Abstract
A 21-year-old man underwent a joint-preserving posterior acetabular resection of metastatic osteosarcoma using a three-dimensional (3D) printed model and intraoperative navigation. The combined application of these advanced technologies can allow for surgical planning of osteotomies involving complex anatomy and help guide resections intraoperatively. They can maximise the achievement of negative oncological margins, preservation of native hip stability and critical neurovascular structures, and optimal postoperative function in an effort to resect all clinically evident disease. For this particular patient, with secondary bony metastases, they allowed for a safe and well-tolerated procedure that ultimately afforded him palliative benefit, improved quality of life and, conceivably, prolonged survival in the setting of a devastating prognosis. Although he, sadly, has since passed away, he survived for over 2 years after initial metastasis with preserved hip stability and the ability to graduate college, stay active and maintain a quality of life that addressed his goals of care.
Keywords: orthopaedics, palliative procedures, palliative care, end of life decisions (palliative care), orthopaedic and trauma surgery
Background
Although osteosarcomas are uncommon tumours overall, they are the most common primary malignancy of bone and have a great degree of morbidity and mortality. Despite advances in chemotherapeutic agents and surgical technique, prognosis remains poor, particularly for patients with relapsed/metastatic disease.
Performing tumour resections within tightly confined and complex anatomical areas, such as the pelvis, makes it challenging to achieve negative oncological margins, acceptable postoperative function, preservation of critical neurovascular structures and minimal perioperative morbidity, mortality and recurrence.1–6
A number of studies have shown that using three-dimensional (3D) printing to replicate the 3D anatomy for preoperative planning can decrease surgical time, operating room time and blood loss; improve hardware placement; and decrease radiation exposure,7–11 and using intraoperative navigation can reduce intralesional resections and improve disease-free and overall survival.6 12–16
When necessary to offer a patient the best chance at a cure or prolonged survival, performing these complex resections safely is of paramount importance. Procedures that may otherwise not have been recommended or possible can be successfully performed with little to no morbidity with the aid of these advanced technologies.
Case presentation
A 21-year-old otherwise healthy, athletic man initially presented with a history of progressive, unexplained right knee pain for 5 months after a basketball injury, underwent an MRI and was referred to orthopaedic oncology for concerns of osteosarcoma. His diagnosis was confirmed in March 2016 after an open biopsy found grade 3 osteoblastic osteosarcoma of the right proximal tibia. His tumour responded poorly to standard neoadjuvant MAP (methotrexate, adriamycin and cisplatin) chemotherapy before undergoing an above-the-knee amputation in June 2016. Following an additional four cycles of adjuvant MAP chemotherapy, a right proximal humerus metastatic lesion was detected in surveillance scans, for which he underwent wide intercalary resection and allograft reconstruction in January 2017, followed by high-dose ifosfamide and etoposide (I/E) chemotherapy. His pathology confirmed negative tumour margins.
Unfortunately, during his fourth cycle of I/E chemotherapy, a restaging whole-body bone scan showed a new focus of uptake within the left posterior inferior acetabulum in March 2017.
Investigations
Positron emission tomography-CT (figures 1 and 2) and CT images (figures 3–5) demonstrated an approximately 1.4 cm focal cortical-based sclerosis within the posterior left ischium not previously seen. Similarly, an MRI of the pelvis with and without contrast documented a focal, well-circumscribed lesion corresponding to the focus of avid tracer uptake on the bone scan without evidence of soft tissue mass (figures 6 and 7).
Figure 1.
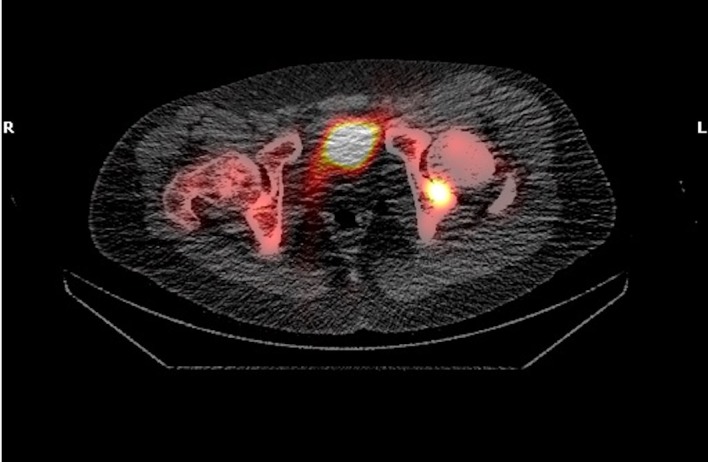
Pelvis positron emission tomography-CT axial view showing focal cortical-based sclerosis within the posterior left ischium.
Figure 2.
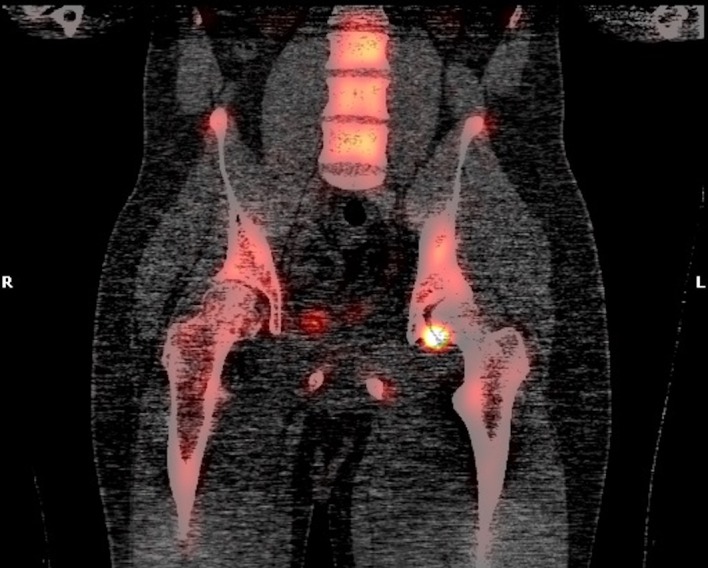
Pelvis positron emission tomography-CT coronal view showing an approximately 1.4 cm focal cortical-based sclerosis within the posterior left ischium.
Figure 3.
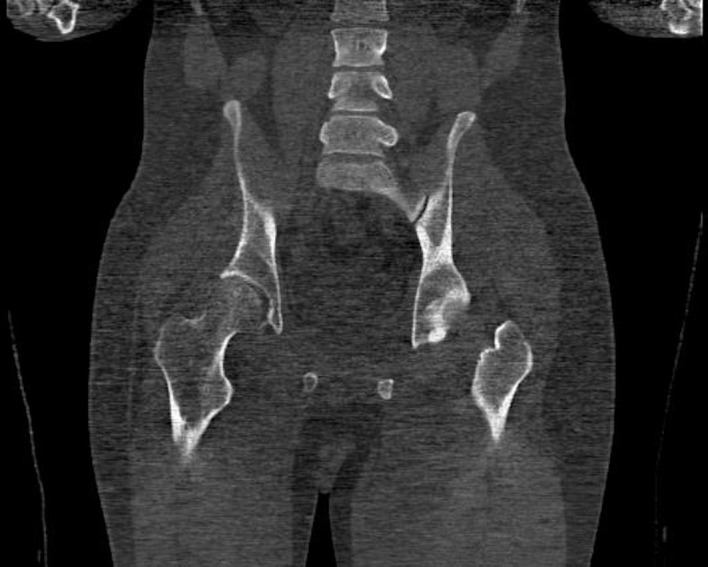
Pelvis CT coronal view showing an approximately 1.4 cm focal cortical-based sclerosis within the posterior left ischium.
Figure 4.
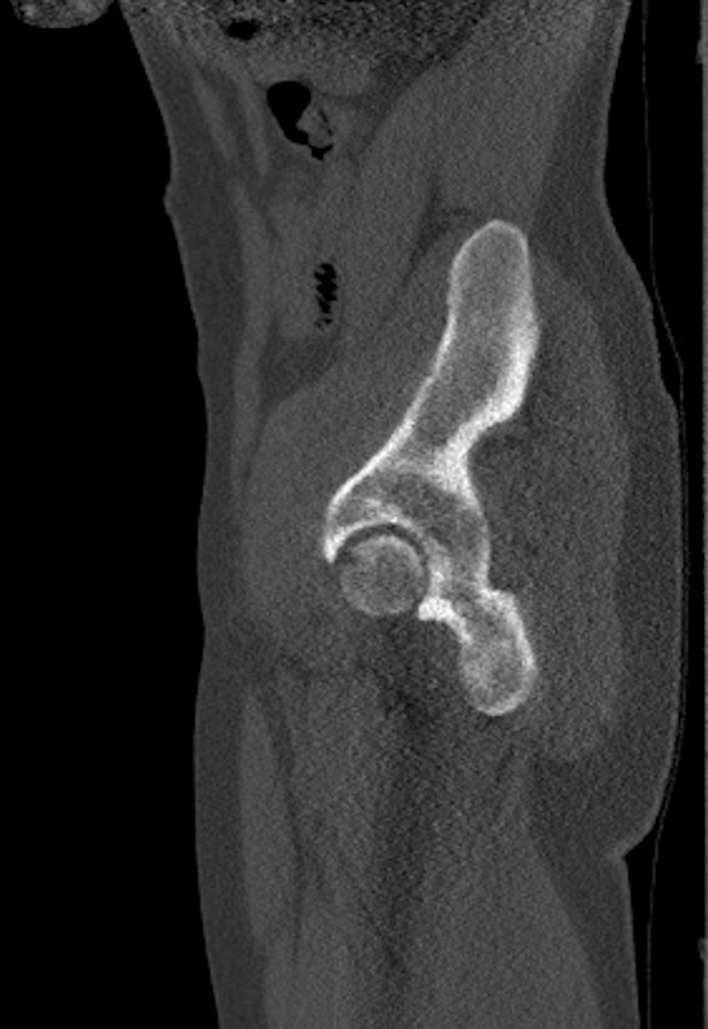
Pelvis CT sagittal view showing an approximately 1.4 cm focal cortical-based sclerosis within the posterior left ischium.
Figure 5.
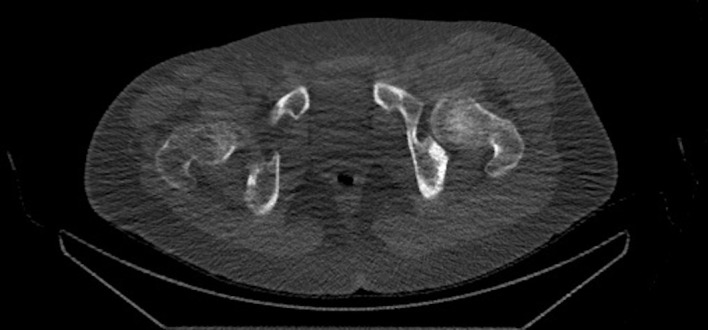
Pelvis CT axial view showing an approximately 1.4 cm focal cortical-based sclerosis within the posterior left ischium.
Figure 6.
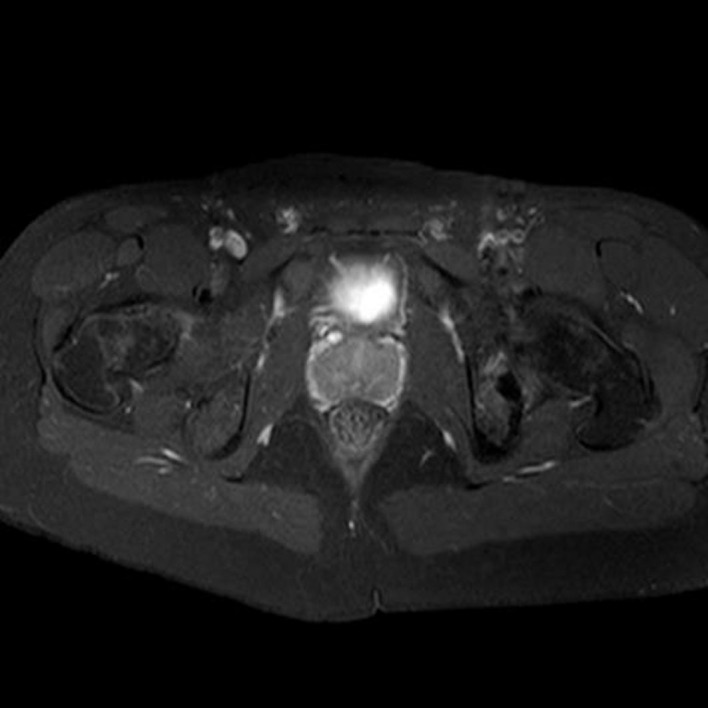
Pelvis T1 MRI with gadolinium contrast axial view demonstrating a focal, well-circumscribed lesion.
Figure 7.
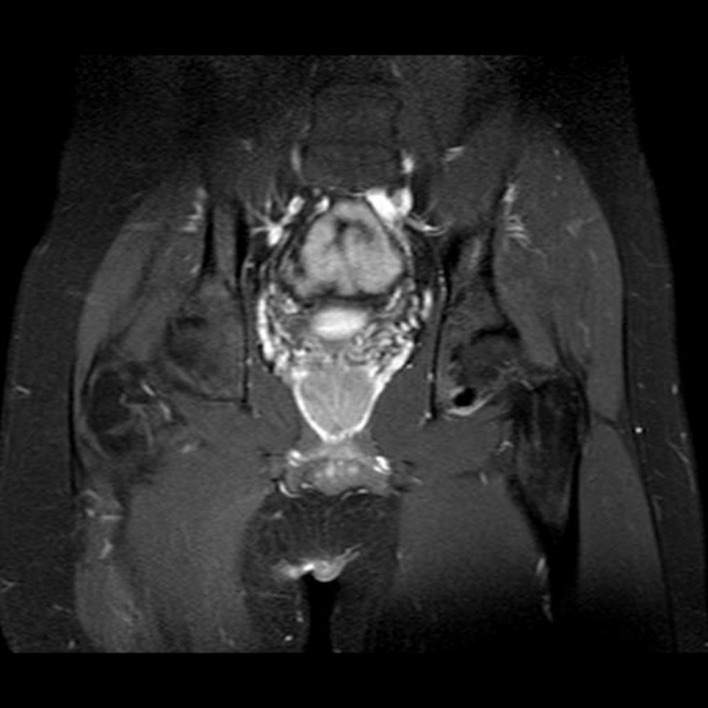
Pelvis T1 MRI with gadolinium contrast coronal view demonstrating a focal, well-circumscribed lesion.
Treatment
Additional cytotoxic agents such as gemcitabine and paclitaxel were discouraged by the patient’s medical oncology team, given the low likelihood of response and likely poor quality of life associated with these therapies.
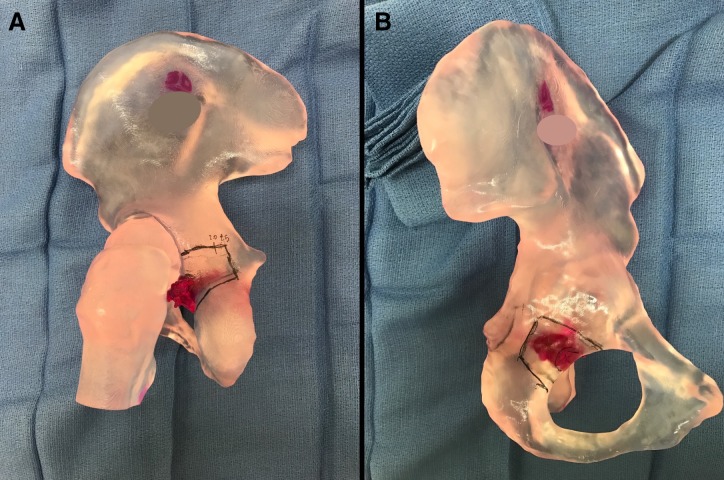
A multidisciplinary team approach with a large team of orthopaedic, medical and radiation oncologists was taken to discuss surgical options, radiation and systemic trials, which were then presented to the patient and his family. One experimental trial, a phase two study of denosumab, permitted patients with the non-measurable disease (according to Response Evaluation Criteria in Solid Tumors or ‘RECIST’ criteria) to enrol given the demonstrated importance of complete surgical remission in the survival of patients with recurrent osteosarcoma.17 18 Thus, the patient decided to pursue resection of the metastatic posterior acetabular lesion. The goal, in doing so, was to eradicate clinically evident disease that would inevitably become debilitating to (a) prolong disease-free and overall survival and (b) offer an experimental adjuvant therapy on a clinical trial that might further improve survival, while maintaining as much hip stability in the process to afford him the best possible quality of life. Given the low likelihood of cure even with ‘aggressive’ resection, optimising safety/minimising morbidity was of foremost concern. A 3D-printed model (printed by medical technology company Onkos Surgical) was therefore used to plan the complex osteotomy that aimed to minimise acetabular bone loss and maximally preserve native hip function and stability (figure 8A,B).
Figure 8.
(A and B) 3D-printed model demonstrating the planned osteotomy.
In April 2017, the patient was taken to the operating room. A Kocher-Langenbeck incision was made over the left hip, extending from distal/lateral to the posterior superior iliac spine (PSIS) to 10 cm distal to the tip of the greater trochanter. The piriformis and short external rotators were reflected to protect the sciatic nerve and the posterior column was exposed. A Medtronic Stealth probe was placed into the PSIS and the Medtronic O-arm was used for an intraoperative CT scan (figure 9). Given the osteoblastic and confined nature of the acetabular lesion, intraoperative navigation allowed for satisfactory image guidance.
Figure 9.
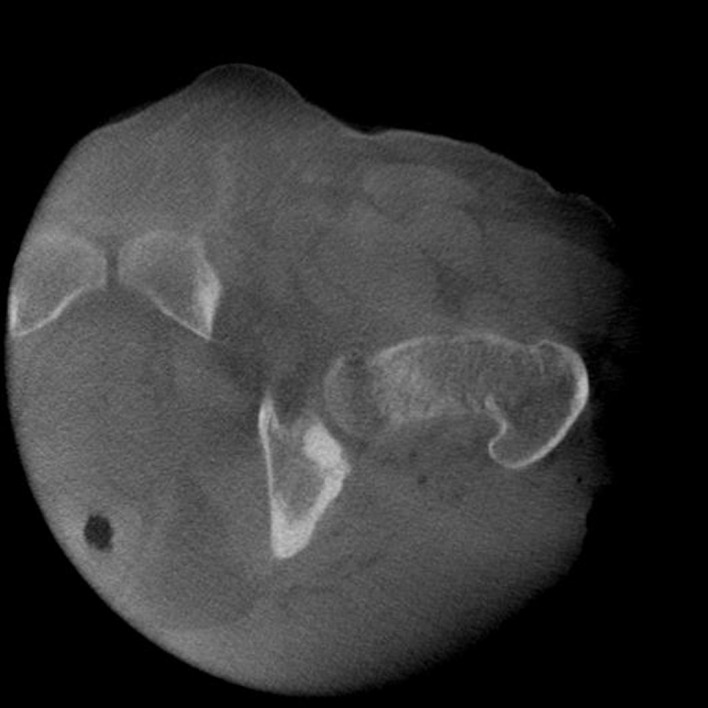
Intraoperative CT by Medtronic O-arm used for Medtronic Stealth navigation.
A Medtronic Stealth navigated drill guide was used to make drill holes along the intended osteotomy template through the ischium, along the posterior column, and transversely to the posterior ilium. A combination of the Misonix BoneScalpel and osteotomes was used to connect the drilled osteotomy template. Following the resection, intraoperative navigation allowed confirmation that the entire lesion was resected with satisfactory margins.
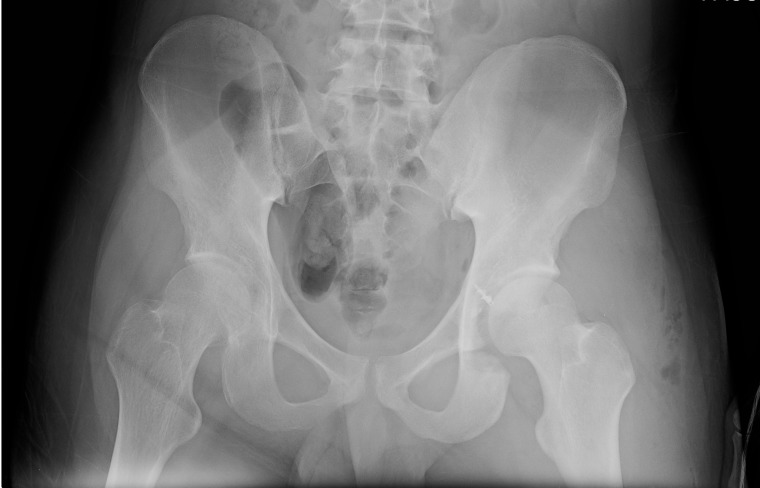
Postoperative radiographs confirmed resection of the left acetabular lesion and a well-located left hip (figure 10). Histological analysis confirmed identical high-grade osteoblastic osteosarcoma with tumour-free margins.
Figure 10.
Postoperative anterior-posterior view of the pelvis.
Outcome and follow-up
Following his surgery, the patient was able to wean off his precautions and return to his athletic and academic pursuits while undergoing surveillance for recurrence of his metastatic disease and being treated on AOST1321 with denosumab. Sadly, at the 5-month postoperative interval, scans showed a significant increase in radiotracer uptake in multiple pelvic areas, lumbar vertebrae, the right humerus and the right second rib (figure 11).
Figure 11.
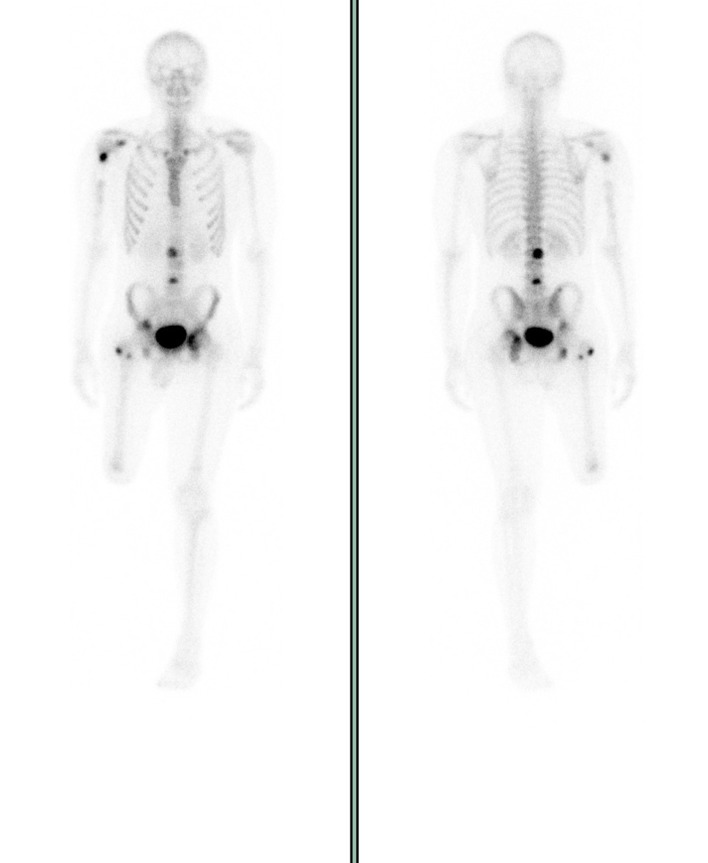
Whole-body bone scan with osteoblastic bone metastases in the right proximal humerus, right coracoid, right proximal femur, right iliac legion, left acetabulum and lumbar (L2 and L4) vertebral bodies.
These sites were radiated in October 2017 and the patient started sorafenib chemotherapy in January 2018. Bone scans in August 2018 showed widespread metastatic disease, at which point he started receiving palliative radiotherapy and dexamethasone to relieve sacral nerve compression. Despite progressive metastatic disease and complications thereof, he had, for the most part, been able to maintain an active lifestyle, lifting weights, until the end of 2018, at which point pain began to limit his physical activity for the first time since receiving his diagnosis nearly 3 years prior. He continued to be extensively involved in the motivational speaking and graduated college as a valedictorian nominee with a BA in psychology before passing away in January 2019.
Discussion
Over the last few decades, the length of survival of patients with osteosarcoma has improved dramatically with medical treatment. However, patients with either primary or relapsed/metastatic osteosarcoma should still be evaluated for surgical resectability, as surgical resection with complete elimination of all detectable metastatic disease is imperative in achieving prolonged survival or cure. Even with complete resection of distant metastases, the survival rate is 16%–27%. Long-term survival is significantly decreased if surgical resection does not achieve wide negative margins, and disease progression and death are inescapable with chemotherapy alone.17–21
Overall survival after recurrence remains poor for patients who relapse, especially for those with skeletal metastases. Bacci et al compared 52 patients with bone metastases with 371 patients who relapsed with lung metastases and found that the 5-year event-free survivals were 11% vs 27%, respectively.17
A total of 235 patients with osteosarcoma of the extremity at Rizzoli who relapsed after neoadjuvant treatments were treated with surgery, surgery plus second-line chemotherapy or only chemotherapy/radiotherapy. All of the 69 surviving patients’ treatment regimens included surgery, suggesting that prolonged survival and, for a few, the cure is possible in patients with relapsing osteosarcoma but only with aggressive treatments that completely remove the recurrence.
In a study of metastatic relapse in osteosarcoma patients, Kempf-Bielack et al found that early relapses (within 1 year) had the worst rates of survival, with approximately 74% of patients (427 of 576 patients) dying a median of 0.9 years after their first relapse. However, the same study also found that the median survival period for patients achieving a second complete remission with macroscopic resection was improved compared with those that did not (2.2 vs. 0.6 years).20 For our patient, given that his primary tumour was not controlled by standard chemotherapy, his twice-relapsed osteosarcoma, and the location of his pelvic metastasis, his prognosis prior to resection was very poor. However, if remission were to be even attempted, repeat complete surgical resection was imperative and, ultimately, within the patient’s goals of care to attempt to extend his length of survival and maintain his quality of life in the setting of a possibly debilitating lesion and devastating prognosis.
In this case, the complex anatomy of the pelvis and the tumour involvement of the acetabulum made it a technically challenging resection. Achieving complete resections in the periacetabular region are especially challenging in that they frequently necessitate further reconstruction to address postresection hip instability and gait disturbances. 3D-printed models represent one tool that has been used to plan complex cases involving difficult-to-visualise anatomy like that of the pelvis. Bagaria et al described four cases in which 3D printing was used in the management of complex fractures, documenting decreased surgical time and anaesthetic dosage, as well as decreased overall operating room (OR) time.7 Ma et al found similar results in a case series describing the use of 3D-printed guiding templates in the surgical management of eight patients (10–25 years of age) undergoing resection of high-grade osteosarcoma of the knee.10 Sheth et al described the successful use of 3D printing for preoperative planning in the setting of recurrent anterior shoulder instability.9
Unfortunately, there is no prior literature evaluating the efficacy of this technology in treating pelvic osteosarcoma lesions of the acetabulum. However, given the aforementioned benefits of using this technology for other conditions and in other locations, we used a 3D-printed model of the patient’s pelvis to facilitate advance planning of a technically challenging osteotomy, allowing for accurate visualisation of the tumour and superior appreciation of the visuospatial relationships with the rest of the pelvis and hip joint in order to plan the intended orientation of a complex multiplanar osteotomy. The use of the 3D model also allowed for a resection that minimised disruption to the native hip joint and maintained hip stability without the need for pelvic reconstruction.
Once the surgical plan was determined using a 3D-printed model, the use of intraoperative navigation allowed for real-time visualisation, localisation and navigation of the excision, allowing for a precise resection that avoided critical structures and achieved negative margins. By creating a precise osteotomy with minimal disruption to the acetabulum to resect the patient’s pelvic lesion with clear margins, we were able to (1) afford the patient his best chance of extended survival time, (2) prevent pathological fracture/functional impairment from a lesion in the periacetabular region and (3) maintain acceptable hip stability by avoiding a larger type of procedure such as total hip arthroplasty or pelvic reconstruction.
A disadvantage of using these technologies is the cost/availability of them. Much of the cost of 3D printing is the upfront cost associated with purchasing the printer itself, which can cost approximately US$3000. Additional costs include paying inhouse staff to convert CT images into 3D files, as well as the software used to do so. Printing models commercially can cost between US$500 and 2000, depending on the material used. Similarly, without an inhouse intraoperative navigation system, using this technology could be logistically challenging and/or financially prohibitive, though we expect the technology to become more available and affordable as its use (and availability of data to support it) increases.
Despite potential disadvantages, our case presentation demonstrates the promising benefits of using new technology to develop a superior planned osteotomy, execute a precise negative-margin resection, and reduce the morbidity of acetabular metastatic osteosarcoma. Currently, there is no existing literature evaluating the efficacy of the combined use of 3D printing and intraoperative navigation in improving morbidity and mortality associated with resection of metastatic acetabular lesions in patients with osteosarcoma. However, this case demonstrates that the usage of this technology, in conjunction with significant chemotherapy treatments, may potentially have improved the extent and quality of this particular patient’s survival period by minimising the likelihood of disease recurrence. It is also possible, then, that this technology may benefit other similar patients, and its usage may be worth considering when financial, logistic and patient-specific circumstances allow.
Learning points.
This is a good example of how relentlessly aggressive high-grade osteosarcoma can be, relapsing multiple times within a few short years despite multimodal therapy, including multiple rounds of chemotherapy, radiation therapy, and surgical resections and reconstructions.
Metastatic lesions must be completely surgically resected in order to achieve the possibility of cure, remission or extended survival.
A patient-centred approach that takes into account the patient’s goals of care in the context of their overall health is imperative in making medical and surgical management decisions that will ultimately determine the length of survival and possible benefit gained from extending that survival.
If surgery is thought to be the next best (or the only) step, it can be done safely with low morbidity and preserved functional quality of life with the use of 3D-printed models and intraoperative imaging to guide complex resections that may otherwise not have been pursued.
Footnotes
Contributors: All authors contributed to the writing of the case report and final article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Next of kin consent obtained.
References
- 1. Ozaki T, Lindner N, Hillmann A, et al. Influence of intralesional surgery on treatment outcome of chondrosarcoma. Cancer 1996;77:1292–7. [DOI] [PubMed] [Google Scholar]
- 2. Ozaki T, Flege S, Kevric M, et al. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol 2003;21:334–41. 10.1200/JCO.2003.01.142 [DOI] [PubMed] [Google Scholar]
- 3. Siebenrock KA, Hertel R, Ganz R. Unexpected resection of soft-tissue sarcoma. Arch Orthop Trauma Surg 2000;120(1-2):65–9. 10.1007/PL00021218 [DOI] [PubMed] [Google Scholar]
- 4. Fiorenza F, Abudu A, Grimer RJ, et al. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br 2002;84:93–9. 10.1302/0301-620X.84B1.0840093 [DOI] [PubMed] [Google Scholar]
- 5. Isakoff MS, Barkauskas DA, Ebb D, et al. Poor Survival for Osteosarcoma of the Pelvis: A Report from the Children’s Oncology Group. Clinical Orthopaedics and Related Research 2012;470:2007–13. 10.1007/s11999-012-2284-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laitinen MK, Parry MC, Albergo JI, et al. Is computer navigation when used in the surgery of iliosacral pelvic bone tumours safer for the patient? Bone Joint J 2017;99-B:261–6. 10.1302/0301-620X.99B2.BJJ-2016-0149.R2 [DOI] [PubMed] [Google Scholar]
- 7. Bagaria V, Deshpande S, Rasalkar DD, et al. Use of rapid prototyping and three-dimensional reconstruction modeling in the management of complex fractures. Eur J Radiol 2011;80:814–20. 10.1016/j.ejrad.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 8. Cherkasskiy L, Caffrey JP, Szewczyk AF, et al. Patient-specific 3D models aid planning for triplane proximal femoral osteotomy in slipped capital femoral epiphysis. J Child Orthop 2017;11:147–53. 10.1302/1863-2548-11-170277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sheth U, Theodoropoulos J, Abouali J. Use of 3-Dimensional printing for preoperative planning in the treatment of recurrent anterior shoulder instability. Arthrosc Tech 2015;4:e311–e316. 10.1016/j.eats.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma L, Zhou Y, Zhu Y, et al. 3D-printed guiding templates for improved osteosarcoma resection. Sci Rep 2016;6:23335 10.1038/srep23335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Furlow B. Medical 3-D Printing. Radiol Technol 2017;88:519CT-537CT. [PubMed] [Google Scholar]
- 12. Cho HS, Oh JH, Han I, et al. The outcomes of navigation-assisted bone tumour surgery: minimum three-year follow-up. J Bone Joint Surg Br 2012;94:1414–20. 10.1302/0301-620X.94B10.28638 [DOI] [PubMed] [Google Scholar]
- 13. Ould-Slimane M, Thong P, Perez A, et al. The role of Intraoperative 3D navigation for pelvic bone tumor resection. Orthop Traumatol Surg Res 2016;102:807–11. 10.1016/j.otsr.2016.03.019 [DOI] [PubMed] [Google Scholar]
- 14. Wong KC, Kumta SM. Computer-assisted tumor surgery in malignant bone tumors. Clin Orthop Relat Res 2013;471:750–61. 10.1007/s11999-012-2557-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, Wen L, Zhang J, et al. Three-dimensional printing and computer navigation assisted hemipelvectomy for en bloc resection of osteochondroma: A case report. Medicine 2017;96:e6414 10.1097/MD.0000000000006414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morris GV, Stevenson JD, Evans S, et al. Navigation in musculoskeletal oncology: an overview. Indian J Orthop 2018;52:22 10.4103/ortho.IJOrtho_205_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bacci G, Briccoli A, Longhi A, et al. Treatment and outcome of recurrent osteosarcoma: experience at Rizzoli in 235 patients initially treated with neoadjuvant chemotherapy. Acta Oncol 2005;44:748–55. 10.1080/02841860500327503 [DOI] [PubMed] [Google Scholar]
- 18. Crompton BD, Goldsby RE, Weinberg VK, et al. Survival after recurrence of osteosarcoma: a 20-year experience at a single institution. Pediatr Blood Cancer 2006;47:255–9. 10.1002/pbc.20580 [DOI] [PubMed] [Google Scholar]
- 19. Jaffe N, Carrasco H, Raymond K, et al. Can cure in patients with osteosarcoma be achieved exclusively with chemotherapy and abrogation of surgery? Cancer 2002;95:2202–10. 10.1002/cncr.10944 [DOI] [PubMed] [Google Scholar]
- 20. Kempf-Bielack B, Bielack SS, Jürgens H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS). J Clin Oncol 2005;23:559–68. 10.1200/JCO.2005.04.063 [DOI] [PubMed] [Google Scholar]
- 21. Chou AJ, Merola PR, Wexler LH, et al. Treatment of osteosarcoma at first recurrence after contemporary therapy: the memorial sloan-kettering cancer center experience. Cancer 2005;104:2214–21. 10.1002/cncr.21417 [DOI] [PubMed] [Google Scholar]