ABSTRACT
Arsenic (As) contamination in subsoil and groundwater is a big problem, especially in many South-East Asian countries. As a staple crop growing under flooded condition in these areas, rice (Oryza sativa L.) becomes a big threat to human health through the food chain since As is highly accumulated in grains. Thus, reducing As accumulation in rice through molecular breeding and identification of rice varieties with low As content are the pressing issues. However, the current understanding on the molecular mechanism of As stress response is still limited for rice. In this study, we performed a comprehensive search for the As-responsive small RNAs (sRNAs) of rice. Briefly, 4,762 and 18,152 sRNAs were identified to be highly activated under As stress in roots and shoots respectively, while 14,603 and 8,308 sRNAs were intensively repressed by As treatment in roots and shoots, respectively. A number of the As-responsive sRNAs found their loci on tRNAs, rRNAs or long non-coding RNAs (lncRNAs). Interestingly, these loci preferentially distributed on the 5ʹ halves of the tRNA, rRNA or lncRNA precursors. Among the above-identified As-responsive sRNAs, 252 Argonaute 1 (AGO1)-enriched sRNAs were extracted for target identification, resulting in 200 pairs of sRNA–protein-coding target interactions. Many targets are functionally involved in the development, stress response, reproduction, or lipid metabolism. Additionally, 56 lncRNAs were discovered to be targeted by nine AGO1-enriched sRNAs, indicating the potential involvement of these lncRNAs in As signaling. Taken together, our results could expand the understanding on the non-coding RNA-mediated As stress response in rice.
KEYWORDS: Rice, small RNA (sRNA), argonaute, long non-coding RNA (lncRNA), arsenic (As), target
Introduction
Arsenic (As) belongs to class I carcinogen metalloid, and it is a toxic threat to human health.1 More seriously, in several South-East Asian countries such as China, India, Thailand and Bangladesh, As becomes a common environmental pollutant in soil and groundwater.2-4 Being of greater concern, rice (Oryza sativa L.) is a staple crop in these areas, and it grows in the paddy fields under flooded condition. Owing to severe As contamination in the paddy soil, As uptake by rice seedlings and its high accumulation in grains present a pressing food safety problem. Specifically, the As accumulation level in the rice grains is higher than ten folds of those in the grains of the other cereals such as wheat and barley,5 leading rice to be the largest food dietary source of As for over half of the world’s population.6,7 Excessive As intake by human beings can lead to gastrointestinal symptoms, severe disturbances of the cardiovascular and central nervous systems, and even to death.8,9 Besides, arsenic is also toxic to plant growth and reproduction by reducing photosynthetic rate,10,11 perturbing carbohydrate metabolism,12 or inducing oxidative stress,13,14 all of which will greatly affect crop yields. Thus, researchers were forced to reduce As accumulation in rice grains through genetic engineering, molecular breeding, or identification of the rice varieties with an inefficient As uptake system. Accomplishment of this hard task requires a well understanding of the molecular regulatory mechanisms of As response, uptake, transportation, accumulation and metabolism in rice. The inorganic As forms, including arsenate [As(V)] and arsenite [As(III)], dominate in soil and groundwater.15 To date, several transcriptome-wide studies have been carried out to profile the molecular response of As-stressed rice at mRNA16-19 or miRNA level.20,21 For the rice growth condition, the submerged soil is predominantly invaded by As(III).15 In this consideration, several experiments were designed under As(III) stress treatment. For example, Yu et al. (2012) investigated the As(III)-responsive transcriptome (including mRNAs and miRNAs) of rice roots and shoots, by using Illumina high-throughput sequencing (HTS) technology.19 As a result, genes responsive to As(III) stress were found to be functionally enriched in heavy metal transportation, jasmonate signaling, or lipid metabolism. And, 14 out of 36 As(III)-responsive miRNAs were suggested to be involved in the regulation of As(III)-responsive genes. More interestingly, the study reported quite distinct response patterns between the root and the shoot transcriptomes under As(III) stress.19
Although several studies focused on the regulatory role of miRNAs in the As stress signaling, the roles of the other small non-coding RNA species remain unclear. In this study, by using the sRNA HTS data reported by Yu et al. (2012),19 a comprehensive search for the As-responsive small RNA (sRNAs) in rice roots and shoots was performed based on strict criteria. As a result, 4,762 and 18,152 sRNAs were identified to be highly activated under As stress in roots and shoots, respectively, while 14,603 and 8,308 sRNAs were intensively repressed by As treatment in roots and shoots, respectively. Among these sRNAs, 59 miRBase-registered miRNAs assigned to 36 different sequences were identified to be As-responsive. Besides, a number of the As-responsive sRNAs found their loci on tRNAs, rRNAs or long non-coding RNAs (lncRNAs). Interestingly, these sRNA loci preferentially distributed on the 5ʹ halves of the tRNA, rRNA or lncRNA precursors. Argonaute (AGO) proteins form the central components of the sRNA-incorporated gene silencing complexes,22 and the sRNAs associated with AGO1 are considered to have a great potential of mediating sequence complementarity-based target cleavages in plants.23 In this regard, a total of 252 AGO1-enriched sRNAs were discovered from the above-identified As-responsive sRNAs, followed by transcriptome-wide target identification. Finally, a target list validated by degradome sequencing data was obtained, including 200 pairs of sRNA–protein-coding target interactions. Based on the functional annotations, a dominant portion of the targets is implicated in development, stress response, reproduction, or lipid metabolism. It indicates that, in addition to the miRNAs, the other As-responsive sRNAs associated with AGO1 might play an important role in As stress signaling that affects rice development and yields. Recently, the lncRNAs emerged as a key regulator of gene expression and chromatin status, and play essential roles in plant developmental processes such as flowering.24 In this study, a total of 56 lncRNAs were discovered to be targeted by nine AGO1-enriched, As-responsive sRNAs, indicating the potential involvement of these lncRNAs in As stress signaling. Taken together, our results could expand the current understanding on the molecular mechanisms of As stress response of rice, especially at the non-coding RNA level.
Results and discussion
Identification of As-responsive sRNAs in rice roots and shoots
The previous study by Yu et al. (2012)19 analyzed the response of rice miRNAs under As(III) (sodium arsenite) stress treatment and presented a gift of sRNA-seq data to the public domain. However, the response of the other sRNAs to As(III) stress remains to be investigated. In this study, four sRNA-seq data sets, including control treatment and 80 µM As(III) treatment for 6 h of both roots and shoots, were retrieved from SRA (Sequence Read Archive), and strict criteria were applied to search for As-responsive sRNAs in rice (see Materials and methods). As a result, a total of 4,762 and 14,603 sRNAs in the roots were identified to be induced and repressed under As treatment, respectively (Data S1 and S2). And, 18,152 and 8,308 sRNAs in the shoots were activated and inhibited by As stress, respectively (Data S3 and S4). Among these, a total of 37 As-responsive sRNAs were identified to share identical sequences with 59 rice miRNAs belonging to 26 miRNA families (Tables 1 and S1). Twelve out of the 26 miRNA families, including miR156, miR159, miR160, miR166, miR171, miR396, miR535, miR812, miR1423, miR1428, miR1850 and miR1862 were reported to be As(III)-responsive in Yu et al.’s study (2012).19 However, the remaining 14 families were not discovered by the previous study, which might result from the different search criteria used in the two studies. Besides, we attributed that a part of the 14 newly identified As-responsive families could originate from the updates of miRBase (from release 17 to release 21).25 Indeed, miR5144, miR5540, miR5542, miR5788, miR5794, miR5814 and miR6251 are not included in release 17 but registered in release 21 of miRBase.
Table 1.
List of rice microRNAs responsive to arsenic stress.
| Small RNA IDs | Corresponding microRNA IDs | Small RNA sequences | CK_root1 | As_root2 | CK_shoot3 | As_shoot4 |
|---|---|---|---|---|---|---|
| shoot-CK-high-6605 | osa-miR169i-5p.2 | UGGUGAUAAGGGUGUAGCUCUG | 0 | 0 | 3.52 | 0 |
| shoot-CK-high-7436 | osa-miR5788 | UGGAUGUGACAUACUCUAGUA | 0 | 0.71 | 1.32 | 0 |
| shoot-CK-high-7605 | osa-miR160f-3p | GCAUUGAGGGAGUCAUGCAGG | 0 | 0 | 1.1 | 0 |
| shoot-CK-high-7700 | osa-miR166i-5p | AAUGCAGUUUGAUCCAAGAUC | 0.38 | 0 | 1.54 | 0 |
| root-CK-high-561 | osa-miR171c-5p | GGAUAUUGGUGCGGUUCAAUC | 1.15 | 0 | 0.44 | 1.18 |
| root-CK-high-756 | osa-miR1862d | ACUAGGUUUGUUUAUUUUGGGACG | 77.15 | 5.7 | 9.45 | 56.46 |
| root-CK-high-823 | osa-miR2871a-3p/b | UAUUUUAGUUUCUAUGGUCAC | 3.45 | 0.24 | 1.32 | 3.33 |
| root-CK-high-831 | osa-miR156c-3p/g-3p | GCUCACUUCUCUCUCUGUCAGC | 287.75 | 6.65 | 9.23 | 43.91 |
| root-CK-high-842 | osa-miR159a.2 | UUGCAUGCCCCAGGAGCUGCA | 2.11 | 0 | 0.88 | 0 |
| root-CK-high-1561 | osa-miR3979-3p | CUUCGGGGGAGGAGAGAAGC | 8.23 | 0.71 | 0 | 0 |
| root-CK-high-1916 | osa-miR3979-5p | UCUCUCUCUCCCUUGAAGGC | 113.34 | 5.23 | 0 | 0 |
| root-CK-high-2472 | osa-miR156a/b-5p/c-5p/d/e/f-5p/g-5p/h-5p/i/j-5p | UGACAGAAGAGAGUGAGCAC | 1817.99 | 26.61 | 58.48 | 50.97 |
| root-CK-high-2970 | osa-miR1871 | AUGGCUCUGAUAUCAUGUUGGUUU | 6.13 | 0.48 | 1.54 | 4.7 |
| root-CK-high-4558 | osa-miR156h-3p/f-3p/l-3p | GCUCACUUCUCUUUCUGUCAGC | 16.85 | 0.24 | 1.32 | 3.92 |
| root-CK-high-4770 | osa-miR1423-5p | AGGCAACUACACGUUGGGCGCUCG | 41.74 | 0.48 | 1.1 | 1.96 |
| root-CK-high-5089 | osa-miR5144-5p | UUCUUGUGCUGCUGAAGAGAC | 6.13 | 0.48 | 5.5 | 3.33 |
| root-CK-high-5714 | osa-miR5540 | UUGUGCGAGAUCGACGGUAUA | 1.34 | 0 | 0 | 0.59 |
| root-CK-high-6140 | osa-miR171f-5p | UGUUGGCAUGGUUCAAUCAAA | 24.12 | 2.38 | 0 | 0 |
| root-CK-high-8453 | osa-miR156b-3p | GCUCACUCUCUAUCUGUCAGC | 19.14 | 1.19 | 0 | 0.59 |
| root-CK-high-9201 | osa-miR156j-3p | GCUCGCUCCUCUUUCUGUCAGC | 9.76 | 0.48 | 10.33 | 15.29 |
| root-CK-high-10257 | osa-miR396a-5p/b-5p | UUCCACAGCUUUCUUGAACUG | 23.74 | 1.43 | 1.1 | 0.39 |
| root-CK-high-11189 | osa-miR169r-3p | UGGCAAGUCUCCUCGGCUACC | 9.57 | 0.95 | 2.42 | 0.39 |
| root-CK-high-11266 | osa-miR166a-5p/e-5p | GGAAUGUUGUCUGGUUCAAGG | 126.16 | 11.17 | 3.08 | 7.25 |
| root-CK-high-11392 | osa-miR1850.1 | UGGAAAGUUGGGAGAUUGGGG | 38.29 | 2.85 | 5.72 | 3.53 |
| root-CK-high-11802 | osa-miR535-3p | GUGCUUUCUCCCGUUGUCACU | 103.76 | 4.75 | 1.98 | 6.08 |
| root-CK-high-11843 | osa-miR5814 | AAUCAAGUUAGGAACCAUGCAAGU | 1.34 | 0 | 0.44 | 0.2 |
| root-CK-high-11997 | osa-miR166b-5p | GGAAUGUUGUCUGGCUCGGGG | 22.59 | 1.19 | 4.18 | 8.43 |
| root-CK-high-12836 | osa-miR397a | UCAUUGAGUGCAGCGUUGAUG | 6.13 | 0.24 | 1.1 | 0.59 |
| root-CK-high-13321 | osa-miR5794 | UGAGGAAUCACUAGUAGUCGU | 47.48 | 3.8 | 11.43 | 68.22 |
| shoot-As-high-1758 | osa-miR1427 | UGCGGAACCGUGCGGUGGCGC | 0 | 0.24 | 0 | 1.57 |
| shoot-As-high-3351 | osa-miR5542 | UUUGAGAAGGUAUCAUGAGAU | 0 | 0 | 0 | 1.96 |
| shoot-As-high-8166 | osa-miR6251 | UGUGUAGCCACAUUGUAAGGG | 0.19 | 0 | 0.66 | 12.94 |
| shoot-As-high-8573 | osa-miR169m/i-5p.1/k/l/j/h | UAGCCAAGGAUGACUUGCCUG | 1.15 | 0.48 | 0.22 | 3.53 |
| shoot-As-high-8704 | osa-miR1863a | AGCUCUGAUACCAUGUUAGAUUAG | 0.57 | 0.48 | 0 | 3.33 |
| shoot-As-high-14795 | osa-miR812i/j/g/h | AAGACGGAUGAUUAAAGUUGGACA | 16.27 | 4.51 | 44.41 | 844.1 |
| root-CK-high-77 | osa-miR1428e-3p | UAAGAUAAUGCCAUGAAUUUG | 3.06 | 0 | 0 | 22.94 |
| shoot-As-high-94 |
Since the sequence length and the 5ʹ terminal nucleotide composition of an sRNA contribute greatly to its final action mode,26 we set out to analyze the sequence characteristics of the As-responsive sRNAs. The result shows that in both roots and shoots, a large portion of the As-responsive sRNAs ranges from 21 to 24 nt, especially enriched in 24 nt (Figure 1(a)). The 5ʹ terminal nucleotides of the As-repressed sRNAs in the shoots are predominantly occupied by C and G, while those of the As-repressed sRNAs in the roots are predominantly occupied by A and G. A higher proportion of the As-induced sRNAs in the shoots starts with 5ʹ A, whereas in the roots a higher proportion of the As-induced sRNAs starts with 5ʹ C (Figure 1(a)). Previous study (2012) reported that at the transcriptome level, the As response patterns were quite distinct between the roots and the shoots.19 Interestingly, our result also presents a similar scene that higher number of As-repressed sRNAs exist in the roots compared to the As-induced sRNAs (4,762 As-induced sRNAs versus 14,603 As-repressed sRNAs in the roots). On the other hand, in the shoots, more sRNAs are activated by As stress (18,152 As-induced sRNAs versus 8,308 As-repressed sRNAs in the shoots). Moreover, only 90 sRNAs were found to be induced by As treatment, and only 194 sRNAs were repressed under As stress in both roots and shoots (Figure 1(b)). Notably, a total of 433 sRNAs contribute to the overlap between the As-induced sRNA population in the roots and the As-repressed sRNA population in shoots, and a total of 848 sRNAs contribute to the overlap between the As-induced sRNA population in shoots and the As-repressed sRNA population in roots (Figure 1(b)). These overlaps indicate that some of the sRNAs display contrary As-responsive patterns between roots and shoots, and proper control of the activities might be important for organ-specific As signal transduction.
Figure 1.
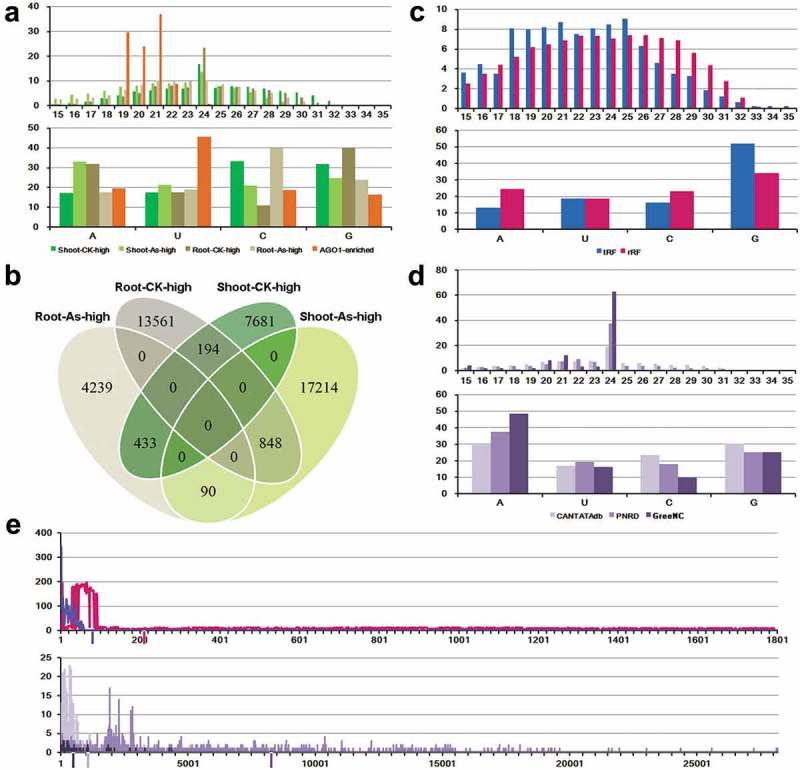
Sequence characteristics of the rice small RNAs responsive to arsenic stress. (a) Sequence length distribution and 5ʹ terminal nucleotide composition of arsenic-responsive small RNAs (sRNAs). Shoot-CK-high: sRNAs repressed in shoots under arsenic stress. Shoot-As-high: sRNAs induced in shoots under arsenic stress. Root-CK-high: sRNAs repressed in roots under arsenic stress. Root-As-high: sRNAs induced in roots under arsenic stress. AGO1-enriched: arsenic-responsive sRNAs that are enriched in AGO1 complexes. (b) Venn diagram showing the overlaps among the four categories of arsenic-responsive sRNAs. (c) Sequence length distribution and 5ʹ terminal nucleotide composition of arsenic-responsive sRNAs mapped onto tRNAs or rRNAs. tRF: arsenic-responsive sRNAs mapped onto tRNAs. rRF: arsenic-responsive sRNAs mapped onto rRNAs. (d) Sequence length distribution and 5ʹ terminal nucleotide composition of arsenic-responsive sRNAs mapped onto the rice long non-coding RNAs (lncRNAs). CANTATAdb: arsenic-responsive sRNAs mapped onto lncRNAs registered in CANTATAdb. PNRD: arsenic-responsive sRNAs mapped onto lncRNAs registered in PNRD. GreeNC: arsenic-responsive sRNAs mapped onto lncRNAs registered in GreeNC. In (a,c,d) the Y axes measure the percentages of the sRNAs. (e) Enrichment of arsenic-responsive sRNA loci towards the 5ʹ half of tRNAs (blue), rRNAs (pink), CANTATAdb-registered lncRNAs (light purple), PNRD-registered lncRNAs (purple), and GreeNC-registered lncRNAs (dark purple). The average length of each RNA species was denoted by the vertical bar with the representative color below the X-axis. Y axes indicate the number of the arsenic-responsive sRNAs mapped onto each position of the tRNAs, rRNAs or lncRNAs.
As-responsive sRNA loci on tRNAs, rRNAs and lncRNAS
In both plants and animals, increasing reports indicated that some of the endogenous sRNAs, defined as tRFs (tRNA-derived RNA fragments) and rRFs (rRNA-derived RNA fragments), were processed from tRNAs and rRNAs, respectively.27-31 Moreover, some of the analyses have demonstrated the regulatory role of tRFs, such as transposable element control32,33 and AGO-mediated target cleavages.34 In this regard, all of the As-responsive sRNAs were mapped onto the rice tRNAs and rRNAs retrieved from RAP-DB.35 As a result, 9,669 unique rRFs and 825 unique tRFs were discovered. Analysis of sequence characteristics shows that a large portion of the tRFs ranges from 18 nt to 25 nt, while a dominant portion of the rRFs distributes within a much wider length range from 18 nt to 29 nt (Figure 1(c)). In addition, higher proportions were observed to begin with 5ʹ G for both tRFs and rRFs (Figure 1(c)). A recent study on tRFs of Arabidopsis thaliana and rice shows that certain tRFs are highly responsive to abiotic stresses such as drought, salt and cold.27 In order to obtain a global view of the impact of As stress on tRF and rRF production, the four sRNA-seq data sets were totally included for mapping onto the rice tRNAs and rRNAs. As a result, 6,302 and 7,523 unique tRFs were identified under the control treatment in roots and shoots, respectively, and 4,058 and 9,651 unique tRFs were identified under the As treatment in roots and shoots, respectively. A total of 57,591 and 53,052 unique rRFs were identified under the control treatment in roots and shoots respectively, and 43,975 and 57,971 unique tRFs were identified under the As treatment in roots and shoots, respectively. Consistent with the first analysis, tRFs are enriched in a narrower length range (18 to 25 nt) than rRFs (18 to 29 nt) (Figure S1). More interestingly, both tRFs and rRFs exhibit distinct As response patterns between roots and shoots. In rice roots, the production of both tRFs and rRFs was repressed by the As treatment, while in shoots, the production of both tRFs and rRFs was activated by As stress (Figure S1).
LncRNAs are emerging to be one novel non-coding RNA species that play critical roles in numerous biological processes in plants.24 Our previous studies indicate that certain structured lncRNAs could serve as the sRNA precursors through the RDR (RNA-dependent RNA polymerase)-independent and DCL1 (Dicer-like 1)-dependent pathway,36,37 while some of the lncRNAs might produce sRNAs through RDR-dependent pathways.38 Here, all of the As-responsive sRNAs were mapped onto the rice lncRNAs derived from CANTATAdb,39 PNRD40 and GreeNC.41 A total of 2,060, 1,440 and 99 unique sRNAs were identified from the lncRNAs registered in CANTATAdb, PNRD and GreeNC respectively. Quite different from those of the tRFs and the rRFs, a significant portion of the lncRNA-derived, As-responsive sRNAs is highly enriched in 24 nt, and 5ʹ A- and 5ʹ G-started sRNAs occupy a dominant portion (Figure 1(d)).
The distribution of As-responsive sRNA loci on the tRNAs, rRNAs and lncRNAs was investigated to check whether there is any hot-spot region for sRNA generation. Intriguingly, the As-responsive sRNA loci were found to preferentially locate on the 5ʹ halves of tRNAs and rRNAs. It is the same case for the lncRNAs (Figure 1(e)). A recent report in mouse and human cells indicate that both tRNAs and rRNAs produce short fragments preferentially at their terminals, and these tRFs and rRFs are generated in an asymmetric manner that predominantly favors either 5ʹ or 3ʹ ends.31 Thus, whether the observed enrichment of the As-responsive sRNA loci on the 5ʹ halves of tRNAs, rRNAs and lncRNAs in rice is a common rule in plants needs further examination.
Identification of protein-coding targets regulated by the As-responsive sRNAs enriched in AGO1
In plants, a great portion of the endogenous sRNAs is implicated in gene silencing pathways after their incorporation into specific RISCs (RNA-induced silencing complexes). Within the RISCs, the AGO proteins contribute to the central components of the RISCs.22 Differential associations with the AGOs could assign the sRNAs into distinct silencing pathways. For example, in plants, many AGO4-associated sRNAs are implicated in epigenetic regulation,42 while those associated with AGO1 have a great potential of performing target cleavages based on high sequence complementarity.23 In this study, based on a defined search criterion (see Materials and methods), AGO1-enriched sRNAs were identified from the As-responsive sRNA population identified above. As a result, a total of 252 AGO1-associated sRNAs were discovered (Data S5). Consistent with the previous report in Arabidopsis thaliana,26 a large portion of the AGO1-associated, As-responsive sRNAs is 21 nt in length (36.90%), and starts with 5ʹ U (45.63%) (Figure 1(a)). However, high percentages of 19-nt (29.76%) and 20-nt (23.81%) sRNAs were found to be associated with AGO1.
In order to extract the potential target transcripts of the AGO1-associated, As-responsive sRNAs, these sRNAs were subject to target prediction by using miRU algorithm43,44 with default parameters. Then, based on the previously proposed criteria,45 the predicted targets were submitted to degradome-seq data-based validation. Finally, target plots (t-plots) were drawn for manual check. As a result, a total of 200 sRNA–target pairs involving 32 AGO1-associated, As-responsive sRNAs (including osa-miR396a-5p and osa-miR396b-5p sharing the identical sequence with root-CK-high-10257) and 146 protein-coding target transcripts were extracted (Table 2, Figure S2 and Data S6). Since the degradome-seq libraries were prepared from different samples (seedlings, leaves and inflorescences of Oryza sativa L. ssp. japonica cv. Nipponbare, and young panicles of Oryza sativa L. ssp. japonica cv. ZH11), the sample-specific regulatory patterns of the above-identified sRNA–target pairs were further investigated based on degradome signal intensity. Interestingly, among these sRNA–target regulatory relationships, some pairs, such as LOC_Os01g59660.1 regulated by shoot-CK-high-5721/root-CK-high-10864 and LOC_Os02g06910.1 targeted by shoot-CK-high-3401 are supported by the cleavage signals widespread in diverse rice samples including seedlings, leaves and inflorescences of Nipponbare, and young panicles of ZH11 (Figure 2(a,b)). Some of the regulatory pairs were specifically identified in only one of the japonica rice cultivars, i.e. Nipponbare or ZH11. For example, the cleavage signals of shoot-As-high-850–LOC_Os01g55040.1 and shoot-As-high-9528/shoot-CK-high-38–LOC_Os02g44370.1 were specifically identified in the leaves of Nipponbare (Figure 2(c,d)), and shoot-CK-high-3401–LOC_Os06g46410.1 was supported by the degradome signatures from the seedlings and inflorescences of Nipponbare (Figure 2(e)). However, the regulation of LOC_Os01g70270.1 by root-CK-high-4920 was only supported by the degradome-seq signatures from the young panicles of ZH11 (Figure 2(f)). Taken together, the results indicate that in addition to the constitutive regulatory relationships, some of the target transcripts are regulated by the AGO1-associated, As-responsive sRNAs in a cultivar- or organ-specific way.
Table 2.
List of arsenic-responsive small RNAs targeting protein-coding transcripts in rice.
| Small RNA IDs | Small RNA sequences | CK_root1 | As_root2 | CK_shoot3 | As_shoot4 | Control5 | AGO1a6 | AGO1b7 | AGO1c8 |
|---|---|---|---|---|---|---|---|---|---|
| root-CK-high-10130 | UAUGACGCUGUUGACUUUUAG | 3.25 | 0 | 1.1 | 1.18 | 8.69 | 141.39 | 2.44 | 32.38 |
| root-CK-high-10257 (osa-miR396a-5p/osa-miR396b-5p) |
UUCCACAGCUUUCUUGAACUG | 23.74 | 1.43 | 1.1 | 0.39 | 108.95 | 1738.59 | 907.64 | 290.36 |
| root-CK-high-10864 | CUUUGGAUUGAAGGGAGCUCU | 7.28 | 0.71 | 17.15 | 5.49 | 17.37 | 638.88 | 3.25 | 415.5 |
| root-CK-high-11406 | UAUUAUAAGACGUUUUGACU | 2.68 | 0 | 2.2 | 2.35 | 5.96 | 46.69 | 1.63 | 33.66 |
| root-CK-high-12952 | UUAACGUUUGACCACUCGUCUU | 1.15 | 0 | 0 | 0 | 0.25 | 2.9 | 0 | 0 |
| root-CK-high-1309 | UAAACAUGUUUGACCGUUCGUC | 1.15 | 0 | 0.22 | 0.59 | 0.5 | 5.01 | 0 | 0 |
| root-CK-high-13603 | UUCAUGUUGAUGUUAGUAGAUU | 5.36 | 0.24 | 3.3 | 2.55 | 1.74 | 17.15 | 0 | 0 |
| root-CK-high-2334 | UUGUUGCUACCUUCCAUGCAGA | 2.49 | 0.24 | 0.22 | 1.18 | 0.25 | 0 | 0 | 7.19 |
| root-CK-high-2928 | UUCUAGCAUUUCCCACAUUCA | 1.34 | 0 | 0 | 0.39 | 0.25 | 0 | 0 | 10.54 |
| root-CK-high-4603 | UGACAGAAGAGAGUGAGCA | 1.15 | 0 | 0 | 0 | 9.68 | 36.14 | 275.46 | 87.88 |
| root-CK-high-4920 | UCUUGACCUUGUAAGACCCAA | 7.28 | 0.24 | 1.1 | 0.78 | 3.72 | 28.22 | 0 | 0 |
| root-CK-high-5263 | AACUGGUACGGACAAGGGG | 2.68 | 0.24 | 1.1 | 2.94 | 0.99 | 0 | 88.57 | 0 |
| root-CK-high-5965 | UGCCUGGCUCCCUGUAUGC | 1.34 | 0 | 0.66 | 0.2 | 0.25 | 0 | 0 | 7.71 |
| root-CK-high-6739 | UUUGGCUUGCAACGUUUGACC | 28.91 | 2.61 | 3.52 | 2.16 | 7.2 | 165.65 | 86.13 | 130.28 |
| root-CK-high-7883 | UUGCAGGUCCGUUGGAUUGGA | 1.53 | 0 | 1.54 | 0.59 | 1.74 | 3.17 | 0 | 11.82 |
| shoot-As-high-16223 | UUUUCACUAAAAAAUAGUCGGC | 0.77 | 0 | 0 | 1.96 | 0.5 | 2.64 | 0 | 0 |
| shoot-As-high-2209 | GGAUGUUUUCAUUAAUCAA | 2.11 | 0.48 | 0.88 | 38.62 | 0.99 | 0 | 42.25 | 0 |
| shoot-As-high-850 | UAGGUGAACCUGCGGAAGGA | 0 | 0 | 0 | 1.57 | 0.5 | 0 | 0 | 2.83 |
| shoot-As-high-9528 | UUGAGCCGUGCCAAUAUCACG | 1.34 | 0.71 | 0.66 | 7.25 | 43.18 | 32.18 | 0.81 | 453.27 |
| shoot-CK-high-1260 | CGGACCAGGCUUCAUUCCUC | 0.38 | 0.24 | 1.32 | 0 | 0 | 0.26 | 0 | 14.39 |
| shoot-CK-high-1263 | CGGACCAGGCUUCAUUCCCC | 81.17 | 47.99 | 15.83 | 0 | 0.5 | 8.7 | 0 | 5.14 |
| shoot-CK-high-2001 | CCACAGGCUUUCUUGAACUG | 1.72 | 0.48 | 11.43 | 0 | 10.67 | 501.45 | 456.66 | 279.57 |
| shoot-CK-high-2855 | UGCACUGCCUCUUCCCUGGC | 0 | 0 | 1.1 | 0 | 2.23 | 32.71 | 91.82 | 74.26 |
| shoot-CK-high-3401 | GAAGCUGCCAGCAUGAUCUGA | 7.66 | 3.8 | 23.09 | 0.2 | 9.18 | 13.98 | 0 | 91.48 |
| shoot-CK-high-38 | GAUUGAGCCGUGCCAAUAUC | 6.51 | 6.65 | 1.54 | 0 | 2.98 | 0 | 0 | 10.02 |
| shoot-CK-high-5721 | UUGGAUUGAAGGGAGCUCUG | 9.19 | 1.66 | 30.34 | 2.74 | 69.49 | 5479.54 | 9979.95 | 7642.19 |
| shoot-CK-high-6015 | AACUGGAAUAAAAGAAUUA | 0 | 0 | 1.1 | 0 | 17.87 | 0 | 94.26 | 11.56 |
| shoot-CK-high-7069 | CGCUUGGUGCAGAUCGGGAC | 18.38 | 6.65 | 26.6 | 1.18 | 1.49 | 4.75 | 0 | 0 |
| shoot-CK-high-7529 | GUUGUCUCAAGCUUGCUGCC | 3.45 | 1.19 | 2.86 | 0.2 | 1.49 | 0 | 119.45 | 0 |
| shoot-CK-high-7947 | UUCCACAGGCUUUCUUGAACUG | 0.96 | 0.24 | 1.98 | 0 | 1.24 | 134.79 | 40.63 | 12.33 |
| root-As-high-992 | CGGCAAGUGAUGUGAACAUU | 0 | 2.38 | 1.54 | 0 | 0 | 3.96 | 0 | 0 |
| shoot-CK-high-1707 |
Note: 1Expression levels in roots under mock treatment. 2Expression levels in roots under arsenic (As) treatment. 3Expression levels in shoots under mock treatment. 4Expression levels in shoots under arsenic treatment. 5Control for “AGO1a”, “AGO1b” and “AGO1c”. 6Accumulation levels in AGO1a. 7Accumulation levels in AGO1b. 8Accumulation levels in AGO1c. All of the expression levels were calculated in RPM (reads per million).
Figure 2.
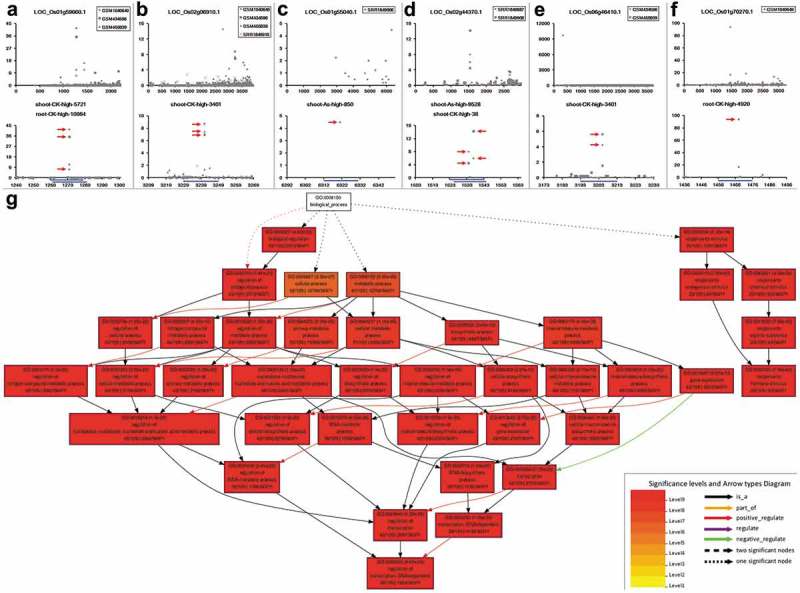
Identification of the protein-coding transcripts targeted by the arsenic-responsive small RNAs. (a–f) showing the examples of small RNA (sRNA)–target regulation validated by degradome-seq data. For each panel, the upper target plot (t-plot) presents the global distribution of degradome signals along the full-length target transcript, with transcript ID and degradome-seq data set IDs shown on the top. The lower t-plot provides a detailed view of degradome signals surrounding the predicted binding site (blue line) of the arsenic-responsive sRNA (ID shown on the top) on the target transcript, which clearly shows the degradome-seq evidence (marked by red arrowheads) supporting the regulatory relationship. For each t-plot, the Y-axis measures the degradome signal intensity in RPM (reads per million), and the X axis indicates the position on the target transcript. (g) Result of GO (Gene ontology) term enrichment analysis. All of the GO terms belong to the “Biological process” category. The red color indicates that the corresponding GO terms are highly enriched in the target transcript list submitted for analysis, when compared to all of the rice transcripts served as a background.
Network construction and functional analysis of the protein-coding targets
GO (Gene Ontology) is a widely used annotation system for functional analysis of protein-coding genes.46,47 Here, to obtain a first functional overlook of the protein-coding targets, the target gene list was sent to agriGO48 for GO term enrichment analysis (see Materials and methods). The results show that the target genes are functionally enriched in “response to chemical stimulus” and “regulation of transcription” under the GO category “biological process” (Figure 2(g)), and enriched in “transcription factor activity” under the GO category “molecular function” (Figure S3). Besides, a significant portion of the target genes encode protein products localized in the nucleus (Figure S4), which is consistent with their transcriptional regulator activity.
Next, a gene regulatory network mediated by the As-responsive sRNAs was constructed based on the sRNA–target list obtained above (Figure S5). The network includes 178 nodes (32 sRNAs and 146 targets) connected by 200 edges. After careful check on the GO annotations of the target transcripts (Table S2), we found that 73 targets were implicated in plant development (Figure 3(a)), 48 targets were involved in stress response (Figure 3(b)), 41 targets played potential roles in reproduction (Figure 3(c)), and 13 targets functioned in lipid metabolism (Figure 3(d)). In this regard, four groups of subnetworks were extracted from the whole network. These subnetworks are valuable for analyzing sRNA-mediated As a response in rice, which could have great impacts on stress signal transduction, development, reproduction and lipid metabolism.
Figure 3.

Subnetworks mediated by the arsenic-responsive small RNAs. According to the target annotations, the subnetworks are potentially involved in plant development (a), stress response (b), reproduction (c), and lipid metabolism (d).
Identification of lncRNAs regulated by the As-responsive sRNAs enriched in AGO1
Recently, the lncRNAs have been uncovered to be a key regulator of gene expression and chromatin status, and have been shown to play essential roles in plant developmental processes such as flowering.24 In order to see whether the AGO1-associated, As-responsive sRNAs could act on lncRNAs, the rice lncRNAs retrieved from three databases, including CANTATAdb,39 PNRD40 and GreeNC41 (see Materials and methods), were subject to target prediction and degradome-seq data-based validation, just as the identification of the protein-coding targets. As a result, 56 lncRNAs were discovered to be targeted by nine sRNAs (Table3, Data S7 and Figure S6), indicating the potential involvement of these lncRNAs in As stress signaling. Based on the signal intensities from different degradome-seq libraries, cultivar- or organ-specific regulation was discovered for certain sRNA – lncRNA pairs, which was similar to the protein-coding targets. For example, root-CK-high-13603-mediated cleavages on lincRNA446 were only detected in the three-week-old seedlings and leaves of Nipponbare (Figure 4(a)), while the regulation of CNT0027061 by root-CK-high-2928 was only discovered in the young panicles of ZH11 (Figure 4(b)). Besides, some of the lncRNAs were regulated by two distinct sRNAs, and the cleavage signals were detected in different degradome-seq libraries. For instance, lincRNA257 was targeted by both root-CK-high-13603 and root-CK-high-2928. The prominent cleavage signal of root-CK-high-13603 was only detected in the leaves of Nipponbare, while the cleavage of lincRNA257 by root-CK-high-2928 was only supported by the degradome-seq library prepared from the young panicles of ZH11 (Figure 4(c)). CNT0027168 was targeted by shoot-As-high-17275 and root-CK-high-1309. The regulation by the former sRNA was only supported by the degradome-seq library of the young panicles of ZH11, while the regulation by the later sRNA was only evidenced from the degradome-seq library of the leaves of Nipponbare (Figure 4(d)). Finally, a regulatory network involving 65 nodes (56 lncRNA targets and nine AGO1-associated, As-responsive sRNAs) connected by 59 edges was established (Figure 4(e)).
Table 3.
List of arsenic-responsive small RNAs targeting long non-coding RNAs in rice.
| Small RNA IDs | Small RNA sequences | CK_root1 | As_root2 | CK_shoot3 | As_shoot4 | Control5 | AGO1a6 | AGO1b7 | AGO1c8 |
|---|---|---|---|---|---|---|---|---|---|
| root-CK-high-10130 | UAUGACGCUGUUGACUUUUAG | 3.25 | 0 | 1.1 | 1.18 | 8.69 | 141.39 | 2.44 | 32.38 |
| root-CK-high-1309 | UAAACAUGUUUGACCGUUCGUC | 1.15 | 0 | 0.22 | 0.59 | 0.5 | 5.01 | 0 | 0 |
| root-CK-high-13603 | UUCAUGUUGAUGUUAGUAGAUU | 5.36 | 0.24 | 3.3 | 2.55 | 1.74 | 17.15 | 0 | 0 |
| root-CK-high-2928 | UUCUAGCAUUUCCCACAUUCA | 1.34 | 0 | 0 | 0.39 | 0.25 | 0 | 0 | 10.54 |
| root-CK-high-4417 | UUAAAUAUGUUUGACCGUUCG | 1.34 | 0 | 0 | 0.39 | 1.49 | 5.8 | 0 | 0 |
| root-CK-high-4603 | UGACAGAAGAGAGUGAGCA | 1.15 | 0 | 0 | 0 | 9.68 | 36.14 | 275.46 | 87.88 |
| shoot-As-high-14 | AUUAAGCCAUGCAUGUGCAAG | 1.53 | 1.19 | 0 | 1.37 | 0.5 | 0 | 30.88 | 0 |
| shoot-As-high-17275 | UGUGAGAAAAAGUCAACGGCG | 0 | 0.48 | 0 | 1.18 | 9.68 | 24 | 56.07 | 70.92 |
| shoot-As-high-2209 | GGAUGUUUUCAUUAAUCAA | 2.11 | 0.48 | 0.88 | 38.62 | 0.99 | 0 | 42.25 | 0 |
Note: 1Expression levels in roots under mock treatment. 2Expression levels in roots under arsenic (As) treatment. 3Expression levels in shoots under mock treatment. 4Expression levels in shoots under arsenic treatment. 5Control for “AGO1a”, “AGO1b” and “AGO1c”. 6Accumulation levels in AGO1a. 7Accumulation levels in AGO1b. 8Accumulation levels in AGO1c. All of the expression levels were calculated in RPM (reads per million).
Figure 4.
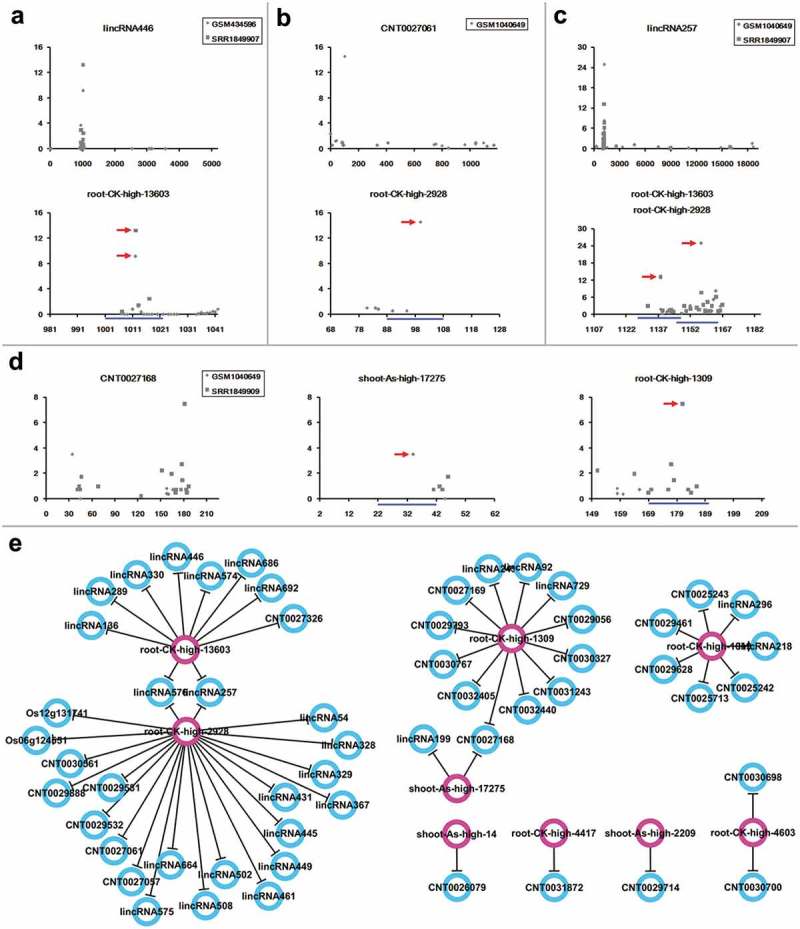
Regulation of long non-coding RNAs by the arsenic-responsive small RNAs. (a–d) show the examples of small RNA (sRNA)–long non-coding RNAs (lncRNAs) regulation validated by degradome-seq data. For each panel, the first target plot (t-plot) presents the global distribution of degradome signals along the full-length lncRNA, with lncRNA ID and degradome-seq data set IDs shown on the top. The remaining t-plot(s) provide(s) a detailed view of degradome signals surrounding the predicted binding site(s) [blue line(s)] of the arsenic-responsive sRNA(s) [ID(s) shown on the top] on the lncRNA. The result clearly shows the degradome-seq evidence (marked by red arrowheads) supporting the regulatory relationship. For each t-plot, the Y-axis measures the degradome signal intensity in RPM (reads per million), and the X axis indicates the position on the lncRNA. (e) Regulatory network constituted by the sRNA–lncRNA pairs supported by degradome-seq data. Pink circles: regulatory sRNAs; blue circles: lncRNA targets.
Conclusions
The thorough understanding of As response, signal transduction and metabolism at molecular level are a prerequisite for developing the rice cultivars with low inorganic arsenic accumulation. In this study, we performed a comprehensive investigation of molecular response of As-stressed rice especially focusing on the non-coding RNAs. Starting from As-responsive sRNA identification, both protein-coding and lncRNA targets were discovered to be regulated by AGO1-associated sRNAs. The results indicate that in addition to the miRNAs, there are hundreds of other sRNAs responsive to As stress, and some of these sRNAs are likely to be implicated in As signaling through target cleavages. The protein-coding targets are functionally enriched in development, stress response, reproduction and lipid metabolism, which could become a future research focus on the regulatory cascades downstream of the AGO1-associated, As-responsive sRNAs. Besides, the lncRNAs targeted by specific As-responsive sRNAs might be also involved in As signaling. Summarily, our results could expand the current understanding of the molecular mechanisms of As stress response of rice, especially at the non-coding RNA level.
Materials and methods
Data sets used in this study
The sRNA sequencing (sRNA-seq) data sets of arsenic stressed rice, including SRR447127 (the roots of 14-day-old seedling under control treatment for), SRR447129 (the roots of 14-day-old seedling under 80 μm sodium arsenite stress for), SRR447132 (the shoots of 14-day-old seedling under control treatment for) and SRR447134 (the shoots of 14-day-old seedling under 80 μm sodium arsenite stress for), were retrieved from SRA (https://www.ncbi.nlm.nih.gov/sra/).49 The sRNA-seq data sets from immunopurified AGO1 complexes, including GSM455965 (total extract, control), GSM455962 (AGO1a), GSM455963 (AGO1b) and GSM455964 (AGO1c), were retrieved from GEO (Gene Expression Omnibus; https://www.ncbi.nlm.nih.gov/geo/).50 Four of the rice degradome sequencing (degradome-seq) data sets, including GSM434596 (seedlings, japonica cv. Nipponbare), GSM455938 (seedlings, japonica cv. Nipponbare), GSM455939 (inflorescences, japonica cv. Nipponbare) and GSM1040649 (young panicles, japonica cv. ZH11 were obtained from GEO. The remaining degradome-seq data sets, including SRR1849887 to SRR1849894 (leaves, japonica cv. Nipponbare), and SRR1849902 to SRR1849910 (leaves, japonica cv. Nipponbare), were retrieved from SRA.
The rice tRNA and rRNA sequences were retrieved from RAP-DB (The Rice Annotation Project Database; http://rapdb.dna.affrc.go.jp/download/archive/irgsp1_rRNA_tRNA.gff.gz).35 The rice lncRNAs were retrieved from three databases, including CANTATAdb (A database of long non-coding RNAs in plants; http://cantata.amu.edu.pl/DOWNLOADS/download_sequence.php?species=oryza),39 PNRD (Plant Non-coding RNA Database; http://structuralbiology.cau.edu.cn/PNRD/download.php),40 and GreeNC (A Wiki-database of plant lncRNAs; http://greenc.sciencedesigners.com/api/db/greenc/species/Oryza_sativa_Japonica_Group/fasta).41 The rice protein-coding transcripts along with their annotations were obtained from RGAP (Rice Genome Annotation Project; http://rice.plantbiology.msu.edu/).51
Search for the As-responsive and AGO1-enriched sRNAs
In order to allow cross-data set comparison, the normalized read count (reads per million; RPM) of an sRNA sequence from a specific library was calculated by dividing the raw count of this sRNA by the total raw counts of the library and then multiplied by 106. By using the normalized sRNA-seq data sets SRR447127 (roots under normal condition) and SRR447129 (roots under arsenic stress), we searched for the As-responsive sRNAs in rice roots based on the following criteria: (1) To be an As-induced sRNAs, the expression level of the sRNA under As treatment should be ten times or more than that under normal growth condition. If the expression of the sRNA could not be detected under normal condition, its expression level under As treatment is required to be 1 RPM at least. (2) To be an sRNA repressed by As stress, the expression level of the sRNA under normal growth treatment should be ten times or more than that under As treatment. If the expression of the sRNA could not be detected under As treatment, its expression level under normal condition is required to be 1 RPM at least. The same criteria were adopted to discover the As-responsive sRNAs in rice shoots by using the normalized sRNA-seq data sets SRR447132 (shoots under normal condition) and SRR447134 (shoots under arsenic stress).
An sRNA is considered to be associated with AGO1, if it fits the following criteria: (1) The accumulation level of the sRNA in GSM455962 (AGO1a), GSM455963 (AGO1b) or GSM455964 (AGO1c) should be at least three times higher than its level in GSM455965 (total extract, control). (2) If the sRNA could not be detected in GSM455965, its accumulation level in GSM455962, GSM455963 or GSM455964 should be 1 RPM at least.
Target prediction and degradome sequencing data-based validation
Target prediction was performed by using miRU algorithm43,44 with default parameters. The degradome sequencing data were utilized to validate the predicted miRNA/sRNA – target pairs. Firstly, in order to allow cross-library comparison, the RPM of a short sequence from a specific degradome library was calculated by dividing the raw count of this sequence by the total counts of the library, and then multiplied by 106. Secondly, all the degradome signatures were mapped onto the predicted target transcripts. Then, the previously proposed criteria45 were applied to extract the potential cleavage sites. Summarily, (1) “Average_Read count_Cleavage site” is the averaged read count (in RPM) of all the degradome signatures (belonging to one library) with their 5ʹ ends mapped to a potential cleavage site; “Average_Read count_Surrounding” is the averaged read count of all the degradome signatures (also belonging to the library) that mapped to the regions surrounding the cleavage site; “Average_Read count_Cleavage site” should be five times or more than “Average_Read count_Surrounding”. (2) Also for the degradome library, among the degradome signatures mapped to a potential cleavage site, the most abundant tag should be amongst the top 12-most-abundant degradome signatures that perfectly mapped to the corresponding transcript. (3) The cleavage site should reside within ninth to tenth, tenth to 11th, or 11th to 12th nucleotide region of the regulatory sRNA. For any degradome library, if the three rules were fulfilled, the potential slicing sites were retained. Finally, both global and local target plots were drawn to perform manual screening, referring to our previous study.52,53 Only the transcripts with cleavage signals easy to be recognized were extracted as the potential miRNA/sRNA – target pairs.
GO (Gene Ontology) term enrichment analysis
GO term enrichment analysis was performed by using “Singular Enrichment Analysis (SEA)” of the online tool agriGO (http://bioinfo.cau.edu.cn/agriGO/analysis.php).48 SEA analysis is designed to identify enriched GO terms in a list of gene identifiers. Fisher exact test was employed as the statistics method by using default parameter setting, and the following parameters were adopted for rice: (1) Singular Enrichment Analysis (SEA); (2) “Oryza sativa MSU6.1 nonTE” for species; (3) “Rice MSU6.1 nonTE transcript ID” for reference.
Funding Statement
This work was funded by the National Natural Science Foundation of China [31601062] and [31571349], the Scientific Research Fund of Hunan Provincial Education Department [18A108], the Natural Science Foundation of Hunan Province, China [2019JJ40114] and [2018JJ3218].
Acknowledgments
We would like to thank all the publicly available datasets and the scientists behind them.
Competing interests
The authors declare that they have no competing interests.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Neubauer O. Arsenical cancer: a review. Br J Cancer. 1947;1:1–11. doi: 10.1038/bjc.1947.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen BP, Liu Z. Transport pathways for arsenic and selenium: a minireview. Environ Int. 2009;35:512–515. doi: 10.1016/j.envint.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuli R, Chakrabarty D, Trivedi PK, Tripathi RD. Recent advances in arsenic accumulation and metabolism in rice. Mol Breed. 2010;26:307–323. doi: 10.1007/s11032-010-9412-6. [DOI] [Google Scholar]
- 4.Fendorf S, Michael HA, van Geen A. Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science. 2010;328:1123–1127. doi: 10.1126/science.1172974. [DOI] [PubMed] [Google Scholar]
- 5.Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol. 2007;41:6854–6859. doi: 10.1021/es070627i. [DOI] [PubMed] [Google Scholar]
- 6.Tsuji JS, Yost LJ, Barraj LM, Scrafford CG, Mink PJ. Use of background inorganic arsenic exposures to provide perspective on risk assessment results. Regul Toxicol Pharmacol. 2007;48:59–68. doi: 10.1016/j.yrtph.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Zhu YG, Williams PN, Meharg AA. Exposure to inorganic arsenic from rice: a global health issue? Environ Pollut. 2008;154:169–171. doi: 10.1016/j.envpol.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Abdul KS, Jayasinghe SS, Chandana EP, Jayasumana C, De Silva PM. Arsenic and human health effects: A review. Environ Toxicol Pharmacol. 2015;40:828–846. doi: 10.1016/j.etap.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Vahter M. Effects of arsenic on maternal and fetal health. Annu Rev Nutr. 2009;29:381–399. doi: 10.1146/annurev-nutr-080508-141102. [DOI] [PubMed] [Google Scholar]
- 10.Azizur Rahman M, Hasegawa H, Mahfuzur Rahman M, Nazrul Islam M, Majid Miah MA, Tasmen A. Effect of arsenic on photosynthesis, growth and yield of five widely cultivated rice (Oryza sativa L.) varieties in Bangladesh. Chemosphere. 2007;67:1072–1079. doi: 10.1016/j.chemosphere.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 11.Rai R, Pandey S, Shrivastava AK, Pandey Rai S. Enhanced photosynthesis and carbon metabolism favor arsenic tolerance in artemisia annua, a medicinal plant as revealed by homology-based proteomics. Int J Proteomics. 2014;2014:163962. doi: 10.1155/2014/163962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jha AB, Dubey RS. Carbohydrate metabolism in growing rice seedlings under arsenic toxicity. J Plant Physiol. 2004;161:867–872. doi: 10.1016/j.jplph.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Dat J, Vandenabeele S, Vranova E, Van Montagu M, Inze D, Van Breusegem F. Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci. 2000;57:779–795. doi: 10.1007/s000180050041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Requejo R, Tena M. Proteome analysis of maize roots reveals that oxidative stress is a main contributing factor to plant arsenic toxicity. Phytochemistry. 2005;66:1519–1528. doi: 10.1016/j.phytochem.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Abedin MJ, Feldmann J, Meharg AA. Uptake kinetics of arsenic species in rice plants. Plant Physiol. 2002;128:1120–1128. doi: 10.1104/pp.010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abercrombie JM, Halfhill MD, Ranjan P, Rao MR, Saxton AM, Yuan JS, Stewart CN. Transcriptional responses of Arabidopsis thaliana plants to As (V) stress. BMC Plant Biol. 2008;8:87. doi: 10.1186/1471-2229-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakrabarty D, Trivedi PK, Misra P, Tiwari M, Shri M, Shukla D, Kumar S, Rai A, Pandey A, Nigam D, et al. Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere. 2009;74:688–702. doi: 10.1016/j.chemosphere.2008.09.082. [DOI] [PubMed] [Google Scholar]
- 18.Norton GJ, Lou-Hing DE, Meharg AA, Price AH. Rice-arsenate interactions in hydroponics: whole genome transcriptional analysis. J Exp Bot. 2008;59:2267–2276. doi: 10.1093/jxb/ern097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu LJ, Luo YF, Liao B, Xie LJ, Chen L, Xiao S, Li JT, Hu SN, Shu WS. Comparative transcriptome analysis of transporters, phytohormone and lipid metabolism pathways in response to arsenic stress in rice (Oryza sativa). New Phytol. 2012;195:97–112. doi: 10.1111/j.1469-8137.2012.04154.x. [DOI] [PubMed] [Google Scholar]
- 20.Pandey C, Raghuram B, Sinha AK, Gupta M. miRNA plays a role in the antagonistic effect of selenium on arsenic stress in rice seedlings. Metallomics. 2015;7:857–866. doi: 10.1039/c5mt00013k. [DOI] [PubMed] [Google Scholar]
- 21.Sharma D, Tiwari M, Lakhwani D, Tripathi RD, Trivedi PK. Differential expression of microRNAs by arsenate and arsenite stress in natural accessions of rice. Metallomics. 2015;7:174–187. doi: 10.1039/c4mt00264d. [DOI] [PubMed] [Google Scholar]
- 22.Vaucheret H. Plant ARGONAUTES. Trends Plant Sci. 2008;13:350–358. doi: 10.1016/j.tplants.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Baumberger N, Baulcombe DC. Arabidopsis ARGONAUTE1 is an RNA slicer that selectively recruits microRNAs and short interfering RNAs. Proc Natl Acad Sci U S A. 2005;102:11928–11933. doi: 10.1073/pnas.0505461102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chekanova JA. Long non-coding RNAs and their functions in plants. Curr Opin Plant Biol. 2015;27:207–216. doi: 10.1016/j.pbi.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mi S, Cai T, Hu Y, Chen Y, Hodges E, Ni F, Wu L, Li S, Zhou H, Long C, et al. Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5ʹ terminal nucleotide. Cell. 2008;133:116–127. doi: 10.1016/j.cell.2008.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alves CS, Vicentini R, Duarte GT, Pinoti VF, Vincentz M, Nogueira FT. Genome-wide identification and characterization of tRNA-derived RNA fragments in land plants. Plant Mol Biol. 2017;93:35–48. doi: 10.1007/s11103-016-0545-9. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Sun Y, Yang X, Wu Z, Guo K, Niu X, Wang Q, Ruan J, Bu W, Gao S. Two featured series of rRNA-derived RNA fragments (rRFs) constitute a novel class of small RNAs. PLoS One. 2017;12:e0176458. doi: 10.1371/journal.pone.0176458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu C, Yu L, Wu B, Ma L, Gou LT, He M, Guo Y, Li ZT, Gao W, Shi H, et al. A sequence of 28S rRNA-derived small RNAs is enriched in mature sperm and various somatic tissues and possibly associates with inflammation. J Mol Cell Biol. 2017;9:256–259. doi: 10.1093/jmcb/mjx016. [DOI] [PubMed] [Google Scholar]
- 30.Cognat V, Morelle G, Megel C, Lalande S, Molinier J, Vincent T, Small I, Duchene AM, Marechal-Drouard L. The nuclear and organellar tRNA-derived RNA fragment population in Arabidopsis thaliana is highly dynamic. Nucleic Acids Res. 2017;45:3460–3472. doi: 10.1093/nar/gkw1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Ender C, Meister G, Moore PS, Chang Y, John B. Extensive terminal and asymmetric processing of small RNAs from rRNAs, snoRNAs, snRNAs, and tRNAs. Nucleic Acids Res. 2012;40:6787–6799. doi: 10.1093/nar/gks307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez G, Choudury SG, Slotkin RK. tRNA-derived small RNAs target transposable element transcripts. Nucleic Acids Res. 2017;45:5142–5152. doi: 10.1093/nar/gkx103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schorn AJ, Gutbrod MJ, LeBlanc C, Martienssen R. LTR-retrotransposon control by tRNA-derived small RNAs. Cell. 2017;170:61–71. doi: 10.1016/j.cell.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loss-Morais G, Waterhouse PM, Margis R. Description of plant tRNA-derived RNA fragments (tRFs) associated with argonaute and identification of their putative targets. Biol Direct. 2013;8:6. doi: 10.1186/1745-6150-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakai H, Lee SS, Tanaka T, Numa H, Kim J, Kawahara Y, Wakimoto H, Yang CC, Iwamoto M, Abe T, et al. Rice Annotation Project Database (RAP-DB): an integrative and interactive database for rice genomics. Plant Cell Physiol. 2013;54:e6. doi: 10.1093/pcp/pcs183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma X, Shao C, Jin Y, Wang H, Meng Y. Long non-coding RNAs: a novel endogenous source for the generation of Dicer-like 1-dependent small RNAs in Arabidopsis thaliana. RNA Biol. 2014;11:373–390. doi: 10.4161/rna.28725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meng Y, Shao C, Wang H, Chen M. Uncovering DCL1-dependent small RNA loci on plant genomes: a structure-based approach. J Exp Bot. 2014;65:395–400. doi: 10.1093/jxb/ert409. [DOI] [PubMed] [Google Scholar]
- 38.Qin J, Ma X, Yi Z, Tang Z, Meng Y. Intronic regions of plant genes potentially encode RDR (RNA-dependent RNA polymerase)-dependent small RNAs. J Exp Bot. 2015;66:1763–1768. doi: 10.1093/jxb/eru542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szczesniak MW, Rosikiewicz W, Makalowska I. CANTATAdb: a collection of plant long non-coding RNAs. Plant Cell Physiol. 2016;57:e8. doi: 10.1093/pcp/pcv201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi X, Zhang Z, Ling Y, Xu W, Su Z. PNRD: a plant non-coding RNA database. Nucleic Acids Res. 2015;43:D982–989. doi: 10.1093/nar/gku1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paytuvi Gallart A, Hermoso Pulido A, Anzar Martinez de Lagran I, Sanseverino W, Aiese Cigliano R. GREENC: a wiki-based database of plant lncRNAs. Nucleic Acids Res. 2016;44:D1161–1166. doi: 10.1093/nar/gkv1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zilberman D, Cao X, Jacobsen SE. ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science. 2003;299:716–719. doi: 10.1126/science.1079695. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y. miRU: an automated plant miRNA target prediction server. Nucleic Acids Res. 2005;33:W701–704. doi: 10.1093/nar/gki383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai X, Zhao PX. psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res. 2011;39:W155–159. doi: 10.1093/nar/gkr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shao C, Chen M, Meng Y. A reversed framework for the identification of microRNA-target pairs in plants. Brief Bioinform. 2013;14:293–301. doi: 10.1093/bib/bbs040. [DOI] [PubMed] [Google Scholar]
- 46.The Gene Ontology Consortium. Expansion of the gene ontology knowledgebase and resources. Nucleic Acids Res. 2017;45:D331–D338. doi: 10.1093/nar/gkw1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du Z, Zhou X, Ling Y, Zhang Z, Su Z. agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010;38:W64–70. doi: 10.1093/nar/gkq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leinonen R, Sugawara H, Shumway M. International nucleotide sequence database C. The sequence read archive. Nucleic Acids Res. 2011;39:D19–21. doi: 10.1093/nar/gkq1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M, Marshall KA, et al. NCBI GEO: archive for high-throughput functional genomic data. Nucleic Acids Res. 2009;37:D885–890. doi: 10.1093/nar/gkn764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawahara Y, de la Bastide M, Hamilton JP, Kanamori H, McCombie WR, Ouyang S, Schwartz DC, Tanaka T, Wu J, Zhou S, et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice. 2013;6:4. doi: 10.1186/1939-8433-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng Y, Shao C, Chen M. Toward microRNA-mediated gene regulatory networks in plants. Brief Bioinform. 2011;12:645–659. doi: 10.1093/bib/bbq091. [DOI] [PubMed] [Google Scholar]
- 53.Yu D, Ma X, Zuo Z, Shao W, Wang H, Meng Y. Bioinformatics resources for deciphering the biogenesis and action pathways of plant small RNAs. Rice. 2017;10:38. doi: 10.1186/s12284-017-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


