Abstract
Exposure of a renal transplant through the abdominal wall is a rare event. A search of the literature reveals only six documented cases which used skin autograft for coverage, with none reported since 1981, and none which used negative-pressure wound therapy (NPWT) to prepare the recipient bed. This case report demonstrates that NPWT followed by split thickness skin graft is a reconstructive option which is feasible in patients who are at high risk for surgical complications in prolonged flap surgery.
Keywords: plastic and reconstructive surgery, renal transplantation, transplantation, urological surgery
Background
The frequency of renal transplantation procedures in North America is increasing annually (National Kidney Foundation 2019; Canadian Institute for Health Information 2017), thus, naturally, the frequency of complications will also rise. Wound healing problems after renal transplant can manifest in many forms, from superficial skin dehiscence to complete fascial dehiscence and allograft exposure. Fascial dehiscence with exposure of the renal allograft is a rare (2%–6%)1 2 but devastating complication which threatens the survival of the graft and patient.3
Renal transplant patients, by the very nature of their immunosuppressive therapies, are at an increased risk of wound breakdown and infection.3 Traditional management options for abdominal fascial dehiscence and allograft exposure can include: secondary intention healing with sterile dressings with or without operative debridement, primary closure with mesh, skin grafting or flap coverage.
Since its introduction in the 1990s, NPWT has been increasingly used to treat open wounds, burns and ulcers, among other conditions.3 Negative-pressure wound therapy (NPWT) increases wound perfusion, reduces oedema, stimulates granulation tissue, decreases bacterial colonisation, removes wound exudate and stimulates biochemical pathways within local cells.4 The portability of NPWT can allow outpatient treatment, which minimises hospital cost.5
The use of NPWT for abdominal fascial dehiscence after renal transplantation was first reported in 2003.6 A 2016 systematic review on NPWT found that its use in this patient population was uncommon, with only 11 case reports published (22 patients in total).5 The risks of NPWT include haemorrhage, enterocutaneous fistula and urinary leakage, secondary to the suction pressure applied to the vascular and ureteric anastomoses.5 However, the authors noted only three instances where urinary leakage had resolved after discontinuing NPWT. There was weak evidence to support the possibility of fistula formation secondary to NPWT, and no instances of haemorrhage or fistula were reported. In addition, NPWT was found to actually resolve urinary leakage in two cases and led to resolution of a lymphocele in another.5
Other reported methods of repair of abdominal fascial dehiscence include use of prosthetic mesh,7 pedicle anterolateral thigh perforator flap8 or split thickness skin autograft alone.9 Using NPWT as an adjunct to prepare the wound bed for skin autograft has never been reported for renal transplant dehiscence. The following case serves as the first example of the use of NPWT to prepare a renal allograft bed for split thickness skin autograft with successful take.
Case presentation
A 70-year-old woman recipient of a deceased-donor renal transplant was referred for abdominal wound dehiscence. This was the patient’s second transplant on the left side. The transplantation was complicated by a perinephric haematoma requiring two separate operative evacuations. At the second evacuation, the tissues were too oedematous to allow primary fascial closure. Thus, a vicryl mesh was used to bridge the fascial defect through the linea semilunaris, after which the skin was closed primarily. At initial consultation the patient was stable on the ward, although she had previously required the intensive care unit to manage postoperative haemorrhagic and cardiogenic shock, thrombocytopaenia, anaemia and atrial fibrillation.
The dehiscence was noted on postoperative day (POD) 23. At this time, the left lower quadrant Gibson incision had an area of marginal incisional necrosis measuring 11×5 cm along the proximal half of the wound, with a small amount of sanguineous drainage. An incisional NPWT device was applied. By POD 34, the area of necrosis had dehisced and the wound then measured 17×7 cm. The vicryl mesh had dehisced on the medial side, but still covered the renal allograft; the dome-shaped graft contours were clearly visible in the wound base. The mesh had turned dark, resembling a black eschar (figure 1). NPWT was continued.
Figure 1.

The abdominal wound prior to debridement. The graft contours are clearly visible. Medial dehiscence of the vicryl mesh can be seen.
By POD 94, the wound had not progressed in terms of wound bed granulation. The kidney was now covered by a dark eschar and was exteriorised. The patient was taken for operative debridement of the eschar, which revealed a healthy renal allograft underneath, and NPWT was reapplied.
Investigations
CT of the abdomen revealed a massive complex perinephric gas-containing haematoma, which was drained prior to wound treatment. The abdominal wound was cultured positive for mixed organisms.
Treatment
On POD 143, the NPWT had cultivated healthy, red granulation tissue and the wound edges had adhered to the perimeter of the renal allograft. The patient was thus brought to the operating room for further debridement and split thickness skin graft (STSG) placement. Wound coverage was obtained with a 0.012-inch non-meshed skin autograft taken from the left thigh. The graft was harvested with a dermatome and sutured into place. Scattered drainage slits were made to allow evacuation of any drainage (figure 2). NPWT was reapplied to help secure the skin graft over the convex renal allograft contour.
Figure 2.
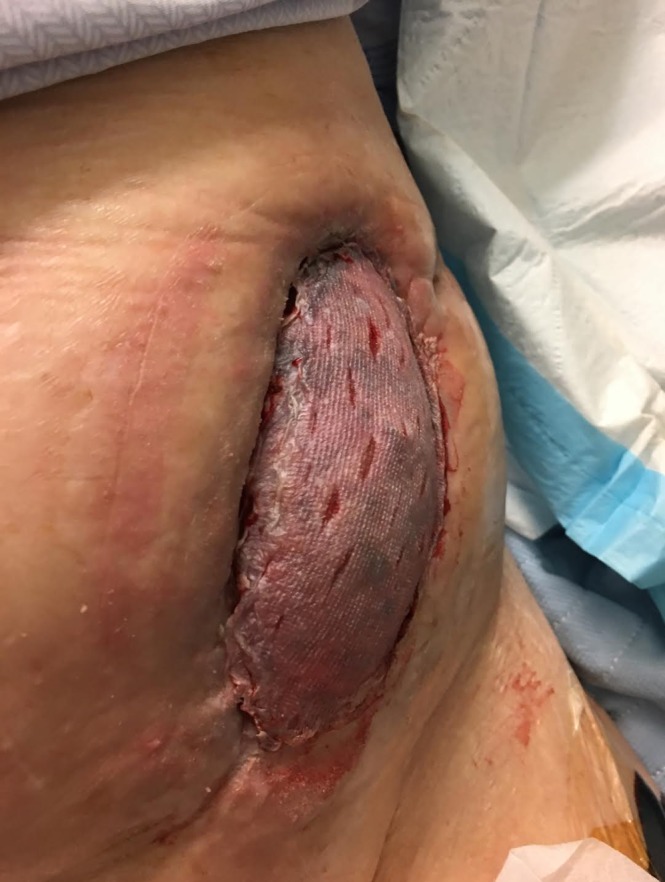
The wound can be seen following split-thickness skin graft placement. Drainage slits have been made in the graft to prevent seroma formation and encourage graft take.
Outcome and follow-up
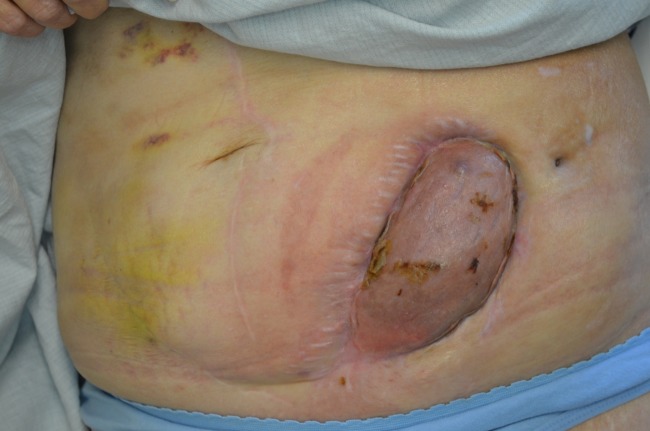
After 7 days of NPWT following the graft placement, there was 100% skin graft take. The NPWT was discontinued 2 weeks following graft placement, at POD 157. The total course of NPWT was 134 days. Treatment was switched to antimicrobial silver dressings biweekly to allow maturation of the skin graft. Figure 3 shows the healed wound.
Figure 3.
The skin graft has healed with full take. The renal allograft is completely covered by the skin graft.
Discussion
The use of a split-thickness autograft to cover a renal allograft is an uncommon event. A search of both the academic and grey literature yielded only one study on the management of post-transplantation renal tissue exposure with skin autografting.9 The study by Paley et al 9 included clinical reports on four patients who had received split-thickness autografts for protruding renal allografts. The grafts were successful in all four patients.9
Paley et al had identified only two previous reports of skin grafting to cover a renal allograft.9 Williams et al 10 successfully used a skin graft to cover one patient’s exteriorised kidney10; Wu et al 11 used a split-thickness graft in an 11-year-old patient with perinephric abscess after transplant, in order to minimise serious drainage.11 The graft was successful, and biopsy indicated that it was well vascularised by the renal cortex.11 All of these studies took place at a time before NPWT was available.
In the present case, the patient’s course was complicated by significant comorbidities, compromised immune status and history of haemorrhagic bleeding requiring two surgical explorations. In addition, previous aortofemoral bypass and bilateral lower extremity vein harvesting for a quadruple coronary artery bypass graft restricted the availability of autologous flaps. Following the guidance of the reconstructive ladder for wound management, the use of NPWT and STSG was preferred over more complex procedures such as a pedicle or free flap. Skin grafting requires less time in the operating room, subjects the patient to less soft tissue trauma, results in a faster recovery time, and works adequately for transplant wound coverage.
A prolonged course of NPWT was used for three reasons. First, NPWT temporised this serious abdominal wound in a systemically unwell patient to allow for granulation tissue to develop. Second, NPWT mechanically flattened the contours of the wound and allowed the abdominal skin edges to adhere to the renal allograft, sealing in the pelvic contents. Third, NPWT promoted granulation tissue to allow for skin graft take. Recognising the appropriate timing and conditions to stop NPWT is very important. It is a clinical decision as to when the wound is optimised by NPWT and ready for skin grafting to further expedite wound closure.
Patient’s perspective.
Overall the surgery itself was good. The recovery was good. I had pain on the second day and that was it. I have not had very much pain with it since then, so it is all healed up now and all is fine. Other than my clothes do not fit because the kidney protrudes. The best part of the experience is that it allowed a little brightness at the end of a very long tunnel that I was in prior to that. I was in the hospital 6 months and it was a very, very long time, and most of that was not for the plastic surgery but I was beginning to think there was nothing outside that hospital. But here I am. I have managed to be out a few times with my friends and that is what makes me happy.
Learning points.
Negative-pressure wound therapy in conjunction with split-thickness skin autografting is a viable option to obtain coverage of a non-healing renal allograft wound.
This method is non-invasive and thus favourable in the context of a high-risk surgical patient or in a patient with poor flap availability.
Skin autograft can successfully take on a renal allograft in a patient on immunosuppressive therapy when a healthy bed of granulation tissue is present.
Acknowledgments
The authors would like to acknowledge Marisa Turkstra, NP, and Julie Orr, NP, for their significant contributions to the wound care management of the patient presented in this case study. Both specialists worked closely with the plastic surgery team while providing excellent care to the patient throughout their course in hospital.
Footnotes
Contributors: All authors provided substantial contributions to the conception or design of the work: MHM and JB conceived the idea of creating the case study and VEM acquired the data for writing of the case study. VEM drafted the initial work. JB and MHM revised it critically for important intellectual content. All authors have given final approval of the version published and agree to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Dean PG, Lund WJ, Larson TS, et al. Wound-healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation 2004;77:1555–61. 10.1097/01.TP.0000123082.31092.53 [DOI] [PubMed] [Google Scholar]
- 2. Flechner SM, Zhou L, Derweesh I, et al. The impact of sirolimus, mycophenolate mofetil, cyclosporine, azathioprine, and steroids on wound healing in 513 kidney-transplant recipients. Transplantation 2003;76:1729–34. 10.1097/01.TP.0000093502.26208.42 [DOI] [PubMed] [Google Scholar]
- 3. Chen X, Liu L, Nie W, et al. Vacuum Sealing Drainage Therapy for Refractory Infectious Wound on 16 Renal Transplant Recipients. Transplant Proc 2018;50:2479–84. 10.1016/j.transproceed.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 4. Banwell PE, Musgrave M. Topical negative pressure therapy: mechanisms and indications. Int Wound J 2004;1:95–106. 10.1111/j.1742-4801.2004.00031.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shrestha BM. Systematic review of the negative pressure wound therapy in kidney transplant recipients. World J Transplant 2016;6:767–73. 10.5500/wjt.v6.i4.767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hodzic J, Adams J, Staehler G, et al. [Vacuum sealing of extensive wound healing disorders after kidney transplantation]. Urologe A 2003;42:1097–100. 10.1007/s00120-003-0299-2 [DOI] [PubMed] [Google Scholar]
- 7. Mehrabi A, Fonouni H, Wente M, et al. Wound complications following kidney and liver transplantation. Clin Transplant 2006;20:97–110. 10.1111/j.1399-0012.2006.00608.x [DOI] [PubMed] [Google Scholar]
- 8. Faizal A, Bujang Safawi E, Sundram M, et al. Soft tissue cover for an exposed transplanted kidney with a pedicled myocutaneous anterolateral thigh perforator and vastus lateralis flap. Urol Int 2011;87:117–9. 10.1159/000324543 [DOI] [PubMed] [Google Scholar]
- 9. Paley D, Spees EK, Zachary JB, et al. Skin autografts to cover exposed renal allografts. Surgery 1981;90:910–3. [PubMed] [Google Scholar]
- 10. Williams G, Birtch AG, Wilson RE, et al. Urological complications of renal transplantation. Br J Urol 1970;42:21–8. 10.1111/j.1464-410X.1970.tb11902.x [DOI] [PubMed] [Google Scholar]
- 11. Wu KT, Gault MH, MacLeany LD. Successful skin graft on renal allograft: report of a case. Can J Surg 1972;12:80–1. [PubMed] [Google Scholar]