Abstract
Cognitive control, the ability to regulate one’s cognition and actions on the basis of over-riding goals, is impaired in many psychiatric conditions. Although control requires the coordinated function of several prefrontal cortical regions, it has been challenging to determine how they work together, in part because doing so requires simultaneous recordings from multiple regions. Here, we provide a précis of cognitive control and describe the beneficial consequences of recent advances in neurosurgical practice that make large-scale prefrontal cortical network recordings possible in humans. Such recordings implicate inter-regional theta (5–8 Hz) local field potential (LFP) synchrony as a key element in cognitive control. Major open questions include how theta might influence other oscillations within these networks, the precise timing of information flow between these regions, and how perturbations such as brain stimulation might demonstrate the causal role of LFP phenomena. We propose that an increased focus on human electrophysiology is essential for an understanding of the neural basis of cognitive control.
Keywords: Cognitive control, Electrophysiology, Conflict, cingulate, Local field potential
Cognitive control and psychiatry
Cognitive control refers to the ability to regulate one’s own cognitive activity and the actions driven by that activity in the presence of overarching goals 1– 3. It is especially important when faced with the need to withhold a response or to countermand a planned thought or action. For example, anyone who has lived with a toddler knows that “inside voice” is different from “outside voice”. The parent needs to teach the child that even something as basic as speaking volume must be linked to a seemingly arbitrary context and that a habit of speaking loudly must be controlled in some circumstances. A more clinically relevant example would come from touching a doorknob in a public building. That action may trigger thoughts of germs and may motivate hand-washing. But a patient with obsessive-compulsive disorder (OCD) undergoing exposure response prevention therapy may choose to deliberately delay hand-washing because of a higher-order goal of practicing not responding to obsessions.
Cognitive control involves three major components 4– 6. First, we must maintain an ever-changing internal mental representation of our long-term goals. Then, we must monitor our interactions with the world and compare the results of those interactions (or the likely results of intended interactions) with our goals and assess how closely they match. Finally, we must adjust our behavior, if need be, to better fit our goals. In other words, our brains function, in an abstract sense, like a closed-loop engineering control system.
A critical element of most control systems is an internal representation of the task and its contingencies. Detection of failure to achieve goals requires a representation of what those goals are; adjustment requires a judgment about which way the adjustment should go. Thus, an important part of control, which we do not focus on here, is the map of task state. There is some evidence that the orbitofrontal cortex (OFC) may be specialized for maintaining this type of representation 7– 9. Another possibility is that maps of state space are more widespread, perhaps in different forms. Such representations, relevant for control, may be stored adjacent to representations needed for the relevant processing (for example, in dorsal anterior cingulate cortex [dACC] 10).
Contexts that elicit control, such as conflict, can have two types of consequences. First, we can try to change course online (that is, adjust our immediate plans so that we do something different) 11– 15. Second, we may change strategies on subsequent trials or encounters. Over short time frames, this process is known as adjustment 16, 17. Over longer time frames, it is known as learning. Both processes are united in that they use monitoring signals to alter stimulus-output mappings. Together, these two forms of adjustment are often known as proactive and reactive control.
Notably, cognitive control overlaps quite a bit with the concept of self-control 3, 18– 21. Self-control, however, is slightly different. Specifically, it can be defined as the deliberate selection of an abstemious option that produces greater long-term benefits when faced with a more tempting option. Thus, it is a cognitive operation that generally requires cognitive control. As the study of one is likely to help shed light on the other, their differences must be kept in mind.
Limits of animal models
Neurophysiological models of cognitive control have been difficult to fully test. For example, major open questions include how theta might influence other oscillations within these networks, the precise timing of information flow between these regions, and how perturbations such as brain stimulation might demonstrate the causal role of local field potential (LFP) phenomena. One major challenge in answering questions like these is the disconnect between results from human and non-human studies. Structurally, we do not know the homology between prefrontal regions in rodent, monkey, and human 22– 24. Most notably, although monkeys appear to have a region homologous to human dACC, the specifics are hotly debated 10, 25. In rodents, portions of dACC may be quite different, and there is likely no homologue of dorsolateral prefrontal cortex (dlPFC) 23, 26. Functionally, the overlaps are unclear as well. For example, dACC is frequently activated in human neuroimaging studies of conflict and associated control-related adjustment. On the other hand, primate neurophysiology studies, which have high temporal and spatial resolution, have generally failed to find single-unit correlates of cognitive conflict in this region 27– 30. The reasons for the disconnect between non-human primate electrode recording studies and human neuroimaging are not clear 31, although the lack of naturalness of the primate tasks may be a contributing factor 32.
This does not mean that non-human animal models are worthless. Indeed, animal models have proven quite useful in understanding the neural basis of, for example, response competition 33– 36, rule switching 37– 40, response inhibition 41, persistence 42– 44, and outcome monitoring 45– 47. These successes, indeed, are foundational in the field of cognitive control. Nonetheless, they do not provide a complete picture of cognitive control and they have some limitations. For example, we lack a clear and non-controversial model of cognitive conflict and the types of control needed for rapid learning, for control related to language, for culture, or arguably for self-control 48.
Consequently, humans are the most important model organism for the study of cognitive control. Unfortunately, the spatial resolution of electro-encephalography (EEG), the most prevalent human electrophysiological technique, may not be conducive to refining the specific functional roles of spatially neighboring PFC regions. It also may not pick up key nodes of the proposed network (for example, OFC 49). Magnetoencephalography, while offering potentially higher resolution, is still far from desired. Finally, functional magnetic resonance imaging, although it is the most widely used method, lacks sufficient spatial and temporal resolution to answer several key questions, such as those related to theta (see below).
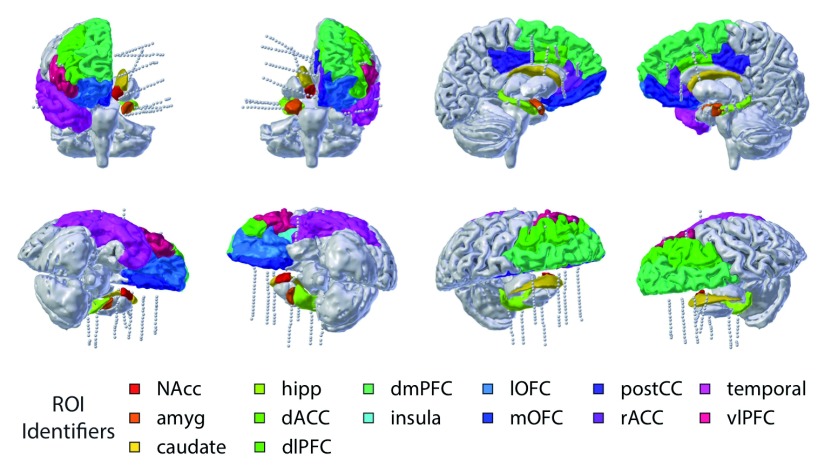
Recent advances in neurosurgery offer the promise of intracranial recordings in humans. Patients with medication-resistant epilepsy often obtain relief from neurosurgery, wherein a seizure-originating focus is removed from their brains. Localizing an individual patient’s focus can require the implantation of temporary monitoring electrodes that effectively triangulate the seizure origin through monitoring of the LFP. Changes in patient and surgeon preference have driven rapid adoption of a specific monitoring technique called stereotactic EEG (stereo-EEG for short), where the monitoring electrodes are long cylindrical shanks placed through very small drill holes in the skull. These electrodes pass through superficial cortex and terminate in the deep brain, often close to the midline ( Figure 1). Thus, these patients may have continuous recordings from multiple PFC components for a period of 1 to 2 weeks. During this time, patients are typically resting in a hospital room, awaiting a seizure event, and are often able to perform psychophysical tasks. These task runs offer a unique opportunity to study cognitive control processes directly in humans. Next, we summarize the relationship between mental illness and cognitive control and then describe some of the benefits of intracranial studies.
Figure 1. An example montage from a human intracranial subject.
dACC, dorsal anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; lOFC, lateral orbitofrontal cortex; mOFC, medial orbitofrontal cortex; NAcc, nucleus accumbens; postCC, posterior cingulate; rACC, rostral anterior cingulate cortex; ROI, region of interest; vlPFC, ventrolateral prefrontal cortex.
The transdiagnostic nature of cognitive control
Deficits in cognitive control have been demonstrated in almost every psychiatric illness (for example, 50– 57). For example, one hallmark of cognitive control is the ability to readily disengage from one train of thought or pattern of action to pursue an alternative. That alternative may be goal-aligned; thus, failure to disengage is often opposed to goals. People with OCD can have trouble disengaging from persistent anxious thoughts and the resulting rituals, people with post-traumatic stress disorder can have trouble disengaging from avoidance behavior driven by fear memories, and people with substance addiction can have trouble disengaging from cravings and the resulting drug-taking. In other words, cognitive control failures are a transdiagnostic phenomenon. Furthermore, such deficits can translate to controlled laboratory tasks of goal-aligned disengagement, such as reversal learning and cognitive conflict tasks 58, 59.
Disorders associated with cognitive control deficits are treatable through cognitive-behavioral therapy (CBT), which seeks to teach patients alternate (more adaptive) behaviors to replace their pre-potent or automatic responses. This could be thought of as providing a second automatic response that, once practiced, is somehow “easy” to select. The success of CBT suggests that control may be a target for remediation and disease treatment. Indeed, both invasive 60 and non-invasive 61 brain stimulation can improve performance on cognitive conflict tasks, the most common laboratory measure of cognitive control. That improvement is associated with changes in neurophysiological signatures of control.
It nevertheless remains difficult to use cognitive control directly as a diagnostic or therapeutic target. Psychiatric disorders are internally heterogeneous 62, 63. Control deficits might be more common among patients with a given diagnosis, but it does not follow that every individual with that diagnosis is guaranteed to have a control deficit. This may explain why all the effects just cited are themselves heterogeneous; for every disorder, there are also studies failing to find cognitive control deficits in a particular patient sample 64. Although this may sound like a case for pessimism, we believe it indicates the potential benefit that can accrue from more fine-grained and precise measures of cognitive control.
Given that control deficits are found across disorders, but heterogeneously, a logical approach would be to identify a test that detects such deficits by studying a large sample of patients with a variety of diagnoses. Two recent studies took the first steps on that road. One applied a comprehensive neurocognitive battery and a variety of psychophysical tasks to 420 patients across mood and anxiety diagnoses, identifying six subgroups through a data-driven clustering approach 65. These subgroups could be discriminated by their performance on a go/no-go task when that performance was expressed relative to age- and sex-corrected healthy volunteer performance. A different approach used the two-step learning paradigm 66, a laboratory task in which subjects must navigate a decision tree to find the most rewarding outcomes. This paradigm has the advantage of a well-accepted parametric behavior modeling approach 67, which, when applied to an individual, yields a single number quantifying capacity for “model-based” control processes. Gillan et al. developed a population norm for this parameter in 1,413 online subjects, establishing a means to quantify individual-level control deficits 66. The authors then showed that subjects with these deficits were more likely to report psychiatric symptoms, particularly those related to perseveration and compulsion.
The anatomic basis of cognitive control
A brief detour into the neuroanatomy of cognitive control reveals a clear overlap between regions associated with control and those associated with psychiatric disease. Psychiatric disorders involve abnormalities in many brain regions, but the most consistent of these are in the prefrontal cortico-basal ganglia network 68. Similarly, cognitive control is closely associated with the prefrontal cortex, so much so that it is sometimes thought to be the defining feature of this large and heterogeneous region 1. This is, of course, overly simplistic, but it is clear that many prefrontal regions are closely involved in cognitive control. Most prominent among these are the dACC and dlPFC. Both neuroimaging and electrophysiological studies, from humans and non-human animals, indicate that these two regions are centrally involved in cognitive control 4, 10, 25. In addition, lesions or damage to these regions tend to produce impairments in control. Other regions that are associated with cognitive control are the ventrolateral prefrontal cortex 69, the dorsomedial PFC (DMPFC) 70, and the OFC 71, 72. Some work even implicates the striatum, although its role is far from clear 38, 54, 73, 74.
Models of prefrontal function ascribe to these regions several features that are critical to cognitive control: (1) they participate in monitoring goal-relevant variables, (2) their activity reflects the evaluation of costs and benefits of choice alternatives, (3) they maintain rule sets and goal-related information in working memory, and (4) they adjust adaptive biases toward more successful behavior. The evidence linking prefrontal cortex to each of these functions is strong. However, the specific assignment of regions to functions remains unclear; indeed, some recent theories hold that it may be impossible to link function to region in a one-to-one manner. Instead, regions may be better described as existing on a hierarchy of control 75– 78.
More formally, cognitive control can be conceptualized as a neural instantiation of ideas from engineering: monitor and controller. These terms originate in engineering control theory and refer to elements of a control system that detect the need for control and implement it, respectively. By this same logic, we may hypothesize that OFC serves as a source of reward information that drives control decisions and that DMPFC serves as a final gateway to motor systems. This speculative assignment of roles to regions offers a suggestion for why dACC appears to be so central: it functions as a hub point for the initiation of control 79– 81. This theory has the potential to integrate several seemingly disparate findings about dACC, including its role in monitoring reward outcomes, its role in choice processes, and its spatial reference frames, especially the frame of actions 82– 88.
Local field potential synchrony may bind frontal regions to achieve control
The broad involvement of dACC, dlPFC, and other prefrontal regions highlights the fact that cognitive control is a network function. This raises a question: how are top-down control signals and bottom-up signals of the need for control routed? Recent work suggests that networks of cognitive control can be established through cross-regional synchronization of oscillations in the LFP. (Some example traces of real patient data are shown in Figure 2). The general notion that LFP synchrony is a mechanism for brain communication is nearly 15 years old 89 and the theory continues to be refined 90– 93. This includes a continued expansion of the definition of “synchrony”, to encompass spike-field coherence 90, 94 measures related purely to the phase of the LFP oscillation, or as coupling between a lower-frequency and higher-frequency oscillation 91, 95– 97. Furthermore, evidence continues to build that LFP synchrony plays a role in long-range brain areal communication. There is particularly strong evidence in sensation- and memory-related research 98, 99.
Figure 2. Example of stereotactic electro-encephalogram recordings—in this case, eight bipolar-referenced channels from the left orbitofrontal cortex (LOF)—during one trial of a cognitive control task.
Example of stereotactic electro-encephalogram recordings—in this case, eight bipolar-referenced channels from the left orbitofrontal cortex (LOF)—during one trial of a cognitive control task.
Oscillations in the theta frequency band (5–8 Hz) are particularly relevant for top-down communication 100. Theta rhythms in the scalp EEG recorded over PFC have long been associated with cognitive control, especially as studied in conflict paradigms 55, 101– 103. In theory, these power increases at the scalp might reflect changes in the synchrony of underlying cortical structures, where greater synchrony of neural firing leads to larger induced dipoles that are more easily observed at the scalp. If this is true, invasive neural recordings during similar tasks should show increases in theta phase synchrony (coherence or phase locking) that precede successful cognitive control.
Human intracranial recording as a tool for studying control
Thus far, invasive human electrophysiology studies support the thesis that LFP synchrony is a key component in fronto-cingulate network formation and function across multiple components of cognitive control. As humans are asked to make decisions according to increasingly abstract rules (that is, to exercise goal-maintenance aspects of control), theta phase synchrony between PFC and motor regions increases 91. Directed analysis shows cross-frequency coupling between these same regions, wherein PFC’s phase drives the amplitude of high-frequency M1 oscillations. A parallel study extended this finding to control during cognitive conflict. After subjects receive error feedback during a cognitive conflict task (signaling an increased need to deploy control), there is directed information transfer from medial to lateral PFC electrodes 104. Furthermore, that information transfer (assessed by mutual information calculations) is strongest between 4 and 8 Hz, the canonical theta band. Using the same task, our group recently showed that this theta synchrony extends well beyond PFC 105. We showed that cognitive control tasks engage pairwise correlations between PFC, cingulate, and subcortical structures and that the majority of high-influence correlations were in the theta band 105. This may reflect a link between control and attention; PFC synchronizes with parietal cortex to entrain parietal oscillations to ongoing intermittent signals, increasing their chance of successful detection 100. The dlPFC-dACC control framework (described above) is also supported by intracranial electrophysiology. A recent study examined single-unit activity in humans during performance of a cognitive conflict task 15. As in macaques, there were only modest neuronal firing rate correlates of conflict in dACC and negligible ones in dlPFC. However, conflict substantially altered spike-phase coherence in dACC and spike-field coherence in dlPFC. The much stronger effects in the LFP domain than in the spiking domain were striking, especially given the failures to find neural correlates of conflict at the unit level in past studies. The authors proposed that the single units may serve as soloists that drive a larger oscillatory choir that in turn drives behavior 15. An open question is whether these networks can be perturbed to demonstrate causality between LFP synchrony and successful control.
Human experimental opportunities are an excellent opportunity for causal testing of cognitive control theories because specific brain regions’ function can be perturbed through electrical stimulation. Although seizures are always a concern in subjects with known epilepsy, many groups have conducted intermittent stimulation experiments in these populations. In those studies, there were no major adverse events and there were several reports of augmented function 106– 109. Arguably, the first report of such an experiment applied to cognitive control was a pair of patients in whom a subjective sense of “the will to persevere” could be evoked through rostral ACC stimulation 110. That study did not quantify the potential control change using a behavioral task, but another recent study did. In psychiatric patients with deep brain stimulation electrodes in the internal capsule, we increased the power of theta rhythms in both lateral and medial PFC and, by doing so, improved subjects’ performance on the multi-source interference task 60. The next step would be defining stimulation patterns that can specifically alter coherence, cross-frequency coupling, or other synchrony metrics. This may be possible by locking stimulation to the phase of a band-limited oscillation, which has recently become possible with advances in real-time signal processing 111– 113.
Conclusions
Cognitive control has long been understood to require the coordinated function of multiple PFC structures. Recent advances in the study of control have begun to dissect what each of these regions contributes, how they collectively detect the need for control, and how they then bias lower-level cognitive processes to achieve a desired result. Access to human intracranial recording sites has allowed great advances in this domain. Specifically, it has allowed confirmation and extension of important theories about the neural basis of control in the organism of interest (humans) rather than in model organisms. This is important because of possible dishomology between human and model organism structure and function in key regions and because of doubt about our ability to elicit human-like control in model organisms. A key next step is linking this network-level recording to formal theories of cognitive control 3, 6. Specific aspects of theoretical/computational models should load onto sub-regions of PFC but this assumption has not yet been tested. Furthermore, if the theta synchrony theory is correct, it should be possible to track sequential flow of a computation through PFC and ACC, for example, through spectrally resolved directed connectivity metrics.
Relatedly, human intracranial opportunities allow direct manipulation of these circuits in the human brain. For instance, as described above, we have verified a role of cortico-striatal circuits in control by manipulating those circuits with deep brain stimulation. Evidence from other cognitive systems suggests that the inter-regional communication necessary for top-down control may require synchronized LFP oscillations. Different groups have found evidence for phase-related measures (for example, coherence), phase-amplitude coupling, and amplitude-amplitude measures (mutual information) as measures of that synchrony. Overall, this hypothesis is plausible but incompletely tested. With rapid advances in recording and stimulation capabilities, particularly for experiments with human volunteers, that will soon change. Furthermore, these experiments often use electrical stimulation to perturb networks of cognitive control, increasingly in activity-dependent closed-loop paradigms. Those approaches offer the prospect not only that we will understand control better in years to come but that such studies will lead directly to new therapies targeting deficits in control.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Matthew R. Roesch, Department of Psychology and Program in Neuroscience and Cognitive Sciences, University of Maryland, College Park, Maryland, USA
Wael Asaad, Departments of Neurosurgery & Neuroscience, Brown University, Providence, RI, 02903, USA
Steve Chang, Department of Psychology and Neuroscience, Kavli Institute for Neuroscience, Yale University, New Haven, Connecticut, USA
Funding Statement
This work was supported by R01 MH118257 (to SRH), DA 038615 (to BYH). ASW acknowledges support from the OneMind Institute and the National Institutes of Health (UH3 NS100548, R21 MH113103, R01 EB026938, R01 MH119384) for work discussed in this article.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. Miller EK, Cohen JD: An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- 2. Botvinick MM, Cohen JD: The computational and neural basis of cognitive control: charted territory and new frontiers. Cogn Sci. 2014;38(6):1249–85. 10.1111/cogs.12126 [DOI] [PubMed] [Google Scholar]
- 3. Shenhav A, Musslick S, Lieder F, et al. : Toward a Rational and Mechanistic Account of Mental Effort. Annu Rev Neurosci. 2017;40:99–124. 10.1146/annurev-neuro-072116-031526 [DOI] [PubMed] [Google Scholar]
- 4. Botvinick M, Braver T: Motivation and cognitive control: from behavior to neural mechanism. Annu Rev Psychol. 2015;66:83–113. 10.1146/annurev-psych-010814-015044 [DOI] [PubMed] [Google Scholar]
- 5. Botvinick M, Nystrom LE, Fissell K, et al. : Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–81. 10.1038/46035 [DOI] [PubMed] [Google Scholar]
- 6. Shenhav A, Botvinick MM, Cohen JD: The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–40. 10.1016/j.neuron.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilson RC, Takahashi YK, Schoenbaum G, et al. : Orbitofrontal cortex as a cognitive map of task space. Neuron. 2014;81(2):267–279. 10.1016/j.neuron.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schuck NW, Cai MB, Wilson RC, et al. : Human Orbitofrontal Cortex Represents a Cognitive Map of State Space. Neuron. 2016;91(6):1402–1412. 10.1016/j.neuron.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wikenheiser AM, Schoenbaum G: Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nat Rev Neurosci. 2016;17(8):513–23. 10.1038/nrn.2016.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heilbronner SR, Hayden BY: Dorsal Anterior Cingulate Cortex: A Bottom-Up View. Annu Rev Neurosci. 2016;39:149–70. 10.1146/annurev-neuro-070815-013952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen X, Scangos KW, Stuphorn V: Supplementary motor area exerts proactive and reactive control of arm movements. J Neurosci. 2010;30(44):14657–75. 10.1523/JNEUROSCI.2669-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boucher L, Palmeri TJ, Logan GD, et al. : Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol Rev. 2007;114(2):376–97. 10.1037/0033-295X.114.2.376 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Aron AR: From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69(12):e55–68. 10.1016/j.biopsych.2010.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Braver TS, Paxton JL, Locke HS, et al. : Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106(18):7351–7356. 10.1073/pnas.0808187106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith EH, Horga G, Yates MJ, et al. : Widespread temporal coding of cognitive control in human prefrontal cortex. Nature Neuroscience. 2019; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heilbronner SR, Hayden BY, Platt ML: Decision salience signals in posterior cingulate cortex. Front Neurosci. 2011;5:55. 10.3389/fnins.2011.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayden BY, Nair AC, McCoy AN, et al. : Posterior cingulate cortex mediates outcome-contingent allocation of behavior. Neuron. 2008;60(1):19–25. 10.1016/j.neuron.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayden BY: Why has evolution not selected for perfect self-control? Philos Trans R Soc Lond B Biol Sci. 2018;374(1766):20180139. 10.1098/rstb.2018.0139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Ridder DT, Lensvelt-Mulders G, Finkenauer C, et al. : Taking stock of self-control: a meta-analysis of how trait self-control relates to a wide range of behaviors. Pers Soc Psychol Rev. 2012;16(1):76–99. 10.1177/1088868311418749 [DOI] [PubMed] [Google Scholar]
- 20. van Horn DHA: Losing control: How and why people fail at self-regulation: R. F. Baumeister, T. D. Heatherton & D. M. Tice. New York: Academic Press, 1994, 307 pp. ($59.95). Clin Psychol Rev. 1995;15(4):367–368. 10.1016/0272-7358(95)90149-3 [DOI] [Google Scholar]
- 21. Buckholtz JW: Social norms, self-control, and the value of antisocial behavior. Curr Opin Behav Sci. 2015;3:122–129. 10.1016/j.cobeha.2015.03.004 [DOI] [Google Scholar]
- 22. Seamans JK, Lapish CC, Durstewitz D: Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res. 2008;14(2–3):249–62. 10.1007/BF03033814 [DOI] [PubMed] [Google Scholar]
- 23. Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, et al. : Circuit-Based Corticostriatal Homologies Between Rat and Primate. Biol Psychiatry. 2016;80(7):509–21. 10.1016/j.biopsych.2016.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carlén M: What constitutes the prefrontal cortex? Science. 2017;358(6362):478–482. 10.1126/science.aan8868 [DOI] [PubMed] [Google Scholar]
- 25. Rushworth MFS, Noonan MP, Boorman ED, et al. : Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70(6):1054–69. 10.1016/j.neuron.2011.05.014 [DOI] [PubMed] [Google Scholar]
- 26. Passingham RE, Wise SP: The Neurobiology of the Prefrontal Cortex. Oxford University Press;2012. Reference Source [Google Scholar]
- 27. Amiez C, Joseph JP, Procyk E: Reward encoding in the monkey anterior cingulate cortex. Cereb Cortex. 2006;16(7):1040–55. 10.1093/cercor/bhj046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ito S: Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302(5642):120–2. 10.1126/science.1087847 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Nakamura K, Roesch MR, Olson CR: Neuronal activity in macaque SEF and ACC during performance of tasks involving conflict. J Neurophysiol. 2005;93(2):884–908. 10.1152/jn.00305.2004 [DOI] [PubMed] [Google Scholar]
- 30. Blanchard TC, Hayden BY: Neurons in dorsal anterior cingulate cortex signal postdecisional variables in a foraging task. J Neurosci. 2014;34(2):646–55. 10.1523/JNEUROSCI.3151-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cole MW, Yeung N, Freiwald WA, et al. : Cingulate cortex: diverging data from humans and monkeys. Trends Neurosci. 2009;32(11):566–74. 10.1016/j.tins.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calhoun AJ, Hayden BY: The foraging brain. Curr Opin Behav Sci. 2015;5:24–31. 10.1016/j.cobeha.2015.07.003 [DOI] [Google Scholar]
- 33. Ebitz RB, Platt ML: Neuronal activity in primate dorsal anterior cingulate cortex signals task conflict and predicts adjustments in pupil-linked arousal. Neuron. 2015;85(3):628–40. 10.1016/j.neuron.2014.12.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bryden DW, Brockett AT, Blume E, et al. : Single Neurons in Anterior Cingulate Cortex Signal the Need to Change Action During Performance of a Stop-change Task that Induces Response Competition. Cereb Cortex. 2019;29(3):1020–1031. 10.1093/cercor/bhy008 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Wang MZ, Hayden BY: Reactivation of associative structure specific outcome responses during prospective evaluation in reward-based choices. Nat Commun. 2017;8:15821. 10.1038/ncomms15821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lauwereyns J, Koizumi M, Sakagami M, et al. : Interference from irrelevant features on visual discrimination by macaques ( Macaca fuscata): a behavioral analogue of the human Stroop effect. J Exp Psychol Anim Behav Process. 2000;26(3):352–7. 10.1037//0097-7403.26.3.352 [DOI] [PubMed] [Google Scholar]
- 37. Bissonette GB, Schoenbaum G, Roesch MR, et al. : Interneurons are necessary for coordinated activity during reversal learning in orbitofrontal cortex. Biol Psychiatry. 2015;77(5):454–64. 10.1016/j.biopsych.2014.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sleezer BJ, Hayden BY: Differential Contributions of Ventral and Dorsal Striatum to Early and Late Phases of Cognitive Set Reconfiguration. J Cogn Neurosci. 2016;28(12):1849–1864. 10.1162/jocn_a_01011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buckley MJ, Mansouri FA, Hoda H, et al. : Dissociable components of rule-guided behavior depend on distinct medial and prefrontal regions. Science. 2009;325(5936):52–8. 10.1126/science.1172377 [DOI] [PubMed] [Google Scholar]
- 40. Womelsdorf T, Johnston K, Vinck M, et al. : Theta-activity in anterior cingulate cortex predicts task rules and their adjustments following errors. Proc Natl Acad Sci U S A. 2010;107(11):5248–53. 10.1073/pnas.0906194107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bryden DW, Roesch MR: Executive control signals in orbitofrontal cortex during response inhibition. J Neurosci. 2015;35(9):3903–14; discussion 707–26. 10.1523/JNEUROSCI.3587-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blanchard TC, Strait CE, Hayden BY: Ramping ensemble activity in dorsal anterior cingulate neurons during persistent commitment to a decision. J Neurophysiol. 2015;114(4):2439–49. 10.1152/jn.00711.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hillman KL, Bilkey DK: Persisting through subjective effort: a key role for the anterior cingulate cortex? Behav Brain Sci. 2013;36(6):691–2. 10.1017/S0140525X13001039 [DOI] [PubMed] [Google Scholar]
- 44. Rudebeck PH, Walton ME, Smyth AN, et al. : Separate neural pathways process different decision costs. Nat Neurosci. 2006;9(9):1161–8. 10.1038/nn1756 [DOI] [PubMed] [Google Scholar]
- 45. Hyman JM, Holroyd CB, Seamans JK: A Novel Neural Prediction Error Found in Anterior Cingulate Cortex Ensembles. Neuron. 2017;95(2):447–456. 10.1016/j.neuron.2017.06.021 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Warren CM, Hyman JM, Seamans JK, et al. : Feedback-related negativity observed in rodent anterior cingulate cortex. J Physiol Paris. 2015;109(1–3):87–94. 10.1016/j.jphysparis.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 47. Ma L, Hyman JM, Phillips AG, et al. : Tracking progress toward a goal in corticostriatal ensembles. J Neurosci. 2014;34(6):2244–53. 10.1523/JNEUROSCI.3834-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Beran MJ: The comparative science of “self-control”: what are we talking about? Front Psychol. 2015;6:51. 10.3389/fpsyg.2015.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Voloh B, Valiante TA, Everling S, et al. : Theta-gamma coordination between anterior cingulate and prefrontal cortex indexes correct attention shifts. Proc Natl Acad Sci U S A. 2015;112(27):8457–62. 10.1073/pnas.1500438112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zilverstand A, Huang AS, Alia-Klein N, et al. : Neuroimaging Impaired Response Inhibition and Salience Attribution in Human Drug Addiction: A Systematic Review. Neuron. 2018;98(5):886–903. 10.1016/j.neuron.2018.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Gruner P, Pittenger C: Cognitive inflexibility in Obsessive-Compulsive Disorder. Neuroscience. 2017;345:243–255. 10.1016/j.neuroscience.2016.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Yang Z, Oathes DJ, Linn KA, et al. : Cognitive Behavioral Therapy Is Associated With Enhanced Cognitive Control Network Activity in Major Depression and Posttraumatic Stress Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3(4):311–319. 10.1016/j.bpsc.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Cavanagh JF, Shackman AJ: Frontal midline theta reflects anxiety and cognitive control: meta-analytic evidence. J Physiol Paris. 2015;109(1–3):3–15. 10.1016/j.jphysparis.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robbins TW, Vaghi MM, Banca P: Obsessive-Compulsive Disorder: Puzzles and Prospects. Neuron. 2019;102(1):27–47. 10.1016/j.neuron.2019.01.046 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Ryman SG, Cavanagh JF, Wertz CJ, et al. : Impaired Midline Theta Power and Connectivity During Proactive Cognitive Control in Schizophrenia. Biol Psychiatry. 2018;84(9):675–683. 10.1016/j.biopsych.2018.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Solomon M, Yoon JH, Ragland JD, et al. : The development of the neural substrates of cognitive control in adolescents with autism spectrum disorders. Biol Psychiatry. 2014;76(5):412–21. 10.1016/j.biopsych.2013.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rodman AM, Jenness JL, Weissman DG, et al. : Neurobiological Markers of Resilience to Depression Following Childhood Maltreatment: The Role of Neural Circuits Supporting the Cognitive Control of Emotion. Biol Psychiatry. 2019;86(6):464–473. 10.1016/j.biopsych.2019.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Izquierdo A, Brigman JL, Radke AK, et al. : The neural basis of reversal learning: An updated perspective. Neuroscience. 2017;345:12–26. 10.1016/j.neuroscience.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Goschke T: Dysfunctions of decision-making and cognitive control as transdiagnostic mechanisms of mental disorders: advances, gaps, and needs in current research. Int J Methods Psychiatr Res. 2014;23 Suppl 1:41–57. 10.1002/mpr.1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Widge AS, Zorowitz S, Basu I, et al. : Deep brain stimulation of the internal capsule enhances human cognitive control and prefrontal cortex function. Nat Commun. 2019;10(1):1536. 10.1038/s41467-019-09557-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dubreuil-Vall L, Chau P, Ruffini G, et al. : tDCS to the left DLPFC modulates cognitive and physiological correlates of executive function in a state-dependent manner. Brain Stimul. 2019; pii: S1935-861X(19)30231-1. 10.1016/j.brs.2019.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cuthbert BN, Insel TR: Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. 10.1186/1741-7015-11-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Widge AS, Malone DA, Jr, Dougherty DD: Closing the Loop on Deep Brain Stimulation for Treatment-Resistant Depression. Front Neurosci. 2018;12:175. 10.3389/fnins.2018.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Paulus MP: Cognitive control in depression and anxiety: Out of control? Curr Opin Behav Sci. 2015;1:113–120. 10.1016/j.cobeha.2014.12.003 [DOI] [Google Scholar]
- 65. Grisanzio KA, Goldstein-Piekarski AN, Wang MY, et al. : Transdiagnostic Symptom Clusters and Associations With Brain, Behavior, and Daily Function in Mood, Anxiety, and Trauma Disorders. JAMA Psychiatry. 2018;75(2):201–209. 10.1001/jamapsychiatry.2017.3951 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 66. Gillan CM, Kosinski M, Whelan R, et al. : Characterizing a psychiatric symptom dimension related to deficits in goal-directed control. eLife. 2016;5: pii: e11305. 10.7554/eLife.11305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Voon V, Reiter A, Sebold M, et al. : Model-Based Control in Dimensional Psychiatry. Biol Psychiatry. 2017;82(6):391–400. 10.1016/j.biopsych.2017.04.006 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Heilbronner SR, Chafee MV: Learning How Neurons Fail Inside of Networks: Nonhuman Primates Provide Critical Data for Psychiatry. Neuron. 2019;102(1):21–26. 10.1016/j.neuron.2019.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Levy BJ, Wagner AD: Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci. 2011;1224(1):40–62. 10.1111/j.1749-6632.2011.05958.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McGuire JT, Botvinick MM: Prefrontal cortex, cognitive control, and the registration of decision costs. Proc Natl Acad Sci U S A. 2010;107(17):7922–6. 10.1073/pnas.0910662107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sleezer BJ, Castagno MD, Hayden BY: Rule Encoding in Orbitofrontal Cortex and Striatum Guides Selection. J Neurosci. 2016;36(44):11223–11237. 10.1523/JNEUROSCI.1766-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sleezer BJ, LoConte GA, Castagno MD, et al. : Neuronal responses support a role for orbitofrontal cortex in cognitive set reconfiguration. Eur J Neurosci. 2017;45(7):940–951. 10.1111/ejn.13532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sharpe MJ, Stalnaker T, Schuck NW, et al. : An Integrated Model of Action Selection: Distinct Modes of Cortical Control of Striatal Decision Making. Annu Rev Psychol. 2019;70:53–76. 10.1146/annurev-psych-010418-102824 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Bissonette GB, Roesch MR: Neurophysiology of rule switching in the corticostriatal circuit. Neuroscience. 2017;345:64–76. 10.1016/j.neuroscience.2016.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Cisek P, Kalaska JF: Neural mechanisms for interacting with a world full of action choices. Annu Rev Neurosci. 2010;33:269–98. 10.1146/annurev.neuro.051508.135409 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Cisek P: Making decisions through a distributed consensus. Curr Opin Neurobiol. 2012;22(6):927–36. 10.1016/j.conb.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 77. Hunt LT, Hayden BY: A distributed, hierarchical and recurrent framework for reward-based choice. Nat Rev Neurosci. 2017;18(3):172–182. 10.1038/nrn.2017.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yoo SBM, Hayden BY: Economic Choice as an Untangling of Options into Actions. Neuron. 2018;99(3):434–447. 10.1016/j.neuron.2018.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Van Hoesen GW, Morecraft RJ, Vogt BA: Connections of the monkey cingulate cortex. Neurobiology of Cingulate Cortex and Limbic Thalamus.See Vogt & Gabriel.1993;249–84. 10.1007/978-1-4899-6704-6_9 [DOI] [Google Scholar]
- 80. Paus T: Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2(6):417–24. 10.1038/35077500 [DOI] [PubMed] [Google Scholar]
- 81. Eisenreich BR, Akaishi R, Hayden BY: Control without Controllers: Toward a Distributed Neuroscience of Executive Control. J Cogn Neurosci. 2017;29(10):1684–1698. 10.1162/jocn_a_01139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Azab H, Hayden BY: Correlates of decisional dynamics in the dorsal anterior cingulate cortex. PLoS Biol. 2017;15(11):e2003091. 10.1371/journal.pbio.2003091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Azab H, Hayden BY: Correlates of economic decisions in the dorsal and subgenual anterior cingulate cortices. Eur J Neurosci. 2018;47(8):979–993. 10.1111/ejn.13865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hunt LT, Malalasekera WMN, de Berker AO, et al. : Triple dissociation of attention and decision computations across prefrontal cortex. Nat Neurosci. 2018;21(10):1471–1481. 10.1038/s41593-018-0239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Matsumoto M, Matsumoto K, Abe H, et al. : Medial prefrontal cell activity signaling prediction errors of action values. Nat Neurosci. 2007;10(5):647–56. 10.1038/nn1890 [DOI] [PubMed] [Google Scholar]
- 86. Quilodran R, Rothé M, Procyk E: Behavioral shifts and action valuation in the anterior cingulate cortex. Neuron. 2008;57(2):314–25. 10.1016/j.neuron.2007.11.031 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Kennerley SW, Walton ME, Behrens TE, et al. : Optimal decision making and the anterior cingulate cortex. Nat Neurosci. 2006;9(7):940–7. 10.1038/nn1724 [DOI] [PubMed] [Google Scholar]
- 88. Strait CE, Sleezer BJ, Blanchard TC, et al. : Neuronal selectivity for spatial positions of offers and choices in five reward regions. J Neurophysiol. 2016;115(3):1098–111. 10.1152/jn.00325.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fries P: A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474–80. 10.1016/j.tics.2005.08.011 [DOI] [PubMed] [Google Scholar]
- 90. Fries P: Rhythms for Cognition: Communication through Coherence. Neuron. 2015;88(1):220–35. 10.1016/j.neuron.2015.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Voytek B, Kayser AS, Badre D, et al. : Oscillatory dynamics coordinating human frontal networks in support of goal maintenance. Nat Neurosci. 2015;18(9):1318–24. 10.1038/nn.4071 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 92. Schalk G: A general framework for dynamic cortical function: the function-through-biased-oscillations (FBO) hypothesis. Front Hum Neurosci. 2015;9:352. 10.3389/fnhum.2015.00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hahn G, Ponce-Alvarez A, Deco G, et al. : Portraits of communication in neuronal networks. Nat Rev Neurosci. 2019;20(2):117–127. 10.1038/s41583-018-0094-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Bastos AM, Schoffelen JM: A Tutorial Review of Functional Connectivity Analysis Methods and Their Interpretational Pitfalls. Front Syst Neurosci. 2016;9:175. 10.3389/fnsys.2015.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nadalin J, Martinet LE, Lo MC, et al. : A statistical modeling framework to assess cross-frequency coupling while accounting for confounding effects. bioRxiv. 2019. 10.1101/519470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Colgin LL: Rhythms of the hippocampal network. Nat Rev Neurosci. 2016;17(4):239–49. 10.1038/nrn.2016.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lisman JE, Jensen O: The θ-γ neural code. Neuron. 2013;77(6):1002–16. 10.1016/j.neuron.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Haegens S, Zion Golumbic E: Rhythmic facilitation of sensory processing: A critical review. Neurosci Biobehav Rev. 2018;86:150–165. 10.1016/j.neubiorev.2017.12.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 99. Miller EK, Lundqvist M, Bastos AM: Working Memory 2.0. Neuron. 2018;100(2):463–475. 10.1016/j.neuron.2018.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 100. Helfrich RF, Knight RT: Oscillatory Dynamics of Prefrontal Cognitive Control. Trends Cogn Sci. 2016;20(12):916–930. 10.1016/j.tics.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cavanagh JF, Frank MJ: Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18(8):414–21. 10.1016/j.tics.2014.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 102. Cohen MX: A neural microcircuit for cognitive conflict detection and signaling. Trends Neurosci. 2014;37(9):480–90. 10.1016/j.tins.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 103. Cohen MX: Midfrontal theta tracks action monitoring over multiple interactive time scales. NeuroImage. 2016;141:262–272. 10.1016/j.neuroimage.2016.07.054 [DOI] [PubMed] [Google Scholar]
- 104. Smith EH, Banks GP, Mikell CB, et al. : Frequency-Dependent Representation of Reinforcement-Related Information in the Human Medial and Lateral Prefrontal Cortex. J Neurosci. 2015;35(48):15827–36. 10.1523/JNEUROSCI.1864-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Provenza NR, Paulk AC, Peled N, et al. : Decoding task engagement from distributed network electrophysiology in humans. J Neural Eng. 2019;16(5):056015. 10.1088/1741-2552/ab2c58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ezzyat Y, Wanda PA, Levy DF, et al. : Closed-loop stimulation of temporal cortex rescues functional networks and improves memory. Nat Commun. 2018;9(1):365. 10.1038/s41467-017-02753-0 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 107. Hampson RE, Song D, Robinson BS, et al. : Developing a hippocampal neural prosthetic to facilitate human memory encoding and recall. J Neural Eng. 2018;15(3):036014. 10.1088/1741-2552/aaaed7 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 108. Saez I, Lin J, Stolk A, et al. : Encoding of Multiple Reward-Related Computations in Transient and Sustained High-Frequency Activity in Human OFC. Curr Biol. 2018;28(18):2889–2899.e3. 10.1016/j.cub.2018.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 109. Widge AS, Ellard KK, Paulk AC, et al. : Treating refractory mental illness with closed-loop brain stimulation: Progress towards a patient-specific transdiagnostic approach. Exp Neurol. 2017;287(Pt 4):461–472. 10.1016/j.expneurol.2016.07.021 [DOI] [PubMed] [Google Scholar]
- 110. Parvizi J, Rangarajan V, Shirer WR, et al. : The will to persevere induced by electrical stimulation of the human cingulate gyrus. Neuron. 2013;80(6):1359–67. 10.1016/j.neuron.2013.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Widge AS, Miller EK: Targeting Cognition and Networks Through Neural Oscillations: Next-Generation Clinical Brain Stimulation. JAMA Psychiatry. 2019;76(7):671–672. 10.1001/jamapsychiatry.2019.0740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Blackwood E, Lo MC, Alik Widge S: Continuous Phase Estimation for Phase-Locked Neural Stimulation Using an Autoregressive Model for Signal Prediction. Conf Proc IEEE Eng Med Biol Soc. 2018;2018:4736–4739. 10.1109/EMBC.2018.8513232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zanos S, Rembado I, Chen D, et al. : Phase-Locked Stimulation during Cortical Beta Oscillations Produces Bidirectional Synaptic Plasticity in Awake Monkeys. Curr Biol. 2018;28(16):2515–2526.e4. 10.1016/j.cub.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation