Abstract
Background
High concentrations of both phosphate and fibroblast growth factor 23 (FGF23) observed in chronic kidney disease (CKD) are associated with an increased risk of cardiovascular morbidity and mortality. Pulse wave velocity (PWV) is a surrogate marker for cardiovascular events and all-cause mortality. It is not known whether a reduction of FGF23 or phosphate alters cardiovascular risk. Sevelamer has shown to have the ability to reduce both phosphate and FGF23 concentrations. Furthermore, reduction of PWV is reported with sevelamer use as well, but it is unclear if this is mediated by decline of phosphate or FGF23. We investigated if sevelamer induced a decline in PWV and if this was associated with a reduction in FGF23.
Methods
In all, 24 normophosphataemic CKD Stage 3 patients started treatment with a fixed dose of sevelamer-carbonate (Renvela®) 2.4 g twice daily, with their usual diet for 8 weeks in a single-arm study. PWV was measured and blood samples were obtained before, during and after washout of treatment with sevelamer. Vascular calcification was quantified using the Kauppila Index (KI). The primary outcome was the change of PWV from baseline to 8 weeks of treatment and the secondary endpoint was the difference of FGF23 following treatment with sevelamer. One of the linear mixed models was used to analyse the association between treatment and outcome. Mediation analysis was performed as a sensitivity analysis. The study was registered in the Dutch trial register (http://www.trialregister.nl: NTR2383).
Results
A total of 18 patients completed 8 weeks of treatment with sevelamer and were analysed. Overall, treatment with sevelamer did not induce a significant reduction of PWV (β = −0.36, P = 0.12). However, in patients with less vascular calcification (lower KI score), there was a statistically significant reduction of PWV, adjusted for mean arterial pressure, after treatment (β = 0.63, P = 0.02). Addition of FGF23 to the model did not alter this association. Mediation analysis yielded similar results. FGF23 did not decrease during treatment with sevelamer.
Conclusion
In this short-term pilot study in normophosphataemic CKD patients, treatment with sevelamer did not improve PWV. In subgroup analysis, however, PWV improved in patients with no or limited abdominal aorta calcifications. This was not associated with a decline of FGF23.
Keywords: cardiovascular risk, chronic kidney disease, FGF23, phosphorus binding therapy, PWV
INTRODUCTION
Chronic kidney disease (CKD) is a major public health concern given its high prevalence and its associated increased risk for cardiovascular morbidity and mortality [1]. This elevated risk cannot be fully explained by traditional Framingham risk factors. Emerging evidence points towards CKD-mineral bone disorder, in particular phosphate and fibroblast growth factor 23 (FGF23) as independent cardiovascular risk factors, in haemodialysis patients [2, 3], as well as in CKD patients [4, 5]. Both factors are associated with left ventricular hypertrophy [6, 7], vascular calcification [8] and disturbed vascular function [9].
An established predictor for cardiovascular outcome is arterial stiffness, as measured by pulse wave velocity (PWV), since it is strongly associated with cardiovascular events and all-cause mortality in hypertensive patients [10, 11] as well as in patients with CKD [12] and end-stage renal disease [13]. PWV is the velocity at which the arterial pulse wave propagates through the arterial system and is a highly reproducible method to assess arterial stiffness [14]. FGF23, a bone-derived hormone involved in controlling serum phosphorus concentrations by enhancing its renal excretion [15, 16], is associated with vascular stiffness and endothelial dysfunction through mechanisms that are currently not fully elucidated [17, 18]. Recent data suggest that vascular endothelium is a direct target for the klotho–FGF23 complex, possibly through the nitric oxide system enhancing vascular stiffness [14, 19, 20]. Vascular stiffness in turn is considered to contribute to increased cardiovascular morbidity [17, 21].
If FGF23 plays a causal role in arterial stiffness, then active targeting FGF23 would be a legitimate goal. Although several studies do demonstrate that FGF23 concentrations can be lowered by the use of non-calcium-based phosphate binders [22–24], it is unknown what would be the impact on intermediate cardiovascular endpoints, such as PWV.
Besides FGF23, hyperphosphataemia is also associated with impaired endothelial function and vascular smooth muscle cell calcification leading to increased arterial stiffness [25–28]. To evaluate the independent role of FGF23 on PWV, we studied CKD patients with normophosphataemia, but increased FGF23.
In this pilot study in patients with CKD Stage 3, we tested whether 8 weeks of treatment with sevelamer-lowered PWV compared with baseline, and if this presumed change was associated with a decline in FGF23 concentration. We also tested if the presence of calcification influenced the effect of sevelamer treatment on PWV.
MATERIALS AND METHODS
Patients and study design
Twenty four CKD Stage 3 [estimated glomerular filtration rate (eGFR) 30–60 mL/min/1.73 m2] patients were recruited in a prospective single-arm pilot study. Additional inclusion criteria were age >18 years, and serum phosphate concentration between 0.9 and 1.49 mmol/L. Exclusion criteria consisted of a known allergy or intolerance for sevelamer-containing drugs, heart failure, current phosphate binder therapy use, unstable kidney function, dependency on tube-feeding or presence of a malabsorption syndrome and a history of kidney transplantation.
Following an observation period of 2 weeks, in all patients, a fixed dose of sevelamer-carbonate (Renvela® provided by Genzyme/Sanofi) 2.4 g twice daily was initiated and continued for 8 weeks. During the duration of the study patients kept their regular diet and further medication was unchanged.
The primary outcome was change of PWV from baseline to 8 weeks of treatment with sevelamer, and the secondary endpoint was change of FGF23 following 8 weeks of treatment. For safety reasons, serum phosphate concentration was measured after 1 week of treatment, and treatment was stopped if serum phosphate declined <0.70 mmol/L, the lower limit of normal. Patients who dropped out of the study were not analysed. This was because effect of treatment could not be evaluated in patients who stopped treatment.
Data collection and measurements
Patient visits were 2 weeks prior to baseline, at baseline (start of sevelamer-carbonate treatment), following 8 weeks of treatment and 2 weeks after cessation of treatment. After 1 week of treatment, a single blood sample was obtained for measurement of serum phosphate. At all other visits, physical examination, laboratory assessment and PWV measurements were performed. Physical examination consisted of measurement of height, weight and an office blood pressure measurement in supine position. Laboratory assessment included creatinine, calcium, phosphorus, parathyroid hormone (PTH) and plasma FGF23. C-terminal FGF23 was measured using sandwich enzyme-linked immunosorbent assay (Immutopics, San Clemente, CA, USA). The intra- and inter-assay coefficient of variation of this assay are <5% and <16%, respectively [29]. eGFR was estimated using the four-point MDRD formula [30]. Twenty-four hour urine samples were collected and creatinine, calcium, phosphate and proteinuria were measured using standard automated techniques. Fractional excretion of phosphate was calculated (urine phosphate× serum creatinine)/(serum phosphate× urine creatinine) and expressed as percentage.
Arterial stiffness was determined by measurement of PWV using the SphygmoCor® device. Transducer pulse waves were measured at the carotid artery and femoral artery (CF-PWV) by a specially trained research nurse following standardized procedures. The SphygmoCor applies an internal general transfer function to calculate measures of arterial stiffness and vascular properties. Three sequences of pulse waves of 10 s were recorded and analysed, but only readings with a standard deviation of <10% of the PWV were kept, averaged and used in the analyses. PWV values <8 m/s are defined as normal. Values above match progressive vascular stiffness of the aorta.
The presence and extent of abdominal aortic calcification were obtained from a lateral lumbar spine X-ray and scored using the Kauppila Index (KI) as previously described (scores 0–3 at each site, with a total range from 0 to 24) [31]. All X-ray images were evaluated by the same expert radiologist.
Information on medication was obtained from questionnaires and cross-checked with medical records.
Statistical analysis
A Generalized Estimating Equations (GEE) model (IBM SPSS Statistics version 22®) was used to compare the PWV and FGF23 concentration within each patient at the different time points of the study. The main analysis was the comparison of PWV during the treatment compared with baseline. Baseline in the analysis was defined as the measurement direct at the start of intervention. The treatment value consisted of the measurement after 8 weeks of treatment. The washout measurement was performed 2 weeks after cessation of therapy. All measurements were included in the model. To evaluate whether the potential effect of the use of sevelamer on PWV was affected by FGF23, FGF23 was added to the model. The analyses for PWV were expanded by stratification for baseline KI (below or above median). Stratification was applied since vascular calcification contributes to arterial stiffness and this component will unlikely be modifiable with short-term intervention as applied in this study.
In addition, we performed a plain mediation analysis (Stata version 14.2®) using a causal step approach to analyse the causal pathway of treatment with sevelamer on PWV and mediation by FGF23. Again, beside the crude analysis, a MAP-adjusted analysis and stratification for KI was performed. Assuming that changes in FGF23 will have in the short term an effect on PWV, we used GEE in this analysis.
The second analysis consisted of a comparison of the FGF23 concentrations at different time points. Again all measurements were included in the model. FGF23 concentrations following 8 weeks of treatment were compared with concentrations at baseline.
Because FGF23 showed a non-normal distribution, FGF23 was log-transformed (natural logarithm) if used as dependent variable. Confounders added to the analysis consisted of known confounders for FGF23 and PWV, which could be subjected to change during study period. For FGF23, these were: eGFR, serum calcium, serum phosphate, PTH, 1,25(OH)2 vitamin D and proteinuria. Possible confounders of PWV were MAP and eGFR. Furthermore, we analysed whether other laboratory parameters changed during the study. If this was the case, then we added those as confounders in the model to evaluate if changes in these parameters had an influence. In addition to these analyses, we evaluated if change of FGF23 (the difference between two subsequent values) was associated with the change of PWV using Pearson’s correlation analysis.
Data are given as mean and standard deviation (± SD) or median with interquartile range (IQR). The threshold for statistical significance was a P-value of 0.05. For all GEE models an exchangeable correlation structure was used.
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki and was approved by the local medical ethics committee, in this case the ‘METc VUmc’ (address; METc VUmc, kamer H-565, Postbus 7057, 1007 MB Amsterdam, +31 20 4445585).
All participants provided informed written consent to participate. The study was registered in the Dutch trial register (http://www.trialregister.nl: NTR2383).
Consent for publication
The study does not contain individual patient’s data, therefore consent for publication is not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
RESULTS
Course of inclusion of patients
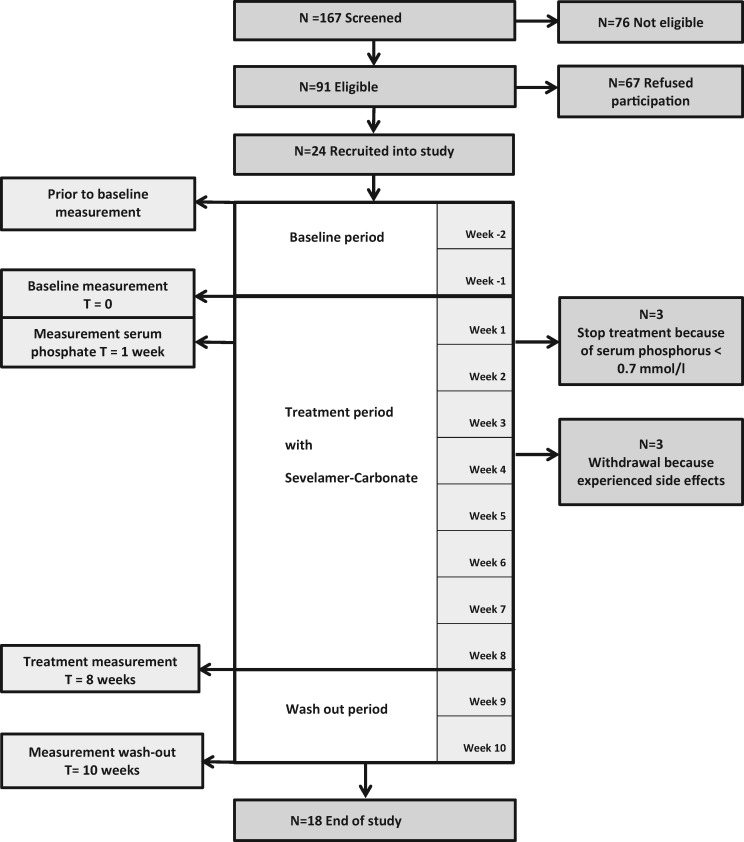
Of 24 patients, 18 patients completed the study (Figure 1). In three patients, treatment was stopped as per protocol after 1 week, because of a phosphate concentration below the threshold of the normal value (0.7 mmol/L), after which it returned to normal. The measured serum phosphate concentrations in these patients were 0.68, 0.42 and 0.64 mmol/L, and all patients were asymptomatic. In addition, three patients stopped treatment because of gastrointestinal side effects possibly related to sevelamer use. Analysis of the effect of treatment with sevelamer on PWV and FGF23 was performed in the remaining 18 patients.
FIGURE 1.
Study consort diagram. Diagram of the number of patients screened, recruited, participated and dropped-out of the study.
Baseline characteristics
Baseline characteristics of all 24 patients who started the study are presented in Supplementary data, Table S1. The mean age of the 18 analysed patients was 52 years and 61% were female (Table 1). Four patients had diabetes (two type 1 and two type 2 diabetes). Mean eGFR was 43.5 (±9.6) mL/min/1.73 m2.
Table 1.
Baseline characteristics of 18 patients treated with sevelamer-carbonate
| Age (years) | 52.0 ± 13.8 |
| Sex (male/female) | 7/11 |
| Diabetes type 1 or 2 (%) | 22 (n = 4) |
| Body mass index (kg/m2) | 27.4 (24.8–32.9) |
| Race (% Caucasian) | 72.2 |
| eGFR (mL/min/1.73 m2) | 43.5 ± 9.6 |
| PWV (m/s) | 9.2 ± 2.3 |
| KI score (0–12) | 1.5 (0.0–8.0) |
| FGF23 (U/L) | 167 (121–234) |
| PTH (pmol/L) | 7.2 (6.1–10.0) |
| 25 OH vitD3 (nmol/L) | 69.6 ± 30.6 |
| 1,25(OH) vitamin D (pmol/L) | 82.6 ± 21.2 |
| Phosphate (mmol/L) | 1.12 ± 0.18 |
| Calcium (mmol/L) | 2.30 ± 0.12 |
| Urinary PO4 excretion (mmol/24 h) | 25.4 (16.4–35.0) |
| Urinary Ca excretion (mmol/ 24 h) | 1.5 (0.9–2.2) |
| Fractional PO4 excretion (%) | 23.5 (14.4–36.4) |
| Proteinuria (g/24 h) | 0.34 (0.0–1.66) |
| Haemoglobin (mmol/L) | 8.1 (7.5–9.2) |
| Albumin (g/L) | 37.0 ± 3.9 |
| Cholesterol (mmol/L) | 4.8 ± 0.8 |
| Low-density lipoprotein cholesterol (mmol/L) | 2.48 ± 0.65 |
| MAP (mmHg) | 99.5 ± 11.8 |
| Smoking (yes/no) | 5/13 |
| Use angiotensin-converting enzyme or angiotensin receptor blocker (%) | 72.2 |
| Use cholecalciferol (%) | 27.8 |
| Use active vitamin D (%) | 33.3 |
Baseline values expressed as mean±SD or median (IQR).
Mean baseline PWV was 9.2 (±2.3) m/s. The KI score ranged from 0 in seven patients to scores above 7 in five patients. The median (IQR) Kauppila score was 1.5 (0.0–8.0). The median (IQR) FGF23 concentration was 167 (121–234) U/L. MAP was 99.5 (±11.8) mmHg. Additional baseline characteristics are depicted in Table 1.
Effect of sevelamer on PWV
Overall PWV did not change during treatment and was 9.2 (±2.3) before treatment and 8.7 (±2.4) m/s after treatment (P = 0.12). Median change of PWV was −0.10 (−0.85 to 0.35) m/s (Table 2 and Supplementary data, Figure S1). This was unchanged after addition of MAP and FGF23 to the model. Also after stratification for baseline FGF23 concentrations above or below the median, the decline of PWV did not differ from baseline to end of treatment (analysis not shown in table). However, when stratified for KI, there was a statistically significant improvement of PWV, after correcting for MAP, in patients with KI scores below the median (Table 2). PWV did not change in patients with higher KI. In the mediation analysis, performed as a sensitivity analysis, an effect of sevelamer on PWV was again found in patients with low Kauppila scores; however, there was no association with possible mediation of FGF23 (analysis not shown). No statistically significant association was found between eGFR or 1,25(OH)2 vitamin D with PWV during treatment (analysis not shown in table).
Table 2.
Effect of 8 weeks treatment with sevelamer-carbonate on PWV (m/s)
| Treatment compared with baseline | β (95% confidence interval) | P-value | |
|---|---|---|---|
| All | |||
| Crude | −0.36 (−0.82 to 0.09) | 0.12 | |
| Model 1: crude + MAP | −0.24 (−0.58 to 0.10) | 0.17 | |
| Model 2: model 1 + FGF23 | −0.22 (−0.58 to 0.13) | 0.22 | |
| KI score <median | |||
| Crude | −0.71 (−1.43 to 0.01) | 0.05 | |
| Model 1: crude + MAP | −0.63 (−1.13 to −0.12) | 0.02 | |
| Model 2: model 1 + FGF23 | −0.58 (−1.04 to −0.12) | 0.01 | |
| KI score >median | |||
| Crude | 0.05 (−0.37 to 0.46) | 0.82 | |
| Model 1: crude + MAP | 0.13 (−0.22 to 0.48) | 0.47 | |
| Model 2: model 1 + FGF23 | 0.28 (−0.07 to 0.62) | 0.11 | |
Values of PWV following treatment compared with PWV baseline. GEE was used to analyse the difference between treatment and baseline PWV values. All PWV measurements (of all time points) were included in the model, depicted is only the difference between treatment and baseline PWV. Stratification for KI score above and below the median was performed.
Effect of sevelamer on phosphate balance, FGF23 and other parameters
The mean reduction of 24-h phosphate excretion from baseline to end of treatment was 9.78 mmol/24 h (P = 0.008), indicating significant reduction of phosphate absorption with sevelamer treatment. Serum phosphate concentration did not change during this period. The median (IQR) change of FGF23 was −1.5 (−33.8 to 16.0) RU/L from baseline to Week 8. This was a non-significant reduction (β = −0.03, 95% confidence interval −0.19 to 0.12; P = 0.68). Additional stratification for FGF23 baseline concentrations above or below the median did not change this absence of effect on FGF23. Besides, FGF23 change did not differ between patients with a reduction of urinary phosphate excretion above or below the median (analysis not shown).
During treatment, there was a statistically significant decline of eGFR, MAP and 1,25(OH)2 vitamin D (Supplementary data, Figure S1), which returned towards baseline values after the washout period. The decline of 1,25(OH)2 vitamin D concentrations appeared in both vitamin D users and non-users. All other baseline parameters did not change significantly (analysis not shown in table).
In the analysis of the association between treatment with sevelamer and FGF23 concentration, adjustment for parameters potentially affecting FGF23 concentrations was performed (Supplementary data, Table S2). After correcting for eGFR and 1,25(OH)2 vitamin D, a statistically significant decline of FGF23 concentration was found following treatment with sevelamer.
To study if any change of FGF23 was associated with a change in PWV, regardless of the effect of sevelamer treatment, measurements from all time points of these two parameters were pooled. The change, or delta, was calculated from all subsequent values of FGF23 and PWV. We found no association between changes of FGF23 with corresponding change in PWV. However, the absolute differences of consecutive values of both PWV and FGF23 were very small, as shown in Supplementary data, Figure S2.
DISCUSSION
Effect of sevelamer on PWV
We hypothesized that a sevelamer-induced reduction of FGF23 would lead to improved vascular function as assessed by PWV. This hypothesis is based on previous observations that sevelamer can lower PWV, and its use is associated with a reduction in FGF23 concentration [22–24]. It is not known if lowering PWV is mediated by a reduction in FGF23 concentrations. Overall, we found no significant improvement in PWV after treatment with sevelamer (Table 2). A secondary analysis in patients with high versus low baseline KI score was performed since increased PWV may in part be the consequence of (non-modifiable) vascular calcification. This revealed noteworthy results. A statistically significant reduction of PWV during treatment with sevelamer was observed in patients with low KI scores after correction for MAP (Table 2). This suggests that in patients with low calcification score, PWV is more amenable for improvement. The latter was also observed in a study in peritoneal dialysis patients in which lower PWV was associated with lower aortic calcification scores and was more likely to change during the observation period [32].
The overall findings of our study are generally in line with those of Chue et al. [33], in which 109 patients with normophosphataemic CKD Stage 3 were randomized to sevelamer or placebo for 40 weeks. Despite the considerably longer intervention period compared with our study, these authors also found no effect of sevelamer on PWV or on FGF23. However, in contrast to our study, in the study of Chue et al. [33], no difference in PWV between placebo and sevelamer was observed when patients with aortic calcifications were excluded. This different outcome may result from the fact that in the study by Chue et al., the PWV results were not corrected for blood pressure, which is a key determinant for PWV.
Previous studies by Takenaka [34] and Othmane et al. [35] studying patients on dialysis with hyperphosphataemia noted a significant decline of PWV following sevelamer treatment. These studies were performed in patients at a different stages of CKD than our study and the duration of treatment in these studies was 6 and 11 months, respectively, compared with 8 weeks in our study. None of these studies measured FGF23. The short-term use of sevelamer in our study, however, was part of our hypothesis that FGF23-dependent arterial stiffness (assessed by PWV) can alter in a short period of time, unless there is outspoken vascular calcification. The possibility for short-term modification of PWV is described in several studies [e.g. studies in which statins or l-arginine (through the nitric oxide pathway) affected PWV within days or weeks] [36–38].
Effect of sevelamer on FGF23
The original premise of our study was that the use of sevelamer would induce a decline in FGF23 concentrations, which subsequently would improve PWV. Sevelamer, like other non–calcium-based phosphate binders, reduces phosphate absorption and has shown to have the potential to reduce FGF23 concentrations as is shown in a number of studies [22–24, 39, 40]. In our study, FGF23 concentrations did not decrease during treatment with sevelamer, despite an effective reduction of phosphate absorption (reflected by a significant decline in 24-h phosphate excretion). The latter indicates that the dosage of the phosphate binder was adequate. The fact that we failed to reduce FGF23 concentrations may, at least in part, be attributed to an unforeseen transient decline in eGFR during the treatment phase. This decline potentially masked the effect of sevelamer treatment on FGF23. Following treatment, there was also a reduction of 1,25(OH)2 vitamin D and MAP, which returned to pre-treatment levels after cessation of sevelamer. A possible explanation is that during the study the patients’ adherence to medication, especially antihypertensive drugs, improved, leading to a decline of MAP which then induced a reduction of eGFR. Interestingly, this phenomenon was also observed in the run-in phase of study of Chue et al. [33]. During that period, where the adherence to sevelamer was the highest, a decline in 1,25(OH)2 vitamin D, blood pressure and eGFR (latter not significant) was observed as well. All in all, our study does not support the concept that phosphate binder therapy in normophosphataemic patients with CKD can lower FGF23.
Influence of FGF23 on PWV
One component of our hypothesis was that the presumed effect of sevelamer on PWV was mediated by FGF23. However, the effect of treatment on PWV did not change by adding FGF23 to the GEE model, excluding FGF23 as confounder in this analysis (Table 2). In the mediation analysis, with the additional sensitivity test performed, an effect of sevelamer on PWV was found; however, this was not affected by FGF23.
Our inability to demonstrate an effect of FGF23 on PWV might be explained by the fact that the absolute change of FGF23 was minimal. Therefore, our study lacks discriminatory power to reveal a potential impact of change of FGF23 on arterial stiffness assessed by PWV. Although it cannot be ruled out that more substantial decline of FGF23 would have improved PWV, Chue et al.’s [33] recent data favour absence of any effect of FGF23 on PWV. In a subgroup analysis of this study, in the patients in whom FGF23 reduction was achieved, they observed that even more profound reduction of FGF23 concentration had no effect on PWV. In addition, in another study, on patients with CKD Stage 3 randomized to either lanthanum carbonate or placebo, the observed decline of FGF23 had no effect on PWV [41]. Finally, in a recent animal study in which mice were treated with sevelamer for 8 weeks, a decline of both FGF23 and PWV was found [42], yet the effect of sevelamer on PWV preceded the reduction of FGF23, and thus the reduction of PWV could also not be attributed to change of FGF23. Altogether, we conclude that although FGF23 is reported to be closely associated to vascular function, our study does not support the hypothesis that lowering FGF23 concentrations could improve PWV.
Strengths and weaknesses of the study
A strength of our study was that we performed the study in normophosphataemic CKD Stage 3 because elevated FGF23 concentrations are observed, yet serum phosphate concentrations are usually still in the normal range at this stage of CKD. This enabled us to limit the potentially confounding effect of normalization of serum phosphate during the intervention period. Moreover in our study, patients had well-controlled blood pressure and so the possible beneficial effect of sevelamer was evaluated in a setting of optimized standard of care. In addition, we measured abdominal calcification score, enabling us to study the potential of effect modification by this structural abnormality of the aorta.
Our study also has several limitations. First, there was no control group. In the study design, however, there was a baseline period, enabling individual patients to serve as their own control. Because of the pilot design, the study was performed in only a small group of patients based on the amount of patients in the earlier study by Oliveira et al. [22]. Although this is a limitation, one could also reflect that if an effect of sevelamer on either FGF23 or PWV is only detectable in a large population, this would also implicate a large number to treat to improve these intermediate endpoints. In this regard, it is encouraging that in the subgroup with low Kauppila scores, a statistically significant effect of treatment on PWV was found. Another limitation of our study is that no attempts were made to standardize intake of phosphate, calcium or other dietary components in the study population. This, however, is a better reflection of the real-life situation. Moreover, by measuring 24-h phosphate excretion, we were able to quantify dietary phosphate intake. In our study, three patients dropped out because of hypophosphataemia. It is possible that in those patients a different effect on PWV during treatment would have occurred. Another possible limitation is that we chose PWV as a proxy for vascular disease. PWV reflects function of the conduit arteries, not of the microcirculation. We cannot exclude that sevelamer treatment may have different effects on other segments of the circulation. Finally, our study was performed in a single centre, which might limit the external validity of our findings.
CONCLUSIONS
In summary, in our study in 18 normophosphataemic patients with CKD Stage 3, treatment with sevelamer did not improve PWV or FGF23. However, in patients with low KI scores, sevelamer improved PWV, but this was not associated with a decline of FGF23. Our findings do not support the assumption of beneficial effects of early treatment of phosphate exposure in order to improve cardiovascular risks in all CKD Stage 3 patients. Future studies aiming to improve cardiovascular risk by modifying PWV through lowering of FGF23 should focus on patients with limited vascular calcification.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the specially trained research nurse for performing the PWV measurements and the radiologist for evaluating the X-ray images.
FUNDING
The present study is investigator-initiated research. An unrestricted grant from Sanofi-Genzyme was available for this study. [Sanofi-Genzyme provided the study medication sevelamer-carbonate (Renvela®) and financed the laboratory measurements. Sanofi did not interfere with the data analysis nor with the draft of the manuscript.]
AUTHORS’ CONTRIBUTIONS
A.B.-d.K. participated in the design of the study, wrote the study protocol, wrote the application for the medical ethical commission, carried out the patients’ recruitment, processed the patient data, carried out the main part of the statistical analysis and wrote the manuscript. F.J.v.I. participated in the statistical analysis and interpretation of the data and helped to draft the manuscript. T.H. helped with the statistical analysis and helped to draft the manuscript. P.M.t.W. participated in the design of the study, wrote the application for the study grant and helped to draft the manuscript. M.G.V. conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
All the authors hereby declare that the results presented in this article have not been published previously in whole or part, except in abstract format. A.B.-d.K received funding for the present study by Sanofi-Genzyme. P.M.t.W. is a member of an advisory board and/or was a speaker at meetings sponsored by AMGEN, MSD, Danone and Baxter. M.G.V. received grants from Abbott BV, reported research funded by Sanofi Nederland BV, Dutch Kidney Foundation and speakers’ fee from Amgen. F.J.v.I. and T.H. reported no disclosures.
REFERENCES
- 1. Go AS, Chertow GM, Fan D. et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 2. Gutiérrez OM, Mannstadt M, Isakova T. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jean G, Terrat JC, Vanel T. et al. High levels of serum fibroblast growth factor (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant 2009; 24: 2792–2796 [DOI] [PubMed] [Google Scholar]
- 4. Isakova T, Xie H, Yang W. et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011; 305: 2432–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kestenbaum B, Sampson JN, Rudser KD. et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 2005; 16: 520–528 [DOI] [PubMed] [Google Scholar]
- 6. Faul C, Amaral AP, Oskouei B. et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zou Jun, Yu Yi, Wu Ping. et al. Serum phosphorus is related to left ventricular remodeling independent of renal function in hospitalized patients with chronic kidney disease. Int J Cardiol 2016; 221: 134–140 [DOI] [PubMed] [Google Scholar]
- 8. Srivaths PR, Goldstein SL, Silverstein DM. et al. Elevated FGF 23 and phosphorus are associated with coronary calcification in hemodialysis patients. Pediatr Nephrol 2011; 26: 945–951 [DOI] [PubMed] [Google Scholar]
- 9. Yilmaz MI, Sonmez A, Saglam M. et al. FGF-23 and vascular dysfunction in patients with Stage 3 and 4 chronic kidney disease. Kidney Int 2010; 78: 679–685 [DOI] [PubMed] [Google Scholar]
- 10. Laurent S, Boutouyrie P, Asmar R. et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37: 1236–1241 [DOI] [PubMed] [Google Scholar]
- 11. Blacher J, Asmar R, Djane S. et al. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension 1999; 33: 1111–1117 [DOI] [PubMed] [Google Scholar]
- 12. Zoungas S, Cameron JD, Kerr PG. et al. Association of carotid intima-medial thickness and indices of arterial stiffness with cardiovascular disease outcomes in CKD. Am J Kidney Dis 2007; 50: 622–630 [DOI] [PubMed] [Google Scholar]
- 13. Blacher J, Safar ME, Guerin AP. et al. Aortic pulse wave velocity index and mortality in end-stage renal disease. Kidney Int 2003; 63: 1852–1860 [DOI] [PubMed] [Google Scholar]
- 14. Tripepi G, Kollerits B, Leonardis D. et al. Competitive interaction between fibroblast growth factor 23 and asymmetric dimethylarginine in patients with CKD. J Am Soc Nephrol 2015; 26: 935–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shimada T, Hasegawa H, Yamazaki Y. et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 2004; 19: 429–435 [DOI] [PubMed] [Google Scholar]
- 16. Saito H, Kusano K, Kinosaki M. et al. Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1alpha, 25-dihydroxyvitamin D3 production. J Biol Chem 2003; 278: 2206–2211 [DOI] [PubMed] [Google Scholar]
- 17. Mirza MA, Larsson A, Lind L. et al. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis 2009; 205: 385–390 [DOI] [PubMed] [Google Scholar]
- 18. Figueiredo VN, Yugar-Toledo JC, Martins LC. et al. Vascular stiffness and endothelial dysfunction: Correlations at different levels of blood pressure. Blood Press 2012; 21: 31–38 [DOI] [PubMed] [Google Scholar]
- 19. Nagai R, Saito Y, Ohyama Y. et al. Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci 2000; 57: 738–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silswal N, Touchberry CD, Daniel DR. et al. FGF23 directly impairs endothelium-dependent vasorelaxation by increasing superoxide levels and reducing nitric oxide bioavailability. Am J Physiol Endocrinol Metab 2014; 307: E426–E436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Isakova T, Gutierrez OM, Wolf M.. A blueprint for randomized trials targeting phosphorus metabolism in chronic kidney disease. Kidney Int 2009; 76: 705–716 [DOI] [PubMed] [Google Scholar]
- 22. Oliveira RB, Cancela ALE, Graciolli FG. et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol 2010; 5: 286–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gonzalez-Parra E, Gonzalez-Casaus ML, Galan A. et al. Lanthanum carbonate reduces FGF23 in chronic kidney disease Stage 3 patients. Nephrol Dial Transplant 2011; 26: 2567–2571 [DOI] [PubMed] [Google Scholar]
- 24. Vlassara H, Uribarri J, Cai W. et al. Effects of sevelamer on HbA1c, inflammation, and advanced glycation end products in diabetic kidney disease. Clin J Am Soc Nephrol 2012; 7: 934–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stevens KK, Patel RK, Mark PB. et al. Phosphate as a cardiovascular risk factor: effects on vascular and endothelial function. Lancet 2015; 385: S10. [DOI] [PubMed] [Google Scholar]
- 26. Shuto E, Taketani Y, Tanaka R. et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol 2009; 20: 1504–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney Int 2009; 75: 890–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Toussaint ND, Lau KK, Strauss BJ. et al. Associations between vascular calcification, arterial stiffness and bone mineral density in chronic kidney disease. Nephrol Dial Transplant 2008; 23: 586–593 [DOI] [PubMed] [Google Scholar]
- 29. Heijboer AC, Levitus M, Vervloet MG. et al. Determination of fibroblast growth factor 23. Ann Clin Biochem 2009; 46: 338–340 [DOI] [PubMed] [Google Scholar]
- 30. Levey AS, Coresh J, Greene T. et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254 [DOI] [PubMed] [Google Scholar]
- 31. Kauppila LI, Polak JF, Cupples LA. et al. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis 1997; 132: 245–250 [DOI] [PubMed] [Google Scholar]
- 32. Tang M, Romann A, Chiarelli G. et al. Vascular stiffness in incident peritoneal dialysis patients over time. Clin Nephrol 2012; 78: 254–262 [DOI] [PubMed] [Google Scholar]
- 33. Chue CD, Townend JN, Moody WE. et al. Cardiovascular effects of sevelamer in stage 3 CKD. J Am Soc Nephrol 2013; 24: 842–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takenaka T, Suzuki H.. New strategy to attenuate pulse wave velocity in haemodialysis patients. Nephrol Dial Transplant 2005; 20: 811–816 [DOI] [PubMed] [Google Scholar]
- 35. Othmane TH, Bakonyi G, Egresits J. et al. Effect of sevelamer on aortic pulse wave velocity in patients on hemodialysis: a prospective observational study. Hemodial Int 2007; 11: S13–S21 [DOI] [PubMed] [Google Scholar]
- 36. Annavarajula SK, Dakshinamurty KV, Naidu MU. et al. The effect of L-arginine on arterial stiffness and oxidative stress in chronic kidney disease. Indian J Nephrol 2012; 22: 340–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Siasos G, Tousoulis D, Vlachopoulos C. et al. Short-term treatment with L-arginine prevents the smoking-induced impairment of endothelial function and vascular elastic properties in young individuals. Int J Cardiol 2008; 126: 394–399 [DOI] [PubMed] [Google Scholar]
- 38. Ballard KD, Lorson L, White CM. et al. Effect of simvastatin on arterial stiffness in patients with statin myalgia. Adv Prev Med 2015; 2015: 351059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yilmaz MI, Sonmez A, Saglam M. et al. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis 2012; 59: 177–185 [DOI] [PubMed] [Google Scholar]
- 40. Koiwa F, Kazama JJ, Tokumoto A. et al. Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients. Ther Apher Dial 2005; 9: 336–339 [DOI] [PubMed] [Google Scholar]
- 41. Seifert ME, de las Fuentes L, Rothstein M. et al. Effects of phosphate binder therapy on vascular stiffness in early-stage chronic kidney disease. Am J Nephrol 2013; 38: 158–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maizel J, Six I, Dupont S. et al. Effects of sevelamer treatment on cardiovascular abnormalities in mice with chronic renal failure. Kidney Int 2013; 84: 491–500 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.