JCM. Cigarette smoking and risk of Atrial Fibrillation: The Rotterdam Study. Am Heart J. 2008;156(6):1163–1169. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain AM, Agarwal SK, Folsom AR, et al. Smoking and Incidence of Atrial Fibrillation: Results from the Atherosclerosis Risk in Communities (ARIC) Study. Hear Rhythm. 2011;8(8):1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu W, Yuan P, Shen Y, Wan R, Hong K. Association of smoking with the risk of incident atrial fibrillation: A meta-analysis of prospective studies. Int J Cardiol. 2016;218:259–266. [DOI] [PubMed] [Google Scholar]
- 6.Pizacani BA, Martin DP, Stark MJ, Koepsell TD, Thompson B, Diehr P. Household Smoking Bans: Which Households Have Them and Do They Work? Prev Med. 2003;36(1):99–107. [DOI] [PubMed] [Google Scholar]
- 7.Dixit S, Pletcher MJ, Vittinghoff E, et al. Secondhand smoke and atrial fibrillation: Data from the Health eHeart Study. Hear Rhythm. 2016;13(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–290. [DOI] [PubMed] [Google Scholar]
- 9.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent Risk Factors for Atrial Fibrillation in a Population-Based Cohort. JAMA. 1994;271(11):840–844. [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. US Dep Heal Hum Serv Centers Dis Control Prev Natl Cent Chronic Dis Prev Heal Promot Off Smok Heal. 2014. [Google Scholar]
- 12.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J Am Coll Cardiol. 2004;43(10):1731–7. [DOI] [PubMed] [Google Scholar]
- 13.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165. [DOI] [PubMed] [Google Scholar]
- 14.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290(1):86–97. [DOI] [PubMed] [Google Scholar]
- 15.Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D’Agostino RB. Secular trends in the prevalence of atrial fibrillation: The Framingham Study. Am Heart J. 1996;131(4):790–795. [DOI] [PubMed] [Google Scholar]
- 16.Haass M, Kübler W. Nicotine and sympathetic neurotransmission. Cardiovasc Drugs Ther. 1997;10(6):657–665. [DOI] [PubMed] [Google Scholar]
- 17.D’Alessandro A, Boeckelmann I, Hammwhoner M, Goette A. Nicotine, cigarette smoking and cardiac arrhythmia: an overview. Eur J Prev Cardiol. 2012;19(3):297–305. [DOI] [PubMed] [Google Scholar]
- 18.Goette A, Lendeckel U, Kuchenbecker A, et al. Cigarette smoking induces atrial fibrosis in humans via nicotine. Heart. 2007;93(9):1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steenland K, Thun M, Lally C, Heath C. Environmental tobacco smoke and coronary heart disease in the American Cancer Society CPS-II cohort. Circulation. 1996;94(4):622–628. [DOI] [PubMed] [Google Scholar]
- 20.He J, Vupputuri S, Allen K, Prerost MR, Hughes J, Whelton PK. Passive smoking and the risk of coronary heart disease--a meta-analysis of epidemiologic studies. N Engl J Med. 1999;340(12):920–926. [DOI] [PubMed] [Google Scholar]
- 21.Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(12):958–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtin SC, Matthews TJ. Smoking Prevalence and Cessation Before and During Pregnancy: Data From the Birth Certificate, 2014. Natl Vital Stat Rep. 2016;65(1):1–14. [PubMed] [Google Scholar]
- 23.Rüge J, Ulbricht S, Schumann A, Rumpf HJ, John U, Meyer C. Intention to quit smoking: is the partner’s smoking status associated with the smoker’s intention to quit? Int J Behav Med. 2008;15(4):328–335. [DOI] [PubMed] [Google Scholar]
- 24.Leonardi-Bee J, Jere ML, Britton J. Exposure to parental and sibling smoking and the risk of smoking uptake in childhood and adolescence: a systematic review and meta-analysis. Thorax. 2011;66(10):847–855. [DOI] [PubMed] [Google Scholar]
- 25.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]