Abstract
Nucleoside diphosphate kinases (Nmes/NDPKs) have been implicated in a multitude of cellular processes including an important role in metastasis suppression and several enzymatic activities have been assigned to the Nme family. Nevertheless, for many of these processes, it has not been possible to establish a strong connection between Nme enzymatic activity and the relevant biological function. We hypothesized that, in addition to its known enzymatic functions, members of the Nme family might also regulate signaling cascades by acting on key signal transducers. Accordingly, here we show that Nme1 directly interacts with the calcium/calmodulin-dependent kinase II (CaMKII). Using purified proteins, we monitored the phosphorylation of a number of CaMKII substrates and determined that at nanomolar levels Nme1 enhances the phosphorylation of T-type substrates; this modulation shifts to inhibition at low micromolar concentrations. Specifically, the autophosphorylation of CaMKII at Thr286 is completely inhibited by 2μM Nme1, a feature that distinguishes Nme1 from other known endogenous CaMKII inhibitors. Importantly, CaMKII inhibition does not require phosphotransfer activity by Nme1 since the kinase-dead Nme1 H118F mutant is as effective as the wild-type form of the enzyme. Our results provide a novel molecular mechanism whereby Nme1 could modulate diverse cellular processes in a manner that is independent of its known enzymatic activities.
Graphical Abstract

Nucleoside diphosphate kinases (Nme/NDPK/Nm23) are ubiquitous proteins best known for their role in nucleotide homeostasis.1 Despite originally being considered housekeeping enzymes,1 later studies demonstrated that the Nme family of proteins are involved in several pathophysiological and cellular processes including cancer and metastasis suppression, endocytosis, intracellular trafficking, cilia function, and transcriptional regulation.2 Several enzymatic activities have been attributed to the Nme family and could help explain their involvement in these diverse cellular functions: nucleoside diphosphate kinase activity, histidine phosphorylation, and 3’−5’ exonuclease activities.3 Nonetheless, the precise molecular mechanisms of Nme action in the majority of these cellular processes remain unknown.2 This gap in knowledge is exemplified by the role of Nme1 in cancer cell motility and metastasis suppression.4 Although Nme1 expression levels are strongly correlated with control of metastatic potential,5 the underlying molecular mechanisms are not yet fully understood.
During our studies of CaMKII function in rodent brain,6 we unexpectedly discovered an interaction between Nme1 and CaMKII, suggesting Nme proteins could regulate CaMKII-dependent signal transduction pathways. CaMKII is a ubiquitous kinase that regulates a multitude of cellular processes including cell cycle and proliferation, cytoskeletal dynamics, and Ca2+ homeostasis.7 CaMKII is activated by a rise in intracellular free calcium, which activates calmodulin. Binding of calmodulin to the autoinhibitory region of CaMKII relieves the inhibition and renders the catalytic domain accessible to substrates.8 Importantly, the frequency and duration of the calcium stimulus determines whether autophosphorylation at Thr286 in the autoinhibitory domain of CaMKII occurs. The autophosphorylated form of the enzyme is active and calcium-independent, allowing CaMKII to remain active long after the termination of the original signal.8
Interestingly, CaMKII activity has also been implicated in cancer and metastasis. CaMKII activity promotes gastric cancer cell metastasis9 while inhibition of CaMKII autophosphorylation prevents breast cancer cell migration and invasion10. These results led to the suggestion that inhibition of CaMKII could represent a promising target for future therapeutics.9, 10 Here we show that Nme1 directly interacts with CaMKII and enhances or inhibits CaMKII kinase activity in a concentration dependent manner, providing an additional molecular mechanism of Nme1 action in the cellular processes described above.
To begin, we sought to determine whether Nme1 and CaMKII interact in a cellular context. We employed a pull-down assay using purified 6xHis-tagged CaMKIIα and adult rat brain lysates, a tissue where Nme1 and CaMKIIα are both expressed8, 11 (Figure 1A). CaMKIIα was immobilized on nickel-nitrilotriacetic acid agarose resin and incubated with rat brain lysate. After washes, proteins bound to the resin were eluted and separated by SDS-PAGE, followed by immunoblotting with relevant antibodies. As shown in Figure 1A (left), Nme1 interacts with immobilized CaMKIIα but not with a control protein, 6xHis-tagged green fluorescent protein (His-GFP).
Figure 1.
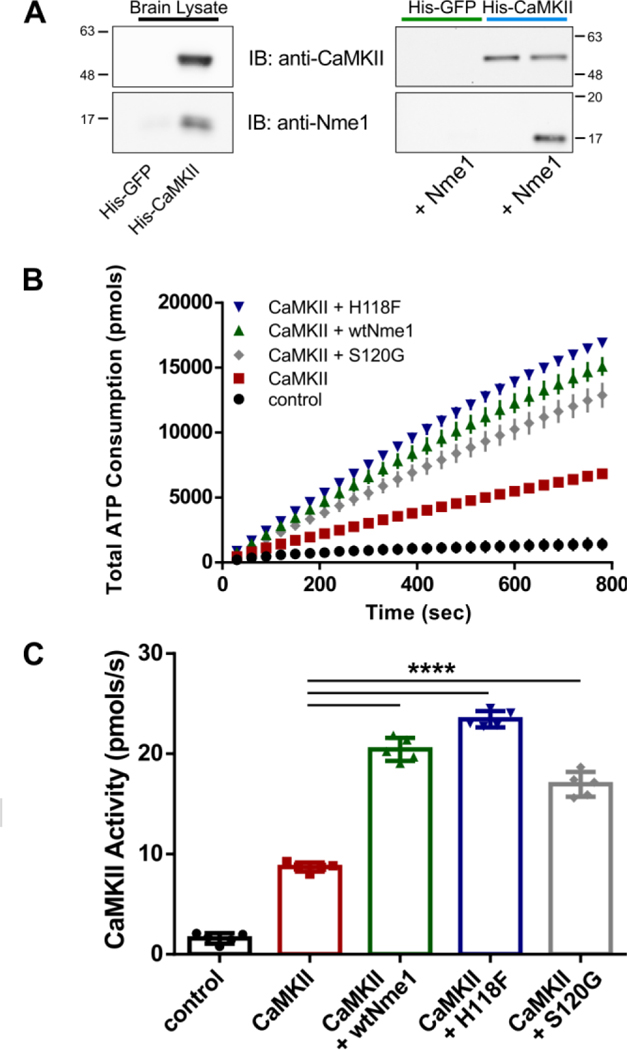
Nme1 enhances CaMKIIα activity. (A) Immobilized GFP or CaMKII were incubated with rat brain lysate (left) or with purified Nme1 protein (right). Retained proteins were separated by SDS-PAGE and immunoblotted (IB) using anti-Nme1 or anti-CaMKIIα antibodies. (B) ATP consumption by CaMKIIα was monitored using the PK/LDH assay and syntide-2 substrate. Samples without CaMKIIα were used as controls. The experiment was repeated in the presence of 500 nM wtNme1 (green arrowhead), Nme1 H118F (blue arrowhead), or Nme1 S120G (gray diamond). (C) CaMKII activity was determined from the slopes of the curves shown in (B) and is summarized using scatter plots (N = 5 ; ****, p < 0.0001, Two-way ANOVA with Tukey’s post hoc test). Although not shown for clarity, the mean value of “CaMKII + wtNme1” is significantly different (p < 0.001) from the values observed for “CaMKII + H118F” and “CaMKII +S120G”.
We next investigated whether Nme1 and CaMKIIα could interact directly. For this, we repeated the experiment using purified Nme1 instead of total rat brain lysate (Figure 1A, right panel). As expected, the control protein His-GFP failed to interact with Nme1. This contrasts with the interaction observed between Nme1 and immobilized His-CaMKIIα. Taken together, these data suggest that Nme1 and CaMKIIα interact directly.
Next, we sought to determine whether Nme1 modulates the kinase activity of CaMKIIα using the classic pyruvate kinase/lactate dehydrogenase (PK/LDH) coupled assay. The assay has been used previously by us and other groups to monitor the activity of purified CaMKII in vitro.6, 12 We found that addition of CaMKIIα to the reaction mixture led to a fast consumption of ATP due to the phosphorylation of the 15 amino acid peptide substrate syntide-2 by CaMKII (Figure 1B,C). When the experiment was repeated in the presence of 500 nM wtNme1, a strong increase in total ATP consumption was detected. The enhanced activity is not due to phosphorylation of syntide-2 by Nme1 nor does it interfere with the conversion of ADP to ATP by PK/LDH (Figure S1). Next, we used a direct kinase assay method by monitoring the incorporation of radiolabeled phosphate from [γ−32P]ATP into the CaMKII peptide substrate syntide-2, a peptide derived from glycogen synthase.6 As shown in Figure S2, addition of 500 nM Nme1 to the reaction mixture led to a significant increase in syntide-2 phosphorylation by CaMKIIα. This observation indicates that Nme1 can act as an enhancer of CaMKII activity.
The phosphotransfer activity of Nme1 is central to many of its reported enzymatic properties.2, 13 Therefore, we began a preliminary characterization of the mechanism of Nme1 enhancement of CaMKIIα activity by asking whether the phosphotransfer activity of Nme1 is required for the effect observed in Figure 1B,C. To this end, we purified a mutant version of Nme1 that lacks all known enzymatic activities14, 15 (Nme1 H118F) and tested its effect on CaMKII activity. When the H118F Nme1 mutant was added to the reaction instead of wtNme1, a slightly greater ATP consumption was observed, suggesting that the enzymatic activities of Nme1 are not required for the enhancement of CaMKII activity. We next employed an Nme1 mutant found in human neuroblastomas (Nme1 S120G) that retains the nucleoside diphosphate kinase activity but is deficient in protein phosphotransfer reactions.15 The S120G mutant also enhanced the activity of CaMKII at 500 nM, although to a lesser extent than wtNme1. Taken together, these results suggest that the enzymatic activity of Nme1 is not required for the stimulatory effect on CaMKII although the nucleoside diphosphate activity may play an inhibitory role towards CaMKII.
We then proceeded to determine the dose-response relationship between Nme1 and CaMKII activity. The PK/LDH assay could not be used for this purpose because we observed that higher concentrations of Nme1 interfered with the linearity of this assay. Therefore, we employed an end-point, luminescent assay that monitors activity of purified kinases by quantifying ATP.16 We varied the Nme1 concentration in the assay from 20 nM to 10 μM using syntide-2 as a substrate (Figure 2A). Interestingly, Nme1 modulation of CaMKII activity is biphasic. At higher nanomolar concentrations Nme1 enhances the kinase activity. However, this enhancement is reversed when micromolar concentrations are reached: Nme1 strongly inhibits CaMKII activity at 4 μM. The inhibition is specific for CaMKII, as Nme1 has no effect on the activity of an unrelated kinase (Akt1) at the same concentration (Figure S3). In addition, Nme1 does not interfere with the end point assay at this concentration (Figure S4).
Figure 2.

Nme1 enhances and inhibits CaMKII activity. (A) The effect of Nme1 concentration on CaMKIIα activity was determined by measurement of ATP consumption during the phosphorylation of the peptide substrate syntide-2 using a luminescent kinase assay. The phosphorylation of syntide-2 obtained in the absence of Nme1 was used as control and is depicted by the dashed gray line (N = 4). (B) CaMKIIα and Ca2+/calmodulin were mixed with GSK3-α and incubated in the absence or presence of 4 μM wtNme1, Nme1 H118F, or Nme1 S120G. The samples were separated by SDS-PAGE and phosphorylation of S21 in GSK3-α was monitored by immunoblotting using a phosphospecific antibody (upper panel). Samples without ATP served as negative controls (N=4). (C) The experiment shown in (B) was repeated in the presence of 500 nM wtNme1, Nme1 H118F, or Nme1 S120G (upper panel) and band intensities quantified (lower panel) (N = 4; ****, p < 0.0001, Two-way ANOVA with Tukey’s post hoc test).
We sought to confirm this result using an independent assay. We monitored the effect of Nme1 on CaMKIIα activity by comparing the phosphorylation of a known CaMKII substrate,6 glycogen synthase kinase-3 alpha (GSK3-α), in the absence and presence of wtNme1 at similar concentrations used in the luminescent assay. The reaction mixtures of the purified proteins were separated by SDS-PAGE and phosphorylation of GSK3-α was detected by immunoblotting using a phospho-GSK3α antibody (Figure 2B). As expected, a band was detected using the phospho-GSK3α antibody when ATP was present in the reaction mixture. The reaction was then repeated in the presence of ATP and wtNme1. In agreement with the observations obtained with the luminescent kinase assay, addition of wtNme1 at 4 μM led to a strong inhibition of GSK3-α phosphorylation, confirming the inhibition of CaMKII by wtNme1.
Next, we asked whether the phosphotransfer or the histidine kinase activities of Nme1 are important for the observed inhibition. As shown in Figure 2B, micromolar concentrations of both Nme1 H118F and S120G mutants inhibit CaMKIIα as effectively as wild type Nme1, suggesting that the enzymatic activities of Nme1 are not required for inhibition of CaMKII. We performed a similar experiment using 500nM wtNme1, Nme1 H118F or Nme1 S120G (Fig. 2C,D). To our surprise, and in contrast to our results with syntide-2. (Fig. 2A), we found that all three Nme1 proteins partially inhibited CaMKII phosphorylation of GSK3-α.
CaMKII substrates have been classified in two categories, S- and T-type,6 based on their binding sites on the kinase. Both syntide-2 and GSK-3 are S-type substrates. Thus far, the endogenous CaMKII inhibitors that have been identified selectively inhibit the phosphorylation of S-type substrates but display weak or no inhibition of T-type substrates.6, 17 Therefore, we sought to determine the effect of Nme1 on CaMKII activity towards T-type substrates. To begin, we used autocamtide-2 as a model for T-type substrate which is derived from the autoregulatory domain of CaMKII and binds to both the S- and T-sites. As previously described, autocamtide-2 was readily phosphorylated by CaMKII (Figure 3A).6 However, when 4 μM Nme1 was added to the reaction mixture ATP consumption was not detected (Figure 3A), suggesting that Nme1 is also an inhibitor of the phosphorylation of T-type substrates at micromolar concentrations.
Figure 3.
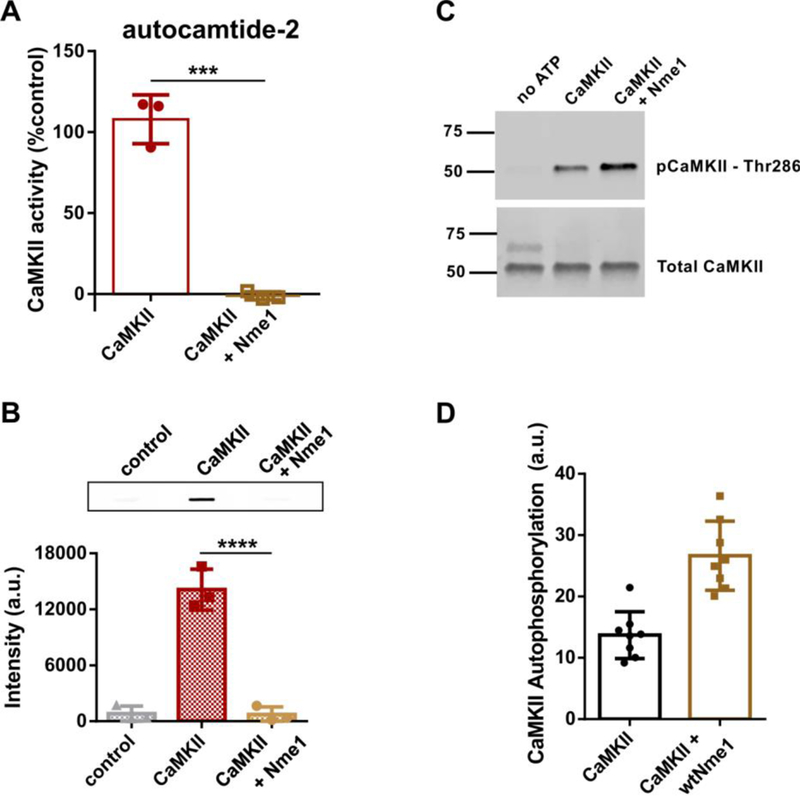
Nme1 is a concentration dependent modulator of CaMKII autophosphorylation. (A) The kinase activity of CaMKIIα was monitored by measurement of total ATP consumption during the phosphorylation of the T-type substrate autocamtide-2 in the absence or presence of Nme1 (4 μM) using the Kinase-Glo luminescent kinase assay (N = 3 ; ****, p < 0.0001, Student’s t-test). (B) Upper panel. CaMKIIα autophosphorylation on residue Thr286 was measured using a phospho-specific CaMKIIα antibody in the absence or presence of 2 μM Nme1. Samples without ATP were used as negative controls. A representative slot-blot is shown. Lower panel: quantification of immunoblots. (N = 3 ; ****, p < 0.0001, Two-way ANOVA with Tukey’s post hoc test). (C) The experiment shown in (B) was repeated in the presence of 500 nM Nme1. A representative blot is shown. (D) Quantification of immunoblots from (C). (N = 8; ****, p < 0.0001, Student’s t-test).
The ability of Nme1 to inhibit T-type substrates suggested the possibility that Nme1 might also block the autophosphorylation of CaMKII since the autoinhibitory domain of CaMKII is a T-type substrate. We monitored the effect of Nme1 on CaMKIIα autophosphorylation by immunoblotting with a Thr286-phospho specific antibody. CaMKIIα was incubated in the presence of ATP and Ca2+/calmodulin and blotted onto a nitrocellulose membrane. As expected, a strong signal was detected when ATP was present in the reaction mix. When the reaction was repeated in the presence of ATP and 2 μM Nme1 the signal detected was not significantly different from the one observed for the negative control (Figure 3B), indicating that Nme1 strongly inhibits the autophosphorylation of CaMKIIα. Finally, we asked if nanomolar concentrations of Nme1 could modulate CaMKII autophosphorylation using the immunoblotting assay. When the reaction was repeated in the presence of 500 nM Nme1, the signal detected was significantly enhanced compared to the negative control (Figure 3C,D). Taken together, our data show that the concentration-dependent effect of Nme1 towards CaMKII activity differs according to the nature of the substrate.
Lastly, we began an examination of the mechanism by which Nme1 inhibits CaMKIIα by asking if Nme1 directly inhibits CaMKII activation or its kinase activity. A block of CaMKIIα activation could occur via inhibition of calmodulin binding to the autoregulatory domain of CaMKIIα. In this scenario, Nme1 could act as a calmodulin scavenger, thus the inhibition at higher concentrations would then occur due to depletion of calmodulin. We tested this possibility by monitoring CaMKII activity in the presence of 4 μM Nme1 at two different calmodulin concentrations, 0.5 and 20 μM: a 40-fold range. As shown in Figure 4A, CaMKII activity was severely reduced in the presence of Nme1 regardless of the calmodulin concentration. Our data support the conclusion that Nme1 inhibition of CaMKII at micromolar concentrations is independent of calmodulin activation of CaMKII. In agreement with this conclusion, we failed to detect a direct interaction between Nme1 and calmodulin (Figure S5).
Figure 4.

Nme1 does not inhibit CaMKII activation. (A) The kinase activity of CaMKIIα was monitored by measurement of total ATP consumption during the phosphorylation of the model peptide syntide-2 in the absence or presence of Nme1 (4 μM) at two different calmodulin concentrations using the Kinase-Glo luminescent kinase assay. Strong inhibition was observed at both calmodulin concentrations indicating Nme1 does not sequester calmodulin (N = 4 ; ****, p < 0.0001, Student’s t-test). (B) Autonomous CaMKII was obtained by pre-incubation with Ca2+/calmodulin and ATP followed by addition of EGTA. Its kinase activity was measured in low free Ca2+ (< 50 nM) in the absence (squares) or presence (circles) of Nme1 (2 μM). The same concentration of EGTA was added during pre-incubation to confirm the presence of autonomous CaMKII activity only. Samples without CaMKII served as negative controls. (C) The slopes of the curves summarized in (B) were quantified and the scatter plots are shown. (N = 6 ; bars indicate standard deviations; ****, p < 0.0001, Two-way ANOVA with Tukey’s post hoc test).
Next, we utilized the PK/LDH coupled assay to determine if Nme1 could inhibit an autonomously active, calmodulin-independent version of CaMKII (Figure 4B,C). We first induced CaMKIIα pre-activation in the absence of Nme1 by incubating the kinase with Ca2+/calmodulin for 2 min, a procedure previously shown to lead to the formation of autonomous CaMKII activity.18 The Ca2+ chelator EGTA was then added to exclude the presence of Ca2+-dependent activity. After incubation, the pre-activated enzyme was immediately added to a solution containing syntide-2 in the presence or absence of Nme1 and the total ATP consumption was measured (Figure 4B,C). Addition of pre-activated CaMKIIα led to a strong phosphorylation of syntide-2 even at low free Ca2+ (< 50 nM) in the solution, as expected. When EGTA was added during the pre-activation no ATP consumption was detected confirming that only the autonomous activity of CaMKII was assayed. However, 2 μM Nme1 strongly inhibited the ATP consumption of autonomous CaMKII. These data demonstrate that Nme1 acts as an inhibitor of the catalytic activity of CaMKII, acting on both the Ca2+-dependent and independent forms of the kinase
In summary, our data demonstrate that Nme1 is a concentration-dependent and substrate-dependent modulator of CaMKII, a major signaling kinase in numerous cell types. In addition, we present evidence that both enhancement and inhibition of CaMKII is independent of the phosphotransfer enzymatic activities attributed to Nme1. Interestingly, the role of Nme1 in metastasis is well correlated with its expression levels while the correlation with its enzymatic activities is still unclear.13, 19, 20 Both CaMKII and Nme1 are ubiquitous enzymes, well expressed in several tissues and therefore it is likely that their interaction is strictly regulated in vivo. Thus, elucidation of the biological function of their interaction will probably require the identification of modulating factors and/or processes.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by NIH grants R01NS065856 (S. Paradis) and R21NS102661 (S. Paradis and M. Marr). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- ATP
adenosine-5’-triphosphate
- CaMKII
Ca2+/calmodulin-dependent kinase II
- GSK3-α
glycogen synthase kinase 3 alpha
- PK/LDH
pyruvate kinase/lactate dehydrogenase
Footnotes
ASSOCIATED CONTENT
Accession Codes:
NME1 P15532
CAMK2A A8K161
The Supporting Information is available free of charge on the ACS Publications website.
Figures S1–S5; Experimental Procedures (PDF)
The authors declare no competing financial interests.
REFERENCES
- (1).Hsu T, Steeg PS, Zollo M, and Wieland T. (2015) Progress on Nme (NDP kinase/Nm23/Awd) gene family-related functions derived from animal model systems: studies on development, cardiovascular disease, and cancer metastasis exemplified. Naunyn Schmiedebergs Arch Pharmacol 388, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Boissan M, Schlattner U, and Lacombe ML (2018) The NDPK/NME superfamily: state of the art. Lab Invest 98, 164–174. [DOI] [PubMed] [Google Scholar]
- (3).Steeg PS, Zollo M, and Wieland T. (2011) A critical evaluation of biochemical activities reported for the nucleoside diphosphate kinase/Nm23/Awd family proteins: opportunities and missteps in understanding their biological functions. Naunyn Schmiedebergs Arch Pharmacol 384, 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Novak M, Jarrett SG, McCorkle JR, Mellon I, and Kaetzel DM (2011) Multiple mechanisms underlie metastasis suppressor function of NM23-H1 in melanoma. Naunyn Schmiedebergs Arch Pharmacol 384, 433–438. [DOI] [PubMed] [Google Scholar]
- (5).Rosengard AM, Krutzsch HC, Shearn A, Biggs JR, Barker E, Margulies IM, King CR, Liotta LA, and Steeg PS (1989) Reduced Nm23/Awd protein in tumour metastasis and aberrant Drosophila development. Nature 342, 177–180. [DOI] [PubMed] [Google Scholar]
- (6).Royer L, Herzog JJ, Kenny K, Tzvetkova B, Cochrane JC, Marr MT 2nd, and Paradis S. (2018) The Ras-like GTPase Rem2 is a potent inhibitor of calcium/calmodulin-dependent kinase II activity. J Biol Chem 293, 14798−14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Wang YY, Zhao R, and Zhe H. (2015) The emerging role of CaMKII in cancer. Oncotarget 6, 11725−11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Hudmon A, and Schulman H. (2002) Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem 71, 473–510. [DOI] [PubMed] [Google Scholar]
- (9).Liu Z, Han G, Cao Y, Wang Y, and Gong H. (2014) Calcium/calmodulindependent protein kinase II enhances metastasis of human gastric cancer by upregulating nuclear factorkappaB and Aktmediated matrix metalloproteinase9 production. Mol Med Rep 10, 2459–2464. [DOI] [PubMed] [Google Scholar]
- (10).Chi M, Evans H, Gilchrist J, Mayhew J, Hoffman A, Pearsall EA, Jankowski H, Brzozowski JS, and Skelding KA (2016) Phosphorylation of calcium/calmodulin-stimulated protein kinase II at T286 enhances invasion and migration of human breast cancer cells. Sci Rep 6, 33132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Carotenuto P, Marino N, Bello AM, D’Angelo A, Di Porzio U, Lombardi D, and Zollo M. (2006) PRUNE and NM23-M1 expression in embryonic and adult mouse brain. J Bioenerg Biomembr 38, 233–246. [DOI] [PubMed] [Google Scholar]
- (12).Chao LH, Pellicena P, Deindl S, Barclay LA, Schulman H, and Kuriyan J. (2010) Intersubunit capture of regulatory segments is a component of cooperative CaMKII activation. Nat Struct Mol Biol 17, 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Khan I, and Steeg PS (2018) The relationship of NM23 (NME) metastasis suppressor histidine phosphorylation to its nucleoside diphosphate kinase, histidine protein kinase and motility suppression activities. Oncotarget 9, 10185–10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Wu Z, Guo L, Ge J, Zhang Z, Wei H, and Zhou Q. (2017) Two serine residues of non-metastasis protein 23-H1 are critical in inhibiting signal transducer and activator of transcription 3 activity in human lung cancer cells. Oncology letters 14, 2475–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Freije JM, Blay P, MacDonald NJ, Manrow RE, and Steeg PS (1997) Site-directed mutation of Nm23-H1. Mutations lacking motility suppressive capacity upon transfection are deficient in histidine-dependent protein phosphotransferase pathways in vitro. J Biol Chem 272, 5525–5532. [DOI] [PubMed] [Google Scholar]
- (16).Tanega C, Shen M, Mott BT, Thomas CJ, MacArthur R, Inglese J, and Auld DS (2009) Comparison of bioluminescent kinase assays using substrate depletion and product formation. Assay Drug Dev Technol 7, 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Chang BH, Mukherji S, and Soderling TR (1998) Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci U S A 95, 10890−10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Hashimoto Y, Schworer CM, Colbran RJ, and Soderling TR (1987) Autophosphorylation of Ca2+/calmodulin-dependent protein kinase II. Effects on total and Ca2+-independent activities and kinetic parameters. J Biol Chem 262, 8051–8055. [PubMed] [Google Scholar]
- (19).Ma D, Luyten GP, Luider TM, Jager MJ, and Niederkorn JY (1996) Association between NM23-H1 gene expression and metastasis of human uveal melanoma in an animal model. Investigative ophthalmology & visual science 37, 2293–2301. [PubMed] [Google Scholar]
- (20).Tokunaga Y, Urano T, Furukawa K, Kondo H, Kanematsu T, and Shiku H. (1993) Reduced expression of nm23-H1, but not of nm23-H2, is concordant with the frequency of lymph-node metastasis of human breast cancer. International journal of cancer 55, 66–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


