ABSTRACT
Mitogen-activated protein kinases (MAPKs) play important roles in plant development and adaptive responses to biotic and abiotic stresses. Recently, a rice MAPK gene, OsMAPK20-5, has been reported to protect rice plants against autotoxicity by suppressing herbivore-induced ethylene and nitric oxide signaling. In this context, we observed that silencing OsMAPK20-5 increased the percentage of leaf roll caused by leaf folder Cnaphalocrocis medinalis and the severity of rice blast caused by Magnaporthe grisea but decreased the severity of sheath blight caused by Rhizoctonia solani. These findings show that silencing OsMAPK20-5 has different effects on rice pests in the field, and these differences have important implications for the evolution and exploitation of resistance strategies in plants.
KEYWORDS: Rice, mitogen-activated protein kinase, Cnaphalocrocis medinalis, Magnaporthe grisea, Rhizoctonia solani, defense response, resistance
Plants have evolved strategies to protect themselves from biotic stresses, including pathogens and herbivorous insects.1,2 Of these defense strategies, induced plant defense is the most important. After recognizing herbivore-related signals, plants activate defense-related signaling pathways mainly mediated by mitogen-activated protein kinase (MAPK), jasmonic acid (JA), salicylic acid (SA), and ethylene.3,4 These pathways collectively regulate the transcription of defense-related genes and the production of defense compounds, allowing the plant to resist these biotic stresses.2,5 MAPK cascades, conserved in all eukaryotes, have been reported to play crucial roles in plant growth and in developing responses to biotic and abiotic stresses.6–8 Recently, we found that OsMAPK20-5, which is induced by the infestation of the gravid brown planthopper (BPH) females, Nilaparvata lugens, negatively regulated ethylene and nitric oxide (NO) accumulation in rice plants following such an infestation, thereby decreasing rice resistance to adult BPH and their oviposited eggs.9 Although this negative regulation by OsMAPK20-5 of ethylene and NO levels is regarded as a strategy plants use to prevent defense response-related autotoxicity, whether and how OsMAPK20-5 influences the performance of other rice pests remains unknown.
Rice, one of the most important staple crops worldwide, suffers heavily from many pests, the most destructive of which in China are the rice planthoppers, BPH and white-backed planthopper Sogatella furcifera, striped stem borer (SSB) Chilo suppressalis, leaf folder (LF) Cnaphalocrocis medinalis, rice blast caused by hemibiotrophic fungus Magnaporthe grisea, and sheath blight caused by necrotrophic fungus Rhizoctonia solani.10,11 Previous studies have revealed that rice has developed different mechanisms against these pests.12–18 For instance, JA- and ethylene-mediated signaling pathways positively regulate the resistance of rice to SSB but negatively mediate its resistance to BPH.16,17 Thus, we investigated the influence of rice lines with silencing of OsMAPK20-5 (ir-MAPK20-5 lines, ir-34, and ir-39), which were obtained by inserting an inverted-repeat orientation (irMAPK20-5) vector into the rice variety Xiushui 11 using Agrobacterium tumefaciens-mediated transformation,9 on these pests in the field.
In this study, plant growth conditions, plant age and lines used for experiments, and the design of the field experiment were the same as those described in Li et al.9 The severity of rice sheath blight and rice blast was investigated at the peak of disease following standard methods described in the International Rice Research Institute.19 For the investigation of damage levels caused by LF, we sampled 15 hills of plants in each plot at each time interval; for each hill, the number of leaves rolled by LF and the total number of leaves were recorded and then the percentage of leaf roll was calculated. The result showed that silencing OsMAPK20-5 decreased the severity of rice sheath blight on modified plants compared to wild-type (WT) plants (Figure 1(a)). In contrast, the percentage of leaf roll caused by LF was significantly higher on ir-MAPK20-5 lines than on WT plants at one investigation time (August 16, 2014), although no difference was observed at two other investigation times (Figure 1(b)). Moreover, the severity of rice blast was obviously higher on ir-MAPK20-5 lines than on WT plants (Figure 1(c)). Previously, OsMAPK20-5 was reported to suppress the production of oviposition-induced ethylene and NO in rice damaged by BPH, thereby decreasing the resistance of rice plants to BPH and protecting them from autotoxicity.9 These findings suggest that silencing OsMAPK20-5 has different ecological consequences for various rice pathogens and herbivores in the field, highlighting the specificity of plant resistance mechanisms in response to different pests.18 It has been well documented that JA, SA, and ethylene all positively regulate the resistance of rice to M. grisea and R. solani.20–22 Moreover, NO has been reported to positively regulate the resistance in beans and tomatoes to R. solani.23,24 These data may explain why silencing OsMAPK20-5 enhances the resistance of rice to R. solani but fails to explain why silencing OsMAPK20-5 decreases resistance to M. grisea. Perhaps silencing OsMAPK20-5 affects defense responses in rice plants differently depending on the pathogens and herbivores the plants confront. An example of a defense strategy that varies according to the pest can be seen in a rice 1-aminocyclopropane-1-carboxylic acid (ACC) synthase gene, OsACS2. Silencing OsACS2 was found to decrease volatile emission from rice plants infested by SSB but enhance volatile release from plants infested by gravid BPH females.25 Further research should elucidate why OsMAPK20-5-mediated defenses have different effects on these rice pests.
Figure 1.
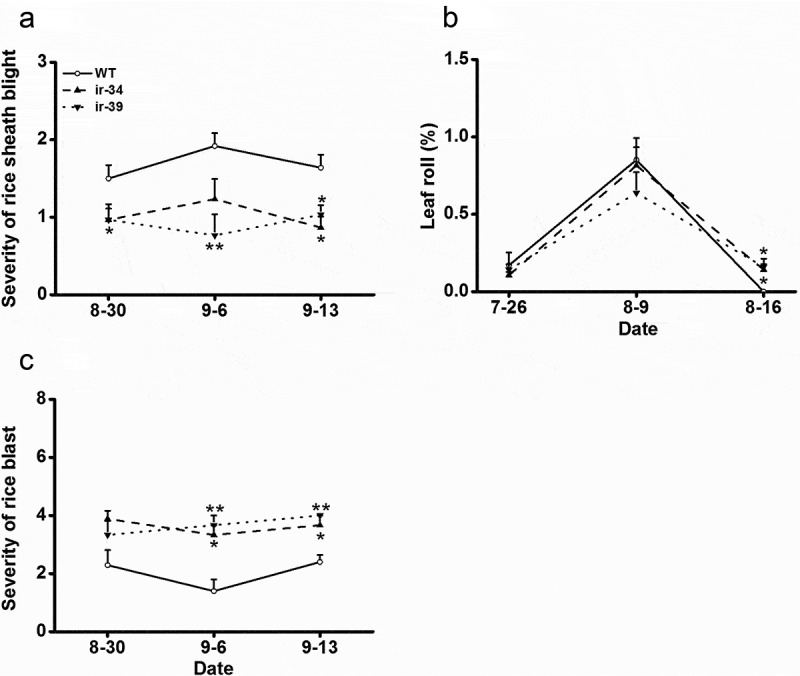
OsMAPK20-5 has different effects on various pests in the field.
Wild-type (WT) and ir-MAPK20-5 (ir-34, ir-39) plants were planted in an experimental field in 2014. (a and c) Mean severity (+SE, n = 3) of rice sheath blight (a) and rice blast (c) on ir-MAPK20-5 and WT plants during the investigation period. (b) Mean percentage (+SE, n = 3) of leaf roll on irMAPK20-5 and WT plants during the investigated period (*p < 0.05; **p < 0.01, Dunnett-t post hoc tests).
In nature, plants are always attacked simultaneously by multiple pathogens and herbivore species. Thus, the result displayed in this study – namely, that OsMAPK20-5-mediated rice defenses had different ecological consequences for various pests – indicates that the evolution of plant resistance strategies may be divergent and community dependent in nature, underlying the importance of the trade-offs plants must make between resistance to various biotic stresses. From the perspective of the application, the different ecological consequences for various pests suggest that it is important and necessary when breeding resistant crop varieties to identify the main pest species in the crop system and to understand their interactions each other and with each crop plant.
Funding Statement
This work was supported by the National Natural Science Foundation of China [31330065];Special Fund for Agro-scientific Research in the Public Interest [201403030]; the National Program of Transgenic Variety Development of China (2016ZX08001-001); and the earmarked fund for China Agriculture Research System (CARS-01-40).
Acknowledgments
We thank Emily Wheeler for editorial assistance. The study was jointly sponsored by the National Natural Science Foundation of China (31330065), the Special Fund for Agro-scientific Research in the Public Interest (201403030), the National Program of Transgenic Variety Development of China (2016ZX08001-001), and the earmarked fund for China Agriculture Research System (CARS-01-40).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kombrink E, Somssich IE.. Defense responses of plants to pathogens. Adv Bot Res. 1995;21:1–3. [Google Scholar]
- 2.Schuman MC, Baldwin IT. The layers of plant responses to insect herbivores. Annu Rev Entomol. 2016;61:373–394. doi: 10.1146/annurev-ento-010715-023851. [DOI] [PubMed] [Google Scholar]
- 3.Erb M, Meldau S, Howe GA. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012;17:250–259. doi: 10.1016/j.tplants.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kazan K, Lyons R. Intervention of phytohormone pathways by pathogen effectors. Plant Cell. 2014;26:2285–2309. doi: 10.1105/tpc.114.125419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hancock RD, Hogenhout S, Foyer CH. Mechanisms of plant-insect interaction. J Exp Bot. 2015;66:421–424. doi: 10.1093/jxb/eru503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Zhang S. Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci. 2015;20:56–64. doi: 10.1016/j.tplants.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Bequette CJ. MAP kinase signaling in plant responses to biotic stress. Doctor of Philosophy in Biological Sciences thesis, University of South Carolina, 2015. [Google Scholar]
- 8.Wimalasekera R, Scherer GFE. Involvement of mitogen-activated protein kinases in abiotic stress responses in plants. Plant Metabol Regul Under Environ Stress. 2018; Chaper 21. 389–395. [Google Scholar]
- 9.Li J, Liu X, Wang Q, Huangfu J, Schuman MC, Lou Y. A group D MAPK protects plants from autotoxicity by suppressing herbivore-induced defense signaling. Plant Physiol. 2019;179:1386–1401. doi: 10.1104/pp.18.01411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ, Thon M, Kulkarni R, Xu JR, Pan H, et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 2005;434:980–986. doi: 10.1038/nature03449. [DOI] [PubMed] [Google Scholar]
- 11.Chai HN, Du YZ, Zhai BP. Characterization of the complete mitochondrial genomes of Cnaphalocrocis medinalis and Chilo suppressalis (Lepidoptera: Pyralidae). Int J Biol Sci. 2012;8:561–579. doi: 10.7150/ijbs.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, Shahzad MF, Zhang Z, Sun H, Han P, Li F, Han Z. Genome-wide analysis reveals the expansion of cytochrome P450 genes with xenobiotic metabolism in rice striped stem borer, Chilo suppressalis. Biochem Biophys Res Commun. 2014;443:756–760. doi: 10.1016/j.bbrc.2013.12.045. [DOI] [PubMed] [Google Scholar]
- 13.Ye GY, Yao HW, Shu QY, Cheng X, Hu C, Xia YW, Gao MW, Altosaar I. High levels of stable resistance in transgenic rice with a cry1Ab gene from Bacillus thuringiensis Berliner to rice leaffolder, Cnaphalocrocis medinalis (Guenée) under field conditions. Crop Prot. 2003;22:171–178. doi: 10.1016/S0261-2194(02)00142-4. [DOI] [Google Scholar]
- 14.Sharma TR, Madhav MS, Singh BK, Shanker P, Jana TK, Dalal V, Pandit A, Singh A, Gaikwad K, Upreti HC, et al. High-resolution mapping, cloning and molecular characterization of the Pi-kh gene of rice, which confers resistance to Magnaporthe grisea. Mol Gen Genomics. 2005;274:569–578. doi: 10.1007/s00438-005-0035-2. [DOI] [PubMed] [Google Scholar]
- 15.Datta K, Velazhahan R, Oliva N, Ona I, Mew T, Khush GS, Muthukrishnan S, Datta SK. Over-expression of the cloned rice thaumatin-like protein (PR-5) gene in transgenic rice plants enhances environmental friendly resistance to Rhizoctonia solani causing sheath blight disease. Theor Appl Genet. 1999;98:1138–1145. doi: 10.1007/s001220051178. [DOI] [Google Scholar]
- 16.Zhou G, Qi J, Ren N, Cheng J, Erb M, Mao B, Lou Y. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 2009;60:638–648. doi: 10.1111/j.1365-313X.2009.03988.x. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Ju H, Zhou G, Zhu C, Erb M, Wang X, Wang P, Lou Y. An EAR-motif-containing ERF transcription factor affects herbivore-induced signaling, defense and resistance in rice. Plant J. 2011;68:583–596. doi: 10.1111/j.1365-313X.2011.04709.x. [DOI] [PubMed] [Google Scholar]
- 18.Stam JM, Kroes A, Li YH, Gols R, van Loon JJA, Poelman EH, Dicke M. Plant interactions with multiple insect herbivores: from community to genes. Annu Rev Plant Biol. 2014;65:689–713. doi: 10.1146/annurev-arplant-050213-035937. [DOI] [PubMed] [Google Scholar]
- 19.IRRI. Standard Evaluation System (SES) INGER, IRRI Philippines 1996; p. 52.
- 20.Xie XZ, Xue YJ, Zhou JJ, Zhang B, Chang H, Takano M. Phytochromes regulate SA and JA signaling pathways in rice and are required for developmentally controlled resistance to Magnaporthe grisea. Mol Plant. 2011;4:688–696. doi: 10.1093/mp/ssr005. [DOI] [PubMed] [Google Scholar]
- 21.Taheri P, Tarighi S. Riboflavin induces resistance in rice against Rhizoctonia solani via jasmonate-mediated priming of phenylpropanoid pathway. J Plant Physiol. 2010;167:201–208. doi: 10.1016/j.jplph.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Helliwell EE, Wang Q, Yang Y. Transgenic rice with inducible ethylene production exhibits broad-spectrum disease resistance to the fungal pathogens Magnaporthe oryzae and Rhizoctonia solani. Plant Biotechnol J. 2013;11:33–42. doi: 10.1111/pbi.12004. [DOI] [PubMed] [Google Scholar]
- 23.Noorbakhsh Z, Taheri P. Nitric oxide: a signaling molecule which activates cell wall-associated defense of tomato against Rhizoctonia solani. Eur J Plant Pathol. 2016;144:551–568. doi: 10.1007/s10658-015-0794-5. [DOI] [Google Scholar]
- 24.Keshavarz-Tohid V, Taheri P, Taghavi SM, Tarighi S. The role of nitric oxide in basal and induced resistance in relation with hydrogen peroxide and antioxidant enzymes. J Plant Physiol. 2016;199:29–38. doi: 10.1016/j.jplph.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Li J, Ju H, Liu X, Erb M, Wang X, Lou Y. Contrasting effects of ethylene biosynthesis on induced plant resistance against a chewing and a piercing-sucking herbivore in rice. Mol Plant. 2014;7:1670–1682. doi: 10.1093/mp/ssu085. [DOI] [PubMed] [Google Scholar]


