ABSTRACT
We recently demonstrated that nitric oxide (NO) accumulation and PHYTOGB1 transcriptional regulation are early components of the regulatory pathway that is activated in tomato roots during the onset of the mycorrhizal symbiosis between Rhizophagus irregularis and tomato roots. We further showed that the mycorrhizal interaction was associated with a specific NO-related signature, different from that triggered by the pathogen Fusarium oxysporum. Here, we extend our investigation by exploring the NO- and PHYTOGB1-related root responses elicited by another root mutualistic endosymbiotic fungus: Trichoderma harzianum T-78. By using T-78 in vitro-grown cultures, we found that T-78 triggered an early and transient burst of NO in tomato roots during the first hours after the interaction. T-78 also elicited the early upregulation of PHYTOGB1, which was maintained during the analyzed timespan. By using glass-house bioassays, we found that in a well-established tomato-T-78 symbiosis, NO root levels were maintained at basal level while PHYTOGB1 expression remained upregulated. Our results demonstrate that the T-78 symbiosis is associated with a rapid and transient burst of NO in the host roots and the transcriptional activation of PHYTOGB1 from early stages of the interaction until the establishment of the symbiosis, most likely to control NO levels and favor the mutualistic symbiosis.
KEYWORDS: Endophyte, nitric oxide, phytoglobin, plant-microbe interaction, symbiosis
Nitric oxide (NO) is a diffusible reactive gaseous molecule involved in the regulation of a wide range of plant developmental processes and defense against biotic and abiotic stresses.1,2 During plant immune responses, NO triggers a reprograming of the expression of defense-related genes, the production of secondary metabolites with antimicrobial properties, and the hypersensitive response.3 More recent evidences further indicate a role of NO in the establishment of plant–microbe mutualistic associations as the rhizobial and mycorrhizal symbioses.4,5 Although the specific role(s) of NO in plant–microbe mutualistic interactions remains largely unexplored, experimental data support a different regulation pattern and functions of NO in plant interaction with beneficial and pathogenic microbes.5–7 NO plant accumulation can be regulated by the activity of plant phytoglobins that may function as NO dioxygenases that catalytically metabolize NO to nitrate.8–10 We recently found that the onset of the arbuscular mycorrhizal (AM) symbiosis between Rhizophagus irregularis and tomato roots is associated with a specific NO-related signaling in the host root, and the transcriptional activation of the tomato NO-inducible phytoglobin gene PHYTOGB1.7 Here, we extend our investigation by exploring the NO- and PHYTOGB1-related root responses elicited by another root mutualistic endosymbiotic fungus: Trichoderma harzianum T-78 (hereafter T-78).11
T-78 triggers a transient burst of NO accumulation and the upregulation of PHYTOGB1 in the host root during early steps of the symbiotic interaction
We first determined whether NO is an early signaling component of the interaction between T-78 (CECT 20714, Spanish collection of type cultures) and tomato roots (Solanum lycopersicum cultivar Moneymaker). To this aim, we analyzed NO levels in tomato roots by using T-78 invitro-grown cultures and the fluorescent indicator for the detection of NO DAF-2 (Merck Biosciences, armstadt, Germany) according to ref. 7. We set up the early interaction experiments by transferring tomato plants previously germinated and grown for 2 wk in hydroponic condition to Petri plates containing the T-78 cultures, as described by ref. 7. At 4, 8, 24, and 48 h after setting up the experiment, plants were harvested and root material was collected. This time span was selected according to our previous findings which evidenced two different peaks of NO plant production in R. irregularis roots during the first 48 h after the contact.7 We detected a transient burst of NO in tomato roots 4 h after the contact with T-78 (Figure 1a). After this NO peak, NO levels returned to basal levels and no differences on NO accumulation were found between control and T-78 roots at 8, 24, and 48 h after the contact. This result indicates that root interaction with T-78 is associated with an early and transient burst of NO. In analogy to our observations, a rapid and transient increase of NO was previously detected in roots of Arabidopsis thaliana following the contact with the mutualistic endosymbiont Trichoderma asperelloides.12 It is remarkable that the root interaction with the AM fungus R. irregularis and with the pathogenic fungus F. oxysporum is similarly associated with an early burst of NO in the host root.7 These results might suggest that the rapid NO burst triggered by T-78 is part of an unspecific early plant response to different fungi, probably as a response to general fungal microbe-associated molecular patterns. However, after this early NO burst, the transient character of the NO burst triggered by T-78 contrasts with the oscillatory pattern of NO accumulation observed during the AM interaction.7 Such differences in the patterns of NO-related signaling might highlight the different colonization strategies followed by these different mutualistic fungal symbionts. The AM symbiosis establishment relies on a continual signaling between the symbionts and on the activation of an extensive genetic and developmental program in both partners.13 In contrast, the strategy followed by T-78 and T. asperelloides to colonize roots is mostly based on the early repression of plant immune responses to scape plant defenses.11,14
Figure 1.

Nitric oxide (NO) accumulation and expression of the tomato phytoglobin genes in tomato roots after early stages of the interaction with Trichoderma harzianum T-78. (a) NO was detected in tomato roots by fluorimetry by using the specific NO detector DAF-2 at 4, 8, 24, and 48 h after the contact with T-78 and in control roots. In vitro-grown cultures of T-78 were used in the experiments. NO levels are reported as the fold increases relative to that of the control roots at each time point ± SE (n = 4 biological replicates). The asterisk indicates a statistically significant difference in comparison to control roots according to Student’s t-test (P < .05). (b) Expression level of PHYTOGB1, PHYTOGB2, and PHYTOGB3 was analyzed in roots of tomato plants at 4, 8, 24, and 48 h after the contact with T-78 and in control roots. Results were normalized to the SlEF gene expression in the same samples. The expression levels are reported as the fold change relative to that of the control plants at each time point ± SE (n = 4 biological replicates). At each time point asterisks indicate significant differences compared to control plants at ***P < .001,**P < .01 and,*P < .05 according to Student’s t-test. Ns: not significant.
We next studied whether T-78 regulates the tomato phytoglobin genes during early stages of the interaction. To this aim, we analyzed the expression of the tomato phytoglobin genes PHYTOGB1, PHYTOGB2, and PHYTOGB3 in tomato roots at 4, 8, 24, and 48 h after the contact by using qPCR and the specific primers according to ref. 7. T-78 triggered the transcriptional activation of the gene PHYTOGB1 in tomato roots already at 4 h after the contact (Figure 1b). PHYTOGB1 remained upregulated in T-78 roots during the entire monitored timespan. By contrast, transcript levels of PHYTOGB2 and PHYTOGB3 were not (or just marginally) affected by T-78. These results indicate that T-78 triggers the transcriptional activation of the phytoglobin gene PHYTOGB1 in the host roots during the early stages of the interaction. Together, our results indicate that during the early stages of the T-78-tomato symbiosis, NO is rapidly and transiently accumulated in the host roots. Moreover, due to the role of PHYTOGB1 on the regulation of NO accumulation in tomato roots,7 our findings further suggest that T-78 induces the upregulation of PHYTOGB1 to control NO levels and evade the activation of plant defenses in order to successfully colonize the host root.
The well-established symbiosis between T-78 and tomato roots is associated with basal root NO levels and the transcriptional activation of the PHYTOGB1 gene
After the initial recognition, T-78 grows externally on the root surface and ingresses into the root cortex where it remains accommodated by the plant as an avirulent symbiont.11,15 The T-78 symbiosis should be finely regulated, assuring benefits to both partners, with the plant receiving protection and more available nutrients and the fungus obtaining organic compounds and a niche for growth.16 In order to investigate whether a well-established T-78 symbiosis is associated with changes on NO accumulation in the host root, we established a glass-house bioassay. Tomato plants were germinated, grown, and inoculated with T-78 as described by ref. 15. After 5 wk, we harvested the plants and measured NO root levels by using DAF-2 according to ref. 7. As shown in Figure 2a, NO levels in roots of T-78-inoculated plants were similar to that observed in roots of control plants. By using qPCR, we found that PHYTOGB1 was strongly upregulated in T-78-colonized roots compared to roots of control plants (Figure 2b). These results indicate that the well-established T-78-tomato symbiosis is associated with the transcriptional activation of PHYTOGB1 in the host root, most likely to control NO levels and maintain the symbiosis. Although to a lesser extent, PHYTOGB2 was also upregulated in roots of T-78 colonized plants, while T-78 colonization did not affect significantly the expression of PHYTOGB3. Though PHYTOGB2 is not an NO-inducible gene in tomato roots,7 several studies indicate that it is involved in stress-related responses and in hormonal signaling.17–19 However, its specific role on plant–microbe interaction remains largely unknown. Altogether, our results show that the T-78–tomato symbiosis is associated with a rapid and transient burst of NO in the host root, and the transcriptional activation of PHYTOGB1 from early stages of the interaction until the establishment of the symbiosis, most likely to control NO levels and favor the mutualistic interaction. Our results further demonstrate that the NO- and PHYTOGB1-related responses elicited by T-78 are different to the ones triggered by the AM fungus R. irregularis and the pathogenic fungus F. oxysporum, suggesting a specificity of the NO-related plant responses according to the specific plant interaction.
Figure 2.
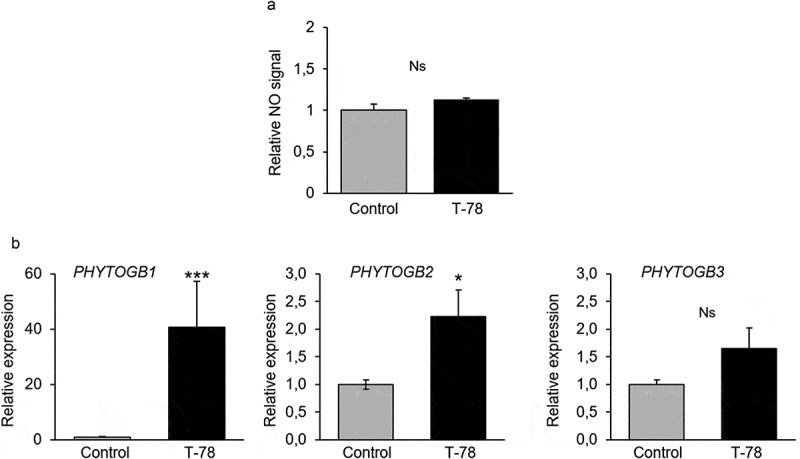
Nitric oxide (NO) accumulation and expression of the tomato phytoglobin genes in tomato roots after the establishment of the plant-Trichoderma harzianum T-78 symbiosis. (a) NO was detected in tomato roots by fluorimetry by using the specific NO detector DAF-2, 5 wk after the inoculation with T-78. Plants were grown in pots in a glass-house. NO level is reported as the fold increase relative to that of the control roots ± SE (n = 4 biological replicates). Ns: not significant. (b) Expression level of PHYTOGB1, PHYTOGB2, and PHYTOGB3 was analyzed in roots of tomato plants 5 wks after the inoculation with T-78 and in control roots. Results were normalized to the SlEF gene expression in the same samples. The expression levels are reported as the fold changes relative to that of the control plants ± SE (n = 4 biological replicates). The asterisks indicate a statistically significant difference in comparison to the control roots at ***P < .001 and *P < .05 according to Student’s t-test. Ns: not significant.
Funding Statement
This research was supported by grants: P12BIO296 from Junta de Andalucía; grant AGL2015-29 64990-C2-1-R from Spanish Ministry of Economy and Competitiveness and grant for Atracting Talent to Salamanca by Salamanca Ciudad de Cultura y de Saberes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Besson-Bard A, Pugin A, Wendehenne D.. New insights into nitric oxide signaling in plants. Annu Rev Plant Biol. 2008;59:1–4. doi: 10.1146/annurev.arplant.59.032607.092830. [DOI] [PubMed] [Google Scholar]
- 2.Domingos P, Prado AM, Wong A, Gehring C, Feijo JA. Nitric oxide: a multitasked signaling gas in plants. Mol Plant. 2015;8:506–520. doi: 10.1016/j.molp.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Mur LAJ, Carver TLW, Prats E. NO way to live; the various roles of nitric oxide in plant-pathogen interactions. J Exp Bot. 2006;57:489–505. doi: 10.1093/jxb/erj052. [DOI] [PubMed] [Google Scholar]
- 4.Hichri I, Boscari A, Castella C, Rovere M, Puppo A, Brouquisse R. Nitric oxide: a multifaceted regulator of the nitrogen-fixing symbiosis. J Exp Bot. 2015;66:2877–2887. doi: 10.1093/jxb/erv051. [DOI] [PubMed] [Google Scholar]
- 5.Martínez-Medina A, Pescador L, Terrón-Camero L, Pozo MJ, Romero-Puertas MC. Nitric oxide shape plant-fungi interactions. J Exp Bot. 2019. doi: 10.1093/jxb/erz289. [DOI] [PubMed] [Google Scholar]
- 6.Nagata M, Murakami E, Shimoda Y, Shimoda-Sasakura F, Kucho K, Suzuki A, Abe M, Higashi S, Uchiumi T. Expression of a class 1 hemoglobin gene and production of nitric oxide in response to symbiotic and pathogenic bacteria in Lotus japonicus. Mol Plant Microbe Interact. 2008;21:1175–1183. doi: 10.1094/MPMI-21-9-1175. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Medina A, Pescador L, Fernandez I, Rodríguez-Serrano M, García JM, Romero-Puertas MC, Pozo MJ. Nitric oxide and phytoglobin PHYTOGB1 are regulatory elements in the Solanum lycopersicum-Rhizophagus irregularis mycorrhizal simbiosis. New Phytol. 2019. doi: 10.1111/nph.15898. [DOI] [PubMed] [Google Scholar]
- 8.Perazzolli M, Dominici P, Romero-Puertas MC, Zago E, Zeier A, Sonoda M, Lamb C, Delledonne M. Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell. 2004;16:2785–2794. doi: 10.1105/tpc.104.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill RD. Non-symbiotic haemoglobins – what’s happening beyond nitric oxide scavenging? AoB PLANTS. 2012:pls004. doi: 10.1093/aobpla/pls004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill R, Hargrove M, Arredondo-Peter R. Phytoglobin: a novel nomenclature for plant globins accepted by the globin community at the 2014 XVIII conference on oxygen-binding and sensing proteins. F1000Res. 2016;5:212. doi: 10.12688/f1000research.8133.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martínez-Medina A, Appels FVW, van Wees SCM. Impact of salicylic acid- and jasmonic acid-regulated defences on root colonization by Trichoderma harzianum T-78. Plant Signal Behav. 2017;3,12:e1345404. doi: 10.1080/15592324.2017.1345404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta KJ, Mur LAJ, Brotman Y. Trichoderma asperelloides suppresses nitric oxide generation elicited by Fusarium oxysporum in Arabidopsis roots. Mol Plant Microbe Interact. 2014;27:307–314. doi: 10.1094/MPMI-06-13-0160-R. [DOI] [PubMed] [Google Scholar]
- 13.MacLean AM, Bravo A, Harrison MJ. Plant signaling and metabolic pathways enabling arbuscular mycorrhizal symbiosis. Plant Cell. 2017;29:2319–2335. doi: 10.1105/tpc.17.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brotman Y, Landau U, Cuadros-Inostroza Á, Tohge T, Fernie AR, Chet I, Viterbo A, Willmitzer L. Trichoderma-plant root colonization: escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 2013;9:e1003221. doi: 10.1371/journal.ppat.1003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez-Medina A, Fernández I, Sánchez-Guzmán MJ, Jung SC, Pascual JA, Pozo MJ. Deciphering the hormonal signalling network behind the systemic resistance induced by Trichoderma harzianum in tomato. Front Plant Sci. 2013;4:206. doi: 10.3389/fpls.2013.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Medina A, Pozo MJ, Cammue BPA, Vos CMF. Belowground defence strategies in plants: the plant-Trichoderma dialogue In: Vos CMF, Kazan K editors. Belowground defence strategies in plants. Switzerland: Springer International Publishing; 2016. p. 301–327. [Google Scholar]
- 17.Trevaskis B, Watts RA, Andersson CR, Llewellyn DJ, Hargrove MS, Olson JS, Dennis ES, Peacock WJ. Two hemoglobin genes in Arabidopsis thaliana: the evolutionary origins of leghemoglobins. Proc Natl Acad Sci U S A. 1997;94:12230–12234. doi: 10.1073/pnas.94.22.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt PW, Watts RA, Trevaskis B, Llewelyn DJ, Burnell J, Dennis ES, Peacock WJ. Expression and evolution of functionally distinct haemoglobin genes in plants. Plant Mol Biol. 2001;47:677–692. [DOI] [PubMed] [Google Scholar]
- 19.Bustos-Sanmamed P, Tovar-Méndez A, Crespi M, Sato S, Tabata S, Becana M. Regulation of nonsymbiotic and truncated hemoglobin genes of Lotus japonicus in plant organs and in response to nitric oxide and hormones. New Phytol. 2011;189:765–776. doi: 10.1111/j.1469-8137.2010.03527.x. [DOI] [PubMed] [Google Scholar]


