Abstract
Background
Comorbidity is common among patients with myocardial infarction (MI). We examined whether comorbidity level modified the single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI)-based prediction of 5-year risk of MI and all-cause death in patients with MI.
Methods
This cohort study included patients with prior MI having a SPECT MPI at Aarhus University Hospital, Denmark, 1999–2011. Using nationwide registries, we obtained information on comorbidity levels (low, moderate, and severe) and outcomes. We computed risk and hazard ratios (HRs) with 95% confidence intervals (CIs) for MI and all-cause death, comparing normal (no defects) versus abnormal scan (reversible and/or fixed defects) using Cox regression adjusting for sex, age, and comorbidity level.
Results
We identified 1,192 patients with MI before SPECT MPI. The 5-year risk for patients with normal versus abnormal scans were 11.7% versus 18.3% for MI, and 8.0% versus 13.2% for all-cause death, respectively. The overall 5-year adjusted HR (aHR) of MI was 1.56 (95% CI: 1.09–2.21), 1.33 (95% CI: 0.82–2.15) with low comorbidity, 1.39 (95% CI: 0.68–2.83) with moderate comorbidity, and 2.53 (95% CI: 1.14–5.62) with severe comorbidity. Similarly, the 5-year aHR for all-cause death was 1.39 (95% CI: 0.90–2.14) overall; 2.33 (95% CI: 0.79–6.84) with low comorbidity, 2.05 (95% CI: 0.69–6.06) with moderate comorbidity, and 1.07 (95% CI: 0.64–1.80) with severe comorbidity.
Conclusion
We conclude that comorbidity level may modify the 5-year risk prediction associated with an abnormal SPECT MPI scan in patients with previous MI.
Keywords: comorbidity, epidemiology, myocardial infarction, myocardial perfusion imaging
Introduction
Single-photon emission computed tomography myocardial perfusion imaging (SPECT MPI) is a non-invasive procedure for risk stratification after myocardial infarction (MI).1,2 With increasing age, the number of patients with chronic disorders are increased.3 Concurrently, life expectancy among MI patients has increased during recent decades due to improvements in treatment and rehabilitation.4,5 Consequently, more MI patients have other chronic diseases, i.e comorbidities.6 The prevalence of comorbidity among MI patients may be increased further due to shared risk factors between cardiovascular disease and other diseases, such as diabetes.7
It has previous been shown that comorbidity level did not affect substantially the association between SPECT MPI scan result and the risk of MI and all-cause death in patients without previous MI or cerebrovascular disease.8 Despite the common presence of comorbid conditions in MI patients, the impact of comorbidity level on the prognosis following a normal versus abnormal SPECT MPI scan in MI patients has to our knowledge not previously been examined. We therefore examined the SPECT MPI-predicted 5-year risk of MI and all-cause death following a normal versus abnormal SPECT MPI scan in patients with previous MI, both overall and according to comorbidity level.
Methods
Setting
The Danish National Health Service provides free universal tax-supported healthcare, guaranteeing unfettered access to general practitioners and hospitals.9 Using the unique 10-digit Civil Personal Register number assigned to each individual who was born in or immigrated to Denmark, it is possible to link all Danish medical registries at the individual level.10
Study population
We conducted this cohort study in Denmark using the Aarhus University Hospital Myocardial Perfusion Imaging (AUH-MPI) Database.8 According to a previously published paper, this database has collected information on all 99mTc-sestamibi SPECT MPI procedures performed at Aarhus University Hospital, Skejby, Denmark from January 1999 until April 2011, and contains information on sex, age, and scan result.8 We used the AUH-MPI Database to identify all adult Danish patients (over the age of 18 years) who had a 99mTc-sestamibi SPECT MPI procedure performed between January 1999 and April 2011. To be eligible for inclusion in this study, patients should have a first-time MI diagnosis within 5 years before the SPECT MPI procedure date, as ascertained from the Danish National Patient Registry (DNPR). The DNPR has recorded information on patients discharged from all non-psychiatric hospitals in Denmark since 1977 and from emergency room and outpatient clinic visits since 1995.11 One primary diagnosis and up to 19 secondary diagnoses are provided for each hospital discharge or outpatient clinical visit, classified according to the International Classification of Diseases, 8th Revision (ICD-8) until the end of 1993 and 10th Revision (ICD-10) thereafter.11 We considered only the first SPECT MPI procedure in the study period for each patient.
The SPECT MPI procedure is performed by combining a stress and a rest scan to evaluate any signs of reversibility, irreversibility, or a combination of both. Stress is applied either physically on an ergometer bicycle or pharmacologically with vasodilating (adenosine or dipyridamole) or beta stimulating (dobutamine) drugs. A normal scan was defined as a scan without defects, and an abnormal scan was defined by the presence of a reversible and/or fixed defect.8 We further subdivided an abnormal scan into 3 groups: a reversible defect, a fixed defect, and a combined defect (reversible and fixed defect).
Outcomes
We used the DNPR11 to identify all inpatient admissions and outpatient clinic contacts with a primary or secondary diagnosis of MI following a SPECT MPI procedure during the study period.
We used the Danish Civil Registration System10 to obtain information on all-cause mortality. This registry has recorded information on date of birth, residence, date of emigration, and exact date of any death for the entire Danish population since 1968, with daily electronic updates.10
Comorbidity
We obtained information on comorbid conditions from inpatient and outpatient hospital diagnoses recorded in the DNPR11 between 1977 and the date of the SPECT MPI procedure. The severity of comorbidity was categorized using Charlson Comorbidity Index (CCI) scores.12 CCI scores have been found to be a valid method for measuring comorbidity,13 also in MI patients.14 We computed the CCI score by summing the weight of the remaining 18 conditions included in the CCI after excluding MI. We then categorized the study population according to the following comorbidity levels: score of 0 (low comorbidity), score of 1 (moderate comorbidity), and score ≥2 (severe comorbidity).
Statistical analysis
We used descriptive statistics to characterize the study population undergoing SPECT MPI according to sex, age, comorbidity level, and cardiovascular morbidity (congestive heart failure, peripheral vascular disease, cerebrovascular disease, and diabetes). Follow-up began on the date of the SPECT MPI procedure or discharge from the hospital admission during which the SPECT MPI procedure was performed. Follow-up continued until the date of MI, death, emigration, 5 years of follow-up, or November 15, 2013, whichever came first.
We used a cumulative incidence method to calculate the absolute risk and risk difference of MI and all-cause death within 30 days (including 0 days and 30 days), 31−365 days (including 31 days, and excluding 365 days), 1−5 years (including 1 year, and excluding 5 years), and 5 years overall following SPECT MPI, and illustrated graphically the risk for all-cause death. Death was considered a competing risk in the analyses of MI.
We assessed the impact of comorbidity on risk of MI and all-cause death in MI patients following a normal versus abnormal SPECT MPI scan by stratifying the analyses by comorbidity level. We used Cox proportional hazards regression to compute 5-year hazard ratios (HRs) with 95% confidence intervals (CIs) for each outcome, comparing an abnormal scan with a normal scan, and adjusting for categories of sex, age (18−49 years, 50−59 years, 60−69 years, and ≥70 years), and comorbidity level (low, moderate, and severe comorbidity).
In addition, we conducted 3 sensitivity analyses. The first analysis restricted the outcome to MI diagnoses during an acute inpatient admission. The second and third analyses were restricted to patients diagnosed with a first-time MI 0–1 year and >1–5 years before the SPECT MPI procedure, respectively, to examine the consistency of associations according to time from infarction. The proportional hazards assumption was assessed graphically by log(-log)-plots and found valid within the 0–5-year period. The ICD codes for conditions included in the CCI and for MI are provided in Table S1 and S2. All statistical analyses were conducted using Stata software 12.1 (StataCorp LP, College Station, Texas, USA).
Table S1.
Diagnosis codes according to the International Classification of Diseases, 8th (ICD-8) and 10th revision (ICD-10)
| Disease | Weight | ICD-8 codes | ICD-10 codes |
|---|---|---|---|
| Myocardial infarction | 1 | 410 | I21, I22, I23 |
| Congestive heart failure | 1 | 427.09, 427.10, 427.11, 427.19, 428.99, 782.49 | I50, I11.0, I13.0, I13.2 |
| Peripheral vascular disease | 1 | 440–445 | I70–I74, I77 |
| Cerebrovascular disease | 1 | 430–438 | I60–I69, G45, G46 |
| Dementia | 1 | 290.09–290.19, 293.09 | F00–F03, F05.1, G30 |
| Chronic pulmonary disease | 1 | 490–493, 515–518 | J40–J47, J60–J67, J68.4, J70.1, J70.3, J84.1, J92.0, J96.1, J98.2, J98.3 |
| Connective tissue disease | 1 | 712, 716, 734, 446, 135.99 | M05, M06, M08, M09, M30–M36, D86 |
| Ulcer disease | 1 | 530.91, 530.98, 531–534 | K22.1, K25–K28 |
| Mild liver disease | 1 | 571, 573.01, 573.04 | B18, K70.0–K70.3, K70.9, K71, K73, K74, K76.0 |
| Diabetes I and II | 1 | 249.00, 249.06, 249.07, 249.09, 250.00, 250.06, 250.07, 250.09 | E10.0, E10.1, E10.9, E11.0, E11.1, E11.9 |
| Hemiplegia | 2 | 334 | G81, G82 |
| Moderate to severe kidney disease | 2 | 403, 404, 580–584, 590.09, 593.19, 753.10–753.19, 792 | I12, I13, N00–N05, N07, N11, N14, N17–N19, Q61 |
| Diabetes with end organ damage | 2 | 249.01–249.05, 249.08, 250.01–250.05, 250.08 | E10.2–E10.8, E11.2–E11.8 |
| Any tumor | 2 | 140–194 | C00–C75 |
| Leukemia | 2 | 204–207 | C91–C95 |
| Lymphoma | 2 | 200–203, 275.59 | C81–C85, C88, C90, C96 |
| Moderate to severe liver disease | 3 | 070.00, 070.02, 070.04, 070.06, 070.08, 573.00, 456.00–456.09 | B15.0, B16.0, B16.2, B19.0, K70.4, K72, K76.6, I85 |
| Metastatic solid tumor | 6 | 195–199 | C76–C80 |
| AIDS | 6 | 079.83 | B21–B24 |
Table S2.
Diagnosis codes for myocardial infarction according to the International Classification of Diseases, 8th (ICD-8) and 10th (ICD-10) revision
| Myocardial infarction | ICD-8 codes | ICD-10 codes |
|---|---|---|
| Prior to the scan | 410 | I21, I22, I23 |
| Outcome | 410 | I21 |
Ethics statement
The study was approved by the Danish Data Protection Agency (record number 2015-57-0002; Aarhus University record number 2016-051-000001-509).
Results
Patient characteristics
We identified 1,192 patients with a first-time MI within 5 years before the SPECT MPI. Among these, 362 patients (30%) had a normal scan and 830 patients (70%) had an abnormal scan (Table 1). Their sex and age distributions are shown in Table 1. A total of 152 patients had reversible scan defects, 403 patients had fixed scan defects, and 275 patients had combined scan defects. Among patients with a normal scan, 54% had a low comorbidity level, and among patients with an abnormal scan, 44% had a low comorbidity level. The prevalence of patients with moderate comorbidity was nearly equally distributed among patients with normal and abnormal scans (25% versus 26%). Patients with a normal scan were less likely to have severe comorbidity than patients with an abnormal scan (21% versus 30%) (Table 1). Patients with a normal scan also were less likely to have had congestive heart failure than patients with an abnormal scan (9% versus 21%). The prevalence of peripheral vascular disease was very similar among patients with normal and abnormal scans (8% versus 9%) (Table 1). The prevalence of cerebrovascular disease and diabetes was lower among patients with a normal scan than among patients with an abnormal scan (8% versus 12%, and 12% versus 15%, respectively) (Table 1).
Table 1.
Patients with normal and abnormal scans by sex, age, comorbidity level, and cardiovascular morbidity
| Characteristics | Normal scan (n=362) | Abnormal scan (n=830) | Total (n=1,192) |
|---|---|---|---|
| Sex | |||
| Female | 165 (46%) | 233 (28%) | 398 (33%) |
| Male | 197 (54%) | 597 (72%) | 794 (67%) |
| Age (years) | |||
| 18−49 | 69 (19%) | 149 (18%) | 218 (18%) |
| 50−59 | 119 (33%) | 236 (28%) | 355 (30%) |
| 60−69 | 95 (26%) | 245 (30%) | 340 (29%) |
| ≥70 | 79 (22%) | 200 (24%) | 279 (23%) |
| Median age (years) | 59 | 60 | 60 |
| Comorbidity level* | |||
| Low | 195 (54%) | 369 (44%) | 564 (47%) |
| Moderate | 89 (25%) | 215 (26%) | 304 (26%) |
| Severe | 78 (21%) | 246 (30%) | 324 (27%) |
| Cardiovascular morbidity | |||
| Congestive heart failure | 34 (9%) | 171 (21%) | 205 (17%) |
| Peripheral vascular disease | 30 (8%) | 78 (9%) | 108 (9%) |
| Cerebrovascular disease | 30 (8%) | 97 (12%) | 127 (11%) |
| Diabetes | 43 (12%) | 126 (15%) | 169 (14%) |
Note: Included are patients with a first-time myocardial infarction within 5 years before the scan. *Levels of comorbidity were based on Charlson Comorbidity Index scores as follows: 0 (low), 1 (moderate), and ≥2 (severe).
The median time from MI to SPECT MPI within the study cohort was 0.9 years (interquartile range (IQR): 0.3 years to 2.2 years).
Outcomes
Within 5 years after SPECT MPI, 190 MIs and 131 all-cause deaths occurred in the cohort (Table 2). Median follow-up time for all-cause death was 5 years (IQR: 4.6 years to 5 years), and for MI the median follow-up time was 5 years (IQR: 3.5 years to 5 years).
Table 2.
Risk and hazard ratio of MI and all-cause death within 5 years following a normal versus abnormal scan, overall and according to comorbidity level
| Outcome | Scan result | No. of events | Risk % (95% CI) | Risk difference % (95% CI) | Hazard ratio (95% CI) | |
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted* | |||||
| Overall | ||||||
| MI | Normal | 41 | 11.7 (8.5 – 15.5) | 6.6 (2.1 – 11.1) | 1 (reference) | 1 (reference) |
| Abnormal | 149 | 18.3 (15.7 – 21.0) | 1.64 (1.16 – 2.31) | 1.56 (1.09 – 2.21) | ||
| All-cause death | Normal | 27 | 8.0 (5.5 – 11.5) | 5.2 (1.4 – 8.9) | 1 (reference) | 1 (reference) |
| Abnormal | 104 | 13.2 (11.0 – 15.7) | 1.66 (1.09 – 2.54) | 1.39 (0.90 – 2.14) | ||
| Low comorbidity (0 points) | ||||||
| MI | Normal | 24 | 12.6 (8.1 – 18.1) | 4.5 (−1.9 – 10.9) | 1 (reference) | 1 (reference) |
| Abnormal | 62 | 17.1 (13.4 – 21.2) | 1.41 (0.88 – 2.25) | 1.33 (0.82 – 2.15) | ||
| All-cause death | Normal | 4 | 2.1 (0.8 – 5.4) | 3.7 (0.5 – 6.8) | 1 (reference) | 1 (reference) |
| Abnormal | 20 | 5.7 (3.7 – 8.8) | 2.64 (0.90 – 7.72) | 2.33 (0.79 – 6.84) | ||
| Moderate comorbidity (1 point) | ||||||
| MI | Normal | 10 | 12.0 (6.1 – 20.0) | 4.6 (−4.2 – 13.3) | 1 (reference) | 1 (reference) |
| Abnormal | 35 | 16.5 (11.9 – 21.8) | 1.45 (0.72 – 2.93) | 1.39 (0.68 – 2.83) | ||
| All-cause death | Normal | 4 | 5.2 (2.0 – 13.5) | 5.1 (−1.5 – 11.6) | 1 (reference) | 1 (reference) |
| Abnormal | 21 | 10.3 (6.8 – 15.4) | 2.04 (0.70 – 5.96) | 2.05 (0.69 – 6.06) | ||
| Severe comorbidity (≥2 points) | ||||||
| MI | Normal | 7 | 9.0 (4.0 – 16.7) | 12.6 (3.8 – 21.4) | 1 (reference) | 1 (reference) |
| Abnormal | 52 | 21.6 (16.7 – 27.1) | 2.48 (1.13 – 5.45) | 2.53 (1.14 – 5.62) | ||
| All-cause death | Normal | 19 | 25.9 (17.3 – 37.7) | 0.8 (−10.9 – 12.4) | 1 (reference) | 1 (reference) |
| Abnormal | 63 | 26.6 (21.5 – 32.8) | 1.03 (0.62 – 1.72) | 1.07 (0.64 – 1.80) | ||
Note: Included are patients with a first-time MI within 5 years before the scan. *Adjusted for sex, age, and comorbidity level in the overall analyses, and sex and age in the analyses stratified on comorbidity level.
Abbreviations: CI, confidence interval; MI, myocardial infarction.
Five-year risk of MI was 11.7% (95% CI: 8.5–15.5) among patients with normal scans, and 18.3% (95% CI: 15.7–21.0) among patients with abnormal scans (Table 2). The corresponding adjusted HR (aHR) in the 5-year period was 1.56 (95% CI: 1.09–2.21) (Table 2). The overall 5-year risk difference is shown in Table 2. Risk estimates within 0–30 days, 31–365 days, and 1–5 years following SPECT MPI are provided in Table 3. Sensitivity analyses restricted to the 112 MI diagnoses during an acute admission supported the overall result, with aHR of 1.35 (95% CI: 0.86–2.12) (Table 4). Using a normal scan as the reference, the aHR for the 5-year period was 1.22 (95% CI: 0.72–2.07) for a reversible defect, 1.58 (95% CI: 1.07–2.33) for a fixed defect, and 1.72 (95% CI: 1.14–2.61) for a combined defect (Table 5). The sensitivity analyses that examined the consistency of associations according to time from MI to SPECT MPI supported the overall result with an aHR of 1.53 (95% CI: 0.95–2.47) for MI patients whose infarction occurred 0–1 year before SPECT MPI (Table S3), and 1.57 (95% CI: 0.93–2.65) for MI patients whose infarction occurred >1–5 years before SPECT MPI (Table S4).
Table 3.
30-day, 31−365-day, and 1−5-year risk of MI and all-cause death following a normal versus abnormal scan
| Time intervals | Scan result | No. of events | Risk % (95% CI) | Risk difference % (95% CI) |
|---|---|---|---|---|
| 0−30 days | ||||
| MI | Normal | 7 | 1.7 (0.6 – 3.6) | −0.1 (−1.8 – 1.6) |
| Abnormal | 14 | 1.6 (0.9 – 2.6) | ||
| All-cause death | Normal | 1 | 0.3 (0.0 – 1.9) | −0.2 (−0.8 – 0.4) |
| Abnormal | 1 | 0.1 (0.0 – 0.9) | ||
| 31−365 days | ||||
| MI | Normal | 15 | 4.0 (2.2 – 6.5) | 3.3 (0.5 – 6.1) |
| Abnormal | 60 | 7.2 (5.6 – 9.2) | ||
| All-cause death | Normal | 6 | 1.7 (0.8 – 3.7) | 0.9 (−0.8 – 2.6) |
| Abnormal | 21 | 2.5 (1.7 – 3.9) | ||
| 1−5 years | ||||
| MI | Normal | 19 | 6.5 (4.0 – 9.7) | 4.1 (0.3 – 7.9) |
| Abnormal | 75 | 10.6 (8.4 – 13.0) | ||
| All-cause death | Normal | 20 | 6.2 (4.0 – 9.5) | 4.6 (1.1 – 8.0) |
| Abnormal | 82 | 10.8 (8.8 – 13.2) | ||
Note: Included are patients with a first-time MI within 5 years before the scan.
Abbreviations: CI, confidence interval; MI, myocardial infarction.
Table 4.
Risk and hazard ratio of MI registered during an acute admission within 5 years following a normal versus abnormal scan
| Outcome | Scan result | No. of events | Risk % (95% CI) | Hazard ratio (95% CI) | |
|---|---|---|---|---|---|
| Unadjusted | Adjusted* | ||||
| MI | Normal | 25 | 7.0 (4.6 – 10.2) | 1 (reference) | 1 (reference) |
| Abnormal | 87 | 10.8 (8.8 – 13.1) | 1.54 (0.98 – 2.40) | 1.35 (0.86 – 2.12) | |
Note: Included are patients with a first-time MI within 5 years before the scan. *Adjusted for sex, age, and comorbidity level.
Abbreviations: CI, confidence interval; MI, myocardial infarction.
Table 5.
Risk and hazard ratio of MI and all-cause death within 5 years following a normal scan, a reversible defect, a fixed defect, and a combined defect. Included are patients with a first-time MI within 5 years before the scan
| Outcome | Scan result | No. of events | Risk % (95% CI) | Hazard ratio (95% CI) | |
|---|---|---|---|---|---|
| Unadjusted | Adjusted* | ||||
| MI | Normal | 41 | 11.7 (8.5 – 15.5) | 1 (reference) | 1 (reference) |
| Reversible defect | 21 | 14.1 (9.1 – 20.2) | 1.25 (0.74 – 2.11) | 1.22 (0.72 – 2.07) | |
| Fixed defect | 74 | 18.4 (14.7 – 22.4) | 1.67 (1.14 – 2.44) | 1.58 (1.07 – 2.33) | |
| Combined defect | 54 | 20.1 (15.5 – 25.1) | 1.81 (1.21 – 2.72) | 1.72 (1.14 – 2.61) | |
| All-cause death | Normal | 27 | 8.0 (5.5 – 11.5) | 1 (reference) | 1 (reference) |
| Reversible defect | 14 | 9.7 (5.9 – 15.9) | 1.21 (0.64 – 2.31) | 0.97 (0.51 – 1.85) | |
| Fixed defect | 49 | 12.6 (9.7 – 16.3) | 1.61 (1.00 – 2.57) | 1.41 (0.87 – 2.27) | |
| Combined defect | 41 | 15.9 (11.9 – 21.0) | 2.00 (1.23 – 3.25) | 1.61 (0.99 – 2.64) | |
Note: *Adjusted for sex, age, and comorbidity level.
Abbreviations: CI, confidence interval; MI, myocardial infarction.
Table S3.
Risk and hazard ratio of MI and all-cause death within 5 years following a normal versus abnormal scan
| Hazard ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Outcome | Scan result | No. of events | Risk % (95% CI) | Risk difference % (95% CI) | Unadjusted | Adjusted* |
| MI | Normal | 22 | 11.4 (7.2 – 16.7) | 8.1 (1.9 – 14.2) | 1 (reference) | 1 (reference) |
| Abnormal | 85 | 19.4 (15.9 – 23.3) | 1.64 (1.03 – 2.62) | 1.53 (0.95 – 2.47) | ||
| All-cause death | Normal | 14 | 7.9 (4.7 – 13.0) | 3.9 (−1.1 – 8.9) | 1 (reference) | 1 (reference) |
| Abnormal | 50 | 11.8 (9.1 – 15.3) | 1.48 (0.82 – 2.67) | 1.19 (0.65 – 2.17) | ||
Note: Included are patients with a first-time MI 0−1 year before the scan. *Adjusted for sex, age, and comorbidity level.
Abbreviations: CI, confidence interval; MI, myocardial infarction.
Table S4.
Risk and hazard ratio of MI and all-cause death within 5 years following a normal versus abnormal scan.
| Hazard ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Outcome | Scan result | No. of events | Risk % (95% CI) | Risk difference % (95% CI) | Unadjusted | Adjusted* |
| MI | Normal | 19 | 11.4 (6.8 – 17.2) | 5.6 (−1.1 – 12.3) | 1 (reference) | 1 (reference) |
| Abnormal | 64 | 17.0 (13.4 – 21.0) | 1.62 (0.97 – 2.70) | 1.57 (0.93 – 2.65) | ||
| All-cause death | Normal | 13 | 8.2 (4.8 – 13.7) | 6.6 (0.9 – 12.2) | 1 (reference) | 1 (reference) |
| Abnormal | 54 | 14.8 (11.5 – 18.8) | 1.88 (1.03 – 3.45) | 1.56 (0.84 – 2.89) | ||
Note: Included are patients with a first-time MI >1−5 years before the scan. *Adjusted for sex, age, and comorbidity level.
Abbreviations: CI, confidence interval; MI, myocardial infarction.
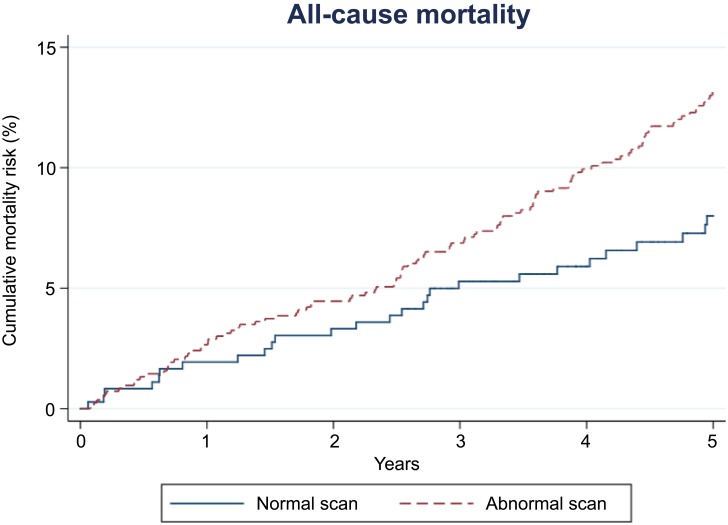
Patients with a normal scan had a 5-year mortality risk of 8.0% (95% CI: 5.5–11.5). In patients with an abnormal scan, this risk was 13.2% (95% CI: 11.0–15.7) (Table 2). The corresponding aHR was 1.39 (95% CI: 0.90–2.14) (Table 2). The cumulative mortality risk is provided in Figure 1. The overall 5-year risk difference is shown in Table 2, and risk estimates within 0–30 days, 31–365 days, and 1–5 years following SPECT MPI are provided in Table 3.
Figure 1.
Estimated cumulative incidence of all-cause mortality risk among patients with normal and abnormal scans.
Using a normal scan as reference, the aHR was 0.97 (95% CI: 0.51–1.85) for a reversible defect, 1.41 (95% CI: 0.87–2.27) for a fixed defect, and 1.61 (95% CI: 0.99–2.64) for a combined defect (Table 5). No substantial differences were seen in the aHRs in sensitivity analyses restricted to patients whose previous MI occurred 0–1 year before SPECT MPI (aHR =1.19; 95% CI: 0.65–2.17) (Table S3) or >1–5 years before SPECT MPI (aHR =1.56; 95% CI: 0.84–2.89) (Table S4), compared to the overall results.
Comorbidity
For all 3 levels of comorbidity, an abnormal scan was associated with an increased 5-year risk of MI compared with a normal scan, as well as considerable risk differences between patients with a normal versus abnormal scan (Table 2). Five-year aHRs comparing an abnormal scan with a normal scan were 1.33 (95% CI: 0.82–2.15) among patients with low comorbidity, 1.39 (95% CI: 0.68–2.83) among patients with moderate comorbidity, and 2.53 (95% CI: 1.14–5.62) among patients with severe comorbidity (Table 2).
Compared with a normal scan, an abnormal scan was associated with an increased 5-year risk of all-cause death among patients with all 3 levels of comorbidity (Table 2). Risk differences according to comorbidity levels were 3.7% (95% CI: 0.5–6.8) for low comorbidity, 5.1% (95% CI: -1.5–11.6) for moderate comorbidity, and 0.8% (95% CI: -10.9–12.4) for severe comorbidity (Table 2). Comparing an abnormal scan with a normal scan, the 5-year aHR was 2.33 (95% CI: 0.79–6.84) among patients with low comorbidity, 2.05 (95% CI: 0.69–6.06) among patients with moderate comorbidity, and 1.07 (95% CI: 0.64–1.80) among patients with severe comorbidity (Table 2).
Discussion
In this cohort study of patients with previous MI, we found that comorbidity level may modify the 5-year risk prediction of an abnormal SPECT MPI scan for MI and all-cause death. The impact of an abnormal scan was strongest for MI in patients with severe comorbidity. For all-cause death, the impact of an abnormal scan was strongest in patients with low and moderate comorbidity.
An important study strength was access to a study population in a setting of a universal free tax-supported health care system. All Danish citizens have universal and free access to general practitioners and hospitals, which minimizes selection bias. Another study strength is complete follow-up of all patients until the date of an outcome, emigration, or end of follow-up.11 Information about the SPECT MPI scan result was obtained before the information on the outcomes. Furthermore, recorded diagnoses in the DNPR are likely to be independent of the SPECT MPI scan result. Any potential misclassification therefore would be non-differential and cannot explain the increased aHRs for MI and all-cause death.
We cannot entirely rule out that some patients with a SPECT MPI scan in the study period were not registered in the AUH-MPI Database. However, we assume that the association between SPECT MPI and outcomes among unregistered patients would not differ from those among registered patients. This should, therefore, not have influenced our results.
The positive predictive values of MI diagnoses and comorbidities included in the CCI have been reported to be high in the DNPR; 97% for first-time MI,15 98% for CCI comorbidities,16 and 88% for an inpatient diagnosis of recurrent MI.15 Mortality data from the Danish Civil Personal Registration System are complete.10 We controlled for other potential predictors (sex, age, and comorbidity level). However, it is possible that other unmeasured variables, such as smoking, hypertension, and obesity, influenced the results. Nevertheless, our primary aim was to assess the predictive ability of SPECT MPI, rather than to examine a possibly causal relation, in which confounding would have been an issue.
Our study was of limited size. Thus, some of our estimates were imprecise and with broad CIs, and we cannot rule out that some of our findings are due to chance. Finally, as we only included MI patients with a SPECT MPI scan, we cannot generalize our findings to MI patients without a SPECT MPI scan.
To our knowledge, no other studies have examined the risk of MI and all-cause death as separate outcomes following an abnormal versus normal SPECT MPI scan in MI patients, but previous studies have examined the risk of MI or death as combined endpoints after MI following SPECT.17,18 A study by Jain et al17 examined the risk of death or reinfarction following dipyridamole thallium testing 3 to 21 days after acute MI with a median follow-up of 18 months in 73 patients aged 65 years and older (mean age 75 years). The authors reported that reversible ischemia was a predictor of death or reinfarction, with a relative risk of 2.51 (95% CI: 1.05–5.96). Stratmann et al18 examined the risk of late cardiac events (non-fatal MI or cardiac death) in 133 men hospitalized with an acute MI who had technetium-99m sestamibi SPECT before hospital discharge. The mean time to a cardiac event after testing was 19 months, and in those patients without an event, follow-up was 39 months. The study found that an isolated fixed defect was a predictor of increased risk for late cardiac events (relative risk of 2.1; 95% CI: 1.1–4.3).
These above-mentioned studies used combined endpoints. Therefore, their results are not completely comparable with our study’s results.
The impact of comorbidity level on risk of MI and all-cause death following a normal versus abnormal SPECT MPI scan has to our knowledge not previously been investigated in patients with MI. Rather, earlier studies examined the risk of adverse events following a normal versus abnormal SPECT MPI in patients with selected chronic conditions, including diabetes, obesity, and end-stage renal disease.19–21 These studies looked at specific chronic conditions rather than different comorbidity levels, and did not restrict their study population to previous MI patients. Their results are therefore not comparable with ours.
Conclusion
In conclusion, comorbidity level may modify the 5-year risk prediction of an abnormal SPECT MPI scan in patients with previous MI. The impact of an abnormal scan was strongest for MI in patients with severe comorbidity, and for all-cause death in patients with low and moderate comorbidity. When SPECT MPI is used as a prognostic tool, the difference in its predictive value across comorbidity levels should be considered.
Supplementary materials
Acknowledgments
The authors acknowledge Professor Henrik Toft Sørensen from the Department of Clinical Epidemiology, Aarhus University, for providing valuable comments to the manuscript.
Disclosure
The Department of Clinical Epidemiology is involved in studies with funding from various companies as research grants to (and administered by) Aarhus University. The authors report no other conflicts of interest in this work.
Supplementary materials
References
- 1.Brown KA, Heller GV, Landin RS, et al. Early dipyridamole (99m)Tc-sestamibi single photon emission computed tomographic imaging 2 to 4 days after acute myocardial infarction predicts in-hospital and postdischarge cardiac events: comparison with submaximal exercise imaging. Circulation. 1999;100(20):2060–2066. doi: 10.1161/01.cir.100.20.2060 [DOI] [PubMed] [Google Scholar]
- 2.Mahmarian JJ, Shaw LJ, Filipchuk NG, et al. A multinational study to establish the value of early adenosine technetium-99m sestamibi myocardial perfusion imaging in identifying a low-risk group for early hospital discharge after acute myocardial infarction. J Am Coll Cardiol. 2006;48(12):2448–2457. doi: 10.1016/j.jacc.2006.07.069 [DOI] [PubMed] [Google Scholar]
- 3.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 4.Fox KAA, Steg PG, Eagle KA, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297(17):1892–1900. doi: 10.1001/jama.297.17.1892 [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356(23):2388–2398. doi: 10.1056/NEJMsa053935 [DOI] [PubMed] [Google Scholar]
- 6.Schmidt M, Jacobsen JB, Lash TL, Bøtker HE, Sørensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ. 2012;344:e356. doi: 10.1136/bmj.e356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyre H, Kahn R, Robertson RM, et al. Preventing cancer, cardiovascular disease, and diabetes: a common agenda for the American Cancer Society, the American Diabetes Association, and the American Heart Association. Circulation. 2004;109(25):3244–3255. doi: 10.1161/01.CIR.0000133321.00456.00 [DOI] [PubMed] [Google Scholar]
- 8.Schelde AB, Schmidt M, Madsen M, et al. Impact of co-morbidity on the risk of first-time myocardial infarction, stroke, or death after single-photon emission computed tomography myocardial perfusion imaging. Am J Cardiol. 2014;114(4):510–515. doi: 10.1016/j.amjcard.2014.05.031 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 2019;11:563–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 11.Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 13.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol. 2003;56(3):221–229. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs DR, Kroenke C, Crow R, et al. PREDICT: a simple risk score for clinical severity and long-term prognosis after hospitalization for acute myocardial infarction or unstable angina: the Minnesota heart survey. Circulation. 1999;100(6):599–607. doi: 10.1161/01.cir.100.6.599 [DOI] [PubMed] [Google Scholar]
- 15.Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6(11):e012832. doi: 10.1136/bmjopen-2016-012832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain S, Baird JB, Fischer KC, Rich MW. Prognostic value of dipyridamole thallium imaging after acute myocardial infarction in older patients. J Am Geriatr Soc. 1999;47(3):295–301. [DOI] [PubMed] [Google Scholar]
- 18.Stratmann HG, Mark AL, Amato M, Wittry MD, Younis LT. Risk stratification with pre-hospital discharge exercise technetium-99m sestamibi myocardial tomography in men after acute myocardial infarction. Am Heart J. 1998;136(1):87–93. doi: 10.1016/s0002-8703(98)70186-3 [DOI] [PubMed] [Google Scholar]
- 19.Boiten HJ, van Domburg RT, Valkema R, Zijlstra F, Schinkel AFL. Dobutamine stress myocardial perfusion imaging: 8-year outcomes in patients with diabetes mellitus. Eur Heart J Cardiovasc Imaging. 2016;17(8):871–876. doi: 10.1093/ehjci/jev351 [DOI] [PubMed] [Google Scholar]
- 20.Korbee RS, Boiten HJ, Ottenhof M, Valkema R, van Domburg RT, Schinkel AFL. What is the value of stress (99m)Tc-tetrofosmin myocardial perfusion imaging for the assessment of very long-term outcome in obese patients? J Nucl Cardiol. 2013;20(2):227–233. doi: 10.1007/s12350-012-9657-z [DOI] [PubMed] [Google Scholar]
- 21.Doukky R, Fughhi I, Campagnoli T, Wassouf M, Ali A. The prognostic value of regadenoson SPECT myocardial perfusion imaging in patients with end-stage renal disease. J Nucl Cardiol. 2017;24(1):112–118. doi: 10.1007/s12350-015-0303-4 [DOI] [PubMed] [Google Scholar]