Abstract
The endothelium is the largest organ in the body and recent studies have shown that the endothelial glycocalyx (eGCX) plays a major role in health and disease states. The integrity of eGCX is vital for homoeostasis and disruption of its structure and function plays a major role in several pathologic conditions. An increased understanding of the numerous pathophysiological roles of eGCX may lead to the development of potential surrogate markers for endothelial injury or novel therapeutic targets. This review provides a state-of-the-art update on the structure and function of the eGCX, emphasizing the current understanding of interorgan crosstalk between the eGCX and other organs that might also contribute to the pathogenesis of kidney diseases.
Keywords: chronic kidney disease, diabetes mellitus, endothelial glycocalyx
INTRODUCTION
The vascular endothelium is the largest organ in the body, forming an interface between the bloodstream and the blood vessel wall. It lines the inside of all blood vessels in the body. The luminal surface of all vascular endothelial cells is covered by the endothelial glycocalyx (eGCX), which comprises membrane-bound negatively charged proteoglycans, glycoproteins, glycolipids and glycosaminoglycans [1]. It provides the building block needed for a strong and functional vascular endothelium. Over the last few years, the potential possible pathophysiological roles of eGCX have been the focus of intense research. Disruption or dysfunction of the eGCX has been associated with disease states such as diabetes, chronic kidney disease (CKD), inflammatory conditions, sepsis, hypernatraemia, hypervolaemia and ischaemia/reperfusion injury. Also, changes in the eGCX have been associated with treatment responses in conditions such as sepsis. In this review we summarize evidence regarding the structure and function of the eGCX, alterations of the eGCX in various diseases states and therapeutic options.
eGCX structure
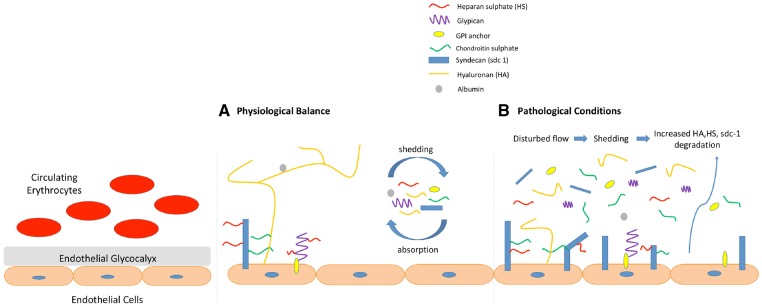
The eGCX lines the luminal surface of the vascular endothelium, interacting with plasma lipids and proteins [2, 3]. The composition and dimensions, permeability and charge of the eGCX interact dynamically with blood flow throughout the vasculature [4, 5]. The eGCX is attached to the endothelial cells through several backbone molecules, mainly proteoglycans and glycoproteins, creating a network in which soluble molecules from either plasma or endothelium are incorporated [1]. Proteoglycans consist of a core protein that can contain one or more negatively charged glycosaminoglycan (GAG) side chains. eGCX proteoglycans are structurally diverse regarding the size of the core protein, the number and type of GAG side chains and attachment to the endothelial cell membrane. Some core proteins such as syndecan are attached to endothelial cells through a transmembrane domain [6]. Others, such as glypican, are connected to the endothelial cell membrane through a glycosylphosphatidylinositol anchor [7], while others, such as perlecans, decorins, versicans, mimecans and biglycans, are secreted after the attachment and modification of GAG side chains [1].
GAGs are characterized by distinct linear disaccharide polymers of variable length. There are five types of GAGs: hyaluronic acid (HA), heparan, chondroitin, keratin and dermatan sulphates. Among these, heparan sulphate is the dominant type, making up 50–90%. Small modifications in the concentration and organization of GAGs can have a great impact on eGCX function since GAGs contain many distinct binding sites for plasma-derived proteins [1].
HA is a much longer GAG than heparan or chondroitin sulphate. HA is secreted on the endothelial surface and linked to the endothelial surface receptor CD 44 in caveolae. It weaves into the eGCX through its interaction with the CD44 receptor. Even if it is not sulphated, as opposed to the other four types of GAGs, it gets a negative charge from hydration properties supplied by carboxyl groups [8]. HA has been found to provide a direct connection between the endothelium and its extracellular matrix and is involved in the maintenance of eGCX integrity. The loss of HA as a part of eGCX degradation is observed to be closely associated with the endothelial dysfunction, which is an early predictor of many diseases such as diabetes, hypertension, atherosclerosis and dyslipidemia [9].
Endothelial glycoproteins also contribute to preserve eGCX stability. Endothelial glycoproteins contain 2–15 sugar residues arranged as branched carbohydrate side chains and comprise adhesion molecules, such as selectins (E and P), integrins and immunoglobulins, and some components of coagulation, haemostasis and fibrinolysis. Molecules secreted by glycoproteins participate in a wide range of eGCX functions, including endothelial cell adhesion, recognition and recruitment from the bloodstream and cell signalling [1] (Figure 1).
FIGURE 1.
Structure and functions of eGCX in physiological and pathological conditions.
Functions of eGCX
eGCX functions are summarized in Table 1. Tissue fluid balance and microvascular fluid exchange have been classically explained by the Starling principle since 1896. However, recent developments in our understanding of eGCX functions have increased the need for revising the Starling principle by incorporating the glycocalyx model. The eGCX is also known as the endothelial gatekeeper, summarizing its main physiological role as being the key determinant of vascular permeability, maintaining the balance between fluid filtration and absorption in the capillary lumen [1, 10].
Table 1.
Functions of eGCX
| Acts as the exclusion zone between blood cells and the endothelium |
| The exact composition varies greatly according to the local microenvironment |
| Plays the main role in transvascular fluid exchange and fluid balance (acts as the ‘molecular sieve’ for plasma proteins, forming the basis of the Starling principle and acting as the origin of the oncotic forces, which control the transcapillary movement of water) |
| Modulates adhesion of inflammatory cells and platelets to the endothelial surface |
| Functions as a sensor and mechanotransducer of the fluid shear forces |
| Maintains blood rheology and damage leads to platelet activation (normally retains anticoagulation factors such as antithrombin) |
| Plays a role in shear stress–dependent nitric oxide production |
| Retains protective enzymes (e.g. superoxide dismutase) |
The eGCX, composed of a negatively charged network of GAGs and proteoglycans, coats the luminal surface of the vascular endothelium. The eGCX is not permeable to large molecules such as dextran, and its neutralization increased rat mesenteric artery permeability for fluorescence-labelled dextrans [11]. The negatively charged GAGs also contribute to prevent albumin transport through the vascular wall barrier [12]. Thus vascular permeability is modified by the molecular size, structure and electrostatic charge of the eGCX [13].
The eGCX also modulates interactions between the vessel wall and blood cells. The negatively charged eGCX repels and is impermeable to red blood cells (RBCs), contributing to ensure the enclosed flow of RBCs in the microcirculation, and weakens the interaction of leucocytes and platelets with the vessel wall, thus controlling platelet and leucocyte adhesion and modulating the haemostatic and inflammatory responses [1, 12, 14]. eGCX adhesion molecules, including intercellular adhesion molecules 1 and 2, platelet/endothelial cell adhesion molecule and vascular cell adhesion molecule (VCAM), behave as ligands for integrins on leucocytes and platelets [1] and are induced by the inflammation. After monocyte and neutrophil adhesion via integrins and selectins, adhesion molecules promote cell rolling and leucocyte extravasation [15]. On the other hand, how the glycocalyx (conversely) inhibits leucocyte adhesion as shown by some studies needs further investigation [16, 17].
The eGCX also controls the interaction with the microenvironment by enabling binding of ligands and enzymes that regulate cell signalling, enzyme modifications and vascular protection. Without being exhaustive, fibroblast growth factors and the lipolytic system [lipoprotein lipase and its ligand low-density lipoprotein (LDL)] are dependent on the interactions of their ligands and receptors within the eGCX [1]. Moreover, the eGCX is a binding site for crucial anticoagulant mediators such as heparin cofactor II, antithrombin III, thrombomodulin and tissue factor pathway inhibitor (TFPI). Heparin cofactor II is a plasma protease inhibitor, inhibiting thrombin when in the presence of eGCX dermatan sulphate. Antithrombin III inhibition of coagulation by lysing thrombin, factor Xa and factor IXa is enhanced when it is bound to eGCX heparan sulphate. The endothelial cell surface protein thrombomodulin contains GAGs (chondroitin sulphate) and is a cofactor for thrombin. TFPI is a single-chain polypeptide that can reversibly inhibit factors VIIa and Xa after binding the eGCX heparan sulphates [1, 18, 19].
The eGCX may also protect the endothelium by binding enzymes that metabolize oxygen radicals, such as extracellular superoxide dismutase. These enzymes maintain nitric oxide (NO) bioavailability, decrease oxidative stress and prevent endothelial dysfunction [1, 18, 19]. Furthermore, the eGCX facilitates the release of NO when exposed to shear stress, thus reducing shear stress by dilating vessels [18–20]. Heparan sulphate proteoglycans sense the shearing force of interstitial flow, resulting in mechanotransduction and activation of specific cellular responses [21]. Most mechanical stress is converted to biochemical signals through the solid phase of the eGCX rather than through its fluid phase. The eGCX is also able to redistribute to perform some of its important actions, thus modulating microvessel haemodynamics [22].
Disruption of the eGCX and its role in pathophysiology
The eGCX may be damaged by exposure to shear and oxidative stress in conditions such as diabetes, sepsis, CKD, hypernatraemia, hypervolaemia and ischaemia/reperfusion injury [12, 23–27]. Here we will discuss the suspected roles of the eGCX in these conditions.
DISEASES
Diabetes and hyperglycaemia
Diabetes mellitus (DM) may be complicated by microvascular (retinopathy, neuropathy, nephropathy) and macrovascular [cardiovascular disease (CVD), peripheral arterial disease, stroke] injury. Chronic hyperglycaemia injures the vessel wall, leading to increased endothelial permeability and impaired NO synthase function. However, the precise cause-and-effect relationship between vascular dysfunction and DM complications are not fully understood [7]. Shedding of the eGCX is thought to contribute to DM-induced vascular dysfunction. Assessment of the systemic glycocalyx volume in 10 healthy subjects by comparing the distribution volume of a glycocalyx-permeable tracer (dextran 40) and a glycocalyx-impermeable tracer showed that the systemic glycocalyx volume was decreased by 50% within 6 h after induction of acute hyperglycaemia [28]. Hyperglycaemia also increased plasma hyaluronan levels and promoted endothelial dysfunction and coagulation activation [28]. The eGCX thickness was 50% lower in DM patients than in healthy controls, while serum hyaluronidase and hyaluronan levels were higher in diabetics [23]. The thinnest eGCX was found in DM patients with microalbuminuria [23]. Furthermore, the eGCX volume was lower in sublingual and retinal vessels from patients with type 2 DM [29]. Overall, these studies revealed that both acute and chronic hyperglycaemia significantly reduced eGCX size, supporting the idea that eGCX disruption may be associated with DM-induced endothelial dysfunction.
Endothelial dysfunction, characterized by faulty vasodilation due to decreased NO availability in response to endothelium-derived relaxing factors, is present in both type 1 and 2 DM patients. It is a well-known risk factor for CVD and is thought to lead to microangiopathy by altering vascular permeability, blood flow and pressure [30–39]. Indeed, endothelial dysfunction predicts early micro- and macrovascular complications of DM. Several other mechanisms may also contribute to endothelial dysfunction in DM, including dyslipidaemia and hypertension. However, hyperglycaemia itself is still thought to be the primary cause of DM-related eGCX disruption and dysfunction [38]. Mediators of hyperglycaemia-induced eGCX damage include reactive oxygen species, advanced glycation end products and the activation of glycocalyx-degrading enzymes such as hyaluronidase (HYAL1) and heparanase [23, 38].
DM-related eGCX shedding correlated with altered serum GAG concentrations, including increased HA, syndecan-1 and chondroitin sulphate and decreased heparan sulphate. HA and syndecan-1 were recently proposed as plasma markers of DM-related eGCX shedding. Plasma syndecan-1 was increased in both type 2 and 1 DM patients with diabetic nephropathy and microalbuminuria. However, further studies are required to determine exactly how DM-induced eGCX loss and dysfunction contributes to vascular injury and dysfunction in DM [38].
Sepsis
Sepsis is a systemic inflammatory response to a documented or presumed infection. The eGCX is damaged early in the course of inflammatory conditions such as systemic inflammatory response syndrome and sepsis. In hamsters, tumour necrosis factor-α (TNF-α), a representative pro-inflammatory cytokine, damaged the eGCX and enhanced macromolecular permeability without increasing leucocyte recruitment [40]. TNF-α induced release of cytokines, heparinase and histamine and proteases by mast cells further disrupted the eGCX [41]. TNF-α induced shedding of syndecan-4 were mediated by matrix metalloproteinase-9 (MMP-9), contributing to eGCX damage [42]. There is also evidence that endothelial signalling is critical to septic glycocalyx degradation via TNF-α [17, 43].
Increased serum eGCX constituent levels correlated with increased mortality in sepsis [41, 44]. Shedding of eGCX constituents, such as endothelial cell adhesion molecules, is thought to induce further inflammation and increase recruitment of leucocytes and platelets contributing to organ dysfunction, such as acute kidney injury (AKI) and respiratory failure [45, 46].
Disruption of the eGCX in sepsis is associated with increased vascular wall permeability to macromolecules, loss of circulating albumin and subsequent fluid extravasation and oedema [7]. Parenteral albumin did not increase survival rates in sepsis patients as compared with crystalloids alone [47]. Together, these studies suggest that while eGCX disruption persists, effective oncotic pressure cannot be restored by administering albumin since large amounts of albumin will continue to leak into the interstitial space [48].
CVD
Despite therapeutic efforts to tackle the main risk factors (dyslipidaemia, high blood pressure, diabetes, obesity, smoking), CVD remains the leading cause of death globally. Atherosclerosis is a major cause of CVD and dysfunction of vasculo-protective endothelium is a key contributor. Exploration of the vasculo-protective properties of the vessel wall against atherosclerosis may identify novel therapeutic approaches [49]. The eGCX is considered to be protective from atherosclerosis, and eGCX shedding is associated with loss of the vasculo-protective properties of the vessel wall [49]. eGCX shedding increases lipid fluxes that lead to lipid deposition in the vessel wall, a characteristic feature of atherosclerosis, interferes with endothelial cell communication and increases inflammatory cell migration to the vessel wall. Thus disruption of the eGCX leads to decreased endothelial NO synthase expression by endothelial cells, promoting vasoconstriction and dysregulation of vascular tone. Emerging eGCX-targeted approaches could potentially be used to restore eGCX and treat early cases of atherosclerosis [50].
In isolated pig heart, near-complete eGCX shedding occurred after reperfusion following 20 min of warm (37°C) no-flow ischaemia, resulting in increased capillary permeability-induced tissue oedema with enhanced coronary perfusion pressure [51]. These results are consistent with human findings of increased plasma levels of eGCX constituents following coronary artery bypass or peripheral arterial surgery [45]. In pig heart, antithrombin significantly decreased post-ischaemic eGCX shedding, vascular leakage/tissue oedema and coronary perfusion pressure. Protection by antithrombin was potentiated by the addition of colloid [51].
Statins are the most used lipid-lowering medications, reducing LDL cholesterol through inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. Statins have pleiotropic actions beyond lowering LDL cholesterol, which depend on inhibition of intracellular signalling requiring HMG-CoA reductase metabolites, such as inflammatory cytokine-induced signalling in endothelial cells, a key driver of eGCX shedding [9]. Additionally, familial hypercholesterolaemia is associated with a profound perturbation of the eGCX. Rosuvastatin for 8 weeks led to the partial recovery of systemic glycocalyx volume in 13 patients with heterozygous familial hypercholesterolaemia [52]. However, uncertainty still exists about whether this is related to a direct statin effect or to improved lipid status [45].
Diazoxide is a potassium channel activator with antihypertensive and hyperglycaemic activities. During coronary artery bypass surgery, diazoxide decreased post-surgical plasma hyaluronan and syndecan-1 levels, which supported the idea that diazoxide treatment may reduce disruption of the eGCX [53].
Oxidative stress is widely recognized as a major contributor to the development of endothelial dysfunction, eGCX disruption and atherosclerosis progression. In experimental metabolic syndrome, combination therapy with olmesartan and azelnidipine successfully reduced oxidative stress via inhibition of nicotinamide adenine dinucleotide phosphate oxidase activity in aortic endothelial cells and improved endothelial function and maintenance of eGCX integrity [54].
CKD
Intact endothelial cell function is essential for normal kidney function. Patients with CKD have increased eGCX shedding, which is associated with endothelial dysfunction. Plasma levels of two major eGCX components, syndecan-1 and hyaluronan, increased steadily across CKD categories [25]. This is thought to be a consequence of increased shedding, although a lack of urinary excretion of immune reactive degradation products could not be entirely ruled out. Supporting the increased shedding hypothesis, plasma syndecan-1 and HA positively correlated with plasma markers of endothelial dysfunction such as von Willebrand factor, soluble fms-like tyrosine kinase-1, angiopoietin-2 and soluble vascular adhesion molecule-1. Consistently, in a rat model of CKD, plasma syndecan-1 inversely correlated with eGCX thickness, which was significantly decreased in aortic endothelial cells from CKD rats [27]. Overall, this study suggests that eGCX integrity and function are compromised in CKD. However, the specific mechanisms underlying the loss of eGCX integrity in CKD are not yet fully understood. In CKD, endothelial dysfunction is widely acknowledged to be a risk factor for atherosclerosis and cardiovascular events [55, 56]. Further supporting the causative link between CKD and endothelial dysfunction, endothelial function improves after kidney transplantation [55, 57, 58].
To investigate the association between renal function and the eGCX dimension, the perfused boundary region (PBR) was measured in control participants, patients with normal kidney function after kidney transplantation, end-stage renal disease patients and patients with interstitial fibrosis/tubular atrophy by using non-invasive sidestream darkfield (SDF) imaging. Serum levels of eGCX components such as syndecan-1, soluble thrombomodulin and the marker of endothelial activation, angiopoietin-2, were also measured [51]. This study indicated that PBR and the serum levels of sydecan-1 and thrombomodulin were elevated in patients with end-stage renal disease or interstitial fibrosis/tubular atrophy compared with the control participants and stable kidney transplant patients. PBR was also found to be positively correlated with angiopoietin-2. Taken together, these results support the idea that patients with reduced renal function (estimated glomerular filtration rate) have a decreased eGCX dimension [59]. Although diabetic nephropathy induces a decrease in eGCX dimension [60], the link between eGCX and end-stage renal disease is still not fully understood. Oltean et al. [61] demonstrated in a mouse study that vascular endothelial growth factor-A (VEGF-A) played a key role in renal endothelial dysfunction. In a murine diabetic nephropathy model, kidney VEGF-A165b was upregulated in mice without a loss of kidney function but not in those with a loss of kidney function. The VEGF-A165b isoform was shown to be involved in the phosphorylation of VEGF receptor 2 on the glomerular endothelial cells, reducing the degree of diabetic nephropathy vascular endothelial growth factor related eGCx damage and improving glomerular permeability. After observing the functional and histologic benefits of administration of VEGF-A165b in animal models with diabetic nephropathy, VEGF-A165b was tested on isolated diabetic human glomeruli. The permeability function of the human glomeruli was also restored with the administration of VEGF-A165b, protecting the eGCx of renal vasculature from the destructive effects of diabetic nephropathy [61].
Inflammation contributes to eGCx damage during kidney disease. Monocyte chemotactic protein-1 [MCP-1/chemokine (C-C motif) ligand 2] is the key chemokine recruiting inflammatory cells such as monocytes and macrophages to the kidney and clinical trials targeting MCP-1 support its pathogenic role in humans [62]. Inhibition of MCP-1 with emapticap pegol (NOX-E36) restored the eGCx barrier dimensions in streptozotocin-induced diabetes in Apoe knockout mice. NOX-E36 decreased eGCx degradation by heparinase, which was regulated by macrophage-secreted cathepsin L. This was associated with decreased albuminuria and kidney inflammation in the absence of changes in systemic haemodynamics [63]. Atrasentan, a selective endothelin A receptor antagonist whose clinical trial for diabetic nephropathy was recently terminated, decreased albuminuria by repairing the eGCX barrier in patients with diabetic nephropathy [64].
Evidence of eGCX degradation was also observed in patients suffering from ischaemia/reperfusion injury during vascular surgery [65]. In another study, ischaemic AKI in renal transplantation from donors after cardiac death (DCD) and living donors was investigated by using SDF imaging of peritubular capillaries and serum levels of syndecan-1 and heparan sulphate. DCD kidneys were reported to have reduced capillary flow and higher serum levels of syndecan-1 and heparan sulphate compared with living donor kidneys with minimal ischaemia. These findings provided strong evidence that the eGCx was degraded due to ischaemia and subsequent reperfusion. The loss of endothelial cells and eGCX integrity could contribute to AKI through decreasing tissue perfusion and more inflammation following reperfusion [66].
Administration of GAG-degrading enzymes (hyaluronidase, heparinase and chondroitinase) to mice resulted in increased glomerular vascular permeability to albumin but not to Ficoll (uncharged polymer), indicating that eGCX degradation led to reduced charge selectivity and proteinuria [67]. These results support a role for eGCX damage and systemic endothelial dysfunction in the genesis of albuminuria [68].
Plasma syndecan-1 and HA levels were higher in dialysis patients than in controls, showing that dialysis patients lost eGCx barrier functions and the severity of the loss was correlated with the level of inflammation. The authors also measured the eGCx dimension using the PBR and checked the serum markers of the eGCX components and endothelial dysfunction, such as E-selectin. Interestingly, no correlation was detected between the variables [26].
Peritoneal dialysis requires a healthy peritoneal membrane. It is thought that both a healthy mesothelium and peritoneal vessels are required to preserve the peritoneal barrier. In peritoneal dialysis patients, SDF imaging of the sublingual microvasculature did not disclose any relationship between PBR assessment of systemic eGCx and peritoneal transport parameters [69]. This is not surprising, given that peritoneal vessels receive the bulk of the peritoneal fluid bio-incompatibility challenge [70]. Thus some of the most toxic and highly reactive peritoneal dialysis fluid metabolites, such as 3,4-dideoxyglucosone-3-ene readily react with peritoneal membrane components and do not reach the systemic circulation [71].
MOLECULES
Albumin
Albumin is the major serum protein and has multiple important functions. Stabilization of eGCX is thought to be one of them. Electrostatic interactions occur between negatively charged GAGs in the eGCX and the positively charged arginine in albumin. It is believed that hypoalbuminaemia could facilitate shedding of the eGCX [72].
Albumin transports multiple small molecules, including free fatty acids and spingosine-1-phosphate (S1P) [73]. Binding of S1P to its receptors resulted in MMP inhibition and eGCX integrity preservation [74]. In this regard, in trauma patients, higher plasma levels of syndecan-1, a sign of eGCX shedding, was significantly correlated with a reduction in plasma colloid osmotic pressure [75]. However, this study sheds no light on whether hypoalbuminaemia facilitated eGCX shedding or eGCX shedding promoted hypoalbuminaemia.
In a recent study, Garsen et al. [76] showed that endothelin-1 induces proteinuria by heparanase-mediated disruption of the glomerular glycocalyx in diabetic nephropathy. In this study, the authors demonstrated that in mice, podocyte-specific knockout of the endothelin receptor prevented the diabetes-induced increase in glomerular heparanase expression, consequent reduction in heparan sulphate expression and eGCX thickness and development of proteinuria observed in wild-type counterparts [76].
Thus the relationship between albumin and eGCX seems to be reciprocal. eGCX disruption may facilitate albumin translocation to the interstitial space, while hypoalbuminaemia itself may disturb the function and structure of the eGCX.
Glomerular endothelium, glomerular basement membrane and podocytes are the three key components of the glomerular filtration barrier (GFB). Pathological albuminuria results from increased passage of albumin through the GFB, which exceeds the capacity of proximal tubular cells to reabsorb albumin. Pathological albuminuria is an important indicator of glomerular filtration dysfunction and, as an early sign of progressive cardiovascular and renal disease, is considered as a criterion to diagnose CKD [77]. While classically considered a marker of podocyte injury, albuminuria has been observed in the absence of podocyte changes, confirming the close integration of GFB components and the potential impact of endothelium and basement membrane injury on glomerular albumin permeability. The endothelial surface layer, comprised of the eGCX and adsorbed plasma constituents, restricts the permeability of glomerular capillaries to albumin. Communication between the glomerular endothelium and podocytes also influenced the contribution of podocytes to the albumin permeability of the GFB [78].
Recently a novel sensitive glomerular permeability assay confirmed that eGCX damage leads to increased albumin glomerular permeability in a mouse model. eGCX dysfunction in diabetic mice was associated with increased urinary albumin excretion. Furthermore, eGCX function was restored by angiopoietin-1 [79]. Patients with albuminuric CKD had widespread loss of the eGCX, which was hypothesized to lead to increased systemic microvascular permeability, linking albuminuria to systemic vascular disease [78, 80].
Tissue sodium deposition
High salt (NaCl) intake has a blood pressure–independent effect on endothelial function, contributing to the development of vascular diseases, whereas the arterial system benefits from lowering salt intake. The eGCX has been proposed as an effective sodium buffer since positively charged sodium ions can be trapped in the eGCX mesh by negatively charged proteoglycans [81, 82]. Quantitative eGCX analyses showed that chronic exposure to high ambient sodium weakened the sodium buffer capacity of eGCX, leading to eGCX disruption through reduction of negatively charged heparan sulphate residues [24]. High sodium levels increase the expression of endothelial sodium channels (EnNaCs) in the plasma membrane. Both the collapse of the eGCX and the increased number of EnNaCs allowed sodium to access endothelial cells more freely. Enhanced sodium fluxes into endothelial cells triggers intracellular signalling that may promote endothelial dysfunction through a reduction in NO release and an increase in mechanical arterial stiffness [24, 83, 84]. Arterial stiffness is a well-characterized contributor to the vasculopathy of CKD [85].
Volume
Volume loading is a blood-sparing procedure commonly used to improve haemodynamics and optimize cardiac output. However, it may lead to adverse effects such as tissue oedema, hypervolaemia, hypertension and heart failure [86]. Hypervolaemia is associated with enhanced release of atrial natriuretic peptide (ANP) and with eGCX collapse [87]. ANP itself was found to disrupt the eGCX through a cyclic guanosine monophosphate–linked proteolytic pathway in patients having coronary bypass surgery [88, 89]. Collapse of the eGCX was associated with detrimental interstitial fluid shifting [86]. From that perspective, plasma levels of eGCX degradation components should be explored for their potential as surrogate markers to assess vascular endothelial injury that may provide information about when to limit uncontrolled fluid resuscitation to prevent interstitial oedema [90].
Furthermore, crystalloids and colloids are the two main types of volume expanders. Research indicates that eGCX has an important role in the differential effects of colloids and crystalloids on plasma volume expansion. Crystalloid was considered to be expanded throughout the intravascular space, whereas colloids had no ability to distribute into the glycocalyx [91–93]. This issue is important since, during fluid resuscitation, the integrity of the eGCX should be considered. For example, recent studies have shown that crystalloid versus colloid infusion makes sense when considering the integrity of the eGCX in critical patients [92, 93].
THERAPEUTIC RESTORATION OF THE EGCX
The eGCX may restore itself within 5–7 days of an insult [94]. Accelerating restoration of the eGCX and protecting it from further damage are promising targets for the treatment of chronic vascular disease, as well as management of patients in critical care settings [44]. To date, there is no established drug directly acting on protection and regeneration of the eGCX [95]. However, some agents have been explored in this regard, including sulodexide, albumin, fresh frozen plasma, glucocorticoids and other agents.
Sulodexide is a mixture of heparin sulphate (80%) and dermatan sulphate (20%) extracted from porcine intestinal mucosa that has been unsuccessfully tested in clinical trials for diabetic nephropathy, but is in clinical use for venous insufficiency [96]. In type 2 diabetic patients, sulodexide restored the components of the eGCX in the retinal circulation within 8 weeks [29]. In rats with carotid artery balloon injury, sulodexide restored the eGCX and reduced damage, blood coagulation, lipid metabolism and local inflammation [97]. Sulodexide also accelerated eGCX restoration, decreased vascular permeability and increased survival in septic mice [98].
Albumin and fresh frozen plasma have several clinical indications. In pig heart transplantation–induced ischaemia/reperfusion, albumin decreased interstitial oedema and leucocyte-to-endothelial interaction within coronary arteries, contributing to preserve eGCX integrity [99]. Albumin is a carrier for several bioactive molecules, including S1P [100]. S1P is considered to protect the eGCX by inhibiting syndecan-1 shedding [101].
Fresh frozen plasma also protects eGCX integrity and preserves syndecan-1. In addition to being a source of albumin, fresh frozen plasma may inhibit various proteases that disrupt the eGCX and may induce the release of preformed syndecan-1 from endothelial cells [102].
Glucocorticoids are widely used as anti-inflammatory agents. They preserve the eGCX precisely through anti-inflammatory actions on leucocytes and by reducing cytokine-induced damage on the vascular barrier [103]. In an isolated pig heart model, administration of hydrocortisone protected the eGCX against ischaemia/reperfusion and TNF‐α infusion [104]. A better characterization of the molecular basis of these effects may reveal new cellular targets for drug development devoid of the extensive adverse effects profile of glucocorticoids.
Several additional approaches have preserved eGCX volume in animal studies, including NO, TNF‐α inhibitors, hyaluronan, TNF–polyethylene glycol, allopurinol, adenosine agonists and heparin [7].
CONCLUSION
Damage to the eGCx during DM and other systemic diseases appears to be a key contributor to endothelial dysfunction. Additional identification of factors regulating the stability and repair of the eGCx under stress conditions and the pathophysiological roles of the eGCx might ultimately lead to the design of new diagnostic and therapeutic strategies for diseases characterized by endothelial dysfunction or vascular injury, including CKD.
ACKNOWLEDGEMENTS
We would like to thank Professors Giuseppe Remuzzi and Daniela Macconi for editing the manuscript. M.K. gratefully acknowledges use of the services and facilities of the Koc University Research Center for Translational Medicine, funded by the Republic of Turkey, Ministry of Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Ministry of Development.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Reitsma S, Slaaf DW, Vink H. et al. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 2007; 454: 345–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gouverneur M, Spaan JAE, Pannekoek H. et al. Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol 2006; 290: H458–H462 [DOI] [PubMed] [Google Scholar]
- 3. Pries AR, Secomb TW, Gaehtgens P.. The endothelial surface layer. Pflugers Arch 2000; 440: 653–666 [DOI] [PubMed] [Google Scholar]
- 4. Lipowsky HH. Microvascular rheology and hemodynamics. Microcirculation 2005; 12: 5–15 [DOI] [PubMed] [Google Scholar]
- 5. Haraldsson B, Nystrom J, Deen WM.. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 2008; 88: 451–487 [DOI] [PubMed] [Google Scholar]
- 6. Gotte M. Syndecans in inflammation. FASEB J 2003; 17: 575–591 [DOI] [PubMed] [Google Scholar]
- 7. Alphonsus CS, Rodseth RN.. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia 2014; 69: 777–784 [DOI] [PubMed] [Google Scholar]
- 8. Pahakis MY, Kosky JR, Dull RO. et al. The role of endothelial glycocalyx components in mechanotransduction of fluid shear stress. Biochem Biophys Res Commun 2007; 355: 228–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wheeler-Jones CP, Farrar CE, Pitsillides AA.. Targeting hyaluronan of the endothelial glycocalyx for therapeutic intervention. Curr Opin Investig Drugs 2010; 11: 997–1006 [PubMed] [Google Scholar]
- 10. van den Berg BM, Nieuwdorp M, Stroes ES. et al. Glycocalyx and endothelial (dys) function: from mice to men. Pharmacol Rep 2006; 58: 75–80 [PubMed] [Google Scholar]
- 11. Haaren PMA, VanBavel E, Vink H. et al. Charge modification of the endothelial surface layer modulates the permeability barrier of isolated rat mesenteric small arteries. Am J Physiol 2005; 289: H2503–H2507 [DOI] [PubMed] [Google Scholar]
- 12. Chelazzi C, Villa G, Mancinelli P. et al. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care 2015; 19: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vink H, Duling BR.. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol 2000; 278: H285–H289 [DOI] [PubMed] [Google Scholar]
- 14. Vink H, Constantinescu AA, Spaan JA.. Oxidized lipoproteins degrade the endothelial surface layer: implications for platelet-endothelial cell adhesion. Circulation 2000; 101: 1500–1502 [DOI] [PubMed] [Google Scholar]
- 15. Ait-Oufella H, Maury E, Lehoux S. et al. The endothelium: physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Med 2010; 36: 1286–1298 [DOI] [PubMed] [Google Scholar]
- 16. Mulivor AW, Lipowsky HH.. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol 2002; 283: H1282–H1291 [DOI] [PubMed] [Google Scholar]
- 17. Schmidt EP, Yang Y, Janssen WJ. et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 2012; 18: 1217–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rubanyi GM, Romero JC, Vanhoutte PM.. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol 1986; 250(6 Pt 2): H1145–H1149 [DOI] [PubMed] [Google Scholar]
- 19. Burke-Gaffney A, Evans TW.. Lest we forget the endothelial glycocalyx in sepsis. Crit Care 2012; 16: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Florian JA, Kosky JR, Ainslie K. et al. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res 2003; 93: e136–e142 [DOI] [PubMed] [Google Scholar]
- 21. Shi Z-D, Wang H, Tarbell JM.. Heparan sulfate proteoglycans mediate interstitial flow mechanotransduction regulating MMP-13 expression and cell motility via FAK-ERK in 3D collagen. PLoS One 2011; 6: e15956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee TC, Long D, Clarke R.. Effect of endothelial glycocalyx layer redistribution upon microvessel poroelastohydrodynamics. J Fluid Mech 2016; 798: 812–852 [Google Scholar]
- 23. Nieuwdorp M, Mooij HL, Kroon J. et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 2006; 55: 1127–1132 [DOI] [PubMed] [Google Scholar]
- 24. Oberleithner H, Peters W, Kusche-Vihrog K. et al. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch 2011; 462: 519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rehm M, Haller M, Orth V. et al. Changes in blood volume and hematocrit during acute preoperative volume loading with 5% albumin or 6% hetastarch solutions in patients before radical hysterectomy. Anesthesiology 2001; 95: 849–856 [DOI] [PubMed] [Google Scholar]
- 26. Vlahu CA, Lemkes BA, Struijk DG. et al. Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol 2012; 23: 1900–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Padberg J-S, Wiesinger A, di Marco GS. et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis 2014; 234: 335–343 [DOI] [PubMed] [Google Scholar]
- 28. Nieuwdorp M, van Haeften TW, Gouverneur MCLG. et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 2006; 55: 480–486 [DOI] [PubMed] [Google Scholar]
- 29. Broekhuizen LN, Lemkes BA, Mooij HL. et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 2010; 53: 2646–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hadi HAR, Carr CS, Al Suwaidi J. . Endothelial dysfunction: cardiovascular risk factors, therapy and outcome. Vasc Health Risk Manag 2005; 1: 183–198 [PMC free article] [PubMed] [Google Scholar]
- 31. Sen N, Ozlu MF, Akgul EO. et al. Elevated plasma asymmetric dimethylarginine level in acute myocardial infarction patients as a predictor of poor prognosis and angiographic impaired reperfusion. Atherosclerosis 2011; 219: 304–310 [DOI] [PubMed] [Google Scholar]
- 32. Kanbay M, Afsar B, Siriopol D. et al. Endostatin in chronic kidney disease: associations with inflammation, vascular abnormalities, cardiovascular events and survival. Eur J Intern Med.2016; 33: 81–87 [DOI] [PubMed] [Google Scholar]
- 33. Ozkok A, Ozkok S, Takir M. et al. Serum heparanase levels are associated with endothelial dysfunction in patients with obstructive sleep apnea. Clin Respir J 2018; 12: 1693–1699 [DOI] [PubMed] [Google Scholar]
- 34. Kanbay M, Afsar B, Siriopol D. et al. Relevance of uric acid and asymmetric dimethylarginine for modeling cardiovascular risk prediction in chronic kidney disease patients. Int Urol Nephrol 2016; 48: 1129–1136 [DOI] [PubMed] [Google Scholar]
- 35. Kanbay M, Yilmaz MI, Sonmez A. et al. Serum uric acid independently predicts cardiovascular events in advanced nephropathy. Am J Nephrol 2012; 36: 324–331 [DOI] [PubMed] [Google Scholar]
- 36. Kanbay M, Yilmaz MI, Sonmez A. et al. Serum uric acid level and endothelial dysfunction in patients with nondiabetic chronic kidney disease. Am J Nephrol 2011; 33: 298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aciksari G, Kavas M, Atici A. et al. Endocan levels and endothelial dysfunction in patients with sarcoidosis. Angiology 2018; 69: 878–883 [DOI] [PubMed] [Google Scholar]
- 38. Dogne S, Flamion B, Caron N.. Endothelial glycocalyx as a shield against diabetic vascular complications: involvement of hyaluronan and hyaluronidases. Arterioscler Thromb Vasc Biol 2018; 38: 1427–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perrin RM, Harper SJ, Bates DO.. A role for the endothelial glycocalyx in regulating microvascular permeability in diabetes mellitus. Cell Biochem Biophys 2007; 49: 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Henry CB, Duling BR.. TNF-α increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 2000; 279: H2815–H2823 [DOI] [PubMed] [Google Scholar]
- 41. Nelson A, Berkestedt I, Schmidtchen A. et al. Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock 2008; 30: 623–627 [DOI] [PubMed] [Google Scholar]
- 42. Ramnath R, Foster RR, Qiu Y. et al. Matrix metalloproteinase 9-mediated shedding of syndecan 4 in response to tumor necrosis factor alpha: a contributor to endothelial cell glycocalyx dysfunction. FASEB J 2014; 28: 4686–4899 [DOI] [PubMed] [Google Scholar]
- 43. Lukasz A, Hillgruber C, Oberleithner H. et al. Endothelial glycocalyx breakdown is mediated by angiopoietin-2. Cardiovasc Res 2017; 113: 671–680 [DOI] [PubMed] [Google Scholar]
- 44. Schott U, Solomon C, Fries D. et al. The endothelial glycocalyx and its disruption, protection and regeneration: a narrative review. Scand J Trauma Resusc Emerg Med 2016; 24: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Becker BF, Chappell D, Bruegger D. et al. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res 2010; 87: 300–310 [DOI] [PubMed] [Google Scholar]
- 46. Martin L, Koczera P, Zechendorf E. et al. The endothelial glycocalyx: new diagnostic and therapeutic approaches in sepsis. BioMed Res Int 2016; 2016: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Caironi P, Tognoni G, Masson S. et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med 2014; 370: 1412–1421 [DOI] [PubMed] [Google Scholar]
- 48. Lira A, Pinsky MR.. Choices in fluid type and volume during resuscitation: impact on patient outcomes. Ann Intensive Care 2014; 4: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nieuwdorp M, Meuwese MC, Vink H. et al. The endothelial glycocalyx: a potential barrier between health and vascular disease. Curr Opin Lipidol 2005; 16: 507–511 [DOI] [PubMed] [Google Scholar]
- 50. Mitra R, O’Neil GL, Harding IC. et al. Glycocalyx in atherosclerosis-relevant endothelium function and as a therapeutic target. Curr Atheroscler Rep 2017; 19: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chappell D, Jacob M, Hofmann-Kiefer K. et al. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc Res 2009; 83: 388–396 [DOI] [PubMed] [Google Scholar]
- 52. Meuwese M, Mooij L, Nieuwdorp H. et al. Partial recovery of the endothelial glycocalyx upon rosuvastatin therapy in patients with heterozygous familial hypercholesterolemia. J Lipid Res 2009; 50: 148–153 [DOI] [PubMed] [Google Scholar]
- 53. Mennander AA, Shalaby A, Oksala N. et al. Diazoxide may protect endothelial glycocalyx integrity during coronary artery bypass grafting. Scand Cardiovasc J 2012; 46: 339–344 [DOI] [PubMed] [Google Scholar]
- 54. Nagasu H, Satoh M, Yorimitsu D. et al. Comparison of combination therapy of olmesartan plus azelnidipine or hydrochlorothiazide on renal and vascular damage in SHR/NDmcr-cp rats. Kidney Blood Press Res 2011; 34: 87–96 [DOI] [PubMed] [Google Scholar]
- 55. Yilmaz MI, Sonmez A, Saglam M. et al. Longitudinal analysis of vascular function and biomarkers of metabolic bone disorders before and after renal transplantation. Am J Nephrol 2013; 37: 126–134 [DOI] [PubMed] [Google Scholar]
- 56. Recio-Mayoral A, Banerjee D, Streather C. et al. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease a cross-sectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis 2011; 216: 446–451 [DOI] [PubMed] [Google Scholar]
- 57. Oflaz H, Turkmen A, Turgut F. et al. Changes in endothelial function before and after renal transplantation. Transplant Int 2006; 19: 333–337 [DOI] [PubMed] [Google Scholar]
- 58. Kocak H, Ceken K, Yavuz A. et al. Effect of renal transplantation on endothelial function in haemodialysis patients. Nephrol Dial Transplant 2006; 21: 203–207 [DOI] [PubMed] [Google Scholar]
- 59. Dane MJC, Khairoun M, Lee DH. et al. Association of kidney function with changes in the endothelial surface layer. Clin J Am Soc Nephrol 2014; 9: 698–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Haraldsson BS. The endothelium as part of the integrative glomerular barrier complex. Kidney Int 2014; 85: 8–11 [DOI] [PubMed] [Google Scholar]
- 61. Oltean S, Qiu Y, Ferguson JK. et al. Vascular endothelial growth factor-A165b is protective and restores endothelial glycocalyx in diabetic nephropathy. J Am Soc Nephrol 2015; 26: 1889–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Perez-Gomez MV, Sanchez-Niño MD, Sanz AB. et al. Targeting inflammation in diabetic kidney disease: early clinical trials. Expert Opin Investig Drugs 2016; 25: 1045–1058 [DOI] [PubMed] [Google Scholar]
- 63. Boels MGS, Koudijs A, Avramut MC. et al. Systemic monocyte chemotactic protein-1 inhibition modifies renal macrophages and restores glomerular endothelial glycocalyx and barrier function in diabetic nephropathy. Am J Pathol 2017; 187: 2430–2340 [DOI] [PubMed] [Google Scholar]
- 64. Boels MGS, Avramut MC, Koudijs A. et al. Atrasentan reduces albuminuria by restoring the glomerular endothelial glycocalyx barrier in diabetic nephropathy. Diabetes 2016; 65: 2429–2439 [DOI] [PubMed] [Google Scholar]
- 65. Rehm M, Bruegger D, Christ F. et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 2007; 116: 1896–1906 [DOI] [PubMed] [Google Scholar]
- 66. Snoeijs MG, Vink H, Voesten N. et al. Acute ischemic injury to the renal microvasculature in human kidney transplantation. Am J Physiol 2010; 299: F1134–F1140 [DOI] [PubMed] [Google Scholar]
- 67. Jeansson M, Haraldsson B.. Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. Am J Physiol Renal Physiol 2006; 290: F111–F116 [DOI] [PubMed] [Google Scholar]
- 68. Obeidat M, Obeidat M, Ballermann BJ.. Glomerular endothelium: a porous sieve and formidable barrier. Exp Cell Res 2012; 318: 964–972 [DOI] [PubMed] [Google Scholar]
- 69. Vlahu CA, Lopes Barreto D, Struijk DG. et al. Is the systemic microvascular endothelial glycocalyx in peritoneal dialysis patients related to peritoneal transport? Nephron Clin Pract 2014; 128: 159–165 [DOI] [PubMed] [Google Scholar]
- 70. Ortiz A, Wieslander A, Linden T. et al. 3,4-DGE is important for side effects in peritoneal dialysis what about its role in diabetes. Curr Med Chem 2006; 13: 2695–1702 [DOI] [PubMed] [Google Scholar]
- 71. Santamaría B, Ucero AC, Reyero A. et al. 3,4-Dideoxyglucosone-3-ene as a mediator of peritoneal demesothelization. Nephrol Dial Transplant 2008; 23: 3307–3015 [DOI] [PubMed] [Google Scholar]
- 72. Adamson RH, Clough G.. Plasma proteins modify the endothelial cell glycocalyx of frog mesenteric microvessels. J Physiol 1992; 445: 473–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Curry F-RE, Adamson RH.. Sphingosine-1-phosphate and the “albumin effect” on rat venular microvessels. FASEB J 2013; 27(1 Suppl): 896 [Google Scholar]
- 74. Zeng Y, Adamson RH, Curry FR. et al. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol 2014; 306: H363–H372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rahbar E, Cardenas JC, Baimukanova G. et al. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med 2015; 13: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Garsen M, Lenoir O, Rops ALWMM. et al. Endothelin-1 induces proteinuria by heparanase-mediated disruption of the glomerular glycocalyx. J Am Soc Nephrol 2016; 27: 3545–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Satchell SC, Tooke JE.. What is the mechanism of microalbuminuria in diabetes: a role for the glomerular endothelium? Diabetologia 2008; 51: 714–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Satchell S. The role of the glomerular endothelium in albumin handling. Nat Rev Nephrol 2013; 9: 717. [DOI] [PubMed] [Google Scholar]
- 79. Desideri S, Onions KL, Qiu Y. et al. A novel assay provides sensitive measurement of physiologically relevant changes in albumin permeability in isolated human and rodent glomeruli. Kidney Int 2018; 93: 1086–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Salmon AHJ, Ferguson JK, Burford JL. et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol 2012; 23: 1339–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Oberleithner H. Two barriers for sodium in vascular endothelium? Ann Med 2012; 44(Suppl 1): S143–S148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Oberleithner H. Sodium selective erythrocyte glycocalyx and salt sensitivity in man. Pflügers Archiv 2015; 467: 1319–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Siegel G, Walter A, Kauschmann A. et al. Anionic biopolymers as blood flow sensors. Biosens Bioelectron 1996; 11: 281–294 [DOI] [PubMed] [Google Scholar]
- 84. Oberleithner H, Riethmuller C, Schillers H. et al. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci USA 2007; 104: 16281–16286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kusche-Vihrog K, Schmitz B, Brand E.. Salt controls endothelial and vascular phenotype. Pflugers Arch 2015; 467: 499–512 [DOI] [PubMed] [Google Scholar]
- 86. Doherty M, Buggy DJ.. Intraoperative fluids: how much is too much? Br J Anaesth 2012; 109: 69–79 [DOI] [PubMed] [Google Scholar]
- 87. Chappell D, Bruegger D, Potzel J. et al. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit Care 2014; 18: 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bruegger D, Schwartz L, Chappell D. et al. Release of atrial natriuretic peptide precedes shedding of the endothelial glycocalyx equally in patients undergoing on- and off-pump coronary artery bypass surgery. Basic Res Cardiol 2011; 106: 1111–1121 [DOI] [PubMed] [Google Scholar]
- 89. Bruegger D, Jacob M, Rehm M. et al. Atrial natriuretic peptide induces shedding of endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol Heart Circ Physiol 2005; 289: H1993–H1999 [DOI] [PubMed] [Google Scholar]
- 90. Guidet B, Ait-Oufella H.. Fluid resuscitation should respect the endothelial glycocalyx layer. Crit Care 2014; 18: 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Woodcock TE. Plasma volume, tissue oedema, and the steady-state Starling principle. BJA Education 2017; 17: 74–78 [Google Scholar]
- 92. Zazzeron L, Gattinoni L, Caironi P.. Role of albumin, starches and gelatins versus crystalloids in volume resuscitation of critically ill patients. Curr Opin Crit Care 2016; 22: 428–436 [DOI] [PubMed] [Google Scholar]
- 93. Woodcock TE, Woodcock TM.. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth 2012; 108: 384–394 [DOI] [PubMed] [Google Scholar]
- 94. Potter DR, Jiang J, Damiano ER.. The recovery time course of the endothelial-cell glycocalyx in vivo and its implications in vitro. Circ Res 2009; 104: 1318–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tarbell JM, Cancel LM.. The glycocalyx and its significance in human medicine. J Intern Med 2016; 280: 97–113 [DOI] [PubMed] [Google Scholar]
- 96. Perez-Gomez M, Sanchez-Niño M, Sanz A. et al. Horizon 2020 in diabetic kidney disease: the clinical trial pipeline for add-on therapies on top of renin angiotensin system blockade. J Clin Med 2015; 4: 1325–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li T, Liu X, Zhao Z. et al. Sulodexide recovers endothelial function through reconstructing glycocalyx in the balloon-injury rat carotid artery model. Oncotarget 2017; 8: 91350–91361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Song JW, Zullo JA, Liveris D. et al. Therapeutic restoration of endothelial glycocalyx in sepsis. J Pharmacol Experimental Therapeutics 2017; 361: 115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Jacob M, Paul O, Mehringer L. et al. Albumin augmentation improves condition of guinea pig hearts after 4 hr of cold ischemia. Transplantation 2009; 87: 956–965 [DOI] [PubMed] [Google Scholar]
- 100. Adamson RH, Clark JF, Radeva M. et al. Albumin modulates S1P delivery from red blood cells in perfused microvessels: mechanism of the protein effect. Am J Physiol 2014; 306: H1011–H1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Zeng Y, Adamson RH, Curry F-RE. et al. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol 2014; 306: H363–H372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Haywood-Watson RJ, Holcomb JB. et al. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One 2011; 6: e23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chappell D, Jacob M, Hofmann-Kiefer K. et al. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology 2007; 107: 776–84 [DOI] [PubMed] [Google Scholar]
- 104. Chappell D, Hofmann-Kiefer K, Jacob M. et al. TNF-α induced shedding of the endothelial glycocalyx is prevented by hydrocortisone and antithrombin. Basic Res Cardiol 2009; 104: 78–89 [DOI] [PubMed] [Google Scholar]