Abstract
Introduction: When nanoparticles (NPs) enter a physiological environment, a coating of biomolecules or biocorona (BC) forms on the surface. Formation of the NP-BC is dependent on NP properties, the physiological environment, and time. The BC influences NP properties and biological interactions such as cellular internalization, immune responses, biodistribution, and others, leading to pharmacological and toxicological consequences. To date, examination of the NP-BC has focused primarily on protein components and healthy conditions. Therefore, we evaluated the protein and lipid content of BCs that formed on physicochemically distinct gold nanoparticles (AuNPs) under healthy and obese conditions. A comprehensive understanding of the NP-BC is necessary for the translation of in vitro toxicity assessments to clinical applications.
Materials and Methods: AuNPs with two coatings (poly-N-vinylpyrrolidone [PVP] or citrate) and diameters (20 or 100 nm) were incubated in pooled human serum, and an integrated proteomic/lipidomic approach was used to evaluate BC composition. Macrophages were utilized to evaluate differential immune responses due to variations in the AuNP-BC.
Results: AuNPs form distinct BCs based on physicochemical properties and the surrounding environment, with the obese BC containing more proteins and fewer lipids than the healthy BC. Differential macrophage inflammatory responses were observed based on AuNP properties and BC composition.
Discussion and Conclusion: Overall, these findings demonstrate that AuNP size and coating, as well as physiological environment, influence the protein and lipid composition of the BC, which impacts cellular responses following exposure. These findings demonstrate that incorporation of BCs representing distinct physiological conditions may enhance the translatability of nanosafety in vitro studies.
Keywords: corona, in vitro, lipids, macrophages, nanotoxicology, obesity
Introduction
Nanoparticles are increasingly utilized in a variety of processes and technologies, impacting manufacturing procedures, medicine, electronics, and consumer products. Gold nanoparticles (AuNPs) are considered a promising candidate for a number of biomedical applications due to their biocompatibility and anti-inflammatory properties.1,2 Currently, gold salts and AuNPs are used in the treatment of arthritis and as an agent for some cancer treatments.3,4 Proposed uses of AuNPs have a broad scope, ranging from photodynamic cancer therapy and targeted drug delivery to their inclusion in vaccines.5–7 The small size of nano-gold enhances therapeutic efficacy compared to conventional methods. Specifically related to size, AuNPs have increased surface area, allowing for the association of larger amounts of drug while also more effectively penetrating biological barriers.
To translate AuNPs to a clinical setting, it is necessary to understand initial interactions between AuNPs and biomolecules that dictate subsequent biological responses. When NPs enter a physiological environment, they form a coating of biomolecules known as a biocorona (BC). The BC imparts a new surface to the NP, altering NP physicochemical properties, influencing interactions with cells, biodistribution, clearance, functionality, and toxicity.8–12 The clinical application of AuNP-based theragnostic approaches requires a comprehensive assessment of the NP-biomolecular interactions forming the BC and their influence on cellular responses.
The formation of the NP-BC is governed by NP physicochemical properties, the biological environment, and time.13–16 The majority of BC research has focused on NP properties (material, charge, size, surface coating, etc.) influencing surface interactions with proteins.14,17–19 However, fewer studies have examined the impact of the biological environment on BC formation. It is known that many factors, such as gender, diet, underlying disease states, and others, can influence the biomolecular profile within the circulation.20–22 Previous research has demonstrated that the changes in circulating biomolecules that occur in hyperlipidemia result in altered iron oxide NP-BC composition, cellular interactions, and toxicological consequences.23
To date, many disease states that likely influence BC formation have not been adequately examined. Chronic underlying disease conditions are increasingly common within our population. Furthermore, individuals suffering from these conditions are known to often be susceptible to exposures and are more likely to receive medical treatments.24,25 Obesity is increasingly prevalent (∼40% of adults in the United States) and predisposes individuals to serious diseases, including heart disease, stroke, apnea, diabetes, cancer, and others.26–28 Obesity is associated with modifications in metabolism and circulating factors, including proteins and lipids.29–32 Currently, little is known about NP-lipid interactions and how this may impact BC formation. Our previous assessment demonstrated that hyperlipidemia alters the protein content of the BC that forms on single-walled carbon nanotubes.33 To safely and effectively utilize AuNPs for theragnostic applications, it is necessary to understand how widespread diseases such as obesity may impact biological responses.
While NP therapeutics have generated significant interest in recent years, there has been difficulty in translating in vitro laboratory findings to clinical application. The BC represents a major challenge for the development and use of NPs in medicine, particularly those designed to act as “stealth” therapeutics meant to avoid immune system detection.34 The translation of cell-based toxicity assessments may be assisted by the incorporation of the BC into study designs. Through the inclusion of the BC, in vitro studies may more accurately depict the in vivo environment and appropriately model NP-cellular interactions and responses. Furthermore, high throughput cell-based screening methods could allow for the evaluation of a number of physiological environments to identify potential susceptible populations to exposure.
In this study, variations in AuNP-BC composition due to differences in physicochemical properties and a common disease condition, obesity, were examined through the utilization of pooled human serum. In addition, a macrophage cell line was used to assess the toxicological implications of these altered BCs. A comprehensive examination of the biomolecules (proteins and lipids) composing the AuNP-BC in prevalent subpopulations will allow for a more complete understanding regarding initial interactions that occur when AuNPs are introduced into biological environments. Ultimately, these results will assist in the utilization of in vitro studies to more effectively evaluate AuNPs before in vivo studies and clinical usage.
Materials and Methods
AuNP characterization
Four AuNPs that differed based on size (diameter 20 or 100 nm) and suspension material (poly-N-vinylpyrrolidone [PVP] or citrate) were purchased (Nanocomposix, San Diego, CA). AuNPs were characterized to verify manufacturer's specifications. The hydrodynamic size, polydispersion index, and ζ-potential (ZetaSizer Nano, Malvern) were assessed in deionized water with AuNPs at a concentration of 25 μg/mL (n = 3/AuNP). The number of AuNPs per mL was determined by NP tracking software (NanoSight, Malvern, Westborough, MA) (n = 3/AuNP).
Human serum characterization
Pooled human sera from healthy or obese individuals (males and females) were purchased from Lee Biosolutions (Maryland Heights, MO). Sera were characterized for traditional lipid end points used in health clinics through commercially available kits to measure triglycerides (Cayman Chemical, Ann Arbor, MI), as well as total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein/very low-density lipoprotein (LDL/VLDL) (Bioassay Systems, Hayward, CA) (n = 5).
Formation of the AuNP BC
BCs were formed on AuNPs as described in our recent publications.8,23 Briefly, AuNPs were incubated for 8 hours at 4°C in 10% serum while being constantly mixed. Specifically, 250 μL of AuNPs (1 mg/mL), 650 μL of deionized water, and 100 μL of healthy or obese pooled human serum (Lee Biosolutions) were combined in a 1.5 mL tube. Following incubation, AuNPs were pelleted by centrifugation at 15,000 g for 10 minutes and washed with phosphate-buffered saline (PBS). A total of three washes with PBS were performed to remove any free biomolecules not associated with the AuNPs. Four replicates of each AuNP-BC were produced for subsequent proteomic analysis. Furthermore, four were produced and combined for lipid screening and library construction. Identification of lipid components of the BC and relative quantification of lipids were performed using three additional replicates.
Assessment of the protein components of the AuNP BC
Protein components of the BC were prepared and analyzed in a manner similar to our previous publications.23 Briefly, proteins were digested using porcine trypsin (0.2 ng/μL) before being concentrated to dryness, resuspended in 0.1% trifluoroacetic acid in water, and purified using UltraMicroSpin columns (The Nest Group, Southborough, MA). Purified peptide samples following concentration were resuspended in 0.1% formic acid in HPLC-grade water for mass spectrometry analysis.
All MS data were acquired using the Dionex UltiMate 3000 RSLC Nano LC System attached to the Q Exactive™ HF Hybrid Quadrupole-Orbitrap Mass Spectrometer (Thermo Scientific, Waltham, MA) with a loading pump at a flow rate of 5 mL/min and settings similar to those which are described in our previous publications.23
Briefly, 1 μg of peptide solution was loaded into the trap column and washed for 5 minutes using 2% acetonitrile in purified water followed by separation of peptides using a 120-minute method. Peptides were eluted at a flow rate of 300 nL/min with a linear gradient of 5% to 30% buffer B in 80 minutes and reaching 45% B in 91 minutes, then 100% of B in 93 minutes. B was held for 7 minutes at 100% before reverting back to 5% B in 100 minutes. After each 120-minute sample run, columns were washed 2× with a linear gradient of 5% to 100% of B to keep them clean and reduce sample carry over before running the next sample.
Analysis of proteomics data
The files from the mass spectrometer were processed using the MaxQuant computational proteomics platform version 1.6.2.10.35 The peak list generated was searched against the Homo sapiens sequences from UNIPROT retrieved on September 19, 2018 and a common contaminant database. The following settings were used for MaxQuant: default Orbitrap parameters, minimum peptide length of seven amino acids, data were analyzed with the iBAQ method and “Match between runs” interval set to 1 minute, protein FDR was set to 1%, enzyme trypsin allowing for two missed cleavage and three modifications per peptide, fixed modifications were Carbamidomethyl (C), and variable modifications were set to Acetyl (Protein N-term) and Oxidation (M).
An in-house script was used to perform the following steps on the MaxQuant results: removed all the common contaminant proteins and generated Venn Diagrams of proteins shared between conditions and particles. The statistical analyses were performed in the R environment (www.cran.r-project.org). A t-test was performed on the iBAQ intensities, and only proteins with p-value <0.05 were used in all analyses. Supplementary Table S1 contains protein data, including identification and average abundance.
Sample preparation for lipid screening of the AuNP BC
Following formation of the BC, lipids were extracted from the particle surface using the Bligh–Dyer lipid extraction method.36 Briefly, AuNPs were centrifuged at 20,000 g for 20 minutes before removal of supernatant followed by the addition of PBS, methanol, and chloroform. Mixtures were then vortexed briefly before the introduction of purified water and additional chloroform, after which they were centrifuged at 16,000 g for 10 minutes. This caused the mixture to separate into the aqueous alcoholic (top) phase and the organic chloroform (bottom) phase. The bottom phase was isolated and concentrated to dryness. Before analysis, samples were resuspended in a 3:6.65:0.35 mixture of methanol, acetonitrile, and ammonium acetate.
Lipid screening data acquisition
Samples were diluted 3.5× in a 3:6.65:0.35 mixture of methanol, acetonitrile, and ammonium acetate. A volume of 8 μL of diluted sample was used for flow injection at a triple quadrupole mass spectrometer (6410 Agilent) equipped with a capillary pump (Agilent 1100 series) and an autosampler (G1377A Agilent). Pump flow rate was set at 20 μL/min, and the machine was pumped with 0.1% formic acid in acetonitrile between sample injections. Capillary voltage on the instrument was 3.5–5 kV, and the gas flow was 5.1 L/min at 300°C.
The multiple reaction monitoring (MRM) profiling approach was used. The MRM profiling is based on chemical functional group screening and flow injection and consists of a “discovery” phase to first identify all lipids and classes present before a “screening” phase, which is used to further determine the presence and relative quantity of lipids found in distinct samples.37–44 The triple quadrupole mass spectrometer was set to monitor lists of MRMs related to different lipid classes: ceramides, phosphatidylcholines and sphingomyelins, phosphatidylethanolamines, phosphatidylglycerols, phosphatidylinositols, phosphatidylserines, cholesteryl esters, triacylglycerols (triglycerides), and the metabolite class of acyl-carnitines. Free fatty acids were monitored only by the parent mass. The parent masses of the MRMs screened were based on the lipid maps, and the product ions were related to class-related fragments (e.g., m/z 184 for Phosphatidylcholine/Spingomyelin [PC/SM] lipids).
Due to the large number of lipid isomers, the many MRMs corresponded to multiple individual lipids, meaning that the number of lipids reported as present in this publication is the lowest theoretical value for the possible number of lipids present. Egg yolk lipid extract was used as a quality control sample to monitor instrument performance. Pure methanol (8 μL) was used to flush the system between the injections of different samples. Supplementary Table S2 contains all lipid data, including identities and average abundance.
Cell culture and toxicity assessment
Macrophages were selected to evaluate BC alterations in NP-cellular interactions due to their presence at sites of NP deposition, as well as their role in recognizing and removing foreign materials. RAW 264.7 macrophages were grown at 37°C and 5% CO2 in a Galaxy 170 S incubator (New Brunswick Scientific, Edison, NJ) using DMEM media with 10% fetal bovine essence and 1% penicillin/streptomycin. Cells were plated in a 96-well plate and allowed to grow to 90% confluency before exposure to AuNPs with or without a healthy or obese BC at 12.5 or 50 μg/mL in serum-free media for 3 or 24 hours.
Cellular viability was assessed using the MTT assay (n = 4/group). BC-induced variations in the inflammatory response were measured following a 24-hour exposure to 50 μg/mL of AuNP through assessment of gene expression of tumor necrosis factor-α (TNF-α), macrophage inflammatory protein-2 (MIP-2), monocyte chemoattractant protein (MCP-1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (control) using real-time reverse transcriptase PCR (n = 6/group). Untreated macrophages in serum-free media were utilized as controls for the MTT assay and assessment of gene expression.
Statistics and biomolecular data analysis
Only proteins found in three of four replicates were counted as present and used in subsequent analyses. Lipids were required to be 30% above the blank in all three replicates to be counted as present and used in subsequent analyses. Identified biomolecules from each AuNP BC were compared, and Venn diagrams were generated using the online tool Venny 2.1.0 (http://bioinfogp.cnb.csic.es/tools/venny) to determine commonalities and differences in the identities of biomolecules within the BC of each AuNP. Biomolecule abundance differences between healthy and obese were determined as significant by a student's t-test; p < 0.05. Comparisons of biomolecule abundance between AuNPs were determined by one-way ANOVA with Tukey post hoc analysis; p < 0.05. Comparisons of macrophage inflammatory responses were also performed by one-way ANOVA with Tukey post hoc analysis; p < 0.05.
Results
Serum and AuNP characterization
Obese serum was found to have elevated triglycerides, HDL, LDL/VLDL, and total cholesterol compared to healthy serum (Table 1). The hydrodynamic size, ζ-potential, and polydispersion index of all four AuNPs were characterized with no BC and following addition of either a healthy BC or obese BC (Table 2). Addition of all BCs increased the hydrodynamic size of the AuNPs, with obese BCs causing a larger increase than healthy BCs (Table 2). The 20 nm PVP AuNP, while following the same pattern, had a smaller hydrodynamic size than the 20 nm citrate coated AuNPs in both healthy and obese serum. Limited polydispersion was observed for all AuNPs with and without a BC (Table 2). ζ-potential of all AuNPs was decreased following addition of either the healthy or obese BC (Table 2).
Table 1.
Characterization of Healthy and Obese Human Serum
| Triglycerides (mg/dL) | HDL (mg/dL) | LDL/VLDL (mg/dL) | Total cholesterol (mg/dL) | |
|---|---|---|---|---|
| Healthy serum | 38.8 ± 0.5 | 26.0 ± 0.6 | 145.4 ± 3.2 | 183.1 ± 1.9 |
| Obese serum | 191.6 ± 3.0a | 56.5 ± 0.8a | 225.2 ± 1.6a | 231.5 ± 0.7a |
Pooled human serum from healthy and obese male and female individuals was characterized for traditional lipid end points used in health clinics through commercially available kits to measure triglycerides, total cholesterol, HDL, and LDL/VLDL. All analyses are reported as mean ± SEM (n = 5/group). Statistical significance was determined by t-test, p < 0.05.
Denotes statistical significant difference between healthy and obese serum (p < 0.05).
HDL, high-density lipoprotein; LDL/VLDL, low-density lipoprotein/very low-density lipoprotein.
Table 2.
Gold Nanoparticle Characterization Following Addition of Biocoronas
| BC | Citrate 20 | Citrate 100 | PVP 20 | PVP 100 |
|---|---|---|---|---|
| Particle concentration (NPs/μg × 108) | ||||
| None | 6.9 ± 1.6 | 3.2 ± 0.3 | 2.8 ± 0.6 | 2.0 ± 0.5 |
| Hydrodynamic size (nm) | ||||
| None | 23.4 ± 0.5 | 103.9 ± 2.0 | 43.9 ± 7.9 | 134.5 ± 3.2 |
| Healthy | 870.9 ± 165.0a | 915.9 ± 157.3a | 134.4 ± 2.3a | 955.8 ± 190.9a |
| Obese | 1303.3 ± 95.0a,b | 1435 ± 104.8a,b | 328.1 ± 53.6a,b | 1420 ± 22.3a,b |
| Polydispersion index | ||||
| None | 0.16 ± 0.02 | 0.09 ± 0.01 | 0.17 ± 0.05 | 0.11 ± 0.08 |
| Healthy | 0.29 ± 0.06a | 0.27 ± 0.07 | 0.07 ± 0.01a | 0.39 ± 0.12a |
| Obese | 0.34 ± 0.02a | 0.14 ± 0.13 | 0.19 ± 0.03b | 0.10 ± 0.05b |
| ζ Potential (mV) | ||||
| None | −35.5 ± 2.6 | −43.0 ± 3.7 | −30.3 ± 2.9 | −28.3 ± 3.8 |
| Healthy | −15.1 ± 2.7a | −7.3 ± 2.2a | −16.5 ± 3.3a | −8.6 ± 1.2a |
| Obese | −13.5 ± 1.7a | −8.2 ± 2.1a | −14.2 ± 2.1a | −5.7 ± 1.8a |
AuNPs were diluted in and incubated for 8 hours at 4°C in 10% pooled human serum. AuNPs were characterized with and without BCs through assessment of ζ-potential, hydrodynamic size, and polydispersion index. Data represent mean ± standard deviation, n = 3–5/group. Statistical significance was determined by one-way ANOVA using Tukey's multiple comparisons test, p < 0.05.
Denotes statistical significance compared to an AuNP with no BC.
Denotes statistical significance compared to an AuNP with healthy BC.
BC, biocorona; NP, nanoparticle.
Protein components of the BC—identification and relative quantification between AuNPs
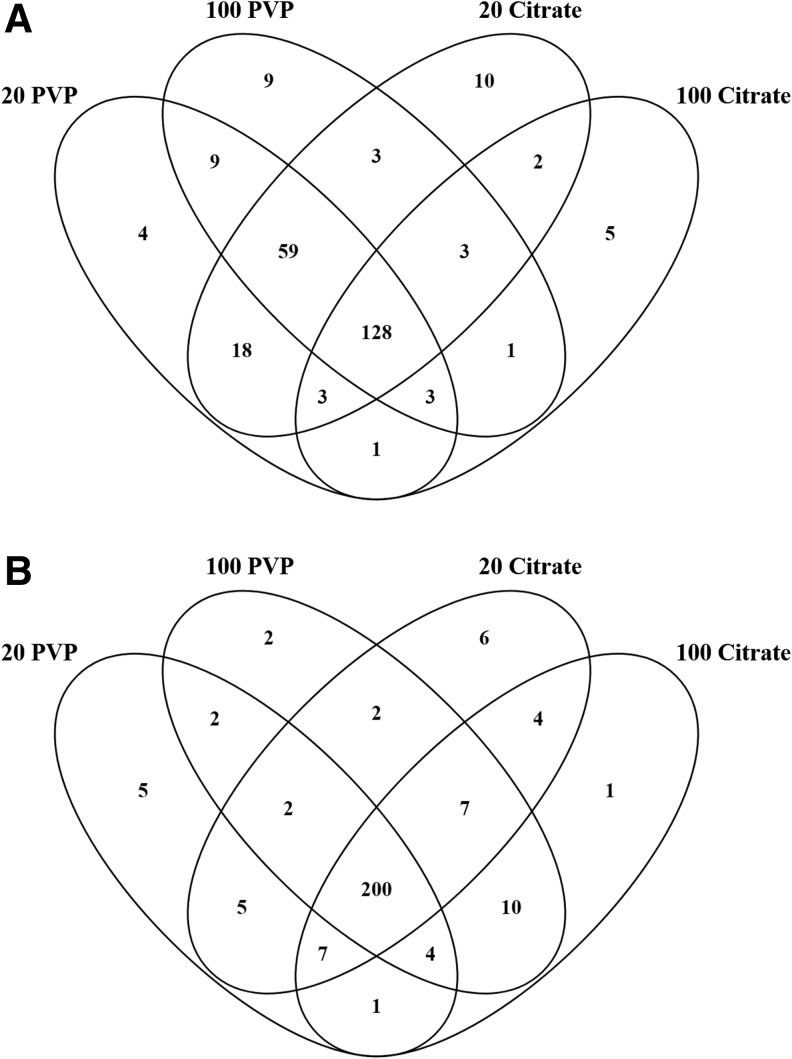
Following incubation in healthy serum, a total of 225, 215, 226, and 146 proteins were found in the BCs of the 20 nm PVP-, 100 nm PVP-, 20 nm citrate-, and 100 nm citrate-coated AuNPs, respectively (Fig. 1A and Supplementary Table S3). In the healthy serum environment, a total of 128 proteins (49.6% of all proteins identified) were shared between all four AuNPs, including apolipoproteins A-1, A-2, A-4, B, C-1, C-2, D, E, as well as gelsolin, complement factor H, coagulation factor XII, plasminogen, prothrombin, fibronectin 1, vitronectin, clusterin, and others. The BCs on 20 nm PVP-, 100 nm PVP-, 20 nm citrate-, and 100 nm citrate-coated AuNPs contained 4, 9, 10, and 5 unique proteins, respectively (Fig. 1A and Supplementary Table S3).
FIG. 1.
Comparison of BC composition of all four particles in healthy or obese serum. Proteins identified from each AuNP BC were compared to proteins in all other AuNP BCs under the same condition, either (A) healthy or (B) obese. Venn diagrams were used to illustrate all comparisons. A comprehensive list of all proteins found in each BC may be found in Supplementary Table S1. List of specific proteins used to generate Venn diagram (A, B) may be found in Supplementary Tables S3 and S4, respectively. AuNPs, gold nanoparticles; BC, biocorona.
A total of 18 proteins were exclusive to the 20 nm AuNPs incubated in healthy serum. Among these 18 were coagulation factor XII, complement factor 9, soluble scavenger receptor, and multimerin-1. There were nine proteins found only in the PVP-coated AuNPs, including insulin-like growth factor 2, ficolin-2, and histone H2A. Only 2 proteins, metalloreductase STEAP3 and epididymis luminal protein 213, were exclusive to the two 20 citrate-coated AuNPs. Cholesterol ester transfer protein was found in the BCs of the two 100 nm AuNPs and not the 20 nm AuNP BCs.
In obese serum, 20 nm PVP-, 100 nm PVP-, 20 nm citrate-, and 100 nm citrate-coated AuNPs adsorbed 226, 229, 233, and 234 proteins, respectively. AuNPs incubated in obese serum formed BCs that shared 200 proteins, or 77.5% of all proteins identified, including apolipoproteins A-I, A-II, A-IV, C-I, C-III, E, and LI, complement components 1, C6, and H, von Willebrand factor, transthyretin, clusterin, gelsolin, and others (Fig. 1B and Supplementary Table S4). All AuNPs were determined to associate unique proteins, with the 20 nm PVP-, 100 nm PVP-, 20 nm citrate-, and 100 nm citrate-coated AuNP BCs containing 5, 2, 6, and 1 exclusive proteins, respectively.
The 20 nm AuNPs contain 5 exclusive proteins, which include retinoic acid receptor responder protein 2 and immunoglobulin heavy variables 1–18 and 3–38, while the 100 nm AuNPs have 10, including β-2-microglobulin and soluble scavenger receptor. The PVP-coated AuNPs exclusively share 2 proteins, ficolin-2 and cDNA FLJ95778 (similar to SERPINA10), while the citrate-coated AuNPs have 4, including serpin peptidase inhibitor and cDNA FLJ56989 (similar to MARCO).
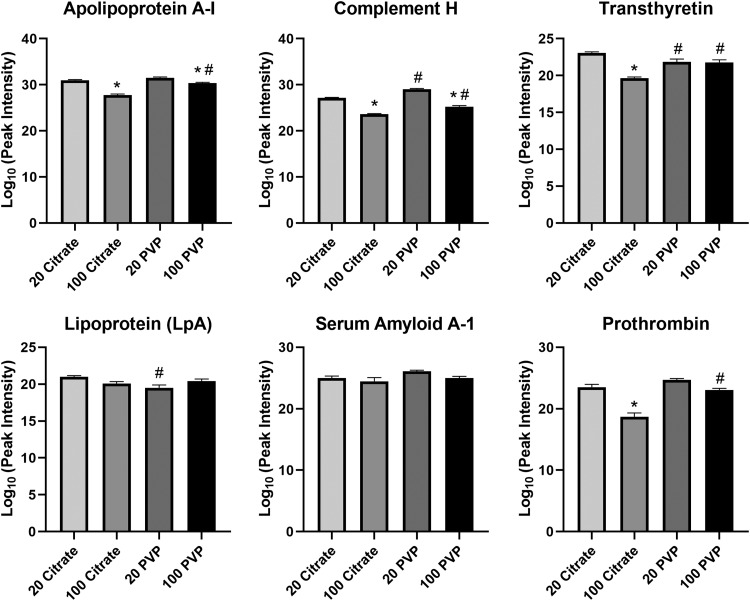
Although the majority of proteins identified were shared between AuNPs, differences in quantity were determined due to variations in AuNP size and surface coating (Fig. 2). For example, in healthy serum, apolipoprotein A-1 and complement H both bind 20 nm AuNPs in greater abundance than their 100 nm counterparts (Fig. 2). Transthyretin and prothrombin associated citrate-coated AuNPs in a size dependent manner (20 nm >100 nm) while equivalently binding PVP-coated AuNPs. The PVP-coated 100 nm AuNPs adsorbed more apolipoprotein A-1, complement H, transthyretin, and prothrombin than the citrate-coated 100 nm AuNPs. There was also more complement H protein on the PVP-coated 20 nm AuNP than the citrate-coated 20 nm AuNP. The opposite was true for transthyretin and lipoprotein-A, both of which were more abundant on the citrate-coated 20 nm AuNPs than the PVP-coated 20 nm AuNPs. All AuNPs were determined to associate similar amounts of serum amyloid A.
FIG. 2.
Relative abundance comparison of selected proteins found in the BCs of all four AuNPs in the healthy condition. Proteins identified as present with the BCs of all four AuNPs in the healthy condition were compared in their abundance. Peak intensities were log10 transformed to convert the raw values to a more readily understandable format. Comparisons were performed by one-way ANOVA with Tukey post hoc analysis (p < 0.05). *Denotes statistical significance compared to the corresponding 20 nm AuNP with the same coating, #denotes statistical significance compared to the corresponding citrate-coated AuNP with the same size.
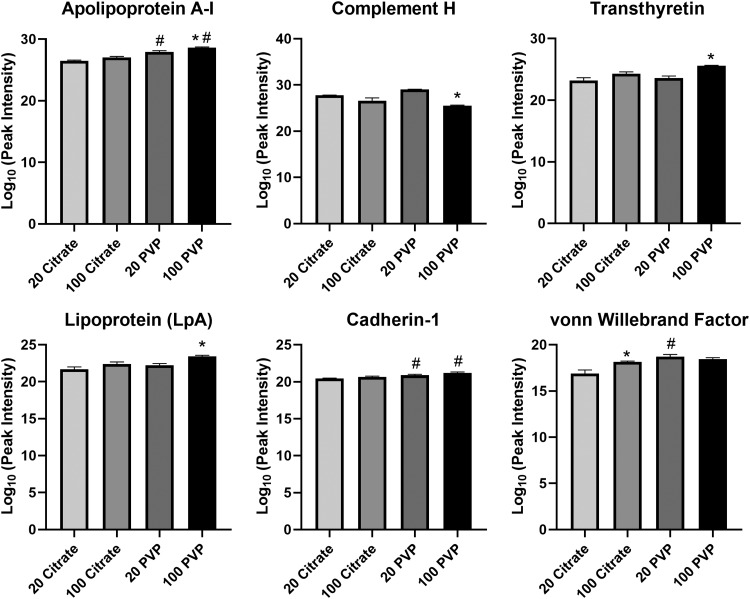
Abundance differences were also observed when AuNPs were incubated in obese serum to form BCs. Apolipoprotein A-1, transthyretin, and lipoprotein-A are more abundant in the 100 nm PVP-coated AuNPs than the 20 nm PVP-coated AuNPs (Fig. 3). The 100 nm citrate-coated AuNP BC contains more von Willebrand factor than the 20 nm citrate-coated AuNP BC. Complement H protein associated more with 20 nm PVP-coated AuNPs than the 100 nm PVP-coated AuNPs. Coating also influenced protein adsorption, as apolipoprotein A-1 and cadherin are more abundant in both sizes of PVP-coated AuNP than their citrate-coated counterparts. Von Willebrand factor is more abundant in the PVP-coated 20 nm AuNP BC than the citrate-coated 20 nm AuNP BC.
FIG. 3.
Relative abundance comparison of selected proteins found in the BCs of all four AuNPs in the obese condition. Proteins identified as present with the BCs of all four AuNPs in the obese condition were compared in their abundance. Peak intensities were log10 transformed to convert the raw values to a more readily understandable format. Comparisons were performed by one-way ANOVA with Tukey post hoc analysis (p < 0.05). *Denotes statistical significance compared to the corresponding 20 nm AuNP with the same coating, #denotes statistical significance compared to the corresponding citrate-coated AuNP with the same size.
Comparison of healthy and obese BCs—protein identities
AuNPs incubated in obese serum generally adsorbed a larger number of proteins than those incubated in healthy serum (Fig. 4 and Supplementary Table S5). The PVP-coated 20 nm AuNPs were the exception, adsorbing an approximately equal number of total proteins between conditions, although it should be noted that proteomic profiles were distinct between conditions, with 35 and 36 proteins exclusive to the healthy and obese conditions, respectively (Fig. 4 and Supplementary Table S5). The pattern of more proteins being present in the obese BCs was more prominent in 100 nm AuNPs than 20 nm AuNPs (Fig. 4). For 100 nm AuNPs, a smaller number of these proteins were shared between the healthy and obese conditions, demonstrating adsorption of distinct proteins due to serum environment.
FIG. 4.
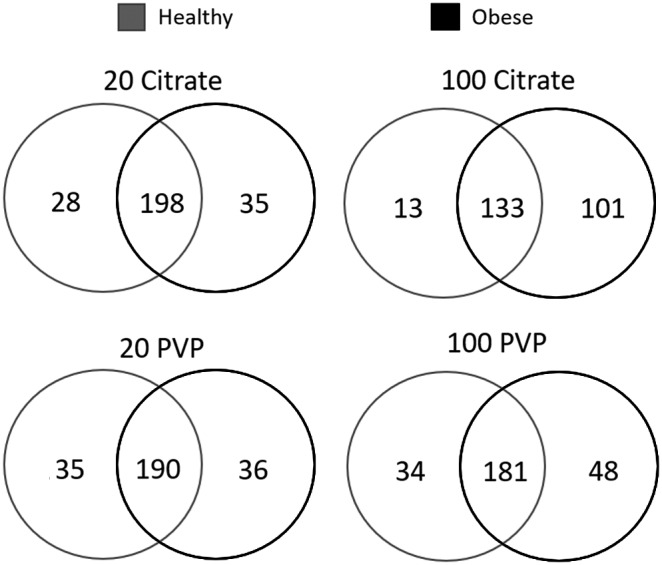
Comparison of BC composition between healthy and obese conditions for all four AuNPs. Proteins identified as present in each AuNP BC formed from healthy serum were compared to proteins within the same AuNP BC formed from obese serum. Venn diagrams were used to illustrate all comparisons. A comprehensive list of all proteins found in each BC is found in Supplementary Table S1. A list of specific proteins used to generate the Venn diagrams in the figure is found in Supplementary Table S5.
Comparison of healthy and obese BCs—relative quantification
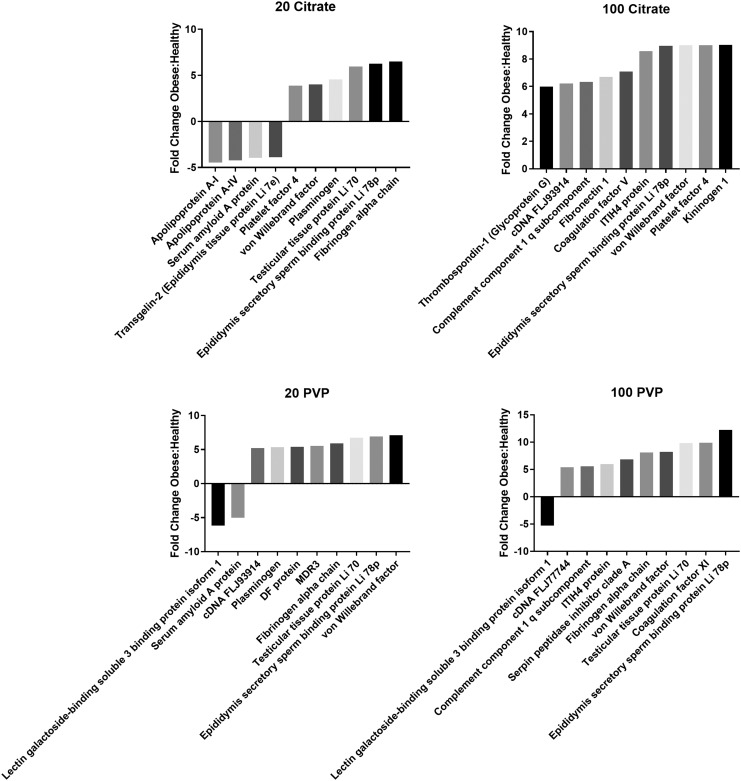
Of the 10 proteins that demonstrated the largest differences between healthy and obese conditions, most were found to be more abundant in the obese condition compared to the healthy (Fig. 5 and Supplementary Table S1). Several proteins were found to be repeated between the 10 most altered proteins for all AuNPs, including von Willebrand factor, which was found as more abundant in the obese BCs for all four AuNPs. Furthermore, fibrinogen alpha chain and testicular tissue protein Li 70 were found as more abundant in the obese BC for all AuNPs except citrate 100 nm. Commonalities were observed based on AuNP properties as well; lectin galactoside-binding soluble 3 binding protein isoform 1 was found as more abundant in the healthy BC for PVP-coated AuNPs, and platelet factor 4 was found as more abundant in the obese BC for citrate-coated AuNPs.
FIG. 5.
Fold changes of proteins between the healthy and obese BCs. Quantities of shared proteins between each AuNP BC are depicted as fold changes. Negative values indicate that the protein was more abundant in healthy conditions, while positive values indicate that the protein was more abundant in obese conditions. Individual graphs depict the 10 most altered proteins in terms of abundance between healthy and obese conditions. Supplementary Table S1 includes a comprehensive list of all protein fold changes between healthy and obese conditions for each AuNP tested.
Furthermore, protein binding was influenced by AuNP size, with Complement Component 1q subcomponent and ITIH4 protein more abundant in the obese BC of 100 nm AuNPs. Both serum amyloid AI protein and plasminogen experienced large fold changes between conditions, with serum amyloid AI being more abundant in the healthy BC and plasminogen being more abundant in the obese BC. Each AuNP had at least two proteins that experienced substantial fold changes between healthy and obese conditions that were unique to that particle, underlying that each particle forms a unique BC despite commonalities between AuNPs.
Lipid components of the AuNP BCs—identification and relative quantification between AuNPs
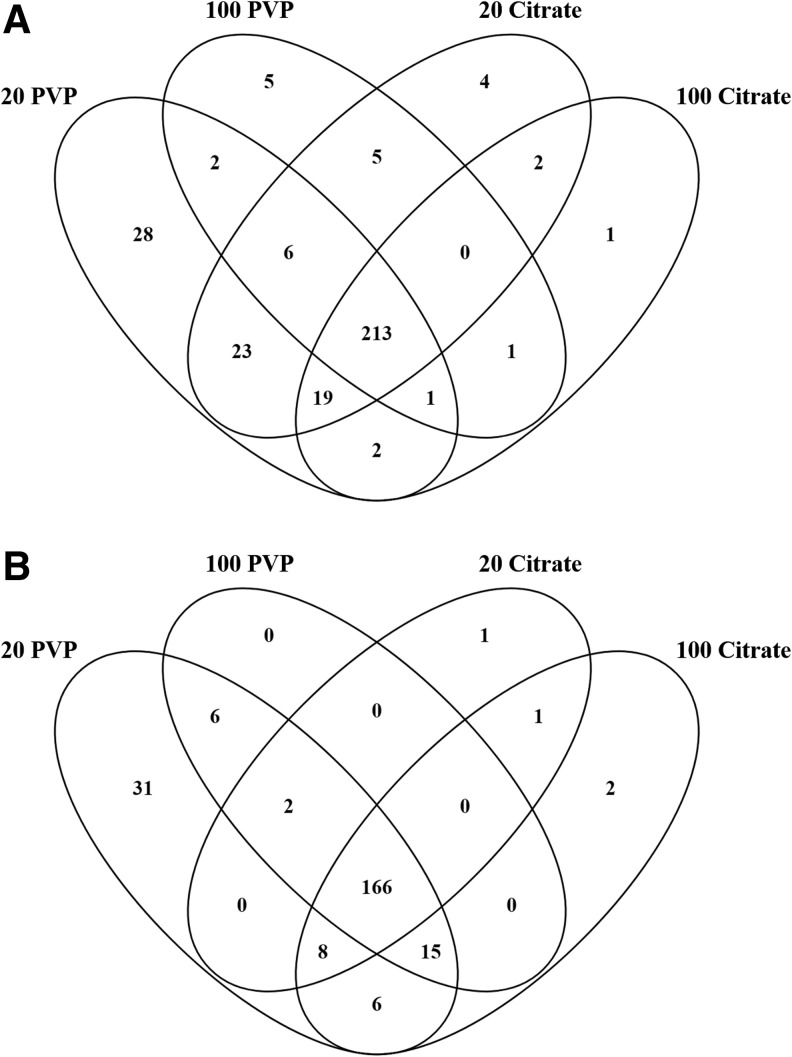
A total of 294, 233, 272, and 239 MRMs related to lipids (hereafter referred to as lipids for simplification) were found in the healthy BCs of the 20 nm PVP-, 100 nm PVP-, 20 nm citrate-, and 100 nm citrate-coated AuNPs, respectively (Fig. 6A and Supplementary Table S6). A total of 213 lipids, or ∼68% of the lipids identified, were shared between all four AuNPs following incubation in healthy serum. These 213 included such lipids as cholest-5-en-3β-yl hexadecanoate, 16:1 cholesteryl ester, phosphatidylcholine (38:8), sphingomyelin (d18:2/22:1), and others. The 20 nm PVP-coated AuNP BC was the most unique, with 28 distinct lipids, while 100 nm PVP-, 20 nm citrate-, and 100 nm citrate-coated AuNPs contained 5, 4, and 1 unique lipids, respectively (Fig. 6A and Supplementary Table S6). A total of 23 lipids were found only in the 20 nm AuNP BCs, including 20:0 campesteryl ester, 22:3 stigmasteryl ester, and ceramide (d18:1/22:0), while 1 lipid [phosphatidylcholine plasmalogen (42:6); phosphatidylcholine (41:7)] was only in the 100 nm AuNP BCs.
FIG. 6.
Comparison of BC composition of all four particles in healthy or obese serum. Lipids identified from each AuNP BC were compared to lipids in all other AuNP BCs under the same condition, either (A) healthy or (B) obese. Venn diagrams were used to illustrate all comparisons. A comprehensive list of all lipids found in each BC is found in Supplementary Table S2. List of specific lipids used to generate Venn diagram (A, B) is found in Supplementary Tables S6 and S7, respectively.
The PVP and citrate coated AuNP BCs each had two lipids which were exclusive to that coating, including 2-Hydroxylauroylcarnitine, 3-hydroxydodecanoylcarnitine for citrate-coated AuNPs, and triglyceride (54:4) fatty acid 18:2 for PVP-coated AuNPs. Size seems to have an influence on the total number of unique lipids adsorbed to the AuNP surface, as both 20 nm AuNPs adsorbed more unique proteins than the 100 nm AuNP of the same coating when incubated in healthy serum.
Within obese serum, the BCs of the 20 nm PVP-, 100 nm PVP-, 20 nm citrate-, and 100 nm citrate-coated AuNPs contained 234, 189, 178, and 198 lipids, respectively, with 166 lipids shared between all four AuNPs. This 166 included lipids such as triglyceride (50:2) fatty acid 16:0, (5Z,8Z)-tetradecadienoylcarnitine, and N-(dodecanoyl)-sphing-4-enine-1-phosphocholine. The 20 nm PVP-, 100 nm PVP-, 20 nm citrate-, and 100 nm citrate-coated AuNPs contain 31, 0, 1, and 2 unique lipids, respectively (Fig. 6B and Supplementary Table S7). No lipids were unique to the 20 or 100 nm AuNPs. Six lipids, including triglyceride (48:1) fatty acid 16:0, were only in the PVP-coated AuNP BCs, and the citrate-coated AuNP BCs share only a single unknown lipid found during acyl-carnitine class screenings.
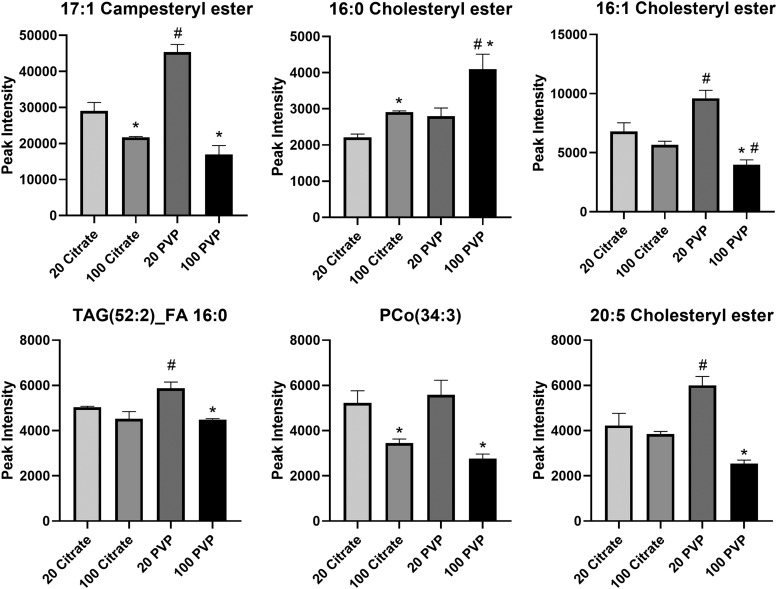
All AuNPs were found to adsorb a number of lipids in common; however, differences in abundance of these shared lipids were determined. In the healthy condition, many lipids were more abundant in the BC of the 20 nm PVP-coated AuNP than the 100 nm PVP-coated AuNP, including 17:1 campesteryl ester, 16:1 cholesteryl ester, triglyceride (52:2) fatty acid 16:0, phosphatidylcholine alkyl ether (34:3), and 20:5 cholesteryl ester (Fig. 7). Conversely, 16:0 cholesteryl ester was more abundant in the 100 nm PVP-coated AuNP BC than the 20 nm PVP-coated AuNP BC.
FIG. 7.
Relative abundance comparison of selected lipids found in the BCs of all four AuNPs in the healthy condition. Lipids identified as present with the BCs of all four AuNPs in the healthy condition were compared in their abundance. Comparisons were performed by one-way ANOVA with Tukey post hoc analysis (p < 0.05). *Denotes statistical significance compared to the corresponding 20 nm AuNP with the same coating, #denotes statistical significance compared to the corresponding citrate-coated AuNP with the same size. FA, fatty acids; PCo, phosphatidylcholine alkyl ether lipids; TAG, triglycerides.
Within the citrate-coated AuNP BCs, 17:1 campesteryl ester and phosphatidylcholine alkyl ether (34:3) were more abundant in the 20 nm AuNP BCs, and 16:0 cholesteryl ester was more abundant in the 100 nm AuNP BC. Coating also dictates the adsorption of lipids, as the PVP-coated 20 nm AuNP BCs tended to contain more 17:1 campesteryl ester, 16:1 cholesteryl ester, triglyceride (52:2) fatty acid 16:0, and 20:5 cholesteryl ester than the citrate-coated 20 nm AuNP BCs. The 16:0 cholesteryl ester was more abundant in the PVP-coated 100 nm AuNP than the citrate-coated 100 nm AuNP, while the opposite pattern was found for 16:1 cholesteryl ester.
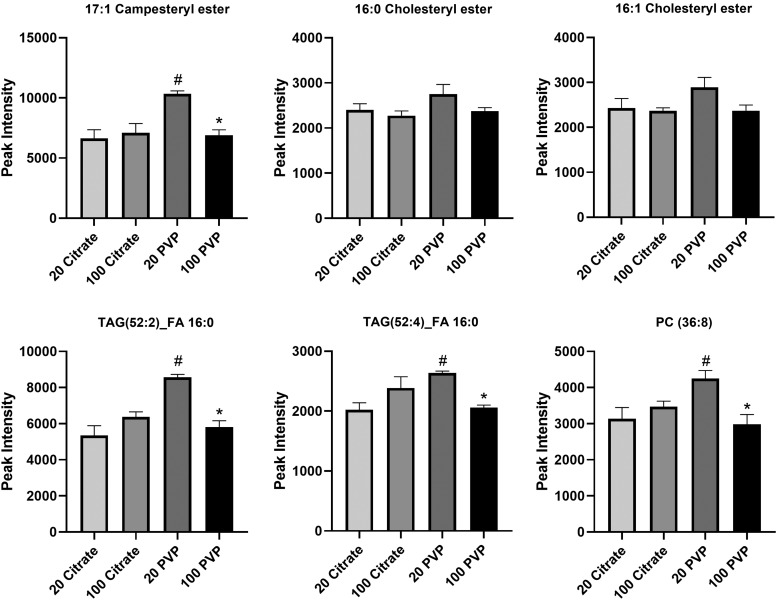
Differences in lipid adsorption were also observed in the obese BCs, although to a lesser extent. The 20 nm PVP-coated AuNP adsorbed more 17:1 campesteryl ester, triglyceride (52:2) fatty acid 16:0, triglyceride (52:4) fatty acid 16:0, and phosphatidylcholine (36:8) than the 100 nm PVP-coated AuNP (Fig. 8). Furthermore, the 20 nm PVP-coated AuNP adsorbed more of each of these lipids than the citrate-coated 20 nm AuNP. No differences between AuNPs were found in the abundances of 16:0 cholesteryl ester or 16:1 cholesteryl ester.
FIG. 8.
Relative abundance comparison of selected lipids found in the BCs of all four AuNPs in the obese condition. Lipids identified as present with the BCs of all four AuNPs in the obese condition were compared in their abundance. Comparisons were performed by one-way ANOVA with Tukey post hoc analysis (p < 0.05). *Denotes statistical significance compared to the corresponding 20 nm AuNP with the same coating, #denotes statistical significance compared to the corresponding citrate-coated AuNP with the same size. PC, phosphatidylcholine lipids.
Comparison of healthy and obese BCs—lipid identities
The identities of lipids found to associate with each AuNP in either healthy or obese serum were compared (Fig. 9). AuNPs incubated in obese serum adsorbed a smaller number of lipids than the corresponding AuNP incubated in healthy serum. This pattern was more prominent in 20 nm AuNPs than 100 nm AuNPs (Fig. 9 and Supplementary Table S8). For 20 nm AuNPs, a larger number of lipids were unique to the healthy condition, indicating the formation of a BC distinct from the BC formed in obese conditions. 100 nm AuNP BCs were more similar between conditions, with similar numbers of lipids shared between conditions and smaller numbers of lipids unique to the healthy BC.
FIG. 9.
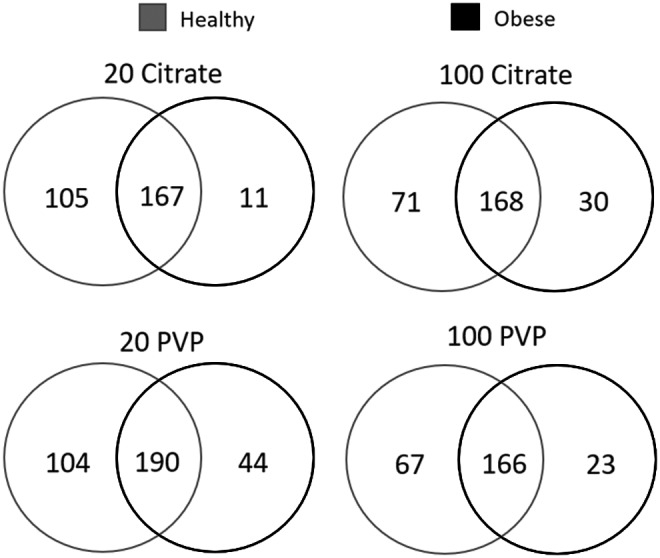
Comparison of BC composition between healthy and obese conditions for all four AuNPs. Lipids identified as present in each AuNP BC formed from healthy serum were compared to lipids within the same AuNP BC formed from obese serum. Venn diagrams were used to illustrate all comparisons. A comprehensive list of all lipids found in each BC is found in Supplementary Table S2. A list of specific lipids used to generate the Venn diagrams in the figure is found in Supplementary Table S8.
Obese and healthy comparisons—relative lipid quantification
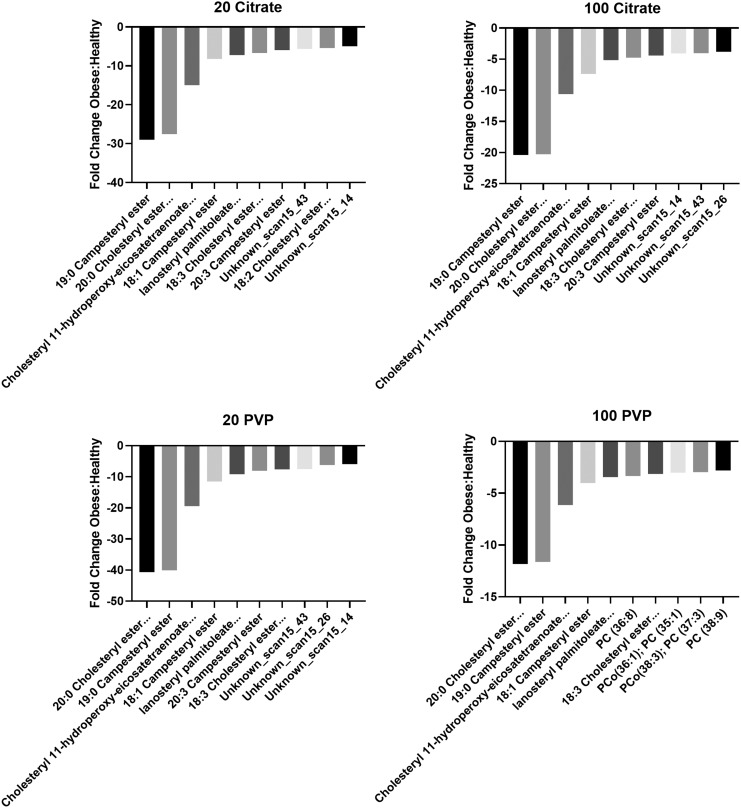
Of the 10 lipids which had the largest fold changes between the healthy and obese BCs, all were more abundant in the healthy BC (Fig. 10 and Supplementary Table S2). These lipids were almost exclusively cholesteryl esters and were highly conserved between AuNPs. Six lipids were shared between all four AuNPs, including 19:0 campesteryl ester; 20:0 cholesteryl ester, 18:0 sitosteryl ester; cholesteryl 11-hydroperoxy-eicosatetraenoate, 22:2 cholesteryl ester, 20:1 stigmasteryl ester, 20:2 sitosteryl ester; 18:1 campesteryl ester; lanosteryl palmitoleate, 18:2 campesteryl ester; and 18:3 cholesteryl ester, 16:2 stigmasteryl ester, 16:3 sitosteryl ester. Three more lipids, specifically 20:3 campesteryl Ester and two unknown lipids detected during the cholesteryl ester class screenings, also had large fold changes between healthy and obese, but only for three AuNP types; the PVP-coated 100 nm AuNP was the exception.
FIG. 10.
Fold changes of lipids between the healthy and obese BCs. Quantities of shared lipids between each AuNP BCs are depicted as fold changes. Negative values indicate that the lipid was more abundant in healthy conditions, while positive values indicate that the lipid was more abundant in obese conditions. Individual graphs depict the 10 most altered lipids in terms of abundance between healthy and obese conditions. Supplementary Table S2 includes a comprehensive list of all lipid fold changes between healthy and obese conditions for each AuNP tested. PC indicates phosphatidylcholine lipids, and PCo indicates phosphatidylcholine alkyl ether lipids. Certain lipid names were shortened for brevity, which are listed in full here. 20:0 Cholesteryl ester… ester indicates 20:0 Cholesteryl ester, 18:0 Sitosteryl ester. Cholesteryl 11-hydroperoxy-eicosatetraenoate… indicates Cholesteryl 11-hydroperoxy-eicosatetraenoate, 22:2 Cholesteryl ester, 20:1 Stigmasteryl ester, 20:2 Sitosteryl ester. Lanosteryl palmitoleate… indicates lanosteryl palmitoleate, 18:2 Campesteryl ester. 18:3 Cholesteryl ester… indicates 18:3 Cholesteryl ester, 16:2 Stigmasteryl ester, 16:3 Sitosteryl ester. 18:2 Cholesteryl ester… ester indicates 18:2 Cholesteryl ester, zymosteryl oleate, 16:1 Stigmasteryl ester, 16:2 Sitosteryl ester.
Only the citrate-coated 20 nm and PVP-coated 100 nm AuNP BCs contained lipids, which were exclusive to one AuNP BC. The 18:2 cholesteryl ester, zymosteryl oleate, 16:1 stigmasteryl ester, 16:2 sitosteryl ester was exclusive to the citrate-coated 20 nm AuNP BC, and phosphatidylcholine (36:8), phosphatidylcholine alkyl ether (36:1); phosphatidylcholine (35:1), phosphatidylcholine alkyl ether (38:3); phosphatidylcholine (37:3); and phosphatidylcholine (38:9) were exclusive to the PVP-coated 100 nm AuNP BC.
BC-induced variations in macrophage viability and inflammatory response
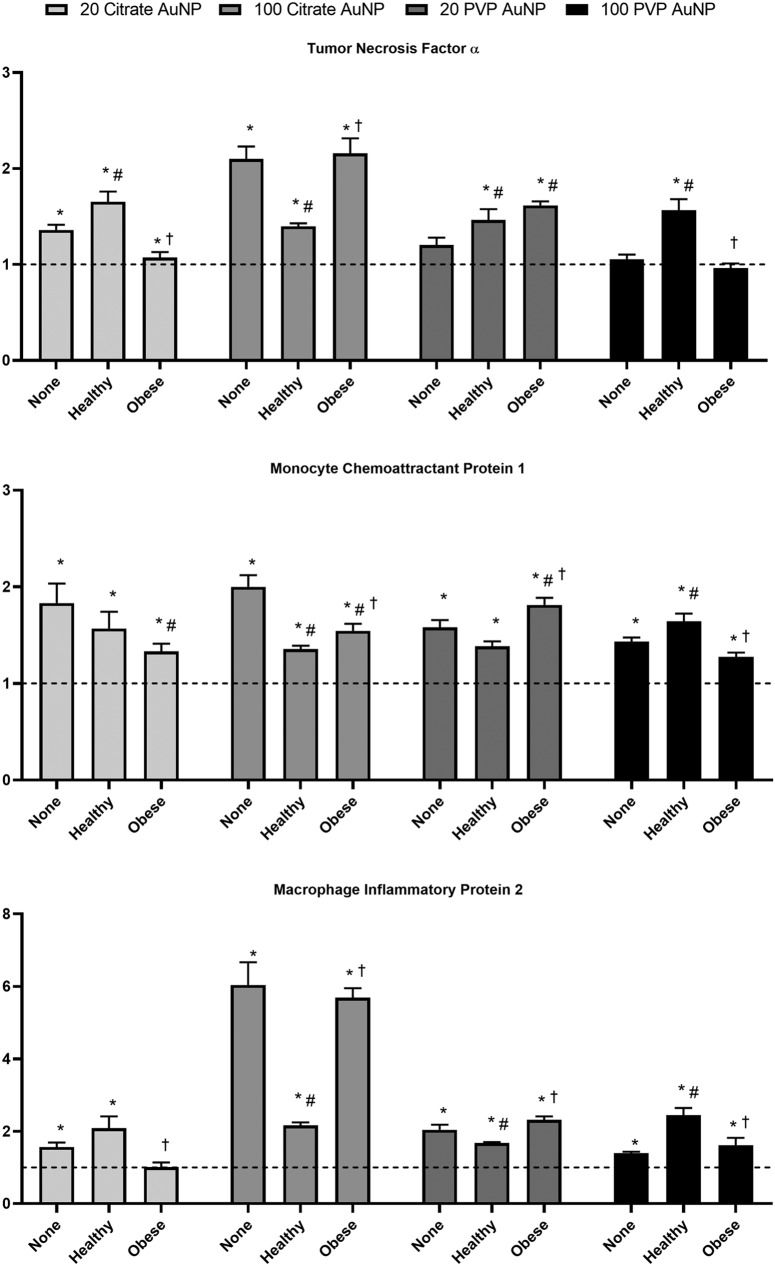
At concentrations (12.5 or 50 μg/mL) and time points (3 or 24 hours), no alterations in cell viability were determined due to AuNP exposures with or without BCs (Supplementary Fig. S1). The citrate-coated 20 nm AuNP without a BC increased expression of TNF-α, MCP-1, and MIP-2 (Fig. 11). The addition of a healthy BC induced similar upregulation of MCP-1 or MIP-2 while increasing expression of TNF-α compared to the 20 nm citrate-coated AuNP without a BC. The obese BC reduced expression of TNF-α and MIP-2 compared to the healthy BC. The addition of the obese BC on 20 nm citrate-coated AuNPs induced MCP-1 expression similar to when a healthy BC was present. All three inflammatory markers were increased by exposure to citrate-coated 100 nm AuNP without a BC. The healthy BC significantly and uniformly reduced expression compared to AuNPs without a BC. The addition of an obese BC caused increased expression of TNF-α and MIP-2 compared to the healthy BC.
FIG. 11.
Changes in gene expression following exposure to AuNPs with or without a healthy or obese BC. Expression of mRNA was assessed relative to serum-free media control cells (untreated) (dotted line). Cells were grown to 90% confluency in a 96-well plate before exposure to 50 μg/mL AuNPs with no BC, a healthy BC, or an obese BC for 24 hours. Gene expression of TNF-α, CCL2, CXCL2, and GAPDH (control) was assessed through PCR to evaluate the inflammatory response induced by AuNP exposure. *Denotes statistical significance compared to the expression of the unexposed control, #denotes statistical significance compared to the AuNP with no BC, and †denotes statistical significance compared to the AuNP with a healthy BC. Comparisons were performed by one-way ANOVA with Tukey post hoc analysis (p < 0.05). GAPDH, glyceraldehyde 3-phosphate dehydrogenase; TNF-α, tumor necrosis factor-α.
Macrophages exposed to PVP-coated 20 nm AuNP without a BC demonstrated increased expression of MCP-1 and MIP-2, although TNF-α was not changed compared to control. The healthy BC caused an increase in TNF-α expression, a decrease in MIP-2 expression, and had no effect on the expression of MCP-1. Compared to the healthy BC, the obese BC caused increases in MCP-1 and MIP-2 expression, but had no effect on TNF-α. The PVP-coated 100 nm AuNP without a BC caused an increase in the expression of MCP-1 and MIP-2, but not TNF-α. The healthy BC caused expression of all three inflammatory markers to increase compared to the AuNP without a BC. The obese BC reduced the expression of all three inflammatory gene markers to levels comparable to the AuNP without a BC.
Discussion
The application of in vitro assessments to evaluate the safety of biomedical NPs and exposures to NPs in general is dependent on understanding the formation of the BC and its impact on cellular interactions and toxicity. Currently, scientific knowledge is lacking regarding how NP physicochemical properties and underlying diseases, such as obesity, impact NP-BC formation. Furthermore, most investigations of the NP-BC have focused primarily on the protein content. Our findings demonstrate variability in the protein and lipid content of the BC due to AuNP physicochemical properties, including size (20 or 100 nm) and coating (PVP or citrate), and obesity. This variability in the BC impacted AuNP properties and cellular responses, which may influence their functionality and use.
NPs are highly modifiable and can be engineered with specific physicochemical properties (size, composition, charge, and surface coating) enabling their usage in a variety of technologies. These properties may be altered by the addition of a BC, impacting NP functionality.45,46 NPs in medicine may be particularly vulnerable to BC-induced alterations in functionality, as the environment in which they are used is not only rich in biomolecules, but highly variable between individuals, as well. Introduction of AuNPs into obese serum resulted in increased hydrodynamic size compared to those in a healthy serum environment. This increase in size is likely due to agglomeration of AuNPs, as demonstrated in previous studies.47 Enhanced agglomeration that may occur in obesity could alter therapeutic functionality of AuNPs by reducing NP surface area and altering size-dependent biodistribution.
The 20 nm PVP-coated AuNPs demonstrated smaller changes in size and polydispersion following addition of the BCs. These findings suggest that 20 nm PVP-coated AuNPs more effectively resisted agglomeration in biological environments compared to larger AuNPs and those coated with citrate. The increased agglomeration observed for citrate coated AuNPs may be due to the dynamic relationship that exists between citrate and the AuNP upon introduction into serum. Specifically, proteins and citrate can associate and dissociate from the NP surface in a concentration-dependent manner, altering agglomeration.48 Based on these findings, the 20 nm PVP-coated AuNPs appear more capable of maintaining their nano-properties in biological environments, potentially assisting in their utilization in biomedicine.
Previous investigations of the NP-BC have focused almost exclusively on protein components.49–51 Our current study attempted to more comprehensively examine the BC by utilizing both proteomic and lipidomic approaches. Use of this combination of methods identified a similar number of proteins and lipids associating with the surface of all AuNPs. This suggests that previous research utilizing only a proteomic approach may not be able to fully establish relationships between NP-BC formation and cellular responses. Incorporation of both approaches provides a fuller understanding of the complexity of the NP-BC and may assist in the understanding of its biological responses. The use of physicochemically-distinct AuNPs allowed for comparisons of two modifiable NP properties (size and coating) that influence BC formation.
Adsorption of proteins and lipids was determined to be influenced by both AuNP size and surface coating. When incubated in healthy serum, 20 nm AuNPs adsorbed more unique proteins and lipids compared to 100 nm AuNPs of the same coating. This indicates that the surface curvature and increased surface area of the 20 nm AuNPs may allow for a more diverse set of biomolecules to associate with the AuNP surface. Unique proteins and lipids were also determined to associate with AuNPs based upon citrate or PVP coating. Specifically, 20 nm PVP AuNPs were determined to bind the greatest number of unique lipids and a higher abundance of many shared lipids compared to other AuNPs. The addition of the lipid-rich BC likely facilitates their resistance to agglomeration, as studies have demonstrated that enhancement of surface lipid content enhances NP suspension stability.52,53
Although all AuNPs demonstrated differential association of proteins and lipids, size appears to be the primary contributor to differences in BC formation. This is similar to a previous work investigating the association of proteins with the surfaces of spherical silver NPs of similar size and coatings following incubation in fetal bovine serum.54 Together, these findings suggest that the association of proteins and also lipids can somewhat be controlled through modifications in NP size and coating. This could allow for the selective removal of biomolecules from the circulation or the ability to utilize the natural BC to assist in NP functionality through alterations in NP targeting and clearance.
To date, the majority of investigations of NP safety have been performed in scenarios utilizing healthy animal and cell culture models. However, a growing portion of the global population exists with some form of underlying disease. Common examples include obesity, hyperlipidemia, metabolic syndrome, diabetes, and cardiovascular disease. Individuals with these diseases are often increasingly sensitive to exposures, demonstrating exacerbated responses, and are more likely to receive medical treatments than healthy individuals.24,25 Obesity underlies a number of other chronic conditions and is known to modify circulating biomolecules, including proteins and lipids.29–32
Our current study demonstrated that protein and lipid components of the AuNP-BC were modified in obese serum environments compared to healthy. Interestingly, the BCs that formed on AuNPs in obese serum were less dependent on NP physicochemical properties compared to BC created in healthy serum. This was demonstrated by an increased percentage of proteins shared by all AuNPs and a less unique lipid profile for each AuNP in obese serum. In addition, the abundance of lipids such as 17:1 campesteryl ester was more variable in healthy conditions than obese. Together, these findings suggest that alterations in BC formation resulting from NP physicochemical properties may be less of a concern in an obese environment, as they associate more with similar biomolecules.
AuNPs in an obese biological environment associate distinct BCs compared to those forming in a healthy environment. Previously, we have demonstrated differences in protein components of the iron oxide NP-BC due to incubation in hyperlipidemic rat serum compared to healthy. This evaluation demonstrated that the hyperlipidemic conditions increased the number of distinct proteins forming the BC. Similar results were observed in our current study, which demonstrated that obesity increased the number and abundance of proteins associating with the AuNP surface. Many of these differences are likely related to disease-related alterations in protein abundance within the serum. Specifically, von Willebrand factor, fibrinogen alpha chain, serum amyloid AI, and inter-alpha-trypsin inhibitor heavy chain-4 are known to be increased in the circulation of individuals suffering from obesity.55–58
Not all changes in protein abundance can be directly explained by disease-related differences in circulating abundance. For instance, complement component 1q was increased on 100 nm citrate- and PVP-coated AuNPs in obese conditions compared to healthy. Complement component 1q is known to remain stable despite body weight and maintains similar levels in obese and anorexic women.59 Recently, it has been shown that lung cancer patients have a greater amount of complement component 1q in their serum BCs than healthy individuals and that this difference may be exploited to produce NP therapeutics with prolonged circulation.60 This indicates that 100 nm AuNPs may remain in circulation for longer periods of time in obese individuals, and perhaps those with other diseases, due to their BCs containing greater amounts of complement component 1q. Furthermore, these data identify complement component 1q as an important element within the diseased BC that should be considered, and potentially utilized, when designing or testing NPs for clinical and therapeutic use.
In addition, the obese BC demonstrated increased amounts of lectin galactoside-binding soluble 3 binding protein isoform 1 on 20 nm and 100 nm PVP-coated AuNPs and platelet factor 4 on 20 nm and 100 nm citrate-coated AuNPs compared to the healthy BC. The association of these proteins with the AuNP may be due to disease-related reductions in the abundance of other biomolecules increasing the ability of these proteins to interact with the surface of AuNPs. Many proteins related to blood clotting and coagulation (platelet factor 4, coagulation factor V, kininogen-1, coagulation factor XI, von Willebrand factor, and others) were more abundant in obese BCs compared to healthy. Obesity is known to cause abnormalities in blood coagulation, and factors involved in the coagulation cascade accumulate at interfaces between biological surfaces and implanted medical devices.61,62 These findings suggest that obesity alters biomolecular content of serum, which may impact protein-NP interactions forming the BC. These interactions may specifically predispose individuals suffering from obesity to dysregulation of coagulation.
Dysregulation of lipids is involved in the progression and development of obesity.63–65 Overall, AuNPs incubated in healthy serum were determined to associate more diverse lipids than those in obese serum. Furthermore, shared lipids of the AuNP-BCs were found to be more abundant in the healthy BC compared to the obese BC. These disease-related variations in lipid accumulation may be due to differences in the association of proteins. Specifically, more distinct proteins were found to absorb to the surface of AuNPs in obese conditions, and they may reduce available surface area for lipid accumulation. The BCs that formed on AuNPs in a healthy environment were found to be rich in cholesteryl esters, whereas the obese BCs had more triglycerides.
The increased amount of cholesteryl esters in the healthy BC was unexpected. Obesity induced by a high-fat diet has been shown to have little to no effect of the serum concentrations of major cholesteryl ester species.29 Therefore, the increase in cholesteryl ester abundance within the healthy BC is not driven purely by short-term dietary-induced obesity. Rather, the increase in cholesteryl esters in the healthy BC may be due to some of the longer term health effects that occur in obese humans over time. Specifically, obese individuals are known to have higher circulating levels of cholesteryl ester transfer protein, which transfers cholesteryl esters from high-density lipoprotein to low- and very-low density lipoproteins.66,67 Individuals who suffer from cardiovascular disease do not convert cholesterol to cholesteryl ester as efficiently as healthy individuals.68 Furthermore, the accumulation of cholesteryl esters within macrophages has been shown to lead to the formation of atherosclerotic plaques, contributing to heart disease.69,70
Therefore, these factors in combination could lead to a sequestration of cholesteryl esters within atherosclerotic plaques, resulting in a lower circulating concentration in obese individuals. As a direct result of this sequestration, fewer cholesteryl esters are available to bind the AuNP surface in obese serum, resulting in a greater abundance in both healthy serum and within the AuNP BC formed from healthy serum. The increased amount of triglycerides within the obese BC was expected and likely is abundance driven, as triglycerides are a dietary lipid class and have been shown, both in our own samples and previous research, to be elevated in obesity.71,72
The disease-related variations in BC formation on AuNPs were determined to impact macrophage responses in cell culture. As expected, none of the AuNPs evaluated caused any significant decreases in macrophage viability. This is consistent with previous research demonstrating the absence of direct cytotoxic effects due to AuNP exposure.2,73 AuNP physicochemical properties and disease-related differences in the BC induced differential gene expression of macrophage inflammatory cytokines. Effects of the healthy and obese BC in terms of inflammatory gene expression were not found to be consistent between AuNPs. This is likely due to the uniqueness of each BC as a result of physicochemical properties of the AuNP. The complexity of the BC implies that it is unlikely that a single protein or lipid governs cellular responses.
A previous evaluation was performed investigating the contribution of bovine serum albumin in the cellular response to the NP-BC.11 This study compared silver NPs with either a full BC formed following incubation in fetal bovine serum or a BC consisting of only bovine serum albumin. It was determined that although albumin was the primary protein present within the BC, its presence did not correlate to cellular effects induced by the full BC. In our current study, healthy and obese BCs demonstrated differential induction of inflammatory gene expression following exposure to AuNPs with different BCs. This variability highlights how disease environments can modify NP surfaces, resulting in altered immune cell responses. Previous research has demonstrated that the addition of a hyperlipidemic BC on iron oxide NPs enhances endothelial cell expression of inflammatory genes and cell adhesion markers.13
Together, these findings demonstrate that the comparative assessment of NP toxicity utilizing in vitro studies performed in serum-free environments where the BC is not able to form may not generate relevant cellular responses. In addition, each NP's ability to instigate immune responses following association of the BC is not similar due to variations in the formation of the BC that may occur due to NP properties and biomolecule content of the biological environment.
Conclusions
While we believe that both the design and conclusions of this study are robust, it is not without limitations. While the use of pooled mixed-gender serum is ideal for making broad, generalizable conclusions, it does not allow for the assessment of individual variability. The pooling of subject serum was random and did not control for factors such as age or preexisting disease. While pooling the serum likely reduced the influence of many extraneous factors, it is unlikely that they were eliminated entirely. Our previous study demonstrated unique variations in the protein content of the BC that forms on iron oxide NPs between individuals based on sex, blood parameters, and exercise.23 Future studies will evaluate specific individual variations in the lipid content of the NP-BC.
In conclusion, our findings demonstrate that physicochemically distinct AuNPs form unique BCs that differ based upon protein and lipid content. Furthermore, underlying disease states, such as obesity, may impact the association of biomolecules with the NPs surface. The addition of these BCs may differentially influence cellular interactions and responses. The incorporation of the BC into in vitro toxicity assessment models may enhance the translatability of results to clinical applications and outcomes of nano-enable therapeutics. The use of the BC also can be an efficient and effective tool for the screening of subpopulation susceptibility in cell culture. A more comprehensive understanding of the initial interactions between the biological environment and the NP surface will allow for the development and expanded usage of NPs in biomedical applications.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the National Institute of Environmental Health Sciences Grant number ES024392, as well as the Ralph W. and Grace M. Showalter Research Trust. The Q Exactive Orbitrap HF mass spectrometer used in this study was purchased through funding from the Office of the Executive Vice President of Research and Partnership of Purdue University.
Supplementary Material
References
- 1. Khan MA, Khan MJ. Nano-gold displayed anti-inflammatory property via NF-kB pathways by suppressing COX-2 activity. Artif Cells Nanomed Biotechnol 2018:46(S1);S1149–S1158 [DOI] [PubMed] [Google Scholar]
- 2. Shukla R, Bansal V, Chaudhary M, et al. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 2005:21;10644–10654 [DOI] [PubMed] [Google Scholar]
- 3. Dykman LA, Khlebtsov NG. Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta Naturae 2011:3;34–55 [PMC free article] [PubMed] [Google Scholar]
- 4. Haume K, Rosa S, Grellet S, et al. Gold nanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol 2016:7;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng Y, Samia AC, Meyers JD, et al. Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer. J Am Chem Soc 2008:130;10643–10647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pati R, Shevtsov M, Sonawane A. Nanoparticle vaccines against infectious diseases. Front Immunol 2018:9;2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stuchinskaya T, Moreno M, Cook MJ, et al. Targeted photodynamic therapy of breast cancer cells using antibody–phthalocyanine–gold nanoparticle conjugates. Photochem Photobiol Sci 2011:10;822–831 [DOI] [PubMed] [Google Scholar]
- 8. Adamson SXF, Lin Z, Chen R, et al. Experimental challenges regarding the in vitro investigation of the nanoparticle-biocorona in disease states. Toxicol In Vitro 2018:51;40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maiorano G, Sabella S, Sorce B, et al. Effects of cell culture media on the protein-Nanoparticle complexes and influence on the cellular response. ACS Nano 2010:4;7481–7491 [DOI] [PubMed] [Google Scholar]
- 10. Monopoli MP, Åberg C, Salvati A, et al. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol 2012:7;779–786 [DOI] [PubMed] [Google Scholar]
- 11. Shannahan JH, Podila R, Brown JM. A hyperspectral and toxicological analysis of protein corona impact on silver nanoparticle properties, intracellular modifications, and macrophage activation. Int J Nanomedicine 2015:10;6509–6520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walczyk D, Bombelli FB, Monopoli MP, et al. What the cell “sees” in bionanoscience. J Am Chem Soc 2010:132;5761–5768 [DOI] [PubMed] [Google Scholar]
- 13. Shannahan JH, Fritz KS, Raghavendra AJ, et al. Disease-induced disparities in formation of the nanoparticle-biocorona and the toxicological consequences. Toxicol Sci 2016:152;406–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neagu M, Piperigkou Z, Karamanou K, et al. Protein bio-corona: Critical issue in immune nanotoxicology. Arch Toxicol 2017:91;1031–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frost R, Langhammer C, Cedervall T. Real-time in situ analysis of biocorona formation and evolution on silica nanoparticles in defined and complex biological environments. Nanoscale 2017:9;3620–3628 [DOI] [PubMed] [Google Scholar]
- 16. Eudald C, Tobias P, Albert D, et al. Time evolution of the nanoparticle protein corona. ACS Nano 2010:4;3623–3632 [DOI] [PubMed] [Google Scholar]
- 17. Walkey CD, Olsen JB, Guo H, et al. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc 2012:134;2139–2147 [DOI] [PubMed] [Google Scholar]
- 18. Lundqvist M, Stigler J, Elia G, et al. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci U S A 2008:105;14265–14270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jedlovszky-hajdu A, Bombelli FB, Monopoli MP, et al. Surface coatings shape the protein corona of SPIONs with relevance to their application in vivo. Langmuir 2012:28;14983–14991 [DOI] [PubMed] [Google Scholar]
- 20. Tran ZV, Weltman A, Glass GV, et al. The effects of exercise on blood lipids and lipoproteins: A meta-analysis of studies. Med Sci Sport Exerc 1983:15;393–402 [PubMed] [Google Scholar]
- 21. Murphy WG. The sex difference in haemoglobin levels in adults—Mechanisms, causes, and consequences. Blood Rev 2014:28;41–47 [DOI] [PubMed] [Google Scholar]
- 22. Jenkins DJ, Wolever TM, Venketeshwer Rao A, et al. Effect on blood lipid of very high intakes of fiber in diets low in saturated fat and cholesterol. N Engl J Med 1993:329;21–26 [DOI] [PubMed] [Google Scholar]
- 23. Kobos LM, Adamson SXF, Evans S, et al. Altered formation of the iron oxide nanoparticle-biocorona due to individual variability and exercise. Environ Toxicol Pharmacol 2018:62;215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dubowsky SD, Suh H, Schwartz J, et al. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect 2006:992;992–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dong GH, Qian Z, Liu M, et al. Obesity enhanced respiratory health effects of ambient air pollution in Chinese children: The Seven Northeastern Cities study. Int J Obes 2013:37;94–100 [DOI] [PubMed] [Google Scholar]
- 26. Adult Obesity Prevalence Maps. Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion. 2019. [cited July 8, 2019]. www.cdc.gov/obesity/data/prevalence-maps.html Last accessed July8, 2019
- 27. Berghöfer A, Pischon T, Reinhold T, et al. Obesity prevalence from a European perspective: A systematic review. BMC Public Health 2008:10;1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flegal KM, Kruszon-moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2019:20782;2284–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eisinger K, Liebisch G, Schmitz G, et al. Lipidomic analysis of serum from high fat diet induced obese mice. Int J Mol Sci 2014:2991–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fjeldborg K, Christiansen T, Bennetzen M, et al. The macrophage-specific serum marker, soluble CD163, is increased in obesity and reduced after dietary-induced. Obesity 2013:21;2437–2443 [DOI] [PubMed] [Google Scholar]
- 31. Park HS, Yul J, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-α and IL-6. Diabetes Res Clin Pract 2005:69;29–35 [DOI] [PubMed] [Google Scholar]
- 32. Suzuki K, Ito Y, Ochiai J, et al. Relationship between obesity and serum markers of oxidative stress and inflammation in Japanese. Asian Pacific J Cancer Prev 2003:4;259–266 [PubMed] [Google Scholar]
- 33. Raghavendra AJ, Fritz K, Fu S, et al. Variations in biocorona formation related to defects in the structure of single walled carbon nanotubes and the hyperlipidemic disease state. Sci Rep 2017:7;8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ke PC, Lin S, Parak WJ, et al. A decade of the protein corona. ACS Nano 2017:11;11773–11776 [DOI] [PubMed] [Google Scholar]
- 35. Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 2008:26;1367–1372 [DOI] [PubMed] [Google Scholar]
- 36. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959:37;911–917 [DOI] [PubMed] [Google Scholar]
- 37. Casey T, Harlow K, Ferreira CR, et al. The potential of identifying replacement gilts by screening for lipid biomarkers in reproductive tract swabs taken at weaning. J Appl Anim Res 2018:46;2119 [Google Scholar]
- 38. Cordeiro FB, Ferreira CR, Sobreira TJP, et al. Multiple reaction monitoring (MRM)-profiling for biomarker discovery applied to human polycystic ovarian syndrome. Rapid Commun Mass Spectrom 2017:31;1462–1470 [DOI] [PubMed] [Google Scholar]
- 39. de Lima CB, Ferreira CR, Milazzottta MP, et al. Comprehensive lipid profiling of early stage oocytes and embryos by MRM profiling. J Mass Spectrom 2018:53;1247–1252 [DOI] [PubMed] [Google Scholar]
- 40. Dhillon J, Ferreira CR, Jose T, et al. Multiple reaction monitoring profiling to assess compliance with an almond consumption intervention. Curr Dev Nutr 2017:1;1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dipali SS, Ferreira CR, Zhou LT, et al. Histologic analysis and lipid profiling reveal reproductive age-associated changes in peri-ovarian adipose tissue. Reprod Biol Endocrinol 2019:6;1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferreira CR, Yannell KE, Mollenhauer B, et al. Chemical profiling of cerebrospinal fluid by multiple reaction monitoring mass spectrometry. Analyst 2016:141;5252–5255 [DOI] [PubMed] [Google Scholar]
- 43. Franco J, Ferreira C, Sobreira TJP, et al. Profiling of epidermal lipids in a mouse model of dermatitis: Identification of potential biomarkers. PLoS One 2018:13;e0196595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yannell KE, Ferreira CR, Tichy SE, et al. Multiple reaction monitoring (MRM)-profiling with biomarker identification by LC-QTOF to characterize coronary artery disease. Analyst 2018:143;5014–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Salvati A, Pitek AS, Monopoli MP, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol 2013:8;137–143 [DOI] [PubMed] [Google Scholar]
- 46. Amiri H, Bordonali L, Lascialfari A, et al. Protein corona affects the relaxivity and MRI contrast efficiency of magnetic nanoparticles. Nanoscale 2013:5;8656–8665 [DOI] [PubMed] [Google Scholar]
- 47. Sharma G, Kodali V, Gaffrey M, et al. Iron oxide nanoparticle agglomeration influences dose rates and modulates oxidative stress-mediated dose-Response profiles in vitro. Nanotoxicology 2014:8;663–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ding F, Radic S, Chen R, et al. Direct observation of a single nanoparticle-ubiquitin corona formation. Nanoscale 2013:5;9162–9169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chandran P, Riviere JE, Monteiro-Riviere NA, et al. Surface chemistry of gold nanoparticles determines the biocorona composition impacting cellular uptake, toxicity and gene expression profiles in human endothelial cells. Nanotoxicology 2017:11;507–519 [DOI] [PubMed] [Google Scholar]
- 50. Lundqvist M, Stigler J, Cedervall T, et al. The evolution of the protein corona around nanoparticles: A test study. ACS Nano 2011:5;7503–7509 [DOI] [PubMed] [Google Scholar]
- 51. Wen Y, Geitner NK, Chen R, et al. Binding of cytoskeletal proteins with silver nanoparticles. RSC Adv 2013:(Cd);22002–22007 [Google Scholar]
- 52. Wu Y, Hudson JS, Lu Q, et al. Coating single-walled carbon nanotubes with phospholipids. J Phys Chem B 2006:110;2475–2478 [DOI] [PubMed] [Google Scholar]
- 53. Ke PC. Fiddling the string of carbon nanotubes with amphiphiles. Phys Chem Chem Phys 2007:9;439–447 [DOI] [PubMed] [Google Scholar]
- 54. Shannahan JH, Lai X, Ke PC, et al. Silver nanoparticle protein corona composition in cell culture media. PLoS One 2013:8;e74001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Doumatey AP, Zhou J, Zhou M, et al. Proinflammatory and lipid biomarkers mediate metabolically healthy obesity: A proteomics study. Obesity 2016:24;1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wiewiora M, Piecuch J, Sedek L, et al. The effects of obesity on CD47 expression in erythrocytes. Cytom Part B Clin Cytom 2017:491;485–491 [DOI] [PubMed] [Google Scholar]
- 57. Zhao Y, He X, Shi X. Association between serum amyloid A and obesity: A meta-analysis and systematic review. Inflamm Res 2010:59;323–334 [DOI] [PubMed] [Google Scholar]
- 58. Blann A, Bushell D, Davies A, et al. von Willebrand factor, the endothelium and obesity. Int J Obes Relat Metab Disord J Int Assoc Study Obes 1993:17;723–725 [PubMed] [Google Scholar]
- 59. Pomeroy C, Mitchell J, Eckert E, et al. Effect of body weight and caloric restriction on serum complement proteins, including Factor D/adipsin: Studies in anorexia nervosa and obesity. Clin Exp Immunol 1997:108;507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ren J, Cai R, Wang J, et al. Precision nanomedicine development based on specific opsonization of human cancer patient-personalized protein coronas. Nano Lett 2019:19;4692–4701 [DOI] [PubMed] [Google Scholar]
- 61. Pergola GO, Pannacciulli N. Coagulation and fibrinolysis abnormalities in obesity. J Endocrinol Invest 2002:25;899–904 [DOI] [PubMed] [Google Scholar]
- 62. Xu L, Bauer J, Siedlecki CA, et al. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf B Biointerfaces 2014:124;49–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Despres J-P. Obesity and lipid metabolism: Relevance of body fat distribution. Curr Opin Lipidol 1991:2;5–15 [Google Scholar]
- 64. Singh SP, Niemczyk M, Saini D, et al. Role of the electrophilic lipid peroxidation product 4-hydroxynonenal in the development and maintenance of obesity in mice. Biochemistry 2008:47;3900–3911 [DOI] [PubMed] [Google Scholar]
- 65. Susumu M, Ikawa M, Okabe M, et al. Dynamic modification of sphingomyelin in lipid microdomains controls development of obesity, fatty liver, and type 2 diabetes. J Biol Chem 2011:286;28544–28555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Arai T, Fujioka S, Nozaki S, et al. Increased plasma cholesteryl ester transfer protein in obese subjects. Arterioscler Thromb 1994:14;1129–1136 [DOI] [PubMed] [Google Scholar]
- 67. Asayama K, Hayashibe H, Dobashi K, et al. Increased serum cholesteryl ester transfer protein in obese children. Obes Res 2002:10;439–446 [DOI] [PubMed] [Google Scholar]
- 68. Gerl MJ, Vaz WLC, Domingues N, et al. Cholesterol is inefficiently converted to cholesteryl esters in the blood of cardiovascular disease patients. Sci Rep 2018:8;14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ghosh S, Zhao B, Bie J, et al. Macrophage cholesteryl ester mobilization and atherosclerosis. Vascul Pharmacol 2011:52;1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kunnert B, Krug H. The composition of cholesterol esters in fatty streaks and atherosclerotic plaques of the human aorta. Atherosclerosis 1971:13;93–101 [DOI] [PubMed] [Google Scholar]
- 71. Hollister LE, Overall JE, Snow HL. Relationship of Obesity to serum triglyceride, cholesterol, and uric acid, and to plasma-glucose levels. Am J Clin Nutr 1967:20;777–782 [DOI] [PubMed] [Google Scholar]
- 72. Nieman D, Henson D, Nehlsen-Cannarella S, et al. Influence of obesity on immune function. J Am Diet Assoc 1999:99;294–299 [DOI] [PubMed] [Google Scholar]
- 73. Anwar A, Janát-Amsbury M, Ray A, et al. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur J Pharm Biopharm 2012:77;417–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.