Abstract
Background:
Prior studies with single photon emission computed tomography myocardial perfusion imaging (SPECT) MPI have shown a survival benefit with early revascularization in patients with >10-12.5% ischemic myocardium. The relationship between positron emission tomography (PET) -derived degree of ischemia, early revascularization, and survival is unknown.
Objectives:
To evaluate the association between percent ischemia on PET MPI, revascularization, and survival.
Methods:
We followed 16,029 unique consecutive patients undergoing Rubidium-82 rest-stress PET MPI from 2010-2016. Patients with known cardiomyopathy and non-diagnostic perfusion results were excluded. Percent ischemic myocardium was estimated from a 17-segment model. Propensity scoring was used to account for non-randomized referral to early revascularization (90 days of PET). A Cox model was developed, adjusting for propensity scores for early revascularization and %ischemia, and an interaction between ischemia and early revascularization was tested.
Results:
Median follow-up was 3.7 years. Overall, 1277 (8%) patients underwent early revascularization and 2,493 (15.6%) died (738 cardiac). Nearly 37% of patients (n = 5,902) had ischemia, with 13.5% (n = 2,160) having ≥ 10%. In propensity-adjusted analyses, there was a significant interaction between ischemia and early revascularization (p <0.001 for all cause and cardiac death), such that patients with greater ischemia had improved survival with early revascularization, with a potential ischemia threshold at 5% (upper limit 95% confidence interval at 10%). There was no differential association between ischemia and early revascularization on death based on history of known CAD (interaction p=0.72).
Conclusion:
In a contemporary cohort of patients undergoing PET MPI, patients with greater ischemia had a survival benefit from early revascularization. On exploratory analyses, this threshold was lower than that previously been reported for SPECT. These findings require future validation in prospective cohorts or trials.
Keywords: Ischemia, survival, myocardial perfusion imaging, positron emission tomography, coronary revascularization
CONDENSED ABSTRACT:
Among 16,029 consecutive patients undergoing PET MPI followed for a median of 3.7 years, 1277 (8%) of whom underwent early revascularization (within 90 days of MPI), proportion of ischemic myocardium on PET MPI may identify patients who have a long term survival benefit with early revascularization. The ischemia threshold above which survival benefit was observed with revascularization was much lower (>5%) than that previously reported for SPECT MPI. These findings support an ischemia-based revascularization strategy.
Introduction
The role of coronary revascularization in the management of patients with stable coronary artery disease (CAD) has been a major area of debate. Two large randomized clinical trials (1,2) have shown that revascularization, compared with aggressive medical therapy, does not reduce major adverse cardiac events or improve survival in patients with stable CAD. While significant CAD in these trials (1, 2) was mainly defined based upon angiographic disease severity without physiologic data, other trials(3,4) have suggested that an invasive physiologic assessment to detect flow-limited coronary disease might be better suited to identify candidates who could benefit from revascularization (5).
Stress myocardial perfusion imaging (MPI) has a well-established role in the diagnosis of physiologically important CAD.(6,7) Perfusion abnormalities on stress MPI are prognostic of worse long-term cardiovascular outcomes and death.(8,9) As such, the magnitude of ischemia has been proposed as a gatekeeper to identify ideal revascularization candidates who have improved long-term cardiovascular and survival outcomes.(10) While a meta-analysis of 5 trials failed to show a benefit of revascularization over medical therapy in a sub-group of patients with myocardial ischemia at baseline,(11) observational studies have identified a threshold of >12.5% ischemic myocardium by SPECT MPI above which revascularization confers a survival benefit.(8,12) These studies,(8,11) included patients tested over 2 decades ago with older imaging technologies and revascularization techniques. Imaging technologies, revascularization practices, and medical therapies are continually evolving; and whether similar benefit exists in a more contemporary cohort studied with contemporary MPI technologies is not known.
Positron Emission Tomography (PET) is increasingly being used for stress MPI, as it has routine attenuation correction reducing the inclusion of artifact masquerading as ischemia, superior image resolution, lower radiation exposure, greater diagnostic accuracy, shorter acquisition times and improved prognostic utility. (13,14) While ischemia on PET MPI has been shown to help in prognostication of high-risk patients,(9,15) whether it could also help guide treatment by identifying patients who receive a therapeutic benefit with revascularization over medical management is not known. In the current study, we evaluated whether PET MPI testing identifies patients who may have a survival benefit from revascularization compared to medical management in a contemporary cohort of patients. Additionally, we explored whether there is a percent ischemia threshold above which patients may derive a survival benefit with early coronary revascularization.
Methods
Study population
We included a total of 19,221 unique consecutive patients who underwent rest/stress rubidium-82 (Rb82) PET MPI within the Saint Luke’s Health System between January 1, 2010 and December 31, 2016. The Saint Luke’s Health System has 4 cardiac-dedicated PET and PET-CT systems at 4 major metropolitan hospitals in Kansas City with fully functioning nuclear cardiology laboratories. Patients with known cardiomyopathy (EF <40%) (n=2846) and non-diagnostic scintigraphic results (n = 346) were excluded. Only the index test was included for patients with multiple MPI tests during the study period. The study protocol was approved by the Institutional Review Board of Saint Luke’s Hospital.
Study variables
Trained laboratory personnel collected information on patient demographics, risk factors, medical history, symptoms, and medications at the time of the MPI test from patient interviews and medical chart review. Patients with a history of myocardial infarction (MI) or prior revascularization with PCI or CABG were defined as having known CAD at the time of testing.
Rb82 PET and PET/CT MPI Imaging Protocols
All patients were studied using a dedicated PET (Siemens ECAT ACCEL) or PET/CT scanner (Siemens Biograph 16 or 64, Nashville, TN) after fasting for at least 6 hours. Patients were asked to withhold caffeine containing beverages for 24 hours prior to the test and beta-blockers, calcium channel blockers, and nitrates on the morning of the test. Line source attenuation was used with the dedicated PET camera and a low dose transmission CT scan for attenuation correction was acquired prior to rest image acquisition with the PET/CT cameras. Initially, after intravenous administration of Rb82 (740-2220 mBq), rest emission images were acquired in list-mode for 5.5 minutes. All patients then underwent pharmacological stress testing using standard doses of regadenoson (n=10,609), dipyridamole (n=5359), adenosine (n=14) or dobutamine (n=47). At peak stress, stress images were acquired in a similar manner after a repeat dose of intravenous Rb82 (740-2220 mBq). Both rest and stress images were ECG-gated. Patient heart rate, blood pressure, and 12-lead ECG were acquired at rest, during, and after pharmacological stress. After acquisition of images, all studies were electronically transmitted to the central nuclear cardiology laboratory where trained nuclear technologists processed and reconstructed the images for interpretation. Commercial software (Imagen Pro, Kansas City, MO) was used for reconstruction and perfusion images were reconstructed from the list mode acquisitions starting 90-120 seconds after the beginning of Rb82 infusions at rest and peak stress.
Analysis of perfusion and gated images
Perfusion images and left ventricular ejection fraction (LVEF) were quantified using commercial software (Cedars Sinai Cardiac Suite/QPET). All images were processed and interpreted at the central nuclear laboratory by experienced physicians semi-quantitatively using a 17-segment model and standard 5-point scoring system (16), and recorded in the database. Global Summed Rest Score (SRS), Summed Stress Score (SSS), and Summed Difference Score (SDS) were calculated from the perfusion images. The percentage of infarcted and ischemic myocardium were calculated from SRS and SDS respectively by dividing by the maximum score of 68 [% ischemic myocardium = (SDS/68)*100; % infarcted myocardium= (SRS/68)*100]. Rest and stress LVEF were calculated from gated myocardial perfusion images acquired with 8-frame gating using the Cedars Sinai QGS software.
Study outcomes
The date of last follow-up was December 31, 2017. Follow-up was censored at date of last office visit if alive or date of death determined from medical chart review and query of the National Death Index (NDI). The primary study endpoint was all-cause mortality, and the secondary study endpoint was cardiac mortality ascertained using cause of death data from NDI (17), which has previously been shown to provide comparable information to independent expert review (18,19).
Early revascularization was defined as revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass surgery (CABG) within 90 days of the MPI test. This information was collected by trained personnel from the cardiac catheterization database and medical chart review during the study period. This was later cross-validated at the end of the study period. Patients not undergoing early revascularization were included in the medical therapy (MT) group.
Statistical analysis
Baseline demographic, clinical risk factors, symptoms and test results were compared between patients who underwent early revascularization and medical management using student t-test or Mann-Whitney U test for normally and non-normally distributed continuous variables respectively and chi-square test for categorical variables.
First, a propensity score was developed to account for selection bias and non-randomized treatment allocation of early revascularization. We created a non-parsimonious logistic regression model to estimate a patient’s likelihood of receiving early revascularization. A total of 27 covariates which could potentially be associated with the decision to revascularize were entered in the propensity score model, including patient demographics (age, gender, body mass index), clinical risk factors (known CAD, hypertension, diabetes mellitus, hyperlipidemia, current smoker, family history of atherosclerotic cardiovascular disease, peripheral vascular disease, cerebrovascular accident, atrial fibrillation, inpatient vs outpatient status at the time of the test, abnormal ECG at baseline), prospectively elicited symptoms (chest pain, dyspnea, and syncope), medications (aspirin, beta-blockers, calcium channel blockers, and statins), stress data (resting rate-pressure product, peak SBP, peak HR, ECG response), gated data (rest LVEF, stress LVEF), perfusion data (percent infarcted myocardium). Missing values of the covariates were imputed (multiple imputation, IVEWare) to ensure that propensity scores could be calculated for all patients. The distribution of propensity scores derived from this model was compared between patients who underwent early revascularization vs. those who did not (medical therapy) visually using density plots (Figure 1) and using propensity-adjusted standardized differences (Online Table 1). Because this analysis sought to explicitly examine the association of percent ischemia (% ischemia), and its interaction with early revascularization, it was not included in the propensity score. Rather, it was added as a separate covariate so that its association with mortality could be directly assessed and interpreted using a Cox proportional hazards model, after adjusting for other potential confounders using the propensity score. Because the propensity score suggested residual imbalance in 5 covariates (Standardized Differences >10%), we also added these characteristics, (sex, diabetes, known CAD, rest LVEF, %infarcted myocardium, as additional covariates to the Cox model for all-cause mortality that included the propensity score). In the second step, Cox proportional hazards regression model was developed to assess the association of percent ischemic myocardium on PET MPI with all-cause mortality, this was adjusted for propensity score, percent ischemic myocardium, early revascularization and additional 5 variables which were imbalanced between the 2 groups (standardized differences >10%) post propensity score generation (sex, diabetes, known CAD, rest LVEF, %infarcted myocardium. A two-way interaction of percent ischemic myocardium (as a continuous and categorical variable with 0-<5%, 5-10%, >10% categories) and early revascularization (as a time-varying covariate) was tested in the final adjusted Cox model using the likelihood ratio test. Cubic splines were specified for all continuous variables (including percent ischemia) to address non-linear associations between these continuous variables and all-cause mortality. Similar analyses were conducted for cardiac cause of mortality as a secondary endpoint using competing risks regression analysis (frailty model) accounting for competing risk of other causes of death. All model assumptions were examined including linearity, collinearity, additivity, and proportional hazards. Additional sensitivity analyses were conducted with adjustment of all 28 factors potentially affecting decision to revascularize as covariates in the Cox model instead of incorporation in a propensity score (Online Table 2) with similar results.
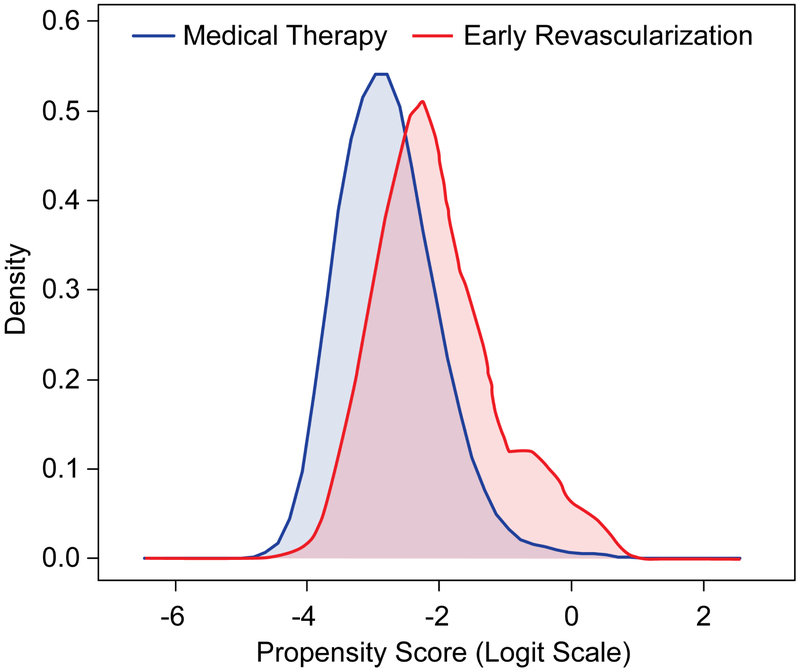
Figure 1: Comparison of propensity scores between the early revascularization and medical therapy groups.
The figure shows large overlap of propensity scores between the 2 groups suggesting a good balance of covariates.
Based on prior data showing differential effect of ischemic myocardium and revascularization in patients with and without known CAD, a three-way interaction term for history of known CAD * percent ischemic myocardium * early revascularization was also tested in the final adjusted Cox model. In case of a significant interaction between percent ischemic myocardium and early revascularization, exploratory analyses plotting spline curves of hazards for death with early revascularization vs. medical therapy across levels of ischemia will be plotted to identify a potential ischemic threshold of survival benefit with early revascularization.
Rest LVEF was missing in 11% of patients; all other covariates were missing in <5% of the study cohort. Missing data was imputed using sequential regression models using the IVEWare software (Ann Arbor, Michigan). Two-sided p-values <0.05 were considered significant. All analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina).
Results
Our study cohort included a total of 16,029 patients, who were followed for a mean of 3.7 ± 2.3 years. There were 2493 (15.6%) deaths. The mean age of the cohort was 68.6 ± 11.9 years, body mass index was 29.6 ± 6.3 kg/m2, 50% were women, and 32.6% had known CAD at baseline (2258 prior MI, 3926 prior PCI, 1933 prior CABG). Hypertension was present in 79.8%, diabetes in 29.3%, and prior stroke/transient ischemic attack in 9.8%. Chest pain was the predominant presenting symptom in about 60% of all patients and half had dyspnea (Table 1).
Table 1:
Baseline characteristics of the study cohort based upon presence of early revascularization (PCI or CABG within 90 days of test)
| Total | 90-day revascularization | P-Value | ||
|---|---|---|---|---|
| n =16029 | Yes n = 1277 |
No n = 14752 |
||
| Demographics and clinical risk factors | ||||
| Age, years | 68.6 ± 11.9 | 69.9 ± 10.8 | 68.5 ± 12.0 | < 0.001 |
| Male Sex | 8008 (50.0%) | 830 (65.0%) | 7178 (48.7%) | < 0.001 |
| Body mass index, kg/m2 | 29.59 ± 6.30 | 29.82 ± 5.77 | 29.57 ± 6.34 | 0.16 |
| Known CAD | 5225 (32.6%) | 647 (50.7%) | 4578 (31.0%) | < 0.001 |
| Prior MI | 2258 (14.1%) | 292 (22.9%) | 1966 (13.3%) | < 0.001 |
| Prior PCI | 3926 (24.5%) | 547 (42.8%) | 3379 (22.9%) | < 0.001 |
| Prior CABG | 1933 (12.1%) | 254 (19.9%) | 1679 (11.4%) | < 0.001 |
| Hypertension | 12794 (79.8%) | 1089 (85.3%) | 11705 (79.3%) | < 0.001 |
| Diabetes Mellitus | 4700 (29.3%) | 525 (41.1%) | 4175 (28.3%) | < 0.001 |
| Hyperlipidemia | 12414 (77.4%) | 1055 (82.6%) | 11359 (77.0%) | < 0.001 |
| Current Smoker | 2150 (13.4%) | 181 (14.2%) | 1969 (13.3%) | 0.41 |
| Family history of CVD | 6304 (39.3%) | 514 (40.3%) | 5790 (39.2%) | 0.48 |
| Peripheral Vascular Disease | 2252 (14.0%) | 237 (18.6%) | 2015 (13.7%) | < 0.001 |
| Cerebrovascular Accident | 1577 (9.8%) | 146 (11.4%) | 1431 (9.7%) | 0.05 |
| Prior Abnormal Calcium Score | 2068 (12.9%) | 98 (7.7%) | 1970 (13.4%) | < 0.001 |
| Atrial Fibrillation | 3184 (19.9%) | 189 (14.8%) | 2995 (20.3%) | < 0.001 |
| Patient Status | 0.52 | |||
| Inpatient | 6042 (37.7%) | 492 (38.5%) | 5550 (37.6%) | |
| Outpatient | 9987 (62.3%) | 785 (61.5%) | 9202 (62.4%) | |
| Abnormal Baseline ECG | 13481 (84.1%) | 1076 (84.3%) | 12405 (84.1%) | 0.87 |
| Symptoms on presentation | ||||
| Chest pain | < 0.001 | |||
| Atypical angina | 9333 (58.3%) | 781 (61.2%) | 8552 (58.0%) | |
| Typical angina | 140 (0.9%) | 31 (2.4%) | 109 (0.7%) | |
| Non-anginal chest pain | 90 (0.6%) | 7 (0.5%) | 83 (0.6%) | |
| None | 6458 (40.3%) | 458 (35.9%) | 6000 (40.7%) | |
| Dyspnea | 7880 (49.2%) | 674 (52.8%) | 7206 (48.8%) | 0.01 |
| Syncope | 1054 (6.6%) | 65 (5.1%) | 989 (6.7%) | 0.03 |
| Baseline Medications | ||||
| Aspirin | 10799 (67.4%) | 942 (73.8%) | 9857 (66.8%) | < 0.001 |
| Beta-blocker | 7236 (45.1%) | 679 (53.2%) | 6557 (44.4%) | < 0.001 |
| Statin | 5852 (36.5%) | 486 (38.1%) | 5366 (36.4%) | 0.23 |
| Calcium Channel Blocker | 3726 (23.2%) | 326 (25.5%) | 3400 (23.0%) | 0.04 |
| Long acting nitrates | 2066 (12.9%) | 251 (19.7%) | 1815 (12.3%) | < 0.001 |
| Stress and Perfusion Results | ||||
| Baseline Heart Rate, bpm | 70.0 ± 13.5 | 70.9 ± 13.4 | 70.0 ± 13.5 | 0.02 |
| Peak Heart Rate, bpm | 89.3 ± 16.5 | 89.2 ± 16.8 | 89.9 ± 16.5 | 0.87 |
| Rest Systolic BP, mmHg | 131.0 (117.0, 146.0) | 135.0 (120.0, 150.5) | 130.0 (117.0, 145.0) | < 0.001* |
| Peak Systolic BP, mmHg | 120.0 (107.0, 135.0) | 123.0 (109.0, 138.0) | 120.0 (107.0, 135.0) | < 0.001* |
| ECG Response | < 0.001 | |||
| Ischemic | 675 (4.2%) | 213 (16.7%) | 462 (3.1%) | |
| Non-Ischemic | 12099 (75.5%) | 722 (56.5%) | 11377 (77.2%) | |
| Non-Diagnostic | 3017 (18.8%) | 317 (24.8%) | 2700 (18.3%) | |
| Equivocal | 229 (1.4%) | 25 (2.0%) | 204 (1.4%) | |
| % Ischemic Myocardium | < 0.001 | |||
| 0% | 10127 (63.2%) | 33 (2.6%) | 10094 (68.4%) | |
| 1-9.9% | 3742 (23.3%) | 271 (21.2%) | 3471 (23.5%) | |
| ≥10% | 2160 (13.5%) | 973 (76.2%) | 1187 (8.0%) | |
| % Scarred Myocardium | 0.0 (0.0, 0.0) | 0.0 (0.0, 4.4) | 0.0 (0.0, 0.0) | < 0.001* |
| Transient ischemic dilation | 2047 (12.8%) | 456 (35.7%) | 1591 (10.8%) | < 0.001 |
| Rest LVEF, % | 61.0 ± 13.7 | 56.2 ± 13.6 | 61.4 ± 13.6 | < 0.001 |
| LVEF Reserve, % | 4.0 (0.0, 8.0) | 0.0 (−5.0, 5.0) | 4.0 (0.0, 8.0) | < 0.001* |
Continuous variables compared using Student's T-test or *Mann-Whitney U test for non-normally distributed variables.
Categorical variables compared using chi-square test.
MI=myocardial infarction, PCI=percutaneous coronary intervention, CABG=coronary artery bypass graft surgery, LVEF=left ventricular ejection fraction, transient ischemic dilation= Stress LV volume/Rest LV volume ratio >1.1
Mean percent ischemic myocardium on PET MPI for the entire study cohort was 3.4 ± 7.1% (Table 1). A total of 5902 (36.8%) patients had ischemia: 3742 (23.3%) with 1-<10% and 2160 (13.5%) with ≥10%.
A total of 1277 (8.0%) patients underwent early revascularization with either PCI (n=1107) or CABG (n=170). Rate of revascularization in patients with ischemia <5%, 5-10% and >10% was 105/12154 (0.9%), 199/1714 (11.6%) and 973/2161 (45%) respectively. Older males with a history of hypertension, diabetes, peripheral vascular disease or cerebrovascular accident were more likely to have early revascularization with PCI or CABG within 90 days of testing (Table 1). Ischemic ECG response, coronary artery calcium score ≥400, greater scarred and ischemic myocardium, transient ischemic dilatation and lower LVEF reserve (stress-rest LVEF) were also associated with early revascularization.
While there were differences in patient characteristics between those who underwent early revascularization vs. not (medical management group) prior to the propensity adjustment (propensity model c-index=0.75), there was a large overlap of propensity scores for patients in both groups (Figure 1 and Online Table 1).
Median follow-up was 3.7 years. In the Cox model for all-cause mortality, adjusted for the propensity score, sex, diabetes, known CAD, rest LVEF, %infarcted myocardium, %ischemic myocardium, and early revascularization as a time-varying covariate, there was a significant interaction (p<0.001as continuous variable and 0.015 as a categorical variable) between percent ischemia and early revascularization (Central Illustration; Online Figure 1), such that patients with higher amounts of ischemic myocardium had a lower hazard of death with early revascularization. The three-way interaction of history of known CAD * %ischemia * early revascularization was not-significant (p=0.719), suggesting that the relationship of ischemia and early revascularization on death was not different in patients with or without a history of prior CAD.
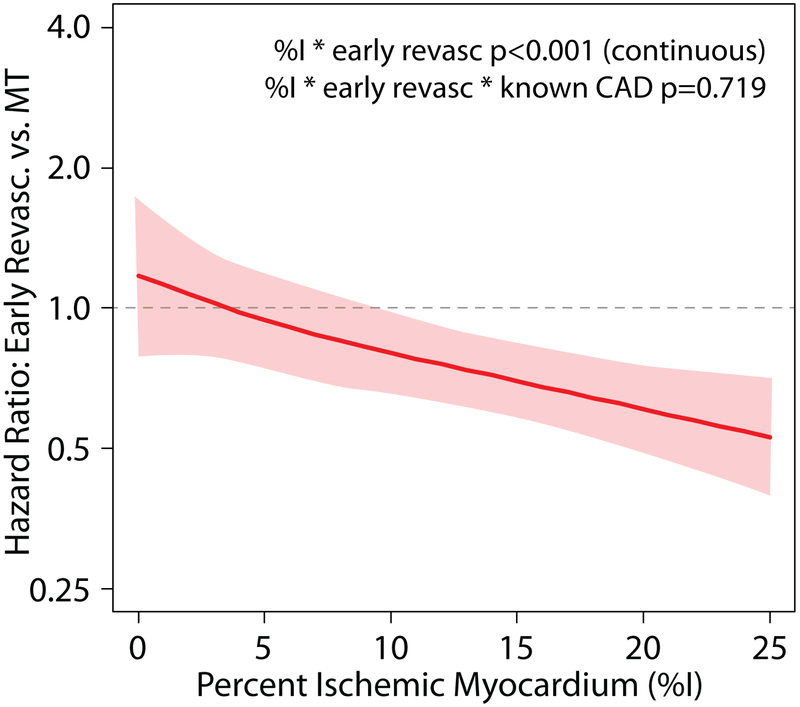
Central Illustration: Ischemia, Revascularization, and Survival: Plot of Hazard Ratios.
All-cause death with early revascularization vs. medical therapy across levels of percent ischemia (analyzed as a continuous variable) on PET MPI. The figure is derived from Cox proportional hazards analysis and shows a patient’s likelihood of survival benefit with coronary revascularization, as compared with medical therapy alone, based on their percent ischemic myocardium on PET MPI testing. The solid line represents point estimates of hazard ratios while the dotted lines represent the upper and lower limits of 95% confidence intervals for hazard ratios. The Cox model was adjusted for propensity score for referral to early revascularization, % ischemic myocardium, early revascularization (as a time-varying covariate), sex, diabetes, known CAD, rest LVEF, %infarcted myocardium.
On plotting the hazards of death with early revascularization vs. MT across levels of ischemia, the point estimates cross unity (HR=1.0 suggesting similar hazard of death with early revascularization and MT) around 5% and upper bounds of 95% confidence intervals cross unity at 10%, suggesting that patients with >10% ischemia have significant survival benefit with early revascularization with > 95% certainty, though patients with lower levels of ischemia (5% or greater) may also benefit with revascularization.
There were 738 cardiac deaths on follow-up. Similar results were seen for outcome of cardiac death (interaction of percent ischemia* early revascularization for cardiac death p <0.001 as a continuous variable, p=0.06 as a categorical variable, 3-way interaction of known CAD *percent ischemia *early revascularization p=0.735). Online Figure 1 presents hazard ratios for cardiac death with early revascularization vs. medical therapy across different levels of ischemia in as a Forest Plot.
Discussion
Among 16,029 patients who underwent PET MPI testing for suspected or known CAD, we found that the effect of ischemic myocardium on long-term survival was modified by revascularization within 90 days of the MPI, such that patients with higher proportion of ischemia on PET MPI had improved survival with early revascularization. The association between inducible ischemia, early revascularization, and survival did not differ by history of prior CAD, supporting the generalizability of these results to all patients undergoing PET MPI. Exploratory analyses suggest that the ischemia threshold above which survival benefit was observed with early revascularization is lower than SPECT MPI, around 5% (upper limit of 95% confidence interval 10%).Collectively, our findings support the use of ischemia on PET MPI to guide post-test management, and improve long-term survival.
The effects of an ischemia-guided revascularization strategy have previously been evaluated. The nuclear sub-studies of the COURAGE and BARI-2D trials showed that revascularization reduces ischemic burden. However, the baseline ischemic burden in these trials has not been shown to be associated with worse outcomes.(20-22) Similarly, a meta-analysis of patients demonstrating ischemia diagnosed using stress ECG, echocardiography, SPECT MPI, or FFR across 5 trials also failed to show a benefit of PCI compared to medical therapy in reducing angina, cardiac events, or death (11). Residual degree of stress abnormal myocardium on serial MPI studies post revascularization was shown to be associated with poor outcomes in the BARI-2D nuclear sub-study, and a trend was noted in COURAGE. (21,22) These nuclear sub-studies did not, however, directly compare outcomes with revascularization and medical therapy by the degree of ischemia on stress testing and were performed in patients with already known obstructive CAD on angiography. It is also important to note that all of these trials were conducted in the era of bare-metal stents.
Our findings significantly extend prior work which examined the interaction of ischemia by SPECT MPI with revascularization on survival. In a cohort of >10,000 patients undergoing dual-isotope SPECT from 1991-1997, Hachamovitch et al. showed that patients with ischemic myocardium greater than 12.5% had a short and long-term survival benefit with early revascularization, in the absence of a large degree of scar.(8,12) The ischemia threshold of 12.5% with SPECT MPI, is based on the proportion of ischemia on SPECT MPI at which the point estimates for survival hazards with early revascularization and medical therapy cross (survival hazard ratio of 1 for early revascularization vs. medical therapy), while the upper bounds of the 95% confidence intervals cross unity at > 15-20% ischemia.(8) We found a similar relationship on PET MPI between ischemia, early revascularization, and survival, but at a much lower threshold of ischemia. In our study, the point estimates of hazards for survival with early revascularization vs. medical therapy cross at around 5% (hazard ratio=1), while upper bounds of 95% confidence intervals cross unity at >10% ischemia. This difference might be related to improved diagnostic accuracy and inherent attenuation correction with PET MPI. Our study shows that threshold for clinically important ischemia may be different depending upon the MPI modality used. If a 10-12.5% ischemia cutoff is used with PET MPI, patients who may benefit from early revascularization may actually be medically managed. While all-cause death was chosen as the primary endpoint to prevent misclassification bias, our findings were similar when cardiac death was studied. Also, in contrast to the Hachamovitch study,(8) we did not find any evidence of differential effect based on percentage of infarcted myocardium or history of CAD. This difference might be secondary to improved revascularization techniques and heart failure and CAD medical therapy in the current era.
Our study has important implications in the current era of value-based imaging, which focuses on improving outcomes at the same or lower cost. A high-value test is one which helps in diagnosis, risk-stratification, and guidance of post-test management. PET MPI has been described as the test of choice in higher risk patients with a greater comorbidity burden who are unable to exercise.(13) In this context, using proportion of inducible ischemia on PET MPI to guide post-test management decisions regarding coronary angiography and revascularization may optimize patient survival and support more efficient resource utilization.
Our study results should be interpreted in the context of the following potential limitations. First, the observational and single-center nature of the study makes it subject to referral bias, unique interpretative accuracy of the PET images by the readers at our institution, and residual confounding. Thus, we cannot infer causality given the observational nature of the study and replication in other settings should be performed. Also, in accordance with current clinical practice, percent ischemia was a major driver of revascularization in our cohort, and residual confounding related to this referral bias could affect the results. However, the primary findings of survival benefit with revascularization in patients with greater proportion of ischemia remained robust with multiple different statistical approaches. Despite the limitations of observational data, we were able to adjust for >30 covariates that may influence referral rates for early revascularization and the relationship between ischemia and mortality. The consecutive enrollment of all patients undergoing MPI for an evaluation of ischemia also supports the generalizability of our findings to patients seen in routine clinical practice, although primarily to those that who are unable to exercise and need pharmacologic stress test assessments. All patients in our study received Rb82 radiotracer agent. As such, results might differ for PET studies done using other radiotracers; however, Rb82 is the most widely used radiotracer especially in United States. Exact percent ischemia thresholds noted in this study might be slightly different at different practices, based on local image interpretation practices, though our images were scored semi-quantitatively per society guidelines (16). Myocardial blood flow measurements were not included in the current study as they were not available for routine clinical use during majority of study period. More than two-thirds of our population did not have known coronary disease at time of PET MPI, thus rates of appropriate medical therapy for coronary artery disease at baseline were low. The type of medical management received by patients during follow-up was unable to be assessed and accounted for.
Conclusions
This study is the largest investigation to date evaluating the relationship between ischemia, treatment with revascularization, and long-term mortality in a contemporary cohort of patients undergoing PET MPI for known or suspected CAD. Patients with higher proportion of ischemia with PET MPI have a survival benefit if revascularized within 90 days of the test. Exploratory analyses suggest the ischemia threshold for benefit with early revascularization with PET MPI might be different and lower than what has been previously reported for SPECT MPI. These results did not differ based on patient history of known CAD prior to MPI. While the results of our observational study are exploratory and require prospective validation in trials such as ISCHEMIA, they suggest an ischemia-based revascularization strategy may improve outcomes in patients with suspected and known CAD.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Patient Care and Procedural Skills: Patients with extensive inducible ischemia detected by positron emission tomographic (PET) myocardial perfusion imaging (MPI) derive survival benefit from revascularization within 90 days of imaging. The proportion of myocardium at risk as detected by this method is less than the amount associated with improved survival when measured by single photon emission computed tomographic (SPECT) MPI. Translational Outlook: Prospective studies are necessary to confirm the extent of ischemia detected by PET MPI that warrants early revascularization to improve long-term survival and define other patient characteristics that should also be considered.
Acknowledgments
Disclosures: Drs. Patel and Al Badarin are supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL110837. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Chan is supported by a grant from the National Heart Blood and Lung Institute (1R01HL123980). Dr. Spertus receives research grant support from Abbott Vascular, Bayer and is the PI of an analytic center for the American College of Cardiology. He serves as a consultant to United Healthcare, Bayer, Janssen, AstraZeneca and Novartis. He has an equity interest in Health Outcomes Sciences. Dr. Bateman receives research grant support from Astellas and GE Healthcare. He serves as a consultant to Curium and to GE Healthcare. He has an equity interest in Cardiovascular Imaging Technologies. Dr. Case receives grant support from Astellas and GE Healthcare. He serves as a consultant to GE Healthcare and has an equity interest in Cardiovascular Imaging Technologies. The other authors report no conflicts.
ABBREVIATIONS
- CAD
coronary artery disease
- MPI
myocardial perfusion imaging
- SPECT
Single Photon Emission Computed Tomography
- PET
Positron Emission Tomography
- Rb82
Rubidium-82
- ECG
electrocardiogram
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boden WE, O'Rourke RA, Teo KK et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–16. [DOI] [PubMed] [Google Scholar]
- 2.Group BDS, Frye RL, August P et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tonino PA, De Bruyne B, Pijls NH et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–24. [DOI] [PubMed] [Google Scholar]
- 4.De Bruyne B, Pijls NH, Kalesan B et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 5.Fractional Flow Reserve-Guided PCI in Stable Coronary Disease. New England Journal of Medicine 2012;367:2355–2356. [DOI] [PubMed] [Google Scholar]
- 6.Fihn SD, Gardin JM, Abrams J et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: Executive Summary. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons J Am Coll Cardiol. 2012;60(24):2564–603. [DOI] [PubMed] [Google Scholar]
- 7.Nesto RW, Kowalchuk GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol 1987;59:23C–30C. [DOI] [PubMed] [Google Scholar]
- 8.Hachamovitch R, Rozanski A, Shaw LJ et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J 2011;32:1012–24. [DOI] [PubMed] [Google Scholar]
- 9.Dorbala S, Hachamovitch R, Curillova Z et al. Incremental prognostic value of gated Rb-82 positron emission tomography myocardial perfusion imaging over clinical variables and rest LVEF. JACC Cardiovasc Imaging 2009;2:846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hachamovitch R Does ischemia burden in stable coronary artery disease effectively identify revascularization candidates? Ischemia burden in stable coronary artery disease effectively identifies revascularization candidates. Circ Cardiovasc Imaging 2015;8:discussion p 8. [DOI] [PubMed] [Google Scholar]
- 11.Stergiopoulos K, Boden WE, Hartigan P et al. Percutaneous coronary intervention outcomes in patients with stable obstructive coronary artery disease and myocardial ischemia: a collaborative meta-analysis of contemporary randomized clinical trials. JAMA Intern Med 2014;174:232–40. [DOI] [PubMed] [Google Scholar]
- 12.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003;107:2900–7. [DOI] [PubMed] [Google Scholar]
- 13.Bateman TM, Dilsizian V, Beanlands RS, DePuey EG, Heller GV, Wolinsky DA. American Society of Nuclear Cardiology and Society of Nuclear Medicine and Molecular Imaging Joint Position Statement on the Clinical Indications for Myocardial Perfusion PET. J Nucl Med 2016;57:1654–1656. [DOI] [PubMed] [Google Scholar]
- 14.Dorbala S, Di Carli MF. Cardiac PET perfusion: prognosis, risk stratification, and clinical management. Semin Nucl Med 2014;44:344–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorbala S, Di Carli MF, Beanlands RS et al. Prognostic value of stress myocardial perfusion positron emission tomography: results from a multicenter observational registry. J Am Coll Cardiol 2013;61:176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dilsizian V, Bacharach SL, Beanlands RS et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol 2016;23:1187–1226. [DOI] [PubMed] [Google Scholar]
- 17. https://www.cdc.gov/nchs/data/ndi/NDI_Users_Guide.pdf.
- 18.Sathiakumar N, Delzell E, Abdalla O. Using the National Death Index to obtain underlying cause of death codes. J Occup Environ Med 1998;40:808–13. [DOI] [PubMed] [Google Scholar]
- 19.Doody MM, Hayes HM, Bilgrad R. Comparability of national death index plus and standard procedures for determining causes of death in epidemiologic studies. Ann Epidemiol 2001;11:46–50. [DOI] [PubMed] [Google Scholar]
- 20.Shaw LJ, Weintraub WS, Maron DJ et al. Baseline stress myocardial perfusion imaging results and outcomes in patients with stable ischemic heart disease randomized to optimal medical therapy with or without percutaneous coronary intervention. Am Heart J 2012;164:243–50. [DOI] [PubMed] [Google Scholar]
- 21.Shaw LJ, Cerqueira MD, Brooks MM et al. Impact of left ventricular function and the extent of ischemia and scar by stress myocardial perfusion imaging on prognosis and therapeutic risk reduction in diabetic patients with coronary artery disease: results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. J Nucl Cardiol 2012;19:658–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw LJ, Berman DS, Maron DJ et al. Optimal Medical Therapy With or Without Percutaneous Coronary Intervention to Reduce Ischemic Burden. Results From the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) Trial Nuclear Substudy 2008;117:1283–1291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.