Abstract
Parainfectious optic neuritis is a very rare cause of acute vision loss. We present a case of a 51-year-old man with a recent upper respiratory tract infection, presumably of viral aetiology, who showed up with complains of painless right eye vision loss, followed by the same symptoms on the left eye 3 weeks later. Ophthalmological examination revealed optic disc swelling (sequential in severity) which was confirmed by optic disc imaging. The remaining evaluations (lumbar puncture, MRI, laboratory and genetic testing) were completely normal. Considering a postviral aetiology, 5-day intravenous methylprednisolone treatment was performed. Follow-up examinations revealed slight visual acuity and visual fields recovery, with subsequent optic disc atrophy and microcystic macular oedema, bilaterally. This case illustrates how important a correct clinical history is to guide a correct diagnosis and posterior management.
Keywords: neuroopthalmology, ophthalmology, retina
Background
Parainfectious optic neuritis is defined when optic nerve involvement occurs after presumably/confirmed systemic infectious disease, particularly of viral aetiology. The pathogenesis behind this disease is still uncertain but an autoimmune mechanism is the most probable cause.1 It is more common in children than in adults but due to its rarity, precise epidemiological characterisation is not possible. Differential diagnosis is extensive, particularly with other subtypes of optic neuropathies. To date, there is limited information about this entity in the literature.
In 2012, Gelfand et al originally described the presence of small vacuoles at the inner nuclear layer (INL) of the retina in patients with multiple sclerosis (MS).2 Since then, this retinal microcystic macular oedema has been linked to several optic neuropathies—besides MS, neuromyelitis optica, Leber’s hereditary optic neuropathy, dominant optic atrophy, isolated relapsing optic neuropathy and glaucoma, for example.3–6 The prevalence of these changes has been reported in only a small fraction of patients with optic neuropathies, ranging between 5% and 25% and its true pathophysiology and clinical significance are still unclear.7
We present a case of bilateral parainfectious (postviral) optic neuritis followed by microcystic macular oedema, describing its clinical features and progression, intending to highlight the relevance of a precise clinical history and an extensive work-up to allow correct diagnosis and treatment which are crucial for visual recovery and long-term prognosis.
Case presentation
A 51-year-old man presented to our ophthalmology department complaining of acute, painless loss of vision in the right eye (OD). The patient referred as the only remarkable medical history an upper respiratory tract infection 2 weeks before visual symptoms onset, treated with oral ibuprofen 400 mg three times a day and oral paracetamol 1000 mg three times a day during 5 days. He denied any relevant ocular or familial medical history and was not taking any medication.
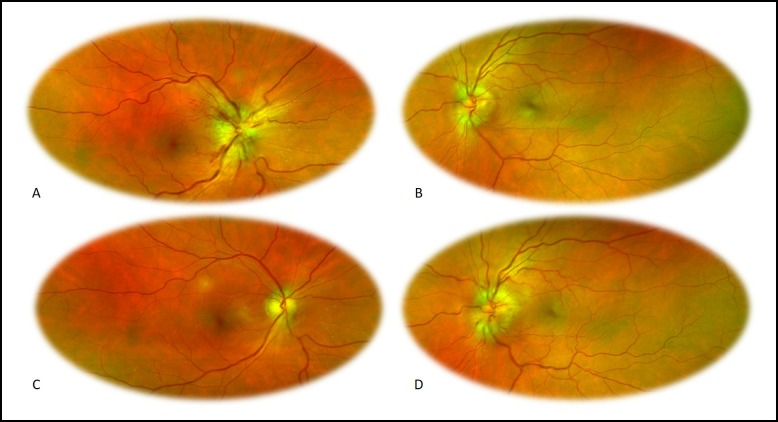
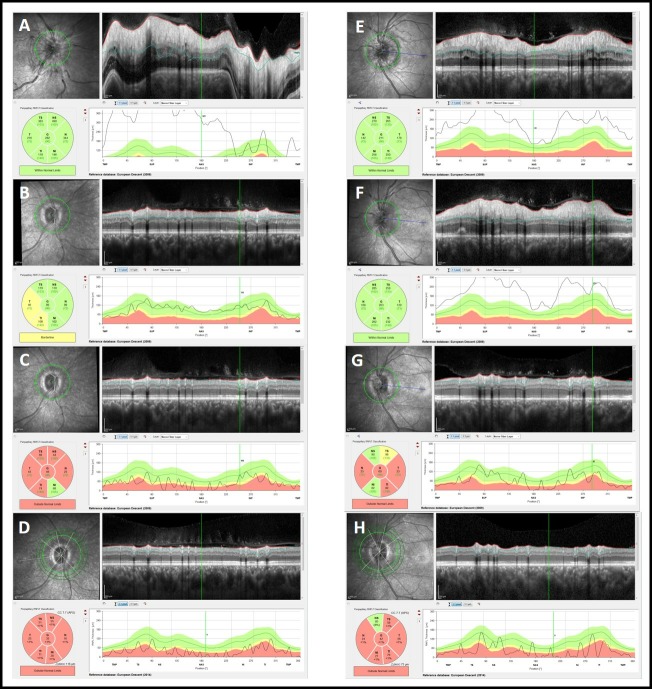
At the first visit, best corrected visual acuity (BCVA) was 20/40 in the OD, 20/20 in the left eye (OS) and a right relative afferent pupillary defect was present. External examination was normal and extraocular motility was full. Anterior segment examination was unremarkable, with an intraocular pressure of 14 mm Hg in both eyes (OU). Dilated funduscopy (figure 1) revealed a right optic disc swelling (2 D), with blurring of all disc margin, retinal nerve fibre layer oedema, visible peripapillary splinter haemorrhages, venous dilatation and tortuosity, with loss of physiologic cup, disc hyperaemia, Paton lines and hard exudates. Spontaneous venous pulsations were present and there were no signs of nerve fibre layer infarct. The left optic disc showed just subtle swelling, with blurred margins and retinal nerve fibre layer oedema, both predominantly superior and inferiorly. No other findings were visible, including vitritis or any signs of vasculitis/retinitis. Optical coherence tomography (OCT) (figure 2) revealed an elevation of peripapillary retinal nerve fibre layer thickness in both eyes, mostly marked in the right eye. On fluorescein angiography, bilateral optic disc hyperfluorescence with right optic disc leakage was present. MRI and the first blood tests (including C reactive protein (CRP) and erythrocyte sedimentation rate) were completely normal.
Figure 1.
Images obtained with Optos ultra-widefield (UWF) optomap imaging technology: (A) right eye retinography, first visit; (B) left eye retinography, first visit; (C) right eye retinography, 3 weeks later; (D) left eye retinography, 3 weeks later.
Figure 2.
Optical coherence tomography (Spectralis, Heidelberg Engineering, Germany, V.6.0) from baseline until last follow-up, revealing peripapillary retinal nerve fibre thickness: (A) right eye, baseline; (B) right eye, 1 month; (C) right eye, 3 months; (D) right eye, 5 months; (E) left eye, baseline; (F) left eye, 1 month; (G) left eye, 3 months; (H) left eye, 5 months.
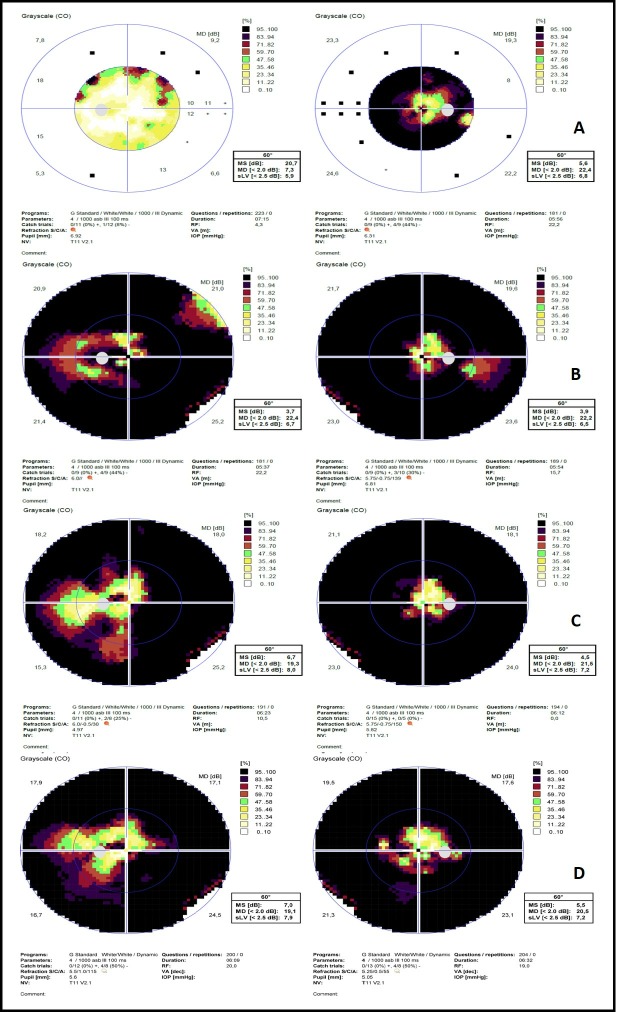
Three weeks later, his BCVA was 20/100 OD and decreased to 20/40 in OS, with a pale right optic disc and a more evident left optic disc swelling (1 D), with blurring of all disc margin, retinal nerve fibre layer oedema, an inferior located peripapillary splinter haemorrhage, venous dilatation and tortuosity, with loss of physiologic cup, disc hyperaemia and Paton lines. Spontaneous venous pulsations were present and there were no signs of hard exudates and nerve fibre layer infarct (figure 1). Standard automated perimetry revealed a significant bilateral visual field constriction, with decreased visual field threshold sensitivity, sparing some central field in both eyes and temporal field in the OD (figure 3).
Figure 3.
Automated static perimetry (Oc Tupus 900, Haag-Streit Diagnostics, Switzerland) showing MD and sLV values: (A) baseline; (B) 1 month; (C) 3 months; (D) 9 months. IOP, intraocular pressure; MD, medium deviation; MS, multiple sclerosis; sLV, square root of loss variance; RF, reliability factor; NV, normal value; VA, visual acuity.
Investigations
Laboratory testing: haematologic counts, coagulation tests, ionogram, erythrocyte sedimentation rate (ESR), C reactive protein and serum angiotensin-converting enzyme levels were normal. Infectious serologic tests were all negative/non-reactive (HIV, Hepatitis C virus, Hepatitis B virus, Epstein-Barr virus, Herpes-Simplex Virus 1, Herpes-Simplex Virus 2, influenzae, varicella zoster, Bartonella quintana, Borrelia burgdorferi, Brucella, toxoplasmosis and syphilis) for acute infection. Antinuclear antibodies, perinuclear antineutrophil cytoplasmic antibodies (p-ANCAs), cytoplasmic ANCAs (c-ANCAs), anticardiolipin antibodies (ACAs), antiphospholipidic antibodies, antiaquaporin 4 (AQ4) antibodies and HLA-B27 were also negative.
Lumbar puncture: cerebrospinal fluid analysis revealed no cytochemical changes and no oligoclonal bands. Viral panel, TPHA, anti-Borrelia burgdorferi antibodies and bacterial direct and cultural tests were negatives. The opening pressure was also normal.
Genetic testing: mtDNA G11778A, G3460A and T14484C mutations were not present.
MRI: encephalic parenchyma without focal lesions, without any signs of demyelinating disease of the central nervous system. No space-occupying lesion was present. Optic nerves were with normal morphology, without T2 hyper sign.
OCT: sequential examinations are shown in figure 2.
Standard automated perimetry: sequential examinations are shown in figure 3.
Treatment
Five-day intravenous methylprednisolone (1000 mg/day)
Outcome and follow-up
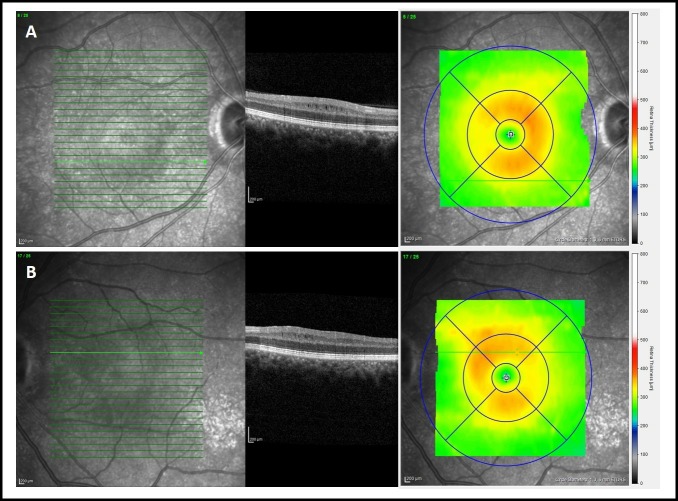
At last follow-up (5 months after first evaluation) BCVA increased to 20/63 in OD and 20/25 in OS. An improvement in visual field defects was also shown. Both eyes had a significantly reduced retinal nerve fibre layer thickness and the presence of microcystic macular oedema exclusively at the INL (figure 4).
Figure 4.
Optical coherence tomography (Spectralis, Heidelberg Engineering, Germany, V.6.0) revealing last follow-up macular thickness due to microcystic oedema: (A) right eye; (B) left eye.
Discussion
In this report, we present a case of simultaneous (but sequential in severity) bilateral optic disc swelling, associated with an important decreased in both eyes’ visual acuity, followed by an improvement after corticosteroids treatment.
Bilateral optic disc swelling represents a diagnostic challenge due to the diversity of possible aetiologies (vascular, neurologic, autoimmune, genetic and infectious). In adults, studies reveal that papilloedema (due to increased intracranial pressure) remains the most frequent cause, generally followed by pseudopapilloedema and optic neuritis.8 9 Both papilloedema and pseudopapilloedema were excluded, the first by MRI findings and the second by optic disc imaging. Considering vascular causes of optic disc swelling, bilateral involvement is very rare. Besides, our patient’s age, the absence of comorbidities and normal laboratory findings excluded both arthritic and non-arthritic optic neuropathies as a possible diagnosis. A genetic cause, like Leber’s hereditary optic neuropathy was also considered but the disease usually manifests between the second and fourth decades, with a subacute presentation. Also, optic disc appearance did not reveal the presence of telangiectatic microangiopathy and genetic testing was negative.
Lastly, after excluding the diseases referred previously, bilateral involvement with the presence of RAPD confirmed optic neuritis as the diagnosis and the possibility of an atypical optic neuritis was explored. Optic neuritis may occur at any age, but clinical characteristics differ by age group. Unlike children, in whom a bilateral presentation is most frequent (60%–70%), in adults, particularly in individuals without any history of an inflammatory or autoimmune disorder, unilateral disease is responsible for 70% of all cases, being most frequent in the female gender.10 The clinical history reported previously clearly does not reveal a typical adult optic neuritis case: our patient had >50 years and presented with a painless vision loss with bilateral involvement and disc swelling—characteristics of atypical optic neuritis.11
To date, due to its rarity, very few case series describe the course, recovery and outcome in adults with bilateral (simultaneous or sequential) optic neuritis.12–14 Unlike unilateral cases, in adults, bilateral ones are more frequent among men but the reasons for this gender asymmetry remain unclear.12–14 Despite being more frequent in children, even in adults some of these cases may have a postviral aetiology.1 12
Parainfectious aetiology is generally accompanied by optic disc swelling, with ocular manifestations showing up days to weeks after infection (between 14 and 28 days according to one report).1 MRI usually reveals optic nerve enhancement, but some cases do not reveal any imaging finding. A viral infection is the most frequent cause, but the causative agent may not be identified. Even in the presence of negative serologies, patients with a history suggesting a preceding infection should be treated appropriately.1 Regarding treatment, there are no defined guidelines, but due to the relatively benign adverse effect profile, a limited course of intravenous corticosteroids is the most chosen option, with or without antiviral coverage as well.1 12 In general, visual recovery is good.
In this particular case, after excluding the most probable causes behind this clinical scenario and taking into account patient’ history of previous upper respiratory tract infection, we consider bilateral parainfectious optic neuritis to be the most probable answer to this diagnostic challenge, proving the relevance of medical history to guide ophthalmologic differential diagnosis.
Finally, last follow-up OCT revealed the presence of microcystic oedema at the INL. This finding has been recently associated not only with several optic neuropathies but also with some retinal entities, like age-related macular degenerations or retinal telangiectasis.15 16 Most published studies reveal a tendency for the oedema to be perifoveal with an annular shape with predilection for the nasal side of the macula, as we found in our patient.7 Its pathogenesis is still on debate due to the scarcity of longitudinal studies. Its relationship with optic disc atrophy, as our patient’s case, suggest retrograde trans-synaptic degeneration as a possible causative factor. The location of Müller cells at the INL makes these cells dysfunction a very promising theory for microcysts production, probably due to a change in potassium channel Kir4.1 and AQ4 regulation.17 Some other authors postulate a role for retinal traction to explain the nasal predominance but many cases of microcystic do not reveal any signs of traction.7 The clinical significance of this finding remains unclear but some papers reveal a relationship between its presence and poorer anatomical (more significant retinal nerve fibre loss) and functional outcome.3–5 To conclude, the 3 weeks delay of treatment onset associated with bilateral microcystic INL oedema may explain our patient outcome, with only a slight improvement in visual acuity and visual fields.
Learning points.
Parainfectious optic neuritis occurs after presumably/confirmed systemic infectious disease (frequently viral), and bilateral involvement is the rule.
There is scarce documentation on the literature about this entity, due to its rarity, which makes this aetiology diagnosis of exclusion.
There are no treatment guidelines but intravenous corticosteroids are the most frequent option.
Further investigation and reports are necessary to elucidate disease pathogenesis and to guide proper management.
Microcystic macular oedema at the inner nuclear layer may happen in a minority of optic neuropathies cases, being probably related to retrograde trans-synaptic degeneration involving Müller cells—its clinical significance is unclear.
Footnotes
Contributors: DH-F: data acquisition, data analysis and paper conception. MEL: data analysis, paper conception. MT: data acquisition. JT-F: data acquisition, data analysis and paper revision.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Rappoport D, Goldenberg-Cohen N, Luckman J, et al. Parainfectious optic neuritis: manifestations in children vs adults. J Neuroophthalmol 2014;34:122–9. 10.1097/WNO.0000000000000113 [DOI] [PubMed] [Google Scholar]
- 2. Gelfand JM, Nolan R, Schwartz DM, et al. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain 2012;135:1786–93. 10.1093/brain/aws098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burggraaff MC, Trieu J, de Vries-Knoppert WA, et al. The clinical spectrum of microcystic macular edema. Invest Ophthalmol Vis Sci 2014;55:952–61. 10.1167/iovs.13-12912 [DOI] [PubMed] [Google Scholar]
- 4. Abegg M, Dysli M, Wolf S, et al. Microcystic macular edema: Retrograde maculopathy caused by optic neuropathy. Ophthalmology 2014;121:142–9. [DOI] [PubMed] [Google Scholar]
- 5. Wolff B, Basdekidou C, Vasseur V, et al. Retinal inner nuclear layer microcystic changes in optic nerve atrophy. Retina 2013;33:2133–8. 10.1097/IAE.0b013e31828e68d0 [DOI] [PubMed] [Google Scholar]
- 6. Kaufhold F, Zimmermann H, Schneider E, et al. Optic neuritis is associated with inner nuclear layer thickening and microcystic macular edema independently of multiple sclerosis. PLoS One 2013;8 10.1371/annotation/f13fb9e2-f441-4e99-bb97-79152da1e74e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bhargava P, Calabresi PA. The expanding spectrum of aetiologies causing retinal microcystic macular change. Brain 2013;136:3212–4. 10.1093/brain/awt295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iijima K, Shimizu K, Ichibe Y. A study of the causes of bilateral optic disc swelling in Japanese patients. Clin Ophthalmol 2014;8:1269–74. 10.2147/OPTH.S61650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hata M, Miyamoto K. Causes and Prognosis of Unilateral and Bilateral Optic Disc Swelling. Neuroophthalmology 2017;41:187–91. 10.1080/01658107.2017.1299766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boomer J, Siatkowski M. Optic neuritis in adults and children. Semin Ophthalmol 2003;18:174–80. 10.1080/08820530390895172 [DOI] [PubMed] [Google Scholar]
- 11. Hoorbakht H, Neuritis O. its Differential Diagnosis and Management. Open Ophthalmol J 2012;6:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de la Cruz J, Kupersmith MJ. Clinical profile of simultaneous bilateral optic neuritis in adults. Br J Ophthalmol 2006;90:551–4. 10.1136/bjo.2005.085399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Du Y, Li K, Yang J, et al. Disc swelling and mild initial visual acuity loss predict a better short-term visual acuity outcome in bilateral acute optic neuritis. J Clin Neurosci 2012;19:1380–2. 10.1016/j.jocn.2011.10.020 [DOI] [PubMed] [Google Scholar]
- 14. Frederiksen JL. Bilateral acute optic neuritis: prospective clinical, MRI, CSF, neurophysiological and HLA findings. Neuro-Ophthalmology 1997;17:175–83. 10.3109/01658109709044663 [DOI] [Google Scholar]
- 15. Cohen SY, Dubois L, Nghiem-Buffet S, et al. Retinal pseudocysts in age-related geographic atrophy. Am J Ophthalmol 2010;150:211–7. 10.1016/j.ajo.2010.02.019 [DOI] [PubMed] [Google Scholar]
- 16. Gaudric A, Ducos de Lahitte G, Cohen SY, et al. Optical coherence tomography in group 2A idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol 2006;124:1410 10.1001/archopht.124.10.1410 [DOI] [PubMed] [Google Scholar]
- 17. Reichenbach A, Wurm A, Pannicke T, et al. Müller cells as players in retinal degeneration and edema. Graefes Arch Clin Exp Ophthalmol 2007;245:627–36. 10.1007/s00417-006-0516-y [DOI] [PubMed] [Google Scholar]