Abstract
The low pressure glow discharges considered in this paper are the hollow cathode (Paschen), and the flat cathode (Grimm). Both discharges have similar voltage—current characteristics which are responsible for their radiation stability. The analytical sample is supplied to the discharge through a sputtering mechanism which provides a stable and non-selective source of particles. Some of the fundamental properties of the glow discharge and sputtering phenomena will be discussed, including the relation between the geometry of the discharge, and the nature and pressure of sustaining gas, and current, on the emission characteristics of the discharges. These will he followed by a description of the conventional instrumentation developed for analytical purposes using the hollow cathode and flat discharge. A description of the hollow cathode developed at the National Bureau of Standards (NBS) will follow. The techniques used for the introduction of various conductive and nonconductive materials into the discharge will be discussed. The use of these discharges will be illustrated with examples taken from the literature and from the measurements performed at NBS. The paper will conclude with a discussion of possible future developments of low pressure glow discharges. A collection of references to works on low pressure glow discharges, containing 690 entries, concludes this work.
Keywords: Grimm discharge, hollow cathode discharge, low pressure glow discharges, Paschen discharge, planar cathode discharge, pulsed discharge
1. Introduction
The energy necessary to excite the radiations from various free particles—atoms, ions, molecules, and free radicals—originating from the analytical sample, and required in analytical emission spectroscopy, can be supplied in a variety of forms according to the excitation source used. Table 1 enumerates these sources which are divided in a somewhat arbitrary manner into two general categories: electrical discharges and thermal sources.
Table 1.
Sources of energy used in analytical emission spectroscopy to excite the radiations from atoms, ions, molecules, and free radicals.
The production of free particles and their excitation through the use of high temperature furnaces or combustion flames is a purely thermal phenomenon. When electrical discharges are used for the same purpose, the production of free particles and their excitation often result from a combined effect of the energy developed in the electric field and thermal energy. An extreme case in this regard is illustrated by the dc arc where the thermal energy is the determining parameter. The other extreme, where the production of free particles and their excitation results from the energy generated in electric fields, is illustrated by the low pressure glow discharges, namely the planar and hollow cathode discharge. Although in this case a certain amount of heat is generated as a result of ion bombardment at the cathode, this thermal energy is only incidental to the process and is not necessary for the production and maintenance of the discharge, for the generation of free particles, and for the subsequent excitation processes which occur in these sources.
The basic conditions required from an excitation source are: capability to be supplied in a controlled manner with the analytical sample in solid, liquid, or gaseous state for both electrically conductive and non-conductivc materials. The excitation source should excite all the chemical species of interest with high sensitivity and stability. Furthermore, interferences due to matrix effects, interelement actions, chemical reactions, and selective energy transfers should be as small as possible. These interferences are associated to a large extent with those excitation sources in which the production and excitation of particles result from thermal energy, where the thermal characteristics of each chemical species such as melting, boiling, and vaporization temperatures, vapor pressure, dissociation, are specific for every chemical species and play a determining role. Self-absorption is also a phenomenon often associated with thermal excitation and is responsible for loss of sensitivity and non-linearity between the emission intensity and actual sample concentration. The processes occurring in the excitation source become even more complex when the energy generated by an electrical field is associated with the thermal energy. From the various sources of excitation mentioned in table 1, the electrical glow discharges produced under low pressure, and in particular the planar cathode and hollow cathode discharges mentioned previously, are less subjected to the processes discussed in the foregoing, and are also practically free of self-absorption.
The sputtering phenomenon, responsible in this case for the production of free particles from the analytical sample, is less subjected to selectivity, and the absence of oxygen from the gas supporting the discharge elimininates the matrix and chemical reactions interferences resulting from the action of oxygen on the sample.
Based on these considerations we have initiated a study of the low pressure glow discharges and some of the results obtained in our preliminary experiments will be discussed in this work together with factual data from the available scientific literature.
2. Production and General Characteristics of Low Pressure Glow Discharges1
Low pressure glow discharges of the type pertinent to our interests here can be produced using a simple cylindrical glass tube provided with two electrodes of adequate shape, an inlet for the gas sustaining the discharge, and a vacuum connection, as illustrated after Francis [105] in figure 1. The tube is filled with a rare gas, say helium or argon, at a pressure between 1 to 20 Torr, and a dc potential V is applied across the electrodes, through a current limiting resistor R. If the dc potential V, is increased by changing the value of the variable resistance R, a small current / is detected by the sensitive meter A. An intermittent discharge, produced in random bursts, is thus observed at very low values of i of the order of 10−18 A. As the potential V is further increased, the current i increases rapidly and rises to values exceeding those determined by the resistance R. Under these conditions a dark self-sustained discharge is produced and the voltage at which this phenomenon is observed is called the breakdown voltage Vb (of the order of 1000 V). Such discharges are called dark discharges or Townsend discharges, and are characterized by currents of the order of 10−6 A under practically constant voltage. This is illustrated in figure 2 by the region AB [257], As the current is further increased, the voltage decreases through a transitional region CD (subnormal glow discharge), and reaches a constant value Vn at the point D. A visible glow discharge is now produced at the normal cathode fall potential Vn (of the order of 200 to 300 V). This potential remains practically constant for large variations of the current from about 10−4 A to about 0.1 A.
Figure 1 –
Characteristics of a low pressure glow discharge. After Francis [105].
Figure 2 –
Voltage-current characteristics of a self-sustained low pressure glow discharge. After Penning [257].
With a further increase of the current the limited cathode area becomes current saturated, whereupon the voltage rises and the discharge enters in the abnormal mode of operation described by portion EF of the curve on figure 2. If the current is increased again, the voltage goes first through a sharp increase followed at F by a transition region and a sudden drop, reaching values of the order of several tens of volts for currents of the order of 10 A; this is the arc discharge. A significant characteristic of this type of discharge is heating of the sustaining gas.
The glow discharge defined by the points D-E-F on figure 2 is of interest to the analytical spectroscopist and can be produced directly by raising the potential V to the value Vn. The appearance of this discharge varies, for the same gas, with the geometry of the electrodes and that of the discharge tube, the distance between the electrodes, the pressure of the sustaining gas, the current, and the nature of the electrodes. These parameters were selected here to produce a discharge which is closely related in its properties to the discharge used in analytical applications.
2.1. Characteristics of the Low Pressure Glow Discharge
The glow discharge described schematically in figure 1 consists of a number of alternating dark and luminous zones. Their existence, disposition, and size depend on the experimental conditions; however, the example chosen here describes the general case of a discharge obtained in a glass tube 30 cm long and 5 cm wide, provided with flat copper electrodes having a diameter of 2.5 cm placed inside the tube 15 cm apart. The gas is helium at a pressure of 10 Torr and the current is 50 to 100 mA. Under these conditions the following zones are observed: at the cathode there is a very thin dark layer called the Aston primary or dark space. This is followed by a weakly luminous cathode layer, and a second dark zone called the Crookes or Hittorf dark space. Following this dark space, and sharply defined toward the cathode, is the strongly luminous negative or cathode glow. The luminous intensity of the cathode glow decreases toward the anode as it merges into another dark space called the Faraday dark space. Between this dark space and the anode there is another luminous zone, called the positive column, separated from the anode by another thin dark space and an anode glow at the surface of the electrode. The variation of the light intensity, electric field, potential, positive space charge density, negative charge density, current density, and gas temperature are described qualitatively in figure 1 [105].
An increase of pressure results in a compression of the cathode dark space, negative glow and Faraday dark space which contract toward the cathode. A decrease of pressure produces a reverse effect, and, if the voltage is not increased, the discharge goes out. A decrease of the distance between the cathode and anode produces a shortening of the anode glow, which disappears altogether where the anode is in the proximity of the cathode. When the anode is near the cathode edge of the negative glow, the voltage necessary to sustain the discharge rises rapidly and the discharge is said to be obstructed. This proves that the positive column is not essential for maintaining the low pressure glow discharge, while the part of the discharge at the cathode, including the Aston and Crookes dark spaces, are indispensable. An increase in voltage results in an increase in the radiation intensity of the discharge, in particular at the cathode, and the definition of the various zones becomes sharper [105].
Unlike the conductivity of electricity in solids, the elementary processes occurring when an electrical current passes through a gas are numerous and complex. They have been summarized by Penning in the diagram from figure 3 [257], From these, the excitation and ionization processes are the most significant in relation to the subject discussed here.
Figure 3.
Diagrammatic summary of several elementary processes occurring in a low pressure glow discharge. Each process originates at the dot and ends at the arrowhead. After Penning [257] [Courtesy of Philips Technical Library and Servire BU; Katwijk aan Zee, Netherlands, Publisher.]
The Aston dark space is characterized by the presence of electrons of low energy originating from the cathode. In the first thin layer of the negative glow these electrons are accelerated sufficiently to excite the particles found in this zone. They lose this energy in the Crookes or Hittorf dark zone. The excitation occurring in the strongly luminous negative glow is produced by the numerous electrons resulting from the ionization process occurring in the cathode dark space as well as by the few faster ones originating from the cathode. Positive ions are also generated in this zone and are attracted toward the cathode. As a result of their impact on the cathode, atoms from this cathode are ejected and reach the glow discharge zone producing a sputtering of the material from which the cathode is made. Under these conditions a mixture of atoms from the supporting gas and from the material from the cathode are always present in the negative glow zone where they are strongly excited by collision with the ions and electrons present there [105].
The Faraday dark space is characterized by the presence of ground-state particles and low energy electrons which have lost this energy in the negative glow zone. After these electrons gain once more sufficient energy, from the electric field which accelerates them toward the anode, they produce the luminous positive zone. A spectroscopic examination of the radiations excited in this zone reveals the presence of the atomic spectra from helium which is the sustaining gas used in this example.
The same examination of the negative glow discharge reveals that, in addition to the radiations from the sustaining gas, strong emissions from the sputtered particles from the cathode are excited and emit radiations from neutral atoms, ions, and molecules. It is this basic property, i.e., the generation of free particles through sputtering and their subsequent excitation by non-therrnal processes in the negative glow region, that makes the low pressure glow discharge a valuable source in analytical spectroscopy.
Further information concerning various characteristics of low pressure glow discharges will be found in references 10, 177, 180, 226, and 252.
3. The Low Pressure Glow Discharge as an Excitation Source in Emission Spectroscopy
3.1. The Planar Discharge
Described diagramatically in figure 1, the actual aspect of this simplest form of low pressure glow discharge is illustrated in figure 4 which was obtained under the conditions described previously. The copper cathode is at left, the copper anode at right, and the principal zones seen are the strongly luminous negative glow at the cathode, followed by the Faraday dark space, the positive column, and the anode glow. The thin Aston and Crookes dark spaces, and the faint cathode layer and the anode dark space cannot be distinguished on this photograph.
Figure 4 –
A low pressure glow discharge produced between two copper disc electrodes in helium at 10 Torr and 100 mA. The cathode is at left, the anode at right.
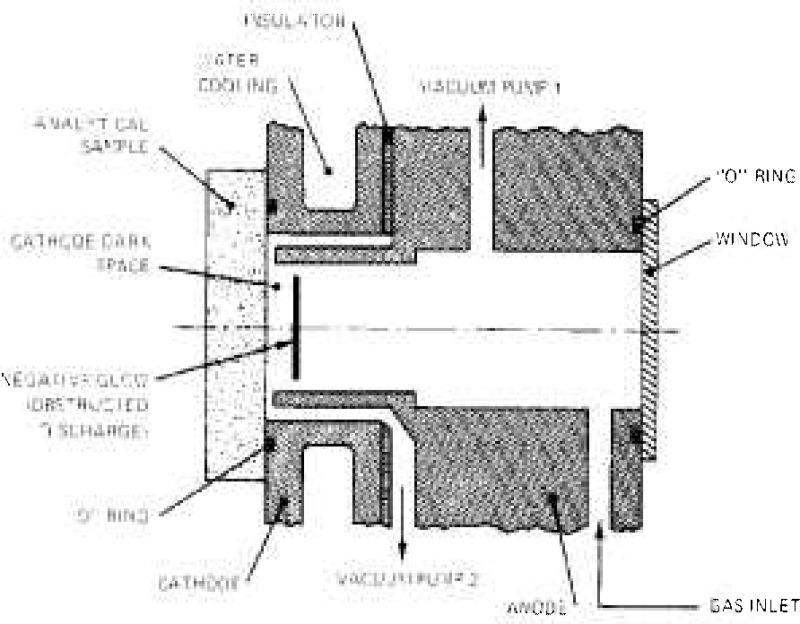
As discussed previously, the zone of interest is the negative glow since the particles sputtered from the cathode, which in this case is the analytical sample, are strongly excited in this zone and produce the spectra of the neutral and ionized atoms together with pertinent molecular spectra. The positive column and the anode glow, which are not needed to produce or maintain the discharge, can be eliminated altogether by bringing the anode electrode near the cathode, and by placing this anode outside the field of examination of the cathode glow. It is from this basic and simplest form of the flat or planar glow discharge that Grimm has developed a discharge tube that is now used routinely as an excitation source in analytical emission spectroscopy [128, 129]; a diagrammatic description of this discharge tube is given in figure 5. It consists of a cylindrical anode and cathode unit, made usually of a copper alloy, and separated by an insulator. The distance between the cathode and the anode is about 0.2 mm. The metallic analytical sample, in the shape of a flat disc, is placed against the cathode provided with a vacuum tight “O” ring, and is in electrical contact with the cathode. The anode is closed with a vacuum tight window. An adequate gas, usually argon or helium, is introduced in the lamp at a pressure of 1 to 20 Torr and flows continuously through the unit. The cathode body is water cooled.
Figure 5 –
Diagrammatic description of the planar low pressure glow discharge developed by Grimm [128, 129].
Under these conditions, when a dc current of 100 to 600 mA and 700 to 2500 V is supplied to the lamp, a glow discharge is produced in front of the cathode, inside the anode space. This discharge is the negative glow described previously in figures 1 and 4. It has the characteristics of an obstructed discharge, and, is suspended free of any material contacts with the two electrodes. being isolated from the walls of the anode by a circular gap, which in fact is the anode dark space, and separated from the cathode by the cathode dark space. The intensely luminous negative glow has a thickness of the order of the mean free pathlength of the particles, and consists mostly of excited particles sputtered continuously and uniformly from the analytical sample and from the supporting gas. The dc current can be supplied to the discharge in an uninterrupted manner or in a pulsed mode. The pulsed mode is generally used with analytical sample subjected to overheating when undergoing the sputtering process.
The glow discharge source developed by Grimm can be obtained from RSV-Präzisionmessgeräte, GmbH Hauptstrasse 60, D-8031 Seefeld 2, West Germany,2 including the source, the vacuum system, and the dc power supply for continuous or pulsed operation. The RSV company is represented in North America by Labscrco Ltd. Unit 8 1100 Invicta Drive, Oakville, Ontario L6H 3K9. A similar low pressure glow discharge source using a planar cathode is manufactured in this country under the name “Cathaquant” by the Spectrogram Corp., 385 State Street, North Haven, CT 06473. A detailed study of the functioning characteristics of this discharge source was made by Grimm [128, 129], Boumans [40], and by Dogan, Laqua, and Massmann [85, 86] and El Alfy, Laqua, and Massmann [93] at the Institut für Spektrochemie und Angewandte Spektroskopie in Dortmund, Germany, and all the data used, and the statements made in the following discussion, are taken from these basic contributions. Further contributions were made by Boumans [41].
The current-voltage characteristic of the discharge is illustrated in figure 6(a) for various pressures, using argon as the carrier gas and nickel for the cathode. This parameter depends, for a given current, also on the type of carrier gas and its pressure and on the type of cathode sample material. The relative intensity of the Ni 3610.46 A line as a function of the current supplied to the lamp and for several argon pressures also is given in figure 6(b), These measurements were made with a 1.5 m direct reading grating spectrometer [129].
Figure 6 –
Voltage-current characteristics of the discharge from figure 5, in argon (a), and (b), the relative intensity of the Ni radiation at λ 3610.46Å as a function of the current supplied to the discharge in argon [129].
The linear relation between the concentration of a chemical element and the corresponding signal intensity obtained with the Grimm glow discharge source is illustrated in figure 7 for Sb in Cu-Pb-Sn(a), Cu in Al(b), Zn in A1(c), and Mn in Al(d) matrices, in comparison with a conventional spark.
Figure 7 –
Relation between the concentration of a chemical element and the corresponding signal intensity obtained in a Grimm glow discharge source in comparison with a spark source for: Sb in Cu-Pb-Sn (A); Cu in Al (B); Zn in Al, Al-Mg, and Al-Mg-Si (C); and Mn in Al; Al-Mg, and Al-Mg-Si (D) samples. After Grimm.
The matrix effect is illustrated in (c) and (d) of figure 7 for Zn and Mn in A1 and in AlMg and AlMgSi alloys, determined with the glow discharge source and a conventional spark source. In all cases the glow discharge was operated in argon at a pressure of 5 to 7 Torrs and a discharge current of 0.15 to 0.25 A. A preburn of 10 to 40 s and an integration time of 3 to 40 s were used for the glow discharge.
The detection sensitivity of the planar glow discharge was determined for several elements by Grimm [129] and is given in table 2.
Table 2.
Detection sensitivity for the glow discharge source after Grimm [129]
| Element | Line Å | Detection limit (ppm) |
|---|---|---|
| Beryllium | 2494.73 | 2 |
| Silicon | 2881.58 | 5 |
| Iron | 3020.64 | 80 |
| Magnesium | 2795.53 | 80 |
| Chromium | 4254.35 | 20 |
| Molybdenum | 3864.11 | 10 |
| Aluminum | 3961.52 | 100 |
| Lead | 4057.83 | 100 |
This detection sensitivity can be increased by looking at the negative glow sidewise [85], or by using the glow-discharge in the hollow cathode mode; this mode of operation will be discussed later in this work.
Further physical characteristics of the glow discharge source were investigated by Dogan, Laqua, and Massmann [85], using a lamp similar to that developed by Grimm, but with additional water cooling of the analytical sample. This provision permits the use of higher currents without overheating the sample.
When a new sample with a fresh surface is submitted to the discharge, the current exhibits a certain instability. This is due to surface impurities and oxides. After the surface has been cleaned through the sputtering process, the discharge stabilizes and “burns” quietly. Aluminum surfaces require a longer cleaning time (40s) while zinc cleans after a shorter preburn (3s) both in argon. The preburn time depends also on the carrier gas and is shorter in krypton than in argon or neon.
The analytical sample is supplied to the discharge as an electrical conducting flat disc with a smooth surface. It is pressed against the cathode which is provided with an “O” ring to insure a vacuum tight seal. The surface sample exposed to the discharge is 0.28 cm2. It is desirable that this surface be representative of the sample, homogeneous, and with particles having a diameter less than 0.1 mm for electrically conducting samples. Non-conducting samples can be mixed with a conducting powder material such as copper, silver, or graphite and pressed to produce the disc-shaped sample.
The amount of material sputtered during the discharge, for the same discharge conditions, i.e., current, gas, pressure, exposed surface, time, depends on the sample material. For instance, the amount of material sputtered in one minute is 0.34 mg for aluminum, 1.0 mg for copper, 3.1 mg for zinc, and smaller amounts for graphite and carbon. When arranged in increasing order, carbon sputters least and is followed by aluminum, iron, steel, copper, brass, and zinc. The amount of sputtered material per unit of time increases with the increase of current. For a power of 90 w the amount of aluminum sputtered is 0.30 mg/min in neon, 0.34 mg/min in argon, and 0.38 mg/min in krypton.
As already mentioned, the spectra excited in a low pressure glow discharge are characterized by the presence of radiations originating from the atoms, ions, molecules, and free radicals from the analytical sample and the carrier gas. The initial emissions originate from the impurities and oxide layers formed at the surface of the sample. After these layers are eroded through the sputtering process, the spectra observed are emitted by the elements which constitute the analytical sample, together with those from the carrier gas, and sometimes with the molecular emission from OH and N2 as impurities. The intensities of these emissions depend on the concentration of the corresponding elements in the sample, the discharge parameters, the geometry of the discharge lamp, and on the carrier gas. In general an increase in current supplied to the discharge increases the radiation intensity. For instance, a twofold increase in current from 60 mA to 120 mA produces a Fivefold increase in the intensity of magnesium emission at λ 2852.13 Å, at an optimum argon pressure of about 8 Torr.
Another property of the discharge is the continuum emission from the carrier gas. For argon this emission is stronger toward the longer wavelengths, while for krypton it is stronger for the short wavelengths.
All the parameters discussed above should be taken into consideration when the Grimm low pressure glow discharge is used for quantitative analyses since they all affect the sensitivity, precision, and accuracy of the measurements.
The essential characteristics of the low pressure glow discharge can be summarized as follows.
The excitation conditions prevailing in the discharge produces for all known chemical species, radiations originating from atoms, ions, and molecules (free radicals) with narrow natural spectral line width and radiations which are, under the experimental conditions described in this work, practically free of self-absorption.
The generation of particles and their supply into the discharge results from a non-thermal and a practically non-selective cathode sputtering process.
The discharge is produced in an inert gas at low pressure and may be used directly as an excitation source in the vacuum ultraviolet.
As a result of its current-voltage characteristics, the discharge is particularly stable.
The discharge is silent.
The use of the Grimm discharge for the quantitative analysis of electrically conductive and non-conductive samples was studied in detail by Dogan, Laqua, and Massmann [86], and by El Alfy, Laqua and Massmann [93], and some of their results will be summarized here. The detection limits measured for several trace elements in aluminum are given in table 3 and were obtained using argon as a carrier gas at a current of 100 mA and 900 V and with a preburn of 45 s and an exposure of 6.5 min. These detection limits compare favorably with those obtained with sparks and interrupted ac arcs.
Table 3.
Detection limits for several elements determined with the Grimm discharge lamp [86].
| Detection Limits in Percent for | ||
|---|---|---|
| Element and wavelength, A* | Medium quartz spectrograph | 3.m grating spectrograph |
| Cu I, 3247.54 | 4.0×105 | 1.5×10−6 |
| Fe I, 3020.64 | 3.0×103 | 2.1 ×10 4 |
| Mg I, 2852.13 | 1.2×10 4 | 8.5×106 |
| Mg lI, 2795.53 | 1.1 ×10 4 | 8.5×106 |
| Mn I, 2794.82 | 1.8 ×10 4 | 1.4×105 |
| Mn II, 2576.10 | 1.1 ×10 4 | 2.2×105 |
| Mn II, 2593.73 | 2.4×10 4 | 4.6 ×10 5 |
| Si I, 2881.58 | 4.0 ×10 4 | 2.7 ×105 |
| Si I. 2516.11 | 1.1 ×10 4 | 9.4 ×106 |
| Ti I, 3653.50 | 1.7×103 | — |
| T1 II, 3349.41 | 3.8×103 | 1 6 ×105 |
| Zn I, 3345.02 | 1.5×103 | 3.6×106 |
As given in lables of Spectral-line Intensities, W 1; Meggers. Ch 11. Corliss and B. F Scribner. NBS Monograph 145. Part 1, 1975
Lower detection limits were observed in krypton for atomic radiations, while neon produced lower detection limits for radiations originating from ions. With argon the detection limits were similar for both atomic and ionic radiations; therefore, this gas presents the best compromise.
The detection limits measured for the same elements in electrically non-conducting materials produced somewhat higher values. In this case the non-conductive sample was mixed with a conducting powder such as copper (reduced) silver, aluminum or graphite in a ratio of up to 1 to 1 sample to metal by volume, and the mixture was pressed (8 to 10 tons /cm2) into a circular disc 1 to 2 mm thick.
Table 4 presents a comparison between analytical data obtained through chemical procedures (Chem.) and by emission spectrometry with the Grimm discharge source (GDS) on a variety of non-conducting sample materials [93]. These data are the average of 10 separate measurements. In this case 50 mg of the pulverized sample was mixed with 950 mg of copper powder (Merck No. 2715) and pressed into a 10 mm diameter disc at a force of at least 8 tons. The standards were obtained by mixing and diluting the oxides of Si, Al, Fe, Ti, Mg. and Mn in a Ca CO3 matrix to produce the desired range of known concentrations. The discharge was operated in argon at a current of 160 mA, using a preburn of 60 to 180 s and an exposure time of 2 to 10 min. The spectra were recorded on photographic plates and the spectral line densities were measured with a micro-densitometer using copper as an internal standard.
Table 4.
Comparison between chemical (Chem.) and spectrometric analyses obtained with the Grimm discharge source (GDS), [93].
| Sample |
Limestonea |
Granita |
Basalta |
Flintclaya |
Argillacious limestoneb |
Pb-Ba-Glassb |
Cementc |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | Chem. | GDS | Chem. | GDS | Chem. | GDS | Chem. | GDS | Chem. | GDS | Chem. | GDS | Chem. | GDS |
| Ca | 34.10 | 34.0 | 0.83 | 0.83 | 4.64 | 4.30 | 0.28 | - | 29.44 | 29.5 | 0.15 | - | 29.50 | 30.5 |
| Si | 3,99 | 4.0 | 33.97 | 34.0 | 22.90 | 23.0 | 27.9(1 | 28.0 | 6.55 | 6.6 | 30.30 | 30.0 | 6.60 | 6.5 |
| Al | 1,27 | 1.30 | 7.27 | 7.50 | 8.73 | 8.5 | 11.10 | 11.0 | 2.21 | 2.20 | 0.10 | 0.12 | 1.56 | 1.60 |
| Fe | 0,69 | 0.68 | 1.48 | 1.50 | 6.81 | 6.9 | 4.85 | 4.75 | 1.10 | 1.08 | 0.03 | 0.27 | 1.12 | 1.10 |
| Mg | 0,45 | 0.45 | 0.26 | 0.30 | 4.49 | 4.50 | 1.18 | 1.20 | 1.30 | 1.30 | 0.02 | - | 0.83 | 0.80 |
| Mn | 0,07 | 0.072 | 0.04 | 0.046 | 0.12 | 0.12 | 0.04 | 0.042 | 0.03 | 0.035 | 0.07 | 0.068 | 0.03 | |
| Ti | 0,08 | 0.078 | 0.13 | 0.15 | 0.69 | 0.67 | 0.56 | 0.58 | 0.01 | 0.012 | 0.007 | - | 0.06 | |
| Na | 0.16 | 0.12 | 2.88 | 2.90 | 3.27 | 3.25 | 0.94 | 0.87 | 4.25 | 4.25 | ||||
| K | 0,32 | 0.35 | 3.87 | 4.10 | 0.17 | 0.17 | 3.18 | 3.40 | 6.96 | 7.0 | ||||
| C | 10.21 | 10.30 | 0.07 | 0.07 | 0.36 | 0.36 | 0.04 | 0.033 | ||||||
Zentral Geologischen Instituts, Berlin.
National Bureau of Standards, Washington.
Centre d’Etudes et de Recherches de I’lndustrie des Lants Hydrauliques, Paris.
These data demonstrate that the Grimm source is capable of performining spectrometric analyses with excellent precision and accuracy, and that the precision is probably related to the inevitable limitations of the photographic plate used as a receptor. Table 4 also shows that the concentration spread for the 10 elements determined extends from about 0.01 to several tens percent, covering the minor and major constituents range.
A modified Grimm discharge is described in references 4, 37, 43, 44, and 223.
The measuring sensitivity of the low pressure planar glow discharge can be increased by producing the discharge in the hollow cathode mode.
3.2. The Hollow Cathode Discharge
3.2.1. Production of the Discharge
The aspect of a low pressure glow discharge is illustrated in its simplest form in figure 4, which was obtained with a single flat cathode. If a second flat cathode is now placed in the discharge tube and the two cathodes are separated by a gap of approximately 25 mm, the discharge produced takes the form observed in figure 8. In this case the discharge follows the outline of the two flat cathodes, and is otherwise identical in structure and properties with the discharge obtained when a single flat cathode is used (Fig. 4).
Figure 8 –
A low pressure glow discharge produced between two flat copper cathodes separated by a gap of 25 mm, and a flat copper anode, in helium at 10 Torr and 100 mA.
If the distance between the two cathodes is decreased to about 8 mm or less, the two separate cathode layers from the preceding figure 8 are seen to coalesce into a single cathode layer having a high radiation intensity, as illustrated in figure 9. This is the hollow cathode discharge. In practice, instead of using two flat cathode electrodes, a cylindrical cathode is used as illustrated in figure 10. This discharge, which occurs inside the cylindrical cavity, can carry currents of the order of several amperes at a cathode fall of several hundred volts and in a relatively cold carrier gas. The distribution of the various zones seen in the planar cathode discharge mode is present in the hollow cathode mode also. The zones are disposed in a circular manner inside the hollow cathode.
Figure 9 –
Same discharge as shown in figure 8 with the two cathodes separated by a gap of 8 mm.
Figure 10 –
Same discharge as shown in figure 9 with a cylindrical cavity as a cathode.
The low pressure hollow cathode discharge source, first described by Paschen in 1916 [253], consisted of a metallic rectangular hollow cathode and a cylindrical anode sealed in a glass vessel filled with helium at a pressure of several Torr (Fig. 11). It was adapted by Schüler and Gollnow to analytical measurements in 1937 [315], and is described in figure 12. It consists of a water cooled anode and cathode unit separated by a glass tube. The gap between the interchangeable hollow cathode and the anode electrode is of 1 mm. The hollow cathode sources used today are all derived from this basic form which was modified to a smaller or larger extent to satisfy the particular requirements of the analyst.
Figure 11 –
Schematic description of the first low pressure hollow cathode discharge developed by Paschen [253].
Figure 12 –
A hollow cathode low pressure glow discharge source developed by Schüler and Gollnow for analytical measurements [315]
3.2.2. Description of an Experimental Hollow Cathode Discharge Source
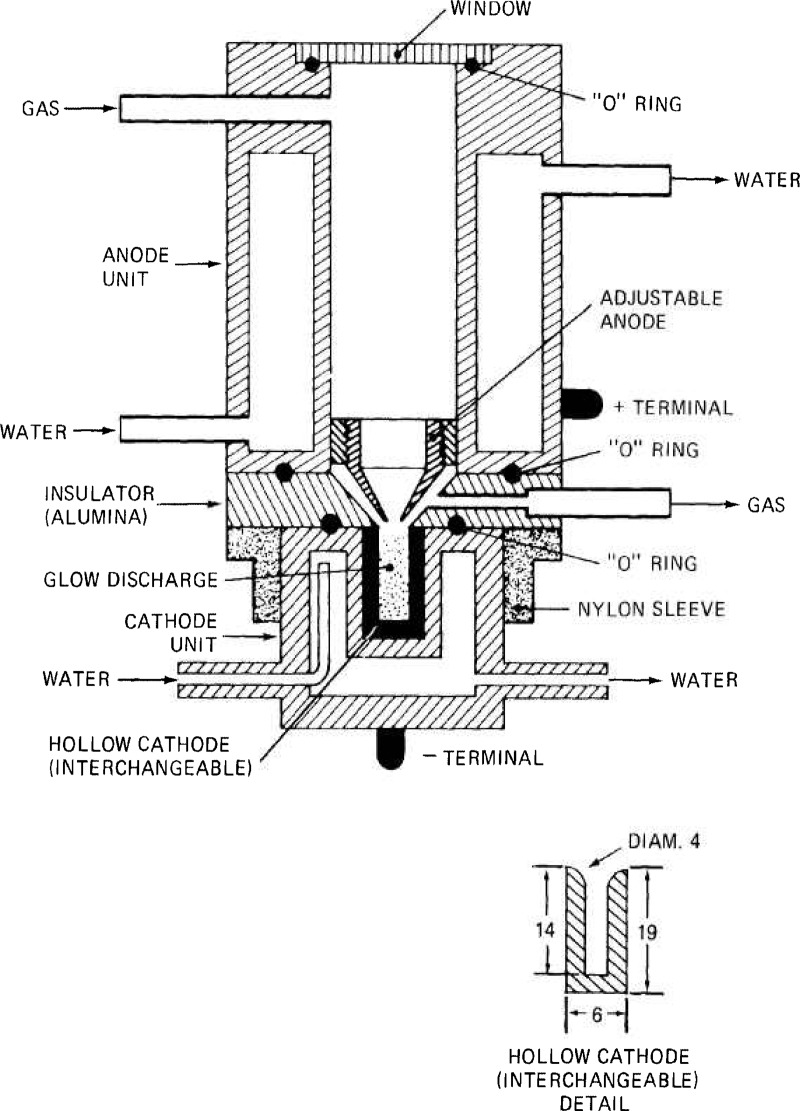
One of the forms developed and used in our laboratory is illustrated schematically in figures 13 and 14. It consists of a water cooled circular anode and cathode units made of brass. The anode is provided with an inlet for the carrier gas, a fused silica window, and a terminal. An adjustable conical ring permits the distance between the anode and cathode to be varied. The cathode unit can accept an interchangeable cylindrical hollow cathode 19 mm long, with an external diameter of 6 mm, provided with a 14 mm deep and 4 mm diameter bore. The anode and cathode are separated by a ring made of 99.7 percent pure alumina provided with a lateral alumina tube for connection to a vacuum pump. The anode, alumina ring and the nylon sleeve are assembled with six nylon screws. The nylon sleeve holds the cathode unit in place, and “O” rings are used to provide a vacuum tight assembly.
Figure 13 –
Experimental hollow cathode source developed in the Center for Analytical Chemistry, National Bureau of Standards. (Dimensions of detail, right, in mm.)
Figure 14 –
Alternate cathode unit (a) used in conjunction with the anode unit of figure 13. The hollow cathode proper is made from the metallic analytical sample and is interchangeable. Another alternate cathode unit, (b), also is used in conjunction with the anode unit from figure 13. The hollow cathode proper is made from two parts: a conical body, provided with a 3 mm orifice, closed by a pellet at the bottom. This pellet is the analytical sample, and can be made from a solid conducting material (metal) or pressed from a metallic powder. Non-conducting materials can be pelleted with a conducting powder such as pure copper, silver or graphite. Dimensions, right, in mm [339].
The nominal dimensions are: length and diameter of anode unit, 65 mm and 76 mm, respectively; length of alumina insulator, 12 mm; and length and diameter of cathode unit, 38 mm and 36 mm, respectively.
The cathode from (b) of figure 14 consists of a conical shaped part provided with an opening of 3 mm, and is made of pure copper in this case. Other pure materials could be used including graphite. The bottom of this conical unit is closed with a pellet 2 mm thick made from the analytical sample. This can be a solid metallic pellet or a pressed disc made of a metallic powder or a nonconducting sample mixed with a conducting powder such as pure copper. This type of hollow cathode was developed and used by Spectroscandia (Nagu, Finland) [339].
An exploded view of the unit is illustrated in figure 15 and the assembled unit is shown in figure 16. Figure 17 shows the unit in front of a Czerny-Turner 1 m grating universal spectrometer which can be operated as a scanning monochromator, a spectrograph, or a multichannel direct reading spectrometer.
Figure 15 –
Exploded view of the hollow cathode source described in figures 13 and 14. From left fused silica window with nylon retaining ring, anode unit, adjustable anode electrode, alumina insulator, nylon sleeve with six nylon assembling bolts, three cylindrical interchangeable hollow cathodes (Fe; Cu; Ag), conical hollow cathode with sample pellet and two cathode units.
Figure 16 –
Assembled hollow cathode source.
Figure 17 –
Hollow cathode unit from figure 16 under functioning conditions on an optical bench in front of a 1 m Czerny-Turner spectrometer. From left: console with power supply for the photomultiplier tube; the preamplifier, amplifier and lock-in amplifier; and the digital voltmeter, analog recorder and time-scaler unit.
The hollow cathode is connected, through a resistance of 1000 Ω and 2000 w, to a dc power supply capable of providing a current of 2.5 A and 2000 V. When the spectrometer is used in the scanning mode, the signal measuring system consists of a photomultiplier followed by a lock-in amplifier, an analog recorder and digital voltmeter. A scaler and timer unit is also available for integration of the signal over a chosen time interval. These parts are contained in the cabinet from figure 17, left. An 8-channel integrating electronic system is used in conjunction with the multichannel functioning mode, and is illustrated in figure 18 at left.
Figure 18 –
Front view of the same hollow cathode unit from figure 17. From left Lock-in light chopper, hollow cathode unit, 8-channel simultaneous direct reading system, console with current and gas monitoring meters and vacuum control valves, and dc power supply for the hollow cathode.
The carrier gas, usually pure argon, helium, or a mixture thereof, is supplied to the hollow cathode from corresponding high pressure bottles. The continuous flow is controlled through pressure regulators at the bottles and is limited by an individual glass capillary tube; the flow is monitored by flowmeters. A duo-seal oil vacuum pump provides the necessary vacuum which is monitored through a sensitive Bourdon-type mechanical gauge, and the hollow cathode source is connected to the gas and vacuum through off-on vacuum valves. The monitoring gauges and the valves and vacuum pump are contained in a cabinet as seen in figure 18. The current-monitoring meters – milliampmeter, voltmeter, and wattmeter–are located at the top of this cabinet which also contains the 1000 Ω, 2000 w resistor.
3.2.3. Characteristics of the Hollow Cathode Discharge Source
The voltage-current characteristics of the discharge were determined using an iron hollow cathode of 3.5 mm inside diameter, at various pressures, in helium, argon, and helium-argon mixture, and the results are given in table 5. From these measurements it can be seen that for a large change in current, from 50 mA to 1600 mA, the corresponding change in voltage is small, from 260 V to 430 V, when argon is used at a pressure of 10 Torr. This current-voltage relation of hollow cathode discharges is responsible for the stability of this excitation source.
Table 5.
Voltage-current characteristics for an iron hollow cathode 3,5 mm diam. in helium and argon at various pressures.
| Current | Volts | |||||||
|---|---|---|---|---|---|---|---|---|
| Helium at | Argon at | Helium-Argon mixture 1:1 | ||||||
| A | 3 Torr | 6 Torr | 10 Torr | 3 Torr | 6 Torr | 10 Torr | 10 Torr | 13 Torr |
| 0.050 | 230 | 240 | 300 | 240 | 280 | 260 | 220 | 280 |
| 0.10 | 230 | 240 | 300 | 240 | 300 | 300 | 230 | 300 |
| 0 20 | 230 | 240 | 300 | 400 | 310 | 310 | 220 | 315 |
| 0.30 | 260 | 240 | 300 | 480 | 315 | 330 | 200 | 340 |
| 0.40 | 320 | 230 | 300 | 540 | 340 | 3.30 | 200 | .360 |
| 0.50 | 540 | 230 | 320 | 620 | 340 | 360 | 210 | 370 |
| 0.60 | 580 | 240 | 310 | 700 | 360 | 380 | 220 | 370 |
| 0.70 | 620 | 240 | 300 | 760 | 380 | 380 | 240–320 | 380 |
| 0.80 | 640 | 260 | 300 | 820 | 390 | .380 | 340 | 380 |
| 0.90 | 680 | 270 | 300 | 870 | 400 | 380 | 360 | 390 |
| 1.00 | 720 | 400 | 300 | 920 | 410 | 380 | 370 | 400 |
| 1.10 | ||||||||
| 1.20 | 770 | 440 | 310 | 1000 | 440 | 400 | 400 | 400 |
| 1.30 | Arc | |||||||
| 1.40 | 820 | 460 | 320 | 480 | 420 | 410 | 400 | |
| 1.50 | ||||||||
| 1.60 | 490 | 340 | 510 | 430 | 440 | 400 | ||
| 1.70 | ||||||||
| 1.80 | Arc | 360 | 540 | 440 | 440 | 400 | ||
Hollow cathodes can be operated at currents from several milliamperes to several amperes. Since the intensity of the radiations excited in these sources is a function of this current, the hollow cathodes used in analytical applications are supplied with currents varying from about 100 mA to about 2 A, the first hollow cathode operated at such high current being described in 1933 by Paschen and Ritschel [256]. Figure 19 describes this source which consists of a metallic cathode made, in this case, of a thick aluminum block 200 mm long provided with a transversal bore of 5 mm. A fused silica container is adapted at each end of this block through conical ground joints, and each is provided with a cylindrical anode, a quartz window, and side tubes for the carrier gas and vacuum connections; the cathode is water cooled. Under these conditions currents of up to 3 A were supplied to the hollow cathode producing an extremely brilliant source of radiation. More recently, high-current hollow cathodes were designed and used for analytical purposes by Maierhofer and associates [196], and by Thornton [362]. The current used with the source illustrated in figures 13 and 17 varied from about 200 mA to 1800 mA according to the experimental conditions.
Figure 19 –
High-current hollow cathode source of Paschen-Ritschel [256].
The development and use of high intensity hollow cathodes is described in references 2, 20, 28, 29, 151, 184, 239, 245, 266, and 279.
The radiation intensities originating from the atoms of various elements, excited in a hollow cathode, are for a given set of experimental conditions a function of the current. This dependence was determined for the source described in figures 13 through 18, using a silver hollow cathode in helium at 3, 6, and 10 Torr pressure, and the results obtained are given in table 6. These data indicate that an increase of current from 50 mA to 1200 mA corresponds to an increase in the relative radiation intensity (PMV) of the Ag 𝜆 3280.68Å emission from 0.30 V to 11.75 V, or a factor of about 40, tapering off from about 1100 mA.
Table 6.
Relative intensity, expressed as photomultiplier voltage (PMV), for Ag λ 3280.68Å as a function of the current (mA) supplied to the hollow cathode source (HC) in helium at various pressures (PHe).
| PMV | |||
|---|---|---|---|
| HC, mA | PHe 3 Torr | PHe 6 Torr | PHe 10 Torr |
| 50 | 0.1l | 0.25 | 0.30 |
| 100 | 0.31 | 0.67 | 0.74 |
| 150 | 0.51 | 0.86 | 1.50 |
| 200 | 0.78 | 1.11 | 1.71 |
| 250 | 1.03 | 1.32 | 1.86 |
| 300 | 1.34 | 1.63 | 2.08 |
| 350 | 1.75 | 1.94 | 2.39 |
| 400 | 2.11 | 2 26 | 2.68 |
| 450 | 2.45 | 2.61 | 3.04 |
| 500 | 2.86 | 3.04 | 3.38 |
| 550 | 3.20 | 3.48 | 3.76 |
| 600 | 3.62 | 3.93 | 4.25 |
| 650 | 4.07 | 4.37 | 4.57 |
| 700 | 4.70 | 4.70 | 4.80 |
| 750 | 5.32 | 5.21 | 5.38 |
| 800 | 5.90 | ||
| 850 | 6.30 | ||
| 900 | 6.82 | ||
| 950 | 7.44 | ||
| 1000 | 9.05 | ||
| 1100 | 10.15 | ||
| 1200 | 11.75 | ||
The spatial distribution of this radiation inside the hollow cathode is illustrated in figure 20, after Büger and Fink [55]. The figure shows the relative intensity profiles obtained in helium at a pressure of 5 Torr and for currents from 40 mA to 1000 mA. The highest intensity was observed in the middle of the negative glow discharge, while close to the walls of the hollow cathode, the radiation intensity decreased to practically zero. This is to be expected since the cathode dark space is located between the wall of the hollow cathode and the negative glow. Similar observations were made earlier by Berezin [23].
Figure 20 –
Radiation intensity distribution in a hollow cathode After Büger and Fink [55].
The radiation stability of the hollow cathode source was determined using copper, silver, and iron hollow cathodes in helium at 10 Torr and for currents of 200, 100, and 200 mA at λ 3273.96Å, 3280.68Å, and 3719.94Å, respectively. The results obtained are assembled in table 7.
Table 7.
Stability of ihe radiation intensity, expressed as photomultiplier signal average (V), determined for a copper (Cu), silver (Ag), and iron (Fe) hollow cathode operated in helium at 10 Torr and a current of 200, 100, and 200 mA. The measurements were taken at time intervals of 100 s after a preburn of 10 min.
| V | |||
|---|---|---|---|
| Time, s | Cu | Ag | Fe |
| 0 | 8.43 | 8.13 | 6.00 |
| 100 | 8.43 | 8.15 | 6.05 |
| 200 | 8.33 | 8.15 | 5.97 |
| 300 | 8.20 | 8.17 | 6.00 |
| 400 | 8.17 | 8.20 | 5.95 |
| 500 | 8.23 | 8.20 | 5.90 |
| 600 | 8.19 | 8.20 | 5.95 |
| 700 | 8.09 | 8.19 | 5.87 |
| 800 | 8.07 | 8.17 | 5.85 |
| 900 |
8.10 |
8.27 |
5.85 |
| Aver | 8.22 | 8.18 | 5.94 |
| σ | 0.13 | 0.04 | 0.07 |
| % σ | 1.6 | 0.5 | 1.2 |
The table 7 data are expressed as averages of photomultiplier voltages. These data were obtained from the tracings produced with the 1 m spectrometer described in figures 17 and 18 and operated in the analog recording mode. A preburn of 10 min was used in all cases. The stability of the photomultiplier with the associated electronics was initially determined using a stable radiation source consisting of a tritium-activated phosphor (half-life 12.5 years). Under these constant illumination conditions, a variation of the photomultiplier signal of 0.3 percent was observed over a period of 60 min. The noise observed during each measurement, expressed as photomultiplier voltage, was on the order of ±0.2 V.
The table 7 data demonstrate the excellent stability of the hollow cathode discharge which is capable of producing and maintaining a radiation intensity over a time period of 20 min with a relative standard deviation of not more than 1.6 percent for a single measurement.
The effect of the sputtering process on the surface of the cathode was studied recently by Jäger and Blum [156] and by Harrison and associates [72] who used copper, stainless steel, and graphite discs in helium, neon, and argon, at various gas pressures and discharge currents and for various sputtering times. After exposure to the discharge, the metal surface was examined with a scanning electron microscope using magnifications up to 5000 times. Different erosion patterns were observed for helium, neon, and argon, indicating a more pronounced change for argon which produced a surface having a “conical structure 20 to 30 microns high,” and a heavier sputtering. When the sputtering time was extended to 15 hours in argon and under a current of 200 mA, the initial cylindrical shape of the hollow cathode was changed progressively into one or several successive bulb-like cavities (also see references 170 and 388). Examination of various parts of the cavity revealed different erosion patterns. It is interesting to compare these results with those obtained by White who studied the potential distribution in an ideal spherical hollow cathode cavity [388].
Jäger and Blum have observed the conical structure mentioned above and have concluded from their study [156], using gold and brass cathodes, that the removal of the sample material depends on crystal structure and orientation, and that there are no signs of surface melting. They also concluded that the preburn time is an essential parameter for obtaining an equilibrated sputtering surface and good analytical results. The preburn time should be established experimentally for every type of sample.
Due to the excitation conditions prevailing in the low pressure glow discharges, the spectra excited in the hollow cathode discharge are characterized by radiations with narrow spectral bandwidth and are practically free of self-absorption under the experimental conditions used to produce and observe the radiations. An example of the spectra excited with the hollow cathode from figures 13 and 14, using a copper and a silver cathode, is illustrated in figure 21 in comparison with the spectra from the same metals excited in a dc arc. The hollow cathode was operated in argon at a pressure of 4 Torr, a current of 500 mA, and an exposure time of 60 s. The de arc was operated at a current of 8 A in air and the exposure time was 5 s. Both spectra were recorded with a 3 m concave grating Eagle spectrograph, using a 6 step rotating sector, on Eastman Kodak 33 plates. The self-reversal of the resonance lines of copper and silver is clearly visible for the radiations excited in the arc, and the lines are wide and ill-defined. The same lines obtained with the hollow cathode are narrow, well-defined, and free of measureable self-absorption.
Figure 21 –
Spectra of Ag and Cu excited in a dc arc and a hollow cathode.
The general aspect of the spectra excited in low pressure glow discharges also exhibit a different intensity distribution when compared with those obtained in conventional arcs and sparks. These differences have led E. W. Salpeter to produce, in collaboration with the RSV Company, an atlas of spectra of the noble gases, He, Ne, Ar, Kr, and Xe from λ 500Å to λ 4000Å, and that of the following chemical elements: Fe, Co, Cr. Mo, Nb, Ni, Ta, Ti, Cu, Be, Ca, Si, Hg, Zn, Pb, Bi, Sb, S, Sn, As, Mg, Cd, Ag, Au, Ir, Pd, Pt, Rh, Ru, Al, Hf, Mn, Se, Te, V, W, and Zr, from λ 1500 Å to λ 4000Å. The excitation source used to produce the spectra was the Grimm glow discharge lamp manufactured by RSV. The atlas was produced in 1971 to 1973 and is divided into five parts. It can be obtained from the Specola Vaticana, Cita di Vaticano, Italy [305].
An atlas of the emission spectrum of uranium excited in a hollow cathode discharge is given in reference 172.
3.2.4. Analytical Applications
The energy, expressed in electron volts (ev), required to excite radiations from the neutral atoms of all known chemical elements, varies from one of the lowest values of 1.426 ev for uranium to the highest value of 21.215 ev for helium. Helium is one of the supporting gases used currently to produce low pressure glow discharges, and is strongly excited in these discharges to emit the radiations from its neutral and the singly ionized atoms (40.811 ev). Hence, the energy available in a hollow cathode discharge is largely sufficient to excite all known chemical species and produce radiations origi-nating from the neutral and ionized atoms as well as from their molecules.
The hollow cathode discharge was initially used by Paschen to produce and study the fundamental characteristics of the spectra of aluminum [254]. Schüler and associates have extended these investigations to the examination of the hyperfine structure of the spectra from rare earths, and of elements available in milligram quantities such as the artificially produced radioactive materials [311, 312, 314, 315, 317, 318, and 322], The excellent spectral characteristics of the discharge are related to the fact that the experimental parameters of the discharge, such as the nature, pressure, and flow of the sustaining gas, current, discharge tube and analytical sample geometry, can all be controlled. This has caused Schüler to recommend its use as a source of excitation in analytical spectroscopy.
Since then, the hollow cathode discharge has been applied extensively to various analytical problems including isotopic analyses and trace element analyses for practically all chemical species, including the halogens, in various matrices, in solid, liquid, and gaseous form, and for electrically conducting and non-conducting materials.
Some of these applications will be discussed here using data available from the scientific literature. Results obtained in our laboratories will also be presented. Further analytical use of the hollow cathode will be found in the numerous publications assembled in Section 5, Collection of References to Works on Low Pressure Glow Discharges, and to its addendum, Subsection 5.1.
The detection sensitivities obtainable with hollow cathodes depend greatly on the experimental parameters; therefore, the data given here should be considered only as an order of magnitude. The detection limits for a number of chemical elements are given in table 8 after Korovin [175]. Further measurements, published by Zilbershtein and associates [398, 399, 400], indicate that an obsolute sensitivity of 3 to 5×10−10g can be obtained for Ag, Mn, and Cu; 6×10−10g for Ga and In; 3 to 5×10−9g for A1 and Ni; and 6 to 7×10−9g for Mg and Fe, in a silicon matrix, using an excitation current of 800 to 900 mA for a burn of 2 min.
Table 8.
Absolute detection limits for 22 chemical elements, expressed in nanograms, after Korovin [175].
| Element | Wavelength, Å | Hollow cathode | Copper spark | dc arc carrier distillation |
|---|---|---|---|---|
| Al | 3092.7 | 10 | 10 | 500 |
| Ag | 3280.7 | 0.03 | 5 | |
| B | 2497.7 | 1 | 10 | 1 |
| Be | 3130.4 | 0.03 | 0.2 | 10 |
| Cd | 2288.0 | 30 | 200 | 7 |
| Co | 3453.5 | 0.3 | 50 | 100 |
| Cr | 2835.6 | 1 | 5 | 300 |
| Cu | 3247.5 | 0.03 | 30 | |
| Fe | 2599.4 | 3 | 50 | 100 |
| Ga | 2943.6 | 0.03 | 100 | |
| K | 7664.9 | 10 | 10 | 200 |
| Li | 6707.8 | 0.1 | 0.2 | 10 |
| Mg | 2852.1 | 0.3(~0.0001) | 1(0.1) | 50 |
| Mn | 2794.8 | 0.03 | 2 | 100 |
| Na | 5889.9 | 0.03 | 10 | 50 |
| Ni | 3050.8 | 1 | 10 | 200 |
| P | 2535.6 | 30 | 2000 | 5000 |
| Pb | 2833.1 | 10 | 5 | 100 |
| Sb | 2528.5 | 100 | 500 | 1000 |
| Si | 2881.6 | 1 | 10 | 300 |
| Sn | 2840.0 | 10 | 100 | |
| Zn | 3345.0 | 3 | 200 | 2000 |
Webb and Webb [381, 382] have used a hollow cathode to determine gases in metals. Their results are given in table 9; the detection limits are given in table 10.
Table 9.
Determination of nitrogen, oxygen, and hydrogen in rnetals [382].
| Steel, NBS Standards | Uranium carbide | Copper | Tungsten | Zirconium | Zircaloy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gas | 8i | 101E | 343 | 125 | A | B | C | D | 1 | 2 | 1 | 2 | 3 | A | B | |
| Nitrogen, certified | 0.018 | 0.039 | 0.074 | 0.002 | 0.051 | 0.037 | 0.037 | 0.022 | ||||||||
| percent found | 0.0175 | 0.034 | 0.072 | 0.0055 | 0.051 | 0.048 | 0.035 | 0.018 | ||||||||
| Oxygen, certified | 0.09 | 0.20 | 0.07 | 0.026 | 290 | 150 | 5.5 | 35 | 22 | |||||||
| percent found | 0.12 | 0.20 | 0.06 | 0.024 | 295 | 160 | 7.3 | 25 | 20 | |||||||
| Hydrogen, certified | 46 | 140 | 150 | |||||||||||||
| ppm found | 46 | 133 | 143 | |||||||||||||
Table 10.
Limit of detection of gases in metals in μg [382].
| Material | Oxygen | Nitrogen | Hydrogen |
|---|---|---|---|
| Steel | 0.35 | 0.55 | |
| Uranium | 1.5 | 2.5 | |
| Carbide | |||
| Tungsten | 0.65 | ||
| Copper | 0.75 | ||
| Zirconium | 0.05 |
A hollow cathode was used by Birks [29] to determine the halogens, fluorine, chlorine, bromine, and iodine, for which the following detection limits were found: 0.25 μg, 1 μg, 5 μg, and 2.5 μg respectively.
Thornton has used a high temperature hollow cathode source similar in design to that described by Webb and Webb [381, 382] to analyze steels and high temperature alloys for trace elements [362]. The samples were loaded in graphite hollow cathodes and were excited in helium with currents from 0.2 A up to 1.4 A and an exposure time of 5 min. The results obtained in the determination of trace elements at the part-per-million level in various metals and alloys are given in tables 11 and 12, in comparison with accepted values. It is interesting to note that the internal standard used in these determinations was helium at λ 2945.11 Å. Various factors which affect the sensitivity and precision as well as matrix interferences are also discussed by Thornton in the same paper.
Table 11.
Analysis of various samples with constant trace-element additions [362].
| Element concentration found, percent | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample No. | Material | Bi | In | Ga* | Sn | Pb | T1 | Sb | Zn* | Ag | Te* | As* | |
| R3393 | Nickel | Accepted value | 0.0009 | 0.0010 | 0.001 | 0.006 | 0.0014 | 0.0014 | 0.0013 | 0.001 | 0.0011 | 0.001 | 0.01 |
| Hollow cathode | 0.0012 | 0.0010 | 0.0012 | 0.006 | 0.0013 | 0.0016 | 0.0018 | 0.0009 | 0.0009 | 0.0012 | 0.014 | ||
| R3394 | Coball | Accepted value | 0.0008 | 0.0011 | 0.001 | 0.006 | 0.0014 | 0.0004 | 0.0020 | 0.001 | 0.0010 | 0.001 | 0.01 |
| Hollow cathode | 0.0010 | 0.0009 | 0.0013 | 0.006 | 0.0012 | 0.0006 | 0.0021 | 0.0007 | 0.0018 | 0.0014 | 0.012 | ||
| R3395 | Iron | Accepted value | 0.0008 | 0.0008 | 0.001 | 0.007 | 0.0009 | 0.0008 | 0.0033 | 0.001 | 0.0010 | 0.001 | 0.01 |
| Hollow cathode | 0.0010 | 0.0009 | 0.0019 | 0.007 | 0.0013 | 0.0010 | 0.0038 | 0.0008 | 0.0011 | 0.0011 | 0.013 | ||
| R3396 | Nickel 80%; | Accepted value | 0.0009 | 0.0008 | 0.001 | 0.006 | 0.0014 | 0.0008 | 0.0016 | 0.001 | 0.0012 | 0.001 | 0.01 |
| chromium 20% | Hollow cathode | 0.0011 | 0.0009 | 0.0009 | 0.005 | 0.0012 | 0.0012 | 0.0018 | 0.0010 | 0.0010 | 0.0010 | 0.009 | |
| R3400 | Nickel 40%; | Accepted value | 0.0008 | 0.0009 | 0.001 | 0.006 | 0.0010 | 0.0008 | 0.0025 | 0.001 | 0.0012 | 0.001 | 0.01 |
| iron 40%; chromium 20% |
Hollow cathode | 0.0011 | 0.0011 | 0.0012 | 0.006 | 0.0012 | 0.0010 | 0.0028 | 0.0009 | 0.0013 | 0.0012 | 0.010 | |
| R3401 | Iron 50%; | Accepted value | 0.0008 | 0.0009 | 0.001 | 0.007 | 0.0011 | 0.0007 | 0.0019 | 0.001 | 0.0011 | 0.001 | 0.01 |
| nickel 30%; cobalt 20% |
Hollow cathode | 0.0009 | 0.0010 | 0.0014 | 0.006 | 0.0011 | 0.0010 | 0.0026 | 0.0007 | 0.0010 | 0.0011 | 0.010 | |
The “accepted” values given are the nominal additions: all other elements were determined by spectrochemical methods.
Table 12.
Hollow-cathode discharge analysis of high temperature alloys [362].
| Element concentration found, percent | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Material | Bi | In | Ga* | Sn | Pb | Tl | Sb | Zn* | Ag | Te* | As* | |
| R3385 | Nickel-base alloy† | Accepted value | 0.0001 | 0.0001 | 0.0002 | 0.009 | 0.0001 | 0.0001 | 0.0004 | 0.0001 | 0.0001 | 0.0001 | 0.01 |
| Hollow cathode | 0.0002 | 0.0002 | 0.0003 | 0.011 | 0.0002 | 0.0002 | 0.0006 | 0.0001 | 0.0001 | 0.0002 | 0.0053 | ||
| R3386 | Nickel-base alloy† | Accepted value | 0.0002 | 0.0002 | 0.0005 | 0.0050 | 0.0002 | 0.0002 | 0.0009 | 0.0002 | 0.0002 | 0.0002 | 0.005 |
| Hollow cathode | 0.0003 | 0.0003 | 0.0006 | 0.0056 | 0.0003 | 0.0003 | 0.0011 | 0.0002 | 0.0002 | 0.0003 | 0.0051 | ||
| R3387 | Nickel-base alloy† | Accepted value | 0.0009 | 0.0009 | 0.002 | 0.0014 | 0.0009 | 0.0009 | 0.0048 | 0.001 | 0.0009 | 0.001 | 0.001 |
| Hollow cathode | 0.0012 | 0.0011 | 0.0026 | 0 0015 | 0.0012 | 0.0011 | 0.0056 | 0.0010 | 0.0009 | 0.0013 | 0.0016 | ||
| R3388 | Nickel-base alloy† | Accepted value | 0.0005 | 0.0005 | 0.001 | 0.0026 | 0.0005 | 0.0004 | 0.0019 | 0.0005 | 0.0005 | 0.0005 | 0.002 |
| Hollow cathode | 0.0006 | 0.0006 | 0.0012 | 0.0025 | 0.0006 | 0.0006 | 0.0022 | 0.0004 | 0.0004 | 0.0007 | 0.0023 | ||
The “accepted” values given are the nominal additions, all other elements were determined by spectrochemteal methods.
Basic composition, percent: nickel 60, chromium 15, coball 15. molybdenum 5, titanium 2 1/2, aluminum 2 1/2
The hollow cathode discharge source described in figures 13, 14, 15, and 16 was used in conjunction with a 3 m concave grating Eagle spectrograph and the gas monitoring and power supply, illustrated in figure 18, to determine Mn, Ni, Co, Cr, Al, Si, and Cu in a series of four steel standards certified by NBS as Standard Reference Material (SRM) 1261 to 1264.
Hollow cathodes machined from each sample were submitted to a discharge of 0.8 A in argon at a pressure of 4 Torr, with a preburn of 60 s followed by an exposure of 120 s. The spectra of the four samples were recorded on a photographic plate (Kodak 33) together with an “unknown” sample which was selected from the four standard samples. The plate was processed in the usual manner and was read on a microdensitometer. Calibration curves were then established from the values found for the standard samples using iron as internal standard, and the values measured for the “unknown” sample were obtained by interpolation from these curves. The resulting data are given in table 13 for the seven elements determined, and indicate that the average uncertainty of the analysis of about 5 percent is within the expected values which can be obtained when photographic plates and procedures are used as photodetectors and data acquisition means.
Table 13.
Determination of seven elements in SRM 1262 using a hollow cathode discharge source.
| Element | Certified value, percent | Found, percent |
|---|---|---|
| Mn | 1.04 | 0.95 |
| Ni | 0.59 | 0.58 |
| Co | 0.30 | 0.305 |
| Cr | 0.30 | 0.28 |
| A1 | 0.095 | 0.087 |
| Si | 0.39 | 0.395 |
| Cu | 0.50 | 0.51 |
The results from the stability tests discussed earlier indicate that an appreciably smaller uncertainty should be obtained when direct measurements are made with multichannel spectrometers using photomultipliers with integration and adequate data acquisition and processing methods available today. Such measurements are being performed at the present time, in association with J. Norris, using a 2 m concave grating multichannel spectrometer and the preliminary results seem to confirm the above statement. However, before the hollow cathode discharge can be used as a routine source of excitation in analytical spectroscopy, further investigations are needed. This author believes that the area requiring detailed studies is that of the sputtering processes occurring in the circumstances characteristic of the hollow cathode discharge mode of operation. The works cited in Section 5 under the title “Sputtering” should provide useful information ori this subject.
4. Future Developments
Future developments in the field of low pressure hollow cathode discharges should aim in particular at increasing the sensitivity of analytical measurements. This sensitivity could be increased by increasing the current supplied to the hollow cathode. Currents from 20 A to 80 A were used by Ahsmann and van Benthem [6] in conjunction with a tantalum hollow cathode of special design. The ion temperature measured in the hot plasma beam produced with this source was of 20000 K to 30000 K for argon and 8000 K for neon.
The properties of high intensity hollow cathodes are discussed in a number of papers [2, 20, 28, 29, 151, 184, 239, 266, 279], and to our knowledge this type of discharge was not used in analytical spectroscopy.
Further increase in the current can be achieved by operating the hollow cathode in the pulsed discharge mode using pulses of the order of microseconds with amplitudes of several thousand amperes and with a repetition rate from several discharges to several hundred discharges per second. Such operating conditions have been described by Kielkopf [166] who used pulse amplitudes between 30 and 1500 A and gas pressures of 5 to 50 Torr in helium. This discharge was used to study the spectrum of triply ionized iron, aluminum, and the triply and quadruply ionized rare earths. Ion temperatures of 12000 K and electron temperatures of 15000 K have been measured for such a discharge. A similar high current pulsed discharge hollow cathode is used currently at the National Bureau of Standards by V. Kaufman to study the emission spectra of various chemical elements in the vacuum ultraviolet.
Pulsed hollow cathode discharges are also described in references 24, 60, 64, 65, 66, 67, 69, 122, 135, 141, 158, 163, and 259.
Superdense hollow cathode discharges have been studied also by Klyarfeld and associates [169] and by Abramovich and associates [1]. Further references to works in this field are assembled in Section 5.
The radiation intensity of a hollow cathode can be increased by providing additional energy to the discharge in the form of an electrical or magnetic field. Thus, van Gelder [114] has designed a cylindrical hollow cathode open at both ends as illustrated in figure 22. Additional excitation is provided by a stream of electrons generated by an auxiliary emitting cathode. Supplementary excitation was achieved also by Human and associates [146, 147, 148] to increase the emission from hollow cathodes, using superimposed direct current and a high frequency field. An intensity gain of two orders of magnitude was obtained by Bodrctsova and associates [31] when a high frequency current was supplied to a hollow cathode. See also reference 35, 36, and 72.
Figure 22 –
High intensity liollnw cathode source after van Gelder [114].
The effect of magnetic fields on hollow cathode discharges was explored by Popovici and Somesan [271, 337, 338], The hollow cathode used by these authors is illustrated schematically in figure 23. It consists of two opposite flat circular discs 20 mm in diameter separated by a gap of 4.5 mm and surrounded by a cylindrical anode 10 mm wide and 30 mm in diameter. The magnetic field, from zero to 3000 Oe, was applied as shown.
Figure 23 –
Description of a low pressure hollow cathode discharge with superimposed magnetic field. After Popovici and Somesan [271, 337, 338]. Dimensions in mm.
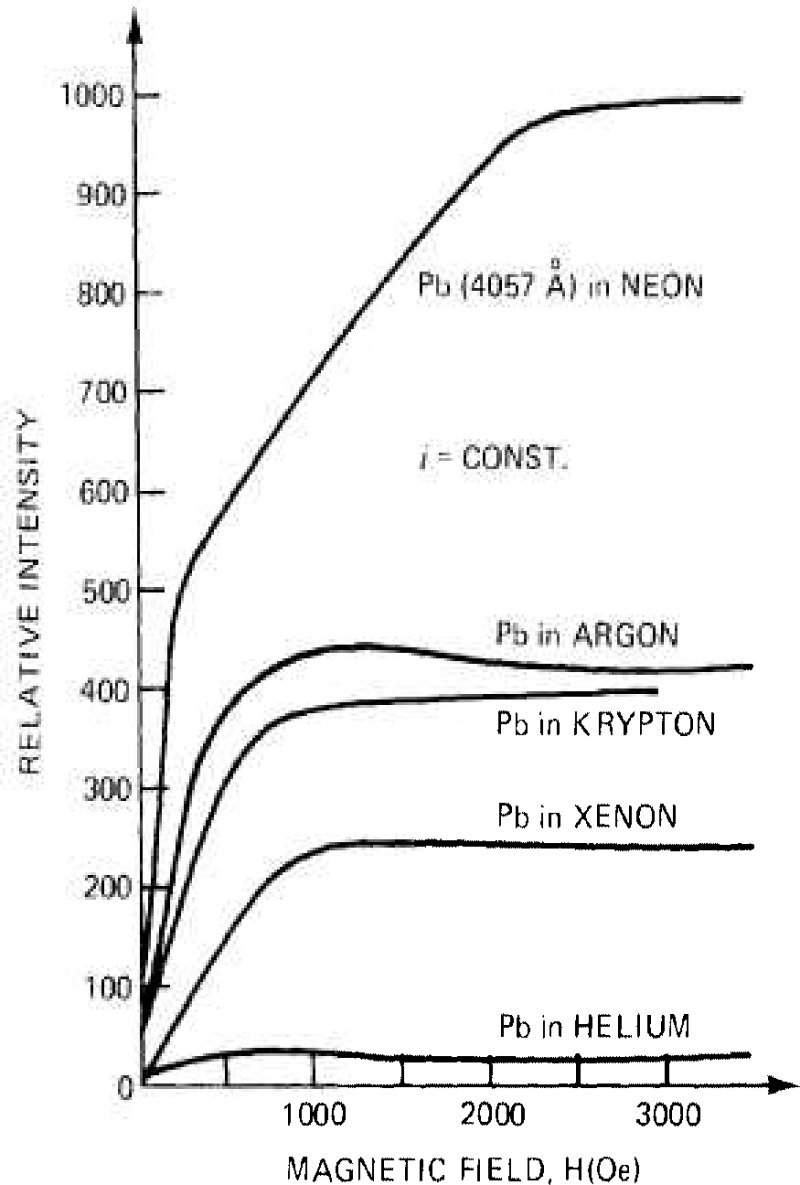
The intensity of the lead radiation at λ 4057.83Å was measured, under a constant current intensity (15 mA/cm2), in Ne, Ar, Kr, Xe, and He, as a function of the magnetic field, and the results from figure 24 show that an increase by a factor of 10 was observed.
Figure 24 –
Intensity of (he lead radiation al λ.4057.83Å excited, under constant current, in a magnetic field and in different noble gases, using the hollow cathode source from figure 23 [271, 337, 338].
This enhancement is significant and, if proved valid for other chemical species, should increase the use of hollow cathode discharges to the analysis of trace elements at the sub-nanogram level.
Further investigations on the effects of magnetic fields on the plasma generated in hollow cathode discharges are discussed in the Addendum to Section 5, Paragraph 15.
The author gratefully acknowledges the permission received from the various scientific journals and publications to use several illustrations and tables reproduced in this work. The sources from which the material was taken are indicated in the text. Further acknowledgment is given to the Center for Analytical Chemistry Text Editing Facility for typing the manuscript.
5. Collection of References to Works on Low Pressure Glow Discharges
The references in the bibliography that follows were selected with an eye toward analytical measurements.
Preceding the alphabetically arranged references is a Brief Subject Index in which the cited works are grouped under 20 subject categories. This index is intended to assist the reader in preliminarily selecting papers in his field of interest. The reader is also furnished a List of Chemical Elements.
The 400 works of the bibliography cover, roughly, the period from 1916 to 1975, In addition to this listing, a second listing offers another 290 citations. Essentially, this addendum covers the years 1975 to 1983.
In all, the base and addendum bibliographies contain 690 citations.
5.1. Brief Subject Index
Glow Discharges: 68, 89, 94, 96, 105, 138, 189, 209, 211, 213, 252, 253, 257, 365, 386.
Atlas of Glow Discharge Spectra: 305.
Wavelength Standard: 71. 341.
Bibliography: 159, 207, 208.
Sputtering: 20, 41, 72, 136, 156, 161, 232, 344, 383, 387.
Fundamental Characteristics: 12, 13, 15, 16, 33, 34, 42, 43, 49, 50, 58, 59, 60, 64, 79, 80, 81, 82, 89, 95, 101, 119, 120, 121, 132, 135, 179, 188, 191, 192, 222, 229, 233, 240, 242, 243, 245, 246, 253, 284, 309, 310, 333, 346, 347, 375, 376, 388, 389, 396.
Excitation Phenomena in Hollow Cathodes: 2, 3, 7, 12,13, 14, 15, 16,17, 19, 23, 24, 32, 35, 36, 37, 38, 39, 51, 53, 62, 67, 75, 79, 80, 81, 84, 87, 89, 91, 99, 108, 112, 117, 118, 120, 121, 124, 131, 133, 134, 137, 144, 145, 158, 162, 166, 169, 173, 187, 188, 191. 192, 195, 199, 224, 225, 229, 230, 231, 242, 244. 246, 247, 248, 249, 250, 251, 253, 254, 255, 256, 258, 270, 271, 272, 276, 278, 280,281a, 285, 286, 302, 304, 306, 307, 308, 311, 314, 325, 329, 330, 336, 340, 346, 347, 350, 353, 355, 356, 357, 368, 369, 371, 373, 384. 388, 391.
Excitation of Molecular Spectra: 62, 277, 283, 313, 316, 319, 320, 321, 323, 324.
Isotopic Analysis and Fine Structure. 8, 9, 30,43,44, 46, 48, 63, 88, 102, 103, 104, 107, 113, 122, 164, 168, 172, 174, 180, 181, 182, 183, 184, 185, 186, 230, 235, 236, 241, 253, 282, 292, 312, 317, 318, 322, 335, 340, 345,364, 365, 366, 367,374, 385, 389, 392, 393, 395, 397.
Instrumental Characteristics: 40, 45, 47, 59, 60, 61, 65, 69, 70, 72, 74, 75, 76, 83. 85, 90, 92, 100,106,109, 110. 115, 125, 126, 127, 141, 143, 149, 150, 158, 159, 160, 163, 165, 178, 190, 193, 194, 200, 201, 202, 204, 209, 211, 212, 214, 215, 227, 237, 261, 263, 264, 268, 269, 273, 274, 275, 281, 293, 294, 315, 318, 322, 326, 327, 328, 334,339, 342, 343, 344, 348, 349, 350, 351, 352, 354, 358,359, 360, 363, 372, 375, 377, 378, 379, 380, 388, 390, 394.
Excitation of Chemical Elements: 5, 10, 25, 26, 27, 28, 32, 40, 41, 53, 67, 69, 97, 98, 117, 118, 124, 125, 173, 175, 190, 193, 194, 203, 218, 262, 263, 264, 266.
Analytical Applications: 5, 11, 21, 22, 25, 26, 27, 29, 40, 41, 52,53, 54, 56,57, 66, 73, 85, 86,92,93,97,98, lift, 122, 123, 138, 139, 140, 142, 149, 150, 151, 152, 153, 154, 155, 157, 163, 164, 171, 176, 177, 197, 198, 205, 206, 209, 210, 211, 212, 214, 216, 217, 219, 220, 221, 223, 225, 226, 227, 228, 234, 236, 238, 239, 241, 247, 248, 249, 250, 259, 260, 265, 266, 267, 279, 288, 289, 290, 291, 294, 295, 296, 297, 298, 299, 300, 301, 303, 314, 315, 331, 332, 362, 370, 380, 381, 382, 394, 398, 399, 400.
Planar Cathode [Grimm Source]: 85, 86, 93, 128, 129, 130, 226, 288.
Hollow Cathode as Atomizer for Atomic Absorption: 124, 152.
High Intensity Hollow Cathodes: 1, 6, 55, 56, 57, 58, 114, 166, 167, 169, 170, 196, 209, 211, 212, 256, 330, 334, 335.
Pulsed Hollow Cathodes: 18, 76, 77, 78, 166, 167, 195, 275, 287, 329.
Hollow Cathode in a Magnetic Field: 271, 337, 338.
Hollow Cathode-RF Discharge Association: 31,146, 147, 148.
High Pressure Glow Discharge: 95, 111, 361.
Lasers and Hollow Cathodes: 4, 10.
5.2. Listing of Chemical Elements
| Reference | Element |
| [5] | Se, As, P, S, I |
| [9] | Pu |
| [11] | nuclear fuels |
| [21] | alloys |
| [22] | S, Cl, F, Br, I |
| [25] | S, Cl, F |
| [26] | S, Cl, F |
| [27] | deuterium |
| [29] | F, Cl, Br, I |
| [48] | Li |
| [52] | F |
| [56] | He, Ar, N, CO2 |
| [57] | I |
| [66] | Cl, F |
| [73] | B |
| [86] | Cu, Fe, Mg, Mn, Si, Ti, Zn |
| [93] | Si, Ca, Mg, Mn, Fe, Al, Ti, Na, K, P, S, C |
| [97] | Mg, Zn, V, Cr |
| [98] | F, Cl, As |
| [116] | F, Cl, B, U Na, K, Rb, Cs |
| [123] | Mo, Ru, Rh, Pd |
| [129] | Be, Si, Fe, Mg, Cr, Mo, Al, Pb |
| [140] | Au |
| [142] | Pb, Cu, B, Sn |
| [151, 153] | Zn, Cu, Mg, Mn, Ca, Sr, Cr, Fe, Co, Ni, Al, Ag, Ga, In, TI |
| [154, 155, 157] | Au |
| [163] | F, Cl |
| [171] | Ar, N |
| [176] | Cl, F |
| [194] | F, Cl, S |
| [197] | As |
| [198] | I |
| [203] | Na, K, Tl, Hg, Fe, Co, Ni |
| [205] | Cd, Mn, Sb, Fe, Mg, Pb, Sn, Ni, Bi, Al, Cu |
| [206] | Li |
| [210] | F, Cl |
| [219, 220, 221] | I |
| [228] | N |
| [234] | Ag, Al, As, B, Be, Bi, Cd, Cr, Cu, In, Mg, Mn, Na, Pb, Sb, Sn, Tl, Zn, Ni, Si |
| [238] | Ag, Cu, Pb, Bi, Cd |
| [239] | Ag, Bi, Cu, As, Ni |
| [259] | Cu, Ag, Mn, Mg, Pb, Bi, Ga, Zn, Cr, Sn, Ni, Sb |
| [260] | Al, Fe, Ca, Co, Si, Mg, Mn, Cu, Na, Ni, Ag, Cr |
| [262] | Cu, In, Sn, Al, Ga, Mg, Ni, Cr, Pb, Mn |
| [266] | V, Mo, Ta, Zr, Ti |
| [267] | Al, Ge, Fe, Bi, Au, In, Co, Mn, Cu, As, Ni, Pb, Ag, Sn, Tl, Cr, Zn, Ga, Sb |
| [283] | P |
| [289, 290, 291] | O |
| [295] | Cd, Zn |
| [296] | Ge, Si |
| [298] | As, Sb |
| [299] | Se, Zn |
| [300] | S, Cd |
| [301] | Bi, Al, B |
| [303] | In, Ge, Cd, Tl, As, Sb, Pb, Sn, Fe, Cu, Ni, Co, Bi, Ag |
| [332] | Bi, Pb, Sn, Cd, Zn, Sb, Cu, Mg, Mn, Fe, Al, Cr, Ni |
| [335] | N, O |
| [362] | Bi, In, Ga, Sn, Pb, Tl, Sb, Zn, Ag, Te, As |
| [370] | N, O |
| [381] | O |
| [382] | O, H, N |
| [393] | N, O, H |
| [398] | Ag, Mn, Cu, Ga, In, Al, Ni, Mg, Fe |
| [399, 400] | Al, Ga, Fe, Ag, Mn, Cu |
5.3. References
- [1].Abramovich L. Y.; Klyarfel’d B. N. and Nastich Y. N.. Ultra-high density glow discharge with a hollow cathode. Zh. Tekhn. Fiz. 36, 714–719 (1966). [Google Scholar]
- [2].Afanas’eva V. L.; Lukin A. V. and Mustafin K. S.. Electron energy distribution in a hollow-cathode discharge in a helium-neon mixture. Soviet Phy.-Tech. Phys. 11, 389–394 (1966). [Google Scholar]
- [3].Afanas’eva V. L.; Lukin A. V. and Mustafin K. S.. Electron energy distribution in a neon-hydrogen mixture in a hollow-cathode discharge. Zh. Tekh. Fiz. 37, 233–235 (1967) (English translation). [Google Scholar]
- [4].Agàrbiceanu L; Agafilei A., Preda A., and Vasiliu V.. Laser effect in a luminescent discharge of a hollow cathode. Rev. Roumaine Phys. 11, 649–650 (1966). [Google Scholar]
- [5].Ahmad C. N. Note on glow’ discharge techniques for selenium, arsenic and other vapours. J. Sci. Instr. 41, 778 (1964). [Google Scholar]
- [6].Ahsmann G. J. M. Jr., and van W. Benthem. A hollow cathode discharge yielding a highly ionized, hot beam. Philips Res. Labs. Rep. (Eindhoven) No. 4112, 11 p. (1966). [Google Scholar]
- [7].Alexeff L; Halchin W., Jones W. D., and Potts J. F.. Plasma-density measurement in a hollow-cathode arc by arc reversal. A EC Accession No. 44864. Rept. No. ORNL-4010, 9 pp (1966).
- [8].Arroe O. H., and Mack J. E.. Hollow-cathode source design for high resolution spectroscopic studies with small samples. J. Opt. Soc. Am. 40, 386–388 (1950). [Google Scholar]
- [9].Artaud J.; Chaput M. and Gerstenkorn S.. Isotopic analysis of plutonium by optical spectroscopy. Comm. Energie At. (France) 9 pp., Rept. No. 1909 (1961). [Google Scholar]
- [10].Asami Y.; Sugawara Y., Tokiwa Y., and Iijrma T.. Metal vapor line spectra in hollow cathode discharge. Scikei Daigaku Kogakubu Kogaku Hokoku, 4, 268–269 (1967). [Google Scholar]
- [11].Aya-Ramirez O. The hollow cathode as the excitation source in emission spectroscopy for trace determinations in nuclear fuels. Report KFK-1496, 86 pp., Center for Nuclear Res., Karlsruhe, Germany: (1971). [Google Scholar]
- [12].Bădăráu E., and Popescu I.. Some problems regarding the phenomena occuring at the cathode of the glow discharge. Rev. Phys. Acad. R. P. Roumaine 5, 41–82 (1960). [Google Scholar]
- [13].Bădăráu E.; Popescu I. and lova I.. Contribution to the mechanism of Doppler effect at the cathode. Ann. Physik 5, 308–326 (1959). [Google Scholar]
- [14].Bădăráu E.; Popoviei C., lova I., and Somcsan M.. Hollow cathode effect in cesium vapor, Ann. Physik 7, 313–320 (1965). [Google Scholar]
- [15].Bădăráu E.; Popoviei C and Somesan M, Mechanism of the hollow cathode effect. Z. Physik. Chem. 230, 90–105 (1965). [Google Scholar]
- [16].Bădăráu E., and Wachter F.. Contribution of photons to the liberation of electrons at the cathode of a glow discharge in mercury. Ann. Physik 7, 418–424 (1961). [Google Scholar]
- [17].Bartholomeyczyk W. On the clean up of noble gases in hollow cathodes and the associated phenomena. Ann. Physik 5, 534–560 (1942–43). [Google Scholar]
- [18].Becart M.; Deprez G and Roig J, Production of spark lines from a hollow cathode tube operating on pulses. Spectrochim. Acta, Suppl. 1957, 342–349. [Google Scholar]
- [19].Becart M., and Marsil M.. Functioning characteristics of a Schüler hollow cathode lamp with two anodes. Compt. Rend. Acad. Sci. (Paris) 261, 3306–3309 (1965). [Google Scholar]
- [20].Behrisch R. Solid materials sputtering through ion bomhardment In Ergebnisse der Exakten Naturwissenschaften, Vol, 35, 297–442 Springer, Berlin, 1964. [Google Scholar]
- [21].Belle C. J., and Johnson J. D.. In-depth compositional profile analysis of alloys using optical emission glow discharge spectrography. Appl. Spectroscopy 27, 118–124 (1973). [Google Scholar]
- [22].Berezin I. A. Determination of sulfur and halogens in solutions with the aid of a hollow cathode. Zavodsk, Lab, 27, 859–861 (1961). [Google Scholar]
- [23].Berezin I. A. Spectral line intensity distribution in hollow cathode Spektroskopiya, Metody i Primenenie, Akad. Nauk SSSR. Sibirsk. Otd. 1964, 60–62. [Google Scholar]
- [24].Berezin I, A. Distribution of spectral line intensities in the hollow cathode. Opt. Spectroscopy 13, 483 (1962). [Google Scholar]
- [25].Berezin I. A., and Aleksandrovich K. V.. Determination of sulfur, chlorine, and fluorine in beryllium oxide by a spectrographic method. Zhur. Anal. Khim. 16, 613–616 (1961). [Google Scholar]
- [26].Berezin I. A.; Degtyareva O. F and Shevchenko P, P. Determination of sulfur, chlorine, and fluorine in the vacuum (ultraviolet) region using a hollow cathode (discharge). Zh. Prikl. Spektroskopii, Akad. Nauk Belorussk. SSR 4, 170–171 (1966). [Google Scholar]
- [27].Berezin I. A., and Sten’gach I. N.. The use of a hollow cathode discharge for the determination of deuterium in titanium. Zh, Anal. Khim. 22, 1897–1898 (1967). [Google Scholar]
- [28].Berezin I. A., and Yanovskaya G. N.. Excitation of iodine in a hollow cathode. Opt. Spektrosk 14, 23–28 (1963). [Google Scholar]
- [29].Birks F. T. The application of the hollow-cathode source to spectrographic analysis. Spectrochim. Acta 6 169–179 (1954). [Google Scholar]
- [30].Blaise J., and Chantrel H.. Hyperfme structure of the arc lines of the mercury spectrum and quadruple polar momentum of 20lHg. J, Phys. Rad. 18, 193–200 (1957). [Google Scholar]
- [31].Bodretsova A. I.; L’Vov B. V. and Mosichev V. I.. Spectral characteristics of a high-frequency discharge in lamps with a hollow electrode, Zh. Prikl. Spektroskopii, Akad. Nauk Belorussk. SSR 4, 207–212 (1966). [Google Scholar]
- [32].Brodretsova A. I; L’Vov B. V., Pavlovskaya E. N., and Prokofev V. K.. Some spectroscopic characteristics of sealed tubes with hollow cathodes of various metals. Zh. Prikl. Spektroskopii, Akad. Nauk Belorussk. SSR 2, 97–104 (1965). [Google Scholar]
- [33].Boeschoten F., and Demeter L, J., Measurements of plasma rotating in a hollow cathode discharge. Plasma Phys, 10 391–409 (1968). [Google Scholar]
- [34].Bogdanova I. P., and Gi-Tkhek Chen. Concentration of excited neon atoms during the discharge in a hollow cathode Opt. Spektrosk. 2, 681–688 (1957). [Google Scholar]
- [35].Borodin V. S., and M Kagan Y.. A study of the hollow cathode discharge. Optics & Spectroscopy 18, 546–547 (1965). [Google Scholar]
- [36].Boiodin V. S., and Kagan Y. M.. The investigation of a hollow cathode discharge. I. Comparison of the electrical characteristics of hollow cathode and positive column discharges. Zh. Tekhn. Fiz. (USSR) 36, 181–185 (1966). [Google Scholar]
- [37].Borodin V. S., and Kagan Y. M.. Excitation of helium lit a hollow-cathode discharge. Optics & Spectroscopy 23, 103–110 (1967). [Google Scholar]
- [38].Borodin V. S., and Kagan Y. M.. Excitation of helium in a hollow cathode discharge. II. Opt. Spektrosk. 23, 357–361 (1967). [Google Scholar]
- [39].Borodin V. S.; Kagan Y. M. and Lyagushchenko R, I, Investigation of a hollow-cathode discharge, II. Zhur, Tekh, Fiz. U, 887–889 (1967). [Enghsh translation.] [Google Scholar]
- [40].Boumans P. W. J. M. Studies of a glow discharge for spectrochemical analysis Proc. 16th Colloquium Spectroscopicum Internationale (1971), Vol. 2, 193–198, Hilger, London. [Google Scholar]
- [41].Boumans P. W. J. M. Studies of sputtering in a glow discharge for spectrochemical analysis. Anal. Chcm. 44, 1219–1228 (1972). [Google Scholar]
- [42].Boyer F., L., and Holt J. F.. Experimental comparison of conventional-hollow and coaxial-hollow cathode low-pressure arcs. AD-659 124, 21 pp. (1967). [Google Scholar]
- [43].Bradley D. J. Anomalous spectral intensity modification in a hollow-cathode discharge. Nature 194 967 (1962). [Google Scholar]
- [44].Brochard J.; Chabbal R., Chantrel H., and Jacquinot P. On the line structure of helium triplets. J. Phys. Rad. 13, 433–437 (1952). [Google Scholar]
- [45].Brody J. K. Some applications of photomultiplier tubes to spectrographic analysis. J. Opt. Soc. Am. 42, 408–415 (1952). [Google Scholar]
- [46].Brody J. K. Spectrochemical research in an atomic energy laboratory. Colloq. Spectros. Intern., 9th, Lyon, 1961, 1, 231–247 (1962). [Google Scholar]
- [47].Brody J. K. Miniature device for starting electrodeless discharge tubes. Rev. Sci. Instr. 36, 710–711 (1965). [Google Scholar]
- [48].Brody J. K.; Fred M and Tomkins F. S., Spectroscopic assay of lithium isotopes. Spectrochim. Acta 6, 383–412 (1954). [Google Scholar]
- [49].Bruce C. E. R. Transition from glow to arc discharge. Nature 161, 521–522 (1948). [Google Scholar]
- [50].Brunei A. Characteristics of the positive zone in a hollow cathode discharge obtained with a probe. Compt. Rend. Acad. Sci. (Paris) 2768,813–816 (1973). [Google Scholar]
- [51].Budick B.; Novick R. and Lurio A.. Light sources for double resonance and level crossing spectroscopy. Applied Optics 4, 229–235(1965). [Google Scholar]
- [52].Buffereau M.; Crehange G. and Poublan J, Study of the use of an electric hollow-cathode discharge as optical excitation sources in the spectrographic determination of fluorine in thorium, uranium and plutonium, Comm. Energie At, (France), Rappt. CKA-R-2436 (1964). [Google Scholar]
- [53].Büger P, A., and W. Fink. Excitation of materials in a hollow cathode. Fresenius Z. Anal, Chem, 244, 121–122 (1969). [Google Scholar]
- [54].Büger P. A., and Fink W.. Analysis of solutions in a hollow cathode. Fresenius Z Anal. Chem. 244, 314–315 (1969). [Google Scholar]
- [55].Büger P. A., and Fink W., Optical investigations in the glow discharge space of a high current hollow cathode, Z, Naturforchung 24a, 105–108 (1969). [Google Scholar]
- [56].Büger P. A.; Maierhofer J. and Reis A.. Quanlitative analysis of gaseous mixtures in a high-current hollow cathode. Frcsenius Z. Anal. Chem, 234, 176–185 (1968). [Google Scholar]
- [57].Buger P. A., and A, Reis. Iodine determination in a high-current hollow cathode Frescrtius Z. Anal. Chem. 235, 181–182 (1968). [Google Scholar]
- [58].Büger P. A., and Scheuermann W.. Measurements of excitation temperatures in the high current hollow cathode. Z. Physik 216, 248–260 (1968). [Google Scholar]
- [59].Burger J, C; Gillies W. and Yamasaki G, K, Performance characteristics of hollow cathode discharge devices for atomic absorption spectroscopy, Westinghouse Electric Corp, (Electronic Tube Div., Elmira, N.Y.), Report ETD-6403 (1964), and ETC-6603 (1966). [Google Scholar]
- [60].Burger J. C; Gillies W and Yamasaki G, K, Hollow cathode discharge devices. Chapter 12 (25 pp.) in Analytical Flame Spectroscopy. Mavrodineanu R., editor. MacMillan; (London: ) Springer; (Berlin-New York: ) 1970. [Google Scholar]
- [61].Burger J. C ; Gillies W and Yamasaki G. K.. Improvements in hollow cathode devices for atomic absorption spectroscopy 6th Annual Meeting Appl Spectry. Soc. Chicago, 3 pp., .May 1967, Available from Westinghouse Electric Co., Elmira, N.Y. 14903 as product Engineering Memo ETD-6702, (1967). [Google Scholar]
- [62].Callomon J. H. Electronic emission spectra of the carbon disulfide ion CS;. Proc. Roy. Soc. (London) A244, 220–244 (1958). [Google Scholar]
- [63].Campbell J. S. Hyperftne structure of fluorine in an arc spectrum. Z. Physik 84,393–401 (1933). [Google Scholar]
- [64].Cano R.; Mattioli M. and Zanfagna B.. Study of the plasma column in a hollow cathode arc. Comm. Energte At. (France) Rappt. CEA 2935. 40 pp. (1966). [Google Scholar]
- [65].Cartwright J, S.; Sebens C. and Slavin W.. Nickel high-brightness lamps At. Absorption Newsletter 5 22–27 (1966). [Google Scholar]
- [66].Chaika M. P. Analysis of low-volatile oxides for halogens. Opt. Spectrosk. 2, 421–425 (1957). [Google Scholar]
- [67].Chebotaev V P. The excitation of neon levels in a neon-hydrogen (hollow-cathode) discharge. Opt, Spektrosk. 20, 21–26 (1966). [Google Scholar]
- [68].Cobine J. D. Gaseous Conductors, 606 pp., Dower, New York, 1958. [Google Scholar]
- [69].Coetzer F. J., and Kessler W. Contribution to the use of hot graphite hollow cathode as a spectrochemical light source. Z. Angew. Phys. 16, 238–243 (1963). (Sec also: Coetzer, F. J., M. Sc. Thesis, 50 pp., Physical Chemistry Institute, Polytechnic, Miinchen, 1963.) [Google Scholar]
- [70].Cordos E., and Malmstadt H. V.. Characteristics of hollow cathode lamp operated m an intermittent high current mode, Anal. Chem, 45, 27–32 (1973). [Google Scholar]
- [71].Crossw’hite H. M.; Dieke G. H. and Legagneur C. S.. Hollow iron cathode discharge as source for wavelength and intensity standards. J. Opt, Soc, Ant. 45, 270–280 (1955). [Google Scholar]
- [72].Daughtrey E. H.; Donohue D. L., Slevin P. J., and W W. Harrison, Surface sputter effects in a hollow cathode discharge. Anal. Chem 47, 683–688 (1975). [Google Scholar]
- [73].Daughtrey E. H., and Harrison W. W.. The determination of boron in solution to sub-ppb concentrations by hollow cathode emission. Anal. Chim. Acta 67, 253–258 (1973) [Google Scholar]
- [74].Daughtrey E, H., and Harrison W. W.. Critical parameters affecting the hollow cathode ion source. Anal. Chem. 47, 1024–1028 (1975). [Google Scholar]
- [75].Davies D. K. An interferometric study of a high-intensity, hollow-cathode source. J. Appl. Phys. 38, 4713–4720(1967). [Google Scholar]
- [76].Dawson J. B., and Ellis D. J.. Pulsed current operation of hollow cathode lamps to increase the intensity of resonance lines for atomic absorption spectroseopy Spectrochim. Acta, Part A 23, 565–569 (1967). [Google Scholar]
- [77].DeJong G. J., and Pieptueier E, H, Time – and wavlength–resolved emission line profiles for pulsed Cu and Ag hollow cathode lamps. Spectrochim. Acta 29B, 159–177 (1974). [Google Scholar]
- [78].DeJong G. J., and Piepmeier E. H. A pulsed arc-glow dis charge hollow cathode lamp. Spectrochim. Acta 29B, 179–190 (1974). [Google Scholar]
- [79].Delcroix J, L.; Minoo H and Trinidade A, R, Establishment of a general rule for an arc discharge with hollow cathode. J. Phys, 29, 605–610(1968). [Google Scholar]
- [80].Delcroix J. L.; Minoo H. and Trinidade A. R., New functioning mode of a hollow cathode arc discharge. Compt. Rend. Acad. Sci. (Paris) 266, 762–764 (1968). [Google Scholar]
- [81].Desai S. K., and Kagan Y. M.. Electrical and optical properties of a hollow-cathode discharge in a mercury-helium mixture. Opt. Spektrosk. 27, 34–41 (1969). [Google Scholar]
- [82].Dieudonne H., and Bril J.. Participation of atoms generated at the cathode to the functioning of a glow discharge Proc. 16th Colloq. Spectroscop. Internat. (1971) Vol. 2, 199–202, Hilger, London. [Google Scholar]
- [83].Dinnin J. I., and Helz A. W., Demountable hot hollow cathode lamp as excitation source in atomic fluorescence flame spectrometry. Anal. Chem. 39, 1489–1491 (1967). [Google Scholar]
- [84].Dobrosavljevic J. S., and Marinkovic M., Study of some excitation characteristics of the discharge in a hot hollow cathode, Spectrochim, Acta 29B, 87–92 (1974). [Google Scholar]
- [85].Dogan M.; Laqua K. and Massmann H.. Spectrochemical analysis with a glow discharge lamp as a light source. I. Electrical properties, disintegration of sample and spectral nature, Spectrochim. Acta 26B, 631–649 (1971). [Google Scholar]
- [86].Dogan M.; Laqua K. and Massmann H. Spectrochemical analysis with a glow discharge lamp as a light source. II. Analytical applications. Spectrochim, Acta 27B, 65–88 (1972). [Google Scholar]
- [87].Donin V. I. Concentration of excited neon atoms in a heliumneon mixture in a hollow-cathode discharge. Zh. Prikl. Spektrosk. 5, 724–729 (1966). [Google Scholar]
- [88].Dontsov Y. P. Isotope shift in the spectrum of molybdenum. Optics and Spectrosc. 8, 236–239 (1960). [Google Scholar]
- [89].Druyvesteyn M. J., and Penning F. M.. The mechanism of electrical discharges in gases at low pressure. Rev. Modern Physics 12, 87–174 (1940) [Google Scholar]
- [90].Dusek J. T. Development and fabrication of a boron absorption tuhe for spectrographic analysis. AEC Accession No. 35858. Rept. No. ANL-7I64, 8 pp. (1966).
- [91].Dushman S. Search for high-efficiency light sources. J. Opt. Soc. Am. 27, 1–24 (1937). [Google Scholar]
- [92].Eichholl H. J., and Voigt R.. Use of a metallic hollow cathode for spectrochemical analyses. Proc. Colloq. Spectros. Intern. 9th, Lyon, 1961, 3, 309–317 (1962). [Google Scholar]
- [93].El Alfy S,: Laqua K. and Massmann H.. Spectrochemical analysis with a glow discharge lamp as a light source. III. Development and description of a universal method for determining the primary components in electrically non-conducting powdered substances. Fresenius Z. Anal. Chem. 263, 1–14 (1973). [Google Scholar]
- [94].von Engel. A.. Glow discharge in Ionized Gases, 2nd edition, p. 217–317. Clarendon Press, Oxford, 1965. [Google Scholar]
- [95].von Engel, A.; Seeliger A. and Steenbeck M. Z.. On the glow discharges at high pressures. Z. Physik 85, 144–160 (1933). [Google Scholar]
- [96].von Engel. A., and Steenbeck M.. Electrical Gas Discharges (in German). 2nd edition (Glow discharges, pp 57–119) Springer, Berlin: (1934). [Google Scholar]
- [97].Erdey L.; Gegus E. and Kocsis E.. Spectroscopic determination of magnesium, zinc, vanadium, and chromium in pure aluminum with use of a hollow electrode. Acta Chim. Acad. Sci. Hung. 11, 277–294 (1957). [Google Scholar]
- [98].Falk H. Spectroscopic determination of halogens and arsenic in glass by using discharge by a hollow cathode. Spectrochim. Acta 21, 423–426 (1965). [Google Scholar]
- [99].Falk H. Optical excitation in a hollow cathode by a negative glow-discharge light. Ann. Physik. 16, 160–173 (1965). [Google Scholar]
- [100].Falk H. A hollow cathode lamp with separated evaporation and excitation spaces as source of excitation in analytical emission spectroscopy. Proc. 14th Colloq. Spectres. Intern., 653–661 (1967). [Google Scholar]
- [101].Fan H. Y. The transition from glow discharge to arc. Phys. Rev. 55, 769–775 (1939). [Google Scholar]
- [102].Fisher R. A., and Fray A. S. A hollow cathode source for the Zeeman effect. Phys. Rev. 56, 675–677 (1939). [Google Scholar]
- [103].Fisher R. A.; Fray A. S. and Platt J. R.. The hollow cathode discharge as a source for Zeeman effect. Phys. Rev 53, 934 (1938). [Google Scholar]
- [104].Fowles G. R. Hyperfine structure and nuclear spins of tungsten and tellurium. Phys. Rev. 78, 744–747 (1950). [Google Scholar]
- [105].Francis G. The glow discharge at low pressure in vol. 22, pp. 53–208 Handbuch derPhysik; Flugge S., ed. Springer; Berlin: (1956). [Google Scholar]
- [106].Frank C. W.; Schrenk W. G. and Meloan C. E.. Feasibility of the iron hollow cathode as a multi-element atomic absorption unit. Anal. Chem. 38, 1005–1008 (1966). [Google Scholar]
- [107].Franklin R., and Steele G. R.. Automatic high-precision uranium isotopic analysis hy emission spectroscopy. Proc. XII Coll. Spect. Int., Exeter, 1965, 498–504. [Google Scholar]
- [108].Frerichs R. Collision of second kind, excitation and recombinations in the glow discharge. Ann. Physik 4, 362–380 (1928). [Google Scholar]
- [109].Galassi M. Atomic absorption sources Flame Notes, Beckman; 1, 10–13 (1966). [Google Scholar]
- [110].Galassi M., and Hell A.. Evaluation of hollow-cathode lamps. Flame Notes. Beckman 1, 28–32 (1966). [Google Scholar]
- [111].Gambling W. A., and Edels H.. The high-pressure glow discharge in air. British J. Appl. Phys. 5, 36–39 (1954). [Google Scholar]
- [112].Gartlein C. W., and Gibhs R. C.. Production of second and third spark spectra in a hollow cathode lamp. Phys. Rev. 38, 1907–1908 (1931). [Google Scholar]
- [113].Gavrilov T. F. Spectral method of isotopic analysis of lithium. Optics and Spectres. 7, 185–187 (1959). [Google Scholar]
- [114].Gelder Z. van. New high-intensity spectral source with a narrow line profile. Appl. Spectrosc. 22, 581–582 (1968). [Google Scholar]
- [115].Gerry E. T., and Rose D. J.. Combined anode-cathode feed of a hollow-cathode arc. J. Appl. Phys. 37, 2725–2726 (1966). [Google Scholar]
- [116].Gillieson A. H., and Birks T. F.. Application of a hollow cathode source for analysis. Congr. Groupe Avance. Method. Anal. Spectrograph. Products Met. 14, 155–173 (1951). [Google Scholar]
- [117].Glad S. The spectrum of singly-ionized carbon, CII. Arkiv Fysik 7, 7–8 (1952). [Google Scholar]
- [118].Glad S. Extension of the analysis of the third spectrum of iron, Felll. Arkiv Fysik 10, 291–294 (1955). [Google Scholar]
- [119].Gofmcistcr V. P., and Kagan Y. M.. Electrical characteristics of a discharge in a hollow cathode in neon. Rev. Roum. Phys. 13, 19–24 (1968). [Google Scholar]
- [120].Gofmeister V. P., and Kagan Y. M.. On the mechanism of excitation in a hollow cathode in neon. Optics and Spectroscopy 25, 185–187 (1968). [Google Scholar]
- [121].Gofmeister V. P., and Kagan Y. M.. Mechanism of excitation in a hollow cathode in argon. Optics and Spectroscopy 26, 379–382 (1969). [Google Scholar]
- [122].Goleb J. A. Application of hollow-cathode discharge tubes to spectrographic analysis. Anal. Instrum. 1965, 229–238 (1966). [Google Scholar]
- [123].Goleb J. A., and Brody J. K.. The analysis of uranium alloys using a hollow cathode. Appl. Spectrosc. 15, 166–170 (1961) [Google Scholar]
- [124].Goleb J. A., and Brody J. K.. Atomic absorption studies using a hollow cathode tube as an absorbing source. Anal Chim. Acta 28, 457–466 (1963). [Google Scholar]
- [125].Goodfellow G. I. Simple interchangeable hollow-cathode lamp for use in atomic-absorption spectrometry. Appl. Spectrosc. 21, 39–42 (1967). [Google Scholar]
- [126].Gordon N. E. Jr., and Cook H. D.. A hollow cathode discharge tube and power supply for routine analysis. Spectrochim. Acta 5, 505 (1953). [Google Scholar]
- [127].Gordon N. E. Jr., and Cook H. D.. A hollow cathode discharge tube and high voltage power supply for routine spectrochemical analysis. Westinghouse Electric Corp. Rept. No. WAPD-T-29, 15 pp. (1953). Nncl. Sci. Ahstr. 11, 11825 (1957).
- [128].Grimm W. Discharge lamp for routine speetrographic analysis. Naturwissenschaften 54, 586 (1967). [DOI] [PubMed] [Google Scholar]
- [129].Grimm W. New glow discharge lamp for optical emission spectra analysis. Spectrochim. Acta, Part B 23, 443454 (1968). [Google Scholar]
- [130].Grimm W. Gas in metals determined by a spectroscopic method Proc. 16th Colloq. Spectroscop. Internat., Vol. 2, 210–212, Hilger, London: (1971). [Google Scholar]
- [131].Gromov V. A. The mechanism of the discharge in a hollow cathode. Opt. Spektrosk. 1, 334–337 (1956). [Google Scholar]
- [132].Gromov V. A., and Ershov A. G.. Distribution of current density in a hollow cathode. Fiz. Sbornik Lvovsk. Univ. 4, 80–83 (1958). [Google Scholar]
- [133].Günther-Schulze A. A new characteristic position in the glow discharge. Z. Physik 30, 175–186 (1924). [Google Scholar]
- [134].Günther-Schulze A. Glow discharge in a hollow cathode. Z. Techn. Physik 1930 (2), 49–54 (1930). [Google Scholar]
- [135].Günther-Schulze A. Electron velocity in insulators at high field strength and its contribution to the theory of electrical discharge. Z. Physik 36, 778–786 (1933). [Google Scholar]
- [136].Günther-Schulze A. Cathodic sputtering and analysis of the physical processes. Vacuum 3, 360–374 (1953). [Google Scholar]
- [137].Günther-Schulze A.; Bar W. and Betz H.. Anode layer and its relation to the phenomena in the positive zone in hydrogen and nitrogen. Z. Physik 109, 293–309 (1938). [Google Scholar]
- [138].Harris C. I., and Mitchell G. P.. Literature survey on the use of the hollow cathode discharge in analytical chemistry. Report No. MR 960, Eindhoven Contract No. 122/0084, 8 pp. (1963).
- [139].Harrison W. W., and Caufield K.. Line sources in absorption spectroscopy. Anal. Chim. Acta 39, 161–166 (1967). [Google Scholar]
- [140].Harrison W. W., and Daughtrey E. H.. Determination of traces of gold by hollow cathode emission. Anal. Chim. Acta 65, 35–40 (1973). [Google Scholar]
- [141].Harrison W. W., and Magee C. W.. Hollow cathode ion source for solids mass spectrometry. Anal. Chem. 46, 461–464 (1974). [Google Scholar]
- [142].Harrison W. W., and Prakash N. J.. Trace element analysis of solutions by hollow cathode excitation. Anal. Chim. Acta 49, 151–159 (1970). [DOI] [PubMed] [Google Scholar]
- [143].Heneage P. Five-element lamp. At. Absorption Newsletter 5, 67 (1966). [Google Scholar]
- [144].Humov E., and Hofmann F. W.. Measurement of absolute radiation intensities in the vacuum-ultraviolet region, J. Opt. Soc. Am. 53, 1259–1265 (1963). [Google Scholar]
- [145].Hirschberg J. G.; Hinnov E. and Hofmann F. W., Spectroscopic investigations of a weakly ionized plasma in a helium hollow cathode discharge. Proc. 6th Conf. Intern. on Ionization Phenomena in Gases. Vol. 2, 359–362 (1963), P. Hubert and E. Cremieu. Alcan. [Google Scholar]
- [146].Human H. G. C., The combined hollow cathode and high frequency discharge as excitation source for atomic fluorescence spectrometry. Spectrochim. Acta 27B, 301–307 (1972). [Google Scholar]
- [147].Human H. G. C., and Butler L. R. P.. High frequency excitation of vapors produced by hollow cathode sputtering, Spectrochim, Acta 25B, 647–656 (1970). [Google Scholar]
- [148].Human H. G. C.; Zeegers P. J. T. and van Elst J. A.. Experimental characteristics of direct current and high frequency boosted hollow cathode lamps. Spectrochim. Acta 29B, 111–119 (1974). [Google Scholar]
- [149].Ivanov N. P Analytical possibilities of a gas discharge tube with a double hollow cathode. Zh. Analit. Khim. 17, 126–128 (1962). [Google Scholar]
- [150].Ivanov N. P., and Andrikanis E. N.. On the analytical use of a gas discharge tube having a double hollow cathode. Zavodsk Lab. 29, 1002–1005 (1963). [Google Scholar]
- [151].Ivanov N. P., and Andrikanis Z. N.. Determination of contaminants in titanium and its compounds, Metody Analiza Khim. Reaktivov i Preparatov, Gos. Kom. Sov. Min. SSSR po Khim. No. 7, 73–76 (1963). [Google Scholar]
- [152].Ivanov N. P., and Gusinskii M. N.. Atomic absorption spectrophotometry with a hollow cathode discharge tube as atomizer. Tr., Vses. Nauch.-Issled. Inst. Khim. Reaktivov Osobo Chist. Khim. Vcshchestu No. 28, 348–359 (1966). [Google Scholar]
- [153].Ivanov N. P.; Nedler V. V. and Andrikanis E. N.. Hot hollow cathode in the analysis of titanium dioxide, Zavodsk. Lab. 27, 836–838 (1961). [Google Scholar]
- [154].Jäeger H. The use of a glow discharge lamp as a light source in spectrometric analysis of gold. Anal. Chim. Acta 58, 57–64 (1972). [Google Scholar]
- [155].Jäeger H. Spectrometric determination of the fineness of gold. Anal. Chim. Acta 60, 303–308 (1972). [Google Scholar]
- [156].Jäeger H., and Blum F.. Some observations on sample sput tering in a glow discharge, Spectrochim. Acta 29B, 73–77 (1974). [Google Scholar]
- [157].Jäeger H., and L., Butler R. P.. The glow discharge source applied to the analysis of gold Proc. 16th Colloq Spectroscop. Internat., Vol. 2, 204–209, Hilgcr, London: (1971). [Google Scholar]
- [158].Jennings W. C.; Noon J. H., Holt E. H., and Buser R. G.. Comparison of hollow cathode and conventional argon ion lasers. Rev. Sci. Instr, 41, 322–326 (1970). [Google Scholar]
- [159].Johnson J. D. Modern Emission Spectroscopy Short Courses. Spectrochemical analysis with low pressure discharges. Glow discharges and demountable hollow cathodes Soc. Applied Spectroscopy Meeting, First Annual Meerlng-FACSS, Atlantic City, N.J. (1974). [Google Scholar]
- [160].Jones W. G., and Walsh A., Hollow-cathode discharges the construction and characteristics of sealed-off tubes for use as spectroscopic light sources. Spectrochim. Acta 16, 249–254 (1960). [Google Scholar]
- [161].Kaminsky M. Atomic and Ionic Impact Phenomena on Metal Surfaces. Springer, Berlin, and Academic Press, New York: (1965). [Google Scholar]
- [162].Kartashev V. G. The deionization of a plasma in a hollow cathode. Radio Engng. Electronic Phys. 11, 491–493 (1966). [Google Scholar]
- [163].Karyakin A. V.; Zakharov E. A. and Krasil’shchik V. Z., Possihle use of a hollow cathode with separated evaporation and excitation regions for the determination of small amounts of halogens in rocks Zh. Anal. Khim. 23, 1418–1420 (1968) [Google Scholar]
- [164].Kashtan M. S.; Sobotovich E. V. and Khlopina T. N.. Enhancement of sensitivity in the spectral isotope analysis of lead. Optics and Spectrosc. 8, 11–13 (1960). [Google Scholar]
- [165].Katskov D. A.; Lebedev G. G. and L’vov B, V.. Spectral characteristics of impulse lamps with hollow cathodes for atomic-absorption measurements. Zh. Prikl. Spektrosk. 10, 215–219 (1969). [Google Scholar]
- [166].Kielkopf J. F. The spectrum of triply ionized gadolinium. Thesis, The Johns Hopkins University (1969). [Google Scholar]
- [167].Kielkopf J. F. Pulsed hollow cathode light source. Spectiochim. Acta 26B, 371–390 (1971). [Google Scholar]
- [168].Kirchhof H. Determination of the isotope ratios in lead samples by atomic absorption. Spectrochim. Acta, Part B24, 235–241 (1969). [Google Scholar]
- [169].Klyarfeld B. N.; Guseva L., G. and Pokrovskaya-Soboleva A. S.. Glow discharge at low pressures and current densities up to 0,1 A/cm2. Soviet Physics. Tcchn. Phys, 11, 520–527 (1966). [Google Scholar]
- [170].Knerr G.; Maierhofer J. and Reis A.. Application of high-current hollow cathode for quantitative analysis of conductors and glasses. Fresenius’ Z. Anal. Chem. 229, 241–255 (1967). [Google Scholar]
- [171].Konovalov V. A., and Frish S. E.. Illumination of the mixture of argon and nitrogen. J. Techn. Phys. (USSR) 4, 523–533 (1934). [Google Scholar]
- [172].Kopfermann H.; Krüger H. and Öhlmann H.. The abnormal fine structure of helium ion line. Z. Physik 126, 760–768 (1949). [Google Scholar]
- [173].Korostyleva I., Investigation A. of the plutonium spectrum under different conditions of excitation in a hollow cathode. Opt. Spectrosk, 17, 469–474 (1964). [Google Scholar]
- [174].Korostyleva L. A., and Striganova G. A.. Isotope shift in the uranium spectrum. Opt. and Spectrosc. 7, 89–90 (1959). [Google Scholar]
- 1175].Korovin Y I. Increasing the sensitivity of determinations by means of discharge in a hollow cathode. Zhur. Anal. Khim. 16, 494–495 (1961). [Google Scholar]
- [176].Korovin Y. I. Spectral determination of chlorine and fluorine in metallic beryllium using discharge in a hollow cathode, Zavodsk. Lab. 31, 45–49 (1965). [Google Scholar]
- [177].Korovin Y. I., and V Kipis L.. The use of a discharge in a hollow cathode to determine impurities in zirconium oxide. Opt. Spektrosk. 5, 334–337 (1958). [Google Scholar]
- [178].Krasilshehik V. Z. Device for working with a hollow cathode. Zavodsk. Lab. 31, 251 (1965). [Google Scholar]
- [179].Krempl H.; Maierhofer J. and Meinel H.. Explanation of rotation-translation equilibrium by temperature measurements in negative glow-discharge light of hollow cathode. Z. Angew. Phys. 22, 171–174 (1967). [Google Scholar]
- [180].Krcye W. C. Interferometric analyses of neon II and argon II spectral lines from a hollow cathode discharge. J. Opt. Soc. Am. 64, 186–196(1974). [Google Scholar]
- [181].Kreye W. €., and Roesler F. L.. Analysis of hollow-cathode-discharge-excited Ar I, Ar II, and Au I spectral line profiles measured with a Fabry-Perol interferometer. J. Opt. Soc. Am. 60, 1100–1108 (1970). [Google Scholar]
- [182].Lee T.; Katz S. and MacIntyre S, A.. The spectrographic determination of uranium 735. V. Routine application of a multiple hollow cathode source assembly and a direct-reading, Littrow grating spectrograph. Appl. Spectry. 16, ‘ 92 96 (1962). [Google Scholar]
- [183].Lee T; Killeen O. P. and MacIntyre S. A.. Spectrographic determination of uranium-235, IV. Using a direct reading, Littrow grating spectrograph, and a hollow cathode. Appl. Spectry. 15, 106–109 (1961). [Google Scholar]
- [184].Lee T., and MacIntyre S. A.. Uranium 235–238 assay on the direct reading optical spectrograph. U.S. At. Energy Comm. T1D. 7531. 30–35 (1957). [Google Scholar]
- [185].Lee T. and MacIntyre S. A.. The spectrographic determination of uranium 235. Part 3 Use of a multiple hollow cathode assembly and a 22 foot direct reading Eagle spectrograph. Appl. Spectry 15, 34–39 (1961). [Google Scholar]
- [186].Lee T., and Rogers L. H.. The spectrographic determination of uranium 235. Part 2. Using a direct reading attachment and a hollow cathode source Appl. Spectry. 15, 3–6 (1961). [Google Scholar]
- [187].Lidsky I., M.; Rothleder S. D.. Rose D. J., Yoshikawa S., Michelson C, and Mackin R. I Jr Highly ionized hollow cathode discharge. J. Appl. Phys. 33, 2490–2497 (1962). [Google Scholar]
- [188].Little P. F., and A. v. Engel. The hollow-cathode effect and the theory of glow discharges. Proc. Roy. Soc. (London) A224, 209–227 (1954). [Google Scholar]
- [189].Llewellyn-Jones F. The Glow Discharge. Methuen, London: (1966). [Google Scholar]
- [190].Lloyd P. D., and Lowe R. M.. On grating techniques for selective modulation of resonance lines from hollow cathode lamps. Spcetrochim Acta 27B, 23–26 (1972). [Google Scholar]
- [191].Lompe A Contribution to the elucidation of the working mechanism of hollow cathodes. Z Physik. 109, 310–311 (1938). [Google Scholar]
- [192].von Lompe, A..; Seeliger R. and Welter E. Investigations on hollow cathodes. Ann. Physik 36 (5), 9–37 (1939). [Google Scholar]
- [193].Lowe R. M The selective modulation of resonance lines from a hollow cathode spectral lamp Spectrochim. Acta 24B, 191–193 (1969). [Google Scholar]
- [194].MacNally J. R.; Harrison G R. and Rowe E.. A hollow cathode source applicable to spectrographic analysis. J Opt. Soc. Am. 37, 93–98 (1947). [DOI] [PubMed] [Google Scholar]
- [195].Mahiett J. M., and Hccart M.. Study of the second UV system of the AlO molecule using a hollow cathode in pulsed current operating mode. Canadian Spectroscopy 13, 95–98 (1968). [Google Scholar]
- [196].Maierhofcr J.; Rcis A. and Setz G.. New high-current metal hollow cathode for Spectrochemical analysis. Z. Instrumentenk. 74, 165–167 (1966). [Google Scholar]
- [197].Maksimov D. E. and Rudnevskii N. K.. Spectral determination of As in Si using a hollow cathode discharge Materialy Ural’sk. Soveshch. po Spektroskopii, 4th, Sverd-lovsk; 1963, 107–108 (1965). [Google Scholar]
- [198].Maksimov D. F . and Rudnevskii N. K.. Spectral determination of iodine in germanium with hollow cathode discharge. Polueh Anal Veshchestv. Osoboi. Chist. Mater. Vses. Konf. Gorky, USSR 1963, 136–138 (1966). [Google Scholar]
- [199].Mandelstam S. L., and Nedler V V.. On the sensitivity of emission Spectrochemical analysis. Spectrochim. Acta 17, 885–894 (1961). [Google Scholar]
- [200].Manning D.C.; Trent D. and Vollmer J. Dual-element Mg-Ca hollow-cathode lamp. At. Absorption Newsletter 4, 234–236 (1965). [Google Scholar]
- [201].Manning D C; Vollmer J. and Fernandez F.. Shielded bismuth hollow cathode lamps. At. Absorption Newsletter 6, 17–18 (1967). [Google Scholar]
- [202].Mattson J. E. Light sources and filter for use in the 130–280Å region. Applied Optics 12, 1394–1396 (1973). [DOI] [PubMed] [Google Scholar]
- [203].Massmann H. Detection limits in spectrochcmical analysis of volatilizable substances in the hollow cathode. Colloq. Speclros. Intern., 9th, Lyon, 1961. 2, 170–182 (1962). [Google Scholar]
- [204].Massmann H. Hollow cathodes for constant intensity ratios of spectra of different elements. Z. lnstrumentenk. 71, 225–229 (1963). [Google Scholar]
- [205].Matić J. S., and Peŝić D. S.. Spectrographic analysis of molybdenum by using a discharge tube with a hollow cathode. Rev. Roumaine Chim. 10 733–739 (1965). [Google Scholar]
- [206].Matić J S., and Peŝić D. S. Spectrographic determination of trace lithium in some refractory oxides by a hollow-cathode discharge tube. Appl. Spectrosc. 22, 63–65 (1968). [Google Scholar]
- [207].Mavrodineanu R Hollow cathode discharge tubes Bibliography on Flame Spectroscopy, National Bureau of Standards Misc. Publ. 281, 140–143 (1967). [Google Scholar]
- [208].Mavrodineanu R. Hollow cathode discharge tubes. Bibliography on Flame Spectroscopy. Chapter 13 in Analytical Flame Spectroscopy, Mavrodineanu R.. editor. MacMillan, London, Springer, New York: (1970) [Google Scholar]
- [209].Mehmet Dogan H. Spectrochcmical analysis with a glow discharge lamp as excitation source. Thesis, Ruhr-University, 90 pp. (1970). [Google Scholar]
- [210].Melamed I. Spectrographic determination of trace amounts of halides. Comm. Energie At. (France), Rcpt. No. 1999, 52 pp. (1961). [Google Scholar]
- [211].Metz N. Development of a hollow cathode for the emission spectral analysis of trace elements in conductive and non-conduetive materials. Ph D. Thesis 116 pp., Technical University München, Germany: (1971). [Google Scholar]
- [212].Metz N., and Maierhofer J.. Experience with a hollow cathode lamp for spectral analyses Proe. 16th Colloq. Spectroscop. Internat., Vol. 2, 227–733, Hilger, London: (1971). [Google Scholar]
- [213].Mierdel G. The glow discharge Handbuch der Experimentalphysik, Vol. 13 313–481, Wien W and Harms F. editors. Akademische Verlagsgesellschaft, Leipzig: 1929. [Google Scholar]
- [214].Milazzo G. Spectrochemical analysis of non-metals in the vacuum ultraviolet hy means of a hollow cathode light source. AD-431,066, 48 pp. (1963).
- [215].Milazzo G. Versatile hollow-cathode light source for spectrochemieal analysis in the vacuum ultraviolet. Appl. Spectrosc. 21, 185–187 (1967). [Google Scholar]
- [216].Milazzo G. Spectrochemical analysis of nonmetals with hollow-cathode light source. U.S. Clearinghouse Fed. Sei. Tech. Inform., AD-821776, 24 pp. (1967). [Google Scholar]
- [217].Milazzo G. Spectrochemical analysis of non-metals with hollow cathode light source. Rept. Rome University, Instituto di Chintica; (Italy: ), 24 pp., DA 91–591-EUC-4052, (1967). [Google Scholar]
- [218].Milazzo G., and Caroli S.. Comparison between the hollow cathode and spark light sources. Chemia Analitycznia 17, 891–897 (1972). [Google Scholar]
- [219].Milazzo G., and Sopranzi M.. Spectrochemical analysis of nonmetals in the vacuum ultraviolet hy means of a hollow cathode light source. NASA Accession No. N65–20930, 32 pp. (1965). [Google Scholar]
- [220].Milazzo G., and Sopranzi M.. Spectrochemical analysis in the vacuum ultraviolet with the hollow-cathode light source. I. Qualitative analysis. Appl. Spectrosc. 21, 172–175 (1967). [Google Scholar]
- [221].Milazzo G., and Sopranzi M.. Spectrochemical analysis in the vacuum ultraviolet with the hollow-cathode light source. II. Quantitative analysis. Appl. Spectrosc. 21, 256–260 (1967). [Google Scholar]
- [222].Minoo H. Gas pressure and electron densities in the active zone of a hollow cathode. Comp. Rend. Acad. Sci (Paris) 272B, 314–317 (1971). [Google Scholar]
- [223].Mitchell G. P., and Harris C. I.. Analytical applications of a hollow-cathode source. Proc. Soe. Anal. Chem. (London) 2, 105–106 (1965). [Google Scholar]
- [224].Mitchell K. B. Spectroscopic studies of ionization in a hollow cathode discharge. J. Opt. Soc. Am. 51, 846–853 (1961). [Google Scholar]
- [225].Mitchell K. B., and D W. Steinhaus. A promising method of identification or spectra using a hollow-cathode discharge. J. Opt. Soc. Am. 47, 118 (1957). [Google Scholar]
- [226].Moal J. Y., and Brossier G.. The Grimm discharge applied to the analysis of natural materials Proc. 16th Colloq. Spectroscop. Internat., Vol. 2, 219–226, Hilger; London: (1971). [Google Scholar]
- [227].Monfils A.: Ottelet I. and Rosen B.. The hollow cathode in spectroanalysis. Ind. Chim. Beige 16, 675–676 (1951). [Google Scholar]
- [228].Monfils A., and Rosen B.. Spectroscopic determination of traces of nitrogen in argon. Rev. Universclle Mines 6 79–81 (1950). [Google Scholar]
- [229].Morse D. L. Plasma rotation in a hollow cathode discharge. Physics of Fluids 8, 517–521 (1965) [Google Scholar]
- [230].Muntenburch H. On Schüler’s hollow cathode as light source for Stark effect investigation. Spectrochim. Acta 16, 1031–1039 (1960). [Google Scholar]
- [231].Murav’ev I. I.; Soldatov A. N, Klimkin V. M., and Yancharina A. M.. Discharge conditions in a hollow cathode for obtaining generation at λ=1.15μ of neon. Izv. Vyssh. Uclieb. Zaved., Fiz. 11, 125–127 (1968). [Google Scholar]
- [232].Musha T. Cathode sputtering in a hollow cathode discharge. J. Pliys. Soc. (Japan) 17 1440–1446(1962). [Google Scholar]
- [233].Musha T. Theory of negative resistance in hollow cathode discharges. J. Phys. Soc. (Japan) 17, 1447–1453 (1962). [Google Scholar]
- [234].Muzgin V. N.: Zolotavin V. L., Gavrilov F. F., and Ulybyshcva L. V.. Use of a discharge tube with a hollow cathode in spectral analysis. Tr. Vses. Nauchn.-Issled. Inst. Standartn. Ohraztsov i Spektral’n. Etalonov 1, 127–133 (1964). [Google Scholar]
- [235].von Naude-Meiring, S.. The quartet structure of the first spark spectrum of mercury 2. Ann. Physik 3, 1–26 (1929). [Google Scholar]
- [236].Newhound K. B., and Fish F H.. Spectroscopic study of small samples in a hollow cathode discharge. Can. J. Phys. 29, 357–361 (1951). [Google Scholar]
- [237].Newburg R. G.; Heroux L. and Hinteregger H. E.. Two light sources for use in the extreme ultravolet. Applied Optics 1, 733–737 (1962). [Google Scholar]
- [238].Novoselov V. A., and Aidarov T. K.. Spectrographic determination of the trace elements Ag, Cu, Pb, Bi, Cd. and A1 in solutions by using a hollow cathode source. Tr. po Khim. i Khim. Technol. 1964, 108–109 (1965). [Google Scholar]
- [239].Novoselov V. A., and Aidarov T. K.. Study of gaseous discharge in a hollow cathode and its application in spectral analysis Materialy Ural’sk. Soveshchn. po Spektroskopii, 4th Sverdlovsk; 1963, 104–106 (publ. 1965). [Google Scholar]
- [240].Oganezov K. A.; Shvangiradze R. R. and Chikhladze Y.. Temperature regime for hot hollow cathodes . Zh. Prikl. Spektrosk. 6 813–815 (1967). [Google Scholar]
- [241].Oganezov K. A.; Chikhladze Y. and Shvangiradze R. R.. Spectral-isotopic method for analyzing gases in solids using a hollow cathode. Izv. Sih. Otd. Akad. Nauk SSSR, Ser. Khim. Nauk No. 4, 141–144 (1967). [Google Scholar]
- [242].Olbers W Stark effect in the sodium arc spectrum. Ann. Physik 5 708–722 (1938). [Google Scholar]
- [243].Ormrod J. H. Test particles in an argon plasma. Canadian Atomic Energy Comm. Rept. AECL-2669, 38 pp. (1967).
- [244].Ostroumenko P. P. Some pcrculiarities of the excitation of the spectral lines of copper in a hollow-cathode discharge. Zh. Prikl. Spektrosk. 5, 581–585 (1966). [Google Scholar]
- [245].Ostroumenko P. P., and Rossikliin V. S.. The temperature of a hollow electrode discharge. Izv. Vysshikh Uchehn. Za-vedenii, Fiz. 8, 17–22 (1965). [Google Scholar]
- [246].Ostroumenko P. P.; Rossikliin V. S. and Tsikora I. L.. Spectroscopic investigation of the formation mechanism of C; in various types of discharges in a CO; atmosphere. Zh. Prikl. Spektroskopii, Akad. Nauk Belorussk. SSR 3, 109–113 (1965). [Google Scholar]
- [247].Pacheva I. Excitation of spectra of barium, cadmium, and copper in a discharge tube with a hollow cathode. Tr. Komis. po Spektroskopii, Akad. Nauk SSSR 2, 229–239 (1964). [Google Scholar]
- [248].Pacheva I. Excitation of barium, copper, und cadmium spectra in a hollow-cathode discharge tube. Bull. Inst. Physique, Sofia 15, 177–184 (1966). [Google Scholar]
- [249].Pacheva I. Excitation of the spectra of barium, cadmium, and copper in a gas-discharge tube with a hollow cathode. Izv. Fiz Inst. Aneb, Bulg. Akad. Nauk 15, 177–184 (1966). [Google Scholar]
- [250].Pacheva I., and Naidenov M.. Spectroscopic investigation of the discharge in a hollow cathode. Izv. Fiz. Inst. ANEB, Bulg. Akad. Nauk 16, 129–133 (1967). [Google Scholar]
- [251].Pahl M., and Kleinmann W.. On the energy homogeneity of ion currents in the glow discharge in a hollow cathode. Ann. Physik 6, 165–177 (1953). [Google Scholar]
- [252].Papoular R. The glow discharge Electrical Phenomena in Gases, 123–140, Iliffe Books, London: (1965). [Google Scholar]
- [253].Paschen F. Bohr’s helium lines. Ann. Physik 50, 901–940 [1916]. [Google Scholar]
- [254].Paschen F. Spark spectrum of aluminum-Part 1. Ann. Physik 71, 142–161 (1923). [Google Scholar]
- [255].Paschen F. The spark spectrum of aluminum-Part 3. Ann. Physik 71 537–561 (1923). [Google Scholar]
- [256].Paschen F., and Ritschl R.. Infrared grating spectrum and spectral laws. Ann. Physik 5, 867–892 (1933). [Google Scholar]
- [257].Penning F. M. Electrical Discharges in Gases. Philips Technical Library, 78 pp., Eindhoven: (1957); Servire BV; Katwijk aan Zee, Netherlands Puhl. [Google Scholar]
- [258].Perry H. F.; Sia R. M. and Burnett C. R.. Helium level populations in a hollow cathode plasma. J. Opt. Soc. Am. 52, 592 (1962). [Google Scholar]
- [259].Pevtsov G. A., and Krasil’shchik V. Z.. Spectral determination of the constituents of chemical concentrates pre-treated on carbon powder as collector. Mctody Analiza Khim. Reaktivov i Preparatov, Gos. Korn. Sov. Min. SSSR po Khim. No. 7, 69–72 (1963). [Google Scholar]
- [260].Pevtsov G. A., and Krasil’shchik V. Z.. The determination of impurities in beryllium oxide by a spectrophotographic method by using a hollow cathode. Zh. Analit. Khim. 19, 1106–1109 (1964). [Google Scholar]
- [261].Pevtsov G. A., and Krasil’shchik V. Z., Separation of evaporation and excitation zones in spectrographic analysis with a hollow cathode. Zh. Analit. Khim. 21, 863–864 (1966). [Google Scholar]
- [262].Pevtsov G. A., and Krasil’shchik V. Z.. Effect of chemical form of trace impurities and third components on the results of spectral analysis with use of a hollow cathode. Tr., Vses. Nauch.-Issled. Inst. Khim. Reaktivov Osobo Chist. Khim. Veshchestv. 29 23–30 (1966). [Google Scholar]
- [263].Pevtsov G. A., and Krasil’shchik V. Z.. New variations of a method for separating the regions of vaporization and excitation in a hollow cathode. Zh. Prikl. Spektrosk. 9, 504–505 (1968). [Google Scholar]
- [264].Pevtsov G. A.; Krasil’schchik V. Z. and Lavkina A. F.. Features of the discharge in a hollow cathode with a membrane separating the region of excitation and evaporation, Zh, Prikl. Spektrosk. 7, 545–549 (1967). [Google Scholar]
- [265].Pevtsov G. A.; Krasil’shchik V. Z and Yakovleva A, F. Analysis of solutions by the dry residue method in a hollow ‘ cathode. Zh. Anal. Khim. 23, 1785–1789 (1968). [Google Scholar]
- [266].Pevtsov G. A.; Krasil’shchik V. Z. and Yakovleva A, F.. Use of chlorination reactions in a hollow cathode for the determination of difficultly volatile elements, Zh. Anal. Khim, 24 234–236 (1969). [Google Scholar]
- [267].Pevtsov G. A.: Manova T. G. and Krasil’shchik V, Z, Spectral determination of trace impurities in alkali and alkaline earth metal salts hy using a gas-discharge tuhe with a hollow cathode. Tr., Vses, Nauch.-lssled. Inst. Khim. Re- aktivov Osobo Chist. Khim, Veshchestv No. 30, 186–201 (1967). [Google Scholar]
- [268].Popham R. E. Studies in flame spectroscopy. A hollow cathode for atomic absorption spectroscopy. Atomic ahsorption characteristics of germanium, gallium, and indium. Effects of phosphate, sulfate, and aluminum on flame spectroscopic determination of alkaline earth metals, calcium in particular, (Kansas State Univ., Manhattan, Kans.) Diss. Abstr B 1968, 29 (6) (1952–3). [Google Scholar]
- [269].Popham R. E., and Schrenk W. G.. Simple demountable hollow cathode for atomic absorption spectroscopy, Appl. Spectrosc. 22, 192–194 (1968). [Google Scholar]
- [270].Popov L. V. Influence of meta.stahle atoms of Hg on the luminescence of Ca in a discharge tube with hollow cathode. J. Exptl. Theoret. Phys. (USSR) 13, 85–92 (1943). [Google Scholar]
- [271].Popovici C., and Somesan M.. On the emission spectrum of the negative glow plasma of a hollow-cathode discharge in (a parallel) magnetic field, Appl. Phys, Letters 8, 103–104 (1966). [Google Scholar]
- [272].Popovici C.; Somesan M. and Nistor V.. Beam-plasma instability in the hollow-cathode discharge. Phys. Letters 22, 587–588 (1966). [Google Scholar]
- [273].Prakash N. J., arid W. W. Harrison. A simple demountable hollow cathode tube for the analysis of solutions. Anal. Chim. Acta 53, 421–427 (1971). [DOI] [PubMed] [Google Scholar]
- [274].Prugger H. Radiation density from radiation sources for atomic absorption and atomic fluorescence analyses, Spectrochim. Acta 24B, 197–206 (1969). [Google Scholar]
- [275].Prugger H.; Grosskopf R. and Torge R., Spectral resonanceline emission in a pulsed hollow cathode lamp, Spectrochim. Acta 26B, 191–200 (1971). [Google Scholar]
- [276].Rasmussen E. The spectra of silver. Kgl, Danske Videnskab, Selskab. Math.-Fys. Medd. 18 32 pp. (1940). [Google Scholar]
- [277].Remy F. Development of a heated graphite hollow-cathode discharge tube for the study of molecular spectra, AD 632129, 17 pp. (1965).
- [278].Remy F. Rotational temperature of the [DUMMY_SPELL ERROR] N2MUN(0–1) band excited in a hollow cathode at controllahle temperature. Bull. Soc. Roy. Sei. Liege. 37, 574–581 (1968). [Google Scholar]
- [279].Rhodes D. R. Hollow cathode discharge tubes, analytical applications, pp, 200–201. in The Encyclopedia of Spectroscopy, Clark G. L. (ed.) Reinhold Publ., New York: (1960). [Google Scholar]
- [280].Richter E. F. von. On the structure of cathodic glow discharges in the proximity and interior of an incandescent hollow cathode. Z. Tech. Physik 17, 306–315 (1936) [Google Scholar]
- [281].Ringhardtz I. New kind of lamp for atomic absorption spectroscopy. Naturwissenschaften 54, 318 (1967). [DOI] [PubMed] [Google Scholar]
- [281a].Risberg P. A review of the term systems foi Na I and K I based on hollow-cathode observations. Ark. Fysik 10, 583–605 (1956). [Google Scholar]
- [282].Ritschl R, The hyperfine structure of arc lines and nuclear momentum of copper Z Physik 79, 1–25 (1932). [Google Scholar]
- [283].Robinson J. W.; Loftin H. P. Jr., and Truitt D.. Construction of demountable hollow-cathode lamps lor stimulating emission of organic compounds. Anal. Chim. Acta 40, 241–250 (1968). [Google Scholar]
- [284].Rohatgi V. K. Electronic and ionic current at the cathode of a hollow cathode discharge. J Appl. Phys. 32, 1173–1174 (1961). [Google Scholar]
- [285].Roig J., and Bercart M.. Functioning of a water cooled hollow cathode discharge in helium, Compt. Rend. Acad Sci. (Paris) 234 1262–1264 (1952). [Google Scholar]
- [286].Roig J., and Becart M.. Functioning characteristics of a water cooled hollow cathode lamp in air or argon at low pressure. Compt Rend. Acad Sci. (Paris) 234, 1606–1608 (1952). [Google Scholar]
- [287].Roig J., and Becart M.. Pulsed current hollow cathode lamp. Production of spark lines. Compt. Rend Acad. Sci. (Paris) 235, 1625–1627 (1952). [Google Scholar]
- [288].Ropert M, Spectrographic E. analysis of non-conducting materials using a glow discharge. Proc. 16th Colloq Spectroscop. Internal., Vol. 2, 214–217. Hilger, London; (1971). [Google Scholar]
- [289].Rosen B New development in the application of a hollow cathode discharge tube designed for the quantitative determination of oxygen in metals. Bull. Soc Appl. Spcctry. 5, 26–27 (1951). [Google Scholar]
- [290].Rosen B. Spectroscopic determination of oxygen in steels. Rev. Universelle Mines 9, 445–454 (1953). [Google Scholar]
- [291].Rosen B., and Ottelet I.. Use of the hollow cathode and the vacuum furnace in spectroanalvsis. Colloque Inter. Spectrographie Strasbourg. October 1950, 155–167, [Google Scholar]
- [292].Rossi G., and Mol M. Isotopic analysis of uranium by an optical spectral method. Ill, Determination of U235/U238 ratios with a hollow cathode source and a direct reading attachment. Spectrochim, Acta 24B, 389–398 (1969). [Google Scholar]
- [293].Rossi G., and Omenetto N.. Demountable water-cooled hollow-cathode lamp for atomic absorption spectroscopy Appl. Spectrosc, 21, 329–331 (1967). [Google Scholar]
- [294].Rossi G., and Omenetto N., Application of a demountable waler-cooled hollow-cathode lamp to atomic-fluorescence spectrometry. Talanta 16, 263–268 (1969). [DOI] [PubMed] [Google Scholar]
- [295].Rudtievskii N. K., and Maksimov D. E.. Use of the hot hollow cathode for the quantitative spectrographic determination of an excess of Cd in Cd sulfide and of Zn in Zti sulfide. Zh. Prikl, Spektroskopii. Akad. Nauk Belorussk. SSR 3, 265–267 (1965). [Google Scholar]
- [296].Rudnevskii N. K., and Maksimov D, E.. Use of a hollow cathode discharge for spectrographic analysis of germanium and silicon. Tr. Khim. Khim. Tekhnol. 1965, 165–167 (1965). [Google Scholar]
- [297].Rudnevskii N. K., and Maksimov D. E.. The use of a hollow cathode discharge for the spectral analytical determination of the substoichiometric part in a binary compound Reinststoffanalytik (Proc, Inti, Symp. Pure Material in Sci. and Technol), Diesden, pp. 285–291 (1965). Akademie-Verlag; Berlin: 1966. [Google Scholar]
- [298].Rudnevskii N. K.; Maksimov D. E. and Burakova L. P.. Hollow-cathode discharge for the spectral determination of ovcr-stoichiomctrie arsenic in gallium arsenide and antimony in indium antimonide. Zh Prikl Spektrosk. 5, 384–385 (1966). [Google Scholar]
- [299].Rudnevskii N. K.: Maksimov D. E., Demarm V. T., and Baldova N. I.. Spectrographic analysis of zinc selenide for excess selenium and zinc impurities. Zh. Prikl. Spektrosk. 9, 156–158 (1968). [Google Scholar]
- [300].Rudnevskii N. K.: Maksimov D. E. and Vysotskii V. V. Spectrographic determination of superstoichiometric amounts of sulfur and cadmium in cadmium sulfide by using a hollow cathode discharge. Zh. Anal. Khim. 22, 1051–1053 (1967). [Google Scholar]
- [301].Rudnevskii N. K.; Tumanova A. N., Kutergina L. V., and Pozdnyakova N. A.. Spectrographic determination of bismuth, aluminum, and boron in white cast iron using a hollow-cathode discharge. Zh. Prikl. Spektrosk. 8, 571–573 (1968). [Google Scholar]
- [302].Russell B. J., and Walsh A.. Resonance radiation from a hollow cathode. Spectrochim. Acta 1959, 883–885. [Google Scholar]
- [303].Ryabkova O. D.; Narbutovskikh T. S. and Katkova D. E.. Spectrographic analysis of solutions. Tr. Ural. Nauch.-Issled. Proekt. Inst. Medn. Prom. No. 9, 349–354 (1966). [Google Scholar]
- [304].von Sails, G.. First spark spectrum of zinc and cadmium. Ann. Physik 76, 142–145 (1925). [Google Scholar]
- [305].Salpeter E. W., and Co R. S. W.. Spectra of glow discharges (Atlas of Spectra in 5 parts). Published by Specola Vaticana, Cita del Vaticano, Italy, 1971–1973. [Google Scholar]
- [306].Sandybaev O. Spectroscopic study of the excitation of atoms in a hollow cathode with two anodes, depending on the supply power. Vestn. Mosk. Univ., Ser. Ill, Fiz. Astron. 21, 9–13 (1966). [Google Scholar]
- [307].Sawyer R. A. Excitation processes in the hollow cathode discharge. Phys. Rev. 36, 44–50 (1930). [Google Scholar]
- [308].Schmidt T. On the quadrupole moment in the nucleus of Ta. Z. Physik 121, 63–65 (1943). [Google Scholar]
- [309].Schoen R., and Holmes J. R.. Temperature measurement in a hollow-cathode discharge. J. Opt. Soc. Am. 44, 402–403 (1954). [Google Scholar]
- [310].Schüler H. On the potential drop of an electrode in a gas discharge tube Z. Physik 22 264–268 (1921). [Google Scholar]
- [311].Schüler H. A new light source and its applicability. Z. Physik 35, 323–337 (1926). [Google Scholar]
- [312].Schüler H. The excitation of spectra for the investigation of hyperfine structures. Z. Physik 59, 149–153 (1930). [Google Scholar]
- [313].Schüler H. Emission spectroscopy of organic substances with the aid of electron impact excitation in the glow discharge. I. Spectrochim. Acta 4, 85–92 (1950). [Google Scholar]
- [314].Schüler H. Possibility of applying the hollow-cathode discharge to spectro-analytical investigations. Colloque Intern. Spectrographic-Strasbourg, October, 1950, 169–171. [Google Scholar]
- [315].Schüler H., and Gollnow H.. A bright glow discharge tube for the spectroscopic examination of small quantities of material. Z. Physik 93,611–619 (1935). [Google Scholar]
- [316].Schüler H.; Gollnow H. and Woeldike A.. Production of emission spectra of organic molecules through electron collisions in a glow discharge. Physik Z. 41, 381–386 (1940). [Google Scholar]
- [317].Schüler H., and Keystone J. E.. Remarks on changes in intensities of hyperfine structure lines. Z. Physik 71, 413–415 (1931). [Google Scholar]
- [318].Schüler H., and Michel A.. About two new hollow cathode discharge tubes. Specktrochim Acta 5, 322–326 (1952). [Google Scholar]
- [319].Schiiler H., and Reinbeck L.. On a method to vary the excitation conditions of organic materials in a glow discharge. Z. Naturforschung 5a, 657–660 (1950) [Google Scholar]
- [320].Schüler H., and Reinbeck L.. On new spectra in the glow discharge of benzene. Z. Naturforschung 6a, 160–165 (1951). [Google Scholar]
- [321].Schüler H., and Reinbeck L.. On the emission spectroscopy of organic materials using electron collision excitation in a glow discharge. II. Spectrochim. Acta 6, 288–301 (1954). [Google Scholar]
- [322].Schuler H, and Schmidt T.. On a liquid air cooled hollow cathode lamp. Z. Physik 96, 485–488 (1935). [Google Scholar]
- [323].Schüler H., and Woeldike A.. Basic contributions to the excitation of organic molecules through electron collisions in a glow discharge. Phys. Z. 42, 390–399 (1941). [Google Scholar]
- [324].Schüler H., and Woeldike A.. Investigations of organic substances by electron excitation in a glow discharge. Phys. Z. 43, 17–22 (1942). [Google Scholar]
- [325].Schüler H., and Wolf K. L.. On the continuous hydrogen spectrum. Z. Physik 33, 42–47 (1925). [Google Scholar]
- [326].Sehens C. R., and Vollmer J. W.. Hollow cathodes for atomic absorption spectroscopy. U.S. Patent 3,412,278.
- [327].Sehens C. R., and Vollmer J. W.. Liquid hollow cathode lamp. U.S. Patent 3,422,301.
- [328].Sehens C.; Vollmer J. and Slavin W.. Multi-element hollow cathode lamps. At. Absorption Newsletter 3, 165–169 (1964). [Google Scholar]
- [329].Sebestyen N. A. Studies of selective modulation in atomic absorption. Spectrochim. Acta 25B, 261–282 (1970). [Google Scholar]
- [330].Setz W., and Maierhofer J.. Radial temperature distribution in the negative glow of a hollow cathode discharge. Z. Angew. Physik 28, 168–173 (1969). [Google Scholar]
- [331].Shteinberg A. N. Use of a hollow cathode source in routine spectral analysis. Zavodsk. Lab. 29, 1084 (1963). [Google Scholar]
- [332].Shteinberg A. N. Spectral analysis of pure metallic tungsten by means of discharge in a hollow cathode. Metody Analiza Khim. Reaktivov i Preparatov, Gos. Korn. Sov. Min. SSSR po Khim. No. 7, 77–81 (1963). [Google Scholar]
- [333].Shteinberg A. N. Static current-voltage and temperature characteristics of the hollow cathode discharge. Optics and Spectrosc. 18, 7–9 (1965). [Google Scholar]
- [334].Shteinberg A. N. Application of hot hollow cathode for emission spectroscopy. Zh. Prikl. Spektroskopii, Akad. Nauk Belorussk. SSR. 2, 385–391 (1965). [Google Scholar]
- [335].Shvangiradze R. R.; Oganezov K. A. and Chikhladze B. Y.. Use of hollow cathode for the determination of gases m solids by the isotopic equilibrium method. Zh. Prikl. Spektroskopii Akad. Nauk Belorussk. SSR. 3, 300–305 (1965). [Google Scholar]
- [336].Sokiryanskii L. F., and Shteinberg A. N.. Kinetics of the introduction of impurities in a discharge plasma in a hot hollow cathode. Zavodsk. Lab. 31, 54–56 (1965). [Google Scholar]
- [337].Somesan M., and Popovici C.. Excitation of 4.38-ev. level of lead in a hollow-cathode discharge in magnetic field. Appl. Phys. Letters 9, 65–67 (1966). [Google Scholar]
- [338].Somesan M., and Popovlel C.. Emission spectra of metal mixtures in hollow cathode discharges in a magnetic field. Rev. Roum. Phys. 13, 155–158 (1968). [Google Scholar]
- [339].Spectroscandia (Nagu, Finland). A new hollow cathode discharge unit for multielement analysis of plane samples. (1975) (Date sheet available from the manufacturer prior to publication: Spectroscandia, Brinkasvägen 1, SF-21660 Nagu, Finland.)
- [340].Stanley R. W., and Dieke G. H.. Interferometric wavelength of iron lines from a hollow cathode discharge. J. Opt. Soc. Am. 45, 280–286 (1955). [Google Scholar]
- [341].Startsev G. P.; Pavlovskaya E. N. and Bodretsova A. I.. Standard source of wavelengths and intensities in the range 2100–5600 Å with a hollow iron electrode. Zh. Prikl. Spektrosk. 1, 102–108 (1964). [Google Scholar]
- [342].Strasheim A., and Butler L. R. P.. A versatile hollow cathode lamp for atomic absorption spectroscopy. Appl. Spectrosc. 16, 109–110 (1962). [Google Scholar]
- [343].Strasheim A., and Butler L. R. P.. A versatile hollow cathode lamp for atomic absorption spectroscopy, p. 26–30 in Spectroscopic Tricks. May L. (ed.). Plenum Press, New York, 1967. [Google Scholar]
- [344].Stuart R. V., and Kramer G. K.. Atomic absorption spectroscopy Tech. Rep. AFML-TR-66–61, 108 pp., May I960. (Litton Systems, Inc.). [Google Scholar]
- [345].Stukenbroeker G. L.; Smith D. D., Werner G. K., and McNally J. R. Jr. Spectro-isotopic assay of lithium. J. Opt. Soc. Am. 42, 383–386 (1952). [DOI] [PubMed] [Google Scholar]
- [346].Stnrges D. J., and Oskam H. J.. Studies of the properties of the hollow cathode glow discharge in helium and neon. J. Appl. Phys. 35, 2887–2894 (1964). [Google Scholar]
- [347].Sturges D. J., and Oskam H. J.. Hollow-cathode glow dischrge in hydrogen and the noble gases. J. Appl. Phys. 37 2405–2412 (1966). [Google Scholar]
- [348].Sugawara M., and Okagaki H.. Hollow-cathode discharge tubes. U.S. Patent No. 3,286,119 (1966). Japan Appl. May 8, 1963.
- [349].Sugawara M.; Okagaki H. and Ikuta Y.. Hollow cathode discharge lamp for emission of atomic resonance lines. U.S. Patent No. 3,242,371 (1966).
- [350].Sugiura Y., and Matoba T.. The use of a lime cathode of carbon in spectroscopy. Astrophys. J. 53, 323–325 (1921). [Google Scholar]
- [351].Sullivan H. M. Hollow-anode lithium lamp. Can. J. Phys. 42, 1695–1699 (1964). [Google Scholar]
- [352].Sullivan J. V., and Walsh A.. High-intensity hollow-cathode lamps. Spectrochim. Acta 21, 721–726 (1965). [Google Scholar]
- [353].Swings P. Some comments on the luminous sources of the future in spectrochemistry. Rev. Universclle Mines 90, 339–341 (1947). [Google Scholar]
- [354].Takahashi M. Application of iron hollow cathode discharge tube to plate calibration. Bunko Kenkyu 13, 102–106 (1965). [Google Scholar]
- [355].Takahashi Y. The first spark spectrum of zinc and cadmium. Ann. Physik 3, 27–48 (1929). [Google Scholar]
- [356].Takahashi Y. The excitation in the negative glow discharge in helium. Ann. Physik 3, 49–57 (1929). [Google Scholar]
- [357].Takatsu K., and Toda T.. Hardening of deposited metals in hollow cathode discharge. Japan J. Appl. Phys. 5, 19–20 (1966). [Google Scholar]
- [358].Tardon S. Discharge tubes with hollow cathode for atomic absorption spectroscopy, and their preparation in the laboratory. Chcm. Prum. 17, 150–154 (1967). [Google Scholar]
- [359].Tardon S.; Stibor B. and Sali J.. Stablilued source for a discharge tube with a hollow cathode. Chem. Listy 60, 1091–1094 (1966). [Google Scholar]
- [360].Thackeray D. Spectrographic light sources. J. Photogr. Sci. 14, 321–328 (1966) [Google Scholar]
- [361].Thomas H., and Heer L.. The current intensity in a glow discharge at atmospheric pressure; a new form of discharge. Z. Techn. Physik 13, 464–470 (1932). [Google Scholar]
- [362].Thornton K. The use of a high temperature hollow cathode lamp for the spectrographic analysis of steels, high temperature alloys and related materials for trace elements. Analyst 94,958–967 (1969). [Google Scholar]
- [363].Tilch T. Regeneration of the noble gas atmosphere of a hollow cathode lamp. Exptl. Tech. Physik 13, 169 (1965). [Google Scholar]
- [364].Tolansky S. The nuclear spin of iodine. 11. Fine structure in the arc spectrum and a fine structure perturbation effect. Proc. Roy. Soc. (London) A152, 663–672 (1935). [Google Scholar]
- [365].Tolansky S. High Resolution Spectroscopy. 291 pp. Methuen & Co. Ltd., London, 1947. [Google Scholar]
- [366].Tolansky S., and Lee E.. Fine structure in the arc spectrum of platinum (A) The nuclear spin of Pt 195. (B) Even isotope displacement. Proc. Roy. Soc. (London) A158, 110–127 (1937). [Google Scholar]
- [367].Török T., and Zaray G.. A low-temperature tandem hollow cathode for the determination of concentration ratios of stable isotopes Proc, 16th Colloq. Spectroscop. Internat. (1971) Vol. 2, 234–238. Hilger, London. [Google Scholar]
- [368].Tsukamoto A. Anomalous change of emission spectral intensity in the hollow cathode discharge. Jap. J. Appl. Phys. 7, 92–93 (1968). [Google Scholar]
- [369].Tsukamoto A. Spectroscopic study of the hollow cathode discharge. J. Sci. Hiroshima Univ., Ser. A-2, 32, 15–28 (1968). [Google Scholar]
- [370].Turovtseva Z. M.; Malyshev V. I. and Noskov A. S.. Determination of nitrogen and oxygen in UF6. Zh. Anal. Khim. 20, 1353–1358 (1965). [Google Scholar]
- [371].Udris Ya.; Guseva L. G. and Chernov V. A.. Some properties of a high-voltage hollow-anode glow discharge. Zhur. Tekh. Fiz. 11, 840–842 (1966). [Google Scholar]
- [372].United Aircraft Corp. (By Ferreira Fernand J). Annular hollow cathode and apparatus using it. French Patent No. 1,455,620, 7 pp. (1966). U.S. Appl. Dec. 10, 1964.
- [373].Urano Y., and Kosasa K.. Spectroscopic studies of a hollow cathode discharge. II. Osaka Kogyo Gijutsu Shikensho Kiho 16, 112–119 (1965), and 14, 47–53 (1963). [Google Scholar]
- [374].Valero F. P. J. Thorium lamps and interferometrically measured thorium wavelengths. J. Opt. Soc. Am. 53, 484–489 (1968). [Google Scholar]
- [375].Van Voorhis C. C, and Shenstone A. G.. Some characteristics of hollow-cathode discharge tubes. Rev. Sci. Instr. 12, 257–261 (1941). [Google Scholar]
- [376].Veith W. The potential fall in a normal glow discharge produced by cathode illumination. Naturwissenschaften 42, 40–41 (1955). [Google Scholar]
- [377].Vollmer J. Bi hollow-cathode lamp. At. Absorption Newsletter 5, 12 (1966). [Google Scholar]
- [378].Vollmer J. Molten Sn hollow-cathode lamps. At. Absorption Newsletter 5, 35 (1966). [Google Scholar]
- [379].Vollmer J. Bismuth-lithium hollow-cathode lamps. U.S. Patent No., U.S. 3,361,925, 4 pp. (1968).
- [380].Walsh A., and Jones W. G.. Atomic spectral lamps. U S. Patent No. 3,089,054 (1963).
- [381].Webb M. S. W., and Webb R. J.. Automatic spectrographic method for the determination of oxygen in steel. Anal. Chim. Acta 33, 138–144 (1965). [Google Scholar]
- [382].Webb M. S. W., and Webb R. J.. Automatic spectrographic determination of gases in metals etc. Anal. Chim. Acta 36, 403–406 (1966). [Google Scholar]
- [383].Wehner G. Sputtering by ion bombardment, Advances in Electronic and Electron Physics, 7, 239–298 (1955). Academic Press, New York. [Google Scholar]
- [384].Wehrli M. On the transition of glow discharge into an arc discharge. Z. Physik 44, 301–318 (1927). [Google Scholar]
- [385].Werner G. K.; Smith D. D., Ovenshine S. J., Rudolph O. B., and McNally J. R. Jr. Further investigations in the spcctro- isotopic assay technique for lithium. J. Opt. Soc. Am. 45, 202–205 (1955). [Google Scholar]
- [386].Weston G. F. The glow discharge. Chapter 3, p. 67–114, in Cold Cathode Glow Discharge Tubes. Iliffe, London: (1968). [Google Scholar]
- [387].Weston G. F. Cathodic sputtering, Chapter 4, p. 115–150, in Cold Cathode Glow Discharge Tubes, Iliffe, London: (1968). [Google Scholar]
- [388].White A. D. New hollow cathode glow discharge. J. Appl. Phys. 30, 711–719 (1959). [Google Scholar]
- [389].Wittke H. Interferometric investigation on a condensed hollow cathode discharge. Z. Physik 116, 547–561 (1940). [Google Scholar]
- [390].Woodriff R. A.; Wheeler G. V. and Ryder W. A.. Hollow-cathode lamp for use in emission and absorption. Appl. Spectrosc. 22, 348–349 (1968). [Google Scholar]
- [391].Yokoyama Y., and Ikeda S.. Atomic absorption spectrometry by a pulsed technique and measurement of half life of copper and magnesium vapors. Spectrochim. Acta 24B, 117–124 (1969). [Google Scholar]
- [392].Zakorina N. A.; Lazeeva G. S. and Petrov A. A.. Application of hollow-cathode discharge tube for spectral-isotopic determination of gases in metals. Vestn. Leningrad. Univ. 21. Ser. Fiz. Khini. No. 4, 38–40 (1966). [Google Scholar]
- [393].Zakorina N. A.; Lazeeva G. S. and Petrov A. A.. Use of a hot hollow cathode for the spectrographic-isotopic determination of gases in metals. Zh. Anal. Khim. 23, 1688–1694 (1968). [Google Scholar]
- [394].Zanzucchi P. J. A study of direct solution analysis with a new hollow-cathode discharge system. 199 pp. Thesis, Univ. of Illinois, Urbana, 1967. [Google Scholar]
- [395].Zhiglinskii A. G., and E., Fafurina N.. Spectral determination of the isotopic composition of magnesium. Zh. Prikl. Spek- trosk. 5, 557–561 (1966). [Google Scholar]
- [396].Zhiglinskii A. G., and Khlopina T. N.. Mechanism of the rise of the gas temperature of a discharge in a cooled hollow cathode. Zh. Prikl. Spektrosk. 8, 562–570 (1968). [Google Scholar]
- [397].Zhiglinskii A. G.; Zaidel A. N. and Petrov A. A.. Spectral analysis of isotope compositions. Industrial Laboratories; 29, 575–577 (1963). [Google Scholar]
- [398].Zil’bershtein K. L.: Kaliteevskii N. I., Razumovskii A. N., and Fedorov Y. F.. The use of a hollow-cathode discharge for analyzing impurities in silicon. Zavodsk. Lab. 28, 43–45 (1962). [Google Scholar]
- [399].Zil’bershtein K. L., and Nikitina O. N.. Sensitivity of the analysis of dry solution residues in a carhon arc and in a discharge tube with a hot hollow cathode. Zh. Prikl. Spektrosk. 6, 576–582 (1967). [Google Scholar]
- [400].Zil’bershtein K. L; Nikitina O. N., Semov M. P., and Legeza S. S. Carbon arc and hollow-cathode discharge as light sources for the analysis of traces of various elements. Izv Sib. Otd. Akad. Nauk SSSR. Ser. Khim. Nauk 1967, 87–90. [Google Scholar]
5.4. Addendum—Preface
The 290 references assembled in the addendum for the most part cover the period from 1975 to 1983. They are in alphabetical order and are numbered in italic type to differentiate them from the 400 references of the base bibliography. Several references dating before 1975 escaped the base bibliography and are included in the addendum. The addendum, like the base listing, is preceded by a Brief Subject Index and a Listing of Chemical elements.
5.5. Brief Subject Index (Add.)
Glow Discharges, General Characteristics, and Reviews: 10, 177, 180, 226, 241, 252.
Atlas of Glow Discharge Spectra: 172.
Sputtering; 53, 129, 230.
Fundamental Characteristics: 5, 12, 23, 27, 30, 46, 59, 61, 75, 76, 77, 91, 93, 97, 100, 101, 114, 115, 117, 123, 144, 159, 165, 169, 171, 186, 217, 219, 220, 236, 243, 256, 257, 258, 267.
Excitation Phenomena in Hollow Cathodes: 9, 14,16, 17,19, 25, 29, 39, 40, 41, 45, 48, 49, 52, 53, 54, 55, 56, 57, 69, 71, 74, 78, 81, 85, 87, 88, 89, 90, 92, 94, 95, 96, 103, 105, 109, 110, 112, 113, 116, 119, 124, 125, 127, 140, 149, 150, 153, 155, 157, 164, 166, 168, 170, 178, 182, 183, 189, 194, 200, 207, 210, 211, 212, 213, 221, 222, 229, 230, 235, 237, 238, 239, 242, 244, 246, 247, 249, 250, 251, 254, 261, 262, 265, 271, 272, 273, 275, 280, 281, 282, 283, 285, 287, 288, 289, 290.
Excitation of Molecular Species: 96, 106, 216, 218, 259, 263, 268, 270.
Isotopic Analysis and Fine Structure: 3, 15, 22, 58, 79, 80, 225, 233, 234, 277.
Intrumental Characteristics: 4, 6, 7, 9, 18, 21, 32, 33, 37, 39, 40, 4L 43, 47, 68, 84, 98, 106 107, 108, 120, 121, 131, 138, 145, 188, 223, 232, 255, 260, 264, 269, 273, 286.
Excitation of Chemical Elements. 1, 3, 6, 26, 34, 64, 65, 73, 86, 102, 118, 166, 167, 170, 172, 173, 252.
Analytical Applications: 33, 34, 38, 42, 44, 45, 50, 104, 120, 126, 128, 132, 133, 134, 136, 139, 147, 148, 152, 154, 174, 175, 176, 179, 189, 190, 191, 192, 193, 195, 196, 197, 198, 199, 201, 202, 203, 204, 205, 206, 208, 209, 214, 215, 223, 224, 228, 234, 245, 270, 274, 276, 288.
Planar Cathode (Grimm Discharge): 4, 37, 43, 44, 223.
Hollow Cathodes in Atomic Absorption: 32, 51, 62, 70, 77, 82, 83, 99, 156, 181, 227, 231, 240.
High Intensity Hollow Cathodes: 2, 20, 28, 29, 151, 184, 239, 245, 266, 279.
Pulsed Hollow Cathodes: 24, 55, 60, 63, 64, 65, 66, 67, 69, 122, 135, 141, 158, 163, 259.
Hollow Cathodes in a Magnetic Field: 11, 13, 31, 111, 130, 137, 142, 143, 146, 185, 187, 191, 192, 193, 196, 197, 199, 200, 201, 205, 206, 207, 224, 229, 230, 242, 248, 278, 279, 284.
Hollow Cathode—RF Discharge Association: 35, 36, 72.
Microhollow Cathode: 50.
5.6. Listing of Chemical Elements (Add.)
| Reference | Element |
| [1] | Al |
| [3] | H |
| [6] | Bi, In |
| [16] | Li |
| [17] | I |
| [19] | Ca |
| [25] | Rare Earths |
| [26] | Rare Earths |
| [34] | Cu, A1 |
| [38] | Ga |
| [49] | He, Cd |
| [51] | As, Se |
| [56] | Ba, Sr |
| [58] | H |
| [64] | Cu, Fe |
| [65] | Zn |
| [66] | A1 |
| [67] | A1 |
| [69] | Cu |
| [70] | Mo, Fe, Si |
| [73] | Sr |
| [75] | Ar |
| [76] | Ar |
| [77] | As |
| [79] | Li |
| [81] | U |
| [87] | Cu |
| [90] | Cs |
| [102] | Hydride |
| [108] | He |
| [109] | He |
| [113] | N, Co |
| [118] | Mg |
| [119] | Mg |
| [122] | Sb, Bi, Ag, Cu |
| [123] | He |
| [128] | Te |
| [133] | Ni, Co |
| [135] | He |
| [136] | Au |
| [137] | Ti |
| [139] | Co, N |
| [140] | He |
| [142] | Alkaline Elements, Mg |
| [143] | Group IV Elements |
| [147] | Ge, Se, Cd |
| [149] | Cd |
| [152] | F, U |
| [164] | Ba, Cd, Cu |
| [166] | Dy |
| [167] | Zn |
| [172] | U |
| [173] | Cd |
| [174] | Al-ammonium Alums |
| [175] | A1 |
| [176] | Ti |
| [178] | U |
| [179] | Graphite |
| [189] | Cd, Na |
| [190] | W. S, P |
| [191] | Alkali Metals |
| [192] | Alkali Earth Elements |
| [194] | In. Ga |
| [198] | W, S, P, Zn, Cd |
| [199] | Cr |
| [201] | Cd |
| [202] | Si, Ge |
| [203] | S, Cd, Se |
| [204] | Si |
| [205] | Rare Earths |
| [206] | Rare Earths |
| [208] | B |
| [209] | Ge |
| [214] | Si |
| [216] | Cr, Ti |
| [218] | Pt |
| [221] | He, Cu, Ag |
| [222] | Cu |
| [223] | Cu |
| [224] | Si |
| [228] | Ga |
| [233] | U-235/U-238 |
| [234] | U-235 |
| [236] | Ne, Zn |
| [237] | Cu, Ag |
| [239] | Cu |
| [244] | Au |
| [245] | Steels, Nickel-base and Ferroall |
| [259] | Xe F, Xe Cl |
| [262] | Sr, Ba |
| [263] | Xe Cl |
| [268] | Cl, Xe Cl |
| [270] | Al, Cu, Ni hydrides and dcuterides |
| [271] | Cu |
| [273] | Am, Cm |
| [274] | Am, Cm |
| [277] | H |
| [283] | Cu |
| [285] | Cu |
5.7. References (Add.)
- [1].Ahmed N. A. G., and Teer D. G.. Cliaraclerisalion of aluminum coatings deposited in a hollow cathode discharge. Thin Solid Films. 80, 49–54 (1981). [Google Scholar]
- [2].Aksenov I. I.; Belous V. A. and Smirnov S, A.. High-current glow discharge with a hollow cathode, Zh. Tekh. Fiz. 45, 1717–24 (1975). [Google Scholar]
- [3].Aladyshkina A. E.: Alroshenko M. P., Zakorina N, A., and Temirkulova N. I. Determination of hydrogen in tantalum by an isotopic-spectral method in a hot hollow cathode. Zavod. Lab. 45, 131–32 (1979). [Google Scholar]
- [4].Alimonti A.; Caroli S. and Senofonte O.. A modified version of the Grimm’s glow discharge lamp for use as a demountable hollow cathode emission source. I. Construction details. Spectrosc. Lett. 13, .W7–12 (1980). [Google Scholar]
- [5].Apel Ch. T.; Keller R. A., Zaiewski Ed. F., and Ensleman Rolf Jr. Optogalvanic effect in a hollow cathode discharge with nonlaser sources. Appl. Opt. 21, 1465–67 (1982). [DOI] [PubMed] [Google Scholar]
- [6].Armannsson H., and Ovenden P. J.. The use of dithizone extraction and atomic absorption spectrometry for the determination of silver and bismuth in rocks and sediments, and of a demountable hollow cathode lamp for the determination of bismuth and indium. Int. J. Environ. Anal. Chem. 8, 127–36(1980). [Google Scholar]
- [7].Ataev A. E.; Litvinov V. S., Nazarova T. B., and Crlapova M. N.. Effect of a hollow cathode for use in metal halide lamps. Svetotekhnika 9, 5–7 (1982). [Google Scholar]
- [8].Ataev A. E.; Voronchev T. A., Kaganov I, L., Ovchukova S. A., and Urlapova M, N.. Study of the effect of a hollow cathode for use in lamps of DRL type. Svetotekhnika 12, 20–21 (1978). [Google Scholar]
- [9].Atnashev Yu. B.; Muzgin V. N. and Gavrilov F. F.. Processes occurring in the gas discharge of a double hollow cathode. Spektrosk. Ee Primen. GeoFiz. Khim. 1975. 19–22. [Google Scholar]
- [10].Bădărău E., and Popescu I.. Gas Ionises, Decharges Electriques dans les Gaz, 334 pp. Dunod, Paris, 1968. [Google Scholar]
- [11].Bădărău E.; Popovici C. and Somesan M.. Amplification of the intensity of discharge current and radiation in hollow cathode discharges in magnetic field. Phenomena Ioniz., Gases, Int. Conf., Contrib. Pap., 8th, 99 pp. Bucharest (1967). [Google Scholar]
- [12].Bakaleinik I. I. Method for determining the electron component of cathode current in gas discharges with a hollow cold cathode. Radiotekh. Elektron. 24, 158–67 (1979). [Google Scholar]
- [13].Barchenko V. T.; Potsar A. A. and Shirshova N. P.. Study of discharge with a hollow cathode in a heterogeneous magnetic field. Izv. Leningr. Elektrotekhn. In-ta. 237, 84–8 (1978). [Google Scholar]
- [14].Batarchukova N. R.; Irikova L. A. and Ptitsyna E. A.. Study of emission from small lamps with a hollow cathode. Tr. Metrol. In-tov SSSR. VNII Metrol, 236/296, 3–6 (1979). [Google Scholar]
- [15].Belobrov I. P.; Zaretskaya N. P. and Levenberg L. S.. Some results of studying a hollow cathode discharge during determination of isotopes, v sb., Geol., Tekh. Razvedki i Tek- hnol. Izuch. Minerul’n. Syr’ya Kazakhstana. 1975, 118–123. [Google Scholar]
- [16].Belobrov I. P., Zaretskaya N. P. and Levenberg L. S.. Some principles of hollow cathode radiation in the lithium resonance line region. Sovrem. Metody Analiza Mineral’n. Syr’ya., Alma-Ata, 1979, 60–3. [Google Scholar]
- [17].Berezm I. A. Anomalous Intensification of iodine spectrographic lines in a hollow cathode discharge. Opt. Spektrosk. 26 855–6 (1969). [Google Scholar]
- [18].Berslund Bo., and Thelin Bo. Demountable double-chamber hollow cathode lamp: a new approach to the determination of trace elements in steel. Analyst (London). 107, 867–71 (1982). [Google Scholar]
- [19].Bevan D. G., and Kirkbright G. F.. The influence of operating parameters on the profile of the calcium 422.7 nm resonance line emitted by a demountable hollow cathode lamp. Appl. Spectry. 30, 162–167 (1976). [Google Scholar]
- [20].Bleekrode R., and Van-Benthem W. Spectroscopic investigations of high-current hollow cathode discharges in flowing nitrogen at low pressures. J. Appl. Phys. 40, 5274–80 (1969). [Google Scholar]
- [21].Boesehoten F.; Kleijn D. J., Komen R., Sens A. F. C.., and Vanlersel A. W. M. . Experiments with a large sized hollow cathode discharge, Part 3. Sci. Tech. Aerosp. Rep. 15, 42 pp. (1977). [Google Scholar]
- [22].Boesehoten F., and Komen R.. On the possibility to separate isotopes by means of a rotating plasma: isotope separation with a hollow cathode discharge. Sci. Tech. Aerosp. Rep. 17, 38 pp. (1977). [Google Scholar]
- [23].Boshuyak B. M.; Zhiglinskii A. G., Kund G. G.., and Khlopina T. N.. Electrical and optical characteristics of a discharge in a cooled hollow cathode. II. Opt. Spektrosk. 33, 1032–6 (1972). [Google Scholar]
- [24].Boshnyak B. M.; Zhiglinskii A. G., and Presnukhina I. P.. Pulsed light source with a hollow cathode. Ural. Konf. Spektrosk., 7th, 1, 19–21 (1971). [Google Scholar]
- [25].Broekaert J. A. C. Spectroscopic measurements at the hollow cathode glow discharge plasma and determination of rare earths by HCE techniques. Colloq. Spectrosc. Int., (Proc.), 18th, 1, 119–24 (1975). [Google Scholar]
- [26].Broekaert J. A. C. Emission spectrographic determination of all rare earths in solutions by hollow cathode excitation. Bull. Soc. Chim. Belg. 85, 261–70 (1976). [Google Scholar]
- [27].Broekaert J. A. C. Determination of rotational temperatures in a transitional type hollow cathode glow discharge. Bull. Soc. Chim. Belg. 86, 895–906 (1977). [Google Scholar]
- [28].Bueger P. A., and Fink W. . Possibilities of analysis in high-energy hollow cathode discharge. Tydskr. Natuurwetensk. 9, 203–19 (1969). [Google Scholar]
- [29].Bueger P. A., and Fink W.. Boltzmann population, excitation temperature, and excitation of material lines in a high-current hollow cathode. Z. Phys. 228, 416–26 (1969). [Google Scholar]
- [30].Bueger P. A., and Fink W.. Ionization and recombination processes in a hollow cathode discharge. Z. Phys. 236, 314–20 (1970). [Google Scholar]
- [31].Bulanin V. V.; Zhilinskii A. P. and Petrov A. V.. Study of an arc discharge plasma with a hollow cathode in a magnetic field. Zh. Tckh. Fiz. 48, 2509–18 (1978). [Google Scholar]
- [32].Burger J. C.; Gillies W. and Yamasaki G. K.. Hollow cathode discharge devices, in Analytical Flame Spectroscopy, pp. 625–49. Editor Mavrodineanu R., Springer, New York, 1970. [Google Scholar]
- [33].Caroli S. Possibilities of the analytical application of hollow cathodes and glow discharges as radiation sources. Kern. Kozl. 56, 391–400 (1981). [Google Scholar]
- [34].Caroli S.; Alimontl A. and Delle Feminine P.. Determination of copper and aluminum in steel by means of hollow cathode and glow discharge light sources: a comparative study. Spectrosc. Lett. 12, 871–86 (1979). [Google Scholar]
- [35].Caroli S.; Alimonti A. and Petrucci F.. The microwave-coupled hollow cathode —a novel radiation source in emission spectroscopy. TrAC, Trends Anal. Chem. (Pers. Ed.) 1, 368–73 (1982). [Google Scholar]
- [36].Caroli S.; Alimonti A. and Petrucci F.. Analytical capabilities of the microwave-coupled hollow cathode discharge in emission spectroscopy. Anal. Chim. Acta 136, 269–76 (1982). [Google Scholar]
- [37].Caroli S.; Alimonti A. and Senofonte O.. A modified version of the Grimm’s glow discharge lamp for use as a demountable hollow cathode emission source. II. The current intensity-voltage characteristic curves. Spectrosc. Lett. 13, 457–69 (1980). [Google Scholar]
- [38].Caroli S.; Alimonti A. and Violante N.. Determination of gallium in biological samples by means of the hollow cathode discharge. Spectrosc. Lett. 13, 313–19 (1980). [Google Scholar]
- [39].Caroli S., and Delle Femmtne P.. Comparative investigations of the hollow cathode and glow discharge light sources: further considerations. Spectrose. Lett. 11, 299–321 (1978) [Google Scholar]
- [40].Caroli S., and Milazzo G. Comparison between hollow cathode, spark excitation, and glow discharge radiation sources. Kem. Kozl. 45, 137–44 (1976). [Google Scholar]
- [41].Caroli S.; Milazzo G. and Benincasa M.. Comparative investigations on the reproducibility of the hollow cathode, glow discharge and spark light sources Spectrose. Lett. 10, 655–76 (1977). [Google Scholar]
- [42].Caroli S., and Senofonte O.. Comparative studies of the hollow cathode and glow discharge radiation sources for aluminum and graphite. Can. J. Spectrose. 25, 73–80 (1980). [Google Scholar]
- [43].Caroli S.; Senofonte O., Alimonti A., and Violante N.. A modified version of the Grimm’s glow discharge lamp for use as a demountable hollow cathode emission source. 111. Blackening as a function of discharge parameters. Spectrose. Lett. 13, 905–31 (1980). [Google Scholar]
- [44].Caroli S.; O Senofonte A. Alimonti, and Zimmer K.. A modified version of the Grimm’s glow discharge lamp for use as a demountable hollow cathode emission source. V. Determination of minor constituents and trace elements in steel. Spectrose. Lett. 14, 575–87 (1981). [Google Scholar]
- [45].Caroli S.; Senofonte O. and Delle Femmine P.. Determination of trace elements in biological material using a hollow cathode discharge: comparative study of matrix effects. Analyst (London) 108, 196–203 (1983). [DOI] [PubMed] [Google Scholar]
- [46].Cellarius C. J.; Dicks L. A. and Turner R.. Determination of the densities and temperatures of two low-energy electron groups in a hollow cathode discharge. Z. Phys. 231,119–127 (1970). [Google Scholar]
- [47].Cervenan L. Study of a glow discharge with a cylindrical hollow cathode. Acta Fac. Rerum Nat. Univ. Comemanae. Phys. 19, 53–69 (1979). [Google Scholar]
- [48].Cervenan L., and Martisovits V.. A study of the radial distribution of the ultraviolet radiaiton from hollow cathode glow discharge. Acta Phys. Slovaca 32, 341–5 (1982). [Google Scholar]
- [49].Cristescu C. P.; Popescu I. M. and Preda A.. Excitation mechanism in the He Cd plasma of a hollow cathode discharge. Rev. Roumaine Phys. 18, 859–865 (1973). [Google Scholar]
- [50].Czakow J. Application of microhollow cathode in spectrographic trace analysis. Kem. Kozl. 45, 159–66 (1976). [Google Scholar]
- [51].Daidoji H.; Ishida N. and Kobayashi M.. The application of arsenic and selenium hollow cathode lamps cooled with water in atomic absorption spectrometry. Bunseki Kagaku 28, 36–40 (1979). [Google Scholar]
- [52].Daughtrey E. H. Jr. Hollow cathode discharge as an emission and ionization source. Ph D. Thesis, 231 pp. Univ. Virginia, Charlottesville, Va. 1974. [Google Scholar]
- [53].Daughtrey E. H. Jr.; Donohue D. L., Slevin P. J., and Harrison W.. Surface sputter effects in a hollow cathode discharge. Anal. Chem. 47, 683–8 (1975). [Google Scholar]
- [54].Delibas M., and Mindreci I, The study of certain excitation characteristics of the discharges in a hollow cathode spectral lamp. An. Stiint. Univ. Al I. Cuza. lasi, Sect, lb, 24, 61–64 (1978). [Google Scholar]
- [55].Deraers D, R., and Ch, D. Allemand. Atomic fluorescence spectrometry with an inductively coupled plasma as atomization cell and pulsed hollow cathode lamps for excitation. Anal. Chem. 53, 1915–21 (1981). [Google Scholar]
- [56].Devyatov A. M.; Fazlaev V. Kh., Mal’Kov M. A., and Volkova L. M.. On mechanism of barium and strontium ions formations in hollow cathode discharge. Proc. Int. Conf. Phenom. loniz. Gases, 13th, 1, 305–6 (1977). [Google Scholar]
- [57].Dobre M., and lova I.. Hollow cathode effect in the geometry of coaxial cylindrical cathodes in monoatomic gas. Stud. Cercet. Fiz. 32, 815–20 (1980). [Google Scholar]
- [58].Dobrosavljevic J., and Pesic D.. Spectrographic-isotopic determination of hydrogen in metals by the hollow cathode discharge technique. Tehnika (Belgrade) 26, 1795–8 (1971). [Google Scholar]
- [59].Dobrosavljevic J, S., and Pesic D. S., Measurements of rotational temperatures in the hollow cathode discharge. Appl. Spectrose. 35, 57–9 (1981). [Google Scholar]
- [60].Dobrosavljevic E, S.; Zhiglinskii A. G. and Khlopina T. N.. Pulsed discharge in a cooled hollow cathode. Bull. Boris Kidric Inst. Nucl. Sci., Phys. 19, 6 pp. (1968). [Google Scholar]
- [61].Doepel R. Empiricism and principles of a theory of hollow cathode discharge. Wiss. Z. Tech. Hochsch., llmenau 15 55–71 (1969). [Google Scholar]
- [62].Drobyshev A, I.; Rish A. M. and Turkin Yu. L. Features of atomization in a cooled hollow cathode discharge for atomic absorption analysis. Vestn. Leningr. Univ., Fiz., Khim. 1982, 117–20. [Google Scholar]
- [63].Drohyshev A. I.; Zhiglinskii A. G. and Turkin Yn. I.. Separation of elements in a pulsed source of light with a hollow cathode. Zh. Prikl. Specktrosk. 19, 620–3 (1973). [Google Scholar]
- [64].Dyulgerova R. Spectral study of copper and iron hollow cathode discharges in pulse mode. Bulg. J. Phys. 4, 459–467 (1977). [Google Scholar]
- [65].Dyulgerova R. Spectroscopical effects arising under applica tion of pulse supply to zinc hollow cathode discharge. Spectrosc. Lett. 10, 727–36 (1977). [Google Scholar]
- [66].Dyulgerova R. Spectroscopic study of aluminum hollow cathode discharge with pulse feeding. Bulg. J. Phys. 4, 569–75 (1977). [Google Scholar]
- [67].Dyulgerova R. Effect of pulse feeding applied to an aluminum hollow cathode discharge. Bulg. J. Phys. 7 90–94 (1980). [Google Scholar]
- [68].Dyulgerova R., and Zhechev D.. Spectroscopical and electrical characteristics of new-construction hollow cathode discharge tube. Spectrose. Lett. 12, 615–29 (1979). [Google Scholar]
- [69].Dyulgerova R.; Zhechev D. and Krasnobaeva N.. Experimental measurement of the intensity of the spectral line Cul 324.7nm and the concentration of copper atoms in a hollow cathode discharge under pulsed and steady-state conditions. Spectrochim. Acta Part B, 35B, 521–26 (1980). [Google Scholar]
- [70].Dyulgerova R.; Zhechev D. and Savova Ts.. New construction of spectral lamps with hollow cathodes for atomic absorption determination of molybdenum, iron, and silicon. EP, Elektropromst. Priborostr. 15, 110–11 (1980). [Google Scholar]
- [71].Falk H., and Lucht H.. Investigation of excitation processes in a hollow cathode discharge by time-resolved measurements in the vacuum u. v. J. Quant. Spectrose. Radiat. Transfer 16 909–17 (1976). [Google Scholar]
- [72].Farnsworth P. B. Excitation processes in an R.F.-boosted, pulsed hollow cathode lamp. Dissertation 163 pp., Univ. of Wisconsin, Madison, Wise., (1981). [Google Scholar]
- [73].Fazlaev V. Kh.; Devyatov A. M. and Makarychev S. V.. Excitation Of strontium atoms in a hollow cathode discharge. Vestn. Mosk. Univ., Ser. 3: fiz., Astron. 20, 81–84 (1979). [Google Scholar]
- [74].Fearn D. G.; Cox A. S., Angela S. and Moffitt D. R.. Investigation of the initiation of hollow cathode discharges. U.S. NT1S, AD Rep. AD-A036079, 48 pp. (1976). [Google Scholar]
- [75].Ferreira C. M., and Delcroix J. L.. Theory of the hollow cathode arc discharge. 1. Transfer of energy in electron cascades to neutrals. Application to argon. J. Phys. (Paris) 36, 1233–40 (1975). [Google Scholar]
- [76].Ferreira C. M., and Delcroix J. L.. Theory of the hollow cathode arc discharge. II. Balance of inctaslables at the cathode interior. Application to argon. J. Phys, (Paris) 36 1241–8 (1975). [Google Scholar]
- [77].Freeman G. H, C.; Outred M. and Morris L. R.. A line profile study of the 193, 76 nm arsenic emission line from lamps used in atomic absorption spectroscopy. Spectrocliiin, Acta Part B, 35B, 687–99 (1980). [Google Scholar]
- [78].Fujii K Spectroscopic study of the negative glow in usual glow and in hollow cathode discharges. Jpn, J, Appl. Phys, 16, 1081–90 (1977). [Google Scholar]
- [79].Fukushima H., Spectroisotopic analysis of lithium using the hollow cathode discharge technique. Bunko Kenkyu 21, 416–22 (1972). [Google Scholar]
- [80].Fukushima H., and Nakajima T.. Isotopic analysis of uranium by optical spectral methods. 1. Determination of uranium-235/uraniuin-238 ratios using a hollow cathode discharge source. Bunko Kenkyu 24, 148–55 (1975). [Google Scholar]
- [81].Gagne J M.; Pianarosa P, Larin G. Saint-Dizier J. P., and Bouchard P. Ionization and excitation of uranium in a hollow cathode lamp. Appl. Opt. 20, 3770–3 (1981). [DOI] [PubMed] [Google Scholar]
- [82].Galassi M. Atomic absorption sources. Flame Notes 1, 10–13 (1966). [Google Scholar]
- [83].Gandrud B., and Skogerboe R. K., Hollow cathode discharge as an atomic absorption medium. Appl. Spectrosc. 25, 243–6 (1971). [Google Scholar]
- [84].Godden M. J. Operating parameters of a hollow cathode discharge melting furnace. Vacuum 23 97–99 (1973). [Google Scholar]
- [85].Gofmeister V. P.; Sh K. Desai and Yu M. Kagan, Excitation mechanism in the hollow cathode discharge in the inert gases and mixtures, Int. Conf. Phenomena Ioniz. Gases, Contrib. Pap., 9th. 167 pp. (1969). [Google Scholar]
- [86].Gorbunova T. M Effect of vapors of metals and their com pounds on the current-voltage characteristics of an uncooled hollow cathode discharge, Izv. Vyssh, Ucheb. Zu- ved., Fiz. 16, 137–8 (1973). [Google Scholar]
- [87].Gorbunova T M Possible determination of copper vapor concentration in a hollow cathode discharge from autoionization line intensity. Izv. Vyssh. Uchebn, Zaved., Fiz. 20, 140–2 (1977). [Google Scholar]
- [88].Gorbunova T M., and Semenova O. P., Intake and radiation of atoms in a discharge with a hot hollow cathode. Zh. Prikl. Spektrosk. 12, 17–20 (1970). [Google Scholar]
- [89].Gorbunova T. M., and Semenova O. P., Mechanism of dischaige with a hot hollow cathode, Zh. Prikl, Spektrosk. 17, 592–7 (1972) [Google Scholar]
- [90].Grdhchko D. P., and Shikh R. B., Study of discharge with a hollow cathode in cesium sapors. TeploFiz. Vys. Temp, 15, 708–11 (1977). [Google Scholar]
- [91].Grechanyi V. G., and Metel A. S.. Effect of boundary conditions on the characteristics of a glow discharge with a hollow cathode, Zh. Tekh, Fiz. 52 442–5 (1982). [Google Scholar]
- [92].Grekova G. V.; Lapshin E., I. and Okhmatovskii G, V. Change in structure of a cathode layer on transition from ail anomalous glow-discharge to a glow discharge with a hollow cathode. Pis’ma Zh. Tekh. Fiz. 1 299–302 (1975). [Google Scholar]
- [93].Grekova G. V.; Lapshin E. I. and Okhmatovskii G. V., Mass composition of ions in a discharge with a hollow cathode. Zh. Tekh. Fiz. 48, 1979–81 (1978). [Google Scholar]
- [94].Grigor’eva O. A ; Karpova E., A. and Yu I. Turkm. Layer-by layer spectral analysis in a hollow cathode discharge. Zh. Prikl. Spektrosk. 33, 240–3 (1980). [Google Scholar]
- [95].Grigor’eva O. A.; Zhiglinskii A. G. and Yu I. Turkm, Discharge m a cooled hollow cathode as a source of light for spectral analysis. Zh. Prikl. Spektrosk 19, 787–90 (1973). [Google Scholar]
- [96].Grove E. L., and Loseke W. A.. Hollow cathode excitation of air-type atmospheres. Can. J.Spectrosc. 18, 83–9 (1973). [Google Scholar]
- [97].Guesterncorn S. Investigations on the properties of hollow cathode lamps. Note Techniques C.F.A.; S.C.A.A 69, 027 (1969). [Google Scholar]
- [98].Hagiwara T., Haiada M. and Tanaka K., Hot hollow cathode discharge for metal analysis. Bunko Kenkyu 18, 141–8 (1969). [Google Scholar]
- [99].Headridge J. B., and Richardson J, Comparison of electrodeless discharge tuhes and hollow cathode lamps in atomic absorption spectroscopy. Lab, Pract, 19, 372–3 (1969). [Google Scholar]
- [100].Helm H Experimental measurements on the current balance at the cathode of a cylindrical hollow cathode glow discharge. Beitr. Plasmaphys, 19, 233–57 (1980). [Google Scholar]
- [101].Helm H.; Howorka F. and M, Pahl, Cathode fall in a cylindncal hollow cathode, Z. Naturforsch. A 27, 1417–25 (1972). [Google Scholar]
- [102].Hershcovitch A., and Prelec K.. Hollow cathode discharge as a plasma source for hydride production. Rev. Sci. Instrum. 52, 1459–62 (1981). [Google Scholar]
- [103].Howard C.; Pillow M. E., Steers E. B. M., and Ward D. W.. Intensities of some spectral lines from hollow cathode lamps. Analyst (London) 108, 145–52 (1983). [Google Scholar]
- [104].Howorka F., Neutral gas analysis by a hollow cathode ion source. Proc. Int. Conf, Ion Souices. 2nd 1973, 92–97. [Google Scholar]
- [105].Howorka F., and Pahl M.. Experimental determination of internal and external parameters of the negative glow plasma of a cylindrical hollow cathode discharge in argon Z. Naturforsch. A 27, 1425–33 (1972). [Google Scholar]
- [106].Howorka K; Scherleitner A., Gieseke V., and Kuen I.. Bakeable hollow cathode for the study of ion-molecule reactions in discharges in gaseous mixtures. Int. J. Mass Spectrom Ion Phys. 32, 321–31 (1980). [Google Scholar]
- [107].lijima T. New type hollow cathode discharge tube with continuously variable voltage. Jpn. J Appl. Phys. 20, L470–L472 (1981). [Google Scholar]
- [108].lijima T. Increase of the helium( + ) 468,6 nm emission from a hollow cathode discharge by restricting the tuner cathode wall. Phys, Lett. A 85A 436–8 (1981). [Google Scholar]
- [109].lijima T. On the radial distribution of the helium (He II) 468.6 nm line intensity in a high voltage hollow cathode discharge tube. Opt, Commun. 45, 56–61 (1983). [Google Scholar]
- [110].Ilic D. B. Low frequency instabilities and plasma turbulence. Report, 143 pp. Inst. Space Res., Stanford Univ., Stanford, Calif. (1973). [Google Scholar]
- [111].lova L. P. Capota and Porumbescu S, On the excitation and ionization mechanism in a hollow cathode discharge in krypton and xenon gases, in the presence or absence of an applied magnetic held. Rev. Roum. Phys. 21, 781–93 (1976). [Google Scholar]
- [112].lova L, Dobrc M. and Katrib S.. Hollow cathode effect in a cylindrical geometry using a helium + hydrogen gas mixture Rev. Roum. Phys. 24, 931–40 (1979). [Google Scholar]
- [113].Ivanov A. P., and Radikov D. N.. Effect of nitrogen on the carbon monoxide band intensity in a hollow cathode discharge Metastah: Sostoyaniya At Mol Melody Ikh Issled. Editor. Koroikov A. 1 1977. 113–117. [Google Scholar]
- [114].Ivashina V. A., and Paleyuk G. M., Determination of the electron temperature, plasma potential, and electron concentration in a gaseous discharge wth a hollow cathode. Spektrosk At. Mol. 1969, 7–9. [Google Scholar]
- [115].Kagan Yu. M.; Lyagushchenko R. I., Taroyan A. S., and Khvorostovskii S. N., Energy distribution of electrons in a hollow cathode, II. Zh. Tekh. Fiz. 43 1488–95 (1973). [Google Scholar]
- [116].Kajzer M., and Sternberg Z. Anamalous line broadening in hollow-cathode discharges. Plot. Colloq. Spectrosc. lilt. 14th 2 663–6 (1967). [Google Scholar]
- [117].Khvorostovskii S. N. Balance of charged particles in a gas discharge plasma with a hollow cathode. Zh. Tekh. Fiz. 50, 1876–85 (1980). [Google Scholar]
- [118].Kidrasov F. Kh. Excitation of magnesium atoms in hollow cathode discharge. J. Phys., Colloq. (Orsey, Fr.) 1979, 121–2. [Google Scholar]
- [119].Kidrasov F. Kh.; Volkova L. M., Devyatov A. M., and Arkhipova L. V.. Spectrnscopic study of a discharge in a magnesium hollow cathode with argon and helium filling. Vestn. Mosk. Univ., Fiz., Asronomyia 15, 563–7 (1974). [Google Scholar]
- [120].Kim M. H.; Li S. C. and Pak W. I.. Preparation and properties of hollow cathode lamp based sinter-cathode. Punsok Hwahak 1982, 36–40. [Google Scholar]
- [121].Kirichenko V. L; Tkachenko V. M. and Tyutyunriik V. B.. Effect of geometric dimensions, material of the cathode, and the type of gas on the optimum pressure region of a glow discharge (tube) with a cylindrical hollow cathode. Zh. Tekh. Fiz. 46, 1857–67 (1976). [Google Scholar]
- [122].Kitagawa K.; Suzuki M.. Aoi N.and Tsuge S.. Analytical and spectral features of atomic magnetooplical rotation spectroscopy (the atomic Faraday effect) of antimony, bismuth, silver, and copper with a hollow cathode lamp operated in a pulse mode. Speetrochim Acta. Part B 36B, 21–34 (1981). [Google Scholar]
- [123].Kohsiek W. Measurement of the electron temperature and density of a helium plasma produced hy a hollow cathode arc discharge. Plasma Phys. 17, 1083–9 (1975). [Google Scholar]
- [124].Kolesov A A., Kolosov P. A, Kruchinin A. M., Smirnov Yu. M., and Chursin A.. Characteristics of a hollow cathode and scattering in an external discharge column. Tr. Mos. F.nerg. In-ta 1979, 74–9. [Google Scholar]
- [125].Kolesov A. A.; Kruchinin A. M., Mel’nikov V. V.. Smirnov Yu. M., and Chursin A.. Study of an external discharge column with a hollow cathode. Fiz. Khim. Obrab. Mater. 1979, 113–15, [Google Scholar]
- [126].Kolev N., and Pacheva I.. Increasing the sensitivity of spectral analysis by using a hollow cathode. God. Inst. Tsvetna Metal., Plovdiv 14, 85–96 (1967). [Google Scholar]
- [127].Kolev N., and Pacheva I.. On the entry of not easily vaporized elements into the hollow cathode discharge plasma. Bulg. J. Phys. 5, 639–45 (1978). [Google Scholar]
- [128].Kolev N.; Vracheva N. and Stefanova M.. Spectrochemical determination of tellurium in pure copper using a hollow cathode discharge. Metalurgiya (Sofia) 34, 28–30 (1979). [Google Scholar]
- [129].Korovin Yu. I., and Kuchumov V. A. Use of a hollow cathode discharge lor atomization of samples. Zh. Prikl. Spektrosk. 14, 778–83 (1971). [Google Scholar]
- [130].Krasil’shchik V. Z. Experimental study of the effect of a magnetic field on the radiation intensity of a gas discharge with a hollow cathode. Tr. Vses. Nauch.-Issled. Inst. Khim. Reaktivov Osobo Chist. Khim. Veshchestv 32, 253–61 (1970). [Google Scholar]
- [131].Krasil’shchik V. Z. Construction of an aparatus for spectral analysis in a hollow cathode. Tr. Vses. Nauch.-Issled, Inst. Khim. Reaktivov Osobo Chist. Khim. Veshchestv 34, 181–4 (1972). [Google Scholar]
- [132].Kiasil’shchik V. Z. F. mission spectral analysis of very pure substances. Report 1. Use of a hollow cathode discharge tube. Melody Anal. Veshchestv 1974, 3–37 [Google Scholar]
- [133].Krasil’shchik V. Z., and Yakovelva A. F.. Analysis of nickel and coball oxides in a hollow cathode discharge tube. Zavod. Lab. 37, 181–2 (1971). [Google Scholar]
- [134].Krasil’shchik V. Z.; Yakovleva A. F. and Shiemberg G. A.. Use of electrolytic enrichment and discharge tube with a hollow cathode for the spectral analysis of high-purity substances. Tr. Vses. Nauch.-lsslcd. Inst. Khim. Reaktivov Osobo Chist. Khim. Veshchestv 34, 156–72 (1972). [Google Scholar]
- [135].Kravchenko V. F.; S Mikhalevskii V. and Papakin V. F.. Excitation of helium lines during a pulsed discharge in a hollow cathode. Zh. Tekh. Fiz 43, 2173–4 (1973). [Google Scholar]
- [136].Kreye W. C. Concentratons of gold atoms in hollow cathode discharge hy resonance line absorption. Phenomena Ioniz. Gases. Int. Conf., Contrib. Pap., 8th Vienna, 100 pp. (1967). [Google Scholar]
- [137].Kruglova L. P.; Maksimov D. E., Rudnevskii N. K., and Shabanova T. M.. Use of hollow cathode discharge in a magnetic field for the analysis of metallic titanium for trace impurities. Poluch. i Analiz Chist. Veshchestv, Gor’kii, 1981, 34–6. [Google Scholar]
- [138].Kucherenko E. T.; Zykova E. V. and Makosevskaya L. N.. Glow discharge with sectionalized hollow cathodes. Ukr. Fiz. Zh. (Russ. Ed.) 17, 2063–5 (1972). [Google Scholar]
- [139].Kudankin A. I., and Radikov D. N.. Effect of carbon monoxide on the intensity of the nitrogen band in a hollow cathode-discharge. Metastab. Soxtoyaniya At. Mol. Metody Ikh Issled. 1977, 102–12. [Google Scholar]
- [140].Kuen I.; Stori H. and Howorka F., Spectroscopic investigations of a hollow cathode discharge in helium. Contrib.-Symp. At. Surf. Phys, 1980, 159–66. [Google Scholar]
- [141].Kureichlk K. P. Study of the stability of hollow cathode lamps operating under pulsed conditions. Zh. Prikl. Spectrosk. 36, 907–12 (1982). [Google Scholar]
- [142].Lazareva L. P., and Rudnevskii A. N., Study of the effect of alkaline elements on the intensity of magnesium lines excitable in a discharge with a hollow cathode in the presence of a magnetic field. Zh Prikl. Speklrosk. 31, 400–3 (1979). [Google Scholar]
- [143].Lazareva L. P.; Shabanova T. M., Maksimov D. E., and Rudnevskii N. K.. Increase in the sensitivity of the determination of Group IV elements in solutions by excitation of the spectrum in the discharge of a hollow cathode with a magnetic field. Fiz.-Khim. Metody Analiza (Gor’kii) 1979, 42–4. [Google Scholar]
- [144].Maerk T. D. Mass speetronietric study of the neutral particles in the negative glow of cylindrical hollow cathode discharge in nitrogen. Z. Naturforsch., Tell A 28, 1397–404 (1973). [Google Scholar]
- [145].Maerk T. D.; Lindinger W., Howorka F., Egger F., Varney R. N., and Pahl M.. Simple bakeablc hollow cathode device for the direct study of plasma constituents. Rev. Sci. Instrum. 43, 1852–3 (1972). [Google Scholar]
- [146].Maksimov D. E.; Lazareva L. P., Nizyakova L. D., and Shabanova T. M.. Emission spectrographic analysis of powdered samples using two-hollow cathode discharge and superimposed magnetic field. Zh. Anal. Khim. 34, 1045–8 (1979). [Google Scholar]
- [147].Maksimov D. E., and Rudnevskii N. K.. Spectrographic analysis of germanium for selenium and cadmium impurities by using a hollow cathode discharge. Tr. Khim. Khim. Tekhnol. 1968, 56–8. [Google Scholar]
- [148].Maksimov D. E., and Rudnevskii A. N., Study and analytical use of the effect of increasing the intensity of lines in a discharge with a hollow cathode. Zh. Prikl. Spektrosk. 34, 406–9 (1981). [Google Scholar]
- [149].Maksimov D. E., and Rudnevskii A. N., Effect of enhancement of line intensity for some elements excited hy hollow cathode discharge with the introduction of cadmium vapor into the plasma. Dokl. Akad. Nauk SSSR 256, 628–31 (1981). [Google Scholar]
- [150].Marakhtanov M. K. Hollow cathode effect in a low-pressure arc discharge, Teplofiz. Vys. Temp. 20, 376–8 (1982). [Google Scholar]
- [151].Mase H; Arai K.. Kawamura W., Wasa S., and Tanabe T., Hollow cylinder cathode for superdense glow discharge. Iongen to Ion o Kiso toshita Oyo Gijutsu, Shinpojumu. 5th, 145–6 (1981), Conf. Proc. [Google Scholar]
- [152].Matic-Dobrosavijevic J. S. Spectrographic determination of fluorine in uranium and its oxides using a discharge tube with a hollow cathode. Glas. Hem. Drus., Beograd 38, 367–74 (1973). [Google Scholar]
- [153].Mehs D. M., and Niernczyk T. M., Excitation temperatures in the hollow cathode discharge. Appl. Spectrosc. 35, 66–9 (1981). [Google Scholar]
- [154].Menge K H.; Maierhofer J. and Reis A.. Spectrographic analysis of solutions with a hollow cathode discharge lamp as excitation source. Messtechmk (Brunswick) 80, 304–6 (1972). [Google Scholar]
- [155].Metyel A. S., and Nastyukha A. I.. Investigation of dissociation processes of some molecules in the hollow cathode glow discharge plasma. Proc. Int. Conf. Phenom. loniz. Gases, 13th. 1 379–80(1977). [Google Scholar]
- [156].Mohamad S. Z., and Pctrakiev A.. Comparative investigations of several sources of light for atomic absorption spectral analysis. Spectrosc. Lett. 14, 47–60 (1981). [Google Scholar]
- [157].Mohammed Y. Contribution to the study of secondary effects of steady discharge in helium. Report. 105 pp. (1980), Toulouse-3 Univ., Toulouse, Fr, [Google Scholar]
- [158].Mokhamad S., and Petkov A. Use of time-resolving high resolution spectroscopy in the investigation of pulse hollow-cathode discharges. J. Phys., Colloq. (Orsay, Fr.), 1979, 195–96. [Google Scholar]
- [159].Moskalev B. I. Structure of plasma inside the hollow cathode of the glow discharge. Int. Conf. Phenomena loniz. Gases. Contrib. Pap., 9th, 166 (1969), Bucharest Rom. [Google Scholar]
- [160].Nehmzow B.; Rutscher A. and Wagner H. E.. Dissociation of water vapor in the hollow cathode glow discharge. J. Phys., Colloq. (Orsay, Fr.) 1979, 55–6. [Google Scholar]
- [161].Novoselov V. A.; Gaikovich S. K. and Aidarov T. K.. Hollow-cathode discharge as a source of light in the vacuum ultraviolet. Prikl. Spektrosk., Mater. Sovcshch., 16th. 1, 63–8 (1969). [Google Scholar]
- [162].Novoselov V. A., and Znamenskii V. B.. Correlations between the intensity of spectral lines, discharge parameters m hollow cathode, and its diameter. Spektrosk., Tr. Sih. Soveshch., 4th 1969, 273–8. [Google Scholar]
- [163].Otruba V.; Jambor J. and Horak J.. Spectral properties of discharge lamps with a hollow cathode during pulsed feeding. Chem. Listy 73, 295–303 (1979). [Google Scholar]
- [164].Pacheva I Excitation of the spectra of barium, cadmium, and copper in a gas-discharge tube with a hollow cathode Izv. Fiz. Inst ANEB, Bulg. Akad. Nauk. 15, 177–84 (1966) [Google Scholar]
- [165].Pacheva I. Inverse population of levels in a hollow cathode discharge. Dokl.-Nats. Konf. At. Spektrosk., 8th, 99–119 (1978). [Google Scholar]
- [166].Pacheva I., and Abadzhieva L.. Effective excitation of the spectrum of dysprosium m a discharge tube with a hollow cathode. Izv. Fiz. Inst. ANEB, Bulg. Akad. Nauk, 16 135–41 (1967). [Google Scholar]
- [167].Pacheva I.; Dyulgerova R., Zhechev D., and Videnova V., Emission lines of zinc from hollow cathode discharges, Bulg. J. Phys. 3 55–63 (1976). [Google Scholar]
- [168].Pacheva I., and Naidenov M.. Spectroscopic investigation of the discharge in a hollow cathode. Izv. Fiz. Inst. ANEB, Bulg. Akad. Nauk. 16, 129–33 (1967). [Google Scholar]
- [169].Pacheva I. Kh.; Pramatarov P. M. and Khristov N. N.. Temperature gradient in the negative glow of a hollow-cathode discharge in helium. Zh. Tekh, Fiz. 42, 2353–6 (1972). [Google Scholar]
- [170].Pacheva I., and Zhechev D.. Mechanism of excitation of the spectra of elements in a hollow cathode tube. Izv. Fiz. Inst. ANEB (At. Nauchnoeksp. Baza), Bulg. Akad. Nauk. 22, 5–11 (1972). [Google Scholar]
- [171].Pahl M.; Lindinger W. and Howorka F.. Mass spectrometry of the negative glow of a cylindrical hollow cathode discharge. Z. Naturforsch. A. 27 678–92 (1972). [Google Scholar]
- [172].Palmer B. A.; Keller R. A. and Englemau R. Jr.. Atlas of uranium emission intensities in a hollow cathode discharge. Report 242 pp. Los Alamos Sci. Lab., Los Alamos. NM: (1980). [Google Scholar]
- [173].Perkov I. A. Hollow cathode discharge in cadmium vapor. Mater. Vses. Konf. Plazmennym Uskorit., 2nd. 1973, 233–4. [Google Scholar]
- [174].Pevtsov G A., and Krasil’shchik V. Z.. Use of a hollow cathode discharge luhe for analyzing aluminum-ammonium alums. Prikl. Spektrosk., Mater. Soveshch., 16th 1, 243–6 (1969). [Google Scholar]
- [175].Pevtsov G. A.; KrasiPshchik V. Z. and Yakovleva A. F.. Spectrographic determination of impurities in aluminum oxide using a hollow cathode discharge. Zavod. Lab. 35, 1340–3 (1969). [Google Scholar]
- [176].Pevtsov G. A.; KrasiPshchik V. Z. and Yakovleva A. F.. Spectrographic determination of impurities in titanium (IV) chloride using a hollow cathode discharge. Zh. Anal. Khim. 25, 580–1, (1970). [Google Scholar]
- [177].Philip C. M. Hollow cathode discharge characteristics. AIAA J. 9, 2191–6 (1971). [Google Scholar]
- [178].Pianarosa P.; Bouchard P., Saint-Dizier J. P., and Gagne J. M.. Density of uranium ions in the 419/20 ground state in a hollow cathode type discharge. Appl. Opt. 22, 1568–72 (1983). [DOI] [PubMed] [Google Scholar]
- [179].Pichugm N. G.; Rudnevskii N.K. and Maksimov D.E.. Use of discharge in a hollow cathode in the spectrographic determination of some impurities in graphite powder. Zh. Anal. Khim. 32, 12–14 (1977) [Google Scholar]
- [180].Pillow M. E. A critical review of spectral and related physical properties of the hollow cathode discharge. Spectrochim. Acta Pari B, 36B, 821–43 (1981). [Google Scholar]
- [181].Pofralidi L. G.; Pofralidi M. G., Talalaev B. M., and A Kogan A.. Use of new designs of light sources (small lamps with a hollow cathode) in atomic-absorption spectral analysis, Tr. G1AP 50, 49–57 (1978). [Google Scholar]
- [182].Popa G.; Sanduloviciu M.. Croitoru P.. and Moldovan C.. Electron beam generation by a hollow cathode discharge, J. Phys., Colloq. (Orsay. Fr.) 1979, 187–8. [Google Scholar]
- [183].Popovici C.: Avram E., Bălăceanu M., Ceauscscu N., Military A., and Dumitrescu A.. The hollow cathode effect in a mixture of hydrogen and nitrogen. Rev. Roum. Phys. 24, 343–7 (1979). [Google Scholar]
- [184].Popovici C.: Balaceanu M., Ceausescu N., and Avram E.. High-voltage hollow cathode discharges. Part I. Physical foundation. Stud. Cercet. Fiz. 32, 889–907 (1980). [Google Scholar]
- [185].Popovici C.; Krejci V. and Stirand O.. Effect of hollow cathode with magnetic field on ionization waves in a neon discharge plasma. Rev. Roum. Phys. 13, 423–6 (1968) [Google Scholar]
- [186].Reshenov S. P., and Antoshkin N. F.. Plasma generation in a hollow arc discharge. Iz.v. Sih. Otd. Akad Nauk SSSR, Ser. Tekh, Nauk 1982, 54–62. [Google Scholar]
- [187].Rocca J I.; Fetzer G. J. and Collins G. J. The effect of an axial magnetic field on the spontaneous emission from an argon hollow cathode discharge. Phys. Lett. A 84A, 118–22 (1981). [Google Scholar]
- [188].Rozsa K. Hollow cathode discharge with variable voltage. Contrib.-Syntp. At. Surf. Phys. 1980, 167–72. [Google Scholar]
- [189].Rudnevskn A. N., and Maksimov D. E., Study of the effect of cadmium on the intensity of sodium lines in a discharge with a hollow cathode, Poluch, i Analiz Chist. Veshchestv. Gor’kii 1981, 37–9. [Google Scholar]
- [190].Rudnevskn N. K ; Maksimov D. E., and Bakhareva L. V.. Spectrographic analysis of tungsten in sulfur and phosphorus impurities using a hollow cathode. Zavod. Lab. 40, 1478–9 (1974). [Google Scholar]
- [191].Ruduevskii N. K.; Maksimov D. E and Lazareva L P Spectrographic determination of alkali metals in solutions by using a hollow cathode discharge in a magnetic field. Zh. Anal. Khim. 29, 1422–4 (1974). [Google Scholar]
- [192].Rudnevskii N. K.; Maksimov D. E. and Lazareva L. P. Study of excitation conditions for the spectrum of alkaline earth elements in a hollow cathode discharge in a magnetic field. Zh. Prikl, Spektrosk. 24, 136–8 (1976). [Google Scholar]
- [193].Rudnevskii N. K.; Maksimov D E., and Lazareva L. P.. Spectral determination of rare earth elements m solutions using a hollow cathode discharge in a magnetic field. Zh. Prikl. Spektiosk. 29, 916–18 (1978). [Google Scholar]
- [194].Rudnevskii N. K.; E Maksimov D., Pichugin N. G., and Khasyanov R. Kh.. Conditions for the excitation of indium and gallium spectra in a discharge with a hollow cathode in different electric power supply systems. Zh, Prikl, Spektrosk. 2(1, 707–9 (1974). [Google Scholar]
- [195].Rudnevskii N. K.; Maksimov D. E. and Shabanova T. M.. Esc of discharge in a hollow cathode in the spectral determination of a superstoichiometnc amount of elements in some binary compounds. Spektrosk., Tr Sib. Soveshch., 4th 1969. 380–2. [Google Scholar]
- [196].Rudnevskii N K.: Maksimov D. E. and Shabanova T. M.. Spectrographic studies and analytical use of a hollow cathode discharge in a magnetic field. Zh. Prikl. Spektrosk. 13, 199–203 (1970). [Google Scholar]
- [197].Rudnevskii N. K.; Maksimov D. E. and Shabanova T. M., Increasing the sensitivity of spectrographic determination of microadditives using a discharge with a hollow cathode in a magnetic field. Ural Konf. Spektrosk., 7th, 1, 12–14 (1970). [Google Scholar]
- [198].Rudnevskn N. K.; Maksimov D. E., Shabanova T. M, and Bakhareva L. V.. Spectrographic analysis of tungsten powder for sulfur, phosphorus, zinc, and cadmium impurities using a hollow cathode discharge, Tr Khim, Khim, Tekhnol. 1973, 102–3. [Google Scholar]
- [199].Rudnevskii N. K.; Maksimov D. E., Shabanova T. M., and Kruglova L. P. Use of a hollow cathode discharge in a magnetic field for the analysis of chromic oxide for impurities, Zh Prikl. Spektrosk. 34, 1114–16 (1981). [Google Scholar]
- [200].Rudnevskii N. K.; Maksimov D. E., Shabanova T, M, and Lazareva L. P.. Discharge in a hollow cathode during the imposition of magnetic fields of different configurations on it. Zavod. Lab. 38, 1338–41 (1972). [Google Scholar]
- [201].Rudnevskii N. K.; Maksimov D. E., Shabanova T. M., and Lazareva L. P.. Spectrographic determination of cadmium in solutions during spectrum excitation in a hollow cathode discharge with the superposition of a magnetic field. Zh. Prikl. Spektrosk. 16, 356–8 (1972). [Google Scholar]
- [202].Rudnevskii N. K.; Maksimov D E., Tumanova A. N., and Shabanova T M., Spectral analysis of semiconductor silicon and germanium by using a hollow cathode discharge. Zh. Prikl. Spektrosk. 37 722–4 (1982). [Google Scholar]
- [203].Rudnevskn N. K.; Maksimov D. E. and Vysotskii V. V.. Use of discharge in a hollow cathode for the quantitative determination of excess sulfur in cadmium sulfide and selenium in cadmium selenide. Spektrosk At. Mol. 1969, 72–4. [Google Scholar]
- [204].Rudnevskii N. K., Pichugin N. G. and Maksimov D. E., Spectral analysis of semiconductor silicon for impurities of hard-to-excite elements in a hollow cathode discharge. Zh. Prikl. Spektrosk. 25, 921–3 (1976). [Google Scholar]
- [205].Rudnevskii N. K.; Pichugin N. G., Maksimov D. E., and Kachan E. E.. Spectral determination of the rare earth elements in solutions using a discharge with a hollow cathode in various power supply regimes with a superimposed magnetic field, Poluch, Anal. Veshchestv Osoboi Chist., (Dokl. Vses. Konf.). 5th 1978, 215–20. [Google Scholar]
- [206].Rudnevskii N. K.; Pichugin N, G., Maksimov D. F, and Kachan E. E.. Spectral determination of rare earth elements in solutions using a discharge with a hollow cathode under different electric supply conditions and with super-imposition of a magnetic field, Poluchcnic i Analiz Veshchestv Osob. Chistoty. M. 1978, 215–20. [Google Scholar]
- [207].Rudnevskii N. K.; Shabanova T. M. and Maksimov D E.. Effeel of a magnetic field on line intensity during spectral excitation in a hollow cathode discharge. Tr. Khim Khim, Tekhnol. 1973, 75–6. [Google Scholar]
- [208].Rudnevskii N. K.; Shabanova T. M.. Maksimov D. E., and Lazaieva L. P., Use of a hollow cathode discharge for analyzing amorphous boron for trace impurities. Zh. Prikl, Spektrosk. 30 1099–101 (1979). [Google Scholar]
- [209].Rudnevskii N. K.; Tumanova A. N., Maksimov D. E., and Lomzilova L. V., Spectrographic determination of some impurities in semiconductor germanium by using a hollow cathode discharge. Zh, Prikl. Spektrosk. 11, 783–6 (1969). [Google Scholar]
- [210].Rybnicek J. Corposcular diagnostics of a hollow cathode discharge. Report, 56 pp. 1978, Inst. Plasma Phys., Cesk. Akad. Ved. Prague, Czech. [Google Scholar]
- [211].Rybnicek J. Corpuscular diagnostics of a hollow cathode discharge, I. Czech. J. Phys. B29, 422–33 (1979) [Google Scholar]
- [212].Ryhnicek J. Corpuscular diagnostics of a hollow cathode discharge. II. Limit angle of inelastic scattering of ions by atoms, Czech. J. Phys B29, 533–44 (1979). [Google Scholar]
- [213].Ryhnicek J. Corpuscular diagnostics of a hollow cathode discharge, III. The metal-ions-regime evolution. Czech. J. Phys. B30, 1307–14 (1980). [Google Scholar]
- [214].Sabatovskaya V. L.; Dzhupii and L. S. Yudelevich I. G., Methods for layer-by-layer spectrocbemical analysis of silicon structures using a discharge with a hot hollow cathode, Nov. Metody Instrument. Analiza Materialov, M. 1979, 19–24. [Google Scholar]
- [215].Sabatovskaya V. L.; Kuzovlev I. A. and Yudelevich I. G.. Lowering the detection limit of microimpurities during the spectrochemical analysis of high-purity substances. Zh. Prikl. Spektrosk. 26, 207–12 (1977). [Google Scholar]
- [216].Tu Sato Tada M., Huang Y. C., and Takei H.. Physical vapor deposition of chromium nitride and titanium nitrides by the hollow cathode discharge process. Thin Solid Films 54, 61–5 (1978). [Google Scholar]
- [217].Schmid. G. Non-thermal equilibrium in a helium hollow cathode discharge. Z. Naturforsch. A 26 1899-906 (1971). [Google Scholar]
- [218].Scullman. R., and P. Cederbalk, The hollow cathode as a source for diatomic platinum molecules. J. Phys. B 10, 3659–64 (1977). [Google Scholar]
- [219].Semenova O. P., and Gorhunova T. M.. Mechanism of discharge from a hot hollow cathode. II. Zh. Prikl, Spektrosk 10 487–92 (1969). [Google Scholar]
- [220].Semenova O. P.; Gorbunova T. M., Bokova N. A., and Sukhanova G. B.. Mechanism of discharge with a hot hollow cathode. Zh. Prikl Spektrosk. 9, 93741 (1968). [Google Scholar]
- [221].Semenova O. P., and Red’Kiria N. V.. Characteristics of the occupation of autoionization states of copper in a discharge with a hollow cathode in helium plus copper and helium plus copper plus silver mixtures, Avtoionizatsion. Yavleniya v Atomnkh. Tr. 2-go Nauch. Seminara, Moskva, 1980, M. 1981, 279–86. [Google Scholar]
- [222].Semenova O. P., and Sukhanova G. B.. Emission characteristics of copper atoms and ions m a hot hollow cathode discharge. Zh Prikl. Spektrosk. 13, 956–60 (1970). [Google Scholar]
- [223].Senoionte O.: S. Caroli and A. Alimonti. A modified version of the Grimm’s glow discharge lamp for use as a demountable hollow cathode emission source. IV. Further data on the behavior of blackening curves for copper. Spectrosc. Lett. 14 195–206 (1981). [Google Scholar]
- [224].Shabanova T. M.; Maksimov D. E. and Rudnevskii N. K.. Atomic emission spectrographic analysis of semiconductor silicon using a hollow cathode discharge in a magnetic field. Tr. Khim. Khim. Tekhnol. 1975, 65–7. [Google Scholar]
- [225].Shvangiradze R. R. Spectral-isotopic method for determining gases in solids using a hollow cathode discharge. Metody Opred. Issled. Sostoyaniya Gazov Met., Vses. Konf., 3rd 1, 94–7 (1973). [Google Scholar]
- [226].Slevin P. J. and Harrison W, W, Hollow cathode discharge as a spectrochemical emission source. Appl. Spectrosc. Rev 10, 201–56 (1975). [Google Scholar]
- [227].Smith K. Ed. Analytical spectroscopy Temperature characteristics of a hollow cathode glow discharge plasma, Characterization of arsenic by atomc absorption spectroscopy in oxy acetylene flames. Determination of iron in copper- aluminum alloys by atomic absorption spectroscopy usable resonance lines of iron. Dissertation, 160 p. Univ. of Iowa, Iowa city, la: (1971). [Google Scholar]
- [228].Solomatin V. S ; Vlasov V. S., Sabatovskaya V. I., and Kuzovlev I. A.. Use of a discharge in a hot hollow cathode for determining impurities in trimethylgalliumctherate. Zavod. Lab. 44, 1346–7 (1978). [Google Scholar]
- [229].Somesan M Self-absorption and cathode sputtering in a hollow cathode discharge in magnetic field, Int, Conf. Phenomena lomz. Gases. Contrib. Pap. 9th 1969, 119 pp. [Google Scholar]
- [230].Somesan M. Self-ahsorption and cathode sputtering in a hollow cathode discharge in a magnetic field. Rev. Roum. Phys. 16, 407–11 (.1971). [Google Scholar]
- [231].Somesan M., and Mihali-Pavelescu G.. Hollow cathode spectral sources and their use in atomic ahsorption spectrophotometry. Studii Cercetari Fiz. 24, 579–92 (1972). [Google Scholar]
- [232].Sommer K.; Thorne A. P. and Learner R. C. M.. An active filter for inert gas lines in a hollow cathode light source. J. Phys. D 16, 233–44 (1983). [Google Scholar]
- [233].Sonobe T.; Ochiai K., Asakura Y.. Tsutsumi K., Aihara W., Takahata Sh., and Shimomura T.. Isotope analysis of uranimu by interference spectroscopy. I. Measurement of uranium-235/uranium-238 ratios by emission with a hollow cathode discharge tube Nippon Genshiryoku Gakkaishi 18, 171–6 (1976). [Google Scholar]
- [234].Sonoda T.; Nakagava I. and Nishihara K.. Spectrographical analysis of uranium-235 with a hollow cathode tube. Bunko Kenkyu 20, 98–104 (1971). [Google Scholar]
- [235].Sugaw’ara M., Murata K., Ohshima T., Motohashi K., and Kobayashi K.. A hollow cathode discharge as a cold uniform plasma source. J. Phy. D. 14 L137–L140 (1981) [Google Scholar]
- [236].Sugawara Y., and Iijima T.. Charge transfer from neon ion to zinc atom within hollow cathode discharge. Seikei Daigaku Kogakubu Kogaku Hokoku 8, 671–2 (1969). [Google Scholar]
- [237].Sukhanova G. B. Mechanism of the population of highly excited states of copper and silver in a hollow cathode discharge. Izv. Vyssh. Uchebn, Zaved., Fiz. 21, 105–8 (1978). [Google Scholar]
- [238].Sukhanova G. B., and Semenova O. P., Nature of the radiation of metal vapors in a hot, hollow cathode discharge. Izv. Vyssh. Ucheb. Zaved., Fiz. 13, 99–102 (1970). [Google Scholar]
- [239].Sukhanova G. B., and Semenova O. P.. Measurement of absolute populations of excited states of copper atoms in a highcurrent discharge with a hollow cathode. Izv. Vyssh. Uchebn. Zaved,. Fiz. 20, 35–40 (1977). [Google Scholar]
- [240].Sullivan J. V., and Van Loon J. C.. A demountable boosted-output spectral lamp for atomic absorption and fluorescence measurements. Anal. Chim. Acta 102, 25–32 (1978). [Google Scholar]
- [241].Szilvassy-Vamos Z. Use of hollow cathode excitation in emission spectral analysis. Gep 25, 155–8 (1973). [Google Scholar]
- [242].Takezaki Y. Experimental study of radial distribution of spectral line intensity emitted from a cylindrical hollow cathode neon discharge in a magnetic field. J. Sci Hiroshima Univ, Ser A; Phys. Chem. 41, 35–58 (1977). [Google Scholar]
- [243].Teixetra M. R., and Rodrigues F. C., The state of equilibrium in a hollow cathode discharge. J. Phys. D 12, 2173–80 (1979). [Google Scholar]
- [244].Teodorovich Z. S., and Semenova O. P.. Emission by gold atoms and ions in a hollow cathode discharge, Izv. Vyssh. Uchebn. Zaved., Fiz. 19, 32–9 (1976). [Google Scholar]
- [245].Thelin Bo. The use of a high temperature hollow cathode lamp for the determination of trace elements in steels, nickel-hase alloys, and ferroalloys by emission spectrometry. Appl. Spectrosc. 35, 302–7 (1981). [Google Scholar]
- [246].Timanyuk V A., and Tkachenko V. M.. Study of optical characterisics of a discharge with a cylindrical hollow cathode in helium. Vestn. Khar’kov. Un-ta 1980, 69–71. [Google Scholar]
- [247].Timanyuk V. A., Tkachenko V. M., and B Tyutyunnik V.. Current amplification in a glow discharge with a hollow plasma cathode. Vestn. Khar’kov. Un-ta 1979, 105–7. [Google Scholar]
- [248].Tkachenko V. M., and Tyutyunnik V. B.. Effect of a magnetic field on a hollow cathode discharge. Opt. Spektrosk. 26, 896–8 (1969). [Google Scholar]
- [249].Tkachenko V. M., and Tyutyunnik V. B.. Glow discharge with a cylindrical hollow cathode in helium. Izv. Vyssh. Ucheb. Zaved. Radioftz. 16, 1759–66 (1973). [Google Scholar]
- [250].Tkachenko V. M., and Tyutyunnik V. B.. Study of plasma parameters in a discharge from a cylindrical hollow cathode in helium. Zh. Tech. Fiz. 46, 1449–58 (1976). [Google Scholar]
- [251].Török T.; Zaray G. and Rehak N., Radial distribution of the gas temperature of a discharge in a cooled hollow cathode. Dokl.-Nats. Konf. Al. Spektrosk., 8th 1978, 120–31. [Google Scholar]
- [252].Török T. Trends of the development of the hollow cathode as radiation source. Kem. Kozl. 57, 307–14 (1982). [Google Scholar]
- [253].Török T., and Zaray G.. Experiments with a low-temperature analytical twin hollow cathode interferometer- spectrometer. III. Relation between the discharge parameters for excitation of helium and argon carrier gases. Spectrochim. Acta, Part B, 33B, 101–13 (1978). [Google Scholar]
- [254].Tsukamoto A. Anomalous change of emission spectral intensity in the hollow cathode discharge. Jap. J. Appl. Phys. 7, 92–3 (1968). [Google Scholar]
- [255].Uramoto J. An accelerated plasma electron beam for vacuum metallurgy. Shinku 20, 170–5 (1977). [Google Scholar]
- [256].Van der Sijde B. Temperature and density profiles of electrons in a hollow cathode argon-arc discharge. J. Quant. Spectrosc. Radial. Transfer 12, 1497–516 (1972). [Google Scholar]
- [257].Van der Sijde B., and Tielemans P. A. W., Temperatures in a hollow cathode argon arc discharge with pressure variation, Contrib, Pap.—lnt, Conf Phenom. Ioniz. Gases, 11th, 1973, 127. [Google Scholar]
- [258].Vaulin E. P. Theoretical principles of heat, mass, and electric charge transfer in hollow cathode units for vacuum melting, Tr. Mosk. Energ. Inst. 462, 25–43 (1980) [Google Scholar]
- [259].Vaselovskii V V. and Naslyukha A. I.. Luminescence of molecules of rare gas halides XeF* and XeCl* formed in a hollow cathode pulse glow discharge plasma. Zh. Prikl. Spektrosk 34, 100–4 (1981). [Google Scholar]
- [260].Vlastnik J.; Luzar O. and Hoiek T., Refilling discharge tubes with hollow cathodes. Hutn. Listy 1, 53–6 (1970). [Google Scholar]
- [261].Volkova L. M.; Devyatov A. M. and Fazlaev V. Kh.. Determination of the temperature and the concentration of strontium atoms and ions in a discharge in a cooled hollow cathode according to the contour of the spectral lines. Vestn. Mosk. Univ., Ser. 3; Fiz., Astron. 18, 20–3 (1977). [Google Scholar]
- [262].Volkova L. M. ; Devyatov A. M. and Fazlaev V. Kh.. Mechanism of the formation of strontium and barium ions in a discharge in a cooled hollow cathode,Vestn, Mosk, Univ., Ser. 3: Fiz., Astron. 23, 16–20 (1982). [Google Scholar]
- [263].Vogel G. A. Analysis of xenon chloride emission in hollow cathode discharge. Report. 64 pp. Number: AF1T/GEP/PH/81–6 (1981).
- [264].Wachter F. and Hutanu G.. Hollow cathode discharges of spherical symmetrical configuration in helium. Rev. Rourn. Phys. 14, 937–43 (1969). [Google Scholar]
- [265].Wagermar H. C. and De Galan L.. Interferometric measurements of atomic line profiles emitted by hollow cathode lamps and by acetylene-nitrous oxide flame. Spectrchim, Acta, Part B 28, 157–77 (1973). [Google Scholar]
- [266].Warner B. E. Investigation of the hollow cathode discharge at high current density. Dissertation 287 pp. Univ. Colorado. Boulder, Co. 1979. [Google Scholar]
- [267].Willins D. J., and Boyd R L F.. Electron emission processes in a hollow cathode discharge. J. Phys. D 6, 1447–54 (1973). [Google Scholar]
- [268].Winegarden J. Measurement of molecular chlorine concentration in a xenon chloride hollow cathode discharge including the effect of molecular hydrogen addition. Report, 102 pp. 1980 Number: AFIT/GEP/PH/80–12. [Google Scholar]
- [269].Witting H, L, Hollow cathode discharge with thermionic cathodes. J. Appl. Phys. 42, 5478–82 (1971). [Google Scholar]
- [270].Wright R. B.; Bates J. K. and Gruen D. M., Matrix-isolation spectroscopy of aluminum, copper, and nickel hydrides and deuterides produced in a hollow cathode discharge. Inorg. Chem. 17, 2275–8 (1978). [Google Scholar]
- [271].Yamashita M., and Hasunuma H., Intensity distribution of spectral lines in a copper hollow cathode. Bunko Kenyu 24, 29–34 (1975). [Google Scholar]
- [272].Yamashita M., and Kimura M., Spatial change of rise time of spectral line intensities in hollow cathode discharge tube. Jpn. J. Appl. Phys. 19, L449–L452 (1980). [Google Scholar]
- [273].Zakharov E. A.; F Myasoedov B., Lebedev I. A., Ozhegov P. I., and Karyakin A, V, Processes for supplying americium and curium to a discharge plasma with a hollow cathode, Zh. Prikl. Spektrosk. 21, 239–43 (1974). [Google Scholar]
- [274].Zakharov E. A.; Myasoedov B. F. and Lebedev I. A., Determination of americium in curium by discharge in a hollow cathode. Zh. Anal. Khim. 30, 1344–8 (1975). [Google Scholar]
- [275].Zakharov F. A.: Myasoedov B, F., Ozhegov P. I., and Karyakin A. V.. Quenching of excited metal atoms in an uncooled hollow cathode. Zh. Prikl. Spektrosk. 21 21–7 (1974). [Google Scholar]
- [276].Zakorinn N. A.; Kohas-Arandu M. M., Orlova N M., and Petrov A. A.. Possible spectrochemical determination of precious metals m ores using a hot hollow cathode. Zh. Prikl. Spektrosk. 31, 404–8 (1979). [Google Scholar]
- [277].Zakorina N. A., and Petrov A. A.. Isotopic spectroscopic analysis of hydrogen in a hot hollow cathode. Zh. Prikl. Spektrosk. 23, 195–200 (1975). [Google Scholar]
- [278].Zarnfir O., and Ionescu-Bujor Th.. Operation of the hollow cathode discharge in an axial magnetic field. Rev. Rom. Phys. 20, 447–52 (1975). [Google Scholar]
- [279].Zarnfir O., and Popovici C., High voltage and low voltage inodes of the hollow cathode discharge in the magnetic field. Contrib. Pap-Int. Corif. Phenom. Ioniz, Gases. 11th 1973, 122, Rom. [Google Scholar]
- [280].Zhechev D. Use of a hollow cathode discharge tube for the determination of the natural spectral line width, Bulg. J. Phys. 3 319–22 (1976) [Google Scholar]
- [281].Zhechev D. Copper excited states formation in a hollow cathode discharge. Opt. Spektrosk. 49, 465–8 (1980). [Google Scholar]
- [282].Zhechev D. Hanle-signal and spectroscopic effects in a conical bottom hollow cathode discharge. Spectrosc Lett. 14, 293–300 (1981). [Google Scholar]
- [283].Zhechev D. On the deformation of Hanle signal of 42P3/2 copper (Cu I) level in hollow cathode discharge. J. Environ. Sci. Health, Part A, A16, 149–56 (1981). [Google Scholar]
- [284].Zhechev D Z., and Chaika M. P.. Hollow cathode discharge radiation in a weak magnetic field, Opl, Spektrosk. 43, 590–1 (1977). [Google Scholar]
- [285].Zhechev D.; Dyulgerova R., and Angelova R.. Radial in-homogeneides in excitation and profile of copper lines in hollow cathode discharge, Spectrosc. Lett. 9,401–10(1976). [Google Scholar]
- [286].Zhechev D.; Komitov L. and Touchev E.. Discharge tube with transparent hollow cathode. Spectrosc. Lett. 11, 423–6 (1978). [Google Scholar]
- [287].Zhiglinskii A. G., and Khlopina T. N.. Electrical and optical characteristics of a discharge in a cooled hollow cathode. I. Opt. Spektrosk. 32, 645–9 (1972). [Google Scholar]
- [288].Zhiglinskii A. G.; Kuchinsii V. V., Milovanov N. P., and Presnukhma I. P. Determination of the concentration of metal atoms using the redistribution of intensity in spectral line hyperfine structure. Opt. Spektrosk. 42, 427–30 (1977). [Google Scholar]
- [289].Zhiglinskii A. G.; Kund G. G. and Morozov A. O.. Holographic study of coherent properties of hollow cathode discharge emission, Opt. Spektrosk. 45, 995–9 (1978). [Google Scholar]
- [290].Zyrnicki W., and Osinska E., Spectroscopic studies of the hollow cathode discharge. Czech. J. Phys B32, 1303–4 (1982). [Google Scholar]
Biography
About the Author: Radu Mavrodineanu’s work has included the development of various spectroscopic sources of excitation in analytical measurements. Now associated with the Bureau as a reemployed annuitant, he was with NBS’ Center for Analytical Chemistry from 1969 through 1978.
Footnotes
Most of the information presented in this section is based on the fundamental works of G. Francis [105] and F. M. Penning [257], the bracketed figures indicating references appearing at the end of this paper. Information was also obtained from other sources [94, 213, 252, 257, 365, 386 and 10].
NOTE: Bracketed numerals in roman type identify the 400 citations assembled in the main body of Section 5, Collection of References to Works on Low Pressure Glow Discharges. Bracketed numerals in italic type identify papers in a 290-citation addendum to this collection, also part of Section 5.
In order to adequately describe materials and experimental procedures. it is occasionally necessary to identify a commercial product by a manufacturer’s name or label. In no instance does such identification imply endorsement by the National Bureau of Standards, nor does it imply that the particular product or equipment is necessarily the best available for that purpose.