ABSTRACT
This study investigated the effects of cold acclimation on circulating fibroblast growth factor 21 (FGF21) levels, as well as its production and signaling in classical brown and white adipose tissues. Male Wistar rats were cold (4°C) acclimatized for 7 days. Subsequently, liver, interscapular and aortic BAT (iBAT and aBAT), and the Sc Ing and epididymal (Epid) white adipose tissues were extracted. Cold acclimation significantly reduced circulating FGF21 and its liver expression. Conversely, FGF21 content increased in iBAT, aBAT and Sc Ing fat depots, along with the expressions of the Fgf21 receptor and the receptor co-factor β-klotho. Cold acclimation increased FGF21 secretion from Sc Ing and Epid adipocytes, although only iBAT and Sc Ing fat depots enhanced ERK1/2 phosphorylation. These findings provide evidence that FGF21 acts in an autocrine/paracrine manner in iBAT and Sc Ing fat depots under cold-acclimating conditions and may contribute to driving depot-specific thermogenic adaptive responses.
KEYWORDS: FGF21, ERK1/2, UCP1, β-klotho, FGFR, thermogenesis, liver, adipose tissue
Introduction
Fibroblast growth factor-21 (FGF21) is a hormone that belongs to a large family of small generally secreted proteins that are involved in diverse actions such as cell growth, cell differentiation, embryonic development,1 and metabolic regulation.2 FGF21 is expressed in several organs, including the liver, brown and white adipose tissues (BAT and WAT, respectively), pancreatic β-cells, and thymus.3 It was initially described as a protein that enhanced glucose uptake in 3T3-L1 and human primary adipocytes by increasing Glut1 mRNA and protein.4 However, it is now well recognized that FGF21 exerts multiple metabolic effects and increases energy expenditure in response to specific stimuli such as cold exposure, high-fat and ketogenic diets, and fasting/refeeding.2 Under conditions of cold-induced thermogenesis, FGF21 up-regulates Ucp1 expression in classical BAT and also in the subcutaneous (Sc) inguinal (Ing) WAT. The latter effect characterizes the ability of FGF21 to induce browning of the Sc Ing WAT, which is actually impaired in mice deficient in FGF21.5 In fact, in mice lacking FGF21 the ability of the WAT to undergo cold-induced browning and acquire multilocular, Ucp1-expressing adipocytes known as beige or brite (“brown-in-white”) adipocytes is markedly impaired.5 Therefore, compelling evidence exists that FGF21 is crucial for the induction of thermogenic adaptive responses in both classical brown and WAT adipocytes upon cold exposure.
In order for FGF21 to signal, it requires the presence of the single-pass transmembrane protein β-klotho and the FGF receptor (FGFR).6,7 FGF21 binds β-klotho at the C terminus and interacts with FGFR through its N terminus.7,8 Thus, activation of the FGFR requires that both termini of FGF21 are intact.9,10 Importantly, β-klotho expression is essential for FGF21 signaling and is the primary determinant of tissue specificity because it limits the action of the hormone to those tissues that express both the receptor and β-klotho, as is the case for BAT and WAT.6,7 Binding of FGF21 to its receptor and the formation of the FGF21/FGFR/β-klotho complex results in autophosphorylation of tyrosine residues on the receptor,11 which subsequently leads to the phosphorylation and activation of MAPK/ERK kinase (MEK)1/2.12 MEK1/2 phosphorylates tyrosine and threonine in extracellular signal-regulated kinase (ERK)1/2, a kinase that has multiple downstream targets.12 There is evidence that FGF21 signals in the brain to activate the sympathetic nervous system (SNS) and induce adipose tissue thermogenesis and that an intact adrenergic system is necessary for FGF21 action.13 However, more recently it has been reported that even in the absence of β3-adrenergic receptors cold-induced thermogenic gene expression (Ucp1, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Pgc1a), deiodinase iodothyronine type II (Dio2), and cell death-inducing DFFA-like effector a (Cidea)) in brown or brite/beige adipose tissues still occurs.14 Furthermore, previous studies have demonstrated that FGF21 can increase thermogenic gene expression (e.g. Ucp1 and Cidea) in isolated primary WAT and BAT adipocytes.5 Therefore, it appears that FGF21 can enhance BAT thermogenesis and induce WAT browning either via SNS activation or through a direct cell-autonomous effect in these tissues.
Based on various rodent studies, it is now well established that the ability of the WAT to undergo browning varies among different fat depots, with the Sc Ing WAT being the most prone to acquiring a brown-like phenotype.15–18 In this context, it is still unclear whether it is a systemic increase or a tissue-specific autocrine/paracrine FGF21 effect that can potentially drive cold-induced browning of WAT. In mouse tissues, the highest level of Fgf21 expression was found in the liver,19 and this organ is considered the main source of circulating FGF21.20 Interestingly, previous studies have reported a reduction in Fgf21 expression in the liver of cold-exposed mice, although this was not accompanied by a reduction in circulating FGF21 levels in these animals.5 Because FGF21 is also produced by BAT, WAT and skeletal muscles,21 it could be that a reduction in liver expression and production did not cause a drop in circulating levels of FGF21 due to a compensatory increase in the production of this hormone by these other tissues. However, it remains to be demonstrated whether other sites of production can compensate for a potentially reduced hepatic production and maintain unaltered circulating levels of FGF21 under cold-acclimating conditions. Furthermore, there has been no clear comparison between BAT and WAT depots with respect to FGF21 expression and content. Of interest are the responses in the Sc Ing and Epid WAT depots and how they compare to each other, and to the classical BAT depots. Because FGF21 treatment induces Ucp1 in Sc Ing but not in Epid fat, differences in the FGF21 signaling may likely exist between these two depots, and this could help explain depot-specific differences with respect to cold-induced WAT browning. In this context, we designed a study to investigate the source of cold-induced FGF21 and examined WAT and BAT depot-specific content, release, and downstream signaling of the FGF21 pathway. We exposed rats to cold (4°C) for 7 days, after that, we extracted the interscapular and aortic BAT (iBAT and aBAT, respectively) and the Sc Ing and Epid fat depots. These tissues were used for the determination of FGF21 content and expression, β-klotho and Fgfr1 expression, and phosphorylation of the downstream FGF21 signaling target ERK1/2. Sc Ing and Epid WAT were also used to isolate adipocytes and assess the release of FGF21 by these cells in vitro. Blood samples were collected on a daily basis to determine potential time-dependent alterations in circulating FGF21 levels, and the livers were extracted to assess Fgf21 gene expression in this organ at the end of the study. Here, we provide novel data regarding the effects of cold acclimation on circulating FGF21 levels, as well as a comparative analysis of FGF21 expression, content, release, and activation of its downstream signaling pathway in classical BAT and WAT depots.
Results
Effects of cold exposure on food intake, plasma NEFA levels, circulating FGF21, and liver Fgf21 expression – At baseline, food intake was similar between the two groups of rats. However, rats that were cold acclimated consumed 47% more food than control animals (Table 1). NEFA levels did not differ between the two groups either at baseline or after 7 days of cold exposure (Table 1). Time course analysis of plasma FGF21 shows that the circulating levels of this hormone progressively reduced throughout the 7-day period of cold exposure, reaching values ~ 40% lower than control after 4 days of cold exposure (Figure 1A). Calculation of area under curve throughout the 7-day period revealed a significant reduction of 31% in circulating FGF21 levels in cold animals in comparison to controls (Figure 1(A,B)). As the liver is the main source of circulating FGF21,20 we also measured its expression in this tissue. In agreement with the plasma data, liver Fgf21 gene expression was markedly reduced (73%) by cold exposure (Figure 1C).
Table 1.
Food intake (g/rat/day) and plasma NEFAs (mmol/l) at baseline and following seven days of cold exposure. Age- and weight-matched animals were either kept at room temperature or cold exposed (4°C) for 7 days.
| Baseline |
7 Days Cold Exposure |
|||
|---|---|---|---|---|
| Control | Cold | Control | Cold | |
| Food Intake (g/rat/day) | 28.68 ± 1.09 | 28.44 ± 0.78 | 27.69 ± 0.87 | 40.91 ± 0.83* |
| NEFA (mmol/l) |
0.268 ± 0.028 | 0.215 ± 0.031 | 0.293 ± 0.022 | 0.236 ± 0.012 |
Food intake and plasma NEFAs were measured prior to (baseline) and on the final day of the cold exposure period. Plasma samples were taken in the fed state. Values are mean ± SEM. Two-way ANOVA, n = 7. *p < 0.05 vs. Baseline Cold and 7 Days Cold Exposure Control.
Figure 1.
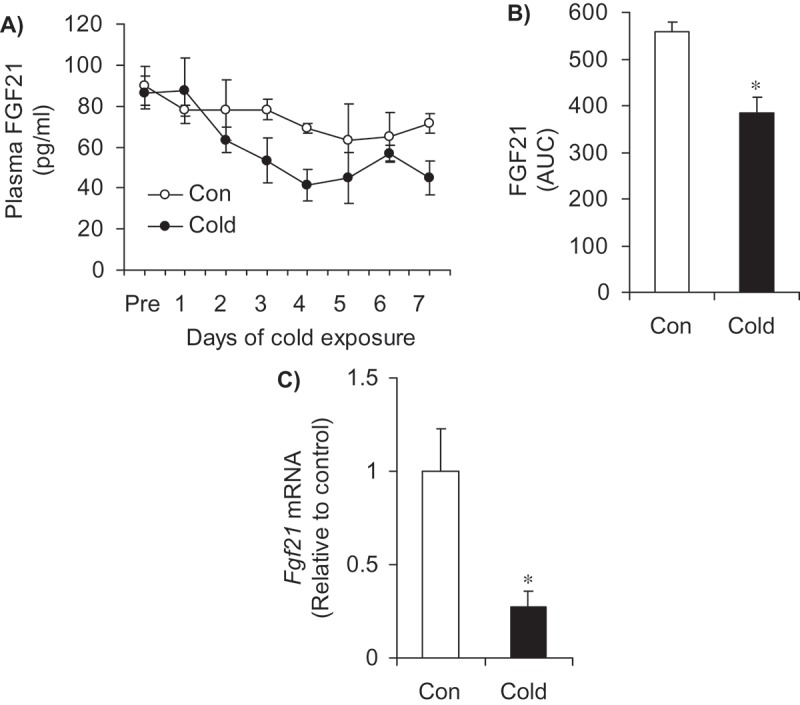
Cold acclimation reduces plasma FGF21 (A and B) and liver gene expression of Fgf21 (C). Age- and weight-matched animals were either kept at room temperature or cold exposed (4°C) for 7 days. Plasma samples were taken daily in the fed state. AUC = area under the curve. *p < 0.05 vs. Con. Unpaired, two-tailed t-test, n = 7 for the plasma data and n = 9 for the gene expression data.
Effects of cold exposure on Fgf21 expression, content, and signaling in iBAT and aBAT – In contrast to the liver, cold exposure significantly increased Fgf21 expression and its content by 11.2- and 1.8-fold, respectively, in the iBAT (Figure 2(A,B)`). In the aBAT, there was no significant alteration in Fgf21 expression; however, there was a 7-fold increase in FGF21 content in this tissue following cold exposure (Figure 2(C,D)). In iBAT, cold exposure significantly elevated the expression of Fgfr1 and β-klotho by 2.3- and 2.5-fold, respectively (Figure 3A), and this was accompanied by a 4.2-fold increase in the phosphorylation of the FGF21 downstream signaling target ERK1/2 (Figure 3B). In contrast to the iBAT, no change in the expression of Fgfr1 was found in aBAT, whereas β-klotho expression was reduced by 50% following cold exposure (Figure 3C). Furthermore, phosphorylation of ERK1/2 was also reduced by 78% in the aBAT of cold-exposed rats (Figure 3D).
Figure 2.
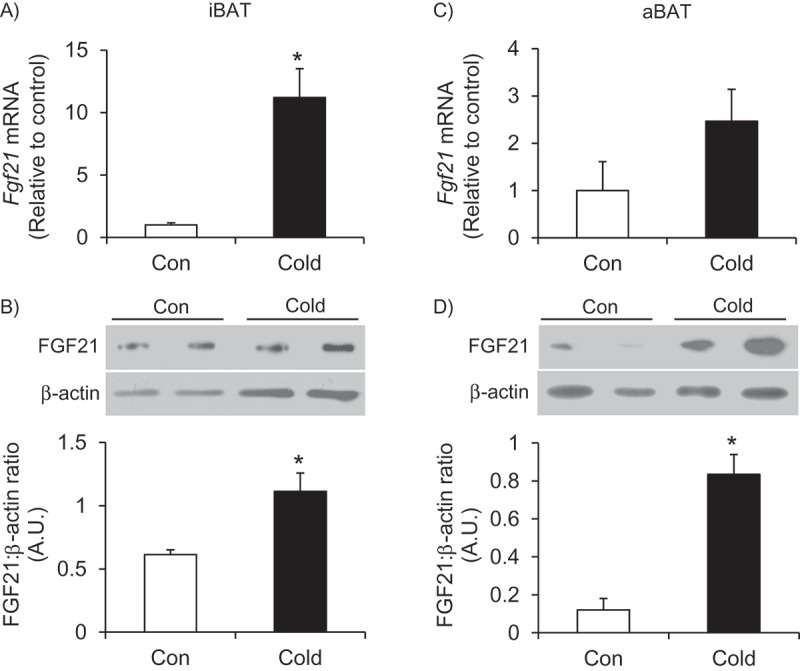
Cold exposure increases the expression and content of FGF21 and the content of FGF21 in the iBAT (A and B) and aBAT (C and D). Age- and weight-matched animals were either kept at room temperature or cold exposed (4°C) for 7 days. *p < 0.05 vs. Con. Unpaired, two-tailed t-test, n = 4–9.
Figure 3.
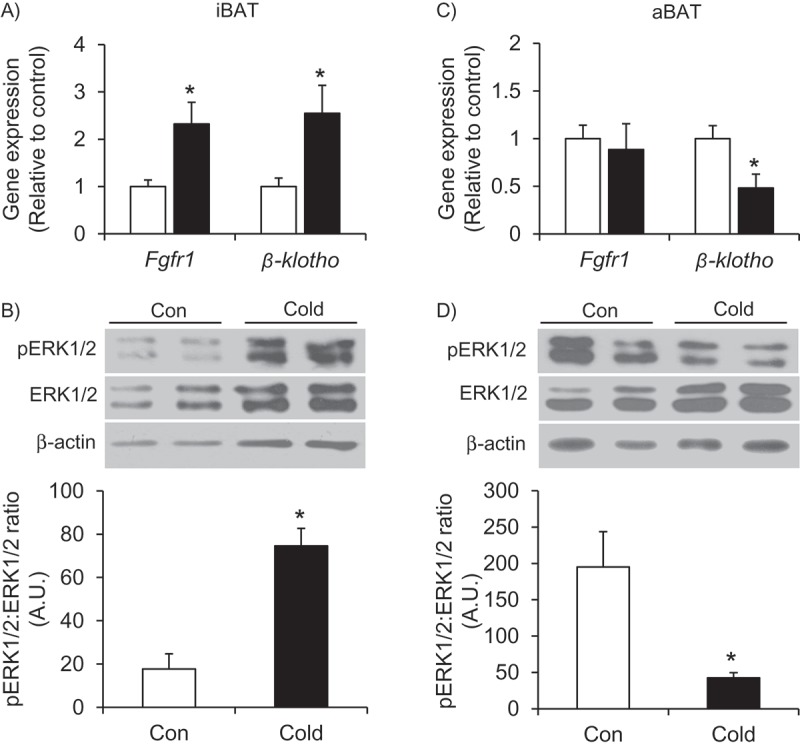
Cold exposure enhances the FGF21 signaling pathway in the iBAT (A and B) but not the aBAT (C and D). Age- and weight-matched animals were either kept at room temperature or cold exposed (4°C) for 7 days. *p < 0.05 vs. Con. Unpaired, two-tailed t-test, n = 4–9.
Effects of cold exposure on the expression, content and secretion of FGF21 in the Sc Ing and Epid WAT – There were significant differences in the expression and content of FGF21 between the two WAT depots. Expression and content of FGF21 was 2-fold greater in the Sc Ing fat of cold-exposed rats compared to control rats (Figure 4(A,C)), whereas in the Epid fat depot these variables did not differ between control and cold acclimating conditions (Figure 4(B,C)). Additionally, the content of FGF21 in the cold-exposed Sc Ing fat was 4-fold greater than the Epid fat (Figure 4C). In agreement with this data, there was a 5-fold increase in the amount of FGF21 secreted from Sc Ing adipocytes following cold exposure (Figure 4D), whereas no significant differences in secreted FGF21 from the Epid adipocytes were found between control and cold conditions (Figure 4E).
Figure 4.
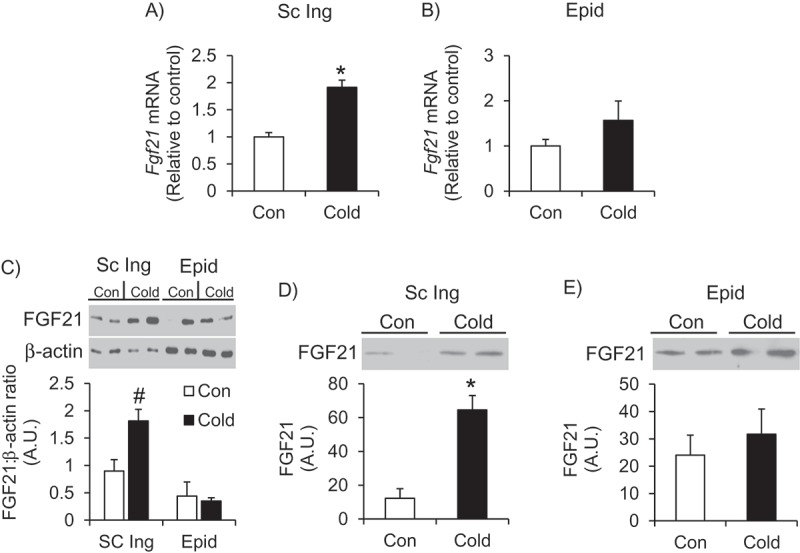
Cold exposure increases the expression, content and secretion of FGF21 in the Sc Ing fat (A, C and D) but not the Epid fat (B, C and E). Age- and weight-matched animals were either kept at room temperature or cold exposed (4°C) for 7 days. *p < 0.05 vs. Con. Unpaired, two-tailed t-test, n = 4–9. #p < 0.05 vs. all other groups. Two-way ANOVA, n = 6.
Effects of cold exposure on the FGF21 signaling pathway in the Sc Ing and Epid WAT – In addition to the increases in Fgf21 expression and content in the Sc Ing WAT, expression of Fgfr1 and β-klotho was also increased 3-fold in this depot following cold exposure (Figure 5A). Phosphorylation of the downstream signaling target ERK1/2 was also increased by 1.6-fold in the Sc Ing WAT upon cold acclimation (Figure 5B). Despite not affecting the expression and content of FGF21 in the Epid fat depot, cold acclimation significantly increased the expression of Fgfr1 and β-kotho by 2.8-fold in this depot (Figure 5C). However, this did not seem to have affected FGF21 signaling because there was no alteration in the phosphorylation of ERK1/2 in the Epid fat following cold exposure (Figure 5D).
Figure 5.
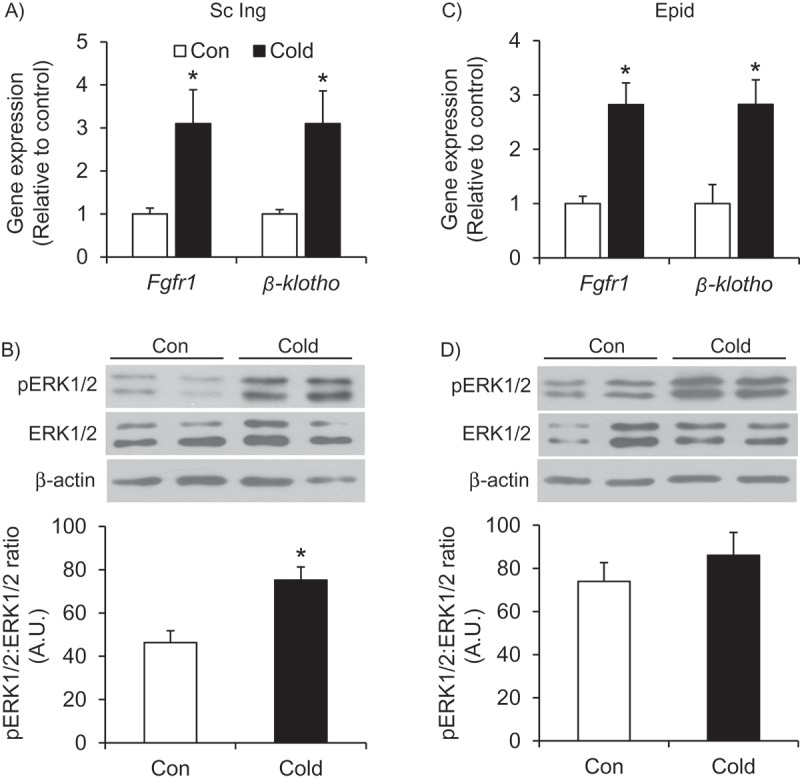
Cold exposure increases the expression of the FGF21 receptor Fgfr1 and the receptor co-factor β-klotho in both the Sc Ing (A) and the Epid (C) fat depots, but only enhances phosphorylation or ERK in the Sc Ing fat (B and D). Age- and weight-matched animals were either kept at room temperature or cold exposed (4°C) for 7 days. *p < 0.05 vs. Con. Unpaired, two-tailed t-test, n = 9.
Discussion
FGF21 is a hormone known to activate the BAT and induce browning of the Sc Ing WAT5,22 and we tested whether or not plasma levels would be increased following cold exposure to facilitate this activation and induction of WAT browning. Here, we report that plasma FGF21 was actually significantly reduced with cold acclimation as a result of its marked reduction in expression in the liver, the organ which is the main source of circulating FGF21.20 This is in contrast to previous results demonstrating an increase in circulating FGF21, despite also showing a reduction in liver Fgf21 expression following prolonged cold exposure.22 Species-specific differences and the length of time of the cold exposure may help to explain these discrepancies. In the liver, FGF21 is upregulated by peroxisome proliferator-activated receptor (PPAR)α, a transcription factor that is activated under fasting conditions, in particular by long-chain FAs that are abundant under these conditions.23,24 Previous work has shown that hepatic Pparα expression is reduced following 24 h of cold exposure (8°C),25 which could explain the reduction in Fgf21 expression we found in the liver. However, we measured Pparα in the livers from cold-acclimated rats,26 but no significant difference was found when compared to control. Even though Pparα expression was not affected by cold acclimation, we cannot rule out the possibility that its content and activity were actually downregulated. In particular because fatty acids are important for PPARα activation,27 and plasma NEFAs did not differ between control and cold acclimated rats, despite the fact that the latter animals ate 47% more than the former ones. Importantly, NEFAs can be rapidly consumed by BAT28 and other peripheral tissues (e.g. skeletal muscles)29,30 to fuel thermogenesis under cold acclimating conditions; therefore, their circulating levels did not increase as a consequence of cold acclimation. As we have previously reported, NEFAs are diverted toward oxidation in skeletal muscles30 and liver,26 which must also have limited their availability to participate in other intracellular pathways. Thus, cold acclimation did not seem to have created a physiological environment that was conducive to PPARα activation or an increase in Fgf21 expression in the liver.
Because the lack of circulating FGF21 limits the ability of the WAT to undergo browning under conditions of cold exposure,5 it could be that FGF21 was being produced within BAT and WAT, where it could exert an autocrine/paracrine effect and induce a thermogenic adaptive response. This would provide an alternative source of FGF21 that is independent of hepatic production. To test this hypothesis, we first investigated two classical BAT depots and then compared the results to those in two typical WAT depots. We found that iBAT exhibited increased expression and content of FGF21 along with enhancement of its downstream signaling pathway with cold exposure, whereas in aBAT only FGF21 content increased. To our knowledge, this is the first study to show this distinct difference in FGF21 signaling between two classical BAT depots. iBAT and aBAT are very similar with respect to their multilocular appearance and UCP1 content;15,31 however, we have previously found higher rates of palmitate and glucose oxidation in aBAT compared to iBAT,15 demonstrating that metabolic differences exist between these two depots. Interestingly, it has been reported that adipocytes from aBAT do not originate from the same Myf5+ lineage as adipocytes from iBAT.31 Furthermore, aBAT (also known as mediastinal BAT or mBAT) does not express Zic1, a gene that is highly expressed in iBAT and frequently used as a marker for classical BAT.32,33 These data provide evidence of a different developmental origin for aBAT compared to iBAT, which could explain the distinct metabolic differences exhibited by aBAT. The perirenal BAT (prBAT) is another classical BAT depot that does not express Zic1.32 Interestingly, both aBAT and prBAT are located more centrally in the rat suggesting that anatomical location of these BAT depots is an important determinant of their metabolic function. Further work is required to determine if prBAT is similar to aBAT in terms of FGF21 signaling and thus different from other classical BAT depots such as iBAT.
We have also found depot-specific differences in Fgf21 expression and signaling in two distinct depots of WAT. In Sc Ing WAT, the depot known to undergo browning, the content of FGF21 and the expressions of Fgf21, Fgfr1 and β-klotho were increased, similarly to iBAT. In contrast, only the expressions of Fgfr1 and β-klotho were increased in Epid WAT, a depot that does not undergo browning. Secretion of FGF21 was also only enhanced in Sc Ing adipocytes following cold exposure. Finally, phosphorylation of ERK1/2 was found in Sc Ing, but not in Epid WAT, even though in the latter tissue the expressions of both Fgfr1 and β-klotho were enhanced with cold exposure. It is possible that there was not a high enough local concentration of FGF21 to stimulate a response in the Epid WAT, since cold exposure did not increase the expression or secretion of FGF21 in this fat depot. This suggests that a potent stimulation might be required for the Epid fat to elicit a downstream response. Indeed, treatment of mice with FGF21 or bFKB1, a recombinant monoclonal antibody that selectively binds the FGFR1-β-klotho complex, resulted in increased expression of Erg-1 (a downstream target of ERK1/2) and increased phosphorylation of ERK1/2, respectively, in the Epid fat.34,35 However, despite eliciting a signaling response, FGF21 or bFKB1 treatment did not increase glucose uptake in the Epid depot, whereas in the Sc Ing depot it elicited both a downstream signaling response and an increase in glucose uptake.34,35 Additionally, it has been shown that in transgenic mice overexpressing Fgf21, Ucp1 expression (an indication of browning) increased in the Sc Ing, but not in the Epid fat depot.35 Collectively these data suggest that a potent stimulation of the Epid fat could elicit a downstream response of the FGF21 signaling pathway, but would likely not result in further metabolic alterations or browning of this tissue.
Importantly, even though phosphorylation of ERK1/2 is often used as an indicator of activation of the FGF21 signaling pathway,4,34 ERK1/2 can also be activated following cold-induced activation of the adrenergic signaling pathway.36,37 Thus, phosphorylation of ERK1/2 may not be the most selective indicator of activation of the FGF21 pathway under cold conditions. Additionally, because the FGF21 signaling pathway has not been fully elucidated, activation of p90 ribosomal S6 kinase (p90RSK) may be of interest to examine. It has been reported that phosphorylation/activation of p90RSK is enhanced in primary mouse brown adipocytes with FGF21 treatment.38 p90RSK can subsequently phosphorylate and activate CREB, which, in turn, enhances the transcription of Pgc1α and Ucp1.38 This is one possible molecular mechanism linking FGF21 with an increase in UCP1 that could be distinctly regulated in the Sc Ing compared to the Epid fat depot and determine depot-specific propensity to browning; however, further research is warranted to test this possibility. Interestingly, recent research has also shown that cold adaptation over 3 weeks occurs independently of FGF21 or UCP1.39 This is in contrast to findings previously reported,5 as well as our findings, and questions the role that FGF21 may have in cold-induced metabolic adaptations. We have hypothesized and provided evidence in our study of FGF21 acting in an autocrine/paracrine manner in the Sc Ing WAT following cold exposure. Even though, FGF21 content was enhanced following 7 days of cold exposure, we cannot rule out other mechanisms that could also be activated in the Sc Ing WAT and contribute to the organism’s ability to adapt to the cold in addition to any FGF21 effects. In this context, evidence of activation of futile cycles in the Sc Ing WAT has been provided40-43 and may comprise the main thermogenic mechanism by which the organism adapts to prolonged cold exposure in the absence of FGF21 and UCP1.
In summary, we provide novel evidence that circulating FGF21 is reduced during cold acclimation because of a marked suppression of its gene expression in the liver. However, this does not prevent FGF21 from acting on iBAT and Sc Ing WAT under cold-acclimating conditions. This is possible because expression and release of FGF21 are increased within iBAT and Sc Ing WAT, allowing for an autocrine/paracrine effect of this hormone that could promote a robust thermogenic response in iBAT and induce browning of the Sc Ing WAT. Conversely, the lack of an increase in Fgf21 expression and release by Epid adipocytes may limit the ability of the Epid fat depot to undergo browning under cold-acclimating conditions. These findings provide a potential explanation for the depot-specific browning response that is observed following cold acclimation.
Materials and methods
Reagents – Type II collagenase was obtained from Sigma (St. Louis, MO, USA). Protease (cOmplete™ Ultra Tablets) and phosphatase (PhosSTOP™) inhibitors were from Roche Diagnostics GmbH (Mannheim, Germany). The HR Series non-esterified fatty acid (NEFA)-HR(2) kit was from Wako Diagnostics (Richmond, VA, USA). The rat FGF21 ELISA was from R&D Systems (Minneapolis, MN, USA). All antibodies were purchased from Cell Signaling (Danvers, MA, USA), except for the FGF21 antibody that was purchased from Abcam (Toronto, ON, Canada).
Animals – Male albino rats (Wistar strain) were housed at 22°C on a 12/12-h light/dark cycle and fed standard laboratory chow (Lab Diet Cat #5012) ad libitum. The protocol containing all animal procedures described in this study was specifically approved by the Committee on the Ethics of Animal Experiments of York University (York University Animal Care Committee, YUACC, permit number 2016–5) and performed strictly in accordance with the YUACC guidelines. All surgery was performed under ketamine/xylazine anesthesia, and all efforts were made to minimize suffering.
Cold exposure – The rats were age- and weight-matched (~ 400 g) and randomly allocated to either the control or cold-exposed group. The rats were housed in individual cages and maintained on a 12/12-h light/dark cycle at 22°C (Con) or 4°C (Cold) for 7 days. Food intake was measured prior to (baseline) and on the final day of the cold exposure period. Blood samples were collected daily always at 9:00 am in the fed state from the saphenous vein, centrifuged for 10 min at 4°C and the plasma stored at −80°C for analysis of NEFAs and FGF21. Upon completion of the protocol, animals were anesthetized (0.4 mg ketamine and 8 mg xylazine per 100 g body weight) in the fed state and the iBAT, aBAT, Epid and SC Ing fat pads as well as the liver were extracted and weighed. A sample of each was flash frozen in liquid nitrogen for quantitative PCR and western blot analysis.
Adipocyte isolation and conditioned media – Adipocyte isolation from the Epid and SC Ing fat pads was performed as previously described.44 Briefly, the adipose tissue was minced in Krebs-Ringer Buffer (0.154 M NaCl, 0.154 M KCl, 0.11 M CaCl2, 0.154 M MgSO4, 0.154 M KH2PO4, 0.154 M NaHCO3, pH 7.4) with 5.5 mM glucose and 30 mM HEPES (KRBH) supplemented with type II collagenase (0.5 mg/ml). Minced tissues were incubated at 37°C with gentle agitation (120 orbital strokes/min) for approximately 25–30 min. The digested tissue was then strained using a nylon mesh and cells were transferred to 50 ml tubes, washed, and resuspended in fresh KRBH. The total cell numbers were determined as previously described45 and an equal number of adipocytes (3.3 x 106/ml) from each depot was incubated in KRBH for a total of 4 h at 37°C. The conditioned media was collected at the end of the incubation period and frozen at −80°C for subsequent analysis of secreted FGF21.
RNA isolation and quantitative PCR – Primers were designed using the software PrimerQuest (IDT) based on probe sequences available at the Affymetrix database (NetAffx™ Analysis Centre, http://www.affymetrix.com/analysis) for each given gene. RNA was extracted from the adipose tissue and liver using TRIzol™ reagent (ThermoFisher Scientific, Mississauga, ON, Canada). Adipose tissue complimentary DNA (cDNA) was made from 5 μg of extracted RNA using the SuperScript II reverse transcriptase (ThermoFisher Scientific, Waltham, MA, USA) and liver cDNA was made from 2 μg of extracted RNA using the ABM EasyScript™ Reverse Transcriptase cDNA synthesis kit (Diamed, Mississauga, ON, Canada), according to the manufacturer’s instructions. Samples were run using 10 μl of ABM EvaGreen qPCR Mastermix (Diamed, Mississauga, ON, Canada) using the following amplification conditions: 95°C (10 min); 40 cycles of 95°C (15 s), 60°C (60 s). Adipose tissue genes were normalized to the control gene GAPDH and liver genes were normalized to the control gene β-actin. Relative differences in gene expression between treatment groups were determined using the ΔΔCt method.46 Values are presented as fold increases relative to the Con group.
Western blotting analysis of content and phosphorylation of proteins, and conditioned media – Adipose tissue collected from the iBAT, aBAT, Epid and SC Ing depots was homogenized in a buffer containing 25 mM Tris-HCl, 25 mM NaCl (pH 7.4), 1 mM MgCl2, 2.7 mM KCl, 1% Triton-X and protease and phosphatase inhibitors (Roche Diagnostics GmbH, Mannheim, Germany). Adipose tissue homogenates were centrifuged, the infranatant collected, and an aliquot was used to measure protein by the Bradford method. Samples were diluted 1:1 (vol:vol) with 2x Laemmli sample buffer and heated to 95°C for 5 min. Samples were then subjected to SDS-PAGE, transferred to PVDF membrane and probed for the proteins of interest. All primary antibodies were used at a dilution of 1:1,000. β-actin was used as a loading control. For the conditioned media, samples were diluted 1:1 (vol:vol) with 2x Laemmli sample buffer, heated to 95°C for 5 min and an equal volume of each sample was subjected to SDS-PAGE. Samples were then transferred to PVDF membrane and subsequently probed for FGF21 (dilution of 1:1,000). Densitometric analyses of western blots were performed using the Scion Image program.
Statistical analyses – Statistical analyses were assessed by unpaired, two-tailed t-test or two-way ANOVA with Bonferroni post-hoc test using the GraphPad Prism statistical software program. Statistical significance was set at p < 0.05.
Funding Statement
This work was supported by Natural Sciences and Engineering Research Council of Canada Grant 311818-2011 (to R.B.C.). D.M.S-K. was supported by a Natural Sciences and Engineering Research Council of Canada Alexander Graham Bell Canada Graduate Scholarship-Doctoral and the Elia Scholarship;Natural Science and Enginnering Research Council of Canada [2016-05358];
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Oulion S, Bertrand S, Escriva H.. Evolution of the FGF gene family. Int J Evol Biol. 2012;2012:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher FM, Maratos-Flier E. Understanding the physiology of FGF21. Annu Rev Physiol. 2016;78:223–241. [DOI] [PubMed] [Google Scholar]
- 3.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher M, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-O M. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A. 2007;104:7432–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-O M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282:26687–26695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yie J, Wang W, Deng L, Tam L-T, Stevens J, Chen MM, Li Y, Xu J, Lindberg R, Hecht R, et al. Understanding the physical interactions in the FGF21/FGFR/β-klotho complex: structural requirements and implications in FGF21 signaling. Chem Biol Drug Des. 2012;79:398–410. [DOI] [PubMed] [Google Scholar]
- 9.Yie J, Hecht R, Patel J, Stevens J, Wang W, Hawkins N, et al. FGF21 N- and C-termini play different roles in receptor interaction and activation. FEBS Lett. 2009;583:19–24. [DOI] [PubMed] [Google Scholar]
- 10.Micanovic R, Raches DW, Dunbar JD, Driver DA, Bina HA, Dickinson CD, Kharitonenkov A. Different roles of N- and C- termini in the functional activity of FGF21. J Cell Physiol. 2009;219:227–234. [DOI] [PubMed] [Google Scholar]
- 11.Su N, Jin M, Chen L. Role of FGF/FGFR signaling in skeletal development and homeostasis: learning from mouse models. Bone Res. 2014;2:14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roskoski R. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66:105–143. [DOI] [PubMed] [Google Scholar]
- 13.Douris N, Stevanovic DM, Fisher Ffolliott M, Cisu TI, Chee MJ, Nguyen NL, Zarebidaki E, Adams AC, Kharitonenkov A, Flier JS, et al. Central fibroblast growth factor 21 browns white fat via sympathetic action in male mice. Endocrinology. 2015;156:2470–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jong JMA, Wouters RTF, Boulet N, Cannon B, Nedergaard J, Petrovic N. The β 3 -adrenergic receptor is dispensable for browning of adipose tissues. Am J Physiol - Endocrinol Metab. 2017;312:E508–18. [DOI] [PubMed] [Google Scholar]
- 15.Wu MV, Bikopoulos G, Hung S, Ceddia RB. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: IMPACT ON WHOLE-BODY ENERGY EXPENDITURE. J Biol Chem. 2014;289:34129–34140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barneda D, Frontini A, Cinti S, Christian M. Dynamic changes in lipid droplet-associated proteins in the “browning” of white adipose tissues. Biochim Biophys Acta. 2013;1831:924–933. [DOI] [PubMed] [Google Scholar]
- 17.Cousin B, Casteilla L, Dani C, Muzzin P, Revelli JP, Penicaud L. Adipose tissues from various anatomical sites are characterized by different patterns of gene expression and regulation. Biochem J. 1993;292:873–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cinti S. Reversible physiological transdifferentiation in the adipose organ. Proc Nutr Soc. 2009;68:340. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492:203–206. [DOI] [PubMed] [Google Scholar]
- 20.Markan K, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh N. FGF21 as a Hepatokine, Adipokine, and Myokine in Metabolism and Diseases. 2014;5:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286:12983–12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–437. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 2007;5:415–425. [DOI] [PubMed] [Google Scholar]
- 25.Shore AM, Karamitri A, Kemp P, Speakman JR, Graham NS, Lomax MA. Cold-induced changes in gene expression in brown adipose tissue, white adipose tissue and liver. PLoS One. 2013;8:e68933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sepa-Kishi DM, Katsnelson G, Bikopoulos G, Iqbal A, Ceddia RB. Cold acclimation reduces hepatic protein kinase B and AMP-activated protein kinase phosphorylation and increases gluconeogenesis in rats. Physiol Rep. 2018;6(1–12):e13592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rui L. Energy Metabolism in the liver. Compr Physiol. 2014; 4(1): 177–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labbe SM, Caron A, Bakan I, Laplante M, Carpentier AC, Lecomte R, Richard D. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J. 2015. DOI: 10.1096/fj.14-266247 [DOI] [PubMed] [Google Scholar]
- 29.Gamu D, Bombardier E, Smith IC, Fajardo VA, Tupling AR. Sarcolipin provides a novel muscle-based mechanism for adaptive thermogenesis. Exerc Sport Sci Rev. 2014;42:136–142. [DOI] [PubMed] [Google Scholar]
- 30.Sepa-Kishi DM, Sotoudeh-Nia Y, Iqbal A, Bikopoulos G, Ceddia RB. Cold acclimation causes fiber type-specific responses in glucose and fat metabolism in rat skeletal muscles. Sci Rep. 2017;7(1–12):15430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Jong JMA, Larsson O, Cannon B, Nedergaard J. A stringent validation of mouse adipose tissue identity markers. Am J Physiol Endocrinol Metab. 2015;ajpendo.00023.2015 DOI: 10.1152/ajpendo.00023.2015. [DOI] [PubMed] [Google Scholar]
- 33.Walden TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. AJP Endocrinol Metab. 2012;302:E19–31. [DOI] [PubMed] [Google Scholar]
- 34.Kolumam G, Chen MZ, Tong R, Zavala-Solorio J, Kates L, van Bruggen N, Ross J, Wyatt SK, Gandham VD, Carano RA, et al. Sustained Brown Fat Stimulation and Insulin Sensitization by a Humanized Bispecific Antibody Agonist for Fibroblast Growth Factor Receptor 1/βKlotho Complex. EBioMedicine. 2015;2(7):730–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding X, Boney-Montoya J, Owen BM, Bookout AL, Coate KC, Mangelsdorf DJ, Kliewer SA.. βKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 2012;16:387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimizu Y, Tanishita T, Minokoshi Y, Shimazu T. Activation of mitogen-activated protein kinase by norepinephrine in brown adipocytes from rats. Endocrinology. 1997;138:248–253. [DOI] [PubMed] [Google Scholar]
- 37.Lindquist JM, Rehnmark S. Ambient temperature regulation of apoptosis in brown adipose tissue Erk1/2 promotes norepineprhine-dependent cell survival. J Biol Chem. 1998;273:30147–30156. [DOI] [PubMed] [Google Scholar]
- 38.Wu A-L, Kolumam G, Stawicki S, Chen Y, Li J, Zavala-Solorio J, Phamluong K, Feng B, Li L, Marsters S, et al. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci Transl Med. 2011;3. [DOI] [PubMed] [Google Scholar]
- 39.Keipert S, Kutschke M, Ost M, Schwarzmayr T, van Schothorst EM, Lamp D, Brachthäuser L, Hamp I, Mazibuko SE, Hartwig S, et al. Long-term cold adaptation does not require FGF21 or UCP1. Cell Metab. 2017;26(2):437–446.e5.. [DOI] [PubMed] [Google Scholar]
- 40.Flachs P, Rossmeisl M, Kuda O, Kopecky J. Stimulation of mitochondrial oxidative capacity in white fat independent of UCP1: A key to lean phenotype. Biochim Biophys Acta - Mol Cell Biol Lipids. 2013;1831:986–1003. [DOI] [PubMed] [Google Scholar]
- 41.Keipert S, Jastroch M. Brite/beige fat and UCP1 — is it thermogenesis? Biochim Biophys Acta - Bioenerg. 2014;1837:1075–1082. [DOI] [PubMed] [Google Scholar]
- 42.Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1-/- mice. J Biol Chem. 2006;281:31894–31908. [DOI] [PubMed] [Google Scholar]
- 43.Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC, et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163:643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaidhu MP, Frontini A, Hung S, Pistor K, Cinti S, Ceddia RB. Chronic AMP-kinase activation with AICAR reduces adiposity by remodeling adipocyte metabolism and increasing leptin sensitivity. J Lipid Res. 2011;52:1702–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fine JB, DiGirolamo M. A simple method to predict cellular density in adipocyte metabolic incubations. Int J Obes Relat Metab Disord. 1997;21:764–768. [DOI] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]


