Abstract
The objective of this study was to examine temporal variations in synovial fluid composition and lubrication following articular fracture. Post-traumatic osteoarthritis (PTOA) was induced by creating an osteochondral fracture in the middle carpal joint of four horses while the contralateral limb served as a sham-operated control. Horses were exercised on a high-speed treadmill, and synovial fluid was collected pre-operatively and at serial timepoints until 75 days post-operatively. Lubricin and hyaluronic acid (HA) concentrations were measured using sandwich ELISAs, and the molecular weight distribution of HA was analyzed via gel electrophoresis. Synovial fluid viscosity and cartilage friction coefficients across all modes of lubrication were measured on days 0, 19, 33, and 61 using a commercial rheometer and a custom tribometer, respectively. HA concentrations were significantly decreased post-operatively, and high molecular weight HA (>6.1MDa) did not recover to pre-operative values by the study termination at day 75. Lubricin concentrations increased after surgery to a greater extent in the OA as compared to sham-operated limbs. Viscosity was significantly reduced after surgery. While boundary and elastoviscous mode friction coefficients did not vary, the transition number, representing the shift between these modes, was lower. Although more pronounced in the OA limbs, similar derangements in HA, HA molecular weight distribution, viscosity, and transition number were observed in the sham-operated limbs, which may be explained by synovial fluid washout during arthroscopy.
Keywords: articular fracture, post-traumatic osteoarthritis, lubricin/PRG4, hyaluronic acid (HA), viscosity
Post-traumatic osteoarthritis (PTOA) accounts for an estimated 12% of symptomatic OA in the lower extremities of adults in the United States.1 In particular, articular fractures have been estimated to increase the risk of PTOA by more than 20-fold.2 The standard-of-care for treating articular fractures by surgically restoring surface congruity and anatomic alignment fails to prevent the progression of OA.3 Traumatic loading has been shown to initiate a cascade of inflammatory events that contribute to the pathogenesis of PTOA and cartilage breakdown via chondrocyte apoptosis and extracellular matrix degradation.4,5 Furthermore, cartilage wear may be exacerbated by a loss of protective cartilage lubricants6 that are downregulated by or made more vulnerable to enzymatic degradation by pro-inflammatory cytokines like TNF-α and IL-1β.7–9 Traumatic joint injuries pose an identifiable risk factor for PTOA, and growing evidence suggests that interventions which are targeted at restoring joint homeostasis and applied acutely after injury may prevent OA development.2
The lubricating capacity of synovial fluid and its lubricant composition have been found to vary widely after traumatic injury. Poor lubrication has been associated with cartilage damage and may play a key role in OA progression both through direct mechanical wear and induction of cell death and apoptosis due to mechanical overloading.6,10,11 Synovial fluid contains two critical cartilage lubricants: lubricin and hyaluronic acid (HA). HA, a linear polymer that imparts synovial fluid with its viscous properties, has been found to decrease in human patients with clinical OA progression and in many animal models of PTOA.12–19 Decreased concentrations and expression of lubricin, a mucinous glycoprotein and cartilage boundary lubricant, have been observed in the rat20,21 and guinea pig22 anterior cruciate ligament tear (ACLT) models, ovine meniscectomy model23 as well as human ACLT.9 Decreased lubricin concentrations in synovial fluid have been linked to inferior boundary lubricating properties.20,22,24
In contrast, several studies evaluating synovial fluid composition following intra-articular fracture have found increased lubricin concentrations. Increased lubricin synovial fluid concentrations have been reported in horses with acute and chronic OA resulting from naturally occurring articular fractures,15,25 surgically-induced models of equine osteochondral fractures,25 human tibial plateau fractures14 and advanced human OA.26 In studies where lubrication was evaluated,14,15 boundary friction coefficients were elevated in synovial fluid from patients with intra-articular fracture. This phenomenon was attributed to decreased levels of HA and was supported by improved lubrication in vitro by supplementing synovial fluid with HA. Nevertheless, the mechanism by which lubrication changes may contribute to PTOA pathogenesis remains unclear. One limitation of most studies is that articular fracture location and the time interval between fracture and synovial fluid sampling were not uniform among subjects. Furthermore, only a single time point after injury was examined. A recent study measured serial synovial fluid lubricin concentrations in horses with surgically-induced osteochondral carpal fragmentation from onset of injury to 70 days post-injury.25 Lubricin concentrations increased following intra-articular fracture, peaking at 14 days post-injury, and gradually returning to baseline by 70 days. In addition to permitting longitudinal synovial fluid sampling, the equine osteochondral fragment model is valuable for its clinical relevance to naturally occurring equine and human OA from injuries such as intra-articular fractures.14 In addition, the equine model has been extensively characterized and used to evaluate OA therapies.27 While serial changes in lubricin levels have been reported in this model, it remains unknown how synovial fluid lubrication and other molecular constituents such as HA affect lubrication over time following intra-articular fracture.
Furthermore, several modes of cartilage lubrication occur throughout the gait cycle,24,28,29 but only boundary mode friction properties of synovial fluid have been characterized following articular fracture. Recently, multiple laboratories have applied the Stribeck framework to cartilage lubrication to map the friction coefficient of cartilage across these modes.24,30–33 These studies have demonstrated that friction scales with sliding speed, viscosity, and inversely with contact load. The Stribeck framework was originally developed to describe the variation in friction coefficient in journal bearings from boundary mode where there is asperity-on-asperity contact to hydrodynamic lubrication in which surfaces are separated by a fluid film greater than the height of the asperities.29 This method involves plotting the coefficient of friction as a function of a dimensionless Sommerfeld number (S) to create a “Stribeck curve,” where S is calculated as: where v is sliding speed, η is viscosity, a is contact width, and FN is normal load.24,29–31 Unlike journal bearings, cartilage is soft and porous and likely unable to sustain a full fluid film. This results in friction coefficients greater than what would be expected for true fluid-film lubrication at high Sommerfeld numbers.24,29 However, cartilage exhibits a similar decrease in friction coefficient with increasing Sommerfeld number, that is, increasing speed or viscosity (Figure 3A) until a plateau in friction coefficient is met at high Sommerfeld numbers, which has been coined as “elastoviscous” lubrication mode.24,29 The origins by which friction decreases is an active area of investigation with research supporting hypotheses such as tribological rehydration,34,35 generation of a pressurized fluid wedge,30,34 and formation of viscous gel layers.24,29
The Stribeck framework has proven to be a powerful tool to describe cartilage lubrication. Importantly, Stribeck analysis has shown that biochemical and mechanical damage to cartilage may result in failure of specific lubrication mechanisms as indicated by measurable changes in boundary and elastoviscous/minimum friction coefficients or transition numbers.30,31 Likewise, changes in lubricant composition (lubricin and HA concentration, HA molecular weight) can dramatically affect the shape of the Stribeck curve and magnitude of friction within certain lubrication regimes. These changes are not detectable when friction is plotted versus only speed. Because increased friction can predispose cartilage to degradation in OA,6,20 it is important to understand how lubricant composition affects cartilage friction coefficients and the transitions from high-friction boundary mode to low-friction elastoviscous mode.
As such, there is a need to understand how articular fracture affects synovial fluid composition and its frictional properties across all modes of lubrication. This information could shed light on the role of lubrication in the pathogenesis of PTOA and reveal new approaches for treatment of articular fractures. Therefore, the goal of this study was to examine temporal changes in lubricin, HA, and synovial fluid lubrication over time in the equine carpal osteochondral fragment model of PTOA.
METHODS
Osteochondral Fragment Model
Following approval by the Cornell University Institutional Animal Care and Use Committee, four Thoroughbred horses (n = 2 castrated males, n = 2 females) aged 2–6 years that were donated for research were used in this study. Horses were maintained in 3.65 m × 3.65 m box stalls. In one randomly assigned limb of each horse, an 8 mm osteochondral fragment was created under arthroscopic guidance in the middle carpal joint while the contralateral forelimb served as a sham-operated control.27 Both limbs were irrigated using a polyionic balanced electrolyte solution (Plasmalyte, Baxter, Inc., Deerfield, IL). Synovial fluid samples were obtained from both limbs at the time of initial arthroscopy (day 0) and at days 5, 9, 12, 19, 26, 33, 61, and 75 post-operatively with aspiration volumes of approximately 2 ml per joint as described previously.36 Synovial fluid samples were centrifuged at 3,000g for 5 min immediately after collection, and synovial fluid supernatants were stored at −80°C until analysis. Prior to the surgery, each horse was acclimated to the treadmill for 1 to 4 days. Horses were exercised on a high-speed treadmill each morning five times weekly for the study duration beginning 2 weeks after surgery. Each session consisted of walking (5 km/h) for 5 min, followed by trotting (16–18 km/h) for 2 min, galloping (28–32 km/h) for 2 min, and ending with 2 min of trotting (16–19 km/h) exercise performed in the morning.
Biochemistry
Synovial fluid composition was analyzed at all time points. As described previously,25 synovial fluid was diluted 1:2,000 in PBS, and lubricin was quantified in duplicate using a peanut agglutinin sandwich ELISA with mAb 9G3 (Cat #: MABT401, Millipore Sigma, Burlington, MA) and purified equine synovial fluid lubricin as the standard. Lectin blots and immunoblots were performed on serial synovial fluid samples from one representative horse using biotinylated peanut agglutinin lectin (Vector Labs, Burlingame, CA), mAb 9G3, and a polyclonal anti-C-terminal lubricin antibody (Cat #: PA3–118, Thermo Fisher Scientific, Waltham, MA), which confirmed that the lubricin measured was full-length and was not degraded post-injury (Figure S-2).
Hyaluronic acid concentrations were measured at all time points in duplicate using a commercially available HA ELISA (Hyaluronan DuoSet ELISA, Cat#: DY3614–05, R&D Systems, Minneapolis, MN).37 For the assay, equine synovial fluid samples from all horses at all time points were diluted 1:80,000 in 5% Tween 20 in PBS. Absorbance was measured at 450 nm with wavelength correction set at 540 nm.
HA molecular weight distribution was determined for all time points by gel electrophoresis similarly to a previously described method.38 Equine SF samples were diluted 1:20 in PBS and were treated overnight with 70 μg/ml proteinase K (Proteinase K, recombinant, PCR grade, Roche Applied Science, Mannheim, Germany). Synovial fluid samples were loaded onto 1% agarose gels. HA standards, Select-HA HiLadder (0.5–1.5 MDa) and Mega-HA Ladder (1.5–6.1 MDa; AMS Biotechnology Limited, Cambridge, MA), were loaded onto each gel to characterize the molecular weight distributions of HA. Electrophoresis was conducted at 50V for 8 h, and gels were stained overnight with 0.005% Stains-All (Sigma-Aldrich, St. Louis, MO) in 50% ethanol. The following day, gels were de-stained in a 10% ethanol solution for 24 h. Gel images were acquired using a Bio-Rad VersaDoc Imaging System (Hercules, CA), combined using the Stitching plugin,39 and relative band intensity was quantified in ImageJ.
Lubrication Analysis
To determine the frictional properties of synovial fluids, samples were tested on a custom tribometer as previously described.32 Briefly, cylindrical cartilage explants (6 mm diameter × 2 mm thickness) were harvested from the patellofemoral groove of neonatal bovine stifles and loaded onto a tribometer in a bath of synovial fluid. Synovial fluid from both sham and injured limbs at days 0, 19, 33, and 61 was tested due to an insufficient volume remaining for analysis at other time points. Explants were compressed to approximately 40% strain against a glass counter-face and permitted to depressurize over the course of 1 h. After reaching an equilibrium normal load, the counter-face was linearly reciprocated at speeds ranging from 0.1–10 mm/s for 2–3 cycles per speed. Simultaneously, a biaxial load recorded the normal and shear loads. For both the forward and reverse directions and at each speed, the friction coefficient was calculated as the mean shear force at the end of sliding when friction had reached an equilibrium value divided by the equilibrium normal load. Care was taken to minimize fluid evaporation during the test, and once the test was completed, synovial fluid was collected and stored at −80°C before evaluating viscosity.
After friction testing, viscosity measurements were performed on the same synovial fluid samples using a commercial rheometer (TA Instruments DHR3 Rheometer, New Castle DE). A flow sweep test where angular velocity varied from 0.1–100 rad/s was performed using a 20 mm parallel plate fixture and gap distance of 500 μm to determine viscosities of synovial fluids from sham and injured limbs. Only synovial fluid collected on days 0, 19, 33, and 61 was tested due to an insufficient volume for analysis at other time points. Viscosity measurements presented are for shear rates of 2 Hz or 0.1 rad/s, which is generally within synovial fluid’s first Newtonian plateau and has previously been shown to appropriately scale Stribeck curves.24
Stribeck Analysis
Lubrication and rheological data were fit to a dimensionless Sommerfeld number, S,
where v is sliding speed, η is viscosity, a is contact width (6 mm), and FN is normal load.24,29 Stribeck curves were generated by plotting friction coefficient as a function of Sommerfeld number. Transition number, St, was calculated by fitting friction coefficients from speeds 0.1–1 mm/s, that is, during the mixed lubrication mode, versus respective Sommerfeld number to the equation24:
in MATLAB using a linear least-squares method (Math-Works, Natick, MA). The boundary friction coefficient, μB, was taken from the Stribeck curves as the upper plateau in friction coefficient at v = 0.1 mm/s and the minimum or elastoviscous mode friction coefficient, μmin, was taken as the lower plateau in friction coefficient at v = 5–10 mm/s.
Statistics
All data were analyzed using a linear mixed effects model to account for the hierarchical nature of the data. The fixed effects in the model included treatment (sham versus OA limb), day, and a treatment day interaction term. Random effects included horse and horse treatment to account for the non-independence of observations. To satisfy model assumptions and normalize data, square root values were used for lubricin ELISA, transition number, and HA 0.5–1.5 MDa gel data, and logarithmic values were used for HA ELISA data. All summary statistics and values shown in figures represent un-transformed data with mean ± SEM. In the case of significant fixed effect terms, post-hoc analysis was performed using Dunnett’s test to compare to day 0 baseline values. p-values <0.05 were considered significant. Statistical analysis was performed using JMP Pro 13.0 software (SAS Institute Inc., Cary, NC).
RESULTS
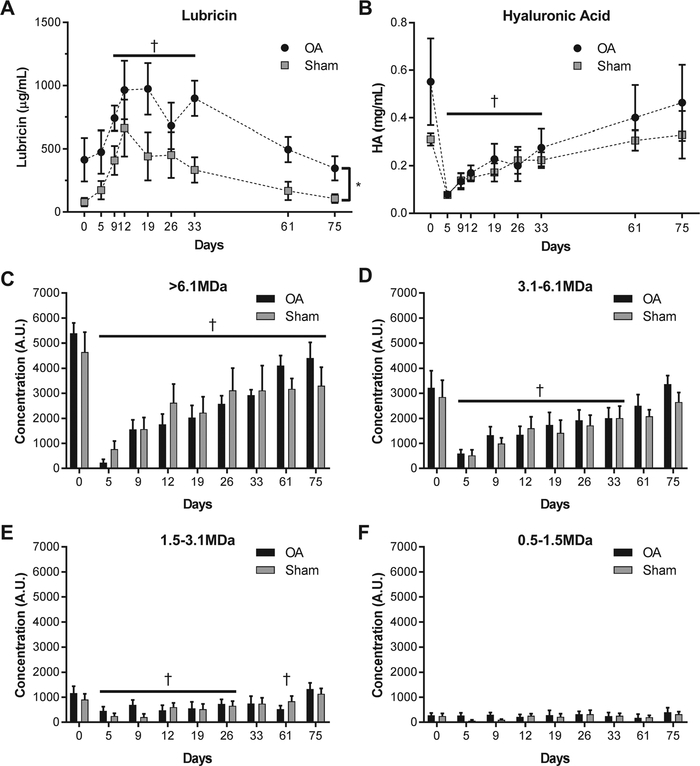
Osteochondral fragmentation resulted in significant changes in the composition of synovial fluid with respect to lubricin, HA, and HA molecular weight distribution. HA concentrations decreased significantly after fracture in both the sham and OA limbs (Figure 1B), with day 5 concentrations approximately 80% lower than day 0 baseline values. Concentrations remained significantly decreased relative to day 0 baseline values until day 33 after surgery. Similarly, high molecular weight HA (>6.1 MDa, Figure 1C) was significantly lower relative to day zero baseline for all time points after injury in both the sham and OA limbs. The 3.1–6.1MDa and 1.5–3.1MDa HA concentrations (Figure 1D,E) were likewise decreased following injury, but recovered to values similar to baseline by day 61 and day 75, respectively. No changes in the concentration of low molecular weight HA (0.5–1.5MDa, Figure 1F) were observed. Lubricin concentrations increased in both OA and sham limbs post-operatively, peaking at 2–3 weeks after injury and remaining elevated until day 61 (Figure 1A). Lubricin concentrations were approximately 2–3× higher in injured than control limbs (p = 0.008).
Figure 1.
Synovial concentration of (a) lubricin increased after injury with levels peaking at 2–3 weeks after injury and not returning toward baseline until day 61 after injury. In contrast, the total concentration of (b) HA decreased after injury, especially the (c) high molecular weight fraction (>6.1 MDa). For the >6.1 MDa fraction, synovial concentrations of HA did not recover to baseline values over the course of the study while the 3.1–6.1 MDa fraction did not recover until 61 days after surgery. Note that † indicates that the combined sham and OA data are significantly different than day 0 at the specified timepoints and *indicates a significant difference between treatment groups across timepoints (p < 0.05, n = 4).
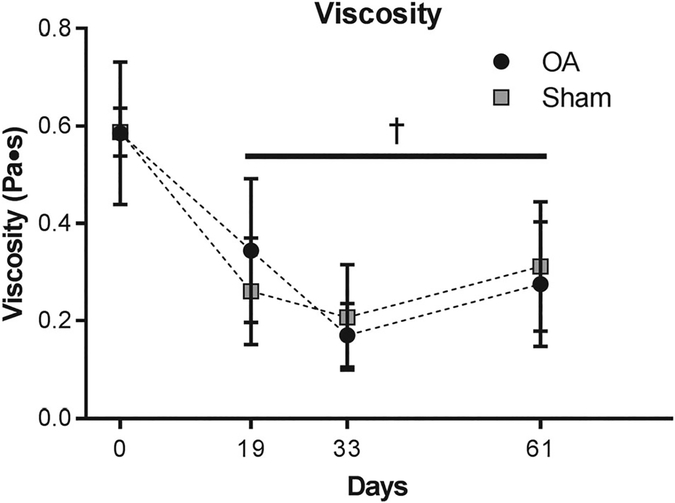
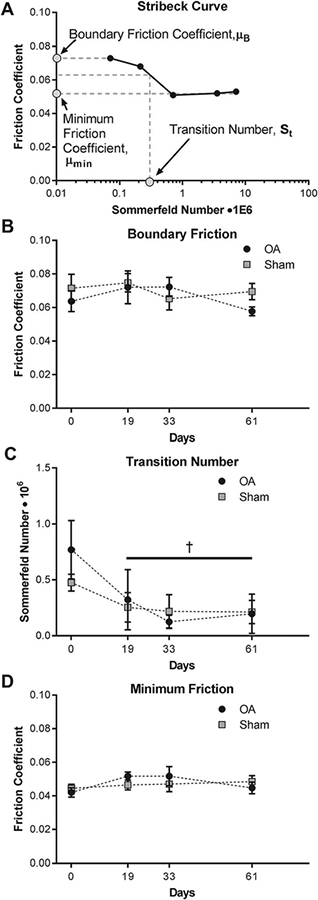
Lubrication analysis revealed distinct changes in synovial fluid with respect to viscosity and lubrication modes, but no significant differences were observed between sham and OA limbs for these measurements (p > 0.05). Compared to baseline values, low shear rate viscosity decreased by nearly half by day 19 and remained significantly lower at days 33 and 61 (Figure 2, p < 0.01). Surprisingly, boundary friction and minimum friction coefficients did not vary over time nor differ between control and OA limbs (Figure 3B, D). Rather, a distinct shift in transition number was observed; in both injured and sham limbs, the transition number was significantly lower at all time points after surgery indicating an earlier transition from boundary mode toward elastoviscous mode as compared to baseline values (Figure 3C).
Figure 2.
Viscosity decreased after injury and did not recover to baseline values. Note that † indicates that the combined sham and OA data are significantly different than day 0 at the specified timepoints (p < 0.05, n = 4).
Figure 3.
(A) Stribeck curves were created by plotting friction coefficients as a function of the Sommerfeld number, which factors in the viscosity of synovial fluid and tribological test parameters including sliding speed, contact width, and normal load. Stribeck curves were used to analyze how lubrication changed over time with respect to (B) boundary lubrication and (D) elastoviscous lubrication and the (C) transition point between these modes. Note that † indicates that the combined sham and OA data are significantly different than day 0 at the specified timepoints (p < 0.05, n = 4).
DISCUSSION
Synovial fluid lubricin, HA, and HA molecular weight distribution were significantly altered following OA induction via osteochondral carpal fracture in the equine model. Interestingly, a reduction in transition number and synovial fluid viscosity were observed after surgery while boundary and minimum friction coefficients remained unchanged. Similar to past results in this model25 and in human and equine articular fractures,14,15 lubricin concentrations were significantly higher after injury. Interestingly, although lubricin concentrations were higher in the limb undergoing fracture, derangements in lubricin, HA and HA molecular weight distribution were paralleled between the injured and sham-operated limbs. This suggests that the loss of HA may be more significantly affected by synovial fluid washout during the arthroscopic procedure as opposed to the carpal fragmentation injury, whereas lubricin expression is induced by intra-articular fragmentation. Total HA concentrations as quantified via ELISA were significantly reduced relative to baseline for 4 weeks after injury. Notably, the high molecular weight HA fractions as quantified via gel electrophoresis were particularly disturbed after fracture; for the highest molecular weight forms (>6.1 MDa), levels remained below baseline for all time points measured after injury (10 weeks) while the 3.1–6.1 MDa fraction remained low for 4 weeks after injury. A concurrent decrease in viscosity of synovial fluid was observed.
With respect to lubrication, no changes in boundary mode and elastoviscous mode friction coefficients were observed over time nor between sham-operated and injured limbs. We have previously determined that maximal boundary lubrication on our custom tribometer was achieved at a concentration of approximately 40 μg/ml of recombinant human lubricin, with concentrations up to 350 μg/ml having no additional effects on friction coefficient.40 Here, we used similar testing conditions, and thus attribute the lack of change in boundary friction coefficients to synovial lubricin concentrations far exceeding 40 μg/ml in the present study. Dose-response relationships between lubrication and lubricin concentration vary among reported studies and may be affected by lubricin source, lubricin quantification methods, and tribometer setup (cartilage-on-cartilage vs. cartilage-on-glass). Notably even between different instruments, saturating concentrations range from 40 to 150 μg/ml lubricin, but friction coefficients at saturation converge toward ~0.10.40,41 This convergence suggests that the median effective dose of lubricin is especially dependent on lubricin source and quantification. Importantly, the dose-response relationship for lubrication of equine cartilage by lubricin has not been previously reported.
Furthermore, the lack of change in elastoviscous friction coefficients despite the decrease in viscosity was surprising. In hard, non-porous materials, low friction coefficients occur as a fluid film develops between articulating surfaces, and this fluid film is promoted by lubricant viscosity. However, for soft, porous materials like cartilage, full fluid-film lubrication is likely not achieved due to fluid flow back into the cartilage matrix and deformation of cartilage asperities.24,29,42 Still, reduced friction with increased sliding speed may be explained by partial fluid film lubrication,29 tribological rehydration,35 and generation of pressurized fluid wedges.30,42 Therefore, we posit two theories as to why elastoviscous friction coefficients did not vary; first, viscosity may play competing roles in lubricating the tissue. While higher viscosity fluids may better support partial fluid film lubrication, tribological rehydration may be hindered due to a reduced ability to flow. Alternatively, minimum friction coefficients may be limited by the properties of cartilage tissue, including its roughness and permeability. Our results agree with a previous study in which viscosity was varied over several orders of magnitude.24 In previous studies of healthy cartilage, similar μmin values were observed when lubricant composition was varied.24 In contrast, an increase in μmin was associated with cartilage impact and surface roughening suggesting that cartilage properties may be crucial to lubrication in this regime.30 Overall, the observed lack of change in elastoviscous friction coefficients despite the decrease in viscosity warrants further investigation into the mechanisms of elastoviscous lubrication.
We observed the most dramatic changes in lubrication in the mixed lubrication regime. Interestingly, the transition number decreased after surgery in both limbs, indicating an earlier transition away from high-friction boundary mode. The effects of lubricin-HA interactions on Stribeck behavior have only recently begun to be investigated,24 and more information is necessary to understand how varying concentrations and overall lubricant viscosities affect cartilage friction regimes. Numerous studies have suggested that lubricin and HA function synergistically to lubricate cartilage.24,43 Although a dose-response Stribeck analysis of lubricin and HA has not yet been conducted, a recent study from our lab has found that both boundary friction coefficients and the transition number were reduced when lubricin was added with HA as compared to HA alone.24 This observation is consistent with the idea that lubricin-HA interactions are facilitated by the elevated lubricin concentrations observed after injury. Such interactions may enhance lubrication through a shift to lower friction at lower sliding speeds.
In contrast to our results, similar studies of synovial fluid after articular fracture have observed greater boundary friction coefficients. In other studies where increased synovial fluid lubricin concentrations were also observed,14,15 greater boundary friction coefficients were reported, leading authors to hypothesize that decreased HA concentrations and a loss of high molecular weight HA may increase friction. In the acute post-injury phase (<3 weeks), Antonacci et al. and Ballard et al. reported a 39% and 100% increase, respectively, in boundary friction coefficients. We similarly observed decreased HA and increased lubricin levels after injury, but no significant changes in boundary lubrication were observed (<8%). The overall magnitude of boundary friction coefficients observed in our study averaged ~0.07, while Ballard et al. and Antonacci et al. reported a range of 0.022 for control synovial fluid to a maximum of 0.044 for injured synovial fluid. One challenge in comparing our results to previous studies is that differing types of tribometers were used; while only stationary contact area configurations were used, here, we employed a cartilage-on-glass system with linear reciprocating motion whereas others used a cartilage-on-cartilage framework with a rotational motion.14,15 Recent investigations using a rotational tribometer have found that boundary lubrication by HA is dependent on the counterface material; relative to saline, a reduction in friction coefficient by HA was only observed in cartilage-cartilage interfaces as compared to cartilage-glass interfaces.44 Notably, other studies have demonstrated that HA and other viscous lubricants do indeed lower friction coefficient in cartilage-on-glass systems.24,30,31,33 Furthermore, the Stribeck framework was not applied in those studies. The Stribeck framework is advantageous because it elucidates the friction regime experienced by cartilage while controlling for viscosity, normal load, and sliding speed, which can shift the system between boundary and elastoviscous friction modes. In other studies,14,19 marked differences in HA molecular weight distribution and concentration, a major driver of synovial fluid viscosity, were reported, but the synovial fluid viscosity was not investigated and only a single, slow rotational speed (0.3 mm/s) was used. Thus, it is possible that the boundary friction coefficients we have reported (at linear speeds of 0.1 mm/s) may not be directly comparable due to differences in testing equipment and definition of boundary regime.
Importantly, HA was significantly diminished after fracture and remained depleted long after injury. This occurred not only in the fractured joint but also, albeit to a lesser extent, in the contralateral sham-operated joint. It is intuitive that HA concentrations would be decreased following arthroscopy and saline lavage. Recent studies have demonstrated that lavage reduces lubricin staining at the cartilage surface45,46 and results in impaired boundary friction properties of cartilage.45,46 However, it was surprising that the loss of high molecular weight HA was sustained for so long following fracture, especially given how quickly lubricin concentrations recovered. One possible explanation is that horses returned to intense treadmill exercise 2 weeks following injury, which may have been a persistent stimulus for HA degradation, impaired HA synthesis or loss of HA due to increased synovial membrane permeability.47 Additionally, the contralateral limb could be affected by systemic effects of the injury or compensatory overloading to protect the injured limb. Further studies are necessary to explore how HA viscosupplementation after articular fracture or arthroscopic lavage may affect synovial fluid lubrication and osteoarthritis development. In an in vitro test, boundary lubrication was restored to normal values when high molecular weight HA was added to synovial fluid samples that showed impaired boundary lubrication and low HA levels.15 Other clinical studies have reported improvements in patient pain scores and joint swelling48 in patients that received viscosupplementation after partial meniscectomy.
Additionally, the elevated lubricin concentrations we observed after injury contribute to a growing body of evidence that lubricin may be modulated differently by species, injury type, exercise, or other factors. Decreased lubricin levels have been reported after anterior cruciate ligament (ACL) tears in humans,9 rat models of ACL tear,8 and guinea pig ACL tear models22 relative to controls. In contrast, in other end-stage human OA studies,26 in an ovine ACLT model19 and equine15,25 and human14 articular fractures, synovial fluid exhibits elevated lubricin concentrations. Several assays, including ELISAs8,9,22,25,26 and semi-quantitative western blots14,15,22,26 have been used to measure lubricin, which could also explain the variations between studies. Most small animal destabilization models of OA have reported decreased synovial fluid lubricin, while increases have been mostly reported in large animal and human cases of articular fracture. Lubricin expression is known to be modulated by exercise,21 mechanical loading,49 and cytokines like TGF-β, TNF-α, and IL-1β.7,50–52 Furthermore, mesenchymal stem cells (MSCs) are known to produce copious amounts of lubricin53,54 and, as such, their release into the joint space due to the osteochondral fragment induction may have led to increased lubricin release into synovial fluid. As many research groups are investigating the therapeutic potential of lubricin to treat osteoarthritis, it is important to understand when it may be indicated for treatment.
This study was not without limitations. Only four horses were evaluated during this study. However, the linear mixed model accounted for serial measurements from each horse, which increased the power of our statistical comparisons. Furthermore, we did not examine expression and enzymatic activity within the joint that resulted in alterations in lubricin and HA concentrations nor the specific biological activity of these molecules. Both molecules have demonstrated anti-inflammatory properties55 and may play important roles in joint homeostasis distinct from their roles in lubrication. Finally, this study used healthy neonatal bovine tissue in combination with the equine synovial fluid samples for measuring lubrication. While this was chosen to enable serial measurements of synovial fluid lubrication throughout OA development, we did not examine the frictional changes in the equine cartilage tissue. Hallmarks of the equine osteochondral fragment model and OA, including proteoglycan loss and surface fibrillation, have been associated with inferior tribological properties.30,31,56
In conclusion, we have shown distinct temporal changes in the concentrations of the critical synovial fluid lubricants, HA and lubricin, in an equine experimental OA model. Importantly, concentrations of HA, especially high molecular weight forms, decreased significantly after surgical intervention and remained below pre-surgery baseline values for weeks after injury. Lubricin concentrations increased after articular fracture in agreement with previous studies in this model and other cases of articular fracture. Interestingly, no changes were observed in boundary mode friction or elastoviscous mode friction coefficients. However, the transition number representing the shift between high-friction boundary mode and low-friction elastoviscous mode was reduced after surgery. This suggests that lubrication was enhanced after fracture. Notably, the sham-operated limb exhibited a similar magnitude of concentration changes in lubricin, HA, and lubricating properties as compared to the control limb. This suggests that non-operated animals or non-operated contralateral limbs should be included in studies using this experimental model that aim to evaluate lubrication outcome parameters. Future studies should focus on elucidating the biological and mechanical roles of lubricin and HA. Despite the wide variation of lubricin and HA in disease, frictional changes, and especially multi-modal friction changes, have been examined for only a narrow subset of concentrations. Altogether, this study indicates that there are distinct time-dependent changes in synovial fluid lubricant composition and frictional properties following articular fracture.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K08AR068469 (HR) and the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1650441 (EF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank the Cornell Statistical Consulting Unit for assistance with model development and statistical analyses. This work made use of the Cornell Center for Materials Research Shared Facilities which are supported through the NSF MRSEC program (DMR-1719875).
Grant sponsor:
National Institute of Arthritis and Musculoskeletal and Skin Diseases; Grant number: K08AR068469; Grant sponsor: Division of Graduate Education; Grant number: DGE-1650441.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
REFERENCES
- 1.Brown TD, Johnston RC, Saltzman CL, et al. 2006. J Orthop Trauma 20:739–744. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DD, Chubinskaya S, Guilak F, et al. 2011. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res 29:802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh JL, Buckwalter J, Gelberman R, et al. 2002. Articular fractures: does an anatomic reduction really change the result? J Bone Joint Surg Am 84–A:1259–1271. [PubMed] [Google Scholar]
- 4.Schenker ML, Mauck RL, Ahn J, et al. 2014. Pathogenesis and prevention of posttraumatic osteoarthritis after intra-articular fracture. J Am Acad Orthop Surg 22:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbone A, Rodeo S. 2017. Review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J Orthop Res 35:397–405. [DOI] [PubMed] [Google Scholar]
- 6.Jay GD, Torres JR, Rhee DK, et al. 2007. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum 56:3662–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SY, Niikura T, Reddi AH. 2008. Superficial zone protein (lubricin) in the different tissue compartments of the knee joint: modulation by transforming growth factor beta 1 and interleukin-1 beta. Tissue Eng Part A 14:1799–1808. [DOI] [PubMed] [Google Scholar]
- 8.Elsaid KA, Machan JT, Waller K, et al. 2009. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting tumor necrosis factor alpha on chondroprotection in an animal model. Arthritis Rheum 60:2997–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsaid KA, Fleming BC, Oksendahl HL, et al. 2008. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum 58:1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waller KA, Zhang LX, Elsaid KA, et al. 2013. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci 110:5852–5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonnevie ED, Delco ML, Bartell LR, et al. 2018. Microscale frictional strains determine chondrocyte fate in loaded cartilage. J Biomech 74:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tulamo RM, Heiskanen T, Salonen M. 1994. Concentration and molecular weight distribution of hyaluronate in synovial fluid from clinically normal horses and horses with diseased joints. Am J Vet Res 55:710–715. [PubMed] [Google Scholar]
- 13.Plickert HDD, Bondzio A, Einspanier R, et al. 2013. Hyaluronic acid concentrations in synovial fluid of dogs with different stages of osteoarthritis. Res Vet Sci 94:728–734. [DOI] [PubMed] [Google Scholar]
- 14.Ballard BL, Antonacci JM, Temple-Wong MM, et al. 2012. Effect of tibial plateau fracture on lubrication function and composition of synovial fluid. J Bone Jt Surgery-American Vol. 94:e64-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonacci JM, Schmidt TA, Serventi LA, et al. 2012. Effects of equine joint injury on boundary lubrication of articular cartilage by synovial fluid: role of hyaluronan. Arthritis Rheum 64:2917–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asari A, Miyauchi S, Sekiguchi T, et al. 1994. Hyaluronan, cartilage destruction and hydrarthrosis in traumatic arthritis. Osteoarthr Cartil 2:79–89. [DOI] [PubMed] [Google Scholar]
- 17.Belcher C, Yaqub R, Fawthrop F, et al. 1997. Synovial fluid chondroitin and keratan sulphate epitopes, glycosaminoglycans, and hyaluronan in arthritic and normal knees. Ann Rheum Dis 56:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Temple-Wong MM, Hansen B, Grissom M, et al. 2010. Effect of knee osteoarthritis on the boundary lubricating molecules and function of human synovial fluid. Trans Orthop Res Soc 35:0340. [Google Scholar]
- 19.Atarod M, Ludwig TE, Frank CB, et al. 2015. Cartilage boundary lubrication of ovine synovial fluid following anterior cruciate ligament transection: a longitudinal study. Osteoarthr Cartil 23:640–647. [DOI] [PubMed] [Google Scholar]
- 20.Elsaid KA, Jay GD, Warman ML, et al. 2005. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum 52:1746–1755. [DOI] [PubMed] [Google Scholar]
- 21.Elsaid KA, Zhang L, Waller K, et al. 2012. The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin’s effect in exercised joints following ACL transection. Osteoarthr Cartil 20:940–948. [DOI] [PubMed] [Google Scholar]
- 22.Teeple E, Elsaid KA, Fleming BC, et al. 2008. Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orthop Res 26:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young AA, McLennan S, Smith MM, et al. 2006. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Ther 8:R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonnevie ED, Galesso D, Secchieri C, et al. 2015. Elastoviscous transitions of articular cartilage reveal a mechanism of synergy between lubricin and hyaluronic acid. PLoS ONE 10:e0143415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reesink HL, Watts AE, Mohammed HO, et al. 2017. Lubricin/proteoglycan 4 increases in both experimental and naturally occurring equine osteoarthritis. Osteoarthr Cartil 25:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neu CP, Reddi AH, Komvopoulos K, et al. 2010. Increased friction coefficient and superficial zone protein expression in patients with advanced osteoarthritis. Arthritis Rheum 62:2680–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIlwraith CW, Frisbie DD, Kawcak CE. 2012. The horse as a model of naturally occurring osteoarthritis. Bone Joint Res. 1:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neu CP, Komvopoulos K, Reddi AH. 2008. The interface of functional biotribology and regenerative medicine in synovial joints. Tissue Eng. Part B Rev. 14:235–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunn AC, Sawyer WG, Angelini TE. 2014. Gemini interfaces in aqueous lubrication with hydrogels. Tribol. Lett 54:59–66. [Google Scholar]
- 30.Bonnevie ED, Delco ML, Galesso D, et al. 2017. Sub-critical impact inhibits the lubricating mechanisms of articular cartilage. J. Biomech 53:64–70. [DOI] [PubMed] [Google Scholar]
- 31.Bonnevie ED, Galesso D, Secchieri C, et al. 2017. Degradation alters the lubrication of articular cartilage by high viscosity, hyaluronic acid-based lubricants. J. Orthop. Res 1456–1464. [DOI] [PubMed] [Google Scholar]
- 32.Gleghorn JP, Bonassar LJ. 2008. Lubrication mode analysis of articular cartilage using Stribeck surfaces. J Biomech 41:1910–1918. [DOI] [PubMed] [Google Scholar]
- 33.Shi L, Sikavitsas VI, Striolo A. 2011. Experimental friction coefficients for bovine cartilage measured with a pin-on-disk tribometer: testing configuration and lubricant effects. Ann Biomed Eng 39:132–146. [DOI] [PubMed] [Google Scholar]
- 34.Burris DL, Moore AC. 2017. Cartilage and joint lubrication: new insights into the role of hydrodynamics. Biotribology 12:8–14. [Google Scholar]
- 35.Moore AC, Burris DL. 2017. Tribological rehydration of cartilage and its potential role in preserving joint health. Osteoarthr Cartil 25:99–107. [DOI] [PubMed] [Google Scholar]
- 36.Reesink HL, Nixon AJ, Su J, et al. 2018. Galectins-1 and −3 increase in equine post-traumatic osteoarthritis. Front Vet Sci 5:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barton KI, Ludwig TE, Achari Y, et al. 2013. Characterization of proteoglycan 4 and hyaluronan composition and lubrication function of ovine synovial fluid following knee surgery. J Orthop Res 31:1549–1554. [DOI] [PubMed] [Google Scholar]
- 38.Lee HG, Cowman MK. 1994. An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Anal Biochem 219:278–287. [DOI] [PubMed] [Google Scholar]
- 39.Preibisch S, Saalfeld S, Tomancak P. 2009. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25:1463–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gleghorn JP, Jones ARC, Flannery CR, et al. 2009. Boundary mode lubrication of articular cartilage by recombinant human lubricin. J Orthop Res 27:771–777. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt TA, Gastelum NS, Nguyen QT, et al. 2007. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum 56:882–891. [DOI] [PubMed] [Google Scholar]
- 42.Graham BT, Moore AC, Burris DL, et al. 2017. Sliding enhances fluid and solute transport into buried articular cartilage contacts. Osteoarthr Cartil 25:2100–2107. [DOI] [PubMed] [Google Scholar]
- 43.Abubacker S, Dorosz SG, Ponjevic D, et al. 2016. Full-length recombinant human proteoglycan 4 interacts with hyaluronan to provide cartilage boundary lubrication. Ann Biomed Eng 44:1128–1137. [DOI] [PubMed] [Google Scholar]
- 44.Abubacker S, McPeak A, Dorosz SG, et al. 2018. Effect of counterface on cartilage boundary lubricating ability by proteoglycan 4 and hyaluronan: cartilage-glass versus cartilage-cartilage. J Orthop Res 36:2923–2931. [DOI] [PubMed] [Google Scholar]
- 45.Teeple E, Karamchedu NP, Larson KM, et al. 2016. Arthroscopic irrigation of the bovine stifle joint increases cartilage surface friction and decreases superficial zone lubricin. J Biomech 49:3106–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stärke F, Awiszus F, Lohmann CH, et al. 2018. The effect of irrigation time and type of irrigation fluid on cartilage surface friction. J Mech Behav Biomed Mater 77:187–191. [DOI] [PubMed] [Google Scholar]
- 47.McCarty WJ, Cheng JC, Hansen BC, et al. 2012. The biophysical mechanisms of altered hyaluronan concentration in synovial fluid after anterior cruciate ligament transection. Arthritis Rheum 64:3993–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waddell DD, Bert JM. 2010. The use of hyaluronan after arthroscopic surgery of the knee. Arthrosc J Arthrosc Relat Surg 26:105–111. [DOI] [PubMed] [Google Scholar]
- 49.Nugent GE, Schmidt TA, Schumacher BL, et al. 2018. Static and dynamic compression regulate cartilage metabolism of PRoteoGlycan 4 (PRG4). Biorheology 43:191–200. [PubMed] [Google Scholar]
- 50.Jones ARC, Flannery CR. 2007. Bioregulation of lubricin expression by growth factors and cytokines. Eur Cell Mater 13:40–45. [DOI] [PubMed] [Google Scholar]
- 51.Alquraini A, Jamal M, Zhang L, et al. 2017. The autocrine role of proteoglycan-4 (PRG4) in modulating osteoarthritic synoviocyte proliferation and expression of matrix degrading enzymes. Arthritis Res Ther 19:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Löfgren M, Svala E, Lindahl A, et al. 2018. Time-dependent changes in gene expression induced in vitro by interleukin-1β in equine articular cartilage. Res Vet Sci 118:466–476. [DOI] [PubMed] [Google Scholar]
- 53.Gleghorn JP, Jones ARC, Flannery CR, et al. 2007. Boundary mode frictional properties of engineered cartilaginous tissues. Eur Cells Mater 14:20–28. [DOI] [PubMed] [Google Scholar]
- 54.Nakagawa Y, Muneta T, Otabe K, et al. 2016. Cartilage derived from bone marrow mesenchymal stem cells expresses lubricin in vitro and In vivo. PLoS ONE 11: e0148777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alquraini A, Garguilo S, D’Souza G, et al. 2015. The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: an anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res Ther 17:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basalo IM, Chen FH, Hung CT, et al. 2006. Frictional response of bovine articular cartilage under creep loading following proteoglycan digestion with chondroitinase ABC. J Biomech Eng 128:131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.