Abstract
Background:
Hexafluoropropylene oxide dimer acid [(HFPO-DA), GenX] is a member of the per- and polyfluoroalkyl substances (PFAS) chemical class, and elevated levels of HFPO-DA have been detected in surface water, air, and treated drinking water in the United States and Europe.
Objectives:
We aimed to characterize the potential maternal and postnatal toxicities of oral HFPO-DA in rats during sexual differentiation. Given that some PFAS activate peroxisome proliferator-activated receptors (PPARs), we sought to assess whether HFPO-DA affects androgen-dependent development or interferes with estrogen, androgen, or glucocorticoid receptor activity.
Methods:
Steroid receptor activity was assessed with a suite of in vitro transactivation assays, and Sprague-Dawley rats were used to assess maternal, fetal, and postnatal effects of HFPO-DA exposure. Dams were dosed daily via oral gavage during male reproductive development (gestation days 14–18). We evaluated fetal testes, maternal and fetal livers, maternal serum clinical chemistry, and reproductive development of F1 animals.
Results:
HFPO-DA exposure resulted in negligible in vitro receptor activity and did not impact testosterone production or expression of genes key to male reproductive development in the fetal testis; however, in vivo exposure during gestation resulted in higher maternal liver weights (), lower maternal serum thyroid hormone and lipid profiles (), and up-regulated gene expression related to PPAR signaling pathways in maternal and fetal livers (). Further, the pilot postnatal study indicated lower female body weight and lower weights of male reproductive tissues in F1 animals.
Conclusions:
HFPO-DA exposure produced multiple effects that were similar to prior toxicity evaluations on PFAS, such as perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), but seen as the result of higher oral doses. The mean dam serum concentration from the lowest dose group was 4-fold greater than the maximum serum concentration detected in a worker in an HFPO-DA manufacturing facility. Research is needed to examine the mechanisms and downstream events linked to the adverse effects of PFAS as are mixture-based studies evaluating multiple PFAS. https://doi.org/10.1289/EHP4372
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a group of high-profile contaminants of emerging concern; the concern is primarily due to extensive research indicating these compounds have extreme environmental persistence (Awad et al. 2011), widespread occurrence (Kaboré et al. 2018; Kannan et al. 2004; Pan et al. 2018), long biological half-lives (Li et al. 2018), and nearly ubiquitous human exposure (Calafat et al. 2007). Further, there is concern for human health effects due to laboratory animal and epidemiological research on both perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). When administered throughout gestation, both PFOS and PFOA have been shown to produce adverse effects in rodent models, including extensive pup mortality and reduced growth rates (Grasty et al. 2003; Lau et al. 2003; Thibodeaux et al. 2003), and their administration is also correlated with increased incidence rates of thyroid dysfunction (Coperchini et al. 2017) and low birth weight (Apelberg et al. 2007) in human populations. Because of the combination of these factors, PFOS was primarily phased out of production by 2002, and subsequently added to Annex B of the Stockholm Convention, and the U.S. EPA has set drinking water health advisories for PFOS and PFOA at (U.S. EPA 2016b). Similarly, beginning in 2006 the major manufacturers of PFOA voluntarily agreed to phase out production by 2015 (U.S. EPA 2006). However, a variety of structural analogs have been developed and utilized as replacement compounds in the production of a range of consumer and industrial products for which fluoropolymers provide desirable characteristics (Wang et al. 2013; Wang et al. 2017b).
Hexafluoropropylene oxide dimer acid [(HFPO-DA), GenX] is a PFAS compound that is used as a polymerization aid in the manufacturing of high-performance fluoropolymers following the phase out of PFOA (Beekman et al. 2016). Recent environmental monitoring studies in North Carolina and the Netherlands have reported elevated levels of HFPO-DA, among other PFAS, in air, groundwater, and surface water sampled within the proximity of manufacturing sites and in drinking water originating from contaminated surface sources (Gebbink et al. 2017; McCord et al. 2018; Strynar et al. 2015; Sun et al. 2016). Despite the extensive in vivo toxicity research available for PFOS and PFOA, relatively little peer-reviewed experimental data exist for HFPO-DA or the other PFAS analogs that have been recently detected. In addition to peer-reviewed studies (Caverly Rae et al. 2015; Gannon et al. 2016; Rushing et al. 2017; Wang et al. 2017a), guideline registration studies from the manufacturer of HFPO-DA are publicly available (https://hero.epa.gov/hero/index.cfm/project/page/project_id/2627); however, even though in utero exposure to PFOS and other PFAS induced extensive neonatal mortality and reduced offspring body weights in rats, similar studies have not been conducted with HFPO-DA to our knowledge. Overall, the paucity of data has led to calls for coordinated efforts to screen and assess the toxicity of the myriad PFAS currently detected in environmental matrices (Bruton and Blum 2017; Wang et al. 2017b).
PFOS and PFOA are known activators of peroxisome proliferator-activated receptors (PPARs), primarily alpha () and gamma () (Vanden Heuvel et al. 2006). HFPO-DA is hypothesized to activate PPARs based on observed up-regulation of PPAR-signaling pathway genes (Wang et al. 2017a), increased markers of liver peroxisome proliferation (DuPont 2008a, 2008b; Rushing et al. 2017), and increased liver weight in mice and/or rats (Caverly Rae et al. 2015; DuPont 2008a, 2008b; Rushing et al. 2017; Wang et al. 2017a). Some phthalate ester metabolites are also PPAR activators (Lapinskas et al. 2005) and in utero exposure reduces gene expression of steroidogenic enzymes and decreases production of testosterone in the testes of male offspring, leading to reproductive tract malformations in rats (Hannas et al. 2011; Mylchreest et al. 2002; Parks et al. 2000; Wilson et al. 2004b). Similarly, Zhao et al. (2014) reported that PFOS reduced testosterone production and impaired fetal rat Leydig cells following in utero exposure. The specific molecular initiating event(s) (MIE) by which PFOS and some phthalate esters produce male reproductive toxicity remain(s) elusive; however, it has been proposed that activation of PPAR, specifically , plays an essential role (Corton and Lapinskas 2005; Gazouli et al. 2002; Nepelska et al. 2015). If this MIE is truly responsible for the anti-androgenic effects of phthalates, then oral exposure to other proposed PPAR agonists, such as HFPO-DA, would be expected to reduce male testis testosterone production in utero and cause male rat reproductive tract malformations, similar to the active phthalates.
In regard to the above concerns, there were two goals for the present study. First, we were interested in identifying whether HFPO-DA, like other PFAS, activates PPAR signaling pathways and, if so, does this lead to a reduction in fetal testis testosterone production resulting in the subsequent increase in the incidence/severity of male reproductive defects. Second, we wanted to leverage these experiments to provide additional relevant in vivo data on the potential for gestational oral HFPO-DA exposure to produce toxic effects in the mother or offspring. We conducted studies with pregnant rats dosed during the specific gestational window critical to masculinization of the male fetal reproductive tract [gestation days (GD) 14–18] (Carruthers and Foster 2005). We evaluated and report on a range of effects primarily related to the maternal and fetal livers, circulating maternal thyroid hormones and lipids, and a single-dose level pilot study on postnatal development. Further, because of prior conflicting reports on the endocrine receptor activity of PFAS and the potential relevance to mammalian reproductive development, we assessed the estrogen, androgen, and glucocorticoid receptor activity (agonism/antagonism) of HFPO-DA using in vitro transcriptional activation assays.
Methods
Dosing Solutions
Dosing solutions were prepared using high-performance liquid chromatography-grade water purchased from Honeywell Research Chemicals and HFPO-DA ammonium salt (CAS: 62037-80-3; Product No.: 2122-3-09; Lot: 00005383) purchased from SynQuest Laboratories. HFPO-DA purity was 100% as determined by the supplier via perchloric acid titration. Dosing was administered once daily via oral gavage at body weight across a range of HFPO-DA/kg-body weight per day (specific doses for different studies reported below). Doses were selected based on data from existing developmental toxicity studies on HFPO-DA in Sprague-Dawley rats. A published study by Caverly Rae et al. (2015) reported was a no observed adverse effect level (NOAEL) and was an upper dose that was tolerated in the rat. Further, an industry guideline prenatal developmental toxicity study by DuPont (2010) reported a NOAEL of and that was overtly toxic to the dam. The doses utilized in the present experiments were chosen to evaluate the reported NOAELs and allow for full dose–response assessment while avoiding overt maternal toxicity at highly elevated doses.
Animals
Time-mated Sprague-Dawley rats [Crl:CD(SD)], approximately 90 d of age, were purchased from Charles River Laboratories and shipped to the National Health and Environmental Effects Research Laboratory at the U.S. EPA in Research Triangle Park, North Carolina, on GD2 (; ). Dams and their offspring were housed individually in clear polycarbonate cages () with heat-treated, laboratory-grade pine shavings and fed NIH07 rodent diet and filtered () municipal tap water ad libitum. Dams were weight-ranked and stratified then randomly assigned to treatment groups to produce similar mean weights and variances. This study was conducted in accordance with a protocol approved by the U.S. EPA National Health and Environmental Effects Research Laboratory’s Institutional Animal Care and Use Committee. Animals were housed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care and maintained at 20–22°C, 45–55% humidity, and a 12:12 h photoperiod (lights off at 1800 hours).
Evaluation of Fetal and Maternal Effects during Gestation
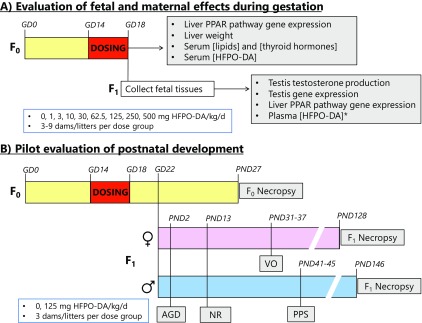
A total of three blocks of 15 dams per block were dosed once daily from GD14–18 with either water vehicle (control) or HFPO-DA to evaluate fetal and maternal effects (Figure 1A). The first block of dams was dosed with control, 62.5, 125, 250, or HFPO-DA ( dams for each). The second and third blocks of dams were dosed with control, 1, 3, 10, or HFPO-DA ( per dose per block). Total sample sizes were for control, for 1, 3, 10, , and for 62.5, 125, 250, and HFPO-DA. In the first two blocks, spanning the entire dose range, we evaluated fetal testis testosterone production, fetal testis gene expression, fetal and maternal liver gene expression, fetal body weight, and maternal serum thyroid hormone and lipid concentrations. In the third block, encompassing the lower dose range utilized here, we collected fetal plasma for measuring HFPO-DA concentrations. Across all three blocks we evaluated maternal weight gain during dosing, reproductive output (number of fetuses and resorptions), maternal serum HFPO-DA concentration, and maternal liver weight at necropsy.
Figure 1.
Schematic diagram of study designs for evaluating maternal, fetal, and postnatal effects of oral gestational hexafluoropropylene oxide dimer acid (HFPO-DA) exposure. Both (A) fetal and (B) postnatal study designs used oral gavage dosing from gestation day (GD) 14–18 at the indicated exposure levels. Fetal plasma HFPO-DA concentration (*) was only evaluated at doses of . AGD, anogenital distance; NR, nipple retention; PND, postnatal day; PPAR, peroxisome proliferator-activated receptor; PPS, preputial separation; VO, vaginal opening.
For the first two blocks, spanning the full dose range, late gestation (GD18) dams were euthanized by decapitation at after the final oral dose [ hours Eastern Standard Time (EST)]. Trunk blood was collected and serum isolated via centrifugation ( for 15 min at 4°C) in vacutainer tubes, transferred to microcentrifuge tubes and stored at . Dam liver weight was recorded and a sample of liver tissue was collected into a polypropylene microcentrifuge tube containing TRIzol Reagent (Invitrogen) on ice. Fetuses were removed and two randomly selected fetuses per litter were weighed. Fetal testes were collected from all male pups with a single testis from the first three males used for determination of ex vivo testosterone production and the remaining testes were homogenized and preserved in TRIzol Reagent for gene expression analysis. The liver was collected from a single, randomly selected fetus per dam/litter for gene expression analysis and transferred to a polypropylene microcentrifuge tube containing TRIzol Reagent (Invitrogen) on ice. Both dam and fetal liver samples were individually homogenized using a Bullet Blender (Next Advance) with zirconium oxide beads, transferred to clean tubes, and stored at prior to RNA extraction (see below). Ex vivo fetal testis testosterone production was measured as previously reported (Wilson et al. 2004b) except the radioimmunoassay (RIA) utilized here was supplied by ALPCO (Catalog No. 72-TESTO-CT2, ALPCO). Briefly, one testis was isolated from each of three separate male fetuses in each litter and incubated in a humidified atmosphere at 37°C for 3 h in of M-199 media (phenol red–free; Hazelton Biologics, Inc.) supplemented with 10% dextran-coated charcoal-stripped fetal bovine serum (Hyclone Laboratories) in 24-well plates under gentle agitation. After incubation, media were removed and stored in siliconized microcentrifuge tubes at until RIA analyses, which were performed according to manufacturer specifications.
Gene expression in fetal testes and fetal/maternal livers was assessed using reverse transcriptase real-time PCR of cDNA synthesized from RNA extracted from sample homogenates. RNA extraction was conducted according to TRIzol Reagent manufacturer specifications using chloroform and isopropanol. Following extraction, RNA was purified using the RNeasy Mini Kit (Catalog No. 74104; Qiagen). RNA concentration and purity (260:280 ratio ) were determined with a NanoDrop 2000 spectrophotometer (Thermo Scientific). For the fetal testes, a 96-well gene array plate was previously custom designed to contain 89 target genes and 3 housekeeping genes, an intra-assay control, a genomic DNA control, a reverse transcriptase control, and a positive PCR control [see Table S1; SABioscience; (Hannas et al. 2012)]. For the fetal and maternal livers, we utilized the Profiler PCR Array for Rat PPAR Targets by Qiagen (Catalog No. 330231 PARN-149Z), which contains 84 target genes relevant to , , and signaling pathways and 5 potential housekeeping genes (see Table S2). PCR reactions were run using RT2 SYBR Green quantitative PCR (qPCR) Master Mix (SABioscience) on an iCycler iQ Real-Time Detection System (Bio-Rad) for fetal testes and on a CFX96 Touch Real-Time Detection System (Bio-Rad) for maternal and fetal livers.
For the third block, dosed with the lower dose range ( HFPO-DA), late gestation (GD18) dams were euthanized by decapitation after the final dose, liver weight was recorded, and trunk blood was collected for serum isolation. Serum was isolated from trunk blood via centrifugation (; 15 min; 4°C) using Becton Dickinson vacutainer tubes and stored in siliconized microcentrifuge tubes at for future analyses. Fetuses were removed and fetal blood was collected from the jugular vein from all fetuses within a litter using heparinized glass capillary tubes. Blood was expelled from capillary tubes using fine-tip disposable transfer pipets into a microcentrifuge tube forming a single composite sample per litter. Fetal blood was then centrifuged at for 15 min at 4°C and plasma was transferred to clean tubes and frozen at .
Maternal sera from all three blocks and fetal plasma from the third block were analyzed for HFPO-DA concentrations similar to previously reported methods (McCord et al. 2018; Reiner et al. 2009; Rushing et al. 2017). Serum or plasma samples () were denatured using formic acid (FA) followed by a cold () acetonitrile (ACN) protein crash. The volumes of FA and ACN varied based on the anticipated concentrations of HFPO-DA in the sample ( FA ACN; FA ACN; FA added, then subsamples removed and crashed with cold ACN). Samples were vortex mixed after FA and ACN additions then centrifuged at for 5 min and the supernatant removed. Sample extracts were separated using a Waters ACQUITY ultra performance liquid chromatograph (UPLC) (Waters Corporation) fitted with a Waters ACQUITY UPLC BEH C18 column (; ; ). Detection was performed using a Waters Quattro Premier XE tandem quadrupole mass spectrometer in negative ionization mode. A stable isotope of HFPO-DA (, Wellington Laboratories) was used as an internal standard for quantitation. Separate calibration curves were prepared for the ranges , , and to account for expected concentration differences between control, offspring (fetus/pup), and dam concentrations across the dose range tested.
Maternal serum samples from the first two blocks were analyzed for thyroid hormones and a standard lipid panel. Total triiodothyronine () and thyroxine () were quantified by radioimmunoassay (RIA) according to manufacturer specifications (IVD Technologies). Thyroid hormone samples were run in duplicate (mean intra-assay coefficient of variation 15.5% for , 11.5% for ), and two calibration standards were run as unknowns with observed concentrations varying from expected by for and for . Thyroid hormone RIA values were considered below detection when specific binding () was ( for and for ) (Sui and Gilbert 2003). Serum total cholesterol, high-density lipoproteins (HDL), low-density lipoproteins (LDL), and triglycerides were quantified using a Beckman Coulter AU480 clinical chemistry analyzer (Beckman Coulter, Inc.) as per manufacturer’s protocol. All reagents were obtained from the instrument manufacturer except for the LDL assay, which was obtained from Diazyme Laboratories.
Pilot Evaluation of Postnatal Development
A single-dose level pilot study utilizing time-mated SD rats was conducted to examine the potential postnatal effects of in utero exposure to HFPO-DA from a similar dosing interval to the fetal studies (Figure 1B). The study consisted of dams exposed to oral daily dosing with either water vehicle or HFPO-DA ( for each) from GD14–18. This dose was selected because it was the highest dose level that did not significantly reduce maternal weight gain during dosing from the fetal evaluation studies. Dams gave birth naturally beginning on the morning of GD22 [i.e., postnatal day (PND) 0]. On PND2 all pups were sexed, weighed, and anogenital distance (AGD) was measured using a Leica MZ6 stereomicroscope (Leica Microsystems) fitted with an ocular micrometer. On PND13, the offspring were sexed, weighed, and evaluated for retention of female-like nipples/areolae. On PND27, the dams were euthanized, uterine implantation sites were scored, pups were weaned to two animals per cage by sex and treatment group, and food was changed to NTP2000 rodent diet. Beginning on PND31 for female offspring and PND41 for male offspring, individuals were evaluated daily for markers of pubertal onset, vaginal opening (VO) for females and balano-preputial separation (BPS) for males.
Beginning at PND128, adult F1 females were weighed, euthanized via decapitation, and examined via necropsy for any reproductive tract malformations and tissue weights were collected for uterus, paired ovaries, liver, paired kidneys, and visceral adipose tissue. Similarly, beginning at PND146 adult F1 males were weighed, euthanized, and examined for reproductive tract malformations and weights were collected for all relevant reproductive tissues. Male necropsy included weights of glans penis, ventral prostate, paired seminal vesicles, paired testes, paired epididymides, levator ani–bulbocavernosus (LABC), paired bulbourethral (Cowper’s) glands, paired kidneys, visceral adipose tissue, and epididymal adipose tissue. After weighing, the left epididymis was separated into two sections, the cauda and the corpus plus caput, and individually minced in M-199 media. Total sperm counts in epididymal sections were measured using a Multisizer 3 Coulter counter (Beckman Coulter).
In Vitro Transcriptional Activation Assays
HFPO-DA was assessed for agonism and antagonism of transcriptional activation for estrogen (ER), androgen (AR), and glucocorticoid receptors (GR). Method details for in vitro transactivation assays for ER (Wilson et al. 2004a), AR (Hartig et al. 2002, 2007), and GR (Conley et al. 2017; Medlock Kakaley et al. 2018) have been previously reported. Briefly, for ER activity we utilized the stably transfected T47D-KBluc cell line [publicly available via American Type Culture Collection (ATCC); CRL-2865] according to protocols provided by ATCC with the modification of Dulbecco’s Modified Eagle Media (DMEM) as the cell culture media instead of Roswell Park Memorial Institute (RPMI) media. We utilized adenoviral transduction to introduce chimp AR (Ad5chAR-g) (Hartig et al. 2007) or human GR (Ad/GR4) (Shih et al. 1991) and a luciferase-based promoter-reporter construct (MMTV-Luc; Ad/mLuc7) (Shih et al. 1991) into CV-1 cells (ATCC CCL-70) to assess GR and AR activity, respectively. For viral transduction, cells were grown to confluence in Petri dishes in 10% dextran-coated charcoal-treated fetal bovine serum RPMI-1640 growth media. Confluent cells were split at a ratio of 1:3 into dishes and inoculated on day 7 () with adenoviral vectors at multiplicities of infection of 1 receptor to 50 reporter constructs. After 24 h incubation with adenoviral vectors, cells were rinsed, resuspended in media, and seeded into assay plates. All assays were run in 96-well plates and luminescence was detected using a BMG Fluostar Omega luminometer (BMG Labtech) following 24-h exposure. HFPO-DA was tested for receptor agonism and antagonism at 10-fold concentration intervals from to (ER) or to (AR and GR). For ER activity, the reference agonist was [(E2) CAS: 50-28-2] and the reference antagonist was ICI-182780 (CAS: 129453-61-8). When assessing ER antagonism, HFPO-DA was competed against E2. For AR activity the reference agonist was dihydrotestosterone [(DHT) CAS: 521-18-6] and the reference antagonist was hydroxyflutamide (CAS: 52806-53-8). When assessing AR antagonism, HFPO-DA was competed against DHT. For GR activity, the reference agonist was dexamethasone [(Dex) CAS: 50-02-2] and the reference antagonist was mifepristone (CAS: 84,371-65-3). When assessing GR antagonism, HFPO-DA was competed against Dex. Cellular cytotoxicity across the dosing range was determined for CV-1 cells utilizing the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye (Mosmann 1983). HFPO-DA was analyzed using biological replicate assay plates (i.e., unique cell passages) with four technical replicates per treatment per plate.
Data Analyses
All values are reported as standard error (SE) and all statistical comparisons were conducted at significance level except for PPAR pathway gene expression, which utilized to detect highly significant analysis of variance (ANOVA) results and to determine pairwise differences of treatment as compared with controls for significant genes. Treatment effects as compared with control were identified using ANOVA in SAS (version 9.4; SAS Institute). Fetal and postnatal data were analyzed using PROC MIXED to correct for the nested effects of individuals within litters (fetus/pup data nested within litter, litter as random variable); dam data were analyzed using PROC GLM. Pairwise comparison of significant ANOVA results was performed using the least squares means (LSMEANS) procedure in SAS. GraphPad Prism (version 7.02; GraphPad, Inc.) was used to generate all figures and to conduct dose–response curve analyses.
Fetal testis and maternal/fetal liver gene expression data were analyzed using the comparative cycle threshold () method. Briefly, delta values were calculated using the equation and normalized to the mean value of the appropriate housekeeping genes. We selected housekeeping genes for each tissue and gene array that did not display a significant (ANOVA ) treatment effect of HFPO-DA exposure (; ; and ). Delta values were then converted to fold-induction by dividing the treated replicate delta by the mean delta of the control replicates for each gene. Fold-induction values were then then -transformed prior to ANOVA.
Fetal testis testosterone production was normalized to the mean control concentration within a given block and analyzed as percentage of control values across blocks. Maternal liver weight was analyzed using body weight as a covariate within PROC GLM followed by pairwise comparison using LSMEANS, this analysis produces linear regressions of body weight versus liver weight for each dose group. Mean female AGD was subtracted from individual male AGD measures to calculate percentage reduction as compared with control.
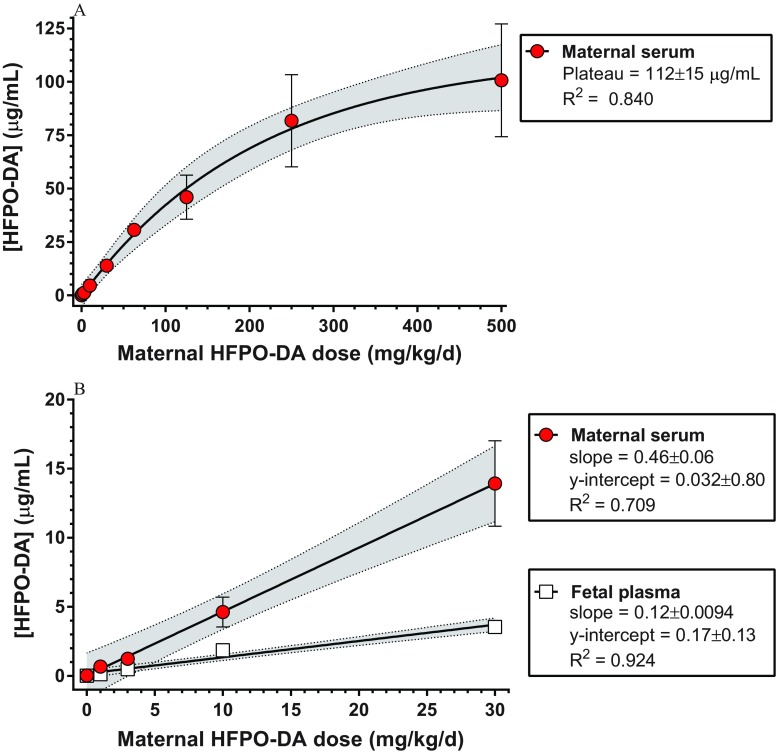
Serum HFPO-DA concentrations in the mother and the fetus were analyzed as a function of oral dose administered to the mother. We utilized nonlinear regression (exponential one-phase association) to describe the increase and saturation of serum HFPO-DA concentrations across the full oral dose range () for maternal serum. Fetal plasma HFPO-DA concentrations were only analyzed in the low-dose range (), which was better described using a linear uptake model. We compared the slopes of the low-dose linear regressions for maternal serum and fetal plasma HFPO-DA concentrations using GraphPad Prism.
Dose–response analyses for the in vitro transactivation assay data and the most sensitive in vivo end points and were conducted using four-parameter logistic regression in GraphPad Prism (constraint to , ). In vitro luminescence data was normalized to background (vehicle control), transformed, and converted to percentage maximum response based on saturating levels of reference agonist. In vivo data were modeled as a function of -transformed internal dose (i.e., dam serum HFPO-DA concentration from GD18), and response data was normalized to control and presented as a percentage. We estimated effect concentrations equivalent to a 5% deviation from control (). Reduction in maternal serum concentration was modeled by ascribing a concentration of one-half of the detection limit (i.e., ; detection limit of ) for the dose groups that were below the detection limit.
Maternal rat serum concentrations were compared with human plasma concentrations from workers in a HFPO-DA manufacturing facility in Dordrecht, Netherlands (DuPont 2017). Human plasma samples represented workers who volunteered to participate in the study with the goal of determining whether there were measurable quantities of HFPO-DA in their blood. Some of the workers were in areas with potential for exposure and others were not (17/24 participants had detectable HFPO-DA levels). Comparisons were made in order to determine how the doses used in the current study relate to likely “worst case” human concentrations based on internal exposure levels rather than comparing exposures across species based upon estimated external dose levels. We calculated the margin of internal exposure (MOIE) as a ratio of maternal rat serum concentration to human plasma concentration for each of the 17 workers with detectable levels (Bessems et al. 2017). MOIEs were calculated using the mean maternal rat serum HFPO-DA concentration from the 1- and dose levels because these represented the lowest oral dose administered and the administered oral dose for the pilot postnatal study.
Results
Fetal Effects from GD14–18 Dosing
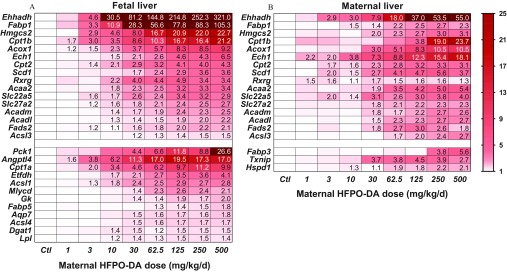
Fetal livers from HFPO-DA–exposed litters displayed highly significant (ANOVA ), dose–responsive up-regulation of 28 different genes in the PPAR signaling pathway arrays (Figure 2A; see also Table S3). Most affected genes were associated with fatty acid metabolism (Acaa2, Acadl, Acadm, Acox1, Acsl1, Acsl3, Acsl4, Cpt1a, Cpt1b, Cpt2, Ehhadh, Etfdh, Fads2, Fabp1, Gk, Hmgcs2, Mlycd, and Scd1). Remaining up-regulated genes were associated with lipid transport (Angptl4, Dgat1, Lpl), adipogenesis (Ech1, Lpl), water transport (Aqp7), insulin signaling (Cpt1a, Dgat1, Pck1), PPAR transcription factors (Rxrg), or PPAR ligand transporters (Fabp1, Fabp5, Slc22a5, Slc27a2). The most highly up-regulated genes included Ehhadh (321-fold), Fabp1 (105-fold), Pck1 (27-fold), Hmgcs2 (23-fold), Cpt1b (21-fold), and Angptl4 (17-fold). Several genes were significantly () up-regulated even at the lowest dose level tested () including Cpt1b, Angptl4, and Acox1.
Figure 2.
Expression of significantly up-regulated genes (ANOVA, ) from peroxisome proliferator-activated receptor (PPAR) signaling pathway gene arrays in (A) fetal ( for control, for treated) and (B) maternal ( for control, for treated) livers following gestation day (GD) 14–18 oral maternal exposure to hexafluoropropylene oxide dimer acid (HFPO-DA). Upper portions (above break) display significantly altered genes common to both fetal and maternal livers, lower portions display genes differentially altered between fetal and maternal livers. Cell values represent significant () dose-level fold-induction values relative to control livers [cells with no value were not significantly different from control (see Table S2 for gene descriptions, and Tables S3 and S6 for complete gene expression data)]. Legend indicates fold-induction compared with control with darker shaded genes more highly expressed. Genes with fold-induction of control were beyond the scale of the legend. Ctl, control.
In contrast to the observed changes in fetal PPAR liver genes, the results for the expression of genes from our custom array for detecting phthalate-like effects in the fetal testis were not significantly different from controls (see Table S4). Further, fetal testis testosterone production was not significantly different from controls at any dose (see Figure S1, Table S5).
Maternal Effects from GD14–18 Dosing
Similar to fetal livers, maternal livers displayed highly up-regulated expression of PPAR signaling pathway–associated genes (Figure 2B; see also Table S6). Overall, the maternal and fetal livers shared up-regulation of 16 genes. The majority of shared, up-regulated genes were associated with fatty acid metabolism (Acaa2, Acadl, Acadm, Acox1, Acsl3, Cpt1b, Cpt2, Ehhadh, Fads2, Fabp1, Hmgcs2, and Scd1). Also similar to the fetal liver, the remaining up-regulated maternal genes were associated with adipogenesis (Ech1), PPAR transcription factors (Rxrg), or PPAR ligand transporters (Slc22a5, Slc27a2). In contrast to the fetal liver, the maternal livers of treated rats did not differ significantly from controls in the expression of Acsl1, Acsl4, Angptl4, Aqp7, Cpt1a, Dgat1, Etfdh, Fabp5, Gk, Lpl, Mlycd, or Pck1; whereas 2 genes associated with cell proliferation (Hspd1, Txnip) and 1 with fatty acid metabolism (Fabp3) were significantly up-regulated in the maternal liver but not the fetal liver. Further, the maternal and fetal livers shared the most highly up-regulated gene (Ehhadh; 55-fold in maternal liver) and both had highly up-regulated Cpt1b expression (24-fold in maternal liver). Only 1 of the shared genes was noticeably more highly up-regulated in the maternal liver than the fetal liver (Ech1; 18-fold vs. 6-fold in maternal and fetal livers, respectively). Overall, the PPAR signaling pathway was up-regulated in both maternal and fetal livers, with both sharing many of the same up-regulated genes; however, the overall profiles of induction were noticeably different between the two life stages, with the fetal liver seemingly displaying greater sensitivity both in terms of the number of genes affected and the degree of up-regulation.
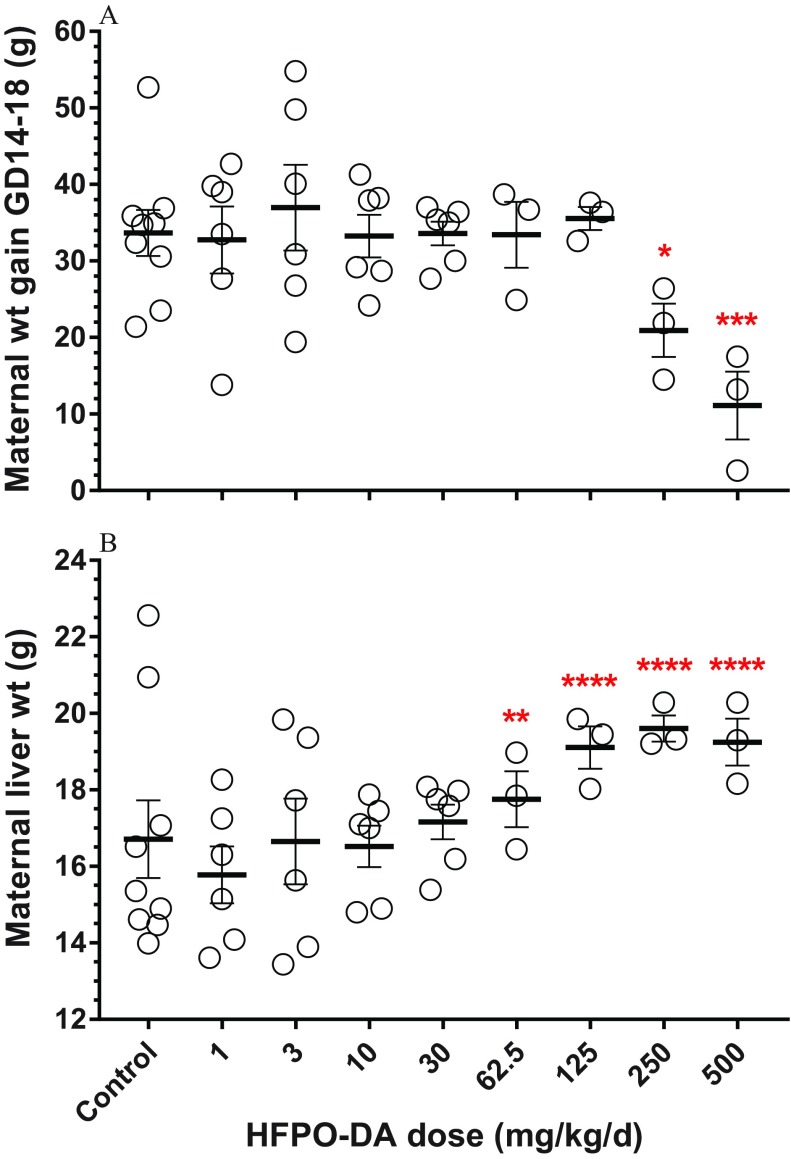
During the GD14–18 dosing window, dams had significantly less body weight gain at the 250- and dose levels compared with controls (ANOVA ; Figure 3A; see also Table S5). On GD18, dams had significantly higher liver weights in the dose groups than controls (ANOVA ; Figure 3B; see also Table S5). There were no significant differences in numbers of live pups, resorptions, or fetal body weight compared with controls (see Table S5).
Figure 3.
(A) Maternal body weight gain during gestation day (GD)14–18 dosing period and (B) maternal liver weight on GD18. Data points represent individual replicates (control, ; , ; , ), bars and whiskers represent standard error, and asterisks represent significant differences compared with control values (*, ; **, ; ***, ; ****, ). Statistical significance was determined using analysis of variance; for liver weight analysis, body weight was included as a covariate.
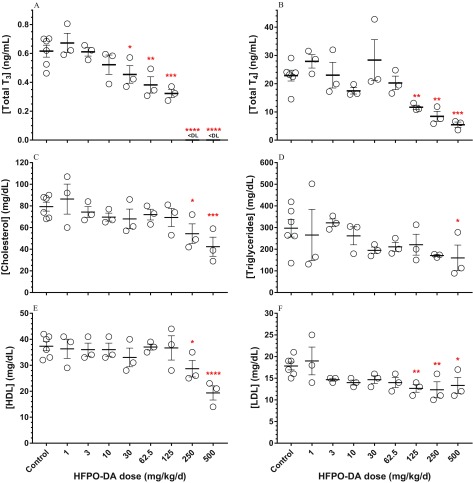
Maternal serum samples displayed dose–responsive decreases in all measures of thyroid hormones and lipids (Figure 4; see also Table S5). Serum triglycerides were significantly lower at , cholesterol and HDL were significantly lower at 250 and , and total and LDL were significantly lower at . The most sensitive end point was serum total , which was significantly lower at and below assay detection levels (i.e., ) in the top two dose levels.
Figure 4.
Concentrations of (A) total triiodothyronine (), (B) total thyroxine (), and lipids [(C) cholesterol, (D) triglycerides, (E) high-density lipoproteins (HDL), and (F) low-density lipoproteins (LDL)] in maternal serum following oral hexafluoropropylene oxide dimer acid (HFPO-DA) dosing from gestation days (GD) 14–18. Dam serum was collected on GD18 approximately 2 h after final oral dose. Data points represent individual replicates (control, ; treated, ), bars and whiskers represent standard error, and asterisks represent significant differences compared with control values using analysis of variance (*, ; **, ; ***, ; ****, ). , values below radioimmunoassay detection limit.
Postnatal Effects from GD14–18 Dosing
In the HFPO-DA pilot postnatal study that utilized GD14–18 dosing, one of three control dams was not pregnant, reducing the sample size to . Control dams and dams dosed with HFPO-DA gave birth to litters with equal numbers of viable pups. On a litter means basis, there were no significant differences for any end point measured through the onset of puberty (see Table S7). On an individual pup basis (as opposed to litter means), female offspring body weight was significantly lower than controls at multiple time points (PND2, PND27, and at VO), indicating a potential trend in growth deficit to investigate in future studies.
Adult males at necropsy had significantly lower tissue weight of the right epididymis on a litter means basis, but no other tissues were affected as compared with controls (see Table S8). On an individual basis, treated male rats had significantly lower tissue weights of the right testis, left testis, paired testes, right epididymis, left epididymis, paired epididymides, and epididymal adipose tissue as compared with controls.
Adult females at necropsy displayed no significant differences in any end point as compared with controls on a litter means basis (see Table S9). On an individual basis, treated female rats had significantly smaller AGD and lower liver weight as compared with controls.
HFPO-DA Concentrations in Maternal Serum and Fetal Plasma
Maternal serum and fetal plasma contained increasing concentrations of HFPO-DA as a function of oral dose following dosing during the GD14–18 experimental window (Figure 5; see also Table S10). Over the full maternal dose range (), uptake appeared to saturate at the higher dose levels and was modeled using exponential one-phase association () with a plateau of (Figure 5A). In the lower dose range (), increases in maternal serum and fetal plasma HFPO-DA concentrations were linear (Figure 5B); however, the maternal slope was significantly greater than the fetal slope with maternal serum HFPO-DA increasing and fetal plasma HFPO-DA concentration increasing for each increase in oral maternal dose ().
Figure 5.
Maternal serum and fetal plasma hexafluoropropylene oxide dimer acid (HFPO-DA) concentrations ( standard error, ; see Table S10) as a function of oral dose following maternal exposure from gestation day (GD) 14–18. Samples were collected on GD18 approximately 2 h after final oral dose. (A) Full maternal dose range modeled using exponential one-phase association and (B) low dose range modeled using linear regression (95% confidence intervals shaded). Fetal plasma was collected only from the low dose range ().
Dose–Response Analyses
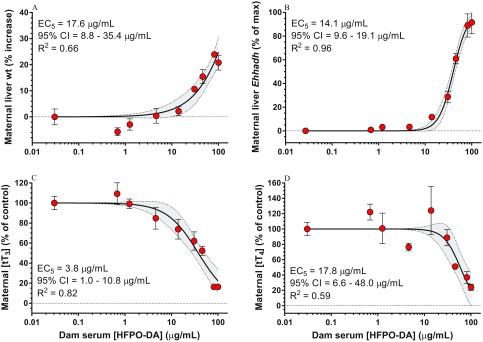
Using maternal serum HFPO-DA concentrations, we estimated effect concentrations for an for the most sensitive end points: maternal liver weight, maternal liver gene expression, and maternal serum [] and [] (Figure 6). Maternal [] was the most sensitive end point with an of (estimated maternal oral dose of using the linear equation from Figure 5) followed by liver Ehhadh expression (), liver weight (), and [] ().
Figure 6.
Dose–response curves (four-parameter logistic regression) and 5% effect estimates [ with 95% confidence intervals (CIs)] for the most sensitive end points [(A) maternal liver weight, (B) maternal liver Ehhadh gene expression, (C) maternal serum total triiodothyronine , and (D) total thyroxine ] as a function of maternal serum hexafluoropropylene oxide dimer acid (HFPO-DA) concentration. Dam serum HFPO-DA concentrations represent those measured on gestation day (GD)18 following GD14–18 dosing. Data points represent standard error, (A) control , , ; (B–D) control, ; treated, .
Comparison of Maternal Rat and Human Internal Exposure Levels
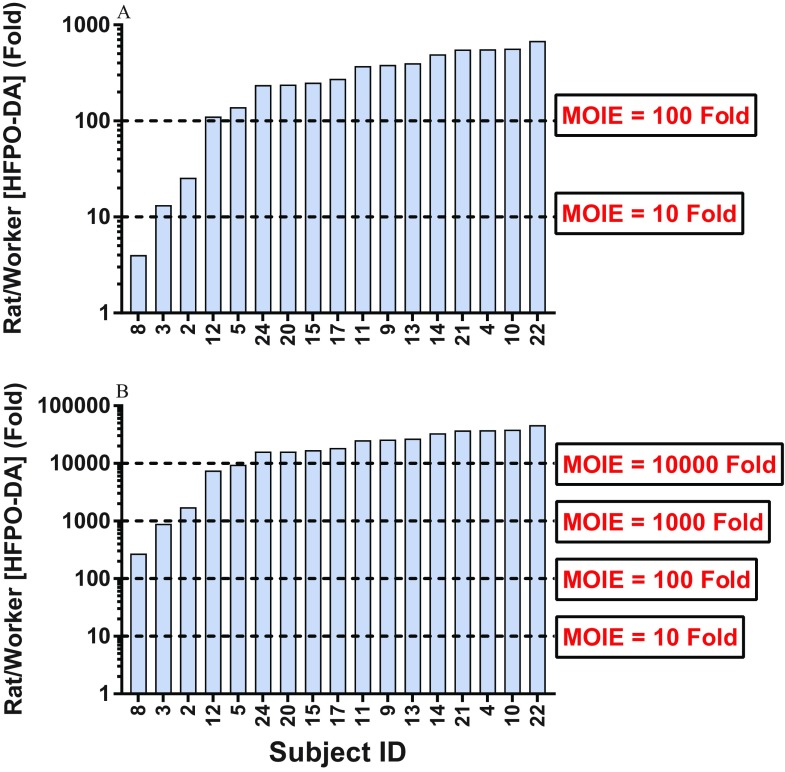
The human worker HFPO-DA plasma concentrations reported by Dupont (2017) ranged from , whereas the mean maternal rat serum concentrations reported here ranged from following a 5-d exposure. At the lowest dose level tested here (), the rat:human MOIEs ranged from 4 to 566 (14/17 MOIEs were ; Figure 7A). Further, at the dose utilized in the postnatal pilot study (), the rat:human MOIEs ranged from 272 to 38,333 (15/17 MOIEs were and 12/17 MOIEs were ; Figure 7B). It is important to note that the maternal rat serum concentrations utilized in this comparison were from short-term (5-d) exposures, whereas the human plasma concentrations were from individuals working in an HFPO-DA manufacturing facility and likely represent chronic exposure levels, but it is unknown whether these concentrations represent a steady state.
Figure 7.
Comparison of mean maternal Sprague-Dawley rat serum hexafluoropropylene oxide dimer acid (HFPO-DA) concentration from (A) 1- and (B) 125-mg/kg per day exposure groups and individual human plasma HFPO-DA concentrations from workers in an HFPO-DA manufacturing facility in the Netherlands (DuPont 2017). Horizontal lines indicate various margins of internal exposure (MOIE) levels as compared with individual worker plasma concentrations.
In Vitro Nuclear Receptor Transactivation
HFPO-DA did not display any estrogenic activity (agonism or antagonism) at concentrations ranging from (see Figure S2). Further, there was no androgen or glucocorticoid receptor agonism at concentrations ranging from . At the very highest dose tested (), which approached the cytotoxic dose of , HFPO-DA exposure did result in a slight glucocorticoid receptor antagonism ( reduction in luciferase expression) and a moderate androgen receptor antagonism ( reduction).
Discussion
The range of adverse effects resulting from oral maternal HFPO-DA exposure reported here are consistent with limited data available for HFPO-DA (Caverly Rae et al. 2015; DuPont 2008a, 2010; Gannon et al. 2016; Rushing et al. 2017; Wang et al. 2017a) and the extensive toxicity literature available for other PFAS, notably PFOS and PFOA [reviewed by ATSDR (2018), ECHA (2014), OECD (2002) and U.S. EPA (2016a)]. We observed up-regulation of genes associated with PPAR signaling pathways, maternal hepatomegaly, reductions in maternal serum lipids and thyroid hormones, and indications of reduced body and tissue weights in F1 animals. All of these effects have been observed following maternal exposure to PFOS/PFOA in laboratory animals and several have been previously observed for HFPO-DA. However, despite extensive PPAR pathway up-regulation, HFPO-DA did not produce any effects that are hallmarks of phthalate syndrome, including reduced fetal testis testosterone production, phthalate-specific fetal testis gene expression changes, reduced AGD on PND2, or male reproductive malformations. This lends support to the hypothesis that the effects of phthalates on male reproductive development are not mediated via the PPAR pathway.
The specific dosing interval utilized in developmental toxicity studies with PFAS is a critical factor for the types of effects that have been described. Grasty et al. (2003) reported significantly increased neonatal mortality and reduced pup weight in Sprague-Dawley rats following gestational PFOS exposure at across a range of 4-d dosing windows. These effects increased in severity as the dosing window moved later in gestation. Further, it was demonstrated that dosing only on GD19–20 was sufficient to produce these effects. Subsequent studies that included dosing during the full gestational period also reported pup mortality and reduced pup body weight. Lau et al. (2003) examined PFOS exposure in the rat and reported significantly increased neonatal mortality shortly after birth () at . Separate studies in Sprague-Dawley rats confirmed the neonatal mortality following gestational exposure to PFOS at (Luebker et al. 2005a, 2005b). Similar results have been reported with other PFAS, primarily PFOA, and in other species, including mice and cynomolgus monkeys [reviewed by Abbott (2015) and Lau et al. (2007)]. In the pilot postnatal study presented here, there was an indication of decreased female pup weight but no effect on pup survival following HFPO-DA exposure from GD14–18 at a relatively high dose (). However, expanding the dosing timeline to include the entire period of fetal development (i.e., GD8 through parturition) appears to reduce neonatal survival and body weight similar to PFOS exposure but at higher oral maternal doses [J.M. Conley and L.E. Gray (personal communication)].
As mentioned above, female pup body weight in the HFPO-DA dose group was significantly lower, on an individual analysis basis, 2 d after birth compared with control animals. Previous studies with laboratory rats have reported stunted growth of surviving pups following PFOS exposure. Lau et al. (2003) reported that pups exposed in utero to PFOS at displayed lower body weights, and Luebker et al. (2005b) reported the same response in all dose levels tested (i.e., ). Overall, reduced pup weight appears to be one of the most sensitive end points in in utero PFAS studies. This effect aligns with multiple epidemiological studies, indicating a negative association between human birth weight and concentrations of PFOS/PFOA [reviewed by Bach et al. (2015) and Negri et al. (2017)] and should be more extensively evaluated for HFPO-DA exposure.
PFAS are known to primarily activate , particularly in the mammalian liver, however other receptors, such as , have also been shown to be activated (Vanden Heuvel et al. 2006). Although the biological significance of induction of PPAR pathway gene expression is not known, it was overall the most sensitive end point in the present studies. Even at the lowest dose tested (), the fetal liver displayed multiple significantly up-regulated genes (Cpt1b, Acox1, Angptl4). Bjork et al. (2008) performed a similar experiment with gestational PFOS exposure in the SD rat (exposed to from GD2 to GD20) and identified 445 genes via microarray that were significantly altered in the fetal liver. Four genes associated with fatty acid metabolism were individually verified using qPCR, 3 of which were also identified as significantly up-regulated in the present study (Acox1, Cpt1a, Cpt1b). Further, maternal PPAR pathway gene expression was almost equally as affected as the fetal livers, however with a notably distinct profile. Wang et al. (2017a) reported up-regulation of PPAR pathway genes in mouse liver following HFPO-DA exposure, whereas Hu et al. (2005) and Martin et al. (2007) performed microarray analyses of adult rat liver gene profiles following oral PFOS and PFOA exposure and reported similar up-regulation of clusters of genes primarily associated with lipid homeostasis. The gene expression profiles reported here indicate that HFPO-DA reached the fetal organs and activated nuclear receptor–mediated cell-signaling pathways and that the profile of expression was different than the maternal gene expression profile. However, the findings are not adequate to definitively conclude that a mechanism of action is operative for the HFPO-DA effects observed here.
In addition to changes in PPAR-mediated gene expression in the maternal liver, we observed a number of alterations to maternal serum lipid and thyroid hormone profiles similar to previous PFAS studies. Luebker et al. (2005b) reported significantly reduced serum cholesterol in pregnant SD rats following PFOS exposure, and Martin et al. (2007) also reported significantly reduced serum cholesterol in adult male SD rats following both PFOS and PFOA exposure. Disruption of maternal rat cholesterol synthesis with a HMG-CoA reductase inhibitor in utero has been shown to induce fetal and neonatal death and retard growth in the absence of maternal toxicity (Henck et al. 1998). It is believed that the majority, if not all, of the cholesterol utilized in the earliest stages of fetal development is derived from the mother, prior to the onset of fetal cholesterol synthesis (Baardman et al. 2013). Further, Martin et al. (2007), Thibodeaux et al. (2003), and Yu et al. (2009) reported significant reductions in serum total and for both PFOS and/or PFOA; however, appeared to be more greatly reduced, whereas in the present study was more affected. Maternal thyroid hormones are critical for fetal neurological development because the mother is the primary source of for the developing brain (Morreale de Escobar et al. 2004) and reduced maternal thyroid hormone concentrations are quantitatively linked to reduced fetal concentrations (O’Shaughnessy et al. 2018). Despite the consistency observed across laboratory rat studies, it is unclear how these results relate to human health effects from PFAS exposure because many epidemiological studies report the opposite patterns or equivocal results (Lau et al. 2007; U.S. EPA 2016a).
Gomis et al. (2018) recently reported on the potential discrepancy in toxicity among a range of PFAS when using orally administered dose as compared with internal dose. By accounting for toxicokinetics in rats across multiple PFAS, the toxicity of some fluorinated alternatives appears to be more equitable to the long-chain PFAS when potency is compared based on internal dose. However, it is important to highlight the substantial toxicokinetic differences between PFOS and HFPO-DA in the rat. In the female rat, HFPO-DA has a reported half-life of following oral exposure to (Gannon et al. 2016) and is not expected to accumulate, whereas PFOS has a reported half-life of following oral exposure to (Chang et al. 2012) and does accumulate. Our samples were collected 2 h after the final oral dose, which is just slightly after the peak serum concentration is achieved in the female rat based on the Gomis et al. (2018) model.
In addition to intraspecies differences in PFAS toxicokinetics, it is also important to note that interspecies differences in absorption, distribution, metabolism, and excretion of PFAS are vast, with half-lives and clearance rates of numerous compounds appearing to be significantly longer in humans and nonhuman primates than in rats/mice (Chang et al. 2012; Olsen et al. 2007). The half-life of HFPO-DA in humans is currently unknown; however, similar to the discussion above, internal dosimetry can potentially reduce uncertainty in cross-species hazard assessment. For comparison, we calculated MOIE values for maternal rat serum concentrations versus plasma samples from humans working in a HFPO-DA manufacturing facility in the Dordrecht, Netherlands (DuPont 2017) (Figure 7). Bessems et al. (2017) originally described the use of MOIE as a physiologically based kinetic modeling approach for reducing uncertainty in the safety assessment of human dermal exposures using oral rodent toxicity data. Comparison of MOIE accounts for species- and route-dependent differences in metabolism between humans and research animals. Here, we utilized a similar calculation to reduce the species-to-species variation in PFAS toxicokinetics and to provide context for the oral doses utilized in terms of known human exposure levels. The highest detected plasma concentration from a worker () was 4-fold lower than the mean maternal rat serum HFPO-DA concentration from the lowest dose level () reported here; whereas the same worker concentration was 272-fold below the mean maternal serum concentration from the dose level () used in the pilot postnatal study presented here. Overall, characterizing toxicokinetics and internal dosimetry for PFAS, including HFPO-DA, can facilitate the determination of the relevance of doses in laboratory animals to human exposures, thereby reducing some of the uncertainty in estimating human health risks from exposure.
The HFPO-DA toxicity profile observed here was highly similar to effects observed in peer-reviewed and industry guideline studies for HFPO-DA as well as in studies conducted for PFOS (among other PFAS). PPAR signaling pathways were activated in maternal and fetal livers and may also be activated in other tissues/organs; however, the effects observed are not necessarily exclusive to , or even PPAR signaling in general (Rosen et al. 2017). The GenX chemicals health assessment is currently undergoing independent, external peer-review in the Office of Water (U.S. EPA). Included in that assessment is a summary of available mode-of-action (MOA) information. Although findings in this study are consistent with other agonists (e.g., increases in liver weight, up-regulation of PPAR pathway target genes), data gaps exist for key events and other mechanisms that might be involved, particularly in other tissues besides those like the liver with high levels. Overall, the findings for HFPO-DA are limited and not adequate to support ascribing a MOA to the multitude of effects seen in this study. Due to the reductions in maternal serum thyroid hormones and lipids observed here, and preliminary studies in our lab, an expanded dosing period that includes the entire period of fetal development may lead to effects on fetal and neonatal development similar to those observed with PFOS and PFOA exposure. Extensive research is needed to investigate the mechanism(s) by which HFPO-DA/PFOS/PFOA produce toxicity, to characterize the toxicokinetics for this and other PFAS in order to better predict toxic effects, and to assess the mixture-based effects of exposure to multiple PFAS compounds given their ubiquitous occurrence.
Supplementary Material
Acknowledgments
We thank B. Hannas (Dow Chemical), V. Sutherland (NIEHS), M. Narotsky (U.S. EPA), K. O’Shaughnessy (U.S. EPA), J. Rogers (U.S. EPA), B. Jacobs (U.S. EPA), J. Strong (U.S. EPA), G. Miller (U.S. EPA), and two anonymous reviewers for reviewing earlier drafts. This work was supported by the U.S. EPA Chemical Safety for Sustainability Research Action Program under the Adverse Outcome Pathway Discovery and Development task. B.S.M. and G.S.T. were supported by the Intramural Research Program of the National Institutes of Health/National Institute of Environmental Health Sciences grants ZIAES102505-09 and ZIAES103316-01.
References
- Abbott BD. 2015. Developmental Toxicity. In: Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. DeWitt JC, ed. Cham, Switzerland:Springer International Publishing, 203–218. [Google Scholar]
- Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, et al. 2007. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect 115(11):1670–1676, PMID: 18008002, 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Research). 2018. Toxicological Profile for Perfluoroalkyls: Draft for Public Comment June 2018. https://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=1117&tid=237 [accessed 7 March 2019].
- Awad E, Zhang X, Bhavsar SP, Petro S, Crozier PW, Reiner EJ, et al. 2011. Long-term environmental fate of perfluorinated compounds after accidental release at Toronto airport. Environ Sci Technol 45(19):8081–8089, PMID: 21774496, 10.1021/es2001985. [DOI] [PubMed] [Google Scholar]
- Baardman ME, Kerstjens-Frederikse WS, Berger RMF, Bakker MK, Hofstra RMW, Plösch T. 2013. The role of maternal-fetal cholesterol transport in early fetal life: current insights. Biol Reprod 88(1):24, PMID: 23153566, 10.1095/biolreprod.112.102442. [DOI] [PubMed] [Google Scholar]
- Bach CC, Bech BH, Brix N, Nohr EA, Bonde JP, Henriksen TB. 2015. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit Rev Toxicol 45(1):53–67, PMID: 25372700, 10.3109/10408444.2014.952400. [DOI] [PubMed] [Google Scholar]
- Beekman M, Zweers P, Muller A, de Vries W, Janssen P, Zeilmaker M. 2016. Evaluation of Substances Used in the GenX Technology by Chemours, Dordrecht. Bilthoven, Netherlands:National Institute for Public Health and the Environment Ministry of Health, Welfare and Sport. [Google Scholar]
- Bessems JGM, Paini A, Gajewska M, Worth A. 2017. The margin of internal exposure (MOIE) concept for dermal risk assessment based on oral toxicity data—a case study with caffeine. Toxicology 392:119–129, PMID: 28288858, 10.1016/j.tox.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JA, Lau C, Chang SC, Butenhoff JL, Wallace KB. 2008. Perfluorooctane sulfonate-induced changes in fetal rat liver gene expression. Toxicology 251(1–3):8–20, PMID: 18692542, 10.1016/j.tox.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Bruton TA, Blum A. 2017. Proposal for coordinated health research in PFAS-contaminated communities in the United States. Environ Health 16(1):120, PMID: 29132367, 10.1186/s12940-017-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. 2007. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect 115(11):1596–1602, PMID: 18007991, 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers CM, Foster PM. 2005. Critical window of male reproductive tract development in rats following gestational exposure to di-n-butyl phthalate. Birth Defects Res B Dev Reprod Toxicol 74(3):277–285, PMID: 15954088, 10.1002/bdrb.20050. [DOI] [PubMed] [Google Scholar]
- Caverly Rae JM, Craig L, Slone TW, Frame SR, Buxton LW, Kennedy GL. 2015. Evaluation of chronic toxicity and carcinogenicity of ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in Sprague–Dawley rats. Toxicol Rep 2:939–949, PMID: 28962433, 10.1016/j.toxrep.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-C, Noker PE, Gorman GS, Gibson SJ, Hart JA, Ehresman DJ, et al. 2012. Comparative pharmacokinetics of perfluorooctanesulfonate (PFOS) in rats, mice, and monkeys. Reprod Toxicol 33(4):428–440, PMID: 21889587, 10.1016/j.reprotox.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Conley JM, Evans N, Cardon MC, Rosenblum L, Iwanowicz LR, Hartig PC, et al. 2017. Occurrence and in vitro bioactivity of estrogen, androgen, and glucocorticoid compounds in a nationwide screen of United States stream waters. Environ Sci Technol 51(9):4781–4791, PMID: 28401766, 10.1021/acs.est.6b06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F, Awwad O, Rotondi M, Santini F, Imbriani M, Chiovato L. 2017. Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA). J Endocrinol Invest 40(2):105–121, PMID: 27837466, 10.1007/s40618-016-0572-z. [DOI] [PubMed] [Google Scholar]
- Corton JC, Lapinskas PJ. 2005. Peroxisome proliferator-activated receptors: mediators of phthalate ester-induced effects in the male reproductive tract? Toxicol Sci 83(1):4–17, PMID: 15496498, 10.1093/toxsci/kfi011. [DOI] [PubMed] [Google Scholar]
- DuPont. 2008a. DuPont-24447: A 28-day oral (gavage) toxicity study of H-28397 in rats with a 28-day recovery. Study #WIL-189205. OECD Guideline 407. Conducted by WIL Research Laboratories, LLC. EI du Pont de Nemours and Company 1-4. U.S. EPA HERO ID: 4221045–4221050.
- DuPont. 2008b. DuPont-24459: A 28-day oral (gavage) toxicity study of H-28397 in mice with a 28-day recovery. Study #WIL-189207. OECD Guideline 407. Conducted by WIL Research Laboratories, LLC. EI du Pont de Nemours and Company 1-4. U.S. EPA HERO ID: 4221051–4221054.
- DuPont. 2010. DuPont-18405-841: An oral (gavage) prenatal developmental toxicity study of H-28548 in rats. OECD Guideline 414. EI du Pont de Nemours and Company. U.S. EPA HERO ID: 4222145.
- DuPont. 2017. DuPont-C30031_516655: determination of HFPO-DA in EDTA human plasma samples. Charles River Laboratories. The Chemours Company. U.S. EPA HERO ID: 4353920.
- ECHA (European Chemicals Agency). 2014. Annex XV Restriction Report: Proposal for a Restriction—Perfluorooctanoic acid (PFOA), PFOA Salts and PFOA-Related Substances. Version 1.0. Helsinki, Finland:ECHA. [Google Scholar]
- Gannon SA, Fasano WJ, Mawn MP, Nabb DL, Buck RC, Buxton LW, et al. 2016. Absorption, distribution, metabolism, excretion, and kinetics of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid ammonium salt following a single dose in rat, mouse, and cynomolgus monkey. Toxicology 340:1–9, PMID: 26743852, 10.1016/j.tox.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Gazouli M, Yao ZX, Boujrad R, Corton JC, Culty M, Papadopoulos V. 2002. Effect of peroxisome proliferators on Leydig cell peripheral-type benzodiazepine receptor gene expression, hormone-stimulated cholesterol transport, and steroidogenesis: role of the peroxisome proliferator-activator receptor alpha. Endocrinology 143(7):2571–2583, PMID: 12072389, 10.1210/endo.143.7.8895. [DOI] [PubMed] [Google Scholar]
- Gebbink WA, van Asseldonk L, van Leeuwen SPJ. 2017. Presence of emerging per- and polyfluoroalkyl substances (PFASs) in river and drinking water near a fluorochemical production plant in the Netherlands. Environ Sci Technol 51(19):11057–11065, PMID: 28853567, 10.1021/acs.est.7b02488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis MI, Vestergren R, Borg D, Cousins IT. 2018. Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives. Environ Int 113:1–9, PMID: 29421396, 10.1016/j.envint.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Grasty RC, Wolf DC, Grey BE, Lau CS, Rogers JM. 2003. Prenatal window of susceptibility to perfluorooctane sulfonate-induced neonatal mortality in the Sprague-Dawley rat. Birth Defects Res B Dev Reprod Toxicol 68(6):465–471, PMID: 14745980, 10.1002/bdrb.10046. [DOI] [PubMed] [Google Scholar]
- Hannas BR, Lambright CS, Furr J, Evans N, Foster PMD, Gray EL, et al. 2012. Genomic biomarkers of phthalate-induced male reproductive developmental toxicity: a targeted RT-PCR array approach for defining relative potency. Toxicol Sci 125(2):544–557, PMID: 22112501, 10.1093/toxsci/kfr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas BR, Lambright CS, Furr J, Howdeshell KL, Wilson VS, Gray LE Jr. 2011. Dose–response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate. Toxicol Sci 123(1):206–216, PMID: 21633115, 10.1093/toxsci/kfr146. [DOI] [PubMed] [Google Scholar]
- Hartig PC, Bobseine KL, Britt BH, Cardon MC, Lambright CR, Wilson VS, et al. 2002. Development of two androgen receptor assays using adenoviral transduction of MMTV-Luc reporter and/or hAR for endocrine screening. Toxicol Sci 66(1):82–90, PMID: 11861975, 10.1093/toxsci/66.1.82. [DOI] [PubMed] [Google Scholar]
- Hartig PC, Cardon MC, Lambright CR, Bobseine KL, Gray LE Jr, Wilson VS. 2007. Substitution of synthetic chimpanzee androgen receptor for human androgen receptor in competitive binding and transcriptional activation assays for EDC screening. Toxicol Lett 174(1–3):89–97, PMID: 17920789, 10.1016/j.toxlet.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Henck JW, Craft WR, Black A, Colgin J, Anderson JA. 1998. Pre- and postnatal toxicity of the HMG-CoA reductase inhibitor atorvastatin in rats. Toxicol Sci 41(1):88–99, PMID: 9520344, 10.1006/toxs.1997.2400. [DOI] [PubMed] [Google Scholar]
- Hu W, Jones PD, Celius T, Giesy JP. 2005. Identification of genes responsive to PFOS using gene expression profiling. Environ Toxicol Pharmacol 19(1):57–70, PMID: 21783462, 10.1016/j.etap.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Kaboré HA, Vo Duy S, Munoz G, Méité L, Desrosiers M, Liu J, et al. 2018. Worldwide drinking water occurrence and levels of newly-identified perfluoroalkyl and polyfluoroalkyl substances. Sci Total Environ 616–617:1089–1100, PMID: 29100694, 10.1016/j.scitotenv.2017.10.210. [DOI] [PubMed] [Google Scholar]
- Kannan KC, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, et al. 2004. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol 38(17):4489–4495, PMID: 15461154, 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- Lapinskas PJ, Brown S, Leesnitzer LM, Blanchard S, Swanson C, Cattley RC, et al. 2005. Role of PPARα in mediating the effects of phthalates and metabolites in the liver. Toxicology 207(1):149–163, PMID: 15590130, 10.1016/j.tox.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99(2):366–394, PMID: 17519394, 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, et al. 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol Sci 74(2):382–392, PMID: 12773772, 10.1093/toxsci/kfg122. [DOI] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, et al. 2018. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 75(1):46–51, PMID: 29133598, 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebker DJ, Case MT, York RG, Moore JA, Hansen KJ, Butenhoff JL. 2005a. Two-generation reproduction and cross-foster studies of perfluorooctanesulfonate (PFOS) in rats. Toxicology 215(1–2):126–148, PMID: 16146667, 10.1016/j.tox.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Luebker DJ, York RG, Hansen KJ, Moore JA, Butenhoff JL. 2005b. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague–Dawley rats: dose–response, and biochemical and pharamacokinetic parameters. Toxicology 215(1–2):149–169, PMID: 16129535, 10.1016/j.tox.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Martin MT, Brennan RJ, Hu W, Ayanoglu E, Lau C, Ren H, et al. 2007. Toxicogenomic study of triazole fungicides and perfluoroalkyl acids in rat livers predicts toxicity and categorizes chemicals based on mechanisms of toxicity. Toxicol Sci 97(2):595–613, PMID: 17383973, 10.1093/toxsci/kfm065. [DOI] [PubMed] [Google Scholar]
- McCord J, Newton S, Strynar M. 2018. Validation of quantitative measurements and semi-quantitative estimates of emerging perfluoroethercarboxylic acids (PFECAs) and hexfluoroprolyene oxide acids (HFPOAs). J Chromatogr A 1551:52–58, PMID: 29628221, 10.1016/j.chroma.2018.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock Kakaley E, Cardon MC, Gray LE, Hartig PC, Wilson VS. 2018. Generalized concentration addition model predicts glucocorticoid activity bioassay responses to environmentally detected receptor-ligand mixtures. Toxicol Sci 168(1):252–263, PMID: 30535411, 10.1093/toxsci/kfy290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. 2004. Role of thyroid hormone during early brain development. Eur J Endocrinol 151(Suppl 3):U25–U37, PMID: 15554884, 10.1530/eje.0.151u025. [DOI] [PubMed] [Google Scholar]
- Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63, PMID: 6606682, 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Wallace DG, Foster PM. 2002. Fetal testosterone insufficiency and abnormal proliferation of Leydig cells and gonocytes in rats exposed to di(n-butyl) phthalate. Reprod Toxicol 16(1):19–28, PMID: 11934529, 10.1016/S0890-6238(01)00201-5. [DOI] [PubMed] [Google Scholar]
- Negri E, Metruccio F, Guercio V, Tosti L, Benfenati E, Bonzi R, et al. 2017. Exposure to PFOA and PFOS and fetal growth: a critical merging of toxicological and epidemiological data. Crit Rev Toxicol 47(6):482–508, PMID: 28617200, 10.1080/10408444.2016.1271972. [DOI] [PubMed] [Google Scholar]
- Nepelska M, Munn S, Landesmann B. 2015. OECD AOP18 - PPARa activation in utero leading to impaired fertility in males. https://aopwiki.org/aops/18 [accessed 18 March 2019].
- O’Shaughnessy KL, Wood CR, Ford RL, Kosian PA, Hotchkiss MG, Degitz SJ, et al. 2018. Thyroid hormone disruption in the fetal and neonatal rat: predictive hormone measures and bioindicators of hormone action in the developing cortex. Toxicol Sci 166(1):163–179, PMID: 30085217, 10.1093/toxsci/kfy190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (Organization for Economic Co-operation and Develoment). 2002. Hazard Assessment of Perfluorooctane Sulfonate (PFOS) and Its Salts. ENV/JM/RD(2002)17/FINAL. http://www.oecd.org/env/ehs/risk-assessment/2382880.pdf [accessed 7 March 2019].
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Zhang H, Cui Q, Sheng N, Yeung LWY, Sun Y, et al. 2018. Worldwide distribution of novel perfluoroether carboxylic and sulfonic acids in surface water. Environ Sci Technol 52(14):7621–7629, PMID: 29749740, 10.1021/acs.est.8b00829. [DOI] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, et al. 2000. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci 58(2):339–349, PMID: 11099646, 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Fenton SE, Lindstrom AB, et al. 2009. Analysis of PFOA in dosed CD1 mice. Part 1. Methods development for the analysis of tissues and fluids from pregnant and lactating mice and their pups. Reprod Toxicol 27(3–4):360–364, PMID: 19028561, 10.1016/j.reprotox.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MB, Das KP, Rooney J, Abbott B, Lau C, Corton JC. 2017. PPARα-independent transcriptional targets of perfluoroalkyl acids revealed by transcript profiling. Toxicology 387:95–107, PMID: 28558994, 10.1016/j.tox.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushing BR, Hu Q, Franklin JN, McMahen R, Dagnino S, Higgins CP, et al. 2017. Evaluation of the immunomodulatory effects of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in C57BL/6 mice. Toxicol Sci 156(1):179–189, PMID: 28115649, 10.1093/toxsci/kfw251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih W, Mears T, Bradley DJ, Parandoosh Z, Weinberger C. 1991. An adenoviral vector system for functional identification of nuclear receptor ligands. Mol Endocrinol 5(2):300–309, PMID: 1645457, 10.1210/mend-5-2-300. [DOI] [PubMed] [Google Scholar]
- Strynar M, Dagnino S, McMahen R, Liang S, Lindstrom A, Andersen E, et al. 2015. Identification of novel perfluoroalkyl ether carboxylic acids (PFECAs) and sulfonic acids (PFESAs) in natural waters using accurate mass time-of-flight mass spectrometry (TOFMS). Environ Sci Technol 49(19):11622–11630, PMID: 26392038, 10.1021/acs.est.5b01215. [DOI] [PubMed] [Google Scholar]
- Sui L, Gilbert ME. 2003. Pre- and postnatal propylthiouracil-induced hypothyroidism impairs synaptic transmission and plasticity in area CA1 of the neonatal rat hippocampus. Endocrinology 144(9):4195–4203, PMID: 12933695, 10.1210/en.2003-0395. [DOI] [PubMed] [Google Scholar]
- Sun M, Arevalo E, Strynar M, Lindstrom A, Richardson M, Kearns B, et al. 2016. Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the Cape Fear River Watershed of North Carolina. Environ Sci Technol Lett 3(12):415–419, 10.1021/acs.estlett.6b00398. [DOI] [Google Scholar]
- Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, et al. 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol Sci 74(2):369–381, PMID: 12773773, 10.1093/toxsci/kfg121. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2006. Risk Management for Per- and Polyfluoroalkyl Substances (PFAS) under TSCA, PFOA Stewardship Program. https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/risk-management-and-polyfluoroalkyl-substances-pfass [accessed 19 February 2019].
- U.S. EPA. 2016a. Health Effects Support Document for Perfluorooctane Sulfonate (PFOS). EPA 822-R-16-002 Washington, DC:U.S. EPA, Office of Water. [Google Scholar]
- U.S. EPA. 2016b. Lifetime health advisories and health effects support documents for perfluorooctanoic acid and perfluorooctane sulfonate. Fed Reg 81:33250–33251. [Google Scholar]
- Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. 2006. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-α, -β, and -γ, liver X receptor-β, and retinoid X receptor-α. Toxicol Sci 92(2):476–489, PMID: 16731579, 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang X, Sheng N, Zhou X, Cui R, Zhang H, et al. 2017a. RNA-sequencing analysis reveals the hepatotoxic mechanism of perfluoroalkyl alternatives, HFPO2 and HFPO4, following exposure in mice. J Appl Toxicol 37(4):436–444, PMID: 27553808, 10.1002/jat.3376. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbühler K. 2013. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ Int 60:242–248, PMID: 24660230, 10.1016/j.envint.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Wang Z, DeWitt JC, Higgins CP, Cousins IT. 2017b. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ Sci Technol 51(5):2508–2518, PMID: 28224793, 10.1021/acs.est.6b04806. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Bobseine K, Gray LE Jr.. 2004a. Development and characterization of a cell line that stably expresses an estrogen-responsive luciferase reporter for the detection of estrogen receptor agonist and antagonists. Toxicol Sci 81(1):69–77, PMID: 15166400, 10.1093/toxsci/kfh180. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright C, Furr J, Ostby J, Wood C, Held G, et al. 2004b. Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicol Lett 146(3):207–215, PMID: 14687758, 10.1016/j.toxlet.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Yu WG, Liu W, Jin YH. 2009. Effects of perfluorooctane sulfonate on rat thyroid hormone biosynthesis and metabolism. Environ Toxicol Chem 28(5):990–996, PMID: 19045937, 10.1897/08-345.1. [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Liu J, Li H, Zhang C, Han P, et al. 2014. Exposure to perfluorooctane sulfonate in utero reduces testosterone production in rat fetal Leydig cells. PLoS One 9(1):e78888, PMID: 24454680, 10.1371/journal.pone.0078888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.