Abstract
Background
Current therapies for anemia of chronic kidney disease (CKD) include administration of supplemental iron (intravenous and/or oral), blood transfusions and replacement of erythropoietin through the administration of recombinant human erythropoietin (rhEPO) and rhEPO analogs, each with limitations. Daprodustat is an orally active, small molecule hypoxia-inducible factor-prolyl hydroxylase inhibitor that is currently in Phase 3 clinical studies. As it is well appreciated that the kidney represents a major route of elimination of many drugs, and daprodustat will be administered to patients with advanced CKD as well as patients with end-stage kidney disease, it is important to characterize the pharmacokinetic profile in these patient populations to safely dose this potential new medicine.
Methods
The primary objective of these studies, conducted under two separate protocols and with identical assessments and procedures, was to characterize the steady-state pharmacokinetics of daprodustat and the six predominant metabolites (i.e. metabolites present in the highest concentration in circulation) in subjects with normal renal function, anemic non-dialysis (ND)-dependent CKD subjects (CKD Stage 3/4) and anemic subjects on either hemodialysis (HD) or peritoneal dialysis (PD). All enrolled subjects were administered daprodustat 5 mg once daily for 14 days (all except HD subjects) or 15 days (for HD subjects). Blood, urine and peritoneal dialysate were collected at various times for measurement of daprodustat, predominant metabolite, erythropoietin and hepcidin levels.
Results
The pharmacokinetic properties of steady-state daprodustat peak plasma concentration (Cmax), area under the plasma daprodustat concentration-time curve (AUC) and the time of Cmax (tmax) were comparable between all cohorts in this study. In addition, there was no clinically relevant difference in these properties in the HD subjects between a dialysis and ND day. For CKD Stage 3/4, HD (dialysis day) and PD subjects, the AUC of all daprodustat metabolites assessed was higher, while the Cmax was slightly higher than that in subjects with normal renal function. Over the course of the 14 or 15 days of daprodustat administration, hemoglobin levels were seen to be relatively stable in the subjects with normal renal function, CKD Stage 3/4 and PD subjects, while HD subjects had a decrease of 1.9 gm/dL. All renally impaired subjects appeared to have similar erythropoietin responses to daprodustat, with approximately a 3-fold increase in these levels. In subjects with minimal to no change in hemoglobin levels, hepcidin levels remained relatively stable. Daprodustat, administered 5 mg once daily for 14–15 days, was generally well tolerated with a safety profile consistent with this patient population.
Conclusion
These studies demonstrated no clinically meaningful change in the pharmacokinetic properties of daprodustat when administered to subjects with various degrees of renal impairment, while for CKD Stage 3/4, HD (dialysis day) and PD subjects, the Cmax and AUC of all daprodustat metabolites assessed were higher than in subjects with normal renal function. Administration of daprodustat in this study appeared to be generally safe and well tolerated.
Keywords: anemia, chronic kidney disease, pharmacokinetics, prolyl hydroxylase inhibitor
INTRODUCTION
Anemia occurs commonly in patients with chronic kidney disease (CKD) as the production of erythropoietin, a hormone produced by the kidneys that supports normal red blood cell production, is typically reduced [1]. Current therapies include administration of supplemental iron (intravenous and/or oral), blood transfusions and replacement of erythropoietin through the administration of recombinant human erythropoietin (rhEPO) and rhEPO analogs [2–4]. While these therapies can increase red blood cell number in these patients, each of these treatments have limitations that impact their use, including:
poor compliance with oral iron therapy due to gastrointestinal intolerance and increased risk of infection and iron overload toxicity with intravenous iron [5];
potential alloimmunization with blood transfusions [6]; and
increased risk of cardiovascular events with rhEPO and rhEPO analogs [7].
Therefore, new therapies with advantages over existing treatments are needed.
Daprodustat (GSK1278863) is an orally active, small molecule hypoxia-inducible factor (HIF)-prolyl hydroxylase inhibitor (PHI) that is currently in Phase 3 clinical studies. HIF-PHIs are an emerging new class of agents under investigation for the treatment of anemia of CKD, which stimulate erythropoiesis through inhibition of HIF-prolyl hydroxylase domain enzymes (PHD1, PHD2, PHD3). This activity results in the accumulation of HIFα transcription factors leading to increased transcription of HIF-responsive genes, stimulating components of the natural response to hypoxia. During hypoxia, the activity of PHD enzymes is reduced, resulting in the accumulation of unhydroxylated HIFα subunits, which dimerize with HIFβ subunits to affect the transcription of HIF-responsive genes, including erythropoietin and others involved in increasing oxygen availability and utilization. Other functions regulated by HIFs include iron metabolism and utilization, angiogenesis, extracellular matrix metabolism, apoptosis, energy and glucose metabolism, vascular tone, cell adhesion and motility [8, 9].
In two separate 24-week clinical studies, daprodustat has demonstrated dose-dependent increases and maintenance in hemoglobin levels in hemodialysis (HD) and non-dialysis (ND)-dependent subjects with anemia of CKD [10, 11]. In ND subjects that were naïve to rhEPO treatment, an oral once daily dose of daprodustat led to the maintenance of hemoglobin levels in the 9–11.5 g/dL range. In HD subjects switched from rhEPO treatment, daprodustat administered once daily maintained hemoglobin levels over the 24-week treatment period. These data suggest that daprodustat may be an alternative to currently available rhEPO and rhEPO analogs for treatment of anemia of CKD.
It is well appreciated that the kidney represents a major route of elimination of many drugs. In instances where drugs are primarily metabolized by the liver, often the metabolites are renally eliminated. As daprodustat is being administered to patients with advanced CKD as well as with end-stage kidney disease (ESKD), it was important to characterize the pharmacokinetic profile in these patient populations to safely dose this potential new medicine. Furthermore, it is important to characterize the pharmacokinetic properties of the metabolites to classify the metabolites as major and minor based on the percent of drug-related material (%DRM) present in the systemic circulation. Therefore, the studies reported here were conducted under two separate protocols and compared the pharmacokinetic parameters of daprodustat and its six predominant metabolites [i.e. metabolites present in the highest concentration in circulation; GSK2391220 (M2), GSK2531403 (M3), GSK2487818 (M4), GSK2506102 (M5), GSK2531398 (M6) and GSK2531401 (M13)] at a clinically relevant dose in subjects with various stages of renal impairment to these parameters in subjects with normal renal function. The proposed metabolism scheme for the six predominant metabolites has been published previously [12].
MATERIALS AND METHODS
Study design and endpoints
The primary objective of these Phase 1 studies, conducted under two separate protocols and with identical assessments and procedures, was to characterize the steady-state pharmacokinetics of daprodustat and the six predominant (i.e. metabolites with the highest circulating concentrations) metabolites in subjects with normal renal function, anemic ND-dependent CKD subjects (CKD Stage 3/4) and anemic subjects on either HD or peritoneal dialysis (PD).
These studies were an open label, parallel-group study design in adult male and female subjects with normal renal function and anemic subjects with impaired renal function (Study NCT02293148 and Study NCT02243306 on ClinicalTrials.gov registry) and were conducted in accordance with Good Clinical Practice guidelines and the 2008 Declaration of Helsinki, and were approved by independent ethics committees. Written informed consent was obtained from each subject before enrollment. These studies were conducted at DaVita Clinical Unit (Minneapolis, MN, USA and Lakewood, CO, USA) and IQVIA (formerly Quintiles, Phase One Services LLC, Overland Park, KS, USA).
Participants
After the initial screening (−28 to −1 days prior to randomization), eligible male and female subjects were enrolled into the study. Subjects were enrolled into four cohorts:
subjects with normal renal function, defined as an estimated creatinine clearance by the Cockcroft–Gault equation ≥90 mL/min/1.73 m2;
subjects with moderate or severe renal impairment with an estimated glomerular filtration rate calculated by the Modification of Diet in Renal Disease Study equation between 30 and 59 mL/min/1.73 m2 for moderate renal impaired subjects and between 15 and 29 mL/min/1.73 m2 for severe renal impaired subjects (CKD Stage 3/4 subjects, respectively);
subjects with ESKD (Stage 5) that were on HD three times weekly for at least 3 months prior to screening (HD subjects); and
subjects with ESKD on PD for at least 2 months prior to screening (PD subjects); subjects could be on either continuous ambulatory PD or automated PD.
For subjects that were considered non-anemic, hemoglobin levels at screening were greater than the lower limit of the reference range for the testing laboratory and ≤16.0 g/dL. For anemic subjects that were rhEPO-naïve, hemoglobin levels were ≤11.5 g/dL, whereas for subjects receiving ongoing rhEPO treatment, hemoglobin levels were ≤12.0 g/dL at screening and ≤11.5 g/dL following discontinuation of rhEPO treatment. Subject eligibility criteria included females of non-child-bearing potential or of child-bearing potential confirmed to be using one of the required contraceptive methods as specified in the protocols.
Exclusion criteria included uncontrolled hypertension (diastolic blood pressure >100 mmHg or systolic blood pressure >160 mmHg at screening), evidence of a significant abnormality on 12-lead electrocardiogram (ECG) at screening (including QTc interval >450 ms), use of prohibited prescription or non-prescription drugs within 7 days (or 14 days if the drug was a potential enzyme inducer) or 5 half-lives (t½), whichever was longer.
Interventions
All enrolled subjects were admitted to the Clinical Unit on the evening of Day 1 (evening prior to dosing) and remained in the Unit until Day 17 for subjects with normal renal function, CKD Stage 3/4 and PD subjects, or Day 18 for HD subjects. Subjects were discharged following the last post-dose assessment, if no clinically significant abnormalities were noted. All investigational products were administered by Clinical Unit staff who confirmed compliance. Subjects attended a follow-up visit 7–14 days following the last dose of daprodustat.
For switching CKD Stage 3/4, HD and PD subjects to daprodustat from their rhEPO treatment, if the subject had a scheduled rhEPO interval ≤7 days, rhEPO treatment was discontinued for at least 7 days prior to daprodustat dosing; for subjects with a scheduled rhEPO interval that was >7 days, rhEPO treatment was discontinued for at least the scheduled interval length prior to administration of daprodustat.
The dose of daprodustat was 5 mg once daily for 14 (all except HD subjects) or 15 days (for HD subjects).
Venous blood sampling for measurement of daprodustat and its predominant metabolite plasma levels occurred pre-dose, then at various times post-dose on Days 1 and 14, while pre-dose samples were taken on Days 3, 7, 12 and 13 for measurement of trough levels for subjects with normal renal function, CKD Stage 3/4 and PD subjects. For HD subjects, venous blood samples were taken pre-dose, and at various times post-dose on Days 14 and 15 and on Days 1, 3, 7, 12 and 13 for measurement of trough levels; Day 14 was an HD day while Day 15 was a non-HD day. Additionally, arteriovenous vascular access blood samples were collected at 3 and 4 h post-dose on Day 14 while subjects were dialyzed for assessment of dialysis clearance of daprodustat and predominant metabolites.
Sample size
As this study was descriptive in nature, no formal statistical comparisons of pharmacokinetic data were performed. Therefore, a sample size of five to eight subjects in each cohort was deemed sufficient to achieve the primary objective.
Assessments
Pharmacokinetic
Blood samples for pharmacokinetic analyses were collected pre-dose and at 0.50, 1, 2, 3, 4, 6, 8, 12, 16, 24, 48 and 72 h post-dose. Plasma analysis was performed under the management of Bioanalytical Science and Toxicokinetics, Drug Metabolism and Pharmacokinetics, GlaxoSmithKline (GSK).
Blood samples were collected into K3 EDTA tubes and immediately placed on water ice. Samples were then centrifuged at 2000g for 10 min; the supernatant plasma was transferred to a Nunc® tube and stored at −20°C before shipment. Samples were shipped frozen to PPD (Middleton, WI, USA), where plasma samples were analyzed for daprodustat and predominant metabolites.
Pharmacokinetic analysis was performed under the management of Clinical Pharmacology Modeling and Simulations, GSK. Plasma daprodustat and metabolites concentration-time data were analyzed by non-compartmental analysis using Model 200 of Phoenix WinNonlin version 6. Calculations were based on the actual sampling times recorded during the study. The pharmacokinetic parameters of interest for each treatment were AUC (0−τ) [area under the concentration–time curve from time zero (pre-dose) to the last measured concentration], Cmax (maximum observed concentration), tmax (time of occurrence of Cmax) and t½ (terminal phase half-life), as data permitted.
%DRM was equal to the AUC of the metabolite divided by the sum of the AUC of all measured metabolites and parent.
Twenty-four hour urine samples for subjects with normal renal function and CKD Stage 3/4 subjects were performed on Day 1 for assessment of renal clearance of daprodustat and its predominant metabolites. For PD subjects, aliquots of peritoneal dialysate samples for analysis of daprodustat and its predominant metabolites were also collected prior to dosing on Day 1, and for 24 h post-dose on Day 14, if possible.
Pharmacodynamic
Blood samples were collected on Day 14 for CKD Stage 3/4 or PD subjects and Days 14 and 15 for HD subjects at pre-dose and at 4, 8 and 12 h post-dose for measurement of plasma hepcidin and erythropoietin. Pre-dose samples were also taken Days 1, 3, 7 and 11 from all CKD Stage 3/4, PD and HD subjects. Hemoglobin levels were measured pre-dose on Days 3, 7 and 11 for all subjects.
Safety and tolerability measures
Safety and tolerability measures included assessment of adverse events (AEs), serious adverse events (SAEs), clinical laboratory findings, vital signs (systolic and diastolic blood pressure and pulse rate), ECGs and concurrent medications. An AE was defined as any untoward medical occurrence in a subject, temporally associated with the use of an investigational product, whether or not considered to be related to the investigational product. An SAE was defined as any untoward medical occurrence that, at any dose, resulted in death, was life-threatening, required hospitalization or prolonged existing hospitalization, resulted in disability/incapacity, was a congenital anomaly/birth defect or was associated with liver injury and impaired liver function. The investigator or site staff was responsible for detecting, documenting and reporting events that met the definition of an AE or SAE.
RESULTS
Subject population
These studies were conducted under two separate protocols: the first protocol enrolled subjects with normal renal function, CKD Stage 3/4 subjects and subjects on chronic HD; the second protocol enrolled subjects on PD. Both protocols employed identical inclusion and exclusion criteria, study procedures and statistical methods. The first study was conducted between 30 August 2011 and 31 August 2013, whereas the second study was conducted between 24 October 2014 and 10 May 2017.
Overall, a total of 30 subjects were enrolled in these studies, with a total of 27 (90%) completing all treatment periods and study assessments as planned. The disposition of the subjects is given as follows:
eight subjects with normal renal function were enrolled and completed the study as planned;
six CKD (four Stage 3 and two Stage 4) subjects were enrolled and all completed the study as planned;
eight HD subjects were enrolled and all completed the study as planned; and
eight PD subjects were enrolled and five completed the study as planned.
The subjects with normal renal function tended to be younger (mean age 38 years) than the subjects with any stage renal impairment (age ranged between 52 and 68 years). Additionally, the subjects with any stage renal impairment also were, on average, anemic with hemoglobin levels ranging between 10.2 and 11.3 g/dL, while the normal renal function cohort had hemoglobin levels within the normal range (mean hemoglobin 14.4 g/dL).
Of the subjects that did not complete the study as planned, one was withdrawn due to an AE (see the ‘Safety results’ section), one was withdrawn at the Investigator’s discretion and one reached a protocol-defined stopping criterion (met hemoglobin stopping criteria of >11.0 g/dL). Demographic and laboratory characteristics of the study population can be found in Table 1.
Table 1.
Demographic and laboratory characteristics of the study population
| Parameter | Normal (n = 8) | CKD Stage 3/4 (n = 6) | HD (n = 8) | PD (n = 8) |
|---|---|---|---|---|
| Male/female | 6/2 | 4/2 | 5/3 | 6/2 |
| Age, years | 38.5 ± 13.3 | 67.8 ± 9.0 | 52.1 ± 10.3 | 55.8 ± 12.1 |
| Height, cm | 174.1 ± 5.7 | 168.2 ± 10.6 | 173.9 ± 6.7 | 168.0 ± 9.7 |
| Weight, kg | 84.2 ± 19.5 | 98.8 ± 15.9 | 99.2 ± 19.6 | 77.2 ± 19.5 |
| BMI, kg/m2 | 27.6 ± 5.6 | 35.4 ± 8.2 | 32.9 ± 6.9 | 27.13 ± 5.46 |
| Hgb, g/dL | 14.4 ± 0.6 | 11.0 ± 1.7 | 11.3 ± 0.7 | 10.2 ± 0.8 |
Values are arithmetic mean ± SD. BMI, body mass index; Hgb, hemoglobin.
Plasma pharmacokinetics
Summarized plasma daprodustat pharmacokinetic parameters following repeat-dose administration are presented in Table 2. In all populations, daprodustat appeared to be readily absorbed with median tmax occurring between 1 and 2 h post-dose, while the apparent t½ of daprodustat was generally between 2 and 3 h following repeat-dose administration. Cmax of daprodustat was similar across the various populations and was highest for the normal renal function group at 96.3 ng/mL, and lowest for PD subjects at 41.5 ng/mL. Similarly, AUC (0−τ) values were comparable between populations, with values between 156 ng·h/mL for the PD subjects and 261 ng·h/mL for HD subjects on an HD day. In the HD cohort, the Cmax and AUC (0−τ) of daprodustat were comparable on a dialysis day versus an ND day. Finally, %DRM varied between populations, with the value highest for the normal renal function subjects, at 54.7%, to 16.0% for PD subjects. The CKD Stage 3/4 subjects and the HD subjects on a dialysis day were similar, at 33.3 and 39.4%, respectively.
Table 2.
Plasma daprodustat pharmacokinetic parameters on Day 14/15
| Parameters | Normal | CKD Stage 3/4 | HD DD | HD ND | PD |
|---|---|---|---|---|---|
| C max, ng/mL | 96.3 (27.0) | 78.0 (24.6) | 82.1 (40.8) | 79.9 (26.0) | 41.5 (20.7) |
| t max, h | 1.00 (1.0–2.0) | 2.00 (0.5–3.3) | 1.55 (0.5–3.3) | 1.00 (0.5–2.0) | 2.00 (1.0–4.0) |
| AUC (0−τ), ng·h/mL | 196 (52.6) | 188 (75.6) | 261 (216.7) | 205 (89.8) | 156 (79.0) |
| t½, h | 2.56 (0.25) | 2.36 (0.39) | 3.01 (1.17) | 18.9 (29.52) | 2.49 (0.95) |
| %DRM | 54.7 (7.45) | 33.3 (15.4) | 39.4 (15.8) | 21.0 (6.66) | 16.0 (7.31) |
Values expressed are arithmetic mean (SD) except tmax, which is given as median (range). DD, dialysis day.
Selected pharmacokinetic parameters of the six predominant metabolites of daprodustat are summarized in Table 3. The occurrence of the peak concentrations of daprodustat metabolites was slower than that of parent for all populations, with the median peak plasma concentrations occurring between 2 and 8 h post-dose. For metabolites M3, M5 and M13, tmax was achieved more slowly in HD subjects on an ND day when compared with normal renal function subjects (median difference ranged from 2 to 5 h). Maximal metabolite concentrations were achieved more rapidly in HD subjects on a dialysis day compared with an ND day (median difference ranged from −0.71 to −6 h). All other differences in tmax were ≤1.5 h. The apparent t½ of the metabolites varied substantially between the subjects with normal renal function and the renally impaired subjects: in subjects with normal renal function, the t½ ranged between 2 and 3 h, while for renally impaired subjects the t½ was up to 35 h (metabolite M13 in HD subjects on a dialysis day); in general, when comparing across all populations, the t½ was the longest for the HD subjects on a dialysis day. Metabolite Cmax values were substantially lower in all subjects when compared with parent, varying between 2.1 and 16.2 ng/mL (2–20% of population-matched parent concentration, respectively). Increases in metabolite Cmax were <2-fold in subjects in any stage of renal impairment compared with subjects with normal renal function. Metabolite AUCs were increased up to 2.84-fold in CKD Stage 3/4 subjects and up to 6.2-fold in HD subjects when compared with subjects with normal renal function. Metabolite AUCs were up to ∼2.22-fold higher and Cmax up to ∼1.7-fold higher in HD subjects on an ND day compared with a dialysis day.
Table 3.
Plasma daprodustat metabolite pharmacokinetic parameters on Day 14/15
| Analyte | Parameter | Normal | CKD Stage 3/4 | HD DD | HD ND | PD |
|---|---|---|---|---|---|---|
| GSK2391220 (M2) | C max (ng/mL) | 8.7 (1.9) | 10.9 (3.6) | 11.3 (6.0) | 15.4 (4.5) | 13.2 (4.5) |
| t max (h) | 3.00 (2.0–4.0) | 4.02 (3.0–4.0) | 2.00 (3.0–6.0) | 4.00 (3.0–6.0) | 4.00 (4.0–6.0) | |
| AUC (0−τ) (ng·h/mL) | 42.4 (10.1) | 98.4 (50.1) | 102.5 (47.0) | 214.5 (84.3) | 191.3 (69.5) | |
| t½ (h) | 3.08 (1.11) | 8.51 (4.47) | 10.02 (2.42) | 8.96 (1.24) | 10.28 (2.29) | |
| %DRM | 11.07 (2.08) | 14.83 (3.08) | 14.86 (3.68) | 19.33 (2.92) | 17.58 (2.59) | |
| GSK2531403 (M3) | C max (ng/mL) | 8.5 (1.8) | 11.4 (3.7) | 12.4 (6.1) | 16.2 (4.6) | 16.1 (6.4) |
| t max (h) | 3.00 (2.0–4.0) | 4.02 (3.0–6.0) | 2.00 (2.0–8.0) | 5.00 (4.0–8.0) | 6.00 (4.0–6.0) | |
| AUC (0−τ) (ng·h/mL) | 43.6 (10.9) | 123.4 (69.6) | 129.1 (60.8) | 268.0 (97.3) | 278.8 (115.9) | |
| t½ (h) | 3.04 (1.03) | 14.48 (11.68) | 15.62 (4.63) | 11.71 (3.20) | 14.98 (4.85) | |
| %DRM | 11.38 (2.23) | 18.44 (4.80) | 18.78 (4.38) | 24.40 (2.70) | 25.13 (5.64) | |
| GSK2487818 (M4) | C max (ng/mL) | 7.1 (1.7) | 8.3 (2.9) | 7.6 (3.6) | 10.7 (2.7) | 9.8 (5.4) |
| t max (h) | 2.00 (2.0–3.0) | 3.50 (2.0–4.0) | 2.00 (2.0–8.0) | 3.50 (2.0–4.0) | 4.00 (3.0–4.0) | |
| AUC (0−τ) (ng·h/mL) | 25.5 (6.0) | 43.4 (20.7) | 43.5 (20.8) | 73.6 (36.4) | 74.4 (31.5) | |
| t½ (h) | 1.78 (0.58) | 3.56 (2.62) | 3.36 (0.79) | 3.95 (1.32) | 3.79 (0.95) | |
| %DRM | 6.60 (0.98) | 6.57 (1.61) | 6.23 (1.20) | 6.56 (1.40) | 6.76 (1.34) | |
| GSK2506102 (M5) | C max (ng/mL) | 2.1 (0.4) | 2.8 (1.0) | 3.0 (1.5) | 3.9 (1.2) | 4.1 (1.5) |
| t max (h) | 3.00 (2.0–4.0) | 4.02 (3.0–6.0) | 2.00 (3.0–6.0) | 6.00 (4.0–8.0) | 4.80 (4.0–6.0) | |
| AUC (0−τ) (ng·h/mL) | 10.7 (2.8) | 34.0 (21.3) | 33.1 (17.5) | 68.6 (25.0) | 74.9 (30.7) | |
| t½ (h) | 2.52 (0.73) | 9.52 (5.40) | 21.3 (9.11) | 13.94 (3.53) | 19.14 (8.10) | |
| %DRM | 2.80 (0.67) | 5.05 (1.71) | 5.19 (1.05) | 6.30 (0.83) | 6.81 (1.34) | |
| GSK2531398 (M6) | C max (ng/mL) | 4.1 (0.9) | 5.1 (1.8) | 4.1 (1.8) | 6.6 (1.7) | 5.9 (2.4) |
| t max (h) | 3.00 (2.0–4.0) | 4.02 (3.0–6.0) | 2.00 (2.0–12.0) | 4.00 (3.0–6.0) | 4.00 (4.0–6.0) | |
| AUC (0−τ) (ng·h/mL) | 18.7 (4.3) | 42.9 (25.9) | 35.4 (17.8) | 78.0 (34.5) | 76.9 (28.1) | |
| t½ (h) | 2.19 (0.51) | 4.78 (1.91) | 7.11 (2.19) | 6.61 (0.93) | 7.32 (2.48) | |
| %DRM | 4.89 (0.87) | 6.31 (1.58) | 5.58 (0.93) | 7.01 (1.10) | 7.05 (0.99) | |
| GSK2531401 (M13) | C max (ng/mL) | 6.0 (1.3) | 7.7 (2.6) | 6.1 (3.7) | 8.2 (3.4) | 11.1 (2.0) |
| t max (h) | 3.00 (3.0–4.0) | 4.02 (3.0–6.0) | 2.00 (0.5–2.2) | 8.00 (6.0–16.0) | 8.00 (6.0–12.0) | |
| AUC (0−τ) (ng·h/mL) | 34.1 (12.2) | 102.1 (54.9) | 77.4 (35.0) | 159.0 (70.2) | 215.6 (41.6) | |
| t½ (h) | 2.73 (0.60) | 10.60 (5.25) | 35.44 (13.27) | 15.28 (2.63) | 27.63 (10.98) | |
| %DRM | 8.55 (2.22) | 15.54 (8.04) | 12.94 (5.42) | 15.42 (7.10) | 20.66 (5.73) |
Values expressed are arithmetic mean (SD) except tmax which is given as median (range). DD, dialysis day.
%DRM for the metabolites were consistently slightly higher for subjects with any stage of renal impairment when compared with subjects with normal renal function, and was typically highest in HD subjects on an ND day and PD subjects.
Urine pharmacokinetics and clearance
Summarized urine daprodustat and its predominant metabolites pharmacokinetic parameters following repeat-dose administration to subjects with normal renal function and CKD Stage 3/4 subjects are presented in Table 4. The proportion of the excreted administered dose of daprodustat >24 h was 0.05% for the subjects with normal renal function and 0.04% for the CKD Stage 3/4 subjects. For the predominant metabolites, the proportion excreted was markedly higher for the subjects with normal renal function (between 1.05 and 4.48%) than for CKD Stage 3/4 subjects (between 0.78 and 3.04%). Renal clearance for the metabolites was approximately 3-fold faster for subjects with normal renal function when compared with the CKD Stage 3/4 subjects. Dialysis clearance in HD subjects was similarly low for daprodustat, but markedly higher for the PD subjects. Finally, dialysis clearance for the metabolites was substantially higher for the HD subjects, while for the PD subjects it tended to be lower than for the HD subjects (Table 4).
Table 4.
Daprodustat and metabolite excretion and clearance on Day 14
| Analyte | Group | % dose excreted in urine in 24 h | Clearance (mL/min) |
|---|---|---|---|
| Daprodustat | Normal | 0.05 (0.0571) | 0.20 (0.18) |
| CKD Stage 3/4 | 0.04 (0.0290) | 0.17 (0.16) | |
| HD | – | −8.50 (9.17) | |
| PD | – | 10.61 | |
| GSK2391220 (M2) | Normal | 3.74 (0.98) | 73.42 (6.69) |
| CKD Stage 3/4 | 2.21 (1.02) | 22.29 (15.33) | |
| HD | – | 182.69 (71.20) | |
| PD | – | 44.46 (48.22) | |
| GSK2531403 (M3) | Normal | 4.48 (1.16) | 86.05 (9.45) |
| CKD Stage 3/4 | 3.04 (1.18) | 26.56 (19.35) | |
| HD | – | 182.56 (69.60) | |
| PD | – | 77.34 (65.91) | |
| GSK2487818 (M4) | Normal | 2.11 (0.43) | 69.57 (5.13) |
| CKD Stage 3/4 | 1.17 (0.77) | 23.73 (16.42) | |
| HD | – | 170.25 (62.44) | |
| PD | – | 17.13 (22.49) | |
| GSK2506102 (M5) | Normal | 1.05 (0.27) | 82.80 (8.83) |
| CKD Stage 3/4 | 0.78 (0.24) | 26.55 (19.43) | |
| HD | – | 169.88 (69.69) | |
| PD | – | 57.87 (59.16) | |
| GSK2531398 (M6) | Normal | 1.57 (0.37) | 70.18 (6.58) |
| CKD Stage 3/4 | 0.97 (0.50) | 23.05 (16.64) | |
| HD | – | 169.13 (65.93) | |
| PD | – | 41.73 (50.17) | |
| GSK2531401 (M13) | Normal | 3.41 (1.17) | 83.79 (8.05) |
| CKD Stage 3/4 | 2.23 (0.69) | 24.24 (16.27) | |
| HD | – | 204.00 (78.66) | |
| PD | – | 72.54 (70.97) |
Values expressed are arithmetic mean (SD). Clearance for normal and CKD Stage 3/4 subjects is renal clearance, and for HD and PD subjects it is dialysis clearance. Clearance for HD subjects was assessed at 3 and 4 h with values for 3 h presented. Clearance for PD subjects was for a 24-h period.
Pharmacodynamics
Hemoglobin
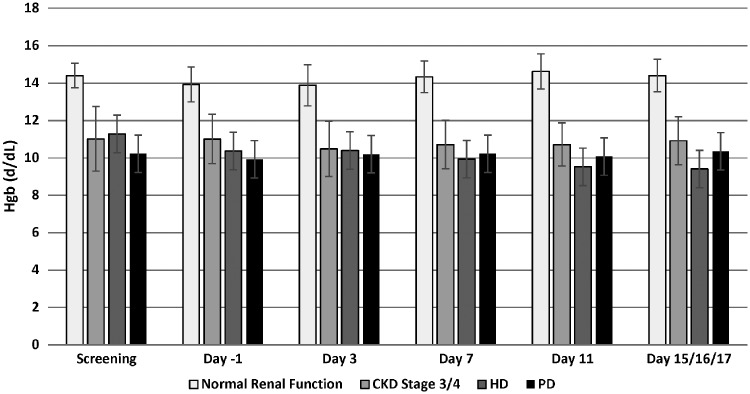
Hemoglobin levels were determined at screening, then Days −1, 3, 7, 11, 15 (for normal renal function and Stage 3/4 subjects), 16 (for HD subjects) or 17 (for PD subjects). Subjects with normal renal function had mean hemoglobin levels within the normal range at screening, and throughout the 14-day dosing period, ranging between 13.9 and 14.6 g/dL. CKD Stage 3/4 subjects were, on average, slightly anemic with hemoglobin levels at screening of 11.0 g/dL. These levels remained, on average, similar through the 14-day dosing period. HD subjects had mean hemoglobin levels at screening similar to CKD Stage 3/4 subjects, with levels of 11.3 g/dL. During the 14-day dosing period, levels were seen to decrease, reaching mean levels of 9.4 g/dL on Day 16. Finally, PD subjects had mean hemoglobin levels at screening of 10.2 g/dL, with levels of 10.4 g/dL on Day 17 (Figure 1).
FIGURE 1.
The effect of daprodustat on hemoglobin (Hgb) levels. Hemoglobin levels were measured at various times during the study. Bars represent arithmetic mean ± SD.
Erythropoietin
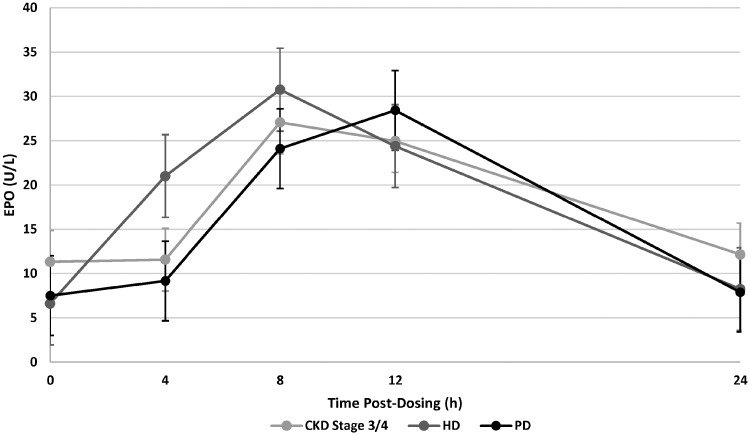
Plasma erythropoietin levels were measured at various times post-dose in CKD Stage 3/4 subjects, HD and PD subjects. Baseline (pre-dose) mean values on Day 14 varied between 6.1 U/L for HD subjects and 11.3 U/L for CKD Stage 3/4 subjects. Following a 5 mg dose of daprodustat, mean erythropoietin levels were observed to increase to the maximal levels between 8 and 12 h post-dose; maximal values varied between 27.1 U/L for CKD Stage 3/4 subjects and 30.8 U/L for HD subjects. Plasma erythropoietin levels returned to near baseline values by 24 h post-dose (Figure 2).
FIGURE 2.
Plasma erythropoietin (EPO) levels following administration of daprodustat. The plasma levels of EPO were measured at various times post-dose of daprodustat. Each point represents the mean±SD.
Hepcidin
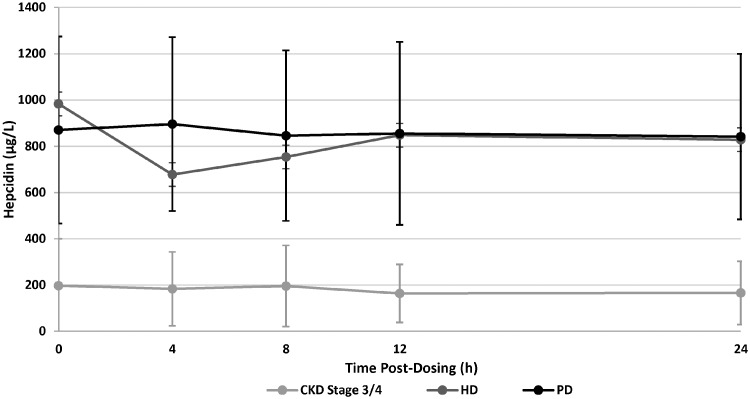
Pre-dose hepcidin values on Day 14 were substantially lower for the CKD Stage 3/4 subjects, with a mean value of 197 µg/L, compared with HD subjects (983 µg/L) or PD subjects (870 µg/L). Hepcidin values were relatively stable throughout the 24-h dosing interval; however, the HD subjects had a slight decrease, to 677 µg/L at 4 h post-dose, which then rose steadily to near baseline values by 12 h post-dose (mean 848 µg/L; Figure 3).
FIGURE 3.
Plasma hepcidin levels following administration of daprodustat. The plasma levels of hepcidin were measured at various times post-dose of daprodustat. Each point represents the mean±SD.
Safety results
There were no deaths or SAEs, or pregnancies, reported in these studies. AEs were collected from Day −1 until the follow-up visit (7–8 days post last dose). No clinically meaningful changes in vital signs (i.e. systolic and diastolic blood pressure and pulse rate) were reported. AEs were reported by 16 of the 30 subjects dosed in the study (53%). A summary of the AEs reported by at least two subjects in any cohort can be found in Table 5.
Table 5.
Summary of AEs reported by more than two subjects
| Preferred term | Normal (n = 8) | CKD Stage 3/4 (n = 6) | HD (n = 8) | PD (n = 8) |
|---|---|---|---|---|
| Nausea | 1 (13) | 1 (17) | 1 (13) | 1 (13) |
| Hypotension | 0 | 1 (17) | 1 (13) | 0 |
| Diarrhea | 1 (13) | 0 | 1 (13) | 2 (25) |
| Headache | 1 (13) | 0 | 1 (13) | 0 |
| Hypoglycemia | 0 | 0 | 0 | 2 (25) |
| Myalgia | 1 (13) | 0 | 0 | 2 (25) |
| Fatigue | 0 | 0 | 1 (13) | 1 (13) |
Numbers in parentheses are the percentage of subjects reporting the AE.
All AEs were mild in intensity except the following (data not shown).
An AE of anemia exacerbation that was moderate in intensity, reported by an HD subject. This subject’s hemoglobin was observed to be 7.8 g/dL on Day 16 ∼24 h following the final 5 mg dose of daprodustat. This subject’s hemoglobin was 11.0 g/dL at screening, and the AE did not lead to withdrawal of the subject.
A severe AE of hypoglycemia reported by a PD subject taking insulin for Type 2 diabetes. The subject with hypoglycemia had a glucose level of 4.5 mmol/L reported 9 h 54 min following administration of daprodustat on Day 14, and resolved 18 min later. This subject was not withdrawn from the study.
A moderate AE of fatigue reported by a PD subject 1 day after the final dose of daprodustat. This subject was not withdrawn.
A moderate AE of influenza reported by a PD subject. This subject was not withdrawn.
Moderate AEs of nausea, fever and myalgia were reported by a PD subject at 7 h following the second dose of daprodustat. Additionally, this subject experienced mild edema of face at 7 h post-dose and mild emesis 19 h post-dose, and was withdrawn from the study. This subject subsequently recovered with all the reported AEs resolved.
DISCUSSION
Several compounds that target the HIF pathway by inhibition of prolyl hydroxylase enzymes are currently in Phase 3 development and may represent a preferred treatment option for anemia of CKD in the future [13]. These compounds, by targeting the oxygen-sensing pathway, trigger the body’s natural response to hypoxia by increasing both the synthesis and secretion of erythropoietin as well as enhancing iron metabolism [14]. In this regard, daprodustat, an orally available small molecule inhibitor of prolyl hydroxylase enzymes, has been shown to be effective in achieving and maintaining hemoglobin levels in anemic CKD subjects, on HD or ND [10, 11].
It is well appreciated that the kidney represents a major route of elimination of many drugs and their metabolites [15]. As such, there are regulatory guidance documents recommending the characterization of the pharmacokinetics of all drugs to provide dosing and administration guidance, including drugs that are not indicated for treatment of renal diseases [16, 17]. As daprodustat is currently in clinical development to treat patients with all stages of CKD, it was critical that the pharmacokinetics of daprodustat and metabolites were characterized in this patient population.
The pharmacokinetic properties of steady-state daprodustat Cmax, AUC and tmax were comparable between all cohorts in this study. In addition, there was no clinically relevant difference in these properties in the HD subjects between a dialysis and ND day, suggesting that daprodustat is not substantially eliminated by HD and therefore can be administered without regard for HD treatment. This is further supported by the results that the renal clearance of daprodustat is minimal; indeed, daprodustat has previously been shown to be primarily metabolized by CYP2C8, as evidenced by the substantial increase in exposure in the presence of a strong CYP2C8 inhibitor [12]. Of the parameters assessed, there was a marked increase in the t½ of daprodustat in HD subjects between a dialysis and ND day. The reason for this increase is not clear; however, there was a high degree of inter-subject variability observed. Additionally, it has been shown that chronic renal impairment can have effects on drug disposition separate from the effects on renal clearance [18]. In particular, accumulation of waste in the blood (i.e. uremic toxins) can affect CYP activity [19]. As this increase in t½ occurred on an ND day, this is a possibility; however, despite the increase in t½, there was no notable change in other pharmacokinetic parameters.
Higher between-subject variability in the pharmacokinetic parameters Cmax and AUC was observed in the HD subjects when compared with the other study populations. The reason for this higher variability is not clear, however, as the blood sampling for bioanalysis of daprodustat and metabolite concentrations was performed on a dialysis day, and dialysis was initiated 2–2.5 h following daprodustat administration, it is possible that the dialysis procedure, with the subsequent effect on fluid volume, may have led to the higher variability. Indeed, pharmacokinetic parameters on Day 15 in this population, an ND day, showed variability comparable to the other populations (data not shown).
Observed daprodustat exposure (Cmax and AUC) was the lowest for the PD subjects than the other groups. The reason for the generally lower pharmacokinetic parameters is not clear, but could be related to the observed lower plasma albumin levels observed in the PD subjects (data not shown). It is well recognized that protein loss occurs in PD [20] and as daprodustat is highly protein bound, lower serum albumin levels may increase the free fraction of daprodustat available for dialysis. In addition, with the higher level of protein loss in PD, protein-bound daprodustat may experience a higher level of elimination. Taken together, this may explain why the observed exposure of daprodustat in the PD subjects was generally lower than the other groups.
For CKD Stage 3/4, HD (dialysis day) and PD subjects, the AUC of all daprodustat metabolites assessed was higher than that in subjects with normal renal function. In addition, for these metabolites, the Cmax was slightly higher in CKD Stage 3/4, HD (dialysis day) and PD subjects when compared with subjects with normal renal function. This is consistent with the role of CYP metabolism to increase the water solubility of drugs to facilitate renal elimination [21]. Classification of metabolites as major or minor is based on the %DRM assessment, where a value of 10% or higher denotes metabolites as major [22]. Based on these findings, the major metabolites of daprodustat in the CKD population (Stage 3/4/5 either ND, HD or PD) are M2 (GSK2391220), M3 (GSK2531403) and M13 (GSK2531401). Of these metabolites, both M2 and M3 are also identified as major in subjects with normal renal function (%DRM ∼11%), while M13 is not (%DRM ∼9%).
As expected for these populations, hemoglobin levels were lower in all anemic subjects with any degree of renal impairment when compared with subjects with normal renal function. Over the course of the 14 or 15 days of daprodustat administration, hemoglobin levels were seen to be relatively stable in the subjects with normal renal function, CKD Stage 3/4 and PD subjects, while HD subjects had a decrease of 1.9 g/dL. Based on the previous study in HD subjects, it appears that 5 mg daily dose of daprodustat is not an effective dose in majority of HD subjects and is lower than the average dose (6–8 mg) to maintain target hemoglobin [11]. Additionally, in this short duration study, the doses of daprodustat were not adjusted based on the subject’s hemoglobin response.
All renally impaired subjects appeared to have similar erythropoietin responses to daprodustat (approximately 3-fold increase). These maximal observed level of erythropoietin in the present study (30.8 U/L in HD subjects) compares favorably favorably to levels observed in individuals that normally reside at sea level and subsequently reside at high altitude (27.9 U/L at 3450 m on Day 1) [23] and are substantially below the levels resulting from therapeutic intravenous doses of the rhEPOs and rhEPO analogs [24]. Finally, as expected for this patient population, hepcidin levels were elevated in the ESKD subjects (both HD and PD), and considerably lower for the CKD Stage 3/4 subjects [25]. Consistent with other reports, in subjects that had minimal to no change in hemoglobin level, there was minimal to no change in hepcidin levels [26].
Administration of 5 mg oral daprodustat once daily for 14 or 15 days was generally well tolerated, with a safety profile consistent with this patient population. No deaths or other SAEs were reported during these studies and no new safety concerns were identified. The most commonly reported AE was nausea, in 3 of 22 subjects (14%), followed by hypotension, diarrhea and headache [reported by 2 of 22 subjects (9%), each]. Nausea was the most commonly reported AE, followed by diarrhea and headache. No clinically meaningful changes over time were observed in hematology, clinical chemistry, urinalysis and vital signs evaluations.
CONCLUSION
These studies demonstrated no clinically meaningful change in the pharmacokinetic properties of daprodustat when administered to patients with various degrees of renal impairment, when compared with subjects with normal renal function. HD did not appear to affect the pharmacokinetic properties, suggesting that daprodustat can be administered without regard to dialysis treatment. A dose of 5 mg given once daily to the anemic CKD and PD populations appeared to maintain hemoglobin levels within the appropriate target range, while the majority of HD population will require higher daprodustat doses to adequately maintain hemoglobin levels by periodic dose adjustments based on hemoglobin levels. A dose of 5 mg given once daily to these patient populations resulted in peak plasma erythropoietin levels consistent with exposure to hypoxia at altitude. Based on these results, it does not appear that the pharmacokinetics of daprodustat are affected by renal function. Finally, in the CKD population, metabolites M2, M3 and M13 are considered major as each of these represented at least 10% of DRM.
ACKNOWLEDGEMENTS
All listed authors met the criteria for authorship set forth by the International Committee for Medical Journal Editors. The authors wish to acknowledge the contribution of Shalini Yapa, Ramiya Ravindranath and the staff at IQVIA (formerly Quintiles, Phase One Services LLC) for their assistance with these studies.
CONFLICT OF INTEREST STATEMENT
All authors were employees of GlaxoSmithKline at the time of conduct of this study. This study was supported by GlaxoSmithKline. S.C., B.C., O.B. and A.R.C. are shareholders of GlaxoSmithKline.
REFERENCES
- 1. Koury MJ. Abnormal erythropoiesis and the pathophysiology of chronic anemia. Blood Rev 2014; 28: 49–66 [DOI] [PubMed] [Google Scholar]
- 2. Fishbane S, Pollack S, Feldman HI. et al. Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988–2004. Clin J Am Soc Nephrol 2009; 4: 57–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gill KS, Muntner P, Lafayette RA. et al. Red blood cell transfusion use in patients with chronic kidney disease. Nephrol Dial Transplant 2013; 28: 1504–1515 [DOI] [PubMed] [Google Scholar]
- 4. Gobe GC, Endre ZH, Johnson DW.. Administration of erythropoietin and its derivatives in renal disease: advantages, mechanisms and concerns. Drug Discov Today Ther Strateg 2007; 4: 79–84 [Google Scholar]
- 5. Agarwal R, Kusek JW, Pappas MK.. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int 2015; 88: 905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alves VM, Martins PRJ, Soares S. et al. Alloimmunization screening after transfusion of red blood cells in a prospective study. Rev Bras Hematol Hemoter 2012; 34: 206–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Szczech LA, Barnhart HX, Inrig JK. et al. Secondary analysis of the CHOIR trial epoetin-α dose and achieved hemoglobin outcomes. Kidney Int 2008; 74: 791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev 2013; 27: 41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmid H, Jelkman W.. Investigational therapies for renal disease-induced anemia. Exp Opin Invest Drugs 2016; 25: 901–916 [DOI] [PubMed] [Google Scholar]
- 10. Holdstock L, Cizman B, Meadowcroft AM. et al. Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants with chronic kidney disease. Clin Kidney J 2019; 12: 129--138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meadowcroft AM, Cizman B, Holdstock L. et al. Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants on hemodialysis. Clin Kidney J 2019; 12: 139--148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson BM, Stier BA, Caltabiano S.. Effect of food and gemfibrozil on the pharmacokinetics of the novel prolyl hydroxylase inhibitor GSK1278863. Clin Pharm Drug Dev 2013; 3: 109–117 [DOI] [PubMed] [Google Scholar]
- 13. Haase VH. Therapeutic targeting of the HIF oxygen-sensing pathway: lessons learned from clinical studies. Exp Cell Res 2017; 356: 160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koury MJ, Haase VJ.. Anaemia in kidney disease: harnessing hypoxia responses for therapy. Nat Rev Nephrol 2015; 11: 394–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miners JO, Yang X, Knights KM. et al. The role of the kidney in drug elimination: transport, metabolism, and the impact of kidney disease on drug clearance. Clin Pharm Therap 2017; 102: 436. [DOI] [PubMed] [Google Scholar]
- 16.FDA. Guidance for Industry (Draft): Pharmacokinetics in Patients with Impaired Renal Function—Study Design, Data Analysis, and Impact on Dosing and Labeling, 2010; https://www.fda.gov/downloads/drugs/guidances/ucm204959.pdf (20 June 2018, date last accessed)
- 17.EMA. European Medicines Agency’s Guidance on the Evaluation of the Pharmacokinetics of Medicinal Products in Patients with Decreased Renal Function, 2014; http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/02/WC500200841.pdf (20 June 2018, date last accessed)
- 18. Zhang Y, Zhang L, Abraham S. et al. Assessment of the impact of renal impairment on systemic exposure of new molecular entities. Clin Pharmacol Ther 2009; 85: 305–311 [DOI] [PubMed] [Google Scholar]
- 19. Dreisbach AW, Lertora JJL.. The effect of chronic renal failure on drug metabolism and transport. Expert Opin Drug Metab Toxicol 2008; 4: 1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balafa O, Halbesma N, Struijk DG. et al. Peritoneal albumin and protein losses do not predict outcome in peritoneal dialysis patients. Clin J Am Soc Nephrol 2011; 6: 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang GWM, Kam PCA.. The physiological and pharmacological roles of cytochrome P450 isoenzymes. Anaesthesia 1999; 54: 42–50 [DOI] [PubMed] [Google Scholar]
- 22.FDA. Safety Testing of Drug Metabolites: Guidance for Industry, 2016. https://www.fda.gov/downloads/Drugs/…/Guidances/ucm079266.pdf (20 June 2018, date last accessed)
- 23. Basu M, Malhotra AS, Pal K. et al. Erythropoietin levels in lowlanders and high-altitude natives at 3450 m. Aviat Space Environ Med 2007; 78: 963–967 [DOI] [PubMed] [Google Scholar]
- 24. Brockmoller J, Kochling J, Weber W. et al. The pharmacokinetics and pharmacodynamics of recombinant human erythropoietin in haemodialysis patients Br J Clin Pharmacol 1992; 34: 499–508 [PMC free article] [PubMed] [Google Scholar]
- 25. Ashby DR, Gale DP, Busbridge M. et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int 2009; 75: 976–981 [DOI] [PubMed] [Google Scholar]
- 26. Mastrogiannaki M, Matak P, Mathieu JRR. et al. Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis. Haematologica 2012; 97: 827–834 [DOI] [PMC free article] [PubMed] [Google Scholar]