Abstract
Stimulant drugs acting at the dopamine transporter (DAT), like cocaine, are widely abused, yet effective medical treatments for this abuse have not been found. Analogs of benztropine that, like cocaine, act at the DAT have effects that differ from cocaine, and in some situations block the behavioral, neurochemical, and reinforcing actions of cocaine. Neurochemical studies of dopamine levels in brain and behavioral studies have demonstrated that benztropine analogs have a relatively slow onset and reduced maximal effects compared to cocaine. Pharmacokinetic studies, however, indicated that the benztropine analogs access the brain at concentrations above their in vitro binding affinities while binding in vivo demonstrates apparent association rates for benztropine analogs lower than that for cocaine. Additionally, the off-target effects of these compounds do not fully explain their differences from cocaine. Initial structure-activity studies indicated that benztropine analogs bind to DAT differently from cocaine and these differences have been supported by site-directed mutagenesis studies of the DAT. In addition, benztropine analog-mediated inhibition of uptake was more resistant to mutations producing inward conformational DAT changes than cocaine analogs. The benztropine analogs have provided new insights into the relation between the molecular and behavioral actions of cocaine, and the diversity of effects produced by dopamine transport inhibitors. Novel interactions of benztropine analogs with the DAT suggest that these drugs may have a pharmacology that would be useful in their development as treatments for cocaine abuse.
I. Introduction
A. The dopamine transporter, a biological target for human diseases and psychostimulant abuse.
Dopamine (DA) neurotransmission subserves a multitude of normal physiological functions in the central nervous system, with many factors affecting DA homeostasis. DA neurotransmission is regulated dynamically at the synaptic level by several mechanisms including negative feedback circuits induced by DA receptor occupancy that modulate neuronal activity, as well as DA synthesis. However, termination of the actions of DA by rapidly reducing its synaptic concentrations is critical. This occurs via metabolic degradation pathways, including monoamine oxidase (MAO), and catechol-oxymethyl-transferase (COMT) enzymes, and by DA uptake (Iversen et al., 2008). DA uptake sites or DA transporters (DATs) are membrane-bound proteins that efficiently transport DA from the extra- to the intra-cellular space, and represent the major mechanism for the rapid termination of DA neurotransmission.
One of the most prominent of the diseases that involve dysfunctional DA neurotransmission is Parkinson’s Disease (PD) in which degeneration of the DA system leads to a reduction in neurotransmission in dopaminergic terminal areas that are important for somatic-motor functions, but also for emotional-affective functions (Lees et al., 2009). Indeed some of the typical symptoms of PD involve a difficulty to initiate movements, as well as depression and apathy (Chaudhuri and Schapira, 2009). Drugs that target the DAT, methylphenidate and d-amphetamine, are clinically effective treatments, and genetic variants in the DAT have been implicated in the etiology ADHD (e.g. Hahn and Blakely, 2007). A DA component is also involved in other diseases involving emotional and affective functions, including schizophrenia, autism, Tourette’s syndrome, and depression (Meisenzahl et al., 2007; Steeves and Fox, 2008; Stein, 2008).
The DAT is also the main target for stimulant drugs of abuse, such as cocaine, amphetamine, methamphetamine, and methylenedioxymethamphetamine (Zhu and Reith 2008). Stimulant abuse and addiction are recognized to be a major public health and socio-economical issues (see for example: Substance Abuse and Mental Health Services Administration, http://www.oas.samhsa.gov) and research efforts over the last two decades have shed light on the neurobiological basis of cocaine dependence (Kalivas, 2007; Nestler, 2005). And while promising new strategies for the development of medical treatments have been reported (e.g. Kharkar et al., 2008; Newman and Katz, 2009; Runyon and Carroll, 2008), cocaine addiction remains a condition for which effective medical treatments have not yet been identified.
Cocaine acts in the CNS at several pharmacological targets. For example, its local anesthetic effects have been well documented together with its effects on Na+ channels (Catterall and Mackie, 2006). However, the main activity contributing to the reinforcing effects of cocaine, and its consequent abuse liability, involves the blockade of plasma membrane monoamine transporters (see review by Carroll et al., 1999). Although cocaine inhibits the transport of dopamine, serotonin, and norepinephrine from the synapse into nerve terminals, blockade of the DAT is considered the main effect through which the pharmacology of cocaine contributes to its behavioral and reinforcing actions (Kuhar et al., 1991; Ritz et al., 1989a).
B. The DAT as a pharmacological target for candidate drugs as psychostimulant abuse medications, the atypical DAT inhibitors benztropine analogs.
It has been hypothesized that drugs blocking the DAT will have reinforcing effects similar to those of cocaine (Kuhar et al., 1991). However, of the several chemical classes of DAT inhibitors synthesized in the last 15–20 years, some have behavioral effects that differ from those of cocaine (Newman and Kulkarni, 2002). Because of these variations in behavioral effects, these “atypical” DAT inhibitors are being actively investigated to find clues that may help in the search for psychostimulant abuse medications.
Agonist or substitution therapies have been successful in the treatment of opioid (Mattick et al., 2009) and nicotine abuse (Stead et al., 2008). As such, drugs that block the DAT, but with lower abuse potential compared to cocaine, have been the focus of many of the drug discovery programs directed at cocaine abuse treatments. One of the most studied compounds, GBR 12909 (Figure 1), which shares some basic pharmacological features with addictive psychostimulants, has preclinical effects suggestive of a clinically effective treatment. Specifically, treatment with GBR12909 decreases cocaine self-administration in animals, at doses that do not affect the behaviors reinforced with food presentation (see review by Rothman et al., 2008). However, the appearance of cardiovascular effects in clinical trials prevented its further development (Vocci et al., 2005).
Figure 1:
Chemical structures of cocaine, GBR 12909 and the BZT analogs referred to in the present manuscript. For a more complete description of the chemistry of the BZT analogs see Newman and Katz (2009).
Several classes of DAT inhibitors that were tested preclinically for their potential as treatments for stimulant abuse have been reviewed elsewhere (e.g. Kharkar et al., 2008; Meltzer, 2008; Prisinzano and Rice, 2008; Runyon and Carroll, 2008). The present chapter focuses on analogs of benztropine (3α-diphenylmethoxytropane, BZT, Figure 1). This parent compound was initially of interest because it shares structural features with both cocaine and GBR 12909 (Figure 1). Therefore, solely from a structural perspective, BZT and its analogs were of interest. Moreover, though BZT is in clinical use for treatment of early stage PD for many years, there are only a few case-reports of its abuse, mainly related to its anticholinergic effects (see for example Grace, 1997). Finally, Colpaert et al. (1979) showed that BZT did not fully substitute in rats trained to discriminate cocaine from saline injections. These considerations suggested that BZT analogs could be of interest for cocaine-abuse treatment, and may have advantages over DAT inhibitors that share cocaine-like preclinical indications of abuse liability. To pursue this possibility, we initiated a program of synthesis and evaluation of BZT analogs. A comprehensive review of the chemistry of these compounds has been recently published (Newman and Katz, 2008). In the present chapter we review preclinical and clinical research that has been conducted on BZT analogs as it relates to the potential of these compounds as medications for cocaine abuse.
C. Definitions.
JJC 1–059: N-(3-((3S,5R)-3,5-dimethyl-4-(3-phenylpropyl)piperazin-1-yl)propyl)-4-fluoro-N-(4-fluorophenyl)aniline
JJC 2–010: 3-(4-(3-(bis(4-fluorophenyl)amino)propyl)piperazin-1-yl)-1-phenylpropan-1-ol
GBR 12909: 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-phenylpropyl)piperazine
WIN 35,428: (–)-2β-carbomethoxy-3β-(4-fluorophenyl)tropane
RTI 371: 3β-(4-methylphenyl)-2β-[3-(4-chlorophenyl)isoxazol-5-yl]tropane
RTI-121: (–)-2β-carboisopropoxy-3β-(4-iodophenyl)tropane
RTI-55: 3β-(4-iodophenyl)tropan-2 beta-carboxylic acid methyl ester
RTI-31: (–)-2β-carbomethoxy-3β-(4’-chlorophenyl)tropane
CP 55940: 2-[(1S,2R,5S)-5-hydroxy-2-(3-hydroxypropyl) cyclohexyl]-5-(2-methyloctan-2-yl)phenol
II. Behavioral studies
A. Stimulation of ambulatory activity
Stimulation of ambulatory activity is one of the common effects produced by psychostimulants after systemic administration. This behavior is likely mediated by the ability of these compounds to interact with DA transmission in specific brain areas related to physiological functions other than control of motor activities (Zahm, 1999). It is interesting to note that local application of psychostimulants in specific dopaminergic terminal fields that are implicated in the subjective effects and abuse liability of psychostimulant drugs increases ambulatory activity (Ikemoto, 2002). Thus, though this behavioral activity is not directly related to the reinforcing effects of these compounds, this behavior provides a relatively simple preclinical model to investigate psychostimulant-like effects of compounds that may have liability for abuse.
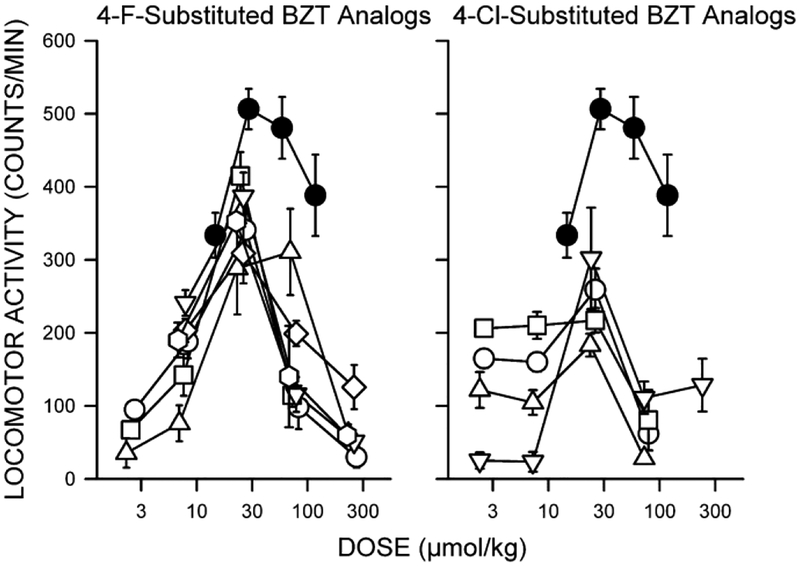
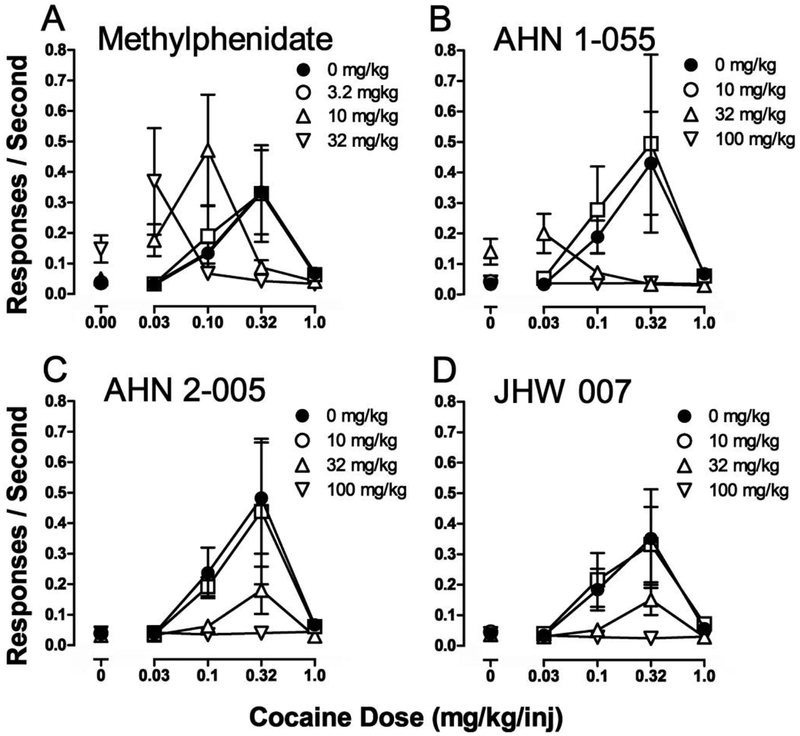
The dose-dependent effects of cocaine and standard DAT inhibitors (e.g., methylphenidate, mazindol, and nomifensine) on stimulation of ambulatory activity can be graphically represented as a bell-shaped, inverted-U curve, with increased stimulation of activity at low to intermediate doses, and a decrease of effects at the larger doses (filled symbols in Figure 2 show cocaine effects). The latter may be the result of the appearance of behaviors other than ambulation, i.e. stereotypies, proconvulsive effects, seizures or convulsions, that are likely incompatible with locomotion. BZT analogs can produce different levels of ambulatory activity, but they most often do not stimulate activity to the same level as that produced by cocaine or cocaine-like drugs (Katz et al., 2001; Katz et al., 1999; Katz et al., 2004; Newman et al., 1994; Newman et al., 1995; Tanda et al., 2005; Tolliver et al., 1999) (Figure 2. For chemical structures see Figure 1). In contrast, standard DA uptake inhibitors, such as cocaine and methylphenidate, have maximal effects that are generally comparable, if relatively restricted time points for measurement are selected, eliminating duration of action as an influence on the measurement of maximal effects (Izenwasser et al., 1994). Thus, the effects of BZT analogs on this behavioral activity were atypically blunted as compared to those of cocaine.
Figure 2:
Dose-dependent effects of 4-F- and 4-Cl-substituted BZT analogs on locomotor activity in mice. Ordinates: horizontal activity counts after drug administration. Abscissae: dose of drug in μmol/kg, log scale. Each point represents the average effect determined in eight mice. The data are from the 30-min period during the first 60 min after drug administration, in which the greatest stimulant effects were obtained. Note that the 4-F-substituted compounds (left panel) were generally more efficacious than the 4-Cl-substituted compounds, and no members of either group had efficacy comparable to that of cocaine. Left panel symbols: Filled circles: cocaine; open circles: 4’-F-BZT; squares: 4’,4”-diF-BZT; triangles: 3’,4’-diCl,4”-F-BZT; downward triangles: 3’,4’-diF-BZT; diamonds: 3’,4”-diF-BZT; hexagons: 4’-Br,4”-F-BZT. Right panel symbols: Filled circles: cocaine; open circles: 4’-Cl-BZT; squares: 4’-Cl-BZT (with the diphenyl-ether system at the asymmetric C3 of the tropane ring, in the equatorial, β, configuration); triangles: 4’,4”-diCl-BZT; downward triangles: 3’,4’-diCl-BZT. See Figure 1 for compound structures. Modified from Katz et al. (1999).
Binding of BZT analogs indicated that they have high-affinity for the DAT and are selective for that target over the other monoamine transporters (Katz et al., 1999), suggesting that the reduced effectiveness of these compounds was not due to a low affinity for the DAT protein. Structure-activity studies indicated that the BZT analogs with a fluoro-substitution in the para-position on either of the phenyl rings were less effective than cocaine, but were most effective among the BZT analogs. Those with chloro-substitutions, had affinities comparable to those of the fluoro-substituted analogs, but were even less effective in stimulating locomotor activity than cocaine (Figure 2).
BZT analogs, when administered before cocaine, can modify cocaine-induced stimulation of ambulatory activity. For example, in rats injected i.p. with 40 mg/kg of 4’-Cl-BZT cocaine (10 mg/kg, i.p.) administered 2 hours later stimulated ambulatory behavior to a greater extent than after saline injection (Tolliver et al., 1999). In contrast, Desai et al. (2005b) showed that the BZT analog, JHW 007 blocked the locomotor-stimulant effects of cocaine. JHW 007 produced a significant DAT occupancy that was not followed by significant cocaine-like stimulant effects. However, the apparent association rate of JHW 007 in vivo was slow. Control mice pretreated with saline showed a dose-dependent stimulation of ambulatory counts by increasing doses of cocaine, with maximum activity shown at 40 mg/kg. Cocaine-induced locomotor activity was completely antagonized in mice pretreated with a 10 mg/kg dose of JHW 007, while another BZT analog, AHN-2005, only reduced the effects of the highest doses of cocaine tested (Desai et al., 2005b). Similarly, a combination of AHN 1–055 with cocaine attenuated the cocaine-induced locomotor stimulation (Velazquez-Sanchez et al., 2009). In contrast, d-amphetamine pretreatment increased the effects of cocaine, suggesting that the absence of an increase in cocaine effects produced by AHN 1–055 was not the result of a “ceiling” being reached, above which further increases could not be obtained. In addition, the possibility that ambulatory activity was reduced through competition with other behaviors, such as focused stereotypies was ruled out. Combined d-amphetamine and cocaine treatments produced both robust locomotion and stereotyped behavior. In contrast, AHN 1–055 did not enhance the stereotyped behavior produced by cocaine alone. These data suggest that the attenuation of the effects of cocaine by AHN 1–055 was not due to the induction of competing behaviors, and that its effects in combination with cocaine are distinctly different from those produced by classic psychostimulants (Velazquez-Sanchez et al., 2009).
B. Cocaine discrimination
Drug-discrimination is a behavioral procedure in which the subjective effects produced by the administration of a drug can be studied in human or animal subjects (Holtzman, 1990). The more minute details of the procedures differ from one experiment to another, but subjects are always trained to emit one response after injection of the training drug and a different response after injection of its vehicle. These responses are only intermittently reinforced, so the only stimulus for the subject regarding which of the two responses will be reinforced is the injection (drug or vehicle) administered before testing (for further details see Holtzman, 1990). The subjective effects of cocaine using this procedure have been extensively studied (Woods et al., 1987), and only drugs that act through brain mechanisms similar to those of cocaine produce cocaine-like responding. Drugs like WIN 35,428, methylphenidate or d-amphetamine will generalize to (or substitute for) the cocaine discriminative stimulus. There is also a correlation between the potencies of various DAT inhibitors in substituting for cocaine and their affinity for the DAT, though the relationship is complicated by the modeling of the binding data for one or two DAT sites (Katz et al., 2000). Further, monoamine uptake inhibitors with affinity primarily for either SERT or NET generally do not fully substitute for the cocaine discriminative stimulus (Baker et al., 1993; Kleven et al., 1990).
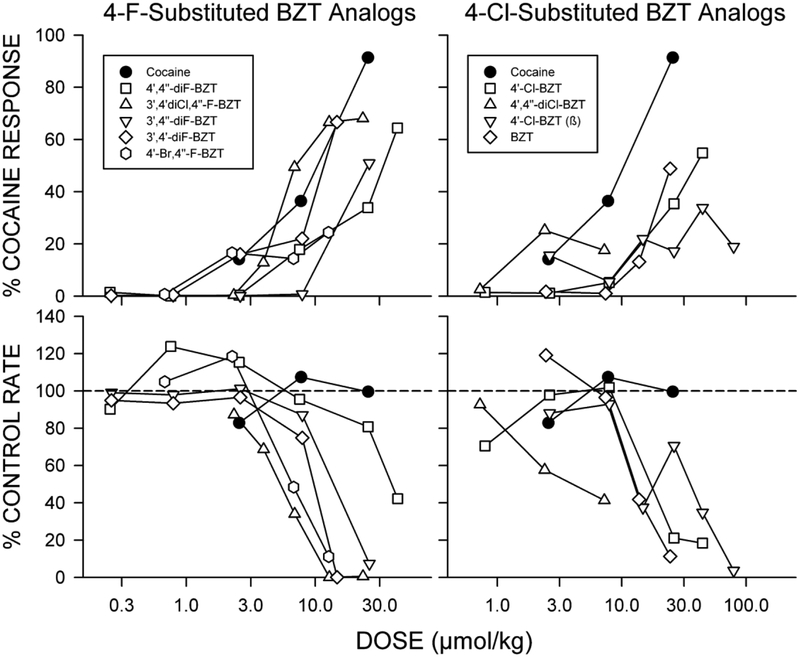
BZT analogs have demonstrated different degrees of effectiveness in substituting for cocaine, and most do not fully substitute for the cocaine discriminative stimulus (see for example Katz et al., 1999) (Figure 3). BZT analogs with para fluoro substituents of the diphenyl ether system are among the more effective, while BZT analogs with other para-substitutions, despite binding affinities comparable to those of the fluoro-substituted compounds (Newman and Katz, 2009), are clearly less effective (Figure 3). It is important to note that the lower efficacy of these compounds in drug-discrimination studies has been suggested as reflecting a slow onset of central effects compared to cocaine or other cocaine-like DAT inhibitors. Thus in some experiments, BZT analogs were administered at different times before testing. However, with the exception of AHN 1–055, none of the BZT analogs studied produced a larger cocaine-like discriminative effect when tested at times from 5–120 min before the session (Katz et al., 1999; Katz et al., 2004). AHN 1–055 fully substituted for cocaine when administered 90 min before testing, but not at other times.
Figure 3:
Effects of BZT analogs in rats trained to discriminate injections of cocaine from saline. Ordinates for top panels: percentage of responses on the cocaine-appropriate key. Ordinates for bottom panels: rates at which responses were emitted (as a percentage of response rate after saline administration). Abscissae: drug dose in μmol/kg (log scale). Each point represents the effect in four to six rats. The percentage of responses emitted on the cocaine-appropriate key was considered unreliable and not plotted if fewer than half of the subjects responded at that dose. Note that the fluoro-substituted compounds (left panels) were generally more effective in substituting for cocaine than the Cl-substituted compounds. See Figure 1 for compound structures. Modifed from Katz et al. (1999).
Due to their reduced effectiveness, BZT analogs have also been tested for their ability to interfere with the discriminative-stimulus effects of cocaine. Combinations of standard DAT inhibitors with cocaine typically resulted in a leftward shift in the cocaine dose-effect curve. In a series of experiments with analogs of BZT substituted with Cl-groups in the 3’-, 4’-, 3’,4’-, and 4’,4’- positions of the diphenylether system, it was shown that despite of their reduced efficacy in substituting for cocaine, these analogs shifted the cocaine discriminative-stimulus dose-effect curve leftward (Katz et al., 2001). In contrast, N-substituted analogs of BZT (e.g. AHN 2–005, JHW 007) did not appreciably shift the cocaine dose-effect curve (Katz et al., 2004). Thus, there are differences among the BZT analogs with regard to their interactions with cocaine.
Studies have implicated nucleus accumbens (NAc) DA transmission in the discriminative-stimulus effects of stimulants (e.g. Callahan et al., 1997; Dworkin and Smith, 1988). Tanda et al. (2006) examined the subjective effects of cocaine and N-substituted BZT analogs in rats discriminating cocaine from saline, and their effects on extracellular DA in the NAc shell. All of the compounds tested dose-dependently (1, 3, and 10 mg/kg i.p.) increased NAc DA levels, however their maximum effects were different and were obtained at different times after injection. Although cocaine and AHN 1–055, dose-dependently generalized with the cocaine discriminative stimulus, AHN 1–055 only did so only at 90 min after injection. Both AHN 2–005 and JHW 007 produced a substitution greater than vehicle, though neither drug fully substituted for cocaine at any dose or time after administration. The subjective effects or cocaine were linearly related to the amount of DA released, and independent of time after injection. However, the BZT analogs were less effective in producing a cocaine-like discriminative stimulus effect even at times and doses that produced a stimulation of DA levels that was equal to or greater than that shown with cocaine to be effective in producing exclusive cocaine-like responding. Other studies have also documented differences in behavioral response to similar concentrations of DA produced by psychostimulants (e.g. Zolkowska et al., 2009). Thus, comparable levels of DA stimulated by the BZT analogs produced less substitution than did cocaine, and the behavioral effects obtained at a given level of DA elevation for BZT analogs depended on the time after injection, suggesting a rapid desensitization to the effects of DA accompanying the administration of BZT analogs.
C). Self-administration
Drug self-administration is a behavioral procedure in which responses of a subject directly produce drug injections. The consistent features of the many variants of the procedure involve training a subject to emit a simple response that produces an intravenous injection of the compound. Often subjects are trained with one known compound, such as cocaine, and tested with various doses of unknown compounds, as well as vehicle. When rates of response are greater than those maintained with vehicle injection, the compound is said to have reinforcing effects (see Katz, 1989, for further details). Behavior maintained by drug self-injection is often considered the gold standard for the study of the reinforcing effects, and thus the abuse liability, of drugs.
The reinforcing effects of cocaine have been extensively documented (Woods et al., 1987). The reinforcing effects of BZT analogs were initially compared to those of cocaine in two studies. In the first, rhesus monkeys were trained to self administer cocaine, and BZT and its 3’-Cl- and 4’-Cl-analogs were subsequently tested. Rates of responding maintained by BZT and its analogs were relatively low compared to those maintained by cocaine (Woolverton et al., 2000) In this study drugs were delivered after each 10th lever press (fixed-ratio 10-response, or FR 10 schedule).
The reinforcing effects of 3’-Cl-, 4’-Cl-, and 3’,4’-diCl-BZT were further tested in a second study, and compared to those of cocaine and GBR 12909 under the FR 10 schedule (Woolverton et al., 2001). The rate of self-administration obtained under the FR schedule with 3’-Cl- and 4’-Cl-BZT, but not with 3’,4’-diCl-BZT was greater than vehicle, but much lower than that maintained by cocaine or by GBR 12909 in the same monkeys. The rate of self-administration of 3’,4’-diCl-BZT was not greater than that maintained by vehicle. In a second part of the study, rhesus monkeys were trained to respond under a progressive-ratio schedule, in which the number of responses required for self administration increases progressively until the subject stops responding. The number of responses completed before the subject stops responding is often considered a measure of the effectiveness of a compound as a reinforcing stimulus (Hodos, 1961). Cocaine and GBR 12909 were the most effective reinforcers, and the BZT analogs were the least efficacious, with the rank order as follows: cocaine> GBR 12909 > 3’-Cl-BZT = 4’-Cl-BZT >3’,4’-diCl-BZT. In a more recent study using rats, progressive-ratio performance maintained by cocaine was greater than that maintained by AHN 1–055, which was only marginally greater than that maintained by vehicle (Ferragud et al., 2009, in press).
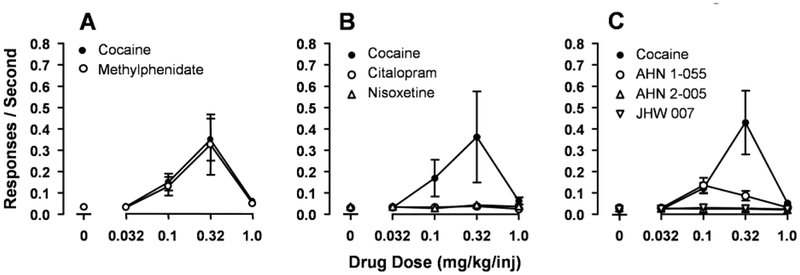
Recently, the reinforcing effects of N-substituted BZT analogs were assessed in rats responding under an FR 5 schedule, and compared to those of standard DAT inhibitors (Hiranita et al., 2009). The DAT inhibitor, methylphenidate, maintained responding like cocaine (Figure 4A), whereas the SERT and NET inhibitors, citalopram and nisoxetine, respectively, did not (Figure 4B). Under the same experimental conditions, neither AHN 2–005 nor JHW 007 maintained rates of responding above those maintained by vehicle (Figure 4C). AHN 1–055 maintained i.v. self-administration behavior, though maximal rates of responding were at a low level as compared to cocaine or methylphenidate (Figure 4C). Reinforcing effects of AHN 1–055 were also demonstrated by Ferragud et al. (2009, in press) using an FR 1-response injection schedule. In addition, after a two-week period in which subjects did not have the opportunity to respond, the responding previously maintained by AHN 1–055 was reduced to the rates previously obtained with vehicle whereas responding previously maintained by cocaine persisted.
Figure 4:
Substitution of different doses of cocaine or other monoamine uptake inhibitors and BZT analogues in rats trained to self-administer cocaine (0.32 mg/kg/injection). Ordinates: responses per second. Abscissae: injection dose (mg/kg/injection). Each point represents the mean (vertical bars represent S.E.M.) of from 6 to 11 subjects. Panel A: Cocaine (filled circles) and methylphenidate (open circles). Panel B: Cocaine (filled circles) and citalopram (open circles) or nisoxetine (triangles). Panel C: Cocaine (filled circles) and AHN 1–055 (open circles), AHN 2–055 (triangles up), or JHW 007 (triangles down). Modified from Hiranita et al. (2009).
Pre-session treatments with methylphenidate (p.o.) shifted the dose-response curve for cocaine self-administration leftward (Figure 5A), suggesting that methylphenidate did not antagonize the reinforcing effects of cocaine, but rather added to them (Hiranita et al., 2009). At variance with methylphenidate, AHN 2–005 and JHW 007 (p.o.) shifted the cocaine dose-response curve downward (Figure 5C and D), suggesting an antagonistic effect of these compounds on cocaine self-administration (Hiranita et al., 2009). The effects of AHN 1–055 were more complex, with intermediate doses of the compound increasing responding at lower doses of cocaine, but decreasing responding for the higher cocaine doses. Also, higher doses of AHN 1–055 shifted the cocaine dose-response curve downward, thus significantly decreasing and antagonizing cocaine maintained self-administration behavior (Figure 5B). Importantly, the BZT analog-induced changes in cocaine self administration were obtained at p.o. doses that had no effects on responding maintained by food reinforcement under conditions similar to those used for the studies of cocaine-maintained responding (Ferragud et al., 2009, in press; Hiranita et al., 2009).
Figure 5:
Effects of presession treatment with methylphenidate and N-substituted BZT analogs on cocaine self-administration. Ordinates: responses per second. Abscissae: injection dose (mg/kg/injection). Each point represents the mean with S.E.M. (n = 6 to 10). Methylphenidate, AHN 1–055, AHN 2–005, or JHW 007 were administered orally at 60, 180, 240, or 300 min before sessions, respectively. Modified from Hiranita et al. (2009).
A slower onset of action as compared to cocaine and other psychostimulants may explain the lower efficacy of BZT analogs as reinforcers. Because of the importance of contingency between the response and the effect of the compound, slow onset of action results in delays in reinforcement. A substantial literature in behavioral science confirms delay in reinforcement as a factor that decreases the effectiveness of all reinforcers (Skinner, 1938) as well as cocaine (e.g. Beardsley and Balster, 1993).
D). Place Conditioning
Place conditioning is a behavioral procedure used to indirectly assess the reinforcing or aversive effects of compounds. As with drug discrimination, the actual details of the procedure can differ from one experiment to the next, but all consist of placing the subject on one side of an enclosure after compound administration, and on the other side after vehicle injection. The texture of floors, illumination and other features of the two sides differ substantially. The majority of published studies proceed in three different phases, starting with a “pre-conditioning” test of how the subject distributes its time on the two sides of the chamber. The second “conditioning” phase involves confining the subject to one of the two sides by closing a door between them. When the subject is confined on one side it is pretreated with a compound, and when confined to the other side it is injected with vehicle. A final “testing” phase follows in which subjects have free access to both sides of the chamber (door open) in a compound-free condition, and the time spent in each side is recorded. It has been repeatedly found that appropriate doses of psychostimulants administered during the conditioning phase increase the time spent by animals in the compound-paired compartment during the testing phase, i.e. place conditioning.
As noted above, one hypothesis for the lower reinforcing efficacy of BZT analogs in self-administration studies as compared to cocaine might result from the delayed onset of their effects. For example, Tanda et al. (2005; 2009) have shown that BZT analogs increase extracellular levels of DA in dialysates from the NAc at a lower rate as compared to cocaine (see Section E below). In self-administration studies, a slow onset of effect would produce a delay of reinforcement that might decrease the rate or amount of behavior maintained. Even a fully effective drug such as cocaine maintains less behavior when its presentation is delayed in a self-administration study (e.g. Balster and Schuster, 1973). In a place conditioning study, the time between compound treatment and placement of the subject in the chamber can be varied to accommodate a delay in onset of effect and thus not alter the effectiveness of the compound.
Place conditioning with BZT analogs administered from immediately to 90 min before conditioning trials was investigated by Li et al. (2005), and results were compared to those obtained with cocaine administration. The effects of cocaine were robust and replicated the type of dose-dependent effects previously reported (see review by Tzschentke, 1998). The N-substituted BZT analogs, AHN 2–005, AHN 1–055, and JHW 007 were less effective and only occasionally produced an increase in time spent in the compound-paired compartment. For example, AHN 2–005 (0.1–10.0 mg/kg) produced a significant place conditioning only at the dose of 3 mg/kg when administered 45 min before the conditioning session. At all other times and doses, the effects of AHN 2–005 were not different from vehicle. Administration of JHW 007 (1.0–10.0 mg/kg) significantly increased time spent in the compound-paired compartment only when the dose of 10 mg/kg .i.p. was administered 45 min before the subject was placed in the conditioning chamber, but not when placed in the chamber immediately or 90 min before trials, or at any of the other doses or times. The N-methyl substituted BZT analog, AHN 1–055, had effects no different from those of saline across a range of doses from 0.3 to 3.0 mg/kg even when administered i.p. up to 90 min before conditioning sessions (Li et al., 2005).
In agreement with Li et al. (2005), in a recent report (Velazquez-Sanchez et al., 2009), AHN 1–055 did not produce place conditioning in mice when administered 1 h before the conditioning sessions using a higher range of doses compared to those used by Li et al. (2005). Velasquez-Sanchez et al. (2009) also showed that AHN 1–055 blocked the conditioning and rewarding effects of cocaine measured in the place conditioning paradigm. These effects of AHN 1–055 paralleled its effects on preventing cocaine-induced early-gene activation (Velasquez-Sanchez et al., 2009) in the nucleus accumbens and dorso-medial striatum, providing a neurobiological correlate to the antagonism of cocaine conditioning by the BZT analog.
Thus, place conditioning experiments with BZT analogs consistently indicate decreased effectiveness compared with cocaine. Further, when the delayed onset of effects of BZT analogs was taken into account, these experiments further suggest that the reduced cocaine-like behavioral effects of BZT analogs could not be entirely accounted for by a slower onset of action.
E. Neurochemistry
Most of the behavioral and reinforcing effects of cocaine have been related to its ability to block the DAT, which consequently leads to increased DA neurotransmission. The effects of cocaine on extracellular DA levels in different dopaminergic terminal areas in brain have been extensively studied using microdialysis (Kuczenski and Segal, 1992; Pontieri et al., 1995; Tanda et al., 1997b) and voltammetry techniques (Greco and Garris, 2003).
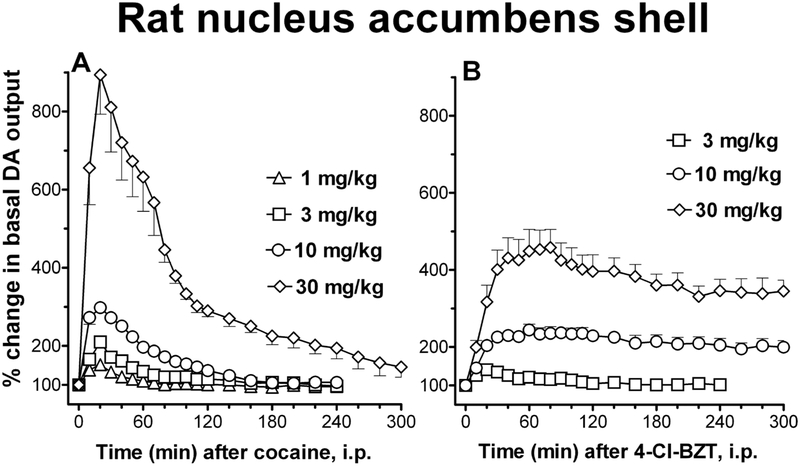
The effects of 4’-Cl-BZT were compared with those of cocaine on stimulation of DA levels in microdialysis studies in rats. One study was performed with probes implanted only in the NAc (Tolliver et al., 1999), and a second study was performed with microdialysis probes implanted in specific DA terminal areas, including the shell and core of the NAc, the medial prefrontal cortex and the dorsal striatum (Tanda et al., 2005). Results from each study show that both cocaine and 4’Cl-BZT dose-dependently stimulated DA levels in all brain regions tested. Across the range of doses studied, cocaine was 2–3-fold more potent than 4’-Cl-BZT in stimulating DA levels in the brain regions studied (Tanda et al., 2005). The differences were most pronounced in the shell of the NAc where cocaine was not only slightly more potent but also more effective than 4’-Cl-BZT (Figure 6). A different pattern was observed in the prefrontal cortex where 4’-Cl-BZT was more effective than cocaine. Whereas the effects of cocaine were rapid and transient, the effects of 4’-Cl-BZT lasted longer with DA levels still significantly elevated more than 5 hours after administration of the higher doses. The long-lasting effects of 4’-Cl-BZT are likely the result of the slow elimination rate of BZT analogs (see below). It should also be noted that although 4’-Cl-BZT effects were longer lasting compared to cocaine, its rate of increase in DA levels was much slower than that for cocaine (Figure 6). A recent study using mice confirms a lower rate of increase in DA levels and a longer duration of action compared to cocaine for the BZT analog, JHW 007 (Tanda et al., 2009). The duration of the effects of JHW 007 on extracellular DA levels was longer than 24 hours at i.p. doses of 10 and 17 mg/kg. The same pattern of activation of DA transmission, lower rate of increase and longer duration of action compared to cocaine, has been also confirmed for 4’−4”-Cl-BZT, MFZ-4–86, and MFZ-4–87 (unpublished observations).
Figure 6:
Time course of effects of systemic administration of cocaine (Panel A) or 4-Cl-BZT (Panel B) on extracellular levels of DA in dialysates from the NAC shell. Results are means (with vertical bars representing S.E.M.) of the amount of DA in 10-min dialysate samples, expressed as percentage of basal values, uncorrected for probe recovery. Modified from Tanda et al. (2005).
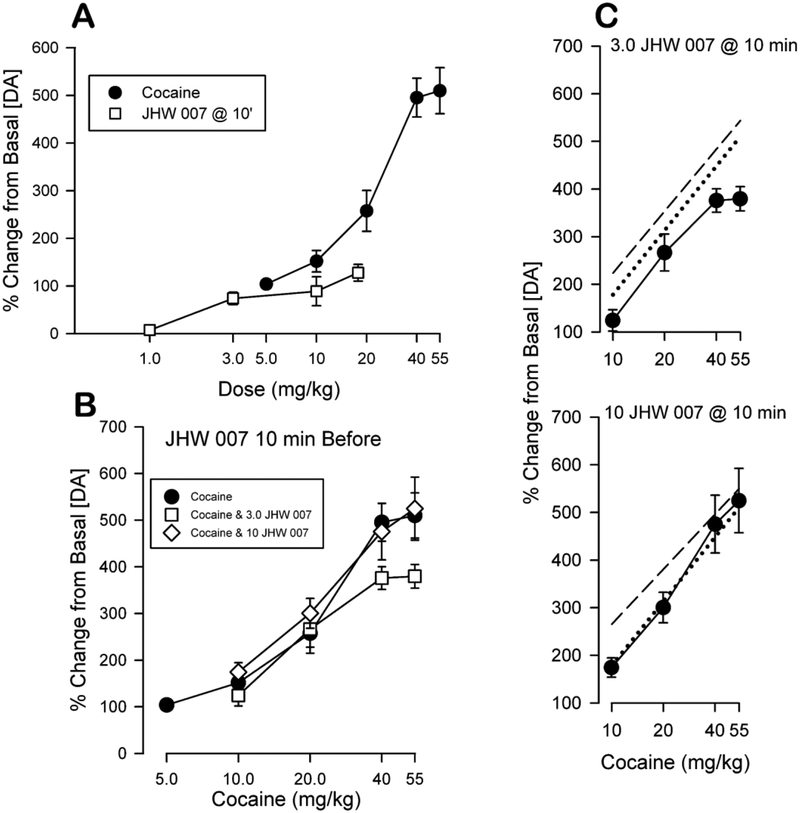
The antagonist effects of JHW 007 on cocaine-induced stimulation of ambulatory activity and on cocaine-maintained self-administration suggest the interaction between JHW 007 and cocaine might involve alteration of cocaine-induced stimulation of extracellular DA levels. For this reason, the effects of combinations of different doses of JHW 007 and cocaine on stimulation of DA levels in the NAc shell were examined in mice and compared with combinations of cocaine and the DAT blocker, WIN 35,428 (Tanda et al., 2009). Isobolographic analysis (Tallarida, 2000; Tallarida, 2007), showed that combinations of cocaine and WIN 35,428 produced an increase in extracellular DA levels that was greater than a simple additive effect. In contrast, the effects of JHW 007 in combination with cocaine were consistently less than additive. At times after injection and at several doses, JHW 007 decreased the effects of cocaine on extracellular DA levels in the NAc shell (Figure 7). These results, showing a less than predicted level of DA stimulated by cocaine, are in agreement with the behavioral effects of combinations of JHW 007 with cocaine (Desai et al., 2005b; Hiranita et al., 2009), and suggest that JHW 007 may reduce the effectiveness of cocaine in stimulating dopamine neurotransmission in brain areas related to its reinforcing actions.
Figure 7:
Effects of combinations of cocaine and JHW 007 at 10 min after injection on extracellular levels of DA. Panel A: Dose-dependent maximal effects of JHW 007 administered 10 min before compared with those of cocaine administered immediately before assessments. Ordinates: change in extracellular DA levels as a percentage of basal values before injection. Abscissae: dose of drug in mg/kg, log scale. Panel B: Observed effects of the combinations. Ordinates: change in extracellular DA levels as a percentage of basal values during the 30-min period after cocaine administration. Abscissae: dose of cocaine in mg/kg, log scale. Panel C: Observed effects compared with the predicted effects of the combinations. Ordinates and abscissae are as in Panel B. The calculated (predicted) additive dose-effect curve for cocaine in the presence of 3.0 (top) or 10.0 (bottom) mg/kg of JHW 007 is shown by the dashed straight line and the effects of cocaine alone are shown by the dotted line. The experimental (obtained) values are shown by the connected circles. Modified from Tanda et al. (2009).
F. Human studies
BZT is the only drug in its class approved for human use, due to its efficacy in early stages of PD, an effect thought to be due to its antimuscarinic effects. There is only one report in which BZT has been evaluated as a potential cocaine-abuse medication in humans (Penetar et al., 2006) in which 16 healthy recreational cocaine users were administered BZT (1, 2 or 4 mg) or placebo 2 hours before the subjects were allowed to self administer cocaine intranasally (0.9 mg/kg). Physiological and subjective measures of cocaine effects were monitored in the ensuing 2 hours. BZT alone did not change cardiovascular parameters or subjective effects evaluated by visual analog scales. In contrast, cocaine produced several subjective responses, most notably an increase in the visual analog scale ratings of “high” and “stimulated” and an increase in heart rate. Pretreatment with BZT, did not modify any effect produced by cocaine. The absence of adverse subjective and physiological effects suggests that higher doses of BZT could be tested in human subjects to better evaluate its therapeutic potential as a medication for cocaine abuse.
III. Pharmacokinetic studies
As noted above, one of the initial hypotheses to explain the lower effectiveness of BZT analogs as compared to cocaine, and the relatively slower onset of effect for behavioral and neurochemical measures, was a slower CNS penetration of BZT analogs. The pharmacokinetic parameters of selected BZT analogs have been studied and compared to those of cocaine (Othman et al., 2007a; Othman et al., 2007b; Raje et al., 2003; Raje et al., 2006). In these studies, performed in rats, compounds were administered i.v. to eliminate differences in rate of absorption from the injection site. The most prominent difference between the BZT analogs and cocaine was the substantially slower disappearance of the BZT analogs from plasma and brain. The longer duration of action described for several BZT analogs with regard to elevation of NAc DA levels can be explained by their lower plasma and brain elimination rates compared to cocaine.
Interestingly, all the BZT analogs studied were found at high concentrations in brain within minutes after injection with initial levels of from 4–15 μg/gram of tissue (Othman et al., 2007a; Othman et al., 2007b; Raje et al., 2003; Raje et al., 2006). The initial compound levels equate to 3–27 μM, depending on the particular compound and exceed the Ki values of 11–30 nM (Newman and Katz, 2009). The relatively fast appearance of the BZT analogs in brain contrasts with the slow onset of their neurochemical and behavioral effects.
IV. Studies of in vivo binding of BZT analogs at the DAT and relationship with behavioral and neurochemical effects
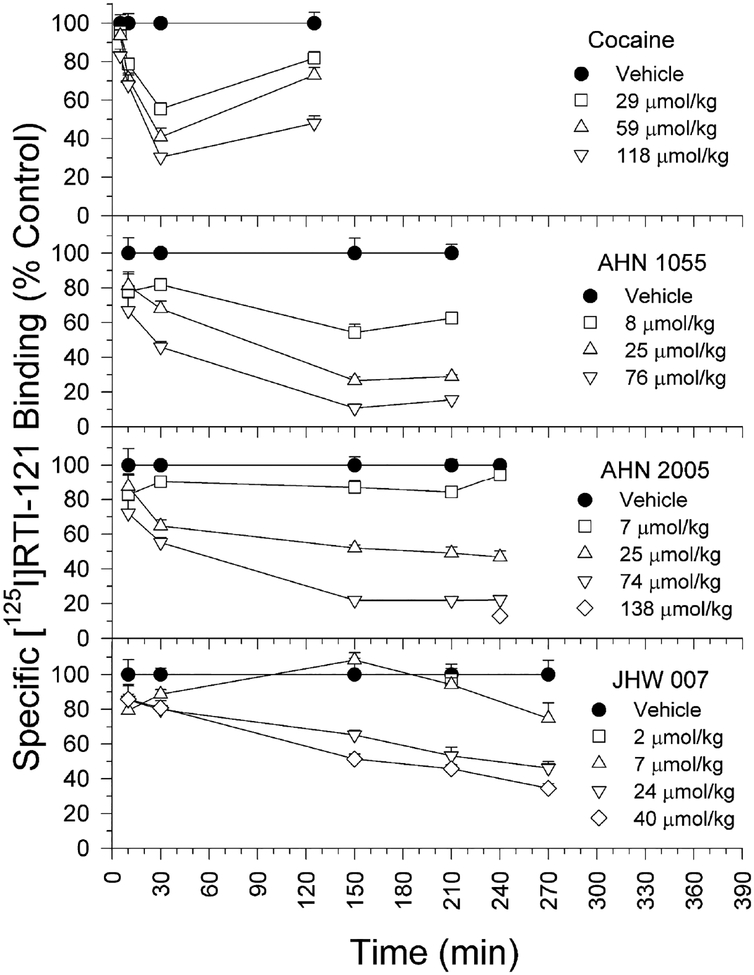
Studies of in vivo displacement of [125I]RTI-121 by cocaine was dose- and time-dependent with a maximum effect 30 min after injection (Desai et al., 2005b). The BZT analogs, AHN 1–055, AHN 2–005, and JHW 007, also displaced [125I]RTI-121 binding in a dose- and time-related manner (Desai et al., 2005a; Desai et al., 2005b). However, AHN 1–055 and AHN 2–005 showed a maximum displacement of [125I]RTI-121 at approximately 150 min after injection, and displacement by JHW 007 did not show a plateau up to 4.5 hours after its administration (Figure 8). Further, there was no evidence of any decrease in the displacement of [125I]RTI-121 by the BZT analogs over the times studied, which follows from the slow elimination rate of BZT analogs described in the pharmacokinetic studies. When the apparent association rate of JHW 007 for the DAT was calculated as a function of the displacement of RTI-121 (as a percentage of specific RTI-121 binding) per minute over the linear portions of the curves, the apparent association of JHW 007 was more than 10-fold lower than that for cocaine (Desai et al., 2005b). A lower association rate for BZT analogs might fully account for the pharmacokinetic findings that they penetrate the CNS relatively rapidly and the in vivo pharmacology showing a slow onset of effects relative to other stimulant drugs such as cocaine.
Figure 8:
Time course of displacement of specific [125I]RTI-121 accumulation in striatum of mice following IP injection of cocaine, AHN 1–055, AHN 2–005, or JHW 007. Ordinates: specific [125I]RTI-121 binding as a percentage of that obtained after vehicle injection. Abscissae: time. For each point the number of replicates was from 5 to 10 or 13. Note that maximal displacement of [125I]RTI-121 was obtained with cocaine at 30 min after injection, and at later times with the other compounds. Modified from Desai et al. (2005a, b).
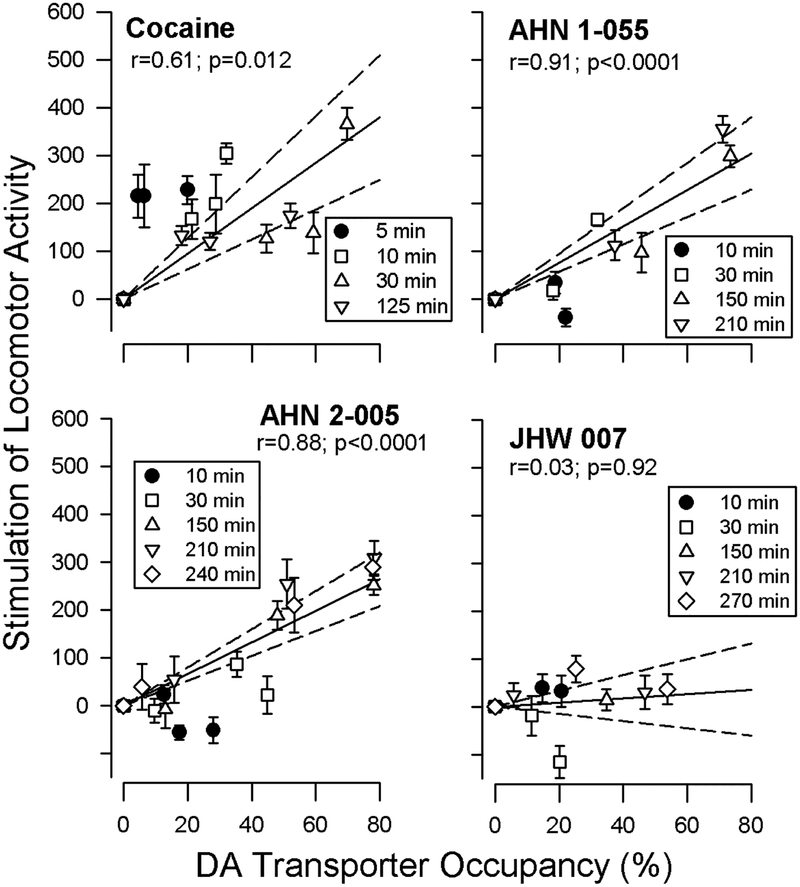
The relationship between DAT occupancy and behavioral effects was assessed by examining the relationship between displacement of RTI-121 and the stimulation of locomotor activity (Desai et al., 2005a; Desai et al., 2005b) (Figure 9). There was a significant relationship between DAT occupancy by cocaine and its stimulation of locomotor activity at the various doses and time points. However, one interesting aspect of this correlation is that a disproportionately greater effect on locomotor activity than could be predicted by DAT occupancy was obtained during the first 5 minutes after cocaine injection. This effect of cocaine makes the association of the two variables unexpectedly poor as compared to previous comparisons of affinity and potency in self administration among various DAT inhibitors (Bergman et al., 1989; Ritz et al., 1987).
Figure 9:
Relationship between dopamine transporter occupancy, determined in studies of displacement of [125I]RTI-121 in striatum, and locomotor-stimulant effects of cocaine, AHN 1–055, AHN 2–005 or JHW 007. Ordinates: difference between mean horizontal activity counts after drug and after saline. Abscissae: percent displacement of [125I]RTI-121. For each drug, the solid line represents the linear regression of percent occupancy of the DA transporter and horizontal locomotor activity when the line is forced to intersect the origin, the point representing no occupancy and no effect. Dashed lines represent 95% confidence limits for the regression lines. Note that the locomotor-stimulant effects of cocaine are less strongly related to DA transporter occupancy than are the effects of AHN 1–055, AHN 2–005, or JHW 007. Modified from Desai et al. (2005a, b).
Differences from these previous outcomes are likely related to the manner in which the binding studies were conducted (e.g. in vivo versus in vitro binding methods; equilibrium versus dynamic aspects of binding; single compound versus comparisons of various compounds, etc.) or the behavioral endpoint (locomotor activity versus self administration). Nonetheless, as the divergence from the regression was observed with data obtained soon after injection, association rate was suggested to play an important role in the stimulant effects of cocaine (e.g. Volkow et al., 2002).
V. Influence of off-target actions of BZT analogs.
The parent compound, BZT, has affinity for M1 muscarinic and H1 histamine receptors. Binding studies performed on BZT analogs have shown that most of them are selective DAT inhibitors as compared to other monoamine transporters, however many have affinity for M1 and H1 receptors (Katz et al., 2001; Katz et al., 2004; Katz et al., 2003). Thus it is possible that sites other than DAT may contribute to the differences between the behavioral effects of BZT analogs as compared to cocaine. Several studies were initiated to examine these possibilities. If the off-target effects of the BZT analogs (those not mediated by the DAT) attenuate their cocaine-like activity, then the effects of cocaine should be antagonized, at least partially, by the administration of a compound selective for the off-target site.
A. Antagonist activities at muscarinic M1 receptors
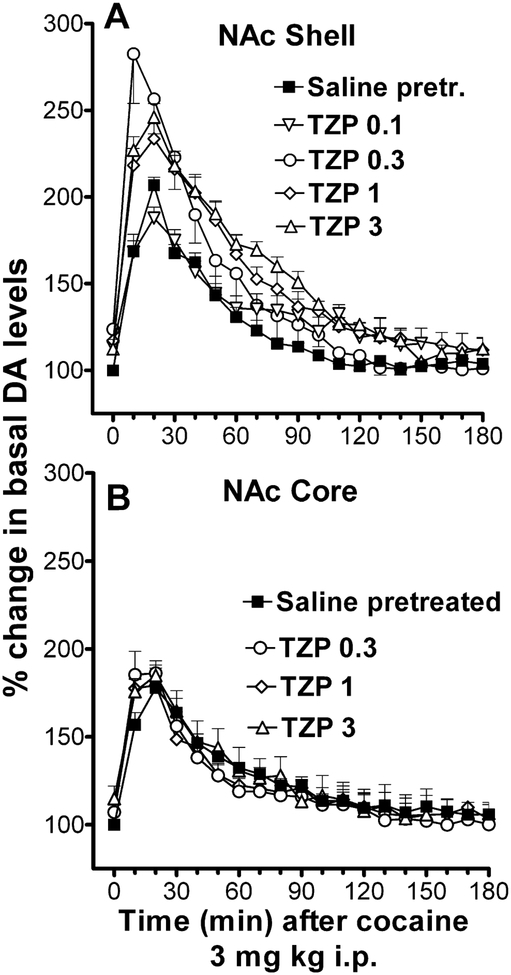
The non-selective anticholinergics, atropine and scopolamine, potentiate several behavioral effects of CNS stimulants (e.g. Carlton and Didamo, 1961; Scheckel and Boff, 1964). This type of interaction was also found for cocaine and either atropine or scopolamine in drug discrimination procedures (Katz et al., 1999). Atropine and scopolamine also potentiated the locomotor stimulant effects of cocaine (Katz et al., 1999). Thus a potentiation, rather than antagonism of cocaine effects occurs with muscarinic antagonists. From these experiments it appears that the muscarinic antagonism produced by some of the BZT analogs does not account for the attenuation their cocaine-like behavioral effects. BZT analogs have a preferential affinity for muscarinic M1 receptors (Tanda et al., 2007) as compared to the M2-M5 receptors, a pattern different from of the nonselective muscarinic antagonists, atropine and scopolamine(see for example Buckley et al., 1989). The muscarinic antagonists trihexyphenidyl and telenzepine that have preferential affinity for muscarinic M1 receptors, were studied in combination with cocaine (Tanda et al., 2007). Both of these antagonists dose-dependently and selectively potentiated the effects of cocaine on DA levels in the shell as compared to the core of the NAc and the medial pre-frontal-cortex (the effects of telenzepine in the NAc are shown in Figure 10). As increases in DA in the shell of the NAc have been related the acute reinforcing effects of cocaine (Pontieri et al., 1995) and other drugs abused by humans (Pontieri et al., 1996; Tanda et al., 2008; Tanda et al., 1997a), the potentiation of the effects of cocaine on DA levels selectively in the NAc shell is in disagreement with the hypothesis that M1 antagonist effects might be responsible for attenuating the reinforcing effects of BZT analogs.
Figure 10:
Time courses for the effects of pretreatments with increasing doses of telenzepine (TZP) on cocaine-stimulated DA extracellular levels in dialysates from the NAc shell (Panel A), and NAc core (Panel B). Cocaine (3.0 mg/kg i.p.) was administered at time=0. Each point represents means (with vertical bars representing SEM) of the amount of DA in 10-min dialysate samples, expressed as percentage of basal values, uncorrected for probe recovery. Modified from Tanda et al. (2007).
It should also be noted that the rate of increase in extracellular DA levels obtained with the combination of M1 receptor antagonists and cocaine is higher than that obtained with cocaine alone (Figure 10). This faster rate of increase is also at variance with that observed for BZT analogs, which is lower as compared to cocaine, indicating that the muscarinic effects of BZT analogs does not account for the different effects on dopaminergic effects. Moreover, in agreement with their ability to potentiate cocaine-induced DA levels telenzepine and trihexyphenidyl can potentiate cocaine-induced subjective effects, producing small leftward shifts in the discriminative-stimulus dose-effects of cocaine (Tanda and Katz, 2007). However, these M1 antagonists differed in their ability to interact with cocaine-induced stimulation of locomotor activity, with trihexyphenidyl potentiating the stimulatory effects of cocaine, and telenzepine attenuating the effects of cocaine (Tanda et al., 2007). Thus, these experiments suggest that affinity of BZT analogs for, and their actions at, muscarinic M1 receptors are unlikely to be the cause of their reduced cocaine-like behavioral and reinforcing effects.
In agreement with these studies, BZT analogs with reduced affinity at muscarinic M1 receptors that retain high affinity and selectivity for the DAT (Agoston et al., 1997b; Kulkarni et al., 2004; Robarge et al., 2000; Zou et al., 2003) have been evaluated (Katz et al., 2004). Of the BZT analogs tested, AHN 1–055 had the highest affinity for the DAT and M1 receptors, 11.8 and 11.6 nM respectively, and it was also among the more effective of the BZT analogs in stimulating ambulatory activity to levels that approached those seen with cocaine. AHN 2–005 and JHW 007 had 29.9 and 24.6 nM affinity for DAT, and 177 and 399 nM for M1 receptors, respectively, but had substantially lower efficacy than cocaine in stimulating locomotor activity or substituting for cocaine as a discriminative stimulus (Katz et al., 2004).
A few studies have also investigated more directly the influence of antagonism at muscarinic receptors on cocaine-induced reinforcing effects. In one study (Ranaldi and Woolverton, 2002), rhesus monkeys were trained to self-administer cocaine under fixed- and progressive-ratio schedules. In most cases, combinations of cocaine and scopolamine maintained less self-administration than did cocaine alone. The authors concluded that anticholinergic actions contribute to the diminished self-administration of BZT analogs relative to cocaine, and suggested that the mechanism involves either antagonism of the reinforcing effect of cocaine or punishment of the cocaine self-administration behavior by the anticholinergic activity.
Wilson and Schuster (1973) found atropine to increase rates of responding maintained by cocaine, a result that is not consistent with punishment by the anticholinergic agent, as punishment would involve a decrease in response rate. Further the atropine-induced increases in response rates were similar to the effect of lowering the cocaine dose. As atropine was administered before the experimental session and independently of responding, it was not functioning as a punishing stimulus. All of these considerations imply a pharmacologically noncompetitive antagonism (if any) of the reinforcing effects of cocaine by anticholinergic effects. Li et al. (2005) evaluated the effects of atropine in a place conditioning procedure. Atropine alone produced a trend toward conditioned place avoidance, and in combination with cocaine there was also a trend towards decreases in effectiveness of cocaine. In contrast to the implications of the Wilson and Schuster (1973) results described above, these trends suggest a behavioral rather than pharmacological basis to the interaction of anticholinergics with the reinforcing effects of cocaine, and further suggest a punishing effect of anticholinergic agents. However, due to the inconsistencies between the Li et al and Wilson and Schuster findings, definitive conclusions regarding the basis for the interaction of anticholinergics with cocaine for reinforcement are not possible at this time.
The hypothesis that the anticholinergic effects of the BZT analogs punish behavior relates specifically to self-administration procedures using the BZT analogs. In contrast, non-competitive pharmacological interactions between cocaine-like and antimuscarinic effects could operate more broadly across the range of behavioral end points. As noted above, there is a range of pharmacological effects that differ between the BZT analogs and cocaine-like DA uptake inhibitors, including drug discrimination, locomotor activity (Katz et al., 1999) and c-Fos expression (Velazquez-Sanchez et al., 2009). The preponderance of data suggests that antimuscarinic effects enhance rather than antagonize many of the behavioral effects of cocaine-like stimulants. However, whether antimuscarinic actions enhance or attenuate any cocaine-like effects of individual BZT analogs will need to be empirically determined, and may depend on the behavioral endpoint and the spectrum of effects of the doses used of the particular BZT analog.
B. Antagonist activities at histamine receptors
Antagonists of histamine receptors have been tested in drug-discrimination studies alone and in combination with various behaviorally effective doses of cocaine in rats trained to discriminate cocaine from saline injections (Campbell et al., 2005). Results from these experiments show that promethazine and triprolidine, selective H1 antagonists, did not modify the subjective effects of cocaine at any dose tested, while other H1 antagonists (e.g., chlorpheniramine and mepyramine) potentiated the subjective effects of cocaine, producing a leftward shift of the cocaine dose-effect curve. The effects of these latter two agents were likely mediated by their activity as DAT inhibitors (Bergman and Spealman, 1986; Tanda et al., 2008; Tuomisto and Tuomisto, 1980). Similarly, the ratios of H1 to DAT affinities for BZT analogs were not significantly related to, and did not predict outcome for their locomotor stimulant effects (Campbell et al., 2005).
As noted, certain histamine receptor antagonists also have actions that are mediated by the DAT, and some of these drugs have psychostimulant-like reinforcing effects in animal models of abuse (Bergman, 1990; Bergman and Spealman, 1986; Wang and Woolverton, 2007; Wang and Woolverton, 2009). In microdialysis studies, i.v. administration of diphenhydramine, and the enantiomers of chlorpheniramine, dose-dependently and selectively elevated DA levels in the shell as compared to the core of the NAc (Tanda et al., 2008), like it has been shown for virtually all drugs abused by humans (Pontieri et al., 1995; Pontieri et al., 1996; Tanda et al., 1997a). However, triprolidine, a selective H1 antagonist, did not modify extracellular DA levels of dopamine. Together these results show that affinity for both H1 and DAT in diphenhydramine and chlorpheniramine does not prevent certain cocaine-like behavioral effects. As this applies to BZT analogs, it suggests that their H1 antagonist actions are probably not responsible for their reduced cocaine-like behavioral effects in animal models of drug abuse.
C. Involvement of other receptors/sites
Most BZT analogs have a high degree of selectivity for the DAT versus SERT and NET while cocaine has similar affinity for DAT and SERT with lower affinity for the NET (Katz et al., 1999; Katz et al., 2004). Thus, the lack of activity of BZT analogs on other monoamine transporters would suggest that agents lacking activity at these sites will be less effective than cocaine in producing effects related to drug abuse. However, several studies have indicated that the self administration of a variety of monoamine transport inhibitors is related to the affinity of compounds for the DAT and not affinity at NET or SERT (Bergman et al., 1989; Ritz et al., 1987). In addition, compounds relatively selective for the DAT retain reinforcing effects (e.g. Howell et al., 2000; Roberts et al., 1999) while selective SERT or NET inhibitors do not show a cocaine-like behavioral profile (e.g. Hiranita et al., 2009; Howell and Byrd, 1995; Woolverton, 1987). Collectively these studies indicate that lack of effects at the SERT and NET does explain the reduced cocaine-like behavioral effects of BZT analogs.
Among other targets that might be involved in the reduced cocaine-like behavioral effects are the sigma receptors. Antagonists of sigma receptors may modulate various effects of cocaine, including stimulation of locomotor activity and reinforcing effects in place conditioning procedures (see Matsumoto et al., 2003, for a review). However, sigma antagonists failed to reduce the self administration of cocaine (Martin-Fardon et al., 2007).
Cannabinoid CB1 receptors have also been implicated in the neurobiology of cocaine addiction (see Arnold, 2005; Tanda, 2007, for review), and (Navarro et al., 2009) have suggested that positive allosteric modulators of the cannabinoid CB1 receptor can contribute to cocaine-antagonist effects of atypical DAT inhibitors. One compound, RTI-371 is a cocaine analog that binds to the DAT but blocks the locomotor stimulation produced by cocaine (Navarro et al., 2009). Screening of RTI-371 at various sites found it to be a positive allosteric modulator of CB1 receptors. RTI-371 and several other DAT-selective inhibitors with atypical actions on locomotor activity, including the BZT analog JHW 007, increased the efficacy of the CB1 agonist, CP 55940 in a calcium mobilization assay. From those results Navarro et al. suggested that enhanced endocannabinoid neurotransmission may contribute to the cocaine-antagonist effects observed with these atypical DAT inhibitors. Consistent with this hypothesis are findings that cannabinoid agonists, including endogenous cannabinoids, reduce spontaneous ambulatory activity through their actions at central cannabinoid CB1 receptors (Ameri, 1999). Activation of D2 receptors by DA released by atypical DAT blockers could enhance endogenous cannabinoid levels (Giuffrida et al., 1999), and the positive allosteric modulation of CB1 receptors by atypical DAT inhibitors might potentiate cannabinoid neurotransmission, that might in turn counteract the stimulatory effects of these atypical DAT blockers, a hypothesis that clearly warrants further study.
VI. Studies of DAT structure as related to its function
Initial structure-activity studies suggested that BZT and its BZT analogs bind to the DAT differently from cocaine and it congeners (Newman et al., 1995), and subsequent site-directed mutagenesis studies of the DAT supported those conclusions. Several studies have demonstrated differences in the effects of mutating the DAT on the binding of BZT or its analogs compared to analogs of cocaine (Chen et al., 2004). For example, mutating aspartate to glutamate at position 79 in the DAT increased the potency of BZT and its analogs in inhibiting DA uptake, whereas cocaine and various classical DAT were unaffected by the mutation (Ukairo et al., 2005). Additionally, the affinity of BZT and GBR 12909 was affected by the concentration of sodium differently from cocaine in several DAT mutants (Chen and Reith, 2004), again suggesting differences from cocaine in how the BZT analogs bind to the DAT. Photoaffinity labeling studies also support the concept of different binding domains for cocaine and its analogs compared to the BZT analogs (Agoston et al., 1997a; Parnas et al., 2008; Vaughan et al., 1999; Vaughan et al., 2007; Zou et al., 2001).
Other evidence supports the notion that the interaction of BZT with the dopamine transporter is different from that of other dopamine uptake inhibitors, exemplified by cocaine. Reith et al. (2001) compared, among other drugs, the abilities of cocaine and BZT to affect the reaction of a methanethiosulfonate (MTS) reagent to various cysteine residues within the human dopamine transporter. A previous study (Ferrer and Javitch, 1998) showed that reaction of MTS reagents with Cys-90 (located within an extracellular loop) increased [3H]WIN 35,428 binding, and cocaine enhanced the reaction of Cys-90 with MTS reagents, resulting in even greater augmentation of [3H]WIN 35,428 binding. In contrast, cocaine decreased the reaction of MTS reagents with Cys-135 and Cys-342 (located within cytoplasmic loops). In contrast to cocaine, BZT had no effect on the reaction of Cys-90 with an MTS reagent, and the decrease in the reaction of Cys-135 with an MTS reagent produced by cocaine was not obtained with BZT (Reith et al., 2001). These results support the idea that different dopamine uptake inhibitors bind to the dopamine transporter in different ways, and suggests that there may be differences in the conformational changes in the transporter produced by this binding.
Loland et al. (2008) compared the effects of DAT inhibitors on the accessibility of MTSET to a cysteine residue inserted into the human DAT (I159C) expressed in COS-7 cells. This cysteine is thought to be inaccessible when the DAT assumes an inwardly facing conformation, but accessible when the DAT assumes an outward conformation. Cocaine potentiated the DAT inhibition produced by MTSET alone, consistent with the induction of a DAT conformation open to the extracellular environment, and thus MTSET. In contrast, BZT analogs protected against the DAT inhibition produced by MTSET, suggesting that these compounds stabilize the DAT in a closed conformation to which the MTSET had limited access.
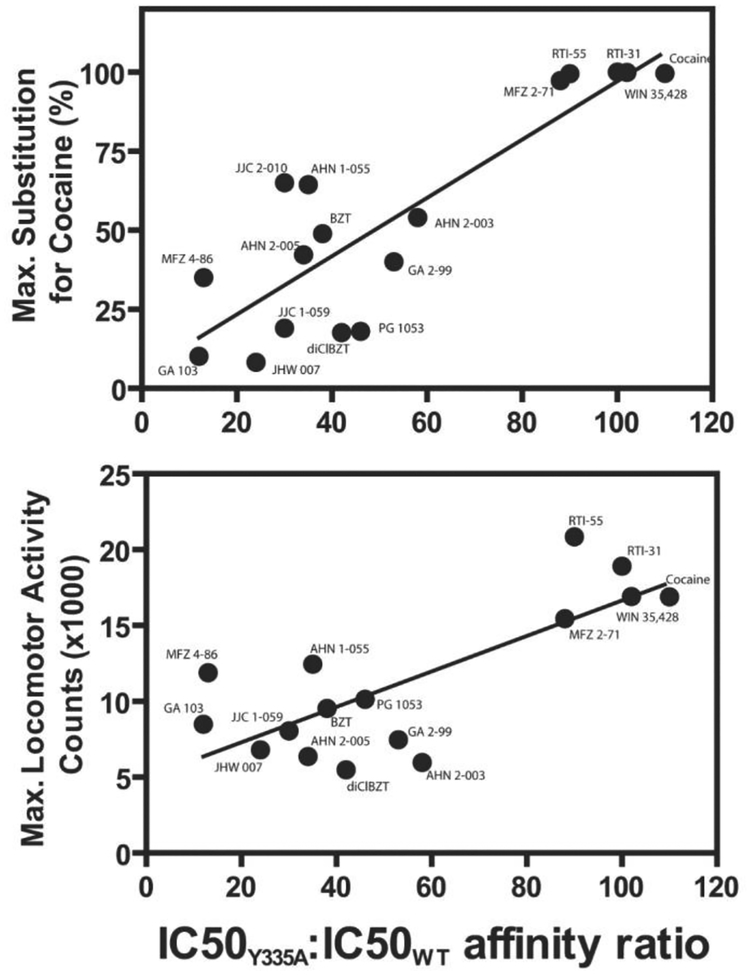
A previous study suggested that Tyr335 in the DAT is critical for regulating the open/closed conformational equilibrium of the DAT (Loland et al., 2002). A Y335A DAT mutation impaired DA transport and decreased the potency of cocaine as an inhibitor of DA uptake. Cocaine and several cocaine analogs were approximately 100-fold less potent as DAT inhibitors in Y335A mutants compared to WT DAT (Loland et al., (2008). In contrast, BZT analogs were only 7–58-fold less potent against the Y335A mutant (Loland et al., 2008). Further, there was a good relationship between the decrease in potencies of the BZT analogs due to the DAT mutation and their effectiveness in stimulating locomotor activity and in substituting for cocaine in rats trained to discriminate cocaine injections from those of saline (Figure 11). One BZT analog, MFZ 2–71, was of particular interest because it showed a Y335A mutation-dependent loss in DAT inhibitory potency that approached that seen with cocaine. In addition, MFZ 2–71, had behavioral effects like those of cocaine; it not only produced a cocaine-like stimulation of locomotor activity, but it also substituted fully in subjects trained to discriminate cocaine from saline injections. Thus the BZT analog MFZ 2–71 shows promise for differentiating structural features of the BZT structure that promote conformational changes of the DAT that may lead to effects unlike those of cocaine, and may indeed contribute to cocaine antagonist actions.
Figure 11:
The relationship between dopamine uptake potency ratios of various dopamine transporter ligands in COS-7 cells transfected with the Y335A mutant and WT DAT (affinity ratio) and behavioral activity. Top Panel: Correlation of affinity ratio and the degree to which the drugs substituted for cocaine in rats trained to discriminate cocaine from vehicle injections. The correlation coefficient (r2) was 0.74 (p<0.0001). Bottom Panel: Correlation of affinity ratio and the maximal locomotor-activity stimulation in mice. The correlation coefficient (r2) was 0.59 (p<0.0005). The affinity ratio was calculated from the IC50 values for inhibition of [3H]dopamine uptake by the compound in COS-7 cells transiently expressing either DAT WT or Y335A. Modified from Loland et al. (2008).
Modeling of the DAT complexed with DA and DAT inhibitors, using as a basis the structure of the bacterial leucine transporter, showed that the binding of DAT inhibitors and substrates (including DA and amphetamine derivatives) overlapped, indicative of a competitive inhibition (Beuming et al., 2008). In contrast to the binding of cocaine, the binding of BZT analogs preserved the hydrogen bond between Asp79 and Tyr156; that bond has been proposed to function as a gate regulating translocation of substrate (Loland et al., 2004). The preservation of the Asp79-Tyr156 bond with the binding of a BZT analog is consistent with the findings of Loland et al. (2008) suggesting different conformational changes induced in the DAT by the binding of cocaine and BZT analogs.
VII. Summary and conclusions
During the last two decades many advances have been made in the understanding of the behavioral and reinforcing effects of cocaine, as well as the neurobiology underlying the effects that lead to its abuse and to addiction. Though a long road remains before a medical treatment against cocaine abuse is available, many new pharmacological tools have been discovered, and among them are the BZT analogs. These compounds have provided new insights into the molecular and behavioral actions of cocaine, and the diversity of effects produced by DAT inhibitors. Several BZT analogs have been used in studies of the molecular structure of the DAT to elucidate the binding domains of DAT inhibitors, and how differences in the interaction of these compounds with DAT contribute to their pharmacological differences. In preclinical studies, different BZT analogs can attenuate or block the behavioral, neurochemical, and/or reinforcing actions of cocaine, and may represent the starting point to develop new medications for the treatment of cocaine addiction.
Acknowledgements.
Work was supported by the Intramural Research Program of the Department of Health and Human Services, National Institute on Drug Abuse, National Institutes of Health.
REFERENCES
- Agoston GE, Vaughan R, Lever JR, Izenwasser S, Terry PD, and Newman AH (1997a). A novel photoaffinity label for the dopamine transporter based on substituted 3 alpha-[bis(4’-fluorophenyl)methoxy]tropane. Bioorganic Medicnal Chemistry Letters 7(23), 3027–3032. [Google Scholar]
- Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH, and Newman AH (1997b). Novel N-substituted 3 alpha-[bis(4’-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem 40(26), 4329–39. [DOI] [PubMed] [Google Scholar]
- Ameri A (1999). The effects of cannabinoids on the brain. Prog Neurobiol 58(4), 315–48. [DOI] [PubMed] [Google Scholar]
- Arnold JC (2005). The role of endocannabinoid transmission in cocaine addiction. Pharmacol Biochem Behav 81(2), 396–406. [DOI] [PubMed] [Google Scholar]
- Baker LE, Riddle EE, Saunders RB, and Appel JB (1993). The role of monoamine uptake in the discriminative stimulus effects of cocaine and related compounds. Behav Pharmacol 4(1), 69–79. [PubMed] [Google Scholar]
- Balster RL, and Schuster CR (1973). Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J Exp Anal Behav 20(1), 119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, and Balster RL (1993). The effects of delay of reinforcement and dose on the self-administration of cocaine and procaine in rhesus monkeys. Drug Alcohol Depend 34(1), 37–43. [DOI] [PubMed] [Google Scholar]
- Bergman J (1990). Psychomotor stimulant effects of the stereoisomers of chlorpheniramine. Psychopharmacology (Berl) 100(1), 132–4. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, and Spealman RD (1989). Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther 251(1), 150–5. [PubMed] [Google Scholar]
- Bergman J, and Spealman RD (1986). Some behavioral effects of histamine H1 antagonists in squirrel monkeys. J Pharmacol Exp Ther 239(1), 104–10. [PubMed] [Google Scholar]
- Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, Newman AH, Javitch JA, Weinstein H, Gether U, and Loland CJ (2008). The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci 11(7), 780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley NJ, Bonner TI, Buckley CM, and Brann MR (1989). Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol 35(4), 469–76. [PubMed] [Google Scholar]
- Callahan PM, De La Garza R 2nd, and Cunningham KA (1997). Mediation of the discriminative stimulus properties of cocaine by mesocorticolimbic dopamine systems. Pharmacol Biochem Behav 57(3), 601–7. [DOI] [PubMed] [Google Scholar]
- Campbell VC, Kopajtic TA, Newman AH, and Katz JL (2005). Assessment of the influence of histaminergic actions on cocaine-like effects of 3alpha-diphenylmethoxytropane analogs. J Pharmacol Exp Ther 315(2), 631–40. [DOI] [PubMed] [Google Scholar]
- Carlton PL, and Didamo P (1961). Augmentation of the behavioral effects of amphetamine by atropine. J Pharmacol Exp Ther 132, 91–6. [PubMed] [Google Scholar]
- Carroll FI, Howell LL, and Kuhar MJ (1999). Pharmacotherapies for treatment of cocaine abuse: preclinical aspects. J Med Chem 42(15), 2721–36. [DOI] [PubMed] [Google Scholar]
- Catterall WA, and Mackie K (2006). Local Anesthetics In “Goodman & Gilman’s 11th Edition, The Pharmacological Basis of Therapeutics” (Brunton L, ed.), pp. 369–386. McGraw Hill Medical Publishing Edition Division, New York. [Google Scholar]
- Chaudhuri KR, and Schapira AH (2009). Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8(5), 464–74. [DOI] [PubMed] [Google Scholar]
- Chen N, and Reith ME (2004). Interaction between dopamine and its transporter: role of intracellular sodium ions and membrane potential. J Neurochem 89(3), 750–65. [DOI] [PubMed] [Google Scholar]
- Chen N, Zhen J, and Reith ME (2004). Mutation of Trp84 and Asp313 of the dopamine transporter reveals similar mode of binding interaction for GBR12909 and benztropine as opposed to cocaine. J Neurochem 89(4), 853–64. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJ, and Janssen PA (1979). Discriminative stimulus properties of cocaine: neuropharmacological characteristics as derived from stimulus generalization experiments. Pharmacol Biochem Behav 10(4), 535–46. [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, French D, Newman AH, and Katz JL (2005a). Relationship between in vivo occupancy at the dopamine transporter and behavioral effects of cocaine, GBR 12909 [1-{2-[bis-(4-fluorophenyl)methoxy]ethyl}−4-(3-phenylpropyl)piperazine], and benztropine analogs. J Pharmacol Exp Ther 315(1), 397–404. [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, Koffarnus M, Newman AH, and Katz JL (2005b). Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci 25(8), 1889–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin SI, and Smith JE (1988). Neurobehavioral pharmacology of cocaine. NIDA Res Monogr 88, 185–98. [PubMed] [Google Scholar]
- Ferragud A, Velázquez-Sánchez C, Hernández-Rabaza V, Nácher A, Merino V, Cardá M, Murga J, and Canales JJ (2009, in press). A Dopamine Transport Inhibitor with Markedly Low Abuse Liability Suppresses Cocaine Self-Administration in the Rat Psychoparmacology. [DOI] [PubMed]
- Ferrer JV, and Javitch JA (1998). Cocaine alters the accessibility of endogenous cysteines in putative extracellular and intracellular loops of the human dopamine transporter. Proc Natl Acad Sci U S A 95(16), 9238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, and Piomelli D (1999). Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci 2(4), 358–63. [DOI] [PubMed] [Google Scholar]
- Grace RF (1997). Benztropine abuse and overdose--case report and review. Adverse Drug React Toxicol Rev 16(2), 103–12. [PubMed] [Google Scholar]
- Greco PG, and Garris PA (2003). In vivo interaction of cocaine with the dopamine transporter as measured by voltammetry. Eur J Pharmacol 479(1–3), 117–25. [DOI] [PubMed] [Google Scholar]
- Hahn MK, and Blakely RD (2007). The functional impact of SLC6 transporter genetic variation. Annu Rev Pharmacol Toxicol 47, 401–41. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Newman AH, and Katz JL (2009). Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther 329(2), 677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W (1961). Progressive ratio as a measure of reward strength. Science 134, 943–4. [DOI] [PubMed] [Google Scholar]
- Holtzman SG (1990). Discriminative stimulus effects of drugs: relationship to potential for abuse In “In: Modern methods in pharmacology, Testing and evaluation of drugs of abuse”, Vol. 6, pp. 193–210. Wiley Liss Inc. [Google Scholar]
- Howell LL, and Byrd LD (1995). Serotonergic modulation of the behavioral effects of cocaine in the squirrel monkey. J Pharmacol Exp Ther 275(3), 1551–9. [PubMed] [Google Scholar]
- Howell LL, Czoty PW, Kuhar MJ, and Carrol FI (2000). Comparative behavioral pharmacology of cocaine and the selective dopamine uptake inhibitor RTI-113 in the squirrel monkey. J Pharmacol Exp Ther 292(2), 521–9. [PubMed] [Google Scholar]
- Ikemoto S (2002). Ventral striatal anatomy of locomotor activity induced by cocaine, D-amphetamine, dopamine and D1/D2 agonists. Neuroscience 113(4), 939–55. [DOI] [PubMed] [Google Scholar]
- Iversen LL, Iversen SD, Bloom FE, and Roth RH (2008). Catecholamines In “Introduction to Neuropsychopharmacology”, pp. 150–213. Oxford University Press, New York. [Google Scholar]
- Izenwasser S, Terry P, Heller B, Witkin JM, and Katz JL (1994). Differential relationships among dopamine transporter affinities and stimulant potencies of various uptake inhibitors. Eur J Pharmacol 263(3), 277–83. [DOI] [PubMed] [Google Scholar]
- Kalivas PW (2007). Neurobiology of cocaine addiction: implications for new pharmacotherapy. Am J Addict 16(2), 71–8. [DOI] [PubMed] [Google Scholar]
- Katz JL (1989). Drugs as reinforcers: Pharmacological and behavioral factors In “The neurobiological basis of reward” (Liebman JM and Cooper SJ, eds.), pp. 164–213. Clarendon Press, Oxford. [Google Scholar]
- Katz JL, Agoston GE, Alling KL, Kline RH, Forster MJ, Woolverton WL, Kopajtic TA, and Newman AH (2001). Dopamine transporter binding without cocaine-like behavioral effects: synthesis and evaluation of benztropine analogs alone and in combination with cocaine in rodents. Psychopharmacology (Berl) 154(4), 362–74. [DOI] [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Kline RH, Allen AC, and Newman AH (1999). Novel 3alpha-diphenylmethoxytropane analogs: selective dopamine uptake inhibitors with behavioral effects distinct from those of cocaine. J Pharmacol Exp Ther 288(1), 302–15. [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, and Terry P (2000). Relationships among dopamine transporter affinities and cocaine-like discriminative-stimulus effects. Psychopharmacology (Berl) 148(1), 90–8. [DOI] [PubMed] [Google Scholar]
- Katz JL, Kopajtic TA, Agoston GE, and Newman AH (2004). Effects of N-substituted analogs of benztropine: diminished cocaine-like effects in dopamine transporter ligands. J Pharmacol Exp Ther 309(2), 650–60. [DOI] [PubMed] [Google Scholar]
- Katz JL, Libby TA, Kopajtic T, Husbands SM, and Newman AH (2003). Behavioral effects of rimcazole analogues alone and in combination with cocaine. Eur J Pharmacol 468(2), 109–19. [DOI] [PubMed] [Google Scholar]
- Kharkar PS, Dutta AK, and Reith MEA (2008). Structure-activity relationship study of piperidine derivatives for dopamine transporters In “Dopamine Transporters, Chemistry, Biology, and Pharmacology” (Trudell ML and Izenwasser S, eds.), pp. 233–264. John Wiley & Sons. [Google Scholar]
- Kleven MS, Anthony EW, and Woolverton WL (1990). Pharmacological characterization of the discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther 254(1), 312–7. [PubMed] [Google Scholar]
- Kuczenski R, and Segal DS (1992). Differential effects of amphetamine and dopamine uptake blockers (cocaine, nomifensine) on caudate and accumbens dialysate dopamine and 3-methoxytyramine. J Pharmacol Exp Ther 262(3), 1085–94. [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, and Boja JW (1991). The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 14(7), 299–302. [DOI] [PubMed] [Google Scholar]
- Kulkarni SS, Grundt P, Kopajtic T, Katz JL, and Newman AH (2004). Structure-activity relationships at monoamine transporters for a series of N-substituted 3alpha-(bis[4-fluorophenyl]methoxy)tropanes: comparative molecular field analysis, synthesis, and pharmacological evaluation. J Med Chem 47(13), 3388–98. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, and Revesz T (2009). Parkinson’s disease. Lancet 373(9680), 2055–66. [DOI] [PubMed] [Google Scholar]
- Li SM, Newman AH, and Katz JL (2005). Place conditioning and locomotor effects of N-substituted, 4’,4”-difluorobenztropine analogs in rats. J Pharmacol Exp Ther 313(3), 1223–30. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K, Sitte HH, Newman AH, Katz JL, and Gether U (2008). Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol 73(3), 813–23. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Granas C, Javitch JA, and Gether U (2004). Identification of intracellular residues in the dopamine transporter critical for regulation of transporter conformation and cocaine binding. J Biol Chem 279(5), 3228–38. [DOI] [PubMed] [Google Scholar]
- Loland CJ, Norregaard L, Litman T, and Gether U (2002). Generation of an activating Zn(2+) switch in the dopamine transporter: mutation of an intracellular tyrosine constitutively alters the conformational equilibrium of the transport cycle. Proc Natl Acad Sci U S A 99(3), 1683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Maurice T, Aujla H, Bowen WD, and Weiss F (2007). Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology 32(9), 1967–73. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Liu Y, Lerner M, Howard EW, and Brackett DJ (2003). Sigma receptors: potential medications development target for anti-cocaine agents. Eur J Pharmacol 469(1–3), 1–12. [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, and Davoli M (2009). Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev (3), CD002209. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Schmitt GJ, Scheuerecker J, and Moller HJ (2007). The role of dopamine for the pathophysiology of schizophrenia. Int Rev Psychiatry 19(4), 337–45. [DOI] [PubMed] [Google Scholar]
- Meltzer PC (2008). Non-nitrogen containing dopamine transporter-uptake inhibitors In “Dopamine Transporters, Chemistry, Biology, and Pharmacology” (Trudell ML and Izenwasser S, eds.), pp. 265–304. John Wiley & Sons. [Google Scholar]
- Navarro HA, Howard JL, Pollard GT, and Carroll FI (2009). Positive allosteric modulation of the human cannabinoid (CB) receptor by RTI-371, a selective inhibitor of the dopamine transporter. Br J Pharmacol 156(7), 1178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2005). The neurobiology of cocaine addiction. Sci Pract Perspect 3(1), 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Allen AC, Izenwasser S, and Katz JL (1994). Novel 3 alpha-(diphenylmethoxy)tropane analogs: potent dopamine uptake inhibitors without cocaine-like behavioral profiles. J Med Chem 37(15), 2258–61. [DOI] [PubMed] [Google Scholar]
- Newman AH, and Katz JL (2008). The Benztropines: Atypical dopamine-uptake inhibitors that provide clues about cocaine’s mechanism at the dopamine transporter In “Dopamine Transporters, Chemistry, Biology, and Pharmacology” (Trudell ML and Izenwasser S, eds.), pp. 171–210. John Wiley & sons. [Google Scholar]
- Newman AH, and Katz JL (2009). Atypical dopamine uptake inhibitors that provide clues about cocaine’s mechanism at the dopamine transporter. Topics in Medicinal Chemistry 4, 95–129. [Google Scholar]
- Newman AH, Kline RH, Allen AC, Izenwasser S, George C, and Katz JL (1995). Novel 4’-substituted and 4’,4”-disubstituted 3 alpha-(diphenylmethoxy)tropane analogs as potent and selective dopamine uptake inhibitors. J Med Chem 38(20), 3933–40. [DOI] [PubMed] [Google Scholar]
- Newman AH, and Kulkarni S (2002). Probes for the dopamine transporter: new leads toward a cocaine-abuse therapeutic--A focus on analogues of benztropine and rimcazole. Med Res Rev 22(5), 429–64. [DOI] [PubMed] [Google Scholar]
- Othman AA, Newman AH, and Eddington ND (2007a). Applicability of the dopamine and rate hypotheses in explaining the differences in behavioral pharmacology of the chloro-benztropine analogs: studies conducted using intracerebral microdialysis and population pharmacodynamic modeling. J Pharmacol Exp Ther 322(2), 760–9. [DOI] [PubMed] [Google Scholar]
- Othman AA, Syed SA, Newman AH, and Eddington ND (2007b). Transport, metabolism, and in vivo population pharmacokinetics of the chloro benztropine analogs, a class of compounds extensively evaluated in animal models of drug abuse. J Pharmacol Exp Ther 320(1), 344–53. [DOI] [PubMed] [Google Scholar]
- Parnas ML, Gaffaney JD, Zou MF, Lever JR, Newman AH, and Vaughan RA (2008). Labeling of dopamine transporter transmembrane domain 1 with the tropane ligand N-[4-(4-azido-3-[125I]iodophenyl)butyl]-2beta-carbomethoxy-3beta-(4-chloro phenyl)tropane implicates proximity of cocaine and substrate active sites. Mol Pharmacol 73(4), 1141–50. [DOI] [PubMed] [Google Scholar]
- Penetar DM, Looby AR, Su Z, Lundahl LH, Eros-Sarnyai M, McNeil JF, and Lukas SE (2006). Benztropine pretreatment does not affect responses to acute cocaine administration in human volunteers. Hum Psychopharmacol 21(8), 549–59. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, and Di Chiara G (1995). Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A 92(26), 12304–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, and Di Chiara G (1996). Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 382(6588), 255–7. [DOI] [PubMed] [Google Scholar]
- Prisinzano TE, and Rice KC (2008). Structure-activity relationshipof GBR 12909 ligands In “Dopamine Transporters, Chemistry, Biology, and Pharmacology” (Trudell ML and Izenwasser S, eds.), pp. 211–232. John Wiley & Sons. [Google Scholar]
- Raje S, Cao J, Newman AH, Gao H, and Eddington ND (2003). Evaluation of the blood-brain barrier transport, population pharmacokinetics, and brain distribution of benztropine analogs and cocaine using in vitro and in vivo techniques. J Pharmacol Exp Ther 307(2), 801–8. [DOI] [PubMed] [Google Scholar]
- Raje S, Cornish J, Newman AH, Cao J, Katz JL, and Eddington ND (2006). Investigation of the potential pharmacokinetic and pharmaco-dynamic drug interaction between AHN 1–055, a potent benztropine analog used for cocaine abuse, and cocaine after dosing in rats using intracerebral microdialysis. Biopharm Drug Dispos 27(5), 229–40. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, and Woolverton WL (2002). Self-administration of cocaine: scopolamine combinations by rhesus monkeys. Psychopharmacology (Berl) 161(4), 442–8. [DOI] [PubMed] [Google Scholar]
- Reith ME, Berfield JL, Wang LC, Ferrer JV, and Javitch JA (2001). The uptake inhibitors cocaine and benztropine differentially alter the conformation of the human dopamine transporter. J Biol Chem 276(31), 29012–8. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Boja JW, George FR, and Kuhar MJ (1989a). Cocaine binding sites related to drug self-administration. NIDA Res Monogr 95, 239–46. [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, and Kuhar MJ (1987). Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237(4819), 1219–23. [DOI] [PubMed] [Google Scholar]
- Robarge MJ, Agoston GE, Izenwasser S, Kopajtic T, George C, Katz JL, and Newman AH (2000). Highly selective chiral N-substituted 3alpha-[bis(4’-fluorophenyl)methoxy]tropane analogues for the dopamine transporter: synthesis and comparative molecular field analysis. J Med Chem 43(6), 1085–93. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Phelan R, Hodges LM, Hodges MM, Bennett B, Childers S, and Davies H (1999). Self-administration of cocaine analogs by rats. Psychopharmacology (Berl) 144(4), 389–97. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Prisinzano TE, and Newman AH (2008). Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharmacol 75(1), 2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon SP, and Carroll FI (2008). Tropane-based dopamine transporter-uptake inhibitors In “Dopamine Transporters, Chemistry, Biology, and Pharmacology” (Trudell ML and S I, eds.), pp. 125–170. John Wiley & Sons. [Google Scholar]
- Scheckel CL, and Boff E (1964). Behavioral Effects of Interacting Imipramine and Other Drugs with D-Amphetamine, Cocaine, and Tetrabenazine. Psychopharmacologia 5, 198–208. [DOI] [PubMed] [Google Scholar]
- Skinner BF (1938). “The Behavior of Organisms: An Experimental Analysis” D. Appleton-Century company, incorporated, New York, London. [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, and Lancaster T (2008). Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev (1), CD000146. [DOI] [PubMed] [Google Scholar]
- Steeves TD, and Fox SH (2008). Neurobiological basis of serotonin-dopamine antagonists in the treatment of Gilles de la Tourette syndrome. Prog Brain Res 172, 495–513. [DOI] [PubMed] [Google Scholar]
- Stein DJ (2008). Depression, anhedonia, and psychomotor symptoms: the role of dopaminergic neurocircuitry. CNS Spectr 13(7), 561–5. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies, DEPARTMENT OF HEALTH AND HUMAN SERVICES (2007). Results from the 2007 National Survey on Drug Use and Health: National Findings In “ http://www.oas.samhsa.gov/nsduh/2k7nsduh/2k7Results.pdf“. [Google Scholar]
- Tallarida RJ (2000). “Drug Synergism and Dose-Effect Data Analysis.” CRC/Chapman-Hall; Boca Raton. [Google Scholar]
- Tallarida RJ (2007). Interactions between drugs and occupied receptors. Pharmacol Ther 113(1), 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G (2007). Modulation of the endocannabinoid system: therapeutic potential against cocaine dependence. Pharmacol Res 56(5), 406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Ebbs A, Newman AH, and Katz JL (2005). Effects of 4’-chloro-3 alpha-(diphenylmethoxy)-tropane on mesostriatal, mesocortical, and mesolimbic dopamine transmission: comparison with effects of cocaine. J Pharmacol Exp Ther 313(2), 613–20. [DOI] [PubMed] [Google Scholar]
- Tanda G, Ebbs A, Newman AH, and Katz JL (2006). Analogs of benztropine as cocaine-abuse medications: drug discrimination and microdialysis studies in rats. In “Abstracts, 37th Annual Meeting of the Society for Neuroscience” Atlanta, GA. [Google Scholar]
- Tanda G, Ebbs AL, Kopajtic TA, Elias LM, Campbell BL, Newman AH, and Katz JL (2007). Effects of muscarinic M1 receptor blockade on cocaine-induced elevations of brain dopamine levels and locomotor behavior in rats. J Pharmacol Exp Ther 321(1), 334–44. [DOI] [PubMed] [Google Scholar]
- Tanda G, and Katz JL (2007). Muscarinic preferential M(1) receptor antagonists enhance the discriminative-stimulus effects of cocaine in rats. Pharmacol Biochem Behav 87(4), 400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Kopajtic TA, and Katz JL (2008). Cocaine-like neurochemical effects of antihistaminic medications. J Neurochem 106(1), 147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Newman A, Ebbs AL, Tronci V, Green J, Tallarida RJ, and Katz JL (2009). Combinations of Cocaine with other Dopamine Uptake Inhibitors: Assessment of Additivity. J Pharmacol Exp Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, and Di Chiara G (1997a). Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science 276(5321), 2048–50. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Frau R, and Di Chiara G (1997b). Contribution of blockade of the noradrenaline carrier to the increase of extracellular dopamine in the rat prefrontal cortex by amphetamine and cocaine. Eur J Neurosci 9(10), 2077–85. [DOI] [PubMed] [Google Scholar]
- Tolliver BK, Newman AH, Katz JL, Ho LB, Fox LM, Hsu K Jr., and Berger SP (1999). Behavioral and neurochemical effects of the dopamine transporter ligand 4-chlorobenztropine alone and in combination with cocaine in vivo. J Pharmacol Exp Ther 289(1), 110–22. [PubMed] [Google Scholar]
- Tuomisto J, and Tuomisto L (1980). Effects of histamine and histamine antagonists on the uptake and release of catecholamines and 5-HT in brain synaptosomes. Med Biol 58(1), 33–7. [PubMed] [Google Scholar]
- Tzschentke TM (1998). Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56(6), 613–72. [DOI] [PubMed] [Google Scholar]
- Ukairo OT, Bondi CD, Newman AH, Kulkarni SS, Kozikowski AP, Pan S, and Surratt CK (2005). Recognition of benztropine by the dopamine transporter (DAT) differs from that of the classical dopamine uptake inhibitors cocaine, methylphenidate, and mazindol as a function of a DAT transmembrane 1 aspartic acid residue. J Pharmacol Exp Ther 314(2), 575–83. [DOI] [PubMed] [Google Scholar]
- Vaughan RA, Agoston GE, Lever JR, and Newman AH (1999). Differential binding of tropane-based photoaffinity ligands on the dopamine transporter. J Neurosci 19(2), 630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan RA, Sakrikar DS, Parnas ML, Adkins S, Foster JD, Duval RA, Lever JR, Kulkarni SS, and Hauck-Newman A (2007). Localization of cocaine analog [125I]RTI 82 irreversible binding to transmembrane domain 6 of the dopamine transporter. J Biol Chem 282(12), 8915–25. [DOI] [PubMed] [Google Scholar]
- Velazquez-Sanchez C, Ferragud A, Hernandez-Rabaza V, Nacher A, Merino V, Carda M, Murga J, and Canales JJ (2009). The Dopamine Uptake Inhibitor 3-[bis(4’-fluorophenyl) metoxy]-tropane Reduces Cocaine-Induced Early-Gene Expression, Locomotor Activity, and Conditioned Reward. Neuropsychopharmacology. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, and Elkashef A (2005). Medication development for addictive disorders: the state of the science. Am J Psychiatry 162(8), 1432–40. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, and Wang GJ (2002). Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol 13(5–6), 355–66. [DOI] [PubMed] [Google Scholar]
- Wang Z, and Woolverton WL (2007). Self-administration of cocaine-antihistamine combinations: super-additive reinforcing effects. Eur J Pharmacol 557(2–3), 159–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, and Woolverton WL (2009). Super-additive interaction of the reinforcing effects of cocaine and H1-antihistamines in rhesus monkeys. Pharmacol Biochem Behav 91(4), 590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MC, and Schuster CR (1973). The effects of stimulants and depressants on cocaine self-administration behavior in the rhesus monkey. Psychopharmacologia 31(4), 291–304. [DOI] [PubMed] [Google Scholar]
- Woods JH, Winger GD, and France CP (1987). Reinforcing and Discriminative Stimulus Effects of Cocaine: analysis of Pharmacological Mechanisms In “Cocaine, Clinical and Biobehavioral Aspects” (Fisher S, Raskin A and Uhlenhuth EH, eds.), pp. 21–65. Oxford University Press, New York & Oxford [Google Scholar]
- Woolverton WL (1987). Evaluation of the role of norepinephrine in the reinforcing effects of psychomotor stimulants in rhesus monkeys. Pharmacol Biochem Behav 26(4), 835–9. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Hecht GS, Agoston GE, Katz JL, and Newman AH (2001). Further studies of the reinforcing effects of benztropine analogs in rhesus monkeys. Psychopharmacology (Berl) 154(4), 375–82. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Rowlett JK, Wilcox KM, Paul IA, Kline RH, Newman AH, and Katz JL (2000). 3’- and 4’-chloro-substituted analogs of benztropine: intravenous self-administration and in vitro radioligand binding studies in rhesus monkeys. Psychopharmacology (Berl) 147(4), 426–35. [DOI] [PubMed] [Google Scholar]
- Zahm DS (1999). Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci 877, 113–28. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, and Baumann MH (2009). Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther 329(2), 738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou MF, Kopajtic T, Katz JL, and Newman AH (2003). Structure-activity relationship comparison of (S)-2beta-substituted 3alpha-(bis[4-fluorophenyl]methoxy)tropanes and (R)-2beta-substituted 3beta-(3,4-dichlorophenyl)tropanes at the dopamine transporter. J Med Chem 46(14), 2908–16. [DOI] [PubMed] [Google Scholar]
- Zou MF, Kopajtic T, Katz JL, Wirtz S, Justice JB Jr., and Newman AH (2001). Novel tropane-based irreversible ligands for the dopamine transporter. J Med Chem 44(25), 4453–61. [DOI] [PubMed] [Google Scholar]