ABSTRACT
White adipose tissue (WAT) nucleated stromal cells (NSC) play important roles in regulation, defense, regeneration and metabolic control. In WAT sites, the proportions and functions of NSC change under diverse physiological or pathologic conditions. We had previously observed the massive anaerobic wasting of glucose to lactate and glycerol in rat epididymal adipocytes. To test site variability, and whether the adipocyte extensive anaerobic metabolism of glucose was found in NSC, we analyzed, in parallel, subcutaneous, mesenteric and epididymal WAT of male adult Wistar rats. Adipocytes and NSC fractions, were isolated, counted and incubated (as well as red blood cells: RBC) with glucose, and their ability to use glucose and produce lactate, glycerol, and free fatty acids was measured. Results were computed taking into account the number of cells present in WAT samples. Cell numbers were found in proportions close to 1:13:100 (respectively, for adipocytes, NSC and RBC) but their volumes followed a reversed pattern: 7,500:10:1. When counting only non-fat cell volumes, the ratios changed dramatically to 100:10:1. RBC contribution to lactate production was practically insignificant. In most samples, NSC produced more lactate than adipocytes did, but only adipocytes secreted glycerol (and fatty acids in smaller amounts). Glucose consumption was also highest in NSC, especially in mesenteric WAT. The heterogeneous NSC showed a practically anaerobic metabolism (like that already observed in adipocytes). Thus, NSC quantitative production of lactate markedly contributed (i.e. more than adipocytes) to WAT global use (wasting) of glucose. We also confirmed that glucose-derived glycerol is exclusively produced by adipocytes.
KEYWORDS: Adipose tissue, glycolysis, lactate, glycerol, adipocytes, stromal cells, glycaemia
Introduction
Adipose tissue, and more especially white adipose tissue (WAT), is one of the most peculiar animal tissues. It has been proposed that its variability in site size, distribution and function is a correlate of its adaptability, diversity of functions and adaptability, constituting, as a whole, a disperse organ.1 After decades of assuming that WAT was largely an energy dump (of triacylglycerols, TAG), the uncovering of its multiple, complex regulatory2 and defense3 functions has increased our attention on this tissue. The direct implication of WAT in the synthesis, regulation and control of hormonal and paracrine signals,4,5 and their relation with inflammation6 clearly indicated a key role in energy metabolism and its pathologies, such as the metabolic syndrome.7
WAT is also implicated in defense, repair and regeneration processes.8 The tissue includes a large number of immune cells, such as macrophages9 and lymphocytes, as well as stromal endothelial cells.10,11 They play a key role in the control of blood flow to WAT itself12 and neighboring tissues.13 By the way, WAT (the adipose organ) also stores TAG and constitutes the main body energy reserve.
Mammalian WAT contains cells of three widely different types: a) adipocytes (large, with huge fat vacuoles), b) blood erythrocytes (very small, non-nucleated), and c) a wide variety of cells, such as those described above, which we opted to describe as ‘nucleated stromal cells’ or NSC, irrespective of their origin and function. This is not a systematic, but a practical experimental grouping of tissue cell fractions. NSC may be attached to adipocytes or other structures; contributing to form a fiber scaffold,14 or remain unattached in WAT interstitial space. The structural and functional variety of these cells is considerable, in accordance with their roles and origins, but their proportions in WAT may change because of’ exposure to external agents, environmental shock, nutrient availability and variety, inducing the activation of defense systems.15 The pathological importance of knowing WAT specific functions is paramount, given its mass, distribution, cell plurality, capacity to change, adapt and respond, and the dramatic consequences observed in human disease, as is the case of metabolic syndrome.16
In spite of the abundance of known antecedents, our knowledge of WAT metabolism remains incomplete and largely limited to a few pathways. This insufficient information is, in part, a consequence of the practical difficulties of establishing comparisons of a quite peculiar tissue, which usually contain between 50–90% fat,17 with as little as 1.3–1.5% of non-fat adipocyte cell volume.18
It is already known that mature WAT behaves, in the presence of glucose and oxygen, practically as an anaerobic tissue,19,20 with low oxygen consumption in vivo21 and a disproportionately high production of lactate22 and glycerol23 with respect to its mass of ‘live cytoplasm’. Primary cultures of adipocytes showed close relationships of lipogenesis, or enhanced glycolytic activity, to the size of the cells.24 In recent papers, we have related enzyme activities, metabolite efflux or gene expression25-27 data to the number of cells, but this procedure requires considerable tissue manipulation: cell isolation, counting and analysis of recovery of the cells.28 This necessary step undoubtedly may hamper the rapid comparative (and quantitative) analysis of WAT sites under different conditions.
Our objective was to determine, using quantitative available methods, the relative capacity of adipocytes, NSC and RBC (red blood cells) present per unit of WAT weight to use glucose, mainly to produce lactate and glycerol. Since this approach could not be done in vivo, we isolated the cells, and incubated them with glucose in primary cultures. Later, we “reconstructed” the WAT ability to use glucose from the number of cells and their glycolytic effectiveness in culture.
Results
Experimental set-up
WAT samples of weight-and age-matched male Wistar rats were pooled in two-rat samples of tissue. Samples were taken from mesenteric (MES), subcutaneous (SC; constituted by both inguinal cordons) and epididymal (EPI) WAT. Adipocytes and NSC suspensions were isolated (and compared with blood-extracted RBC) in primary culture incubations with 7 mM or 14 mM glucose during 24 h as explained under Methods. Changes in glucose, lactate, glycerol and NEFA (non-esterified fatty acids) in the medium were analyzed. Cell numbers were estimated for all samples and used in the calculations, compared with those observed in the collagenase-WAT digests.
WAT cell proportions
The proportions (number, size) of cells (adipocytes, NSC, RBC) in the samples extracted from SC, MES and EPI WAT sites were analyzed in order to refer the experimental data to the original WAT sample, and to be able to compare and compute the results obtained from different incubation wells.
Table 1 shows the number of cells (adipocytes, NSC and RBC) present in 1 g of WAT from SC, MES or EPI WAT sites. No statistically significant differences in adipocyte or RBC numbers were observed, but in EPI WAT, the number of NSC per g was about half that found in MES, SC WAT showed values in between. No differences were found in the mean cell volume for NSC. However, MES WAT adipocytes were smaller than those of EPI WAT (had about half of their volume).
Table 1.
WAT site cell characteristics of young male Wistar rats.
| parameter | units | SC WAT | MES WAT | EPI WAT | p |
|---|---|---|---|---|---|
| cell counts | |||||
| adipocyte | cellsx106/g WAT | 3.19 ± 0.84 | 4.59 ± 0.98 | 2.90 ± 0.35 | NS |
| nucleated stromal cells | cellsx106/g WAT | 43.8 ± 7.7 | 59.8 ± 3.8 | 27.9 ± 7.3 | 0.0220 |
| red blood cells | cellsx106/g WAT | 108 ± 51 | 236 ± 57 | 150 ± 61 | NS |
| cell fragments | 106/g WAT | 151 ± 50 | 103 ± 22 | 105 ± 18 | NS |
| WAT adipocytes recovery | % | 70.4 ± 7.6 | 83.0 ± 8.6 | 77.2 ± 0.1 | – |
| cell volumes | |||||
| adipocyte volume | pL/cell | 251 ± 30 | 159 ± 33 | 309 ± 7 | 0.0065 |
| nucleated stromal cells | fL/cell | 419 ± 28 | 255 ± 79 | 256 ± 44 | NS |
| red blood cells | fL/cell | 32.4 ± 1.9 | 32.4 ± 1.9 | 32.4 ± 1.9 | – |
| red blood cells | % stromal cells | 62.8 ± 12.3 | 79.4 ± 3.3 | 84.4 ± 2.9 | NS |
| cell fragments and droplets | fL/fragment | <5 | – | ||
| other tissue data | |||||
| debris (dry weight) | mg/g WAT | 37 | 59 | 22 | – |
| water in intact tissue | mg/g WAT | 220 ± 63 | 160 ± 37 | 64.4 ± 11.2 | NS |
| water in tissue minus debris | mg/g WAT | 213 ± 62 | 152 ± 41 | 62.9 ± 10.2 | NS |
| fat in intact tissue | mg/g WAT | 740 ± 20 | 744 ± 24 | 871 ± 12 | 0.0014 |
| fat in tissue minus debris | mg/g WAT | 713 ± 29 | 701 ± 62 | 852 ± 1 | 0.0440 |
| tissue density | g/g WAT | 0.9246 ± 0.0130 | 0.9208 ± 0.0129 | 0.9282 ± 0.0048 | NS |
| fat density | g/mL WAT | 0.922 ± 0.022 | – | ||
| debris + interstitial space | µl/g WAT | 237 ± 97 | 275 ± 92 | 145 ± 106 | – |
The data are the mean ± SD of different [N: PG = 2; MES = 3; SC = 4] 2-rat tissue pools. Statistical significance of the differences between groups (1-way anova). The column p represents the p values corresponding to the effect of WAT site. NS = p > 0.05
The number of RBC may indicate a rough approximation to the volume of blood vessels present in the tissue, despite exsanguination. Assuming a standard hematocrit value of 45%, the number of RBCs found in MES WAT represent about 17 µL of blood per g of fresh tissue, whilst the values for SC WAT and EPI WAT were 7.8 µL/g and 10.6 µL/g, respectively. The data agree with a higher blood irrigation of MES WAT compared with the other sites.29 Despite their different localization and function, MES and SC WAT seem more structurally closer than when compared with EPI WAT: a) the ratio of mean values for nucleated cell volumes (adipocytes/NSC) was 624 and 600, respectively, for MES and SC, but double, 1207 for EPI WAT. b) The ratio of nucleated cell numbers (NSC/adipocytes) was 13.7 for SC, 13.0 for MES and 9.6 for EPI. Nevertheless, the closeness of these data suggest that under standard (i.e. no inflammatory conditions, as is the case), the relationship of NSC and adipocyte numbers, despite their sizes, were rather uniform.
The proportion of fat in WAT agrees with the larger adipocyte size in the EPI site, which translates into significant differences in the proportion of tissue triacylglycerol (TAG) reserves. Curiously, this results in probably smaller (no statistical calculation was done because the data were derived from measurements obtained from transformed data) interstitial space for EPI WAT compared with the other two smaller-cell WAT sites. The proportion of tissue water (but also that of debris + interstitial space) was lowest in EPI WAT, which had the highest fat content, the reverse being true for SC WAT. However, the density of the tissue from different sites was practically the same and close to the actual density of fat (all measured at 22ºC).
RBC lactate production
Since we could not isolate the RBCs from WAT collagenase digests, we simply counted their numbers, and then related their ability to metabolize glucose to results obtained using RBC taken from blood (or simply using blood as ‘suspension’ of RBCs). Since we assumed that all RBCs behaved equally irrespective of WAT site, we used the analysis of glucose consumption or lactate production simply to time of incubation, initial glucose and number of cells, containing a calculated value for lactate production in each WAT site sample.
Figure 1 presents the production of lactate by fresh blood RBC incubated under the same conditions than the cells extracted from WAT. The number of RBC present in the actual stromal cell suspensions used were in a range comparable to the intermediate concentration of cells depicted here. The results obtained with washed cells were not significantly different, and are not shown. The production of lactate was marked and depended on incubation time and number of cells. The production of lactate was not significantly altered by glucose concentration in the 7/14 mM range studied (data not shown).
Figure 1.
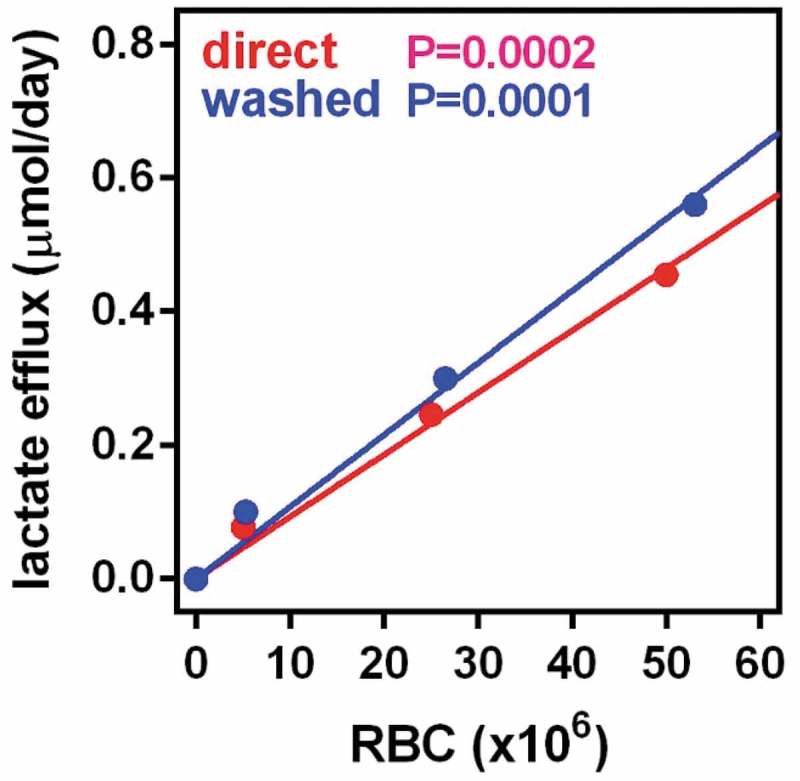
Production of lactate by RBCs incubated in medium with 14 mM glucose The data are the mean ± sem of 4 samples of adult rat blood. The blue dots and line coprrespond to samples of blood washed and processed in the same way than tissue extracts. Red dots and line are the results of direct incubation of whole fresh blood, The X axis represents the the number of RBC per well and the Y axis the amount of lactate released in micromoles per day. The p values represented in the Figure correspond to the statistical significance if the correlation between cell numbers and lactate efflux. No statistically significant differences were appreciated between the values for fresh blood and washed RBCs.
Glucose uptake and metabolite efflux from incubated adipocytes and NSC
Samples from SC, MES and EPI WAT adipocytes and NSC were incubated for 24 h in 7 mM or 14 mM (initial values) of glucose. Lactate, glycerol and NEFA efflux were measured in the medium, as well as the disappearance of glucose, and used to determine the metabolic change per cell that will allow the estimation of global WAT capacity to use glucose.
Table 2 shows the glucose uptake and lactate, glycerol and NEFA efflux from incubated adipocytes, NSC and RBC. The values for NSC shown are the result of discounting the values of the incubated crude cell preparations minus the calculated contribution of RBCs. The only statistically significant difference between groups (taking as comparative factors site and initial medium glucose) was the effect of WAT site on glucose uptake, with maximal values in MES-WAT. No significant effects of glucose concentration were observed on lactate efflux of adipocytes or NSC. The lactate efflux of RBC was practically the equivalent of the glucose taken up, as expected. The relationships between glucose consumption and lactate efflux were closer to carbon equivalence in NSC than in adipocytes.
Table 2.
WAT site cell glucose uptake and metabolite efflux of young male Wistar rats.
| cell type & units (per cell and 24 h) |
SC WAT |
MES WAT |
EPI WAT |
||||
|---|---|---|---|---|---|---|---|
| 7 mM | 14mM | 7 mM | 14mM | 7 mM | 14mM | ||
| glucose uptake | |||||||
| adipocytes | pmol | 4.56 ± 0.68 | 4.94 ± 0.58 | 7.81 ± 0.68 | 8.73 ± 0.99 | 3.36 ± 0.52 | 4.02 ± 1.95 |
| NSC | pmol | 0.344 ± 0.085 | 0.63 ± 0.29 | 0.447 ± 0.077 | 0.811 ± 0.082 | 0,784 ± 0.150 | 1.06 ± 0.45 |
| RBC | fmol | 5.71 ± 1.70 [*] | |||||
| lactate efflux | |||||||
| adipocytes | pmol | 3.63 ± 0.56 | 3.90 ± 0.42 | 3.93 ± 0.91 | 3.88 ± 1.20 | 2.47 ± 0.48 | 2.81 ± 0.42 |
| NSC | pmol | 0.647 ± 0.214 | 0.504 ± 0.183 | 0.796 ± 0.141 | 0.876 ± 0.196 | 0.550 ± 0.222 | 0.564 ± 0.217 |
| RBC | fmol | 11.4 ± 3.4 [*] | |||||
| glycerol efflux | |||||||
| adipocytes | pmol | 1.56 ± 0.35 | 1.52 ± 0.35 | 2.26 ± 0.42 | 2.40 ± 0.33 | 1.71 ± 0.16 | 1.95 ± 0.21 |
| NSC | fmol/ | not detected | |||||
| NEFA efflux | |||||||
| adipocytes | pmol | 0.401 ± 0.109 | 0.385 ± 0.119 | 0,752 ± 0.407 | 0.360 ± 1.67 | 0.242 ± 0.038 | 0.178 ± 0.023 |
| NSC | pmol | not detected | |||||
The data are the mean ± sem of samples (N = 4; N = 3 for 14 mM glucose in MES and EPI), each corresponding to two pooled rat tissues; [*] calculated value (from data shown in Figure 1). Statistical significance of the differences between groups (2-way ANOVA). The only significant (p = 0.0008) relationship was that of glucose uptake by adipocytes with respect to WAT site.
The release of either glycerol or NEFA by NSC to the medium were negligible (small values, not significantly different from zero). The same can be said (as expected) for RBC.
When the individual cell data were adjusted by the number of cells of each group, in order to show the composite estimated values corresponding to all cells present in 1 g of WAT, we obtained the results depicted in Figure 2. These data reflect a minimal quantitative importance of the RBC fraction both for glucose uptake and lactate efflux. Consequently, all further comparisons were limited to NSC and adipocytes. With respect to glucose uptake, as well as lactate and glycerol efflux, adipocytes showed a clear effect of site, with higher values in MES WAT. This effect was also significant for lactate efflux on NSC, with, again, a higher production of lactate in MES WAT. No significant changes were observed in NEFA efflux (only different from zero in adipocytes). However, glycerol efflux rates were different for the sites studied, the highest values being those of MES adipocytes.
Figure 2.
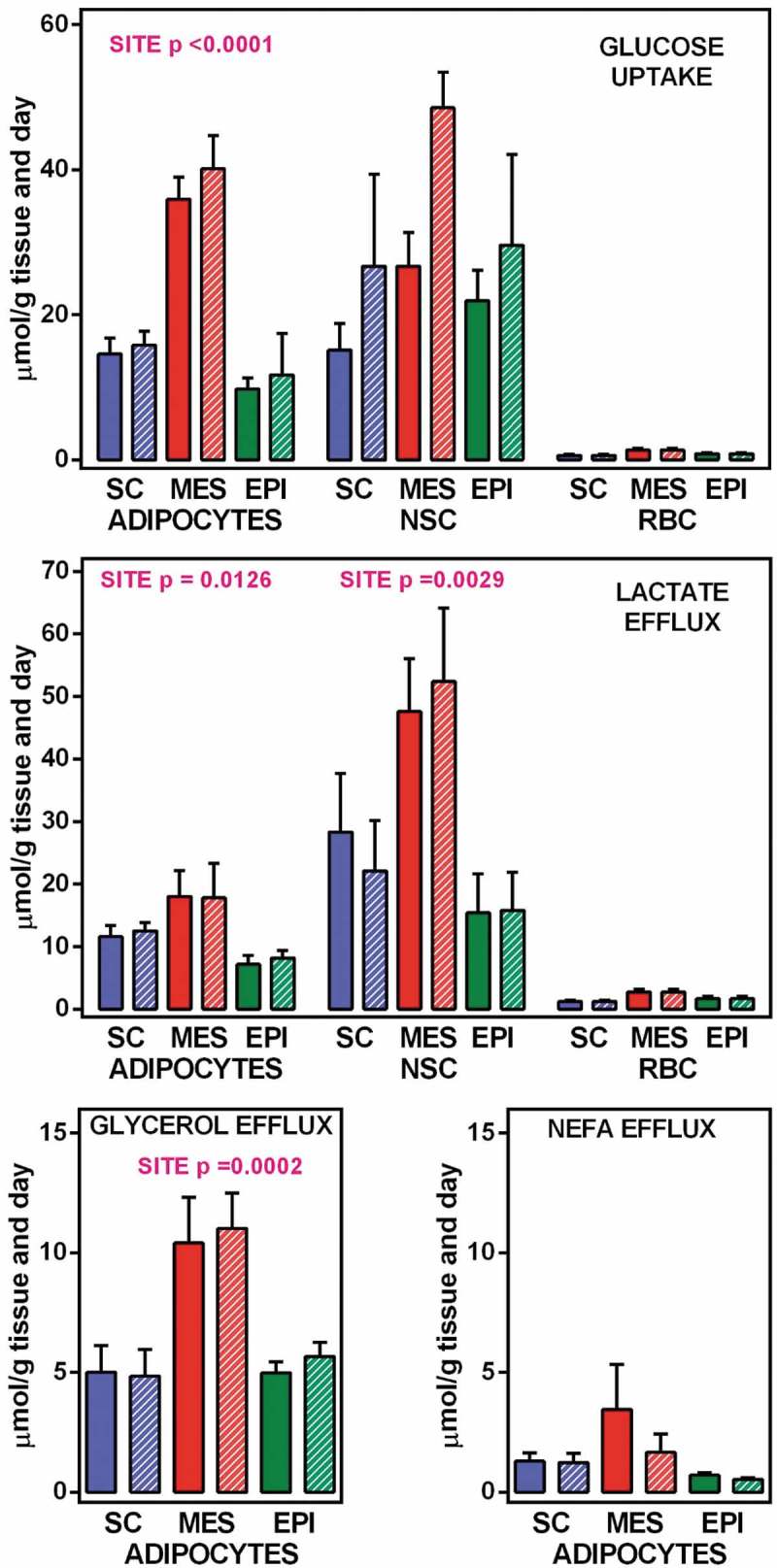
Glucose uptake, and lactate, glycerol and NEFA efflux from WAT component cells corresponding to 1 g of tissue SC = subcutaneous WAT (blue); MES = mesenteric WAT (red); EPI = epidydimal WAT (green). Full columns = 7 mM glucose, white-dashed columns = 14 mM glucose. The data are the mean ± sem of two-rat pooled samples (N = 4; N = 3 for 14 mM glucose in MES and EPI) obtained from adult male rats. The data for glucose uptake and lactate efflux correspond to the number of adipocytes, NSC and RBC contained in 1 g of tissue (Table 1) multiplied by their individual uptake/efflux data (Table 2). The data for glycerol and NEFA correspond exclusively to the adipocytes present in 1 g of tissue, since no efflux of either was observed in NSC or RBC. Statistical significance of the differences between groups (2-way ANOVA): the data for site in adipocytes and NSC are indicated in the Figure itself, the absence of data indicates that the differences, if any, were not significant (P > 0.05); no significant differences were observed for the effect of initial glucose concentration.
Taken as a whole, and contrary to what we expected, in all three sites, lactate efflux by NSC was higher than that produced by the adipocytes. It is remarkable to add, that there were no statistically significant effects of initial glucose concentration on any of the parameters analyzed.
Comparative gene expression in WAT sites
After incubation, adipocytes and NSC suspensions were used to extract their RNA, which was used to analyze the expression of a number of glycolytic and lipogenic enzymes, in the different WAT sites studied. The number of copies per cell in each cell fraction was then used to calculate the global ‘power’ of expressed proteins in adipocytes and NSC of the three WAT sites analyzed.
Table 3 presents the number of copies per cell of the specific mRNA for genes related to the production of 3C substrates from glucose. All genes, except Pdk4 and adipocyte Hsl and Lpl showed a significant effect of site in their expression. In general, the highest values were found in MES WAT
Table 3.
WAT site expression (in specific mRNA copies per cell) of genes related with glycolysis and glycerol metabolism of young male Wistar rats.
| gene | cells | SC WAT |
MES WAT |
EPI WAT |
p |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7 mM | 14mM | 7 mM | 14mM | 7 mM | 14mM | site | glucose | |||
| Glut1 | glucose transporter 1 | adipocytes | 75.7 ± 5.0 | 128 ± 14* | 304 ± 56* | 287 ± 61 | 111 ± 11 | 123 ± 22 | <0.0001 | NS |
| NCS | 20.0 ± 4.8 | 26.3 ± 9.2* | 31.1 ± 4.3* | 52.6 ± 5.6* | 16.3 ± 5.6* | 23.3 ± 5.5 | 0.0050 | 0.0322 | ||
| Hk1 | hexokinase 1 | adipocytes | 172 ± 15 | 222 ± 29* | 488 ± 52* | 500 ± 35* | 343 ± 54 | 394 ± 108 | 0.0011 | NS |
| NCS | 11.9 ± 2.6 | 6.71 ± 4.04 | 26.0 ± 1.6 | 32.4 ± 0.1* | 18.8 ± 6.7 | 20.5 ± 4.7 | 0.0007 | NS | ||
| Ldha | type a lactate dehydrogenase | adipocytes | 1003 ± 196 | 1274 ± 202* | 2271 ± 260* | 1963 ± 214 | 1959 ± 157 | 1988 ± 154 | 0.0003 | NS |
| NCS | 95.6 ± 11.6 | 77.8 ± 11.8* | 222 ± 9* | 265 ± 38* | 189 ± 20 | 168 ± 13 | <0.0001 | NS | ||
| Ldhb | type b lactate dehydrogenase | adipocytes | 164 ± 21 | 176 ± 8* | 332 ± 62* | 362 ± 51* | 339 ± 32 | 340 ± 23 | 0.0003 | NS |
| NCS | 1.15 ± 0.18 | 0.66 ± 0.37* | 3.63 ± 0.48* | 1.96 ± 0.69* | 3.94 ± 0.48 | 3.66 ± 0.32 | <0.0001 | 0.0345 | ||
| Pfkl | phospho-fructo-kinase liver | adipocytes | 131 ± 11 | 195 ± 24 | 392 ± 73* | 404 ± 24* | 170 ± 29 | 182 ± 46 | <0.0001 | NS |
| NCS | 15.1 ± 2.6 | 7.56 ± 3.24 | 34.1 ± 1.2 | 41.2 ± 7.3* | 17.2 ± 4.8 | 16.9 ± 3.3 | <0.0001 | NS | ||
| Phgdh | 3-phospho-glycerate DH | adipocytes | 17.5 ± 1.5 | 25.5 ± 3.7* | 52.1 ± 11.6* | 58.0 ± 9.8* | 42.3 ± 4.7 | 44.0 ± 9.2 | 0.0013 | NS |
| NCS | 2,64 ± 0.33 | 2.44 ± 0.53* | 7.23 ± 0.96 | 8.14 ± 0.81* | 5.40 ± 1.46 | 5.67 ± 1.60 | 0.0013 | NS | ||
| Gpam | glycerol-3P acyl-transferase | adipocytes | 15.6 ± 1.1 | 19.3 ± 4.7* | 58.3 ± 10.7* | 54.7 ± 15.6 | 38.8 ± 2.4* | 34.7 ± 10.7* | 0.0018 | NS |
| NCS | 0.478 ± 0.100 | 0.439 ± 0.115* | 1.12 ± 0.25 | 1.32 ± 0.54* | 0.982 ± 0.137 | 0.904 ± 0.136 | 0.0298 | NS | ||
| Pdk4 | pyruvate DH kinase 4 | adipocytes | 4.76 ± 2.36 | 5.16 ± 2.52* | 19.9 ± 15.9* | 9.50 ± 1.81* | 2.83 ± 0.92 | 2.72 ± 0.85 | NS | NS |
| NCS | 0.061 ± 0.007* | 0.024 ± 0.024* | 0.050 ± 0.016 | 0.047 ± 0.002* | 0.063 ± 0.009* | 0.013 ± 0.003* | NS | NS | ||
| Fas | fatty acid synthase | adipocytes | 97.2 ± 6.3 | 191 ± 30* | 302 ± 54* | 478 ± 84* | 181 ± 27 | 241 ± 63 | 0.0004 | 0.0166 |
| NCS | 5.42 ± 0.83 | 3.49 ± 0.99* | 11.2 ± 1.8 | 19.7 ± 5.1* | 8.62 ± 1.35 | 10.4 ± 1.9 | 0.0012 | NS | ||
| Hsl | hormone-sensitive lipase | adipocytes | 45.0 ± 5.5 | 38.4 ± 5.5* | 48.6 ± 8.6* | 48.4 ± 6.5 | 98.3 ± 34.1 | 109 ± 54 | NS | NS |
| NCS | 0.739 ± 0.282 | 0.364 ± 0.079 | 0.966 ± 0.182 | 1.01 ± 0.15* | 0.566 ± 0.050* | 0.599 ± 0.011 | 0.0195 | NS | ||
| Atgl | adipose TAG lipase | adipocytes | 218 ± 20 | 320 ± 45 | 488 ± 65* | 560 ± 56* | 547 ± 71 | 465 ± 74* | 0.0005 | NS |
| NCS | 5.69 ± 0.85 | 5.22 ± 0.67* | 11.8 ± 2.8 | 18.4 ± 1.03* | 6.08 ± 1.48 | 6.08 ± 0.92 | <0.0001 | NS | ||
| Lpl | lipoprotein lipase | adipocytes | 859 ± 42 | 1106 ± 228* | 1437 ± 222* | 1537 ± 238* | 1466 ± 262 | 1681 ± 358 | NS | NS |
| stromal | 2.62 ± 0.87 | 0.89 ± 0.20* | 3.50 ± 0.38 | 3.51 ± 1.37* | 3.32 ± 0.42 | 3.53 ± 0.26 | 0.0412 | NS | ||
The data are the mean ± sem of N = 4 different (* N = 3) two- rat pooled samples. Data in italics were obtained with 30 or more cycles. Statistical significance of the differences between groups (2-way ANOVA). The far right columns show the p values for site and initial glucose concentration. NS = p > 0.05
The effects of glucose were much more limited than those of site, the only changes observed were in NSC Glut1 and Ldhb of NSC, and in adipocyte Fas. No other effect was observed, and those listed were close to the limit of significance. Some of the data presented may be partially artefactual, because of the low number of copies counted, in some cases lower than 1 per cell. This is probably a consequence of the NSC being a complex mixture of quite different types of cells, a condition magnified in this case by the different proportions of NSC found in different sites per unit of tissue mass. The lowest values of copies per cell in NSC were for genes related to lipid metabolism. (Gpam, Hsl, Fas, Atgl and Lpl), but included a critical regulatory enzyme Pdk4 and the lactate dehydrogenase isozyme Ldhb, much more abundant in adipocytes. These data suggest a probable different regulation of lactate dehydrogenase in adipocytes and the NSC as a whole.
In a way parallel to the proportional representation of cells’ effects on medium metabolites, Figures 3 and 4 show the contribution of adipocytes and NSC to the total number of copies for the genes studied per g of WAT. The contribution of the low number of adipocytes compared with the NSC were similar for most glucose-lactate metabolism-related genes (Figure 3). In the six genes depicted: Glut1, Hk1, Pfkm, Phgdh, Ldha and Ldhb, the effect of ‘type of cell (NSC or adipocyte) was statistically significant, and in all cases and in both cell types the effect of site was also significant. In addition, in NSC, Glut 1 and Ldhb showed a significant effect of glucose. In most cases MES WAT showed the highest contributions (and SC the lowest) both in adipocytes and NSC, with global gene expression values markedly parallel in all cases except for lactate dehydrogenase b (Ldhb), which expression was much lower than that of the a isozyme (Ldha), showing even lower values for NSC compared with adipocytes. This case being the only ‘discordance’ in that series of data.
Figure 3.
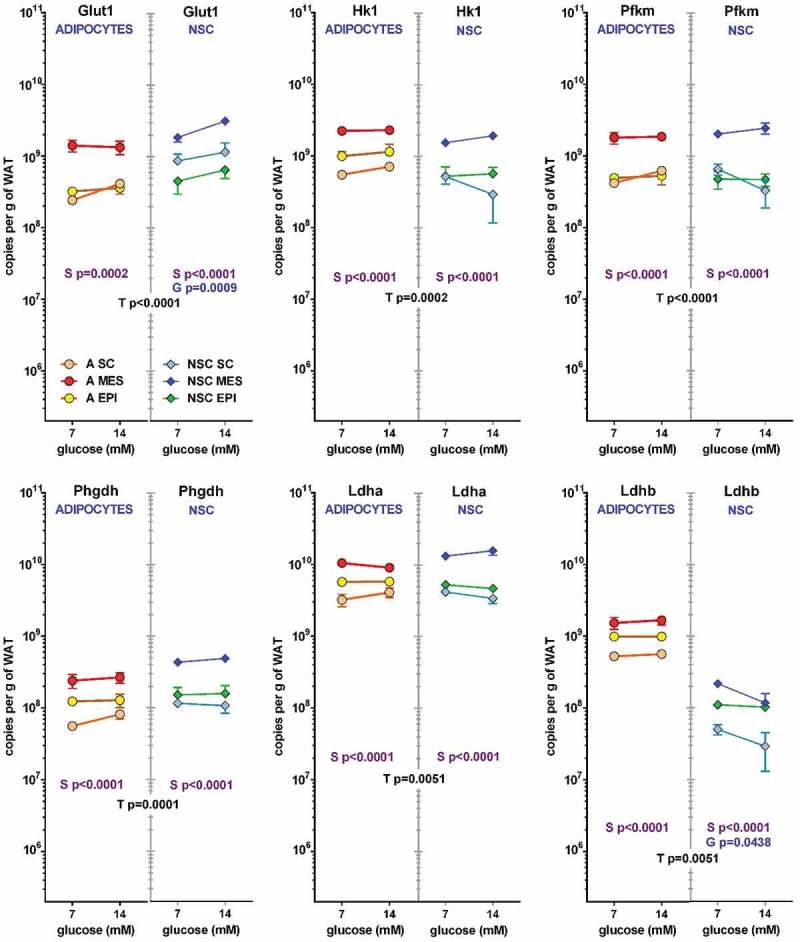
Number of copies of glycolysis-related genes in adipocytes and NSC contained in 1 g of WAT from different sites (SC, MES and EPI) or male adult rats The data correspond to the mean ± sem of two-rat pooled samples (N = 4 or N = 3 in the groups indicated in Table 3). The data have been presented in a of magnitude logarithmic scale (with more than five orders of magnitude span) for easy direct comparison of the abundance of all gene transcripts. The values shown were calculated from the data presented in Tables 1 and 3. Dot and line colours are represented in the Figure: Adipocytes: orange A SC, red A MES, yellow A EPI; NSC: light blue NSC SC, blue NSC-EPI, green NSC-EPI., Statistical significance of the differences between groups (3-way ANOVA). T: corresponds to differences between the ‘type’ or cell (adipocyte vs. NSC), in black, S: refers to the differences between ‘sites’ (SC, MES, EPI) within the same cell group, in purple, and G represents the statistically significant differences in expression induced by the initial ‘glucose’ concentrations, in blue.
The trend shown by Ldhb was partially repeated (Figure 4) in Gpam, Pdk4, Hsl, Atgl, and Lpl, which represent a general, albeit limited, view of lipid metabolism. In all genes studied, the effect of type of cell was, again, fairly patent, this being in part a consequence of different cell size, even allowing for the inert vacuole mass of TAG. The effects of site were significant for Gpam, Fas, Atgl and Lpl, for both cell types, as well as Hsl in NSC. In adipocytes, the effect of initial glucose concentration was observed only in Fas. The gene expression values per unit of tissue weight were clearly higher in adipocytes than in NSC, one-to-two orders of magnitude lower. The primacy of MES was maintained, but the differences between SC and EPI WAT were less marked, interchanging positions, in clear divergence with the glycolytic data of Figure 3. In adipocytes, Lpl expression in EPI WAT was more than one order of magnitude lower than in SC and MES. The expression of Pdk4, normally low, was even lower in NSC, tending to decrease with glucose concentration (the results were not significant), which tend to agree with the reverse trend on Fas, since lipogenesis requires the formation of acetyl-CoA and the decrease in Pdk4 hints at a lower inhibition of pyruvate dehydrogenase.
Figure 4.
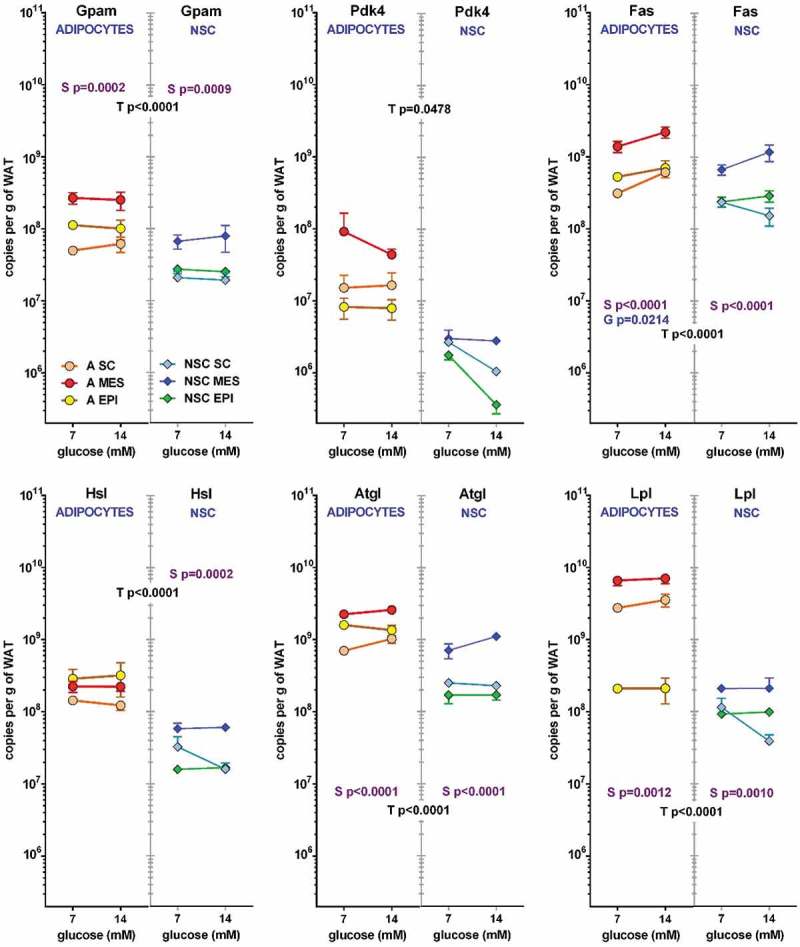
Number of copies of glycerol and lipid metabolism-related genes in adipocytes and NSC contained in 1 g of WAT from different sites (SC, MES and EPI) or male adult rats The data correspond to the mean ± sem of 4 different two-pooled rat samples. All conventions are the same described for Figure 3.
Discussion
In WAT, the global importance of NSC has been widely recognized, especially its implication in questions of regulation,30 defense,31 regeneration32 and even metabolic control33 via hormones and cytokines. However, and despite its also accepted variety and diversity depending on WAT site, to our knowledge, no actual analysis of its overall metabolic contribution to the handling of substrates has been so far analyzed nor established. The results presented here are only the tip of an iceberg that needs to be acknowledged, measured, known and its true magnitude ascertained.
The heterogeneous structure of WAT prevented a direct in vivo approach. Consequently, we had to introduce an initial tissue fractioning step: separating adipocytes from the rest of WAT cells, and analyzing their capacity to use glucose and yield 3-carbon substrates. We are aware that this is only an approximation to the in vivo situation, since cells in primary culture do not behave in the same way than incorporated in a stromal organization within a living tissue.
We are aware that the methodology we used should be refined further to gain sufficient insight on the quantitative role of NSC in substrate handling, but the basic concepts have been already established,26 and the isolation, incubation, viability and analyses needed are available, often being standard and commonly used. The data presented here continues what has been developed in a series of preceding methodological and quantitative studies that drove us to the findings shown here.18-20,26-29,34
The deconstruction-isolated cell- analysis-reconstructive computing of results is not a substitute for the in vivo (unfeasible) analysis, but at least may provide an approximate idea of the metabolic potential of this widely diverse group of cells. The data presented, support the idea that WAT’s own metabolism is even more complex than usually assumed. Adipocytes represent less than 10% of nucleated cells, despite each containing about 10-fold the ‘live cell’ volume of NSC, overall, a few microliters per gram of WAT.28 It has been assumed that WAT reason to exist is essentially defined by adipocytes and their TAG stores,35 metabolic activity being centered on lipogenesis from glucose and lipolysis of TAG. This may be largely true for the largest and more deposit-prone sites, such as EPI WAT, but not as much for the more metabolically active MES WAT36 or the smaller control-related deposits such as perivascular37 or pericardic38 depots. However, and with respect to glycolysis to lactate our data show that NSC glycolytic activity was higher than that of adipocytes in all three sites studied. Nevertheless, the difference was even higher for the most active WAT, MES.
The data obtained for the NSC almost complete glycolytic conversion of glucose to lactate strongly support their anaerobic orientation, even in the presence of abundant oxygen. In this case, both NSC and adipocytes (and even RBCs) tend to convert glucose to 3C-units, largely lactate, responsible for a large share of rodent cell energy supply39
The question is to know whether the observed glycolytic function is intrinsic or adopted. It may be postulated that the presence of large adipocytes, in which most of their cytoplasm was just a thin layer between the plasma membrane and the fat vacuole, too far away from mitochondria27 for efficient oxidative metabolism and lipogenesis to take place;26 and thus becoming, necessarily, glycolytic,40 despite having a sufficient availability of oxygen. There is a long list of WAT NSC types, and their dependence on oxygen is patent when studied from other sources.41 Thus, it is difficult to attribute this WAT-inbred trend simply to an adaptation to relative anaerobiosis, in part due to restricted blood flow (oxygen supply) to the tissue,21 but more to the ability to survive with the limited amount of ATP that glycolysis provides. The high lactate production was unrelated to high medium oxygen, in a parallel way to that shown by adipocytes.27,42 This relative independence with respect to oxygen agrees with the limited WAT oxygen consumption in vivo21 and supports massive energy-inefficient use of glucose for glycolysis to lactate instead of its complete oxidation
In studies of non-adipocyte WAT cells, lactate production has been often associated to hypoxia.43 The results presented here complicate the widely acknowledged relationship of hypoxia in WAT with the negative consequences of inflammation.44 How can hypoxia become a key factor eliciting a pathologic inflammatory response in a tissue which ‘main’ cells, adipocytes, live off the ATP generated by glycolysis,19,27 limiting oxygen use largely for lipogenesis but not for energy45 ? Now, we have found that the ‘other’ WAT cells, NSC, a diverse mixture of structural, epithelial, immune system cells, stem cells, fibroblasts and others, may thrive, as a whole, also using the energy inefficient glycolytic pathway instead of the more efficient mitochondrial oxidative metabolism. In addition, these small cells do not have the geometric constrictions of adipocytes (distance of most zones of cytopkasm to mitochondria). These data clearly hint at a “whole WAT” adaptation to low oxygen and/or the purpose to convert excess glucose to 3C fragments, which runs in parallel for adipocytes and NSC. This trend is maintained even when oxygen is widely available (but glucose is available too), and may represent a true distinguishing WAT characteristic.
Regulation of WAT blood supply has been postulated as a mechanism of defense against excess substrate availability;12 but it reduces too the oxygen supply, which may result in hypoxia, even counting with the Bohr effect, which facilitates the release of oxygen from RBC when lactate is high.46 Probably, the adaptation to low oxygen renders blood oxygen supply as secondary for WAT (few cells, low intrinsic needs) leaving blood flow control as – essentially – a regulatory factor for substrate supply.47 Our data, presented here also hint to MES WAT having a higher blood irrigation48 than the other sites, but – again – lactate production is highest in that site.
The gearing of adipocytes and NSC for glycolysis results fully apparent in Figure 3, with a marked parallelism on the quantitative expression of these two ‘halves’ of active WAT: adipocytes and NSC. A number of differences (lipid metabolism, lactate dehydrogenase ratio) show their different origin and function; but the fact that both participate of WAT glycolytic activity raises the question of the metabolic importance of these cells with respect to whole-body energy handling. It must be taken into account that the mass of the adipose organ is one of the largest for any organ or system in the body.
The capability to thrive with low oxygen may be of paramount importance for a tissue implicated in tasks of regeneration and defense, being able to grow in hypoxic niches where angiogenesis is a critical tool for repair and normalization.49 The parallelism of this facet of WAT and tumors ends here, since the density of substrate and oxygen consumption per unit of weight are quite different, as are the rates of glucose consumption (i.e. the Warburg Effect50) and the unique property of WAT to produce large amounts of glycerol alone, without the concurrence of NEFA excretion.26 Here we have found that this is a peculiarity of adipocytes, since NSC did not release either glycerol or NEFA to the medium.
Glycerol levels and uptake are not as strictly regulated as is glucose by insulin, thus WAT breaking up of glucose to 3C units has the advantage to eliminate excess glucose (and thus lower glycemia)51 without creating a problem of energy supply to the brain, which can be sustained by both lactate and glycerol.52,53
In sum, we found that NSC can match the glycolytic activity of adipocytes, being responsible for a significant portion (which may be a major part) of glycolytic conversion of blood glucose to lactate, a main substrate used to sustain a sizeable part of the energy needs of most body cells.39 However, the efflux and gene expression data suggest that NSC do not participate in WAT active glycerol metabolism. Thus, we also conclude that glycerol is the exclusive product of adipocytes, and postulate that its fate is probably to provide energy to other tissues, with, perhaps, a more direct relationship with the brain, avid consumer of this polyol53 and lactate54 for energy. There are marked differences between WAT sites, with mesenteric WAT playing a more active role, probably related to its location besides the intestine, for glucose disposal and glycerogenesis.
Methods
Rats and sampling
All animal handling procedures and the experimental setup were in accordance with the animal handling guidelines of the corresponding European and Catalan Authorities. The Committee on Animal Experimentation of the University of Barcelona specifically authorized the procedures used in the present study.
Adult male Wistar rats (Janvier, Le Genest-Saint Isle, France), aged 11 weeks when killed, were used. The animals weighed 426 ± 12 g and were kept in two-rat cages with wood shards as bedding material, at 21-22ºC, and 50–60% relative humidity; lights were on from 08:00 to 20:00. They had unrestricted access to water and standard rat chow (#2014, Teklad Diets, Madison, WI USA).
The rats (N = 12) were killed, under isoflurane anesthesia, by exsanguination from the exposed aorta. They were dissected, and samples of epididymal (EP), mesenteric (MES) and subcutaneous (SC; both inguinal cordons) WAT were extracted. In order to obtain sufficient material, same weight samples from the same-site of two size-matched rats were pooled, minced with scissors and thoroughly mixed before further processing.
WAT cell isolation and preparations
Samples of whole WAT were reserved and used for lipid and water content analysis as well as for density estimation as previously described.28 Cells were isolated55 at 37ºC for 1 h in a shaking bath using collagenase (LS004196, type I, from Worthington Biomedical, Lakewood NJ USA) in 2.5 volumes of modified Krebs-Henseleit buffer. Then, the cell suspension was filtered through a double layer of nylon hose. The retained debris was recovered and weighed. The cell suspension was transferred to vertical syringes and left standing for 5–6 minutes at room temperature. Adipocytes formed an upper layer, floating over a liquid phase. The latter was slowly drained from the syringe and the upper adipocyte mass was left in it as previously described. The adipocyte layer was gently suspended again in fresh incubation buffer (free of collagenase) and the process of mixing and draining was repeated twice, discarding the washing fluids.49 Aliquots of the adipocyte layer were used for cell size estimation, lipid content (for analysis of recovery), and for incubation as described below.26
The first washing contained most of the non-attached stromal cells; it was filtered through a 100 µm mesh to remove debris. The filtrate was centrifuged 8 min at 500 × g. The NSC were then re-suspended, and used directly for incubations, cell number estimation and analysis of red blood cell content, as previously described.28 All cell preparations were maintained at room temperature (c. 22ºC), and manipulated for a time as short as possible; adipocytes were used immediately after the final washing.
The stromal cell space in the isolated cell suspension was used to relate their numbers and volumes to initial tissue weight; it was estimated as the sum of the volume of the lower phase of adipocyte separation (extracted in the syringe), plus a part of the volume of the adipocyte phase not occupied by the adipocytes themselves. This latter volume was the difference between the volume of the phase and that of adipocytes, calculated from their numbers and cell volumes.28 Obviously, the first separation of adipocytes and stromal cells left a high number of the latter mixed with adipocytes. The three successive washings resulted in the presence (calculated) of, at most, 0.1% of the initial free stromal cells in the final washed adipocyte fraction (down from an initial 7.3%). This assumption does not take into account stromal cells bound, retained or attached to the larger adipocytes.
Measurement of isolated cell parameters
Adipocyte suspensions were examined using a Neubauer chamber (#717,810 Neubauer improved bright line, Brand Gmbh, Wertheim, Germany) following the procedure already described by us.28 At least 16 fields for sample were photographed using an inverted microscope. Cells were identified, counted, and their size analyzed (under the conditions used, all cells adopted a spheroid form), using the FIJI ImageJ software (http://imagej.nih.gov/ij/).56
Non.adipocyte stromal cells (i.e. including RBCs) were analyzed in each sample with the Scepter 2.0 cell counter (EDM Millipore Corp, Billerica, MA USA) hand-held cell sizer, using two different cell-range tips for the Scepter: Sensor 40, for 3–18 µm particles’ size (PHCC40050, Merck Millipore, Darmstadt, Germany) and Sensor 60, for 6–36 µm particles’ size (PHCC60050, Merck MIllipore). RBCs were estimated from the counting of particles with volumes between 25 fL and 60 fL, since these limits included 90% of total RBC (unpublished data). The larger particles were considered to be nucleated stromal cells (NSC), as previously described.28 Particles smaller than 25 fL were also counted; they were considered to be, essentially, fat droplets and other small cell or fiber agglomerate debris.
Cell incubation procedures
The complete procedure has been described previously by us;26,28,29 in short: Cell incubations were carried out using 12-well plates (#734-2324VWR International BVBA/Sprl., Leuven Belgium) filled with 1.7 ml of DMEM (#11966-DMEM-no glucose; Gibco, Thermo-Fisher Scientific, Waltham MA USA), supplemented with 30 mL/L fetal bovine serum (FBS, Gibco). The medium also contained 25 mM hepes (Sigma-Aldrich), 2mM glutamine (Lonza Biowhittaker, Radnor, PA USA), 1 mM pyruvate (Gibco), 30 mg/mL delipidated bovine serum albumin (Millipore Calbiochem, MA USA) and 100 nM adenosine, 100 U/mL penicillin plus 100 mg/L streptomycin (both from Sigma-Aldrich). The cells were supplemented with glucose (Sigma-Aldrich) to a final concentration of either 7 mM or 14 mM.
Each well received 400 µL of the corresponding cell suspension; after initial sampling, the final incubation volume was 2.0 mL. The cells were incubated at 37°C in a chamber ventilated with air supplemented with CO2 (5%), which gave a theoretical dissolved pO2 of 20 kPa.19 The cells were incubated for 24 h without any further intervention. Then, the wells’ contents were transferred with a pipette to small polypropylene tubes, which, in the case of adipocytes, were left standing for 5 min to pipette out the infranatant and immediately use in situ the adipocyte fraction for RNA extraction. In the case of NSC (containing the original tissue RBCs), the tubes were centrifuged for 8 min at 500 × g. Supernatant medium was extracted, and the cell precipitates (RBC and NSC) were immediately used for RNA extraction. All supernatants were aliquoted, frozen and stored at −20ºC until processed. The gene expression data was analyzed only in the 24 h samples, after incubation and harvesting, and were used to compare the different effects of exposure to glucose concentration. No time zero expression data (i.e. basal state, equivalent to that of initial WAT) were available, since the incubation started with cells just isolated after collagenase incubation and separation, and thus not kept under uniform conditions, and far away from those in the tissue. After one day of incubation, the cells had time to establish a higher degree of gene expression uniformity (with glucose, oxygen and other nutrients available) in which the only different variable was the initial glucose concentration.
Analysis of metabolites in the medium
Medium glucose was measured using a glucose oxidase-peroxidase kit (#11,504, Biosystems, Barcelona Spain) to which we added 740 nkat/mL mutarrotase (porcine kidney, 136A5000, Calzyme, St Louis, MO USA).57 Lactate was measured with kit 1,001,330 (Spinreact, Sant Esteve d’en Bas, Spain); glycerol was estimated with kit #F6428 (Sigma-Aldrich). NEFA were measured using kit NEFA-HR (Wako Life Sciences, Mountain View, CA USA). Data for medium metabolites was referred to the number of cells in the well.
Estimation of RBC lactate production under the conditions of incubation
The variable presence (despite the rats being exsanguinated) of RBC in the samples (affecting cell counts), and their glycolytic nature (affecting glucose uptake and lactate output) were possible sources of interference for the analysis of non-adipocyte production of lactate. Since no practical method was available to remove RBCs from the non-adipocyte cell preparations without producing unknown damage or modification to the other cells, we opted for the estimation of the eventual capability of RBCs to produce lactate using directly RBCs (from blood) alone. Two different preparations of RBCs were obtained from the pooled fresh (heparinized) blood of two ‘WAT donors’. In one, blood was suspended (diluted) in WAT cell isolation medium; then, the cells were centrifuged and washed in incubation medium and re-suspended (i.e. treated as cells isolated from WAT). This suspension was compared with the direct dilution of blood in incubation medium. Using the Scepter cell counter as described above, the number of cells in both suspensions were counted. Three cell concentrations were prepared: 5, 25 and 50 million cells per well. The RBCs were then incubated under the same conditions than the other cells for up to 48 h. The cells were harvested and discarded, and the media lactate was measured using the procedure described above. The glucose (and lactate) present in the RBC carried in the NSC fraction was at least one order of magnitude below the attomol/cell range; consequently, its eventual influence on the zero-time measurements of lactate and glucose was practically unmeasurable, i.e. negligible
Gene expression analysis
Total cell RNA was extracted from all the harvested cells using the Tripure reagent (Roche Applied Science, Indianapolis IN USA). RNA content was quantified in a ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington DE USA). RNA samples were reverse transcribed using oligo-dT primers (Gene Link, Westchester, NY USA) and the MMLV reverse transcriptase (Promega, Madison, WI USA) system.
Real-time PCR (RT-PCR) amplification was carried out using 10 μL amplification mixtures containing Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA USA), 4 ng of reverse-transcribed RNA and 150 nmol of primers. Reactions were run on an ABI PRISM 7900 HT detection system (Applied Biosystems) using a fluorescent threshold manually set to 0.5 for all runs.
A semi-quantitative approach for the estimation of the concentration of specific gene mRNAs per unit of tissue weight was used.58 Arbp was the charge control gene.59 We initially expressed the data as the number of transcript copies per nucleated cell.
The genes analyzed and a list of primers used are presented in Table 4.
Table 4.
List of primers used.
| gene | protein | direction | sequences | bp |
|---|---|---|---|---|
| Glut-1 | glucose transporter type 1, erythrocyte/brain | 5ʹ > | GCTCGGGTATCGTCAACACG | 97 |
| > 3’ | ATGCCAGCCAGACCAATGAG | |||
| Hk1 | hexokinase type 1 | 5ʹ > | TGGATGGGACGCTCTACAAA | 100 |
| > 3’ | GACAGGAGGAAGGACACGGTA | |||
| Ldha | L-lactate dehydrogenase a | 5ʹ > | AAAGGCTGGGAGTTCATCCA | 96 |
| > 3’ | CGGCGACATTCACACCACT | |||
| Ldhb | L-lactate dehydrogenase b | 5ʹ > | GCGAGAACTGGAAGGAGGTG | 145 |
| > 3’ | GGGTGAATCCGAGAGAGGTTT | |||
| Pfkl | phospho-fructokinase, liver, b-type | 5ʹ > | CAGCCACCATCAGCAACAAT | 90 |
| > 3’ | TGCGGTCACAACTCTCCATT | |||
| Pfkm | phospho-fructokinase, muscle | 5ʹ > | CATCCCATTTGTGGTCATTCC | 149 |
| > 3’ | TAAACACTCGCCGCTTGGT | |||
| Phgdh | phospho-glycerate dehydrogenase | 5ʹ > | CTGAACGGGAAGACACTGGGAA | 138 |
| > 3’ | AACACCAAAGGAGGCAGCGA | |||
| Gpam | glycerol-3-phosphate acyl-transferase, mitochondrial | 5ʹ > | GGTGAGGAGCAGCGTGATT | 129 |
| > 3’ | GTGGACAAAGATGGCAGCAG | |||
| Pdk4 | pyruvate dehydrogenase kinase, isoenzyme 4 | 5ʹ > | CTGCTCCAACGCCTGTGAT | 142 |
| > 3’ | GCATCTGTCCCATAGCCTGA | |||
| Fas | fatty acid synthase | 5ʹ > | CCCGTTGGAGGTGTCTTCA | 117 |
| > 3’ | AAGGTTCAGGGTGCCATTGT | |||
| Hsl | lipase, hormone sensitive | 5ʹ > | TCCTCTGCTTCTCCCTCTCG | 108 |
| > 3’ | ATGGTCCTCCGTCTCTGTCC | |||
| Atgl | adipose triacylglycerol lipase | 5ʹ > | CACCAACACCAGCATCCAAT | 120 |
| > 3’ | CGAAGTCCATCTCGGTAGCC | |||
| Lpl | lipoprotein lipase | 5ʹ > | TGGCGTGGCAGGAAGTCT | 116 |
| > 3’ | CCGCATCATCAGGAGAAAGG | |||
| Arbp | 0S acidic ribosomal phospho-protein PO [housekeeping gene] | 5ʹ > | CCTTCTCCTTCGGGCTGAT | 122 |
| > 3’ | CACATTGCGGACACCCTCTA |
Data presentation and statistical procedures
Since a main objective of the study was to evaluate the eventual contribution of non-adipocyte cells to the efflux of glycolytic and lipolytic efflux, we presented the data in two complementary forms. The direct values of substrate efflux (uptake for glucose) and number of copies of specific protein mRNAs were presented per cell (in Tables), whereas, these same values corrected by the number of cells present in each tissue cell fraction per unit of whole tissue weight were shown in Figures. This way, in a theoretically reconstituted WAT each cell type: adipocytes, NSC and RBC showed its global comparative effect on each of the parameters measured, making approximate comparisons possible. This presentation also partly circumvented the problem of statistical comparisons of composite data (Figures), since the statistical power of Table data comparisons was more reliable despite the limited number of samples used.
Statistical analyses and the establishment of significant differences between groups (one- and two-way ANOVAs) were done with the Statgraphics Centurion XVI program (Statpoint Technologies, The Plains, VA USA).
Funding Statement
This study was not specifically funded by any financing Agency. Part of the expenses were covered by the Authors themselves; the University of Barcelona provided funds for complementary work. Dr. F. Rotondo and Dr. A.C.Ho-Palma had pre-doctoral fellowships from the Governments of Catalonia and Peru, respectively. Dr. M.M. Romero contract was supported by the CIBER-OBN Research Web of the Government of Spain.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Cinti S. The adipose organ: morphological perspectives of adipose tissues. Proc Nutr Soc. 2001;60:319–328. [DOI] [PubMed] [Google Scholar]
- 2.Luo LP, Liu ML. Adipose tissue in control of metabolism. J Endocrinol. 2016;231:R77–R99. doi: 10.1530/JOE-16-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saillan-Barreau C, Cousin B, Andre M, Villena P, Casteilla L, Pénicaud L. Human adipose cells as candidates in defense and tissue remodeling phenomena. Biochem Biophys Res Commun. 2003;309:502–505. [DOI] [PubMed] [Google Scholar]
- 4.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nature Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 5.Booth A, Magnuson A, Fouts J, Foster MT. Adipose tissue: an endocrine organ playing a role in metabolic regulation. Horm Mol Biol Clin Investig. 2016;26:25–42. doi: 10.1515/hmbci-2015-0073. [DOI] [PubMed] [Google Scholar]
- 6.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. [DOI] [PubMed] [Google Scholar]
- 7.Bremer AA, Devaraj S, Afify A, Jialal, I. Adipose tissue dysregulation in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E1782–E1788. doi: 10.1210/jc.2011-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Im GI. Adipose stem cells and skeletal repair. Histol Histopathol. 2013;28:557–564. doi: 10.14670/HH-28.557. [DOI] [PubMed] [Google Scholar]
- 9.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Art Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maumus M, Peyrafitte JA, d’Angelo R, Fournier-Wirth C, Bouloumié A, Casteilla L, Sengenès C, Bourin P. Native human adipose stromal cells: localization, morphology and phenotype. Int J Obesity. 2011;35:1141–1153. doi: 10.1038/ijo.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frontini A, Giordano A, Cinti S. Endothelial cells of adipose tissues. A niche of adipogenesis. Cell Cycle. 2012;11:2765–2766. doi: 10.4161/cc.21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alemany M. Regulation of adipose tissue energy availability through blood flow control in the metabolic syndrome. Free Radical Biol Med. 2012;52:2108–2119. doi: 10.1016/j.freeradbiomed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atherosc Thromb. 2010;17:115–130. doi: 10.5551/jat.1735. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima I, Yamaguchi T, Ozutsumi K, Aso H. Adipose tissue extracellular matrix: newly organized by adipocytes during differentiation. Differentiation. 1998;63:193–200. doi: 10.1111/j.1432-0436.1998.00193.x. [DOI] [PubMed] [Google Scholar]
- 15.Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells. 2014;37:365–371. doi: 10.14348/molcells.2014.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome. A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 17.Romero MM, Roy S, Pouillot K, Feito M, Esteve M, Grasa MM, Fernández-López J-A, Alemany M, Remesar X. Treatment of rats with a self-selected hyperlipidic diet, increases the lipid content of the main adipose tissue sites in a proportion similar to that of the lipids in the rest of organs and tissues. PLoS One. 2014;9:e90995. doi: 10.1371/journal.pone.0090995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho-Palma AC, Rotondo F, Romero MM, Memmolo S, Remesar X, Fernández-López JA, Alemany M. A method for the measurement of lactate, glycerol and fatty acids production from 14C-glucose in primary cultures of rat epididymal adipocytes. Anal Meth. 2016;8:7873–7885. doi: 10.1039/C6AY01244B. [DOI] [Google Scholar]
- 19.Sabater D, Arriarán S, Romero MM, Agnelli S, Remesar X, Fernández-López JA, Alemany M. Cultured 3T3L1 adipocytes dispose of excess medium glucose as lactate under abundant oxygen availability. Sci Rep. 2014;4:3663. doi: 10.1038/srep03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero MM, Sabater D, Fernández-López JA, Remesar X, Alemany M. Glycerol production from glucose and fructose by 3T3-L1 cells: A mechanism of adipocyte defense from excess substrate. PLoS One. 2015;10:e0139502. doi: 10.1371/journal.pone.0139502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hodson L, Humphreys SM, Karpe F, Frayn KN. Metabolic signatures of human adipose tissue hypoxia in obesity. Diabetes. 2013;62:1417–1425. doi: 10.2337/db12-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiGirolamo M, Newby FD, Lovejoy J. Lactate production in adipose tissue: A regulated function with extra-adipose implications. FASEB J. 1992;6:2405–2412. [DOI] [PubMed] [Google Scholar]
- 23.Jansson PA, Larsson A, Smith U, Lönnroth P. Glycerol production in subcutaneous adipose tissue of lean and obese humans. J Clin Invest. 1992;89:1610–1617. doi: 10.1172/JCI115756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts R, Hodson L, Dennis AL, Neville MJ, Humphreys SM, Harnden KE, Micklem KJ, Frayn KN. Markers of de novo lipogenesis in adipose tissue: associations with small adipocytes and insulin sensitivity in humans. Diabetologia. 2009;52:882–890. doi: 10.1007/s00125-009-1300-4. [DOI] [PubMed] [Google Scholar]
- 25.Arriarán S, Agnelli S, Remesar X, Fernández-López J-A, Alemany M. The urea cycle of rat white adipose tissue. RSC Adv. 2015;5:93403–93414. doi: 10.1039/C5RA16398F. [DOI] [Google Scholar]
- 26.Rotondo F, Ho-Palma AC, Remesar X, Fernandez-Lopez JA, Romero MM, Alemany M. Glycerol is synthesized and secreted by adipocytes to dispose of excess glucose, via glycerogenesis and increased acyl-glycerol turnover. Sci Rep. 2017;7:8983. doi: 10.1038/s41598-017-09450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho-Palma AC, Rotondo F, Romero MM, Fernández-López JA, Remesar X, Alemany M. Use of 14C-glucose by primary cultures of mature rat epididymal adipocytes. Marked release of lactate and glycerol, but limited lipogenesis in the absence of external stimuli. Adipocyte. 2018;7:204–217. doi: 10.1080/21623945.2018.1460020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotondo F, Romero MM, Ho-Palma AC, Remesar X, Fernandez-Lopez JA, Alemany M. Quantitative analysis of rat adipose tissue cell recovery, and non-fat cell volume, in primary cell cultures. PeerJ. 2016;4:e2725. doi: 10.7717/peerj.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rotondo F, Ho-Palma AC, Remesar X, Fernandez-Lopez JA, Romero MM, Alemany M. Effect of sex and glucose handling by adipocytes isolated from rat subcutaneous, mesenteric and perigonadal adipose tissue. PeerJ. 2018;6:e5440. doi: 10.7717/peerj.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu MY, Guo L, Liu Y, Pei Y, Li N, Jin MM, Ma LC, Li ZB, Sun BR, Li CL. Adipose stromal-vascular fraction-derived paracrine factors regulate adipogenesis. Mol Cell Biochem. 2014;385:115–123. doi: 10.1007/s11010-013-1820-6. [DOI] [PubMed] [Google Scholar]
- 31.Bowles AC, Wise RM, Gerstein BY, Thomas RC, Ogelman R, Febbo I, Bunnell BA. Immunomodulatory effects of adipose stromal vascular fraction cells promote alternative activation macrophages to repair tissue damage. Stem Cells. 2017;35:2198–2207. doi: 10.1002/stem.2689. [DOI] [PubMed] [Google Scholar]
- 32.Qomi RT, Sheykhhasan M. Adipose-derived stromal cell in regenerative medicine: A review. World J Stem Cells. 2017;9:107–117. doi: 10.4252/wjsc.v9.i8.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meissburger B, Perdikari A, Moest H, Müller S, Geiger M, Wolfrum C. Regulation of adipogenesis by paracrine factors from adipose stromal-vascular fraction - a link to fat depot-specific differences. Biochim Biophys Acta-Mol Cell Biol Lipids. 2016;1861:1121–1131. doi: 10.1016/j.bbalip.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Arriarán S, Agnelli S, Sabater D, Remesar X, Fernández-López JA, Alemany M. Evidences of basal lactate production in the main white adipose tissue sites of rats. Effects of sex and a cafeteria diet. PLoS One. 2015;10:e0119572. doi: 10.1371/journal.pone.0119572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koutsari C, Ali AH, Mundi MS, Jensen MD. Storage of circulating free fatty acid in adipose tissue of postabsorptive humans. Quantitative measures and implications for body fat distribution. Diabetes. 2011;60:2032–2040. doi: 10.2337/db11-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Oliveira CC, Acedo SC, Pedrazzoli J, Saad MJ, Gambero A. Depot-specific alterations to insulin signaling in mesenteric adipose tissue during intestinal inflammatory response. Int Immunopharmacol. 2009;9:396–402. doi: 10.1016/j.intimp.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 37.Meijer RI, Bakker W, Alta CLAF, Sipkema P, Yudkin JS, Viollet B, Richter EA, Smulders YM, van Hinsbergh VWM, Serne EH, et al. Perivascular adipose tissue control of insulin-induced vasoreactivity in muscle is impaired in db/db mice. Diabetes. 2013;62:590–598. doi: 10.2337/db11-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi TY, Ahmadi N, Sourayanezhad S, Zeb I, Budoff MJ. Relation of vascular stiffness with epicardial and pericardial adipose tissues, and coronary atherosclerosis. Atherosclerosis. 2013;229:118–123. doi: 10.1016/j.atherosclerosis.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 39.Hui S, Ghergurovich JM, Morscher RJ, Jang C, Teng X, Lu W, Esparza LA, Reya T, Le Z, Yanxiang Guo J, et al. Glucose feeds the tca cycle via circulating lactate. Nature. 2017;551:115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crandall DL, Fried SK, Francendese AA, Nickel M, DiGirolamo M. Lactate release from isolated rat adipocytes: influence of cell size, glucose concentration; insulin and epinephrine. Horm Metab Res. 1983;15:326–329. doi: 10.1055/s-2007-1018710. [DOI] [PubMed] [Google Scholar]
- 41.Kellner J, Sivajothi S, McNiece I. Differential properties of human stromal cells from bone marrow, adipose, liver and cardiac tissues. Cytother. 2015;17:1514–1523. doi: 10.1016/j.jcyt.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Faintrenie G, Géloën A. Lactate production by white adipocytes in relation to insulin sensitivity. Am J Physiol. 1996;270:C1061–C1066. doi: 10.1152/ajpcell.1996.270.4.C1061. [DOI] [PubMed] [Google Scholar]
- 43.Sahlin K. Lactate formation and tissue hypoxia. J Appl Physiol. 1989;67:2640. doi: 10.1152/jappl.1989.67.6.2640a. [DOI] [PubMed] [Google Scholar]
- 44.Taylor CT, Kent BD, Crinion SJ, McNicholas WT, Ryan S. Human adipocytes are highly sensitive to intermittent hypoxia induced nf-kappab activity and subsequent inflammatory gene expression. Biochem Biophys Res Commun. 2014;447:660–665. doi: 10.1016/j.bbrc.2014.04.062. [DOI] [PubMed] [Google Scholar]
- 45.Kaaman M, Sparks LM, van Harmelen V, Smith SR, Sjolin E, Dahlman I, Arner P. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia. 2007;50:2526–2533. doi: 10.1007/s00125-007-0818-6. [DOI] [PubMed] [Google Scholar]
- 46.Bohr C, Hasselbalch K, Krogh A. Über einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensäurespannung des Blutes auf dessen Sauerstoffbindung übt [about a biologically important influence that the carbonic acid tension of the blood exerts on its oxygen binding]. Scand Arch Physiol. 1904;16:402–412. doi: 10.1111/j.1748-1716.1904.tb01382.x. [DOI] [Google Scholar]
- 47.Alemany M. The defense of adipose tissue against excess substrate-induced hyperthrophia: immune system cell infiltration and arrested metabolic activity. J Clin Endocrinol Metab. 2011;96:66–68. doi: 10.1210/jc.2010-2541. [DOI] [PubMed] [Google Scholar]
- 48.Sabater D, Agnelli S, Arriarán S, Romero MM, Fernández-López JA, Alemany M, Remesar X. Cafeteria diet induce changes in blood flow that are more related with heat dissipation than energy accretion. PeerJ. 2016;4:e2302. doi: 10.7717/peerj.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eto H, Suga H, Inoue K, Aoi N, Kato H, Araki J, Doi K, Higashino T, Yoshimura K. Adipose injury-associated factors mitigate hypoxia in ischemic tissues through activation of adipose-derived stem/progenitor/stromal cells and induction of angiogenesis. Am J Pathol. 2011;178:2322–2332. doi: 10.1016/j.ajpath.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nature Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 51.Freund G. The metabolic effects of glycerol administered to diabetic patients. Arch Int Med. 1968;121:123–129. doi: 10.1001/archinte.1968.03640020011003. [DOI] [PubMed] [Google Scholar]
- 52.Schurr A, Payne RS, Miller JJ, Rigor BM. Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: an in vivo study. Brain Res. 1977;744:105–111. doi: 10.1016/S0006-8993(96)01106-7. [DOI] [PubMed] [Google Scholar]
- 53.Tildon JT, Roeder LM. Glycerol oxidation in rat brain: subcellular localization and kinetic characteristics. J Neurosci Res. 1980;5:7–17. doi: 10.1002/jnr.490050103. [DOI] [PubMed] [Google Scholar]
- 54.Bouzier-Sore A-K, Voisin P, Bouchaud V, Bezancon E, Franconi J-M, Pellerin L. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: A comparative NMR study. Eur J Neurosci. 2006;24:1687–1694. doi: 10.1111/j.1460-9568.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- 55.Rodbell M. Metabolism of isolated fat cells .I. Effects of hormones on glucose metabolism + lipolysis. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- 56.Baviskar SN. A quick & automated method for measuring cell area using imagej. Am Biol Teach. 2011;73:554–556. doi: 10.1525/abt.2011.73.9.9. [DOI] [Google Scholar]
- 57.Oliva L, Baron C, Fernández-López J-A, Remesar X, Alemany M. Marked increase in rat red blood cell membrane protein glycosylation by one-month treatment with a cafeteria diet. PeerJ. 2015;3:e1101. doi: 10.7717/peerj.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romero MM, Grasa MM, Esteve M, Fernández-López JA, Alemany M, Semiquantitative RT. -PCR measurement of gene expression in rat tissues including a correction for varying cell size and number. Nutr Metab. 2007;4:26. doi: 10.1186/1743-7075-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bamias G, Goukos D, Laouidi E, Balla IG, Siakavellas SI, Daikos GL, Ladas SD. Comparative study of candidate housekeeping genes for quantification of target gene messenger RNA expression by real-time PCR in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2840–2847. doi: 10.1097/01.MIB.0000435440.22484.e8. [DOI] [PubMed] [Google Scholar]


