Abstract
A 17-year-old male presented thrombotic microangiopathy (TMA) at 6 months of age with arterial hypertension, anemia, thrombocytopenia and kidney injury improving with plasma infusions. Fourteen years later, he was diagnosed with severe arterial hypertension, increase in serum creatinine and chronic TMA on kidney biopsy. Eculizumab was started and after 18 months of treatment, he persisted with hypertension, decline in renal function and proteinuria. Genetic analysis demonstrated mutation in diacylglycerol kinase epsilon (DGKe). Complement blockade was stopped. This case of late diagnosis of DGKe nephropathy highlights the importance of genetic testing in patients presenting TMA during the first year of life.
Keywords: atypical hemolytic uremic syndrome, DGKe, eculizumab, plasma exchange, thrombotic microangiopathy
CASE DESCRIPTION
A 17-year-old male patient presented recurrent episodes of thrombotic microangiopathy (TMA), characterized by microangiopathic hemolytic anemia, thrombocytopenia and organ damage.
The first episode occurred at 6 months of age with arterial hypertension, anemia, thrombocytopenia and kidney injury that required dialysis. After plasma infusions, he recovered renal function and was discharged. At the age of 2 years, he presented a new episode of TMA, but recovered faster with plasma infusions and did not require dialysis. At the age of 14 years, he returned with severe hypertension, increase in serum creatinine and fluctuation in platelet count. Two years later, a renal biopsy demonstrated chronic TMA (Figure 1).
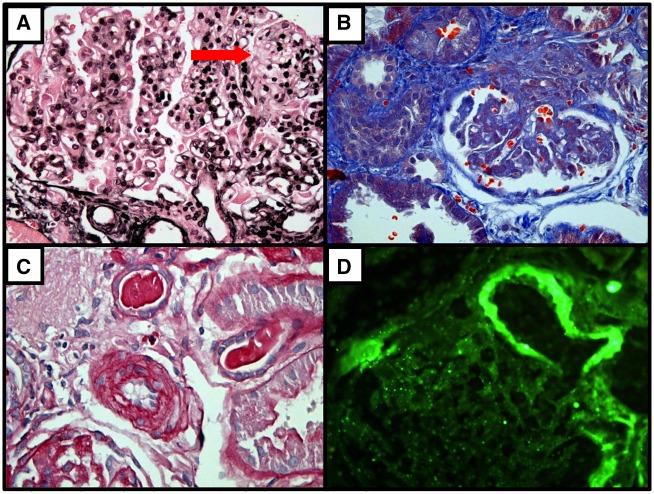
FIGURE 1.
First Renal Biopsy at age 16 years (before treatment with eculizumab), February 6th, 2016. (A) - Reduplication of glomerular basement membrane and mesangiolysis - arrow (silver, x400); (B) - Corrugated and reduplication of glomerular basement membrane (Gömöri’s trichrome, x400); (C) - Intimal thickening of small artery (periodic acid -Schiff, x400); (D) - Immunofluorescence microscopy shows arterial wall staining for C3 (x400).
After secondary causes for TMA were excluded and a normal activity of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 ruled out thrombotic thrombocytopenic purpura, the diagnosis of atypical hemolytic uremic syndrome (aHUS) was made.
Eculizumab was started in the Food and Drig Administration-approved regimen for atypical Hemolytic Uremic Syndrome (aHUS). After 18 months of treatment, despite hematological improvement, the patient persisted with hypertension (four classes of anti-hypertensive drugs), with progressive decline in renal function and increase in proteinuria. Genetic analysis (Centogene®, Rostock, Germany) was positive for an homozygous deletion in the diacylglycerol kinase epsilon (DGKe) gene—p.356AspLysfs*6 (c.1069_1071del), a new mutation in the last amino acid of the catalytic domain of the protein, considered pathogenic according to American College of Medical Genetics classification [1]. A second renal biopsy was performed with worsening of interstitial fibrosis and severe vasculopathy (Figure 2). Eculizumab was stopped and 3 months later kidney function was stable (Table 1).
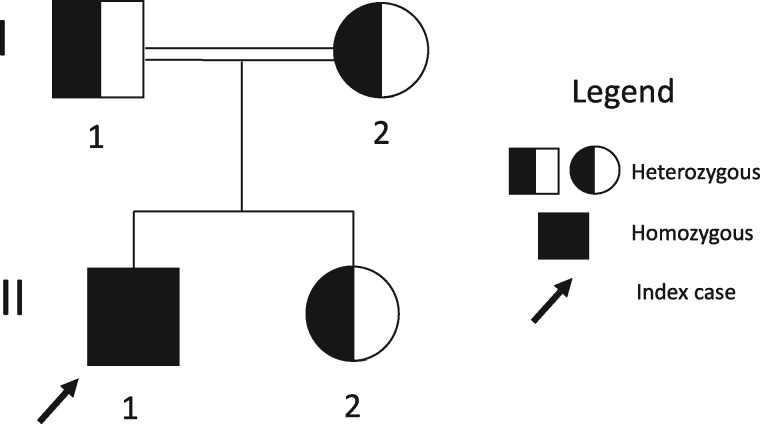
FIGURE 3.
Family Pedigree. Index case (II-1) developed first symptoms of aHUS at six months. His genetic diagnosis prompted the analysis of the family members, uncovering the presence of the variant p.356AspLysfs*6(c.1069_107del) in all of them.
Table 1.
Evolution of hematological and renal parameters of the index patient before and after complement blockade with eculizumab
| Date |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2002 | 2014 | June 2016 | Oct 2016 | Nov 2016 | 2017 | Jan 2018 | Feb 2018 | March 2018 | April 2018 | June 2018 | Aug 2018 | |
| Time point | First mani- festation | Second mani- festation | Resume follow-up | First biopsy | First eculi- zumab infusion | First month treatment | Ongoing eculi- zumab | Ongoing eculi- zumab | Genetic testing | Second biopsy and eculizumab stop | Follow-up after eculizumab stop | Last follow-up | |
| Hb (mg/dL) | 12.4 | 11.2 | 10.9 | 12.2 | 10.6 | ||||||||
| Platelets (×103/mm3) | 199 | 130 | 148 | 203 | 178 | ||||||||
| LDH (U/L) | 166 | 169 | 154 | 182 | |||||||||
| SCr (mg/dL) | 1.8 | 2.4 | 2.0 | 2.4 | 2.3 | ||||||||
| Proteinuria | 3+ | 1.1 g/24 h | 3.6 g/24 h | 2.4 g/ 24 h | 2.2 g/24 h | ||||||||
Manif, manifestation; ecu, eculizumab; LDH, lactic dehydrogenase (upper normal limit 248 U/L); Hb, hemoglobin.
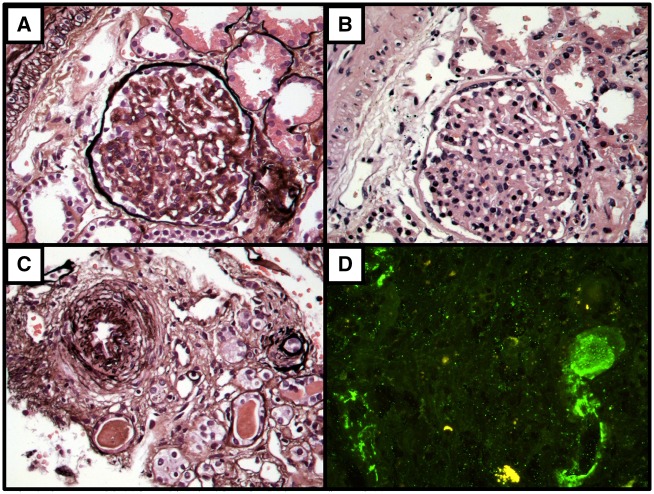
FIGURE 2.
Second Renal biopsy: After 18 months of eculizumab treatment- April 4th, 2018. (A) Corrugated and reduplication of glomerular basement membrane and mesangial expansion -(silver, x400). (B) Glomerular mesangial expansion (hematoxylin and eosin, x400). (C) Intimal thickening of small artery (silver, x400). (D) Immunofluorescence microscopy shows arterial wall staining for C3 (x400).
His parents are consanguineous and carry the variant DGKe p.356AspLysfs*6 (c.1069_1071del) in heterozygosis, as does his younger sister (Figure 3). They are being followed up and the father presents hypertension and a creatinine of 1.3 mg/dL, with no proteinuria.
DISCUSSION
aHUS is characterized by thrombosis in the microvasculature. The central pathophysiology in at least 50–60% of patients is the overactivation of the alternative complement pathway. The introduction of the terminal complement blocker eculizumab changes the perspective of these patients. However, 30–40% of patients do not have an identified genetic variant in complement genes [2].
Lemaire et al. [3] reported recessive loss-of-function mutations in DGKe as the cause of aHUS in some pediatric cases presenting in the first year of life, establishing a different pathophysiological mechanism for TMA that does not involve complement.
In 2017, Azukaitis et al. [4] analyzed data of 44 patients with DGKe nephropathy, and the authors presented a case for whom eculizumab was not able to prevent TMA relapse. Two patients who had relapses of TMA while on eculizumab had been published by Lemaire et al. [3], and case reports of eculizumab and DGKe are summarized in Table 2. Initially, DGKe TMA was considered a complement independent condition. Recent assays support the concept of complement activation (C3 consumption and deposition on biopsy, alternative pathway activation) in a subset of patients, and there are a few patients who also present mutations in complement regulator genes. It still remains unclear whether complement activation is a primary or secondary event in DGKe nephropathy, and which patients could benefit from complement blockade. In Azukaitis et al.’s [4] review, 16 patients recovered completely without specific therapy, and only 1 progressed to end-stage renal disease.
Table 2.
Case reports of patients with TMA and DGKe mutation treated with eculizumab
| References | Number of patients | Age at treatment | Genetic findings | Response to eculizumab |
|---|---|---|---|---|
| Lemaire et al. [3] | 13 | <1 year | Homozygous and compound heterozygous mutations in DGKe | Two patients presented relapse of TMA during treatment |
| Sanchez Chinchilla et al. [5] | 4 | <1 year | Homozygous and compound heterozygous mutations in DGKe | Complete recovery in the patient with DGKe and C3 mutation |
| Three patients also had mutations in thrombomodulin or C3 | ||||
| Miyata et al. [6] | 1 | 4 months | Compound heterozygous mutation in DGKe and a missense polymorphism in CFH | Complete recovery with eculizumab after plasma-resistant TMA |
| Negative anti-Factor H antibody | ||||
| Basak et al. [7] | 1 | 17 years | Missense heterozygous variant in exon 2 DGKe and large homozygous deletion in CFHR1–CFHR3 | Complete recovery |
C3, complement component 3; CFH, complement factor H; CFHR1–CFHR3, complement factor H-related proteins 1 and 3; TMA, thrombotic micorangiopathy.
Our patient presented the first episode of TMA before 1 year of age as described in other reports. Both renal biopsies were in agreement with the description in Mele et al. [8] of patients with DGKe mutations—histological features of chronic TMA, including endothelial cell swelling, glomerular hypercellularity, mesangial cell interposition within endothelial cells and split glomerular basement membrane. He presented with difficult-to-control hypertension, chronic kidney disease and proteinuria that did not improve after 18 months of eculizumab, probably because complement blockade was started in the late phase. Nevertheless, there was hematological improvement and we believe that the patient benefited from the treatment during his overt TMA presentation.
In conclusion, although eculizumab is the treatment of choice for aHUS, we point out the importance of genetic testing, especially in patients who present TMA before the first year of age. This is also true for patients who do not meet the expected outcomes with terminal complement blockade. Despite good outcomes reported in the literature in a few patients, in our patient with DGKe mutation, eculizumab did not prevent kidney progression or improved hypertension control.
FUNDING
There was no funding for the paper. Genetic analysis of the Index Case was funded by Alexion Pharma and Genetic analysis of the family was provided by the Pesquero Lab, UNIFESP, Brazil.
CONFLICT OF INTEREST STATEMENT
The authors confirm that the data presented here have not been published elsewhere except in abstract form at scientific meetings.
REFERENCES
- 1. Richards S, Aziz N, Bale S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015: 17: 405–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schaefer F, Ardissino G, Ariceta G. et al. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int 2018; 94: 408–418 [DOI] [PubMed] [Google Scholar]
- 3. Lemaire M, Frémeaux-Bacchi V, Schaefer F. et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet 2013; 45: 531–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Azukaitis K, Simkova E, Majid MA. et al. The phenotypic spectrum of nephropathies associated with mutations in diacylglycerol kinase epsilon. J Am Soc Nephrol 2017; 28: 3066–3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanchez Chinchilla D, Pinto S, Hoppe B. et al. Complement mutations in diacylglycerol kinase-epsilon-associated atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 2014; 9: 1611–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miyata T, Uchida Y, Ohta T. et al. Atypical haemolytic uraemic syndrome in a Japanese patient with DGKE genetic mutations. Thromb Haemost 2015; 114: 862–863 [DOI] [PubMed] [Google Scholar]
- 7. Basak R, Wang X, Keane C. et al. Atypical presentation of atypical haemolytic uraemic syndrome. BMJ Case Rep 2018; 2018: pii: bcr-2017-222560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mele C, Lemaire M, Iatropoulos P. et al. Characterization of a new DGKE intronic mutation in genetically unsolved cases of familial atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 2015; 10: 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]