Abstract
Background:
Phytoestrogens, naturally occurring plant chemicals, have long been thought to confer beneficial effects on human cardiovascular and metabolic health. However, recent epidemiological studies, have yielded conflicting outcomes, in which phytoestrogen consumption was both positively and negatively correlated with adiposity. Interestingly, several dietary phytoestrogens are known to stimulate or inhibit the activity of the peroxisome proliferator-activated receptor gamma (), a key physiological regulator of adipogenesis.
Objective:
The objective of this study was to test the hypothesis that the pro- or anti-adipogenic activity of phytoestrogen chemicals is related to the ability to activate in adipocytes.
Methods:
The effects of resveratrol and the soy isoflavones genistein and daidzein on adipogenesis were examined in cell-based assays using the 3T3-L1 cell model. In parallel, ligand-mediated alterations in target gene expression were measured by quantitative polymerase chain reaction. The agonist/antagonist activities of phytoestrogens on were further assessed by quantifying their ability to affect recruitment of transcriptional cofactors to the receptor.
Results:
Resveratrol displayed significant anti-adipogenic activities as exhibited by the ability to antagonize -dependent adipocyte differentiation, down-regulate genes involved in lipid metabolism, block cofactor recruitment to , and antagonize the effects of the agonist rosiglitazone. In contrast, genistein and daidzein functioned as agonists while also displaying pro-adipogenic activities.
Conclusions:
These data provide biological evidence that the pro- or anti-obesity effects of phytoestrogens are related to their relative agonist/antagonist activity on . Thus, -activation assays may enable the screening of dietary components and identification of agents with adipogenic activities. https://doi.org/10.1289/EHP3444
Introduction
In the past several decades there has been increasing interest over the impact of natural dietary chemicals on human health. Indeed, several plant-derived compounds—particularly, phytoestrogens—function as endocrine disrupting chemicals (EDCs) by binding estrogen receptors (ERs) and interfering with sex hormone signaling (McLachlan 2001; Roccisano and Henneberg 2012).
Unlike synthetic EDCs such as pesticides (e.g., DDT) and plasticizers (e.g., bisphenol A), which are frequented associated with adverse reproductive effects (Schug et al. 2016), phytoestrogens are widely thought to confer a range of beneficial effects. These include estrogenic effects in bone and female reproductive tissues, where phytoestrogens may alleviate the symptoms of menopause and provide protection against certain types of cancers (Patisaul and Jefferson 2010). In addition to reproductive tissues, there is emerging evidence that exposure to dietary phytoestrogens may positively impact the function of multiple organ systems and have a key influential role on normal metabolic processes (Casals-Casas and Desvergne 2011; Diamanti-Kandarakis et al. 2009). The observed beneficial effects of phytoestrogens include improved insulin sensitivity and cholesterol homeostasis as shown using the INS-1E rat insulinoma cell line (Mellbye et al. 2015), decreased atherosclerosis in postmenopausal women (Myasoedova et al. 2016), a decline in tumor reoccurrence in breast cancer survivors (Messina 2014), and a reduction in ischemic heart disease with enhanced neurological recovery following stroke as shown in a mouse model (Kim et al. 2015). Interestingly, dietary phytoestrogen consumption has been both positively (Newbold 2011; Penza et al. 2006) and negatively (Cederroth and Nef 2009; Rietjens et al. 2017) associated with body fat content and obesity in experimental studies and positively correlated with obesity in humans (Roccisano and Henneberg 2012; Tang-Péronard et al. 2011), suggesting that multiple factors are at play.
The physiological actions of steroid hormones and certain other hydrophilic molecules are mediated through a family of nuclear receptors that function as ligand-activated transcription factors in target cells (Hall et al. 2001; Mangelsdorf et al. 1995). Phytoestrogens manifest their biological and pathological activities through binding to nuclear receptors, modulating their transcriptional responses, and interfering with the signaling pathways regulated by endogenous receptor ligands (McLachlan 2001). In the nuclear receptor field, the majority of studies on phytoestrogens to date have described the ability of many of these agents to modulate ER function and to alter the biology of ER-positive tumors in the breast and other reproductive tissues (Rietjens et al. 2017). However, it is now clear that the nuclear receptor peroxisome proliferator-activated receptor-gamma () is also a target of phytoestrogen action (le Maire et al. 2009; Maloney and Waxman 1999). Indeed, several abundant dietary phytoestrogens are known to augment or disrupt signaling by binding to the receptor and modulating its transcriptional activity, thereby effectively interfering with downstream -mediated biological processes (Bility et al. 2004; Corton and Lapinskas 2005; Hurst and Waxman 2003; Lampen et al. 2003; Schlezinger et al. 2004). Specifically, genistein and daidzein (isoflavones), which are enriched in soy and other legumes, and resveratrol, which is found in grapes and berries, have been described as modulators (Wang et al. 2014).
In the pharmaceutical sciences, is known as the target of thiazolidinedione antidiabetic drugs and clinical trials have shown that the activated receptor vastly improves insulin sensitivity and blood glucose homeostasis (Chiarelli and Di Marzio 2008). However, the major established physiological role of is in fat storage, where the receptor functions as the master regulator of adipogenesis (Lehrke et al. 2005; Spiegelman 1998). The ability of phytoestrogens to modulate activity in multiple cell and tissue types has been established, and previous studies have shown that isoflavones and numerous other phytoestrogens bind directly to (Calleri et al. 2014; Salam et al. 2008; Shen et al. 2006). However, the potential link between phytoestrogens and the adipogenic functions of has not been well studied. This relationship is likely significant given that epidemiological studies demonstrate a strong correlation between dietary phytoestrogen exposure, body fat content, and obesity (Elobeid et al. 2010; Ghosh and Skinner 2007; Jungbauer and Medjakovic 2014; Ørgaard and Jensen 2008; Tang-Péronard et al. 2011).
Several phytoestrogen ligands for , including genistein and resveratrol, are consumed in significant quantities worldwide (Amiot et al. 2016; Cao et al. 2009; Patisaul and Jefferson 2010; Roccisano and Henneberg 2012). This observation prompted a closer look at the relationship between the ability of a phytoestrogen to activate and the subsequent effects on adipogenesis. The current study tests the hypothesis that the pro- or anti-adipogenic effects of resveratrol—and of the soy isoflavones genistein and daidzein—are correlated with their ability to activate or inhibit in adipocytes. Using mammalian adipocytes as a model, the objectives of this study were to (a) evaluate the relative agonist/antagonist activities of phytoestrogens on , (b) characterize the ability of phytoestrogens to promote adipogenesis by activation of , and (c) determine effects of these agents on classical gene expression and receptor–cofactor interactions.
Methods
Biochemicals
Quantitative polymerase chain reaction (qPCR) reagents were obtained from BIO-RAD. Rosiglitazone, genistein, and daidzein were purchased from Sigma. The -specific antagonist GlaxoWellcome9662 (GW9662) was a gift from GlaxoSmithKline Pharmaceuticals. AdipoRed assay reagent was bought from Lonza, and Hoechst 33342 from Sigma. Adipocyte differentiation kits (Adipogenesis Assay Kit item no. 10006908) were purchased from Cayman Chemical Company. Fugene 6 transfection agent and the Dual-Luciferase® Reporter (DLR™) assay system were purchased from Promega. qPCR oligos were obtained from Invitrogen.
Mammalian Cell Culture
3T3-L1 (murine fibroblast) cells, human adipose-derived mesenchymal stem cells, and HeLa (human cervical carcinoma) cells were obtained from American Type Culture Collection (ATCC). 3T3-L1 cells were maintained in Dulbecco’s Modified Eagle’s Medium (MEM) supplemented with 10% bovine serum (Invitrogen). Human adipose-derived mesenchymal stem cells were maintained in low-serum media purchased as a mesenchymal stem cell growth kit (ATCC: PCS-500-040TM). HeLa cells were maintained in MEM supplemented with 10% fetal bovine serum (FBS), glutamine, nonessential amino acids, and sodium pyruvate (Invitrogen). The cells were grown in a humidified 37°C incubator with 5% carbon dioxide.
Concentrations of Ligands
Ligands were prepared from powder in ethanol in , , , and a stocks for serial dilutions. Ligands in ethanol were stored at . Serial dilutions of the stock were used to generate the dose–response studies shown in Figure 1A–C, which included doses ranging from . These concentrations are relevant to those found in humans; several studies of formula-fed infants have detected average plasma levels of genistein and daidzein of at least and , respectively (Cao et al. 2009; Irvine et al. 1998). Likewise in adults, isoflavone levels have been reported to exceed in individuals consuming a soy-rich diet (Gardner et al. 2009). Furthermore, the range spans the concentrations of genistein and daidzein that have been shown to bind and activate (Ricketts et al. 2005; Salam et al. 2008; Shen et al. 2006).
Figure 1.
transcriptional activity was measured in the presence of agonist rosiglitazone, antagonist GW9662, or phytoestrogen ligands. HeLa cells were transiently transfected with a expression plasmid, PEPCK-Luc reporter vector, and a luciferase normalization control. Following transfection, cells were treated with vehicle (Veh) or concentrations ranging from to of the following ligands: Rosiglitazone (Rosi), Genistein (Gen), Daidzein (Daid), or Resveratrol (Resv). After 36 h, cells were harvested and Dual-Luciferase assays were performed. Each value was normalized to the internal luciferase control. Each data point is the average of three independent experiments; individual experiments included four technical replicates used for each treatment. Data points represent means . (A) Agonist mode: Cells were treated with Veh or concentrations ranging from to of the following ligands: Rosi, Gen, Daid, or Resv. (B) Antagonist mode: To evaluate antagonism of Rosi-stimulated activity, Resv and antagonist GW9662 were used at to in the presence or absence of Rosi. (C) Antagonist mode: To evaluate antagonism of Gen- or Daid-stimulated activity, these ligands were used at to in the presence or absence of GW9662. (D) Augmentative effects of co-administration of Rosi () and Gen or Daid (). * for comparison between Veh and each treatment. (E) Agonist mode on DR1-Luc reporter. Following transfection with the expression plasmid, DR1-Luc reporter vector, and a luciferase normalization control, cells were treated for 36 h with Veh or Rosi, Gen, Daid, Resv, or GW9662. * for comparison between Veh and each treatment.
Various concentrations of the stocks (1:1,000 dilutions of the , , and stocks in media) were used (Figure 3A). In the remaining majority of experiments, 1:1,000 dilutions of the stock to a final concentration of was used. Ethanol was used as a vehicle at a 1:1,000 dilution in culture media at a final concentration of 0.1%. The final ethanol concentration for all ligand treatments was also 0.1%.
Figure 3.
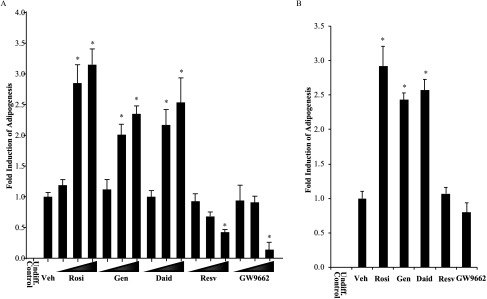
Pro- and anti-adipogenic effects of phytoestrogens in mammalian adipocytes. (A) 3T3L1 mouse pre-adipocytes were grown to confluence and then induced to differentiate using an 8-d protocol (see the “Methods” section). Cells were co-incubated with vehicle (Veh) or various concentrations (, , ) of agonist Rosiglitazone (Rosi), Genistein (Gen), Daidzein (Daid), Resveratrol (Resv), or antagonist GW9662 during the entire differentiation period with the media replaced every 2 d. After 8 d, cells were assayed for adipogenesis using a quantitative fluorescent assay (AdipoRed™ Adipogenesis Assay Reagent). Cells were co-stained with nuclear dye Hoechst 33342, and lipid accumulation values were calculated between treatments by normalizing lipid intensity values to Hoechst intensity. Graphical values for each treatment represent comparison with Vehicle (set at 1). Each data point is the average of three independent experiments; individual experiments included four technical replicates used for each treatment. An undifferentiated control (Undiff. Control) sample is included for comparison. Bars represent . * for comparison between Veh and each treatment. (B) Human mesenchymal stem cells were grown to confluence and induced to differentiate for 8 d. Cells were co-treated throughout with Veh or Rosi, Gen, Daid, Resv, or GW9662. Results were assayed and normalized as described above. * for comparison between Veh and each treatment.
Adipocyte Differentiation Assays
activation in pre-adipocytes is required for the route of differentiation into mature adipocytes (Tontonoz et al. 1994). A key aspect of the process is the transformation of cells from spindly fibroblasts into spherical cells containing cytoplasmic fat globules that can imaged microscopically. Fibroblasts can be differentiated in vitro in a manner that reenacts most of the features characteristic of adipogenesis in vivo. 3T3-L1 cells are a well-characterized mouse embryonic fibroblast (pre-adipocyte) cell line routinely used to study adipocyte differentiation. When induced, differentiation of 3T3-L1 cells into mature adipocytes occurs within 7–9 d.
3T3-L1 cells were differentiated using a standard methylisobutylxanthine, dexamethasone, insulin (MDI) differentiation protocol (Zebisch et al. 2012). The insulin, 3-isobutyl-1-methylxanthine (IBMX), and dexamethasone reagents (at 1,000× stock solutions) were included in the adipocyte kits purchased from Cayman Chemical Company. Briefly, for microscopic imaging studies, of cells were plated in 8-well chamber slides at a density of in MEM supplemented with 10% bovine serum. For quantitative adipogenesis assays, cells were plated in 96-well plates in media. The cells were allowed to adhere overnight and either used the next day or allowed to grow an additional day until reaching 90% confluence. On experimental day 1, cells were incubated with MEM supplemented with 10% FBS, insulin, IBMX, and dexamethasone in the absence or presence of rosiglitazone, GW9662, and/or phytoestrogen ligands. On days 4 and 6, the cells were administered MEM supplemented with 10% FBS and insulin in the presence or absence of rosiglitazone, GW9662, and/or phytoestrogen ligands. For the imaging studies, on day 8 the cells were fixed and stained with Oil Red O according to the manufacturer’s instructions. All of the reagents used in the staining, as described below, were part of the adipocyte differentiation assay kit (Cayman Chemical Company). Briefly, the medium was aspirated and cells were incubated in lipid droplet assay fixative for 15 min. The cells were next incubated with a wash solution for 5 min and then repeated. The wells were allowed to dry completely before adding Oil Red O working solution for 20 min. The cells were then washed twice for 5 min, and coverslips were mounted. Slides were viewed and photographed at 200× magnification using a Zeiss Axio Scope.A1 light microscope. For the quantitative studies, on day 8 the cells were incubated with AdipoRed and Hoechst reagent and assayed according to the manufacturers’ protocols. Briefly, the medium was removed and each well was washed with phosphate buffered saline (PBS). The cells were fixed in 2% paraformaldehyde for 30 min, washed with PBS, and incubated with AdipoRed assay reagent; after 10 min, fluorescence was read () using a Spectramax i3X plate reader. The cells were co-incubated for 30 min with the nuclear dye Hoechst 33342 and read at Ex330/Em470 in order to quantitate the DNA content to allow for correction for cell number variances between samples. Lipid accumulation values were calculated between treatments by normalizing lipid intensity values to Hoechst intensity. All assays were performed in quadruplicate replicates, and a minimum of three independent studies were performed in each instance.
RNA Isolation and Quantitative PCR
For RNA analysis in pre-adipocytes, cells were plated at 60% confluency in 6-well plates in growth medium. After 24 h, the medium was replaced with fresh media supplemented with 0.01% ethanol (vehicle), rosiglitazone, genistein, daidzein, or resveratrol, in the presence or absence of the -specific antagonist GW9662 (Leesnitzer et al. 2002). The cells were harvested after 24 h. For RNA analysis in mature adipocytes, the cells were differentiated for 7 d and then treated as described above. See the figure captions for details on the concentrations of agents used in specific experiments.
RNA was isolated from cultured 3T3-L1 cells using the RNeasy® kit (Qiagen). Five hundred nanograms of RNA was reverse transcribed using the BioRad iScript™ cDNA synthesis kit. The ABI PRISM® 7900HT Real-Time PCR System was used to amplify and quantitate levels of cDNAs amplified from target genes. qPCR reactions were performed using of cDNA, gene-specific primers, and 2× SYBR Green. Data are the (SDs) of triplicate determinations. All experiments were repeated a minimum of three times.
Adipocyte gene expression was assessed using genes that fit the following criteria: (a) they were detectable in a quantitative manner in the 3T3L1 cell model; (b) based on literature, they had been shown to display induction by rosiglitazone, and (c) they possessed established roles in lipid metabolism. These criteria permitted focus on -dependent adipogenic effects of dietary phytoestrogens. A handful of genes met the selection criteria, including fatty acid-binding protein (FABP), PEPCK (phosphoenolpyruvate carboxykinase) and HRASLS3 (Hras-like-suppressor 3) (Hummasti et al. 2008; Tontonoz and Spiegelman 2008).
The following primers were used: FABP, 5′-AAGGTGAAGAGCATCATAACCCT-3′ (for) and 5′-TCACGCCTTTCATAACACATTCC-3′ (rev); HRASLS5, 5′-CAAGCTGGATGGCACATACCT-3′ (for) and 5′-AGTTCCCCTCAATTAGGCTGT-3′(rev); PEPCK, 5′-AGCATTCAACGCCAGGTTC-3′ (for) and 5′-CGAGTCTGTCAGTTCAATACCAA-3′ (rev); and GAPDH 5′-AGGTCGGTGTGAACGGATTTG-3′ (for) and 5′-GGGGTCGTTGATGGCAACA-3′ (rev).
Small Interfering RNA Treatment and Analysis
For small interfering RNA (siRNA) targeting of expression in adipocytes, 3T3L1 cells were plated in 6-well plates at a density of . After 1–2 d, when 90% confluence was achieved, cells were induced to differentiate for 8 d using the MDI differentiation protocol described above. Adipocyte differentiation was confirmed by fixing and staining with Oil Red O. Differentiated cells were trypsinized, washed with PBS, counted and transfected with siRNA duplexes by electroporation using the Nucleofector™ II system (Lonza). The siRNA utilized targeted murine ( and ; Dharmacon ON-TARGETplus™ J-040712-05-0010 and J-040712-05-0010); or a control nontargeting siRNA duplex (Control si-RNA; Dharmacon D-001810-01-20). Each sample representing a single well was electroporated with siRNA cells in a total volume of Nucleofection™ solution in disposable electroporation cuvettes. The samples were removed from the cuvettes using pre-warmed medium MEM with 10% FBS, and replated in 6-well plates in an additional prewarmed media. After 48 h, cells were then treated with ligands for 24 h, total RNA was harvested, and cDNA was prepared and used for quantitative gene expression analysis as described above.
Plasmids
, a mammalian expression vector, a gift from Dr. Bruce Spiegelman, contains the full-length human , as described previously (Mettu et al. 2007). The PEPCK ()-luciferase and DR1-Luc reporter constructs have also been described previously (Mettu et al. 2007). pM and VP16 expression vectors were obtained from Clontech. and pM-ASC-2 contain the yeast Gal4 transcription factor DNA binding domain fused to the nuclear receptor–interacting region of the coactivators and ASC-2, respectively. contains full-length human fused to the VP16 activation domain, and 5x-GAL4-TATA-Luc contains five tandem binding sites for the yeast Gal4 transcription factor, as described previously (Mettu et al. 2007). The pRL-CMV renilla luciferase normalization vector (Promega Corp.) has also been described previously (Hall and Korach 2002).
Transient Transfection Assays
The effects of different ligands on transcriptional activity was assessed in cultured cells. HeLa cells (human cervical carcinoma) were selected for these studies because they lack expression of ERs and other nuclear receptors that respond to phytoestrogens. These cells, therefore, provide a relatively clean background for the analysis of -specific responses. HeLa cells were plated in 24-well plates, 16 h before transfection at a density of . DNA was introduced into the cells using Fugene 6. Each well received of reporter (PEPCK-Luc or DR1-Luc), of expression plasmid (pcDNA3.1nV5-hPPARy1), and of the pRL-CMV renilla luciferase normalization vector (Promega Corp.). Before transfection, cells were washed with PBS, and phenol red–free MEM containing 10% charcoal-stripped fetal calf serum (FCS; HyClone Laboratories, Inc.) was added to each well. The cells were incubated with the DNA/fugene mix for 6 h and then ligands in phenol red–free MEM were added to the cells and incubated for 36 h. Mammalian two-hybrid assays were performed as follows: HeLa cells were plated in 24-well plates, 16 h before transfection at a density of . DNA was introduced into the cells using Fugene 6. In standard mammalian two-hybrid assays, of reporter (5x-GAL4- TATA-Luc), of , of pM or pM (Gal4DBD)-ASC-2, and of the pRL-CMV renilla luciferase normalization vector were used for each well. Before transfection, the cells were washed with PBS, and of phenol red–free MEM containing 10% charcoal-stripped FCS was added to each well. The cells were incubated with the DNA/fugene mix for 6 h, and then ligands in of phenol red–free MEM were added to the cells and incubated for 36 h.
All transcriptional and mammalian two-hybrid experiments were analyzed by luciferase assays, which were performed using the Dual-Luciferase Reporter assay system according to the manufacturer’s protocols (Promega Corp.). Each value was normalized to the renilla luciferase control, and each data point generated was the average of triplicate determinations. All experiments were repeated three times.
Statistical Analysis
All experiments in this study were repeated a minimum of three independent times, and results are expressed as . For adipogenesis assays, three to six biological replicates were used in each experiment; for transfection assays, three biological replicates were used in each experiment; and for RT-PCR, three biological replicates were used in each experiment. In all experiments, statistical comparisons were made between control and treatments using two-sample t-tests. Differences of were considered significant. Statistical analysis of data in experiments involving comparison of multiple groups (Figures 4 and 5) included two-way analysis of variance with multiple comparisons using Fisher’s least significant difference.
Figure 4.
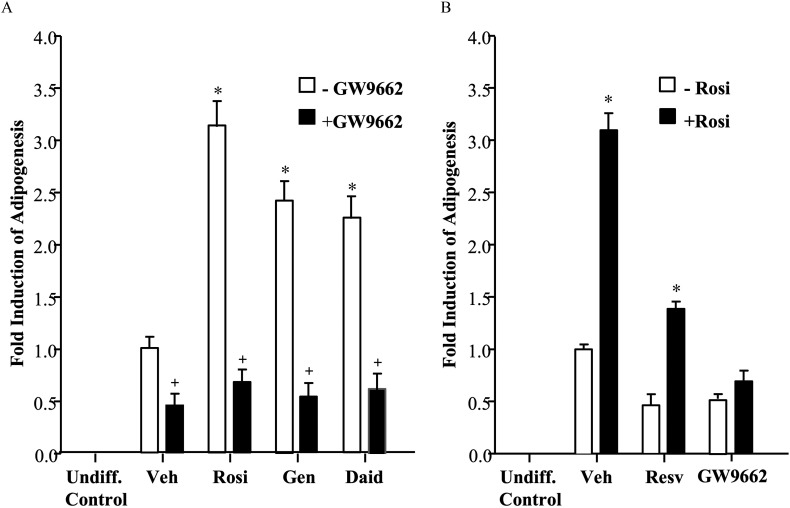
Adipocyte differentiation in the presence of agonist rosiglitazone or phytoestrogens with or without the antagonist GW9662. Adipocyte differentiation assays: 3T3L1 mouse pre-adipocytes were grown to confluence and then induced to differentiate. After 8 d, cells were assayed for adipogenesis using a quantitative fluorescent assay (AdipoRed™ Adipogenesis Assay Reagent). Cells were co-stained with nuclear dye Hoechst 33342, and lipid accumulation values were calculated between treatments by normalizing lipid intensity values to Hoechst intensity. Graphical values for each treatment represent comparison with Vehicle (set at 1). Each data point is the average of three independent experiments; individual experiments included four technical replicates used for each treatment. An undifferentiated control (Undiff. Control) sample is included for comparison. (A) Cells were co-incubated with vehicle (Veh) or agonist Rosiglitazone (Rosi), Genistein (Gen), or Daidzein (Daid) in the absence or presence of antagonist GW9662 during the entire differentiation period with the media replaced every 2 d. Bars represent . * for comparison between Veh and each treatment. + for comparison between each treatment alone and each . (B) Cells were co-incubated with vehicle (Veh) Resveratrol (Resv), or GW9662 in the absence or presence of Rosiglitazone (Rosi) during differentiation, and assayed as described above. Bars represent . * for comparison between each treatment and each .
Figure 5.
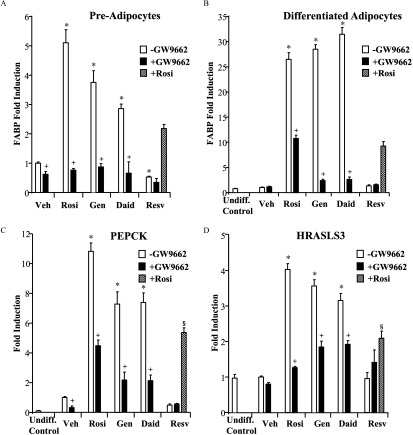
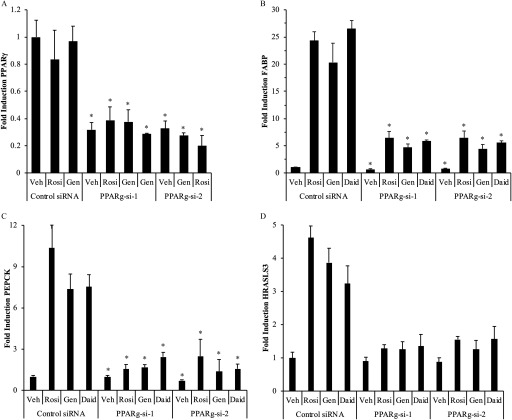
Real-time PCR of PPARγ target genes in 3T3L1 pre-adipocytes and differentiated adipocytes in the presence of agonist rosiglitazone or phytoestrogens with or without the antagonist GW9662. 3T3L1 cells were induced to differentiate for 8 d; differentiation was confirmed by staining with Oil Red O. Pre-adipocytes or differentiated adipocytes were treated for 24 h with vehicle (Veh) or of ligands: Rosiglitazone (Rosi), Genistein (Gen), Daidzein (Daid), or Resveratrol (Resv) in the presence or absence of of the antagonist GW9662 or Rosi where indicated. Total RNA was harvested, and cDNA was prepared and used as a template for gene expression analysis by real-time PCR. (A) FABP expression in pre-adipocytes. (B) FABP, (C) PEPCK, and (D) HRASLS3 expression in mature adipocytes. An undifferentiated control (Undiff. Control) was run in parallel. Each data point is the average of three independent experiments; individual experiments included three technical replicates used for each treatment. Bars represent . For A–D: * for comparison between Veh and each single treatment (Rosi, Gen, Daid, Resv). + for comparison between each treatment alone and each . § for comparison between each and each .
Results
Effect of Genistein, Daidzein, and Resveratrol on Transcriptional Activity
regulates adipogenesis by altering gene expression, an action that requires the transcriptional activity of the receptor. Thus, the ability of phytoestrogens to activate transcriptional activity was first examined. HeLa cells were transfected with a expression vector and a luciferase reporter containing the promoter from the -responsive phosphoenolpyruvate carboxykinase gene (PEPCK-Luc). The cells were treated with the agonist rosiglitazone or isoflavone phytoestrogens (genistein, daidzein), including doses ranging from . Transcriptional responses to ligands were quantified using a luminescent assay (see the “Methods” section). Not surprisingly, rosiglitazone was a potent agonist (half maximal effective concentration, ), reaching maximal efficacy at a pharmacological concentration of (Figure 1A). Genistein and daidzein were less potent in the assay ( and , respectively), likely reflecting their lower binding affinities for compared with rosiglitazone, as reported previously (Salam et al. 2008; Shen et al. 2006). However, both agents displayed significant agonist activity; they were approximately 70% efficacious as compared with rosiglitazone. No significant differences were observed when the isoform was used in the assays (data not shown).
In parallel, the effect of resveratrol on transcriptional activity was assessed. Compared with the isoflavones, resveratrol lacked agonist activity, and further, functioned as an inverse agonist in that cells treated with resveratrol exhibited basal receptor activity of 50% of that of cells treated with vehicle (Figure 1A). To examine the antagonist mode, the cells were treated with increasing concentrations of resveratrol or -specific antagonist GW9662 (Alavanja et al. 2003), together with a constant, activating concentration of rosiglitazone. GW9662 was a potent and efficacious inhibitor, as expected (half maximal inhibitory concentration, ), reflecting its high affinity for the receptor (Alavanja et al. 2003). Notably, treatment with resveratrol resulted in rosiglitazone-activated activity that was 60% of that of cells treated with rosiglitazone alone (Figure 1B), demonstrating efficacy as an inhibitor, albeit less potent () than the high-affinity antagonist GW9662. GW9662 was also capable of inhibiting genistein- and daidzein-activated transcriptional activity (Figure 1C). In contrast, as shown in Figure 1D, genistein and daidzein lacked antagonist activity and further demonstrated their agonist nature by moderately augmenting the effects of rosiglitazone when added at concentrations yielding maximal efficacy (Figure 1A) (e.g., rosiglitazone and genistein or daidzein). Agonist activity of isoflavone phytoestrogens compared with resveratrol was further observed on an alternative and simple promoter context, DR1-Luc (Figure 1E).
Effect of Genistein, Daidzein, and Resveratrol on Adipogenesis in 3T3L1 Cells
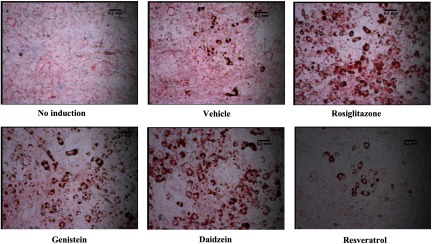
To test the effects of phytoestrogens on adipogenesis, 3T3-L1 cells were plated in microscope chamber slides and differentiation was induced using an established 8-d protocol as part of an adipocyte differentiation assay kit (Cayman Chemical Company). On days 1, 4, and 6 of the assay, the cells were administered vehicle, ligands rosiglitazone or GW9662, or the phytoestrogens genistein, daidzein, or resveratrol. On day 8, lipid droplets were fixed and stained using Oil Red O according to the manufacturer’s instructions. Microscopic imaging results from a typical experiment are shown in Figure 2. As expected, in control wells lacking induction media, the cells remained fibroblastic in appearance. In contrast, induced, vehicle-treated wells contained abundant rounded, differentiated cells as exhibited by lipid staining. Compared with vehicle, the cells incubated with genistein or daidzein appeared to contain more mature adipocytes, similar to that seen with rosiglitazone. In contrast, cells in the resveratrol-treated wells appeared similar to those of the administered vehicle (Figure 2).
Figure 2.
Adipogenesis in the presence of the agonist rosiglitazone or phytoestrogen ligands. Adipocyte differentiation assays: 3T3L1 mouse pre-adipocytes were grown to confluence. Adipocyte differentiation was induced using an 8-d protocol (see the “Methods” section). On days 1, 4, and 6 of the assay, cells were treated with vehicle, rosiglitazone () or phytoestrogens (). On day 8, adipocyte differentiation and lipid accumulation were assayed by fixing and staining lipid droplets using Oil Red O. An undifferentiated control (no induction) is included for comparison. Microscopic imaging is at 200× magnification.
Pro- and Anti-Adipogenic Effects of Phytoestrogens in Mammalian Adipocytes
The next objective was to obtain a quantitative assessment of phytoestrogen exposure on adipogenesis. 3T3-L1 cells were plated in 96-well plates, and adipocyte differentiation was induced as described above. On days 1, 4, and 6 of the assay, the cells were administered vehicle, ligands rosiglitazone or GW9662, or phytoestrogens at concentrations ranging from , which are physiologically relevant and within the ranges shown to bind and activate (Biasutto et al. 2010; Calleri et al. 2014; Cao et al. 2009; Fanti et al. 1999; Ricketts et al. 2005). On day 8, adipocyte differentiation was quantified using a fluorescent dye for intracellular lipids. Undifferentiated fibroblasts displayed an undetectable signal in the assay; however, differentiated adipocytes treated with vehicle yielded a robust response, set at 1 for comparison (Figure 3A). Compared with vehicle, cells treated with isoflavone phytoestrogens displayed a concentration-dependent 2.5- to 3-fold higher induction in adipogenesis that was comparable to that of rosiglitazone. In contrast, resveratrol lacked stimulatory effects, and at micromolar concentrations functioned as an inverse agonist, resulting in adipogenesis that measured below basal levels. Treatment of cells with antagonist GW9662, at , also resulted in lower induction of adipogenesis compared with vehicle control. This observation is consistent with the known requirement of at least some basal receptor activity for adipose differentiation (Barak et al. 1999; Rosen et al. 1999), which was less preserved at the dose. Collectively, the results shown in Figures 2 and 3 indicate that, similar to rosiglitazone, the agonist activity of isoflavone phytoestrogens on transcription was correlated with the ability to induce adipogenesis. In contrast, resveratrol was inhibitory in both regards, behaving similarly to the antagonist GW9662.
To determine whether the adipogenic effects of phytoestrogens could be recapitulated in cells more relevant to humans, adipogenesis assays were performed in human adipose-derived mesenchymal stem cells (ATCC® PCS500011™). This self-propagating, pluripotent cell line is capable of differentiation into mature human adipocytes. Using these cells at confluence, differentiation assays were performed in the presence of ligands as described above. On days 1, 4, and 6 of the assay, the cells were administered vehicle, rosiglitazone, phytoestrogens, or GW9662. On day 8, adipocyte differentiation was quantified by staining with a fluorescent dye for intracellular lipids and with a second fluorescent agent for cell quantification. As seen in differentiated mouse adipocytes, the human cells displayed a pro-adipogenic response to rosiglitazone and the isoflavone phytoestrogens (Figure 3B).
and Adipogenic Effects of Phytoestrogens
To determine whether positive or inhibitory effects of phytoestrogens on adipogenesis are mediated at least in part through , pharmacological modification of activity with co-administration of agonist rosiglitazone or antagonist GW9662 was used to assess phytoestrogen dependence on the intact receptor for observed adipogenic effects. GW9662 was administered at , a concentration at which it functioned as an antagonist of other ligands but did not block adipogenesis altogether (Figures 1B and 3). Quantitative adipocyte differentiation assays were performed using rosiglitazone, genistein, and daidzein at throughout the differentiation protocol, as described above. Treatment of cells with each of the ligands resulted in a 2.5- to 3-fold greater induction of adipogenesis compared with vehicle, and this activity was completely attenuated by co-administration of GW9662, indicating receptor-dependence (Figure 4A). When administered alone, GW9662 and resveratrol treatment resulted in lower basal levels of adipogenesis compared with vehicle controls, an effect that was reversed by rosiglitazone (Figure 4B), suggesting dependence.
Effect of Phytoestrogens on the Expression of Target Genes in Pre-Adipocytes and Differentiated Adipocytes
The effect of phytoestrogens on the expression of target genes during adipocyte differentiation was next examined. FABP expression in 3T3L1 pre-adipocytes and differentiated adipocytes was evaluated by real-time PCR. As expected, treatment with rosiglitazone resulted in significantly higher levels of FABP expression (5-fold) in pre-adipocytes, and even higher levels (26.5-fold) in differentiated cells (Figure 5A,B). Strikingly, isoflavone phytoestrogens mirrored rosiglitazone, with pre- and post-differentiation induction levels of FABP 3 to 4-fold and 28-fold, respectively. These activities were attenuated by antagonist GW9662. In contrast, cells treated with resveratrol had lower FABP expression levels than those treated with vehicle. Furthermore, when co-administered with rosiglitazone, resveratrol behaved as an antagonist, as cells treated with both rosiglitazone and resveratrol had FABP expression that was less than 50% of that observed with the agonist-activated receptor.
Similar studies were carried out to examine phytoestrogen regulation of PEPCK and HRASLS3. No ligand-dependent differences in the expression of either gene were detected in pre-adipocytes (data not shown). Therefore, the results of this analysis were obtained from differentiated adipocytes. As seen for FABP, cells treated with rosiglitazone, genistein, and daidzein had higher expression levels of PEPCK and HRASLS3, and the effects of these agents were lowered by co-treatment with GW9662 (Figure 5C,D). Likewise, treatment of cells with resveratrol did not result in higher PEPCK and HRASLS3 expression. Furthermore, when cells were co-treated with resveratrol and rosiglitazone, levels of gene expression decreased from that seen with rosiglitazone alone.
Effect of siRNAs Knockdown of on Phytoestrogen Induction of Target Genes in Adipocytes
To further confirm the requirement for in phytoestrogen-mediated gene regulation, the expression of FABP in differentiated adipocytes was examined in the presence of siRNAs targeting the predominant murine adipocyte isoform as well as the second isoform, . The cells were treated with either of two siRNA duplexes ( and ). A reduction in mRNA was examined using qPCR analysis. An approximately 65–70% reduction in receptor expression was observed with each of the siRNAs in the absence and presence of ligands, whereas no effect was seen in cells receiving a control, nontargeting siRNA duplex (Figure 6A).
Figure 6.
target gene expression in 3T3L1 cells following siRNA reduction in expression and treatment with agonist rosiglitazone or phytoestrogens. (A) siRNA reduction in expression in adipocytes. 3T3L1 cells were induced to differentiate for 7 d; differentiation was confirmed by staining with Oil Red O. Differentiated cells were treated for 48 h with siRNA duplexes targeting murine and ( and ) or a control nontargeting siRNA duplex (Control si-RNA). Cells were then treated with ligands for 24 h, total RNA was harvested, and cDNA was prepared and used for gene expression analysis. The expression of (B) FABP, (C) PEPCK, and (D) HRASLS3 in 3T3L1 differentiated adipocytes was evaluated by real-time PCR. 3T3L1 cells were induced to differentiate for 7 d and treated with siRNAs as described above. After 48 h, cells were treated for 24 h with vehicle (Veh) or Rosiglitazone (Rosi), Genistein (Gen), or Daidzein (Daid), or Resveratrol (Resv). Total RNA was harvested, and cDNA was prepared and used for gene expression analysis. Each data point is the average of at least three independent experiments; individual experiments included three technical replicates used for each treatment. Bars represent . For A: * for comparison between the Veh-treated Control si-RNA sample and the and samples for all treatments. For B–D: * for comparison between each siRNA and each or .
In the analysis of FABP expression, ligand-dependent gene induction was observed in both the control and si-RNA samples (Figure 6B). However, the total levels of FABP in the receptor-targeted samples were reduced significantly to approximately 25% of that of control. Similar reduction in ligand-dependent induction of the regulated genes PEPCK and HRASLS3 was observed when the receptor was silenced (Figure 6C,D).
Interaction of with the Transcriptional Coactivators and ASC-2 in the Presence of Phytoestrogens
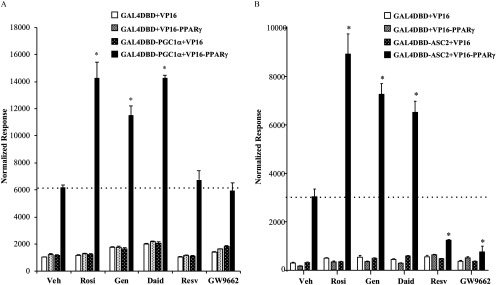
Transcriptional cofactors are required for proper activity (Powell et al. 2007); therefore, it is predicted that phytoestrogens may interfere with action in adipocytes by modifying the formation of receptor–cofactor complexes. To test this hypothesis, a mammalian two-hybrid assay was used to assess the ability of phytoestrogens to facilitate an interaction between the transcriptional activation function 2 domain of and the NR-interacting domain nuclear receptor-box (NR-box) of the coactivators coactivator-1 alpha () activating signal cointegrator 2 (ASC-2). These coactivators are known to interact with in an agonist-dependent manner, and further, are known to play a role in energy metabolism (Liang and Ward 2006; Mahajan and Samuels 2008). The mammalian two-hybrid assay utilized in this study is a validated method used previously to study ligand-mediated cofactor recruitment by PPAR receptors (Hall and McDonnell 2007; Mettu et al. 2007).
HeLa cells were transfected with (NR-Box) or pM-ASC-2 (NR-Box), containing the yeast Gal4 transcription factor DNA-binding domain fused to the NR-Box of the respective coactivator, together with a plasmid. The latter is a chimera of the strong herpes simplex virus VP16 activation domain fused to the N-terminus of full-length . Transcriptional readout, a measurement of protein–protein interactions in the assay, was obtained by cotransfection of a luciferase reporter vector containing five tandem Gal4 binding sites (5x-Gal4Luc). Cells transfected with either one or both of the control vectors (pM, VP16) yielded a relatively low signal that was not altered by ligand, setting a low baseline for the assay (Figure 7). A significant level of and ASC-2 interaction with was observed in the absence of ligand (Figure 7A,B). This is not surprising given that, in the absence of an added activating ligand, PPARs may reside in an active conformation (Barak et al. 1999; Hall and McDonnell 2007; Rosen et al. 1999; Shao et al. 1998; Werman et al. 1997; Wu et al. 2003). Despite the high basal level of coactivator binding to genistein and daidzein were able to enhance the interaction in a manner comparable to the full agonist rosiglitazone (Figure 7A,B). Like GW9662, resveratrol decreased the receptor-ASC-2 interactions below baseline (Figure 7B), consistent with the inverse agonist activity on PPARγ displayed in transcriptional assays (Figure 1B).
Figure 7.
The interaction of with the transcriptional coactivators and ASC-2 in the presence of agonist rosiglitazone, phytoestrogens, or the antagonist GW9662. HeLa cells were transiently transfected with and (A) or (B) Gal4DBD-ASC-2 expression plasmid or empty controls (VP16, Gal4DBD) together with a 5xGAL4 luciferase reporter and a luciferase normalization control. Following transfection, cells were treated with vehicle (Veh) or of Rosiglitazone (Rosi), Genistein (Gen), Daidzein (Daid), Resveratrol (Resv), or antagonist GW9662. After 36 h, cells were harvested and Dual-Luciferase assays were performed. Each value was normalized to the internal luciferase control. Each data point is the average of four independent experiments; individual experiments included three technical replicates used for each treatment. Bars represent . The dotted line on each graph indicates the basal level of coactivator interaction with for comparison with ligand-mediated interactions. * for comparison between Veh and each treatment.
Discussion
The current statistics associated with obesity reflect both its importance in human health and the ongoing initiatives in understanding the causes and treatment of the disease. An estimated 35–40% of U.S. adults are obese (Flegal et al. 2016). Unfortunately, obese individuals are at a high risk for the leading causes of preventable death: heart disease, stroke, type 2 diabetes, and certain types of cancer (CDC 2015). A better understanding of the biological causes of obesity will be important to continue the progress medical science is making toward prevention and treatment of this disease.
Pro- and Anti-Adipogenic Activities of Phytoestrogens
The current study provides evidence that phytoestrogens have both positive and negative effects on adipogenesis, depending on the chemical. The soy isoflavones genistein and daidzein, at concentrations found in humans (Cao et al. 2009; Gardner et al. 2009; Irvine et al. 1998), were pro-adipogenic, as exhibited by their ability to increase the population of mature fat cells in culture and expression of adipogenic genes. Interestingly, these observations are consistent with data from the World Health Organization, where a significant positive correlation between soy consumption and obesity was revealed in a study of 167 countries (Roccisano and Henneberg 2012). Genistein was also shown to promote adipogenesis and adipose tissue weight in male mice when they were administered doses comparable to those consumed by humans who eat a balanced diet (Penza et al. 2006). Likewise, a recent report also concluded that quantities of genistein found in a balanced diet were sufficient to promote both adipogenesis and expression of lipid metabolism genes (Zanella et al. 2015).
In the past decade, health protective effects of resveratrol are being widely reported, and resveratrol consumption has soared (Hobson 2010). This movement was driven by an observation called “The French Paradox,” which links wine consumption to cardioprotection in those on a high-fat, high-caloric diet (Ferrières 2004). Our findings support the belief that resveratrol may have health benefits and protect against obesity. Specifically, resveratrol was able to reduce basal levels of adipogenesis as well as to antagonize the effects of the pro-adipogenic agent rosiglitazone. In line with our observations, others have recently shown that resveratrol can inhibit differentiation of cultured adipocytes (Zhang et al. 2012).
The concept of resveratrol as an anti-obesity agent has also been supported by recent studies in both animals and humans. In mice, resveratrol was found to oppose the effects of a high-fat diet by increasing metabolism and insulin sensitivity (Baur et al. 2006). Furthermore, resveratrol was shown to protect against ovariectomy-associated weight gain in a mouse model of menopause (Sharma et al. 2017). Clinical studies of 11 obese men have also documented the beneficial effects of resveratrol on adipose tissue morphology (Konings et al. 2014) and metabolic rate (Timmers et al. 2011). Resveratrol has not received U.S. Food and Drug Administration approval to date; however, nutritional supplements have been on the market for at least 12 y.
Mechanism by Which Phytoestrogens Mediate Adipogenic Activity
One of the most important observations made in this study was that the effects of phytoestrogens on adipocyte differentiation are correlated with their ability to activate . The isoflavone phytoestrogens examined function as bona fide agonists of via their ability to induce target genes in a receptor-dependent manner as well as promote an activating conformational change in the receptor that facilitates coactivator recruitment. These activities were attenuated by co-administration of a -specific antagonist, demonstrating that these effects were indeed mediated through the receptor. With regard to genistein and daidzein, their pro-adipogenic activities appeared to require receptor activation and -dependent induction of genes involved in lipid metabolism. Although isoflavones are known agonists, our study demonstrates that the agonist activity of isoflavone phytoestrogens on is important for their observed adipogenic effects.
In contrast, our findings also suggest that resveratrol can be classified as a antagonist, as this agent displayed antagonist activity on receptor-dependent gene expression, and when -bound, appeared to lack or even inhibit the ability of the receptor to adopt a transcriptionally active conformation. Equally important, was the observation that the anti-adipogenic effects of resveratrol are reversed by rosiglitazone, suggesting that resveratrol reduces adipogenesis through inhibition of . Both basal and agonist-activated receptor functions were attenuated by resveratrol, including transcriptional response, gene expression, and coactivator recruitment. One question that remains is whether resveratrol antagonist activity involves direct action on receptor activity. Classical antagonists suppress basal and ligand-activated receptor activity by recruiting transcriptional corepressors (Ohashi et al. 2015). It is therefore reasonable that the anti-adipogenic actions of resveratrol may involve enhanced corepressor association with . However, one study showed that resveratrol can target protein for destruction via the ubiquitin–proteasome system, and the authors postulated that this could be the means by which the ligand manifests antagonist activity (Floyd et al. 2008). It is clear that resveratrol binds within the ligand-binding pocket of the receptor (Calleri et al. 2014), but whether the antagonist activity involves receptor–cofactor interactions and/or down-regulation of receptor levels remains to be determined. An additional consideration is the possible interaction of resveratrol-bound with other nuclear receptors. Although the observed effects of resveratrol on adipogenesis appear to involve , resveratrol is known to bind and modulate the activity of other nuclear receptors in adipocytes, including ERs and the arylhydrocarbon receptor. Thus, it will be interesting to determine the relative contributions of each receptor to the resveratrol responses as well as potential crosstalk as an additional regulatory mechanism.
Concentration and Sex-Dependent Activities of Phytoestrogens
Previous studies have shown that some of the biological activities of genistein and resveratrol are concentration dependent. Examination of the effects of resveratrol on the vasculature revealed that high doses are atherogenic in rabbits (Wilson et al. 1996), whereas dietary levels provide protection against atherosclerosis in humans, rodents, and cell models (Mukherjee et al. 2010). With regard to adiposity, dietary levels of soy were associated with increased adiposity compared with pharmacological levels (Penza et al. 2006; Zanella et al. 2015).
In addition to concentration differences, sex-dependent effects of phytoestrogens have also been reported. For example, a positive association between genistein consumption and obesity has been repeatedly observed in male rodents (Chirumbolo 2015; Penza et al. 2006; Zanella et al. 2015), whereas female rats fed a lifelong diet enriched with soy exhibited significantly lower body weight, less visceral fat, and smaller adipocytes (Kurrat et al. 2015). One explanation for the sex-dependent effects is the likelihood that phytoestrogens may be also activating ERs in adipose tissue. Unlike , activated ERs are anti-adipogenic (Davis et al. 2013). Thus, it is possible that gender differences in circulating estrogen levels may impact the relative effect of phytoestrogens on ERs or in males versus females, and perhaps disrupt the balance between the somewhat differing biological actions of the two receptors’ responses.
Conclusions
The present studies demonstrate that the pro- or anti-adipogenic effects of phytoestrogens were related to their ability to activate or inhibit signaling in adipocytes. These findings provide a possible mechanistic explanation for epidemiological studies that associate soy consumption with adiposity and resveratrol with lean body mass. Given the current challenges in obesity treatment and prevention, it will be important to further characterize the adipogenic activities of phytochemicals and the potential impact of their consumption toward human health.
Supplementary Material
References
- Alavanja MC, Samanic C, Dosemeci M, Lubin J, Tarone R, Lynch CF, et al. 2003. Use of agricultural pesticides and prostate cancer risk in the Agricultural Health Study cohort. Am J Epidemiol 157(9):800–814, PMID: 12727674, 10.1093/aje/kwg040. [DOI] [PubMed] [Google Scholar]
- Amiot MJ, Riva C, Vinet A. 2016. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev 17(7):573–586, PMID: 27079631, 10.1111/obr.12409. [DOI] [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, et al. 1999. PPARγ is required for placental, cardiac, and adipose tissue development. Mol Cell 4(4):585–595, PMID: 10549290, 10.1016/S1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. 2006. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444(7117):337–342, PMID: 17086191, 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasutto L, Marotta E, Garbisa S, Zoratti M, Paradisi C. 2010. Determination of quercetin and resveratrol in whole blood—implications for bioavailability studies. Molecules 15(9):6570–6579PMID: 20877244, 10.3390/molecules15096570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bility MT, Thompson JT, McKee RH, David RM, Butala JH, Vanden Heuvel JP, et al. 2004. Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol Sci 82(1):170–182, PMID: 15310864, 10.1093/toxsci/kfh253. [DOI] [PubMed] [Google Scholar]
- Calleri E, Pochetti G, Dossou KSS, Laghezza A, Montanari R, Capelli D, et al. 2014. Resveratrol and its metabolites bind to PPARs. Chembiochem 15(8):1154–1160, PMID: 24796862, 10.1002/cbic.201300754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, et al. 2009. Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. J Expo Sci Environ Epidemiol 19(2):223–234, PMID: 18665197, 10.1038/jes.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals-Casas C, Desvergne B. 2011. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol 73(1):135–162, PMID: 21054169, 10.1146/annurev-physiol-012110-142200. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2015. Overweight & obesity. http://www.cdc.gov/obesity/data/adult.html. [accessed 17 May 2016].
- Cederroth CR, Nef S. 2009. Soy, phytoestrogens and metabolism: a review. Mol Cell Endocrinol 304(1–2):30–42, PMID: 19433245, 10.1016/j.mce.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Chiarelli F, Di Marzio D. 2008. Peroxisome proliferator-activated receptor-γ agonists and diabetes: current evidence and future perspectives. Vasc Health Risk Manag 4(2):297–304, PMID: 18561505, 10.2147/VHRM.S993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirumbolo S. 2015. Genistein as a nature-derived PPAR agonist in adipogenesis and weight gain. Eur J Nutr 54(3):489–491, PMID: 25662822, 10.1007/s00394-015-0848-7. [DOI] [PubMed] [Google Scholar]
- Corton JC, Lapinskas PJ. 2005. Peroxisome proliferator-activated receptors: mediators of phthalate ester-induced effects in the male reproductive tract? Toxicol Sci 83(1):4–17, PMID: 15496498, 10.1093/toxsci/kfi011. [DOI] [PubMed] [Google Scholar]
- Davis KE, Neinast MD, Sun K, Skiles WM, Bills JD, Zehr JA, et al. 2013. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol Metab 2(3):227–242, PMID: 24049737, 10.1016/j.molmet.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 30(4):293–342, PMID: 19502515, 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elobeid MA, Padilla MA, Brock DW, Ruden DM, Allison DB. 2010. Endocrine disruptors and obesity: an examination of selected persistent organic pollutants in the NHANES 1999–2002 data. Int J Environ Res Public Health 7(7):2988–3005, PMID: 20717554, 10.3390/ijerph7072988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti P, Sawaya BP, Custer LJ, Franke AA. 1999. Serum levels and metabolic clearance of the isoflavones genistein and daidzein in hemodialysis patients. J Am Soc Nephrol 10(4):864–871, PMID: 10203372. [DOI] [PubMed] [Google Scholar]
- Ferrières J. 2004. The French paradox: lessons for other countries. Heart 90(1):107–111, PMID: 14676260, 10.1136/heart.90.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. 2016. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 315(21):2284–2291, PMID: 27272580, 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd ZE, Wang ZQ, Kilroy G, Cefalu WT. 2008. Modulation of peroxisome proliferator–activated receptor γ stability and transcriptional activity in adipocytes by resveratrol. Metabolism 57(7 Suppl 1):S32–S38, PMID: 18555852, 10.1016/j.metabol.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner CD, Chatterjee LM, Franke AA. 2009. Effects of isoflavone supplements vs. soy foods on blood concentrations of genistein and daidzein in adults. J Nutr Biochem 20(3):227–234, PMID: 18602820, 10.1016/j.jnutbio.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Skinner MA. 2007. Polyphenols from fruits and vegetables in weight management and obesity control. In: Obesity: Epidemiology, Pathophysiology, and Prevention. Bagchi D, Preuss HG, eds. Boca Raton:CRC Press, 321–337. [Google Scholar]
- Hall JM, Couse JF, Korach KS. 2001. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276(40):36869–36872, PMID: 11459850, 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- Hall JM, Korach KS. 2002. Analysis of the molecular mechanisms of human estrogen receptors α and β reveals differential specificity in target promoter regulation by xenoestrogens. J Biol Chem 277(46):44455–44461, PMID: 12200415, 10.1074/jbc.M200849200. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. 2007. The molecular mechanisms underlying the proinflammatory actions of thiazolidinediones in human macrophages. Mol Endocrinol 21(8):1756–1768, PMID: 17488971, 10.1210/me.2007-0060. [DOI] [PubMed] [Google Scholar]
- Hobson K. 2010. Resveratrol and CoQ10 supplements are popular—but unproven. https://health.usnews.com/health-news/managing-your-healthcare/heart/articles/2010/02/12/resveratrol-and-coq10-supplements-are-popularbut-unproven [accessed 20 January 2019].
- Hummasti S, Hong C, Bensinger SJ, Tontonoz P. 2008. HRASLS3 is a PPARγ-selective target gene that promotes adipocyte differentiation. J Lipid Res 49(12):2535–2544, PMID: 18664718, 10.1194/jlr.M800269-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ. 2003. Activation of PPARα and PPARγ by environmental phthalate monoesters. Toxicol Sci 74(2):297–308, PMID: 12805656, 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- Irvine CH, Shand N, Fitzpatrick MG, Alexander SL. 1998. Daily intake and urinary excretion of genistein and daidzein by infants fed soy- or dairy-based infant formulas. Am J Clin Nutr 68(6 Suppl):1462S–1465S, PMID: 9848517, 10.1093/ajcn/68.6.1462S. [DOI] [PubMed] [Google Scholar]
- Jungbauer A, Medjakovic S. 2014. Phytoestrogens and the metabolic syndrome. J Steroid Biochem Mol Biol 139:277–289, PMID: 23318879, 10.1016/j.jsbmb.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Kim E, Woo MS, Qin L, Ma T, Beltran CD, Bao Y, et al. 2015. Daidzein augments cholesterol homeostasis via ApoE to promote functional recovery in chronic stroke. J Neurosci 35(45):15113–15126, PMID: 26558782, 10.1523/JNEUROSCI.2890-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings E, Timmers S, Boekschoten MV, Goossens GH, Jocken JW, Afman LA, et al. 2014. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int J Obes (Lond) 38(3):470–473, PMID: 23958793, 10.1038/ijo.2013.155. [DOI] [PubMed] [Google Scholar]
- Kurrat A, Blei T, Kluxen FM, Mueller DR, Piechotta M, Soukup ST, et al. 2015. Lifelong exposure to dietary isoflavones reduces risk of obesity in ovariectomized Wistar rats. Mol Nutr Food Res 59(12):2407–2418, PMID: 26346629, 10.1002/mnfr.201500240. [DOI] [PubMed] [Google Scholar]
- Lampen A, Zimnik S, Nau H. 2003. Teratogenic phthalate esters and metabolites activate the nuclear receptors PPARs and induce differentiation of F9 cells. Toxicol Appl Pharmacol 188(1):14–23, PMID: 12668118, 10.1016/S0041-008X(03)00014-0. [DOI] [PubMed] [Google Scholar]
- le Maire A, Grimaldi M, Roecklin D, Dagnino S, Vivat-Hannah V, Balaguer P, et al. 2009. Activation of RXR-PPAR heterodimers by organotin environmental endocrine disruptors. EMBO Rep 10(4):367–373, PMID: 19270714, 10.1038/embor.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, et al. 2002. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 41(21):6640–6650, PMID: 12022867, 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- Lehrke M, Pascual G, Glass CK, Lazar MA. 2005. Gaining weight: the Keystone Symposium on PPAR and LXR. Genes Dev 19(15):1737–1742, PMID: 16077002, 10.1101/gad.1341005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Ward WF. 2006. PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ 30(4):145–151, PMID: 17108241, 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- Mahajan MA, Samuels HH. 2008. Nuclear receptor coactivator/coregulator NCoA6(NRC) is a pleiotropic coregulator involved in transcription, cell survival, growth and development. Nucl Recept Signal 6:e002, PMID: 18301782, 10.1621/nrs.06002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney EK, Waxman DJ. 1999. trans-Activation of PPARα and PPARγ by structurally diverse environmental chemicals. Toxicol Appl Pharmacol 161(2):209–218, PMID: 10581215, 10.1006/taap.1999.8809. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, et al. 1995. The nuclear receptor superfamily: the second decade. Cell 83(6):835–839, PMID: 8521507, 10.1016/0092-8674(95)90199-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan JA. 2001. Environmental signaling: what embryos and evolution teach us about endocrine disrupting chemicals. Endocr Rev 22(3):319–341, PMID: 11399747, 10.1210/edrv.22.3.0432. [DOI] [PubMed] [Google Scholar]
- Mellbye FB, Jeppesen PB, Hermansen K, Gregersen S. 2015. Cafestol, a bioactive substance in coffee, stimulates insulin secretion and increases glucose uptake in muscle cells: studies in vitro. J Nat Prod 78(10):2447–2451, PMID: 26465380, 10.1021/acs.jnatprod.5b00481. [DOI] [PubMed] [Google Scholar]
- Messina M. 2014. Soy foods, isoflavones, and the health of postmenopausal women. Am J Clin Nutr 100 (Suppl 1):423S–430S, PMID: 24898224, 10.3945/ajcn.113.071464. [DOI] [PubMed] [Google Scholar]
- Mettu NB, Stanley TB, Dwyer MA, Jansen MS, Allen JE, Hall JM, et al. 2007. The nuclear receptor-coactivator interaction surface as a target for peptide antagonists of the peroxisome proliferator-activated receptors. Mol Endocrinol 21(10):2361–2377, PMID: 17595321, 10.1210/me.2007-0201. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Dudley JI, Das DK. 2010. Dose-dependency of resveratrol in providing health benefits. Dose Response 8(4):478–500, PMID: 21191486, 10.2203/dose-response.09-015.Mukherjee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myasoedova VA, Kirichenko TV, Melnichenko AA, Orekhova VA, Ravani A, Poggio P, et al. 2016. Anti-atherosclerotic effects of a phytoestrogen-rich herbal preparation in postmenopausal women. Int J Mol Sci 17(8):E1318, PMID: 27529226, 10.3390/ijms17081318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR. 2011. Contribution of endocrine disrupting chemicals to the obesity epidemic: consequences of developmental exposure. In: Multi-System Endocrine Disruption. Bourguignon JP, Jégou B., Kerdelhué B., Toppari J., Christen Y, eds. Berlin, Germany:Springer-Verlag, 101–112. [Google Scholar]
- Ohashi M, Gamo K, Tanaka Y, Waki M, Beniyama Y, Matsuno K, et al. 2015. Structural design and synthesis of arylalkynyl amide-type peroxisome proliferator-activated receptor γ (PPARγ)-selective antagonists based on the helix12-folding inhibition hypothesis. Eur J Med Chem 90:53–67, PMID: 25461311, 10.1016/j.ejmech.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Ørgaard A, Jensen L. 2008. The effects of soy isoflavones on obesity. Exp Biol Med (Maywood) 233(9):1066–1080, PMID: 18535167, 10.3181/0712-MR-347. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Jefferson W. 2010. The pros and cons of phytoestrogens. Front Neuroendocrinol 31(4):400–419, PMID: 20347861, 10.1016/j.yfrne.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penza M, Montani C, Romani A, Vignolini P, Pampaloni B, Tanini A, et al. 2006. Genistein affects adipose tissue deposition in a dose-dependent and gender-specific manner. Endocrinology 147(12):5740–5751, PMID: 16959845, 10.1210/en.2006-0365. [DOI] [PubMed] [Google Scholar]
- Powell E, Kuhn P, Xu W. 2007. Nuclear receptor cofactors in PPARγ-mediated adipogenesis and adipocyte energy metabolism. PPAR Res 2007:53843, PMID: 17389765, 10.1155/2007/53843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts ML, Moore DD, Banz WJ, Mezei O, Shay NF. 2005. Molecular mechanisms of action of the soy isoflavones includes activation of promiscuous nuclear receptors. A review. J Nutr Biochem 16(6):321–330, PMID: 15936643, 10.1016/j.jnutbio.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Rietjens IMCM, Louisse J, Beekmann K. 2017. The potential health effects of dietary phytoestrogens. Br J Pharmacol 174(11):1263–1280, PMID: 27723080, 10.1111/bph.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccisano D, Henneberg M. 2012. Soy consumption and obesity. Food Nutr Sci 3(2):17532, 10.4236/fns.2012.32038. [DOI] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. 1999. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4(4):611–617, PMID: 10549292, 10.1016/S1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Salam NK, Huang THW, Kota BP, Kim MS, Li Y, Hibbs DE. 2008. Novel PPAR-γ agonists identified from a natural product library: a virtual screening, induced-fit docking and biological assay study. Chem Biol Drug Des 71(1):57–70, PMID: 18086153, 10.1111/j.1747-0285.2007.00606.x. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Howard GJ, Hurst CH, Emberley JK, Waxman DJ, Webster T, et al. 2004. Environmental and endogenous peroxisome proliferator-activated receptor γ agonists induce bone marrow B cell growth arrest and apoptosis: interactions between mono(2-ethylhexyl)phthalate, 9-cis-retinoic acid, and 15-deoxy-Δ12,14-prostaglandin J2. J Immunol 173(5):3165–3177, PMID: 15322177, 10.4049/jimmunol.173.5.3165. [DOI] [PubMed] [Google Scholar]
- Schug TT, Johnson AF, Birnbaum LS, Colborn T, Guillette LJ Jr, Crews DP, et al. 2016. Minireview: endocrine disruptors: past lessons and future directions. Mol Endocrinol 30(8):833–847, PMID: 27477640, 10.1210/me.2016-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D, Rangwala SM, Bailey ST, Krakow SL, Reginato MJ, Lazar MA. 1998. Interdomain communication regulating ligand binding by PPAR-γ. Nature 396(6709):377–380, PMID: 9845075, 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- Sharma R, Sharma NK, Thungapathra M. 2017. Resveratrol regulates body weight in healthy and ovariectomized rats. Nutr Metab (Lond) 14:30, PMID: 28413432, 10.1186/s12986-017-0183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P, Liu MH, Ng TY, Chan YH, Yong EL. 2006. Differential effects of isoflavones, from Astragalus membranaceus and Pueraria thomsonii, on the activation of PPARα, PPARγ, and adipocyte differentiation in vitro. J Nutr 136(4):899–905, PMID: 16549448, 10.1093/jn/136.4.899. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM. 1998. PPAR-γ: adipogenic regulator and thiazolidinedione receptor. Diabetes 47(4):507–514, PMID: 9568680, 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- Tang-Péronard JL, Andersen HR, Jensen TK, Heitmann BL. 2011. Endocrine-disrupting chemicals and obesity development in humans: a review. Obes Rev 12(8):622–636, PMID: 21457182, 10.1111/j.1467-789X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. 2011. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab 14(5):612–622, PMID: 22055504, 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. 1994. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 79(7):1147–1156, PMID: 8001151, 10.1016/0092-8674(94)90006-X. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. 2008. Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem 77:289–312, PMID: 18518822, 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Wang L, Waltenberger B, Pferschy-Wenzig EM, Blunder M, Liu X, Malainer C, et al. 2014. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochem Pharmacol 92(1):73–89, PMID: 25083916, 10.1016/j.bcp.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werman A, Hollenberg A, Solanes G, Bjorbaek C, Vidal-Puig AJ, Flier JS. 1997. Ligand-independent activation domain in the N terminus of peroxisome proliferator-activated receptor gamma (PPARγ). Differential activity of PPARγ1 and -2 isoforms and influence of insulin. J Biol Chem 272(32):20230–20235, PMID: 9242701, 10.1074/jbc.272.32.20230. [DOI] [PubMed] [Google Scholar]
- Wilson T, Knight TJ, Beitz DC, Lewis DS, Engen RL. 1996. Resveratrol promotes atherosclerosis in hypercholesterolemic rabbits. Life Sci 59(1):PL15–PL21, PMID: 8684261, 10.1016/0024-3205(96)00260-3. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chin WW, Wang Y, Burris TP. 2003. Ligand and coactivator identity determines the requirement of the charge clamp for coactivation of the peroxisome proliferator-activated receptor γ. J Biol Chem 278(10):8637–8644, PMID: 12502716, 10.1074/jbc.M210910200. [DOI] [PubMed] [Google Scholar]
- Zanella I, Marrazzo E, Biasiotto G, Penza M, Romani A, Vignolini P, et al. 2015. Soy and the soy isoflavone genistein promote adipose tissue development in male mice on a low-fat diet. Eur J Nutr 54(7):1095–1107, PMID: 25341395, 10.1007/s00394-014-0786-9. [DOI] [PubMed] [Google Scholar]
- Zebisch K, Voigt V, Wabitsch M, Brandsch M. 2012. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal Biochem 425(1):88–90, PMID: 22425542, 10.1016/j.ab.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Zhang XH, Huang B, Choi SK, Seo JS. 2012. Anti-obesity effect of resveratrol-amplified grape skin extracts on 3T3-L1 adipocytes differentiation. Nutr Res Pract 6(4):286–293, PMID: 22977681, 10.4162/nrp.2012.6.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



![Figure 1A shows a line graph with standard error of mean plotting fold response on the y-axis across minus Log [M] Ligand on the x-axis for Rosiglitazone, Genistein, Daidzein, and Resveratrol. Figure 1B shows a line graph with standard error of mean plotting fold response on the y-axis across minus Log [M] Ligand on the x-axis for Resveratrol, GW9662, Rosiglitazone plus Resveratrol, and Rosiglitazone plus GW9662. Figure 1C shows a line graph with standard error of mean plotting fold response on the y-axis across minus Log [M] Ligand on the x-axis for Genistein, Genistein plus GW9662, Daidzein, and Daidzein plus GW9662. Figure 1D shows a bar graph with standard error of mean plotting fold response on the y-axis across treatment with vehicle, Genistein, and Daidzein on the x-axis for minus Rosiglitazone and plus Rosiglitazone. Figure 1E shows a bar graph with standard error of mean plotting fold response on the y-axis across treatment with Vehicle, Rosiglitazone, Genistein, Daidzein, and Resveratrol on the x-axis.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/7722/6768326/82aa310750f6/ehp3444_f1.jpg)