Abstract
Background:
The effects of exposure to fine particulate matter () during wildland fires are not well understood in comparison with exposures from other sources.
Objectives:
We examined the cardiopulmonary effects of short-term exposure to on smoke days in the United States to evaluate whether health effects are consistent with those during non-smoke days.
Methods:
We examined cardiopulmonary hospitalizations among adults y of age, in U.S. counties () within of 123 large wildfires during 2008–2010. We evaluated associations during smoke and non-smoke days and examined variability with respect to modeled and observed exposure metrics. Poisson regression was used to estimate county-specific effects at lag days 0–6 (L0–6), adjusted for day of week, temperature, humidity, and seasonal trend. We used meta-analyses to combine county-specific effects and estimate overall percentage differences in hospitalizations expressed per increase in .
Results:
Exposure to , on all days and locations, was associated with increased hospitalizations on smoke and non-smoke days using modeled exposure metrics. The estimated effects persisted across multiple lags, with a percentage increase of 1.08% [95% confidence interval (CI): 0.28, 1.89] on smoke days and 0.67% (95% CI: , 1.44) on non-smoke days for respiratory and 0.61% (95% CI: 0.09, 1.14) on smoke days and 0.69% (95% CI: 0.19, 1.2) on non-smoke days for cardiovascular outcomes on L1. For asthma-related hospitalizations, the percentage increase was greater on smoke days [6.9% (95% CI: 3.71, 10.11)] than non-smoke days [1.34% (95% CI: , 3.77)] on L1.
Conclusions:
The increased risk of -related cardiopulmonary hospitalizations was similar on smoke and non-smoke days across multiple lags and exposure metrics, whereas risk for asthma-related hospitalizations was higher during smoke days. https://doi.org/10.1289/EHP3860
Introduction
Smoke from wildland fires has become one of the leading sources of short-term exposure to poor air quality in the United States (U.S. EPA 2018). Older adults are thought to be susceptible to the effects of poor air quality, not only due to the physiological processes associated with older age, but also due to a higher prevalence of preexisting conditions that may be exacerbated by these exposures (Sacks et al. 2011). Although significant scientific evidence is available concerning the health effects of air pollution exposure, the health effects of wildland fire smoke exposure in this population are not well characterized.
Potential differences in the associations between respiratory and cardiovascular effects and exposure to wildland fire smoke, can help identify whether equivalent health risk messages apply during wildfire events as with other periods of high air pollution. Among the air pollutants found in smoke, fine particulate matter () is of the highest concern for human health (Kim et al. 2015). The U.S. EPA concluded, in its evaluation of the scientific literature on the effects of air pollution, that there is a causal relationship between both short- and long-term exposure and cardiovascular effects and that there is likely to be a causal relationship between both short- and long-term exposure and respiratory effects (U.S. EPA 2009). Most particulate matter is a by-product of combustion, and the fuel and conditions of combustion determine the size and chemical composition of the mixture, which in turn determines the toxicity at exposure (Adams et al. 2015). Previous epidemiologic studies of during wildland fire-smoke events have reported primarily positive and consistent associations with respiratory effects (Delfino et al. 2009; Liu et al. 2017; Moore et al. 2006; Mott et al. 2005; Rappold et al. 2011), whereas evidence of cardiovascular effects have been less consistent (Delfino et al. 2009; Liu et al. 2015; Moore et al. 2006; Rappold et al. 2011; Reid et al. 2016).
Comparing the risk associated with ambient as a result of wildland fire smoke and ambient from other sources is challenging for several reasons. Recent reviews of the health effects due to exposure during smoke episodes (Fann et al. 2018; Liu et al. 2015) have noted not only large differences in exposure, but also several methodological differences between studies. Most studies evaluating the health effects related to wildland fire smoke have examined a single wildfire at a time, in a relatively short timeframe, and within a small geographic area near the point of origin of the fire (e.g., Delfino et al. 2009; Kochi et al. 2012; Rappold et al. 2011). In contrast, health studies examining the effects of all sources of rely on years of daily concentrations and health data and are often conducted in the most populated areas (i.e., large metropolitan areas) (Kim et al. 2015).
The methods to quantify exposure and pollutant data also add to the challenge of studying health effects associated with in wildland fire smoke. A number of studies have relied on air quality monitors, which may be far from the point of origin of the fire (Liu et al. 2015). These air quality monitors are typically located in more densely populated areas and may represent a population with baseline characteristics that are different from the study population. In addition, at the high concentrations observed during periods of intense wildland fire smoke, these monitors have been reported to malfunction (Landis et al. 2017). In more recent studies, chemical transport models (Bell 2006), dispersion models, and satellite images of smoke (Black et al. 2017; Gan et al. 2017; Henderson et al. 2011; Liu et al. 2015; Rappold et al. 2011, 2017) have been used to estimate due to wildland fires or exposure to smoke. Chemical transport and dispersion models provide spatially and temporally resolved exposure estimates in areas without monitors, yet they can contain errors due to misspecification of the model or input variables. A few studies (Gan et al. 2017; Henderson et al. 2011) have previously compared the health effects of wildland fire smoke using multiple exposure metrics. Because there is no gold standard exposure metric, it is important to examine the variability of health risk estimates with respect to multiple metrics of exposure, particularly in studies that examine the relationships between health outcomes and wildland fire exposure.
In light of these challenges, the primary objective of this study was to evaluate the consistency of the associations between short-term exposures and cardiopulmonary hospitalizations during smoke and non-smoke events. The secondary objective was to examine the variability of the estimated associations with respect to different exposure metrics. Specifically, we evaluated associations between cardiopulmonary hospitalizations among Medicare recipients y of age, and exposure to during and outside wildland fire smoke periods across 692 counties. For these counties, we used daily concentrations measured at the monitoring sites and the Community Multiscale Air Quality (CMAQ) model (https://www.epa.gov/cmaq) over the 3-year period of 2008 through 2010.
Methods
County Selection and Study Period
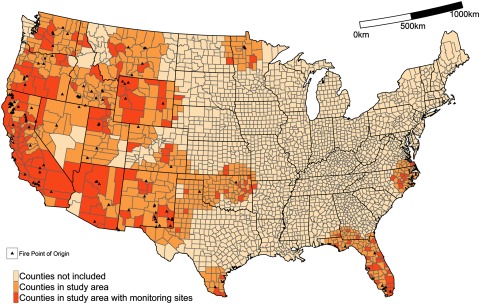
To study the associations between cardiopulmonary hospitalizations and exposure to , we restricted the analysis to counties near at least one large () wildland fire in the 3-year period of 2008 through 2010. During this period, there were 123 large wildland fires (71 in 2008, 30 in 2009, and 22 in 2010) in the contiguous, mostly Western United States. A total of 692 counties, within of these fires, were selected for the study population (Figure 1). Within these counties, we considered daily concentrations of total that on non-smoke days included emissions from non-fire sources, whereas on smoke days, total additionally included wildland fire sources of emissions. All counties in the study area could have been affected by smoke from multiple fires. Wildfire points of origin and burned area parameters were obtained from the Geospatial Multi-Agency Coordination (GeoMAC) website (http://rmgsc.cr.usgs.gov/outgoing/GeoMAC/historic_fire_data/) and the Department of the Interior’s Federal Fire Occurrence website (https://wildfire.cr.usgs.gov/firehistory/).
Figure 1.
Counties included in analysis, 2008–2010.
The centroid of all highlighted counties () were located within of a wildfire point of origin (, denoted by a triangle), of which 178 counties (25.7%) had monitoring sites.
Hospital Admissions
We acquired inpatient hospital admissions data using the Medicare Provider Analysis and Review (MEDPAR) files from the Center for Medicare and Medicaid Services (CMS). MEDPAR contains 100% of Medicare beneficiaries using hospital inpatient services. We restricted our study population to include hospital admissions occurring during the 3-y period of 2008 through 2010 among those y of age because over 93% of those y of age in the United States are covered by Medicare (West et al. 2014).
Daily counts of hospitalizations for cardiopulmonary health outcomes were aggregated to the county of residence. Using principal ICD-9-CM diagnosis codes [International Statistical Classification of Diseases and Related Health Problems, Ninth Revision, Clinical Modification (DHHS 1980)], we defined three health end points: a) respiratory [RSP; ICD-9-CM codes: 480–486 (pneumonia), 490–496 (chronic obstructive pulmonary disease and allied conditions), and 507 (pneumonitis due to solids/liquids)]; b) asthma, bronchitis, and wheezing [ABW; codes 493 (asthma), 490 (bronchitis), and 786.07 (wheezing)]; and c) all-cause cardiovascular disease [CVD; codes 390–448 (diseases of the circulatory system, excluding diseases of veins, lymphatics, and other diseases of the circulatory system)]. Repeat hospitalizations by the same individual were considered independent events. Institutional review board (IRB) approval was obtained from the University of North Carolina at Chapel Hill. Aggregation of MEDPAR data was done in Python (version 2.7.11; Python Software Foundation).
Assessment of Exposure
We used two sources of data: concentrations were a) measured at monitoring stations across the United States and b) estimated using CMAQ. Measured concentrations were obtained from the U.S. EPA’s Air Quality System. These measurements were available every 1, 3, or 6 d, depending on the location, from over 4,000 monitoring sites in the United States (https://www.epa.gov/outdoor-air-quality-data). Measured concentrations captured total concentrations from all sources, including wildland fire and other sources, and were labeled . Daily (24-h average) county-level averages were calculated (Bell et al. 2007; Schwartz 2000) and available for 178 out of 692 (25.7%) counties, or 79,298 county-days during the study period. Using the CMAQ framework, we simulated air quality with and without emissions from wildland fires on a grid (for more details, see Fann et al. 2018 and Rappold et al. 2017). CMAQ estimates with fire emissions included emissions from wildland fires and from all other sources and were labeled as . The CMAQ estimates without wildfire emissions included only non-wildland fire emission sources and were labeled as . County-level CMAQ estimates of exposure were calculated using area-weighted estimates (24-h averages) for all 692 counties, or 758,432 county-days. The difference between these two model runs was the wildland fire-specific contribution of .
We defined the variable SmokeDay as an indicator when was greater than . Smoke days were defined the same way for all analyses because stationary monitors provide total ambient concentrations and cannot distinguish between wildfire and other sources. Overall, 28,118 county-days were considered smoke days, or 4% of the total. In a sensitivity analysis, we also examined the impact of a lower threshold on the robustness of estimates.
We summarized the associations between the ambient and cardiopulmonary hospitalization on smoke () and non-smoke days (), using three distinct exposure metrics, including: a) CMAQ estimated concentrations on all days and in all counties (); b) monitored data (), which are available on fewer number of days and counties than ; and c) CMAQ estimates in locations and times where monitoring data were available () to compare estimated health effects on the same days and in the same counties. As such, the latter two metrics were based on the same days and locations ( and ), whereas the other two utilize CMAQ data ( and ) at the same locations but not on the same days.
Statistical Analysis
The associations between daily counts of hospitalizations and concentrations were examined using a quasi-Poisson regression model for each county separately:
where s and t index counties and days respectively, stands for , , or , SmokeDay is a binary indicator of smoke days, and DOW stands for day of week. To account for seasonality and to control for unmeasured confounders that vary with seasons, we included a natural spline for date (Day) with 18 degrees of freedom (df; 6 per year). To account for confounding by co-occurring weather conditions, we controlled for daily temperature (Temperature) and relative humidity (Humidity), averaged over L0–2, using natural splines with 3 df allowing for nonlinear effects on the outcome. Temperature and relative humidity were obtained from the National Climate Data Center (http://www.ncdc.noaa.gov).
To examine the statistical difference in risk on health outcomes between smoke and non-smoke days, we used the interaction coefficient . The percentage difference in cardiopulmonary hospitalizations for a increase in was examined at single-day lags ranging from the day of exposure [lag day 0 (L0)] up through 6 d postexposure (L6) (Braga et al. 2001). Estimated relative risks were expressed as percentage difference in hospitalization rates per increase in on both smoke and non-smoke days (). For percentage differences in hospitalizations associated with on smoke and non-smoke days, we used estimable function in the gmodels R package (Warnes et al. 2015). Briefly, on non-smoke days the coefficient was equivalent to , whereas for smoke days, we used and .
It was expected that daily rates of hospitalizations would differ from county to county, primarily due to differences in population size. Therefore, we assessed the relationship in each county separately and conducted a random effects meta-analysis to estimate the average percentage difference in hospitalizations associated with on smoke and non-smoke days. This analysis was performed using the rma function in the metafor package (Viechtbauer 2010). All analyses were performed using R (version 3.1.2, unless otherwise specified; R Development Core Team).
Sensitivity Analyses
We tested the sensitivity of our results by restricting data sources in three separate ways and using an alternative modeling technique. First, we restricted our results to include only counties with over 10,000 individuals y of age. Next, we lowered the threshold for smoke days by replacing our current threshold of (), with a threshold of (). Third, we explored variations in risk estimates between regions of the United States. Although many large wildfires occur in the Western part of the United States, they are also frequent in the Southeast, where they burn through different types of vegetation under different meteorological conditions (Figure 1). We restricted our analysis to specifically examine counties from Western Region states (Arizona, California, Colorado, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, and Wyoming) or from Southeastern Region states (Alabama, Arkansas, Florida, Georgia, North Carolina, and Virginia). Last, we summarized the cumulative effect, across multiple lags, following exposure on cardiopulmonary hospitalizations on smoke and non-smoke days. For this we used an unrestricted distributed lag model [dlnm R package (Gasparrini 2011)] and exposure metric. The other two exposure metrics did not have data available every day for a cumulative effect analysis because they were based on monitored data.
Results
A total of 1,032,268 RSP, 82,463 ABW, and 2,558,602 CVD hospitalizations occurred during the study period among Medicare recipients y of age (Table 1). The average daily rate of hospitalizations was greater on non-smoke than on smoke days (Table 1).
Table 1.
Total number of hospital admissions between 1 January 2008 and 31 December 2010.
| Outcome | Total hospital admissions (n) | Daily average rates across counties (per 100,000 people y of age) | |||
|---|---|---|---|---|---|
| Mean | Median | Range | Interquartile range | ||
| Respiratory | |||||
| All days | 1,038,598 | 10.0 | 8.8 | 0.9–106.4 | 5.8 |
| Smoke days | 39,335 | 9.0 | 6.8 | 0–197.6 | 8.5 |
| Non-smoke days | 999,263 | 10.0 | 10.6 | 0.9–106.5 | 5.7 |
| Asthma, bronchitis, and wheezing | |||||
| All days | 82,982 | 0.6 | 0.5 | 0–3.4 | 0.5 |
| Smoke days | 3,114 | 0.5 | 0 | 0–31.7 | 0.7 |
| Non-smoke days | 79,868 | 0.6 | 0.6 | 0–3.4 | 0.4 |
| All-cause cardiovascular | |||||
| All days | 2,569,398 | 20.8 | 19.8 | 4.6–98.8 | 10.2 |
| Smoke days | 99,439 | 22.1 | 19.4 | 0–282.5 | 13.8 |
| Non-smoke days | 2,469,959 | 20.8 | 19.8 | 4.8–98.9 | 10.2 |
Note: All days: 758,432 county-days; smoke days: 28,118 county-days; non-smoke days: 730,314 county-days.
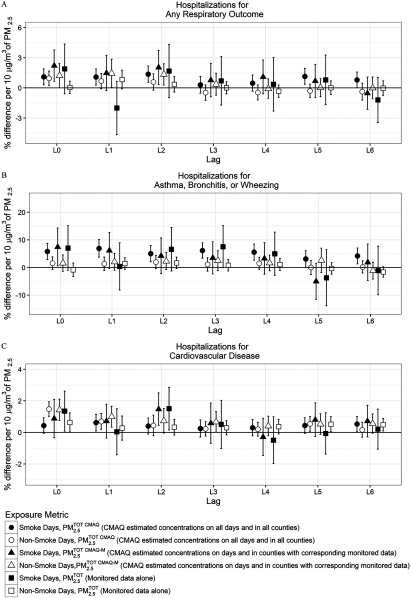
We observed an increased risk for respiratory and all-cause cardiovascular hospitalizations on both smoke and non-smoke days (Figure 2; see also Table S1). The effects were consistently positive across L0–2 for for RSP and CVD hospitalizations. The confidence intervals (CIs) for all cardiopulmonary hospitalizations were wider on smoke days compared with non-smoke days given that there were far fewer smoke days () compared with non-smoke days () (4%) in our 3-year (692 counties and 1,096 d) study period.
Figure 2.
Percentage difference and 95% confidence intervals in hospitalizations during 2008–2010, among U.S. Medicare recipients y of age per increase in , lag day 0 to lag day 6. Smoke days are defined as having a wildfire-specific contribution , and non-smoke days as wildfire-specific contribution . Associations are estimated using a single lag model for the interaction between (, , or ) and SmokeDay adjusting for day of the week, day [natural spline with 6 degrees of freedom (df) per year], temperature (natural spline with 3 df), and relative humidity (natural spline with 3 df) for each county, followed by a meta-analysis. Using 595 counties, 341 counties, and 607 counties were used in the meta-analyses for (A) respiratory; (B) asthma, bronchitis, and wheezing; (C) and cardiovascular hospitalizations, respectively. Using the other metrics ( or ) 134 counties, 92 counties, and 137 counties were used in the meta-analyses for (A) respiratory; (B) asthma, bronchitis, and wheezing; (C) and cardiovascular hospitalizations, respectively. The y-axes limits differ between hospitalization types. (See Table S1 for corresponding numeric data.)
For RSP hospitalizations, the associations with were positive across all Lon smoke days and for L0–2 on non-smoke days (Figure 2; see also Table S1). Furthermore, the associations were statistically significant (defined as a 95% CI excluding 0) at L0–2 and 5–6 on smoke days and at L0 on non-smoke days. At L1, the associations were 1.08% (95% CI: 0.28, 1.89) and 0.67% (, 1.44) on smoke and non-smoke days, respectively. The differences between the corresponding estimates on smoke and non-smoke days were not statistically significant at for any lags (see Table S1).
For ABW hospitalizations, the associations with were generally positive across all lags but higher on smoke days (Figure 2; see also Table S1). For example, at lag 1 the associations per a increase in were 5.78% (2.85, 8.71) on smoke days and 1.55% (, 3.85) on non-smoke days. Point estimates of the association were consistently higher on smoke days, and significant interactions () were observed at L2 and L4 (see Table S1).
Using , there was a statistically significant percentage increase in CVD hospitalizations at L1 on smoke days and L0–1 on non-smoke days (Figure 2; see also Table S1). At L1, the associations were 0.61% (95% CI: 0.09, 1.14) on smoke days and 0.69% (95% CI: 0.19, 1.20) on non-smoke days. Except at L0, the point estimates were similar in magnitude between smoke and non-smoke days, and no statistically significant interactions were found at any L(see Table S1).
Comparing CMAQ (either or ) metrics to monitors (), we observed differences in risk for both sets of days. On smoke days, increased risks in hospitalizations per of were broadly in agreement with respect to direction and magnitude, with the greatest consistency between the three metrics at L0 for all outcomes. On non-smoke days, the estimated effects using CMAQ exposure estimates ( and ) were generally higher in magnitude than when using monitoring data () but confidence intervals generally overlapped. In addition, the higher effect estimate found with CMAQ could have occurred because the range of CMAQ exposure estimates was more compressed than the monitoring data on those days. A narrower range of exposure with the same outcome data generally leads to a higher magnitude effect estimate. On non-smoke days, the effect of was the least consistent at L0 across three exposure metrics but in close agreement for later lags. We also note that monitoring data () on smoke days had, by far, the highest uncertainty, followed by CMAQ on days and in locations with corresponding monitoring data (). However, the metric including all days and all counties () resulted in the most statistically significant outcomes (Figure 2; see also Table S1).
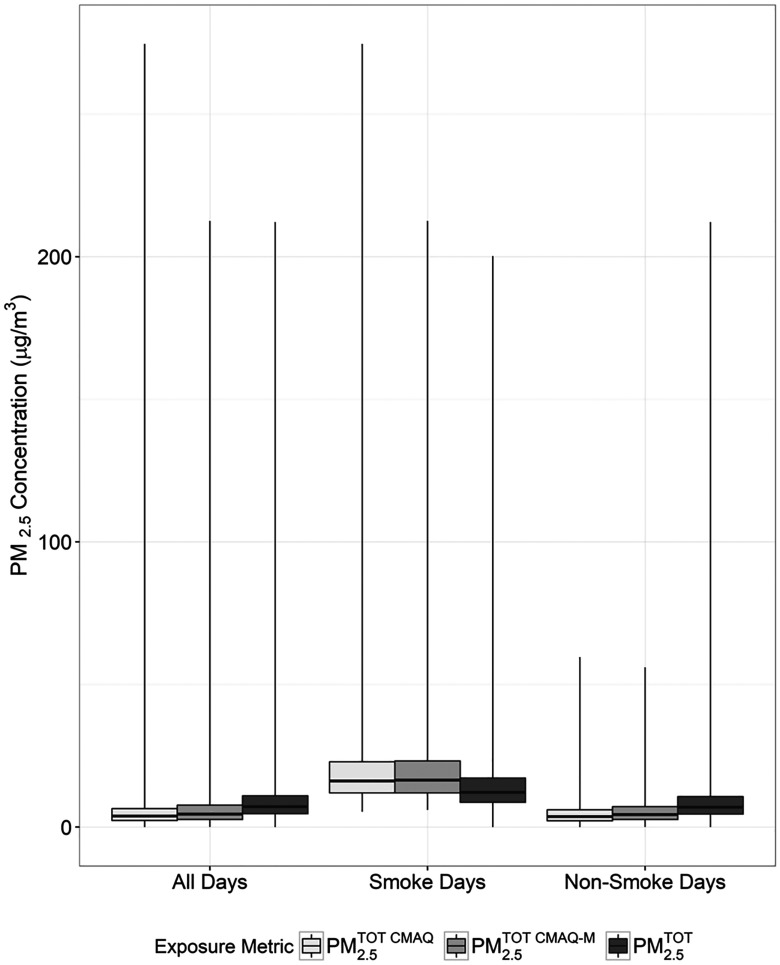
Median concentrations were higher than and . However, on smoke days, the median concentrations were lower than those of the other two metrics (Figure 3; see also Table S2). Furthermore, comparing concentrations from the metrics on days with monitoring data, the median Pearson correlations between and by county were 0.49 and 0.50, on all days and non-smoke days, respectively, but 0.36 on smoke days. When and were averaged across counties, estimates underpredicted concentrations on all days and non-smoke days, yet overpredicted on smoke days (see Figure S1).
Figure 3.
Summary of concentrations (), overall and by smoke and non-smoke days. : CMAQ estimated concentrations on all days and in all counties, : CMAQ estimated concentrations on days and in counties with corresponding monitored data, : Monitored data alone. Smoke days defined as wildfire-specific contribution and on-smoke days defined as wildfire-specific contribution . The horizontal line within each box represents the median, whereas the ends of the box correspond to the 25th and 75th percentiles. The lines extending from the box correspond to the minimum and maximum. (See Table S2 for corresponding numeric data.)
The number of counties included in the meta-analyses for each hospital end point varied. Counties whose models did not converge nor had any warnings due to small daily counts or lack of discordant exposures were removed from the analysis. Therefore, models using had 607 counties included in the meta-analysis for CVD, 592 counties for RSP, and 341 counties for ABW, out of a total of 692 counties. Alternatively, models using either or had 137 counties included for CVD, 134 counties for RSP, and 92 counties for ABW, out of a total of 178 counties.
Sensitivity analyses were conducted by restricting the analysis to counties with larger populations of Medicare enrollees () by lowering the threshold () for defining smoke days using , conducting a regional analysis among Western and Southeastern counties, and using distributed lag models. Restricting by population size left 185 counties, of which 120 (64.9%) had corresponding monitoring data (see Figure S2) but had minimal impact on our estimates (see Figure S3). However, when using a lower threshold for smoke days (), we detected similar effects for respiratory and cardiovascular hospitalizations compared with the results presented for a threshold of (see Figure S4). However, the effects for asthma, bronchitis, and wheezing using the lower threshold were lower and did not differ between smoke and non-smoke days. Results were similar when comparing Western and Southeastern regions (see Figure S5) although the confidence intervals were wider for the Southeast subanalyses. There were far fewer county-days in the Southeast (, ) compared with the Western Region (, ) with . Using an unrestricted distributed lag demonstrated that the percentage increase in respiratory hospitalizations persisted through L2 on both smoke and non-smoke days (see Figure S6). For hospitalizations related to asthma, bronchitis, and wheezing, the effect was present only on L0 on smoke days, which is consistent with an acute reaction such as asthma, in response to increased concentrations of wildland fire-related . A consistent percentage increase in cardiovascular hospitalizations was present through L6 on both smoke and non-smoke days.
Discussion
This study examined the relationship between daily counts of cardiopulmonary hospitalizations and exposure to in a susceptible population of older adults on days with and without smoke from wildland fires. The percentage difference in all-cause cardiovascular and respiratory hospitalizations associated with a increase related to wildland fire smoke (smoke days) was similar to the percentage difference associated with a increase in non-wildfire related (non-smoke days). However, for asthma, bronchitis, and wheezing hospitalizations, the association with was greater during periods of wildland fire smoke compared with non-smoke ambient . When considering multiple exposure metrics, more statistically significant associations with were observed using the CMAQ metric accounting for all days and locations () compared with the other two metrics ( and ).
Our results were consistent on smoke and non-smoke days for respiratory and all-cause cardiovascular hospitalizations. For respiratory hospitalizations, we observed similar percentage differences for L0–2 on both smoke and non-smoke days. However, for cardiovascular outcomes, change in hospitalizations was similar on smoke and non-smoke days for L1–5. Yet, at L0, the magnitude of the percentage increase in cardiovascular hospitalizations was higher on non-smoke days compared with smoke days using total metric but not for the other two metrics (Figure 2; see also Table S1). When considering a more specific outcome such as asthma, bronchitis, and wheezing, we consistently observed a higher percentage increase in hospitalizations on smoke days compared with non-smoke days. This suggests that, in the older population, smoke-related is more likely to trigger acute responses, such as asthma, bronchitis, and wheezing, compared with during non-smoke events, but that the risk of respiratory and all-cause cardiovascular hospitalizations is consistent during smoke and non-smoke events. It is possible that significantly higher associations between and hospitalizations related to asthma on smoke days is due higher levels of , which would be consistent with the results of Liu et al. (2017) and Gan et al. (2017).
The literature has suggested that wildfire-specific can be a trigger for asthma symptoms severe enough to require emergency care (Haikerwal et al. 2016). In fact, the risks for asthma and chronic obstructive pulmonary disease (COPD) seem to be the highest among the elderly ( y of age) during wildland fire smoke episodes (Gan et al. 2017; Mott et al. 2005; Rappold et al. 2011). A wildfire episode is often associated a sudden increase of fine particulate matter, which may be more likely to result in an acute reaction in the respiratory tract and lead to hospitalization among the older population (Black et al. 2017).
Despite the strong evidence regarding the effects of wildfire-specific on respiratory outcomes (Delfino et al. 2009; Liu et al. 2017; Moore et al. 2006; Mott et al. 2005; Rappold et al. 2011) and of all-source exposures on cardiovascular disease (Brook et al. 2010; U.S. EPA 2009), there have been inconsistent results regarding the effect of wildfire-specific exposure on cardiovascular outcomes (Delfino et al. 2009; Dennekamp et al. 2015; Liu et al. 2017; Rappold et al. 2011). There are several possible reasons for this. In comparison with respiratory hospitalizations, far fewer wildfire health studies have included cardiovascular outcomes (Black et al. 2017; Liu et al. 2015; Reid et al. 2016). The majority of studies evaluating CVD outcomes define CVD very broadly by including principal diagnosis codes ICD-9-CM codes 390–459. In our study, we used a slightly more specific set of ICD-9-CM codes (390–448), which excluded diseases of veins and lymphatics and other diseases of the circulatory system (codes 451–459). Some studies have defined CVD even more specifically, such as including only ICD-9-CM codes related to ischemic heart disease (410–413 or 410–414) (Delfino et al. 2009; Haikerwal et al. 2015; Morgan et al. 2010). This increased specificity has led to stronger conclusions in some studies (Haikerwal et al. 2015; Martin et al. 2013; Rappold et al. 2011), yet increased specificity has not resulted in an association in other studies (Delfino et al. 2009; Moore et al. 2006). In addition, the higher risk of acute respiratory impacts such as asthma, bronchitis, or wheezing during smoke days relative to non-smoke days may also shed light on the lack of consistency in findings about cardiovascular outcomes in other studies. Namely, hospitalizations for acute respiratory reactions and some cardiovascular outcomes (e.g., cardiac arrest) in this susceptible population may lead to removing individuals from the risk pool available for CVD outcomes because they are no longer continuously exposed to smoke and are medicated. In addition, there could also be a difference in particle size distribution between wildfire and industrial , such as fewer ultrafine particles in the former.
In contrast to several studies that have examined one fire at a time, a recent study by Liu et al. (2017) evaluated the effect of wildfire-specific and cardiopulmonary outcomes in 561 counties in the Western United States among adults over 65 y of age. The authors defined exposure as consecutive days (smoke wave) of high wildfire-specific () using a chemical transport model. Liu et al. (2017) found a 7.2% (95% CI: 0.25, 15.0) increase in risk for respiratory hospital admissions but no effect for CVD hospital admissions with wildfire-specific . The present study found a 1.10% (95% CI: 0.31, 1.89) increase in respiratory hospitalizations on smoke days and a 0.96% (95% CI: 0.25, 1.67) increase in respiratory hospitalizations on non-smoke days and a 0.43% (95% CI: , 0.92) increase in CVD hospitalizations on smoke days and a 1.47% (95% CI: 1.01, 1.93) increase in CVD hospitalizations on non-smoke days for each increase in , using CMAQ () at L0. The effect size of our estimates was smaller, but the study by Liu et al. (2017) examined effects after 2 consecutive days of smoke and our threshold for smoke days was also considerably lower. Because the study by Liu et al. (2017) and the present study used different exposure models, it is difficult to say with certainty how comparable the two are. If the exposures estimated are roughly the same, then a 10-unit increase in would be comparable within the two studies. To aid in future research, we have made our exposure data publicly available on our website.
Previous health studies of wildfire smoke exposure have compared effect estimates when using different exposure metrics (Gan et al. 2017; Henderson et al. 2011), but they focused on small geographic areas. A study by Henderson et al. (2011) evaluated the relationship between respiratory and cardiovascular all-age hospital admissions and from ambient monitors as well as predicted from a chemical transport model. The study, conducted in British Columbia, Canada, observed a 5% increase in the odds of respiratory hospitalizations [ 1.05 (95% CI: 1.00, 1.10)] for a 1-standard deviation (SD) () increase in characterized using monitors and an 11% [ 1.11 (95% CI: 1.04, 1.18)] increase in the odds of respiratory hospitalizations for a 1-SD () increase in , according to their chemical transport model, representing smoke-related only. Henderson et al. (2011) did not observe increases in cardiovascular hospitalizations; however, they did not focus on the most susceptible populations, such as older adults or those with preexisting conditions (Wettstein et al. 2018) not did they examine the health effects of , which have been suggested to be more important than the health effects of . Another study estimating the effects of smoke on hospital admissions, in the state of Washington, directly compared a chemical transport model, stationary monitors, and a hybrid model (Gan et al. 2017). Here, they found an 8% [ 1.076 (95% CI: 1.019, 1.136) for the hybrid model] increase in the odds of hospital admissions for asthma for a increase in . Similar results were observed for the other metrics used in Gan study. The present analysis demonstrated similar observations with a 5.78% (95% CI: 2.85, 8.71) increase in hospitalizations for asthma, bronchitis, and wheezing using , a 7.44% (95% CI: 0.62, 14.30) increase using , and a 7.03% (95% CI: , 15.13) increase using on smoke days for a increase in at L0 (Figure 2; see also Table S1). Gan et al. (2017) observed differences in the effects for COPD, depending on the smoke-estimation method (11%, 1.11 (95% CI: 1.03, 1.18)] using monitoring data, (, ) 0.99 (95% CI: 0.93, 1.05)] using the chemical model, and [9%, 1.09 (95% CI: 1.03, 1.15)] using the hybrid model. These differences are important because COPD is most prevalent among the elderly ( y of age) and is often conflated with diagnoses for asthma among this age group (Gillman and Douglass 2012). Gan et al. (2017) did not find an effect when examining CVD hospitalizations among those y of age. Although the magnitude of the percentage difference in hospitalizations varied according to the three metrics, similar conclusions regarding risk can be made.
Although several studies have examined the effect of wildfire smoke and cardiopulmonary hospitalizations, many have relied on different data sources, time periods, and study populations, making direct comparisons challenging. Here, we analyzed the impact of using three different exposure metrics, which may shed some light as to how wildfire-specific may be detected in space and time. Recall that the majority of effect estimates generated based on monitoring data ( and ) were not statistically significant, whereas estimates using only CMAQ data () were mostly positive and statistically significant (Figure 2; see also Table S1). This may imply that the sparsity of monitors over space and, particularly, time has an impact on the overall conclusions drawn from the data. Therefore, it is possible that calibrating models by monitoring data could have an impact on the results as well. In our analysis, we chose to not calibrate our CMAQ data for this reason, yet both Gan et al. (2017) and Liu et al. (2017) used some form of spatial smoothing when calibrating, which could explain some of the differences in results between the studies. However, because none of the studies used the same populations, it is difficult to tease apart any distinct reasons for observing different results. In addition, CMAQ is often less accurate at a finer geographic scale and monitor data is mostly unavailable for unpopulated areas.
Notwithstanding the challenges to analyzing the effects of wildland fire smoke-related , our analysis had several strengths. First, we included all Medicare enrollees from 2008–2010, y of age, who were hospitalized for respiratory, asthma, bronchitis, wheezing, or all-cause cardiovascular outcomes (). Second, we examined the effects of daily exposures considering smoke from all fires that affected the study population, whereas the majority of studies analyzing the effects of wildland fire smoke and health effects evaluate one fire at a time (Liu et al. 2015). Third, we also compared the risk of on smoke and non-smoke days in the same population, rather than across different populations. Last, this analysis focused on effects among the elderly, previously identified as a susceptible subgroup, especially concerning outcomes associated with wildland fire smoke (Bell et al. 2013; Delfino et al. 2009; Kochi et al. 2012; Liu et al. 2015; Sacks et al. 2011).
The findings observed in this study should be considered in light of several limitations. In the analysis, all hospitalizations were treated as independent over time, including repeat hospitalizations by the same individual. Exposure misclassification might also be a concern because the location provided in MEDPAR reflects a patient’s home, not necessarily the area where they may have been exposed to wildland fire smoke. The study also focused on the susceptible population of those y of age but did not characterize health impacts in other susceptible populations. In addition, some counties are large, especially in the Western Region (Figure 1), and county-averaged exposure may not adequately capture the exposure. In addition, one could examine an alternative model to estimate the effects of both and leading to a separate risk coefficient for fire-emitted and non–fire-emitted . Although there is a clear value in knowing these risks, such a model would require an assumption that the effects of the two types of particles are additive and could lead to a violation of the no-multiple-treatment assumption or even to interference. For example, the model would assume that the effect of does not depend on the level or composition of . In addition, zero inflated exposure, , can make it difficult to identify the effect of . Finally, one must consider possible misclassifications that occur because of the multiple types of monitors used near the fire with various precisions and that these monitors can malfunction during heavy smoke events. Considering our results and the limitations of our study, the consistency of health effects should be investigated in other cohorts, particularity with respect to the use of multiple exposure metrics.
Conclusions
Our findings suggest that , during smoke days, is more likely to trigger strong, acute responses such as asthma, bronchitis, and wheezing, compared with non-smoke days, in the older population. We also observed statistically significant associations among respiratory and all-cause cardiovascular hospitalizations and short-term exposures on both smoke and non-smoke days across multiple lags following exposure, and these were not statistically different. These findings suggest that respiratory and all-cause cardiovascular health effects of from wildland fire smoke in the older population are consistent with the effects of non-wildland fire smoke .
Supplementary Material
Acknowledgments
We thank G. Pouliot and L. Neas for their assistance in using the Community Multiscale Air Quality (CMAQ) model and Center for Medicare and Medicaid Services data. We also thank K. Rappazzo, L. Wei, and J. Sacks for their editorial help.
References
- Adams K, Greenbaum DS, Shaikh R, van Erp AM, Russell AG. 2015. Particulate matter components, sources, and health: systematic approaches to testing effects. J Air Waste Manag Assoc 65(5):544–558, PMID: 25947313, 10.1080/10962247.2014.1001884. [DOI] [PubMed] [Google Scholar]
- Bell ML. 2006. The use of ambient air quality modeling to estimate individual and population exposure for human health research: a case study of ozone in the Northern Georgia Region of the United States. Environ Int 32(5):586–593, PMID: 16516968, 10.1016/j.envint.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Bell ML, Dominici F, Ebisu K, Zeger SL, Samet JM. 2007. Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environ Health Perspect 115(7):989–995, PMID: 17637911, 10.1289/ehp.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Zanobetti A, Dominici F. 2013. Evidence on vulnerability and susceptibility to health risks associated with short-term exposure to particulate matter: a systematic review and meta-analysis. Am J Epidemiol 178(6):865–876, PMID: 23887042, 10.1093/aje/kwt090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C, Tesfaigzi Y, Bassein JA, Miller LA. 2017. Wildfire smoke exposure and human health: significant gaps in research for a growing public health issue. Environ Toxicol Pharmacol 55:186–195, PMID: 28892756, 10.1016/j.etap.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga ALF, Zanobetti A, Schwartz J. 2001. The lag structure between particulate air pollution and respiratory and cardiovascular deaths in 10 US cities. J Occup Environ Med 43(11):927–933, PMID: 11725331, 10.1097/00043764-200111000-00001. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. 2010. Particulate matter air pollution and cardiovascular disease. Circulation 121(21):2331–2378, PMID: 20458016, 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Brummel S, Wu J, Stern H, Ostro B, Lipsett M, et al. 2009. The relationship of respiratory and cardiovascular hospital admissions to the southern California wildfires of 2003. Occup Environ Med 66(3):189–197, PMID: 19017694, 10.1136/oem.2008.041376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennekamp M, Straney LD, Erbas B, Abramson MJ, Keywood M, Smith K, et al. 2015. Forest fire smoke exposures and out-of-hospital cardiac arrests in Melbourne, Australia: a case-crossover study. Environ Health Perspect 123(10):959–964, PMID: 25794411, 10.1289/ehp.1408436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHHS (U.S. Department of Health and Human Services). 1980. International Statistical Classification of Diseases, Injuries, and Causes of Death. Ninth revision. Clinical modification. DHHS No. (PHS) 80-1260. Washington, DC:DHHS. [Google Scholar]
- Fann N, Alman B, Broome RA, Morgan GG, Johnston FH, Pouliot G, et al. 2018. The health impacts and economic value of wildland fire episodes in the U.S.: 2008–2012. Sci Total Environ 610–611:802–809, PMID: 28826118, 10.1016/j.scitotenv.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan RW, Ford B, Lassman W, Pfister G, Vaidyanathan A, Fischer E, et al. 2017. Comparison of wildfire smoke estimation methods and associations with cardiopulmonary‐related hospital admissions. Geohealth 1(3):122–136, PMID: 28868515, 10.1002/2017GH000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. 2011. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw 43(8):1–20, PMID: 22003319, 10.18637/jss.v043.i08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman A, Douglass JA. 2012. Asthma in the elderly. Asia Pac Allergy 2(2):101–108, PMID: 22701859, 10.5415/apallergy.2012.2.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haikerwal A, Akram M, Del Monaco A, Smith K, Sim MR, Meyer M, et al. 2015. Impact of fine particulate matter (PM2.5) exposure during wildfires on cardiovascular health outcomes. J Am Heart Assoc 4(7):e001653, PMID: 26178402, 10.1161/JAHA.114.001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haikerwal A, Akram M, Sim MR, Meyer M, Abramson MJ, Dennekamp M. 2016. Fine particulate matter (PM2.5) exposure during a prolonged wildfire period and emergency department visits for asthma. Respirology 21(1):88–94, PMID: 26346113, 10.1111/resp.12613. [DOI] [PubMed] [Google Scholar]
- Henderson SB, Brauer M, MacNab YC, Kennedy SM. 2011. Three measures of forest fire smoke exposure and their associations with respiratory and cardiovascular health outcomes in a population-based cohort. Environ Health Perspect 119(9):1266–1271, PMID: 21659039, 10.1289/ehp.1002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-H, Kabir E, Kabir S. 2015. A review on the human health impact of airborne particulate matter. Environ Int 74:136–143, PMID: 25454230, 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Kochi I, Champ PA, Loomis JB, Donovan GH. 2012. Valuing mortality impacts of smoke exposure from major southern California wildfires. J Forest Econ 18(1):61–75, 10.1016/j.jfe.2011.10.002. [DOI] [Google Scholar]
- Landis MS, Pancras JP, Graney JR, White EM, Edgerton ES, Legge A, et al. 2017. Source apportionment of ambient fine and coarse particulate matter at the Fort McKay community site, in the Athabasca Oil Sands Region, Alberta, Canada. Sci Total Environ 584–585:105–117, PMID: 28147291, 10.1016/j.scitotenv.2017.01.110. [DOI] [PubMed] [Google Scholar]
- Liu JC, Pereira G, Uhl SA, Bravo MA, Bell ML. 2015. A systematic review of the physical health impacts from non-occupational exposure to wildfire smoke. Environ Res 136:120–132, PMID: 25460628, 10.1016/j.envres.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JC, Wilson A, Mickley LJ, Dominici F, Ebisu K, Wang Y, et al. 2017. Wildfire-specific fine particulate matter and risk of hospital admissions in urban and rural counties. Epidemiology 28(1):77–85, PMID: 27648592, 10.1097/EDE.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KL, Hanigan IC, Morgan GG, Henderson SB, Johnston FH. 2013. Air pollution from bushfires and their association with hospital admissions in Sydney, Newcastle and Wollongong, Australia 1994–2007. Aust N Z J Public Health 37(3):238–243, PMID: 23731106, 10.1111/1753-6405.12065. [DOI] [PubMed] [Google Scholar]
- Moore D, Copes R, Fisk R, Joy R, Chan K, Brauer M. 2006. Population health effects of air quality changes due to forest fires in British Columbia in 2003: estimates from physician-visit billing data. Can J Public Health 97(2):105–108, PMID: 16619995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan G, Sheppeard V, Khalaj B, Ayyar A, Lincoln D, Jalaludin B, et al. 2010. Effects of bushfire smoke on daily mortality and hospital admissions in Sydney, Australia. Epidemiology 21(1):47–55, PMID: 19907335, 10.1097/EDE.0b013e3181c15d5a. [DOI] [PubMed] [Google Scholar]
- Mott JA, Mannino DM, Alverson CJ, Kiyu A, Hashim J, Lee T, et al. 2005. Cardiorespiratory hospitalizations associated with smoke exposure during the 1997 Southeast Asian forest fires. Int J Hyg Environ Health 208(1–2):75–85, PMID: 15881981, 10.1016/j.ijheh.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Rappold AG, Reyes J, Pouliot G, Cascio WE, Diaz-Sanchez D. 2017. Community vulnerability to health impacts of wildland fire smoke exposure. Environ Sci Technol 51(12):6674–6682, PMID: 28493694, 10.1021/acs.est.6b06200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold AG, Stone SL, Cascio WE, Neas LM, Kilaru VJ, Carraway MS, et al. 2011. Peat bog wildfire smoke exposure in rural North Carolina is associated with cardiopulmonary emergency department visits assessed through syndromic surveillance. Environ Health Perspect 119(10):1415–1420, PMID: 21705297, 10.1289/ehp.1003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CE, Brauer M, Johnston FH, Jerrett M, Balmes JR, Elliott CT. 2016. Critical review of health impacts of wildfire smoke exposure. Environ Health Perspect 124(9):1334–1343, PMID: 27082891, 10.1289/ehp.1409277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JD, Stanek LW, Luben TJ, Johns DO, Buckley BJ, Brown JS, et al. 2011. Particulate matter–induced health effects: who is susceptible? Environ Health Perspect 119(4):446–454, PMID: 20961824, 10.1289/ehp.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. 2000. Assessing confounding, effect modification, and thresholds in the association between ambient particles and daily deaths. Environ Health Perspect 108(6):563–568, PMID: 10856032, 10.1289/ehp.00108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2009. Integrated Science Assessment (ISA) For Particulate Matter (Final Report). EPA/600/R-08/139F. Washington, DC:U.S. EPA. [PubMed] [Google Scholar]
- U.S. EPA. 2018. 2014 National Emissions Inventory, Version 2: Technical Support Document. https://www.epa.gov/sites/production/files/2018-07/documents/nei2014v2_tsd_05jul2018.pdf [accessed 4 March 2019].
- Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J Stat Softw 36(3):1–48, 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- Warnes GR, Bolker B, Lumley T, Johnson. RC. 2015. Gmodels: various R programming tools for model fitting., Part R package version 2.16.2. Funded by the Intramural Research Program, of the NIH, National Cancer Institute and Center for Cancer Research under NCI Contract NO1-CO-12400.
- West LA, Cole S, Goodkind D, He W. 2014. 65+ in the United States: 2010. Washington, DC:U.S. Census Bureau. [Google Scholar]
- Wettstein Z, Hoshiko S, Fahimi J, Harrison R, Cascio WE, Rappold AG. 2018. Cardiovascular and cerebrovascular emergency department visits associated with wildfire smoke exposure in California in 2015. J Am Heart Assoc 7(8):e007492, PMID: 29643111, 10.1161/JAHA.117.007492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.