ABSTRACT
Alternative splicing largely enhanced the diversity of transcriptome and proteome in eukaryas. Along with technological development, more and more genes are reported to be alternatively spliced during mRNA maturation. Here, I report the alternative splicing of SKU5-Similar 3 (SKS3) and its special splicing site in Arabidopsis. SKS3 was predicted to be alternatively transcribed into two variants, SKS3.1 and SKS3.2, which encoded a GPI-anchored protein and a soluble secretory protein, respectively. But, according to experimental data, instead of SKS3.2, a novel variant, SKS3.3, which encodes a protein with a transmembrane region at its C-terminus, was demonstrated. Interestingly, it exhibites a different organ-specific expression pattern with SKS3.1, and an unusual intron splicing site not following ‘GT-AG’ rule or any reported rule.
KEYWORDS: GPI-anchored protein (GPI-AP), Alternative Splicing (AS), splicing site, Long non-coding RNA (lncRNA), Arabidopsis
Introduction
In eukaryas, precursor messenger RNA (pre-mRNA) is transcribed directly from genomic DNA, and then introns are removed, and exons are spliced to be mature mRNA for translation.1–3 The splicing of pre-mRNA is not always unique, but could be alternatively, which makes one gene transcribes diverse variants, and encodes various proteins.4 It largely enhanced the diversity of transcriptome and proteome.5,6 Along with the technological development, >95–100% of human genes,3 and up to 60% of intron-containing genes of Arabidopsis genes,1,2,5,7 are found alternatively spliced and produce at least two alternative variants. The alternative splicing processes are catalyzed by the protein-RNA complexes, spliceosomes, which could recognize the 5’, 3’ splice sites and the branch point of introns.8–10 This recognition and splicing mechanism results in “GT-AG” rule that, most introns start from “GT” and end at “AG”.4,8
Interestingly, the alternative splicing is alternatively that, its occurrence and expression pattern could be regulated by various abiotic stresses,11–15 developmental stages,16 or different organs.17 Although the exact mechanism hasn’t been revealed thoroughly yet,18 a few reports indicated its importance during development, such as, the different alternative variants of HAB1 play opposite roles during ABA signaling in Arabidopsis.19
SKU5-Similar 3 (SKS3) belongs to a subgroup of SKU5-Similar gene family that encode GPI-anchored proteins and are redundantly essential for cell polar expansion and cell wall synthesis of roots in Arabidopsis. SKS3 is predicted to alternatively encode asoluble secretory protein and aplasma membrane attached GPI-anchored protein.20 In this study, I report an unexpected organ-specific alternatively spliced variant of SKS3 in Arabidopsis, which could encode a plasma membrane attached protein with transmembrane region at C-terminus, besides the predicted variant encoding a GPI-anchored protein. Interestingly, its splicing site seems unique, which does not follow the “GT-AG” rule, or any other reported rules. But due to the short repeated “ATCCATC” localizing close to the borders of spliced intron, the exact site could not be identified.
Results
Predicted alternative splicing of SKS3 gene
According to NCBI (https://www.ncbi.nlm.nih.gov/) and TAIR (http://www.arabidopsis.org/) database, two transcriptional variants, SKS3.1 and SKS3.2 are predicated to be transcribed from SKS3 gene. SKS3.1 encodes aGPI-anchoredprotein precursor containing 589 amino acid residues, which could be modified with a glycosylphosphatidylinositol modification at C-terminus; and SKS3.2 encodes asecretoryprotein precursor containing 614 amino acid residues. Gene structures of SKS3.1 and SKS3.2 show that, the predicted alternative splicing occurs at the last exon where the STOP codon of SKS3.1 is removed together with the last intron of SKS3.2 (Figure1).
Figure 1.

Gene structures of SKS3.1 and SKS3.2 predicted by NCBI and TAIR database. Arrows showed the translation direction; exons are in dark grey; untraslated region (UTR) is in light grey; and introns are shown as black lines.
Observed alternative splicing of SKS3 gene in vivo
In Arabidopsis, SKS3 variants were cloned from cDNA generated from different organs. Surprisingly, instead of SKS3.2, a novel variant, SKS3.3, was identified, and exhibit a different expression pattern with SKS3.1 (Figure2(a)). SKS3.3 encodes a shorter precursor containing 314 amino acid residues predicted to attach to plasma membrane through its C-terminal transmembrane region (Figure2(b)). Gene structure of SKS3.3 exhibits an unusual alternative splicing with a long intron cleavage including the whole 6th, 7th and 8th exons, partly 5th and 9th exons, and all introns between them(Figure2(c)).
Figure 2.
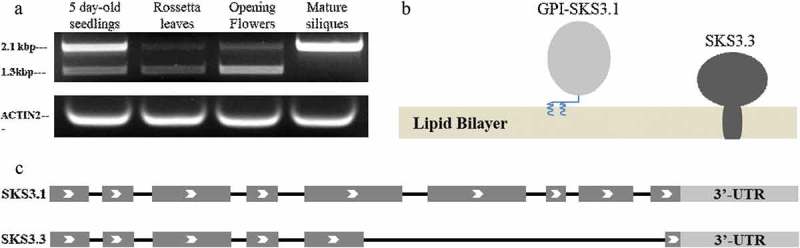
Alternatively splicing of SKS3 gene in Arabidopsis. (a), two SKS3 variants were amplified from cDNA extracted from different organs of Arabidopsis; Actin was utilized as reference and primers were indicated in methods; (b), predicted GPI-anchored SKS3.1 and transmembrane SKS3.3; (c). gene structure of SKS3.1 and SKS3.3. Arrows show the translation direction; exons are in dark grey; untranslated region (UTR) is in light grey, and introns are shown as black lines.
The novel splicing site within “ATCCATC” of SKS3.3
Interestingly, instead of “GT-AG” rule, the borders of the spliced intron do not follow any reported splicing rule, but an unusual “ATCCATC” repeat was found close to the splicing site (Figure 3). However, due to the presence of this repeat, the exact splicing site of SKS3. 3 could not be recognized yet, but limited within the short repeat “ATCCATC’’.
Figure 3.

Splicing site of SKS3.3 variant. Arrows showed the translation direction; exons are in dark grey; untranslated region (UTR) is in light grey, and introns are shown as black lines, the alternatively spliced intron is in red blank and the sequence close to splicing site is labeled in red, and the “ATCCATC” repeats are underlined.
Discussion
Alternative splicing largely enhances the diversity of transcriptome and proteome, which allows one gene to encode various proteins. According to NCBI and TAIR databases, SKS3 gene produces two transcriptional variants, SKS3.1 and SKS3.2, which encode a GPI-anchored protein and a secretory protein respectively. But instead of SKS3.2, experimental data reveals another transcriptional variant, SKS3.3, which encodes a C-terminal transmembrane protein and exhibites different expression pattern with SKS3.1 in various organs. It indicates the high complexity of alternative splicing in Arabidopsis.
Both GPI-anchored SKS3.1 and the smaller C-terminal transmembrane SKS3.3 are expected to attach to the external surface of plasma membrane. Differently, GPI anchoring is much more than a linkage to attach protein on plasma membrane, but plays essential roles in protein sorting and signaling transduction. It suggests a potential functional diversity of SKS3.1 and SKS3.3 protein.
Interestingly, AT5G01745 encoding along non-coding RNA (lncRNA) was found reveresly at 3’-terminus of SKS3, overlapping with the alternative splicing site of SKS3.3 variant (Figure4). LncRNA has been reported to be involved in alternative splicing, potentially through forming double-strand to prevent the recognition and splicing from spliceosome.21–25 It suggests the potential involvement of lncRNA in the unusual alternative splicing of SKS3, and it would be very interesting to further investigate the connection between the alternative splicing and the lncRNA, and the regulation of this lncRNA.
Figure 4.
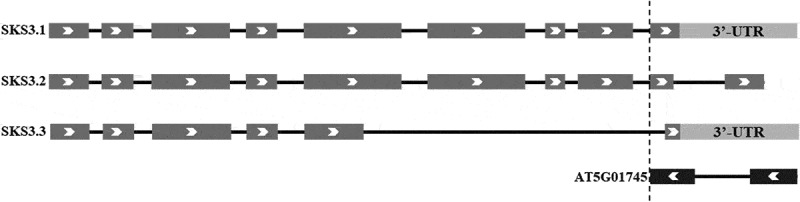
Transcriptional variants of SKS3, and the lncRNA encoding gene AT5G01745 reversely localized at 3’-terminus of SKS3. Arrows show the translation direction; exons are in dark grey; untranslated region (UTR) is in light grey, and introns are shown as black lines.
Generally, due to the specific recognition and splicing by spliceosomes, the vast majority of introns start from “GT” and end at “AG”,4,8–10 with a few exceptions, such as starting from “GC” and ending at “AG”.5 Interestingly, unusual intron borders were identified in SKS3.3variant. But due to the presence of the repeated “ATCCATC” close to it, the splicing site of SKS3.3 could only be limited within these short repeats. It would be very interesting to investigate the exact splicng site and its potential regulation by the lncRNA.
Methods
RNA extraction and semi-quantitative RT-PCR
Total RNA was extract from 5-day-old seedlings, rossetta leaves, whole opening flowers and mature siliques from Arabidopsis, and cDNAs were synthesized by TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgene).
Primers
Primers utilized for semi-quantitative PCR: SKS3-F, TTTTCTCCATTTTCACTCACTGCT; SKS3-R, CTAATATGATATCCGATCCCGGTT; Actin-F, GTTAGCAACTGGGATGATATGG; Actin-R, CAGCACCAATCGTGATGACTTGCCC.
Acknowledgement
I would like to thank the support by FAFU-UCR Joint Center for Horticultural Biology and Metabolomics, Haixia Institute of Science and Technology, Fujian Agriculture and Forestry University.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Bush SJ, Chen L, Tovar-Corona JM, Urrutia AO.. Alternative splicing and the evolution of phenotypic novelty. Philos Trans R Soc B Biol Sci. 2017;372:1–7. doi: 10.1098/rstb.2015.0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy ASN, Marquez Y, Kalyna M, Barta A.. Complexity of the alternative splicing landscape in plants. Plant Cell. 2013;25:3657–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y, Rio DC, Biology S, Biology C. Mechanisms and regulation of alternative pre-mRNA splicing. Annu Rev Biochem. 2015:291–323. doi: 10.1146/annurev-biochem-060614-034316.Mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelemen O, Convertini P, Zhang Z, Wen Y, Shen M, Falaleeva M, Stamm S. Function of alternative splicing. Gene. 2013;514:1–30. doi: 10.1016/j.gene.2012.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filichkin SA, Priest HD, Givan SA, Shen R, Bryant DW, Fox SE, Wong W-K, Mockler TC. Genome-wide mapping of alternative splicing in Arabidopsis thaliana. Genome. 2010:45–58. doi: 10.1101/gr.093302.109.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Severing EI, van Dijk ADJ, Van Ham RCHJ. Assessing the contribution of alternative splicing to proteome diversity in Arabidopsis thaliana using proteomics data. BMC Plant Biol. 2011;11:82. doi: 10.1186/1471-2229-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Gerstein M, Snyder M. RNA-seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gooding C, Godoy LMF, Mann M. A class of human exons with predicted distant branch points revealed by analysis of AG dinucleotide exclusion zones. Genome Biol. 2006;7:1–19. doi: 10.1186/gb-2006-7-8-R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behzadnia N, Lucas M, Cooke LA, Vyle JS, de la Cruz F, Moncalián G. Composition and three-dimensional EM structure of double affinity-purified, human prespliceosomal A complexes. EMBO J. 2007;26:1737–1748. doi: 10.1038/sj.emboj.7601806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai Q, Tuttle N, Novak T, Li N-S, Lu J, Koodathingal P, Dai Q, Staley JP, Piccirilli JA. RNA catalyses nuclear pre-mRNA splicing. Nature. 2013;503:229–234. doi: 10.1038/nature12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu G, Li W, Zhang F, Guo W. RNA-seq analysis reveals alternative splicing under salt stress in cotton, Gossypium davidsonii. BMC Genomics. 2018;19:1–15. doi: 10.1186/s12864-018-4449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leviatan N, Alkan N, Leshkowitz D, Fluhr R. Genome-wide survey of cold stress regulated alternative splicing in Arabidopsis thaliana with tiling microarray. PLoS One. 2013;8:e66511. doi: 10.1371/journal.pone.0066511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W, Lin W-D, Ray P, Lan P, Schmidt W. Genome-wide detection of condition-sensitive alternative splicing in Arabidopsis roots. Plant Physiol. 2013;162:1750–1763. doi: 10.1104/pp.113.217778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeoh LM, Goodman CD, Hall NE, van Dooren GG, McFadden GI, Ralph SA. A serine-arginine-rich (SR) splicing factor modulates alternative splicing of over a thousand genes in Toxoplasma gondii. Nucleic Acids Res. 2015;43:4661–4675. doi: 10.1093/nar/gkv311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon YJ, Park MJ, Kim SG, Baldwin IT, Park CM. Alternative splicing and nonsense-mediated decay of circadian clock genes under environmental stress conditions in Arabidopsis. BMC Plant Biol. 2014;14:1–15. doi: 10.1186/1471-2229-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deschênes M, Chabot B. The emerging role of alternative splicing in senescence and aging. Aging Cell. 2017;16:918–933. doi: 10.1111/acel.2017.16.issue-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loraine AE, McCormick S, Estrada A, Patel K, Qin P. RNA-seq of Arabidopsis pollen uncovers novel transcription and alternative splicing. Plant Physiol. 2013;162:1092–1109. doi: 10.1104/pp.112.211441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui Z, Tong A, Huo Y, Yan Z, Yang W, Yang X, Wang -X-X. SKIP controls flowering time via the alternative splicing of SEF pre-mRNA in Arabidopsis. BMC Biol. 2017;15:1–17. doi: 10.1186/s12915-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Jeong K, Vassiliou CC, Shin CS, Page RH, Avalos CE, Wang H-J, Pines A. ABA signalling is fine-tuned by antagonistic HAB1 variants. Nat Commun. 2015;6:8138. doi: 10.1038/ncomms9965. [DOI] [PubMed] [Google Scholar]
- 20.Zhou K. GPI-anchored SKS proteins regulate root development through controlling cell polar expansion and cell wall synthesis. Biochem Biophys Res Commun. 2019;509:119–124. doi: 10.1016/j.bbrc.2018.12.081. [DOI] [PubMed] [Google Scholar]
- 21.Chekanova JA. Long non-coding RNAs and their functions in plants. Curr Opin Plant Biol. 2015;27:207–216. doi: 10.1016/j.pbi.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Bardou F, Ariel F, Simpson C, Romero-Barrios N, Laporte P, Balzergue S, Brown JS, Crespi M. Long noncoding RNA modulates alternative splicing regulators in Arabidopsis. Dev Cell. 2014;30:166–176. doi: 10.1016/j.devcel.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Severing E, Faino L, Jamge S, Busscher M, Kuijer-Zhang Y, Bellinazzo F, Busscher-Lange J, Fernández V, Angenent GC, Immink RGH, et al. Arabidopsis thaliana ambient temperature responsive lncRNAs. BMC Plant Biol. 2018;18:1–10. doi: 10.1186/s12870-018-1362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Z, Saito A, Kusaka S, Kuniyoshi K, Shimomura Y. Identification and characterization of novel lncRNAs in Arabidopsis thaliana. Biochem Biophys Res Commun. 2017;488:348–354. doi: 10.1016/j.bbrc.2017.05.067. [DOI] [PubMed] [Google Scholar]
- 25.Ma L, Bajic VB, Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10:924–933. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]


