Abstract
The wide applications of lithium intercalating complex metal oxides in energy storage devices call for a better understanding of their environmental impact at the end of their life cycle. In this study, we examine the biological impact of a panel of nanoscale lithium nickel manganese cobalt oxides (LixNiyMnzCo1−y−zO2, 0 < x, y, z < 1, abbreviated to NMCs) to a model Gram-positive bacterium, Bacillus subtilis, in terms of cellular respiration and growth. A highly sensitive single-cell gel electrophoresis method is also applied for the first time to understand the genotoxicity of these nanomaterials to bacterial cells. Results from these assays indicate that the free Ni and Co ions released from the incongruent dissolution of the NMC material in B. subtilis growth medium induced both hindered growth and cellular respiration. More remarkably, the DNA damage induced by the combination of the two ions in solution is comparable to that induced by the NMC material, which suggests that the free Ni and Co ions are responsible for the toxicity observed. A material redesign by enriching Mn is also presented. The combined approaches of evaluating their impact on bacterial growth, respiration, and DNA damage at a single-cell level, as well as other phenotypical changes allows us to probe the nanomaterials and bacterial cells from a mechanistic prospective, and provides a useful means to an understanding of bacterial response to new potential environmental stressors.
Introduction
As the effort to reduce greenhouse gas emission continues, electric vehicles have expanded from a technological novelty to consumer products, largely enabled by the development of lithium ion battery technology.1,2 Lithium intercalation compounds, often used in the cathodes of these batteries, are under active development in order to improve on energy density, charging current, power, and safety, and to reduce cost.3–5 Recently, these complex metal oxides have also demonstrated potential in catalysis applications.6
Lithium nickel manganese cobalt oxides (LixNiyMnz-Co1−y−zO2, 0 < x, y, z < 1), known as NMCs, are a class of complex metal oxides that share the same layered material structure as the commercialized lithium cobalt oxide (LCO). They offer high energy density, improved stability and more cycle times.7 The most common composition of LixNi1/3Mn1/3Co1/3O2 (NMC-111) (x = 1 indicating full lithiation) has been used in commercial electric vehicles, such as the Nissan Leaf and BMW i3.1 The framework of the material features nanoscale layers that enable improved lithium diffusion, better wettability, and good process ability.2,7 Mechanical stress from repeated charging and discharging cycles has also resulted in nanometer scale fractures.8,9 In addition to the NMC-111 composition, by varying the ratios of the starting material, researchers have been able to develop other compositions of NMCs, as well as explore their functions in an effort to boost performance and reduce costs.10 For instance, Ni-enriched NMCs, e.g. NMC-622, have shown promising performance enhancement as the cathode material and is going into full scale production.1,7,11
In spite of the active research in the development of high performing complex metal oxides, due to the lack of infrastructure and incentives for recycling, the majority of these materials will end up in the environment in landfills or aquatic systems at the end of their usage.12–14 Therefore, in order to understand the potential interactions of these nanomaterials with environmental biological models, a few recent studies have focused on the impacts of these nanosheets with model lipid membranes,15 a model Gram-negative bacterium, Shewanella oneidensis,8,16 and a model multicellular aquatic organism, Daphnia magna.17 These early studies have indicated metal ions released from nanosheet dissolution as the primary means of toxicity to some biological species.
Building on the earlier findings, and in order to gain a molecular level insight into the toxicity mechanism, in this study, we examine the impacts of NMC on metabolic respiration and DNA damage to a Gram-positive model bacterium, Bacillus subtilis. In addition to the fact that B. subtilis is one of the most well-studied gram positive bacterial models, the wild type strain SB491 used in this work is commonly found in soils and is critical to many nutrient cycles in the environment. Low concentrations of metal ions are often required by bacteria as essential nutrients. However, high levels of metal ions may lead to non-specific binding interactions with proteins and cellular receptors, resulting in the disruption of biochemical pathways, DNA damage, and toxicity to bacteria.18 In particular, while Ni and Co ions from NMC nanosheets have brought environmental concerns,14 studies have shown that some bacterial species may play a role in metal remediation in the environment through biosorption.19,20 Therefore, we are interested in examining the interactions of these complex metal oxides with a model bacterial species.
Bacteria are generally considered a trophic group to be highly impacted by metal-based nanomaterials based on prior studies.8,16,21 This study aims to broaden the understanding of the impact of nanoscale layered lithium inter-calating materials on the environment using an environmentally relevant model bacterium, B. subtilis, to provide molecular insights into the mechanism of toxicity. The biological impacts of the NMC material, as well as the dissolved ions, will be assessed in terms of bacterial respiration, growth, and DNA breakage. Upon understanding the sources of toxicity, an effort to redesign the NMC material in order to reduce its toxicity is also reported.
Experimental
Synthesis and characterization of Li-intercalating battery materials
NMC nanosheets were synthesized using previously reported methods, and the same batch of Mn-enriched NMC nano-sheets used in the present study was also previously used in other publications.8 Briefly, the metal hydroxides are made via controlled precipitation. The hydroxides are then converted into the lithiated oxides by heating in a molten salt flux, leading to nanoflakes approximately 5–7 nm thick and with lateral dimensions of approximately 80–120 nm diameter.
The material composition was characterized from solution analysis of acid digested particles in aqua regia (a 3: 1 v/v mixture of 37% HCl and 70% v/v HNO3) for 2 h. All solutions were diluted in ultrapure water prior to analysis with inductively coupled plasma-optical emission spectroscopy (ICPOES, PerkinElmer Optima 2000). All standards were prepared in a similar acid-based matrix and was purchased as certified reference materials from Sigma Aldrich. The ion concentrations were measured using three analytical replicates and ratios of the elements were used to calculate the ultimate stoichiometries. The ICP-OES measurements indicated that the synthesized materials had the following stoichiometries: Li0.27Ni0.33Mn0.34Co0.33O2 (33% Mn NMC), Li0.61Ni0.23Mn0.55Co0.22O2 (55% Mn NMC) and Li0.52Ni0.14Mn0.72Co0.14O2 (72%Mn NMC).
Crystal phases of the materials were characterized using powder X-ray diffraction (P-XRD). The synthesized powders were each deposited onto a SiO2 zero-diffraction plate (MTI Corp) and then flattened down gently using a spatula to obtain an even and smooth surface of powder. To prevent loss of material during this characterization step, no other attempts were made to lower the background coming from the degree of evenness in the deposition. A Bruker D8 Advance P-XRD with a Cu-Kα source was used. The resulting reflections characterized were roughly indexed to the <mi> space group as previously reported for the crystal phase of these lithium intercalation materials.22,23 The powder XRD spectrum for Li0.27Ni0.33Mn0.34Co0.33O2 (NMC 111) can be seen in Fig. S1† while the P-XRD spectrum for the Mn-rich NMCs can be found in previously published work.16
The morphology of the synthesized materials was characterized using scanning electron microscopy (SEM, Leo Supra55 VP instrument, standard In-Lens detector at 1 kV incident electron energy). Each sample was prepared via dropcasting (methanolic solution of powder) onto boron-doped Si wafers. A large field of view containing clusters of particles was selected for each particle type to show the overall morphology of a large number of particles. Representative SEM images of the material used in this study are shown in Fig. S2.†
Dissolution studies with ICP-OES
To characterize the concentration of ions leached from NMC-111 in a bacterial growth medium, suspensions of 5 and 50 mg L−1 NMC-111 were prepared in triplicate and magnetically stirred at 37 °C to mimic the biological exposure of B. subtilis to NMC-111. At 48 h, the aqueous phase was isolated using centrifugation (4696 × g for 20 min) followed by additional ultracentrifugation of the resulting supernatant (288 000 × g for 2 h, Beckman Coulter Optima Ultracentrifuge, SW-41 Ti Rotor). A Malvern Zetasizer NanoZS dynamic light scattering instrument was used to assess the effectiveness of sedimentation of particles to ensure no particle was present in the supernatant. The concentrations of released ions in the supernatants were then analyzed using ICP-OES.
Bacterial culture and nanoparticle exposure
B. subtilis SB491 was purchased from the Bacillus Genetic Stock Center (Columbus, OH). A frozen bacterial stock was inoculated on a lysogeny broth (LB) agar plate at 37 °C overnight. A colony was then suspended in a minimal growth medium (11.6 mM NaCl, 4.0 mM KCl, 1.4 mM MgCl2, 2.8 mM Na2SO4, 2.8 mM NH4Cl, 88.1 μM Na2HPO4, 50.5 μM CaCl2, 10 mM HEPES, and 10 mM dextrose) to reach the stationary phase with an absorbance range of 0.15–0.2 at 600 nm (OD600) in a visible spectrometer (SpectroVis® Plus, Vernier Software and Technology) before exposure to nanoparticle suspensions.
To prepare a nanoparticle stock solution, the NMC cathode material was suspended in the growth medium and sonicated for 10 minutes at 70 W. Bacterial culture at the stationary phase was then diluted to an OD600 value of 0.02 into fresh medium containing NMC suspension or ionic counterparts. The cultures were incubated at 37 °C to grow.
B. subtilis cell growth and O2 uptake
Bacterial growth in the presence of NMC or ions was assessed in a 96-well plate. Stationary phase bacterial culture in minimal medium was diluted 1: 1 (v/v) into NMC suspension or ionic solutions in individual wells. The plate was then incubated at 37 °C in a plate reader (Synergy 2 Multi-Mode Reader, BioTek, Winooski, VT), and the absorbance at 630 nm was recorded every 1 hour interval for 24 hours.
Bacterial respiration upon exposure to the battery material was assessed by monitoring the O2 uptake of the cells in a 16-vessel respirometer system as reported earlier (Respirometry Systems and Applications, Inc., Springdale, AR).8,24 In each 125 mL isobaric vessel, 100 mL of B. subtilis culture was grown in growth medium containing the battery material or corresponding amounts of dissolved ions. CO2 from bacterial respiration was removed with a capsule of 1 M KOH in the vessel, while O2 delivered to the vessel was monitored with a pressure sensor.
Single cell electrophoresis for DNA damage of B. subtilis
Single cell electrophoresis on nanoparticle or metal ion-treated B. subtilis cells was conducted following the protocols published earlier with modifications.25,26 Ten microliters of the nanoparticle-treated cell suspension was mixed with 100 μL of 0.5% low-melting agarose (LMA) solution. Immediately after mixing, 40 μL of the suspension was pipetted onto a well of a comet assay microscope slide (Travigen®) and spread evenly. The gel was allowed to solidify at 4 °C. A lysozyme-LMA layer containing 0.5% of lysozyme solution was placed on top of the first layer of gel and was incubated at 4 °C to solidify. The assembled slide was then transferred to a 37 °C incubator for 30 minutes. Each slide was then immersed in a lysing solution (2.5 M NaCl, 100.0 mM EDTA,10.0 mM Tris-HCl, and 1% sodium N-lauryl sarcosine, 1% Triton® X-100, pH 10.0) at room temperature for 1 hour in the dark, followed by a 2 hour incubation in an enzyme digestion solution (2.5 M NaCl, 10.0 mM EDTA, 10.0 mM Tris-HCl, and 0.5 mg mL−1 proteinase K, pH 7.4) at 37 °C. Electrophoresis was performed in a neutral electrophoresis buffer (sodium acetate and Tris, pH 9.0) at 12 V for 30 minutes. The slide was then washed and dehydrated in 1.0 M ammonium acetate in ethanol followed by absolute ethanol and was left at room temperature to dry in the dark. Freshly prepared DMSO solution (5% DMSO and 10 mM NaH2PO4) was used to rehydrate the stack of microgel. The DNA was then stained with 20.0 μL of 1.0 μM YOYO-1 in 5% DMSO. The slide was left to air dry in the dark for 5 minutes before coverslips were placed. Each stack of microgel was imaged under a fluorescence microscope (100×, λex = 491 nm, λem = 509 nm). ImageJ software was used to analyze the DNA tail lengths.
B. subtilis spore and surfactin secretion analysis
Positive control for spore formation was carried out by growing B. subtilis in Difco Sporulation Medium (DSM) (8 g Difco® bacto nutrient broth, 1 g KCl, and 0.12 g MgSO4·7H2O in 1 L MQ water, pH 7.6). After autoclaving and prior to use, 1.0 mL each of sterile solutions of 1.0 M Ca(NO3)2, 0.010 M MnCl2, and 1.0 mM FeSO4 were added to the medium. B. subtilis colonies from an LB agar plate were inoculated in DSM and incubated at 37 °C and 250 × g for approximately 48 hours.
To image spores in both spore-positive control and nanoparticle-treated cultures, optical microscopy with the differential staining method was used according to an established method.27 Once cells were harvested by centrifugation at 750 × g for 10 minutes and resuspended in ultrapure water, a 10 μL aliquot was placed on a glass slide and allowed to air dry. B. subtilis on the microscope slide was then heat fixed and covered with a piece of filter paper flooded with 5% malachite green, and placed above boiling water for 5 minutes. After malachite green was rinsed off with water, the B. subtilis cells were counterstained with0.25% safranin O for 90 seconds, rinsed with water, and allowed to air dry. Samples were imaged under an oil immersion lens at 100×. With this method, spores were stained greenish blue, and vegetative cells were stained red. Bright-field microscopic images were then analyzed manually by counting the total number of cells and the number of spores, and a spore-to-cell ratio was reported.
Surfactin secretion from B. subtilis in a high Mn2+ environment was investigated by LCMS (UHD Accurate-Mass Q-TOF, Agilent 6540) from B. subtilis cultures grown in different growth media at 37 °C for 48 hours. The negative control cultures were grown in LB medium (2.5 g LB in 100 mL ultrapure water). For a high Mn2+ growth medium, LB doped with 0.072 mg L−1 Mn2+ was used. Five-microliter samples of supernatants from bacterial cultures after centrifugation (3220 × g, 4 °C, 10 min) were separated on a reverse phase C18 column (Agilent, Eclipse Plus, 2.1 × 50 mm, 1.8 μm) and analyzed by electrospray ionization (positive ion mode, gas temp 325 °C, source/fragmentor 100 V, capillary voltage 4000 V, 100–1700 m/z). Samples were separated with an isocratic elution of 60% solvent A (95: 5 H2O: ACN with 0.1% ammonium acetate) at 0.40 mL min−1 for 1 min followed by a linear gradient of 40–70% solvent B (95 : 5 ACN : H2O with 0.1% ammonium acetate) over 15 minutes, then a linear gradient of 70–100% B for 2 minutes, and then isocratic elution at 100% B for 2 min. The identity of surfactin was confirmed by comparing the exact mass, retention time, and fragmentation spectra (35 V) to a purchased surfactin standard (Sigma Aldrich). The sample mass spectra of surfactin are shown in Fig. S3.† The data were analyzed with the Agilent Mass Hunter Quantitative Analysis suite. The extracted ion chromatograms were integrated and the area of the peak was normalized to the OD600 value of each culture.
Results and discussion
Dissolution and solution speciation analysis
An earlier study evaluating the toxicity mechanism of NMC to a Gram-negative model bacterium, Shewanella oneidensis, has indicated that the ions dissolved from NMC, in particular, the dissolved Ni and Co ions, are largely responsible for the toxicity to the bacterium, and the dissolution reached equilibrium within an hour.8 Therefore, it is important to characterize the dissolution behavior of NMC in B. subtilis growth medium. ICP-OES analysis of the supernatant from a 5 mg L−1 NMC suspension, shown in Fig. 1(a), indicates that significantly higher concentrations of Li and Ni ions were released compared to those of Mn and Co, in agreement with the incongruent dissolution of such a MO2 nanosheet framework reported earlier.8,28 Molar concentration analysis of the species also indicates that a higher percentage of Ni (26.3%) was dissolved from the NMC bulk material, in comparison to Mn(3.8%) and Co (9.0%).
Fig. 1.
(a) Dissolved ion concentrations from a 5 mg L−1 NMC suspension in B. subtilis minimal growth medium over 48 hours by ICP-OES analysis (error bars indicate the standard deviation, n = 3); (b) speciation simulation of solution species (patterned bars indicating free ions).
To better understand the solution chemistry of NMC dissolution in B. subtilis growth medium, we performed a solution speciation simulation using Visual MINTEQ (https://vminteq.lwr.kth.se/), a chemical solution equilibrium model. Fig. 1(b) shows the major species present (>1%) as a percentage of each dissolved ion from Fig. 1(a) in the given minimal medium composition for B. subtilis. The patterned bars indicate the free ion form of each metal. Therefore, the simulation shows that for all four elements, more than 85% of each stayed in solution as free 2+ ions.
Impact of NMC on B. subtilis growth and respiration
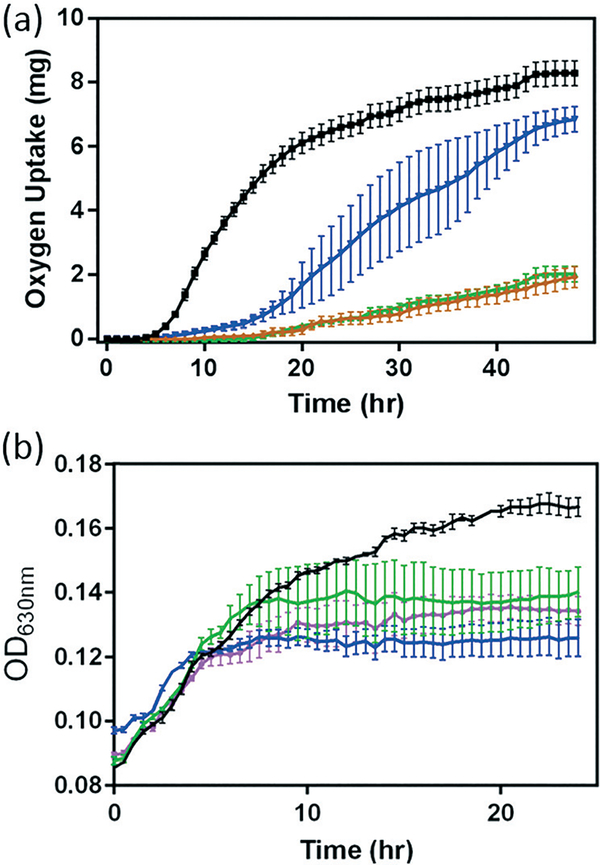
The impact of NMC nanomaterial on B. subtilis was investigated with respirometry and growth curves. Respiration, a functional parameter which assesses the metabolic activity of bacterial cultures, probes a different end-point compared to the growth assay examining optical turbidity that resulted from changes in biomass. Respirometry monitors the oxygen uptake by bacterial cultures in pressure-controlled vessels for an extended period of time.8,16,24 In the absence of a nanomaterial, the respiration profile (black symbol in Fig. 2) of the B. subtilis culture resembles that of a healthy growth curve, because the amount of O2 consumed by the bacterial culture is proportional to the number of metabolically active cells. However, in the presence of 5 mg L−1 NMC nanoparticles, the respirometry curve is significantly altered. Instead of a distinctive lag–exponential–stationary phase profile, the respirometry curve is rather flat with a gradual increase in O2 consumption. In contrast to the delayed respiration response in S. oneidensis with 5 mg L−1 NMC, where the cell culture eventually still reached a similar level of O2 uptake level,8 when incubated with NMC, B. subtilis never reached the same level of O2 consumption, even when monitored beyond 48 hours. This observation indicates that B. subtilis and S. oneidensis have different responses to NMC toxicity. At the same dosage level, while NMC resulted in delays in oxygen uptake during S. oneidensis growth, it severely suppressed this metabolic activity in B. subtilis.
Fig. 2.
Impacts on bacterial respiration (a) and growth (b) of B. subtilis in minimal growth medium without (black) and with 5 mg L−1 NMC (red). Error bars represent the standard deviation from three replicates.
To confirm the observed suppression in respiration, growth curves of B. subtilis in the presence of 5 mg L−1 NMC were also collected at OD630. We note that the presence of NMC as a colloid suspension could introduce optical interference to OD measurements, and the extent of interference was not additive in the presence of bacterial cells. Therefore, simple background subtraction of NMC suspension may not have fully corrected for this background. Fig. 2(b) shows that, in agreement with respirometry results, 5 mg L−1 NMC in growth medium hinders the growth of B. subtilis. The cultures are unable to recover to the same optical density as the control cultures.
Since previous studies have demonstrated that dissolved metal ions from NMC, especially Ni2+ and Co2+, are toxic to S. oneidensis, we investigate if this finding is also applicable to B. subtilis. Fig. 3 shows the responses of both B. subtilis growth and respiration to the 5 mg L−1 NMC suspension and the corresponding Ni and Co ions released from 5 mg L−1 NMC according to the ICP-OES measurements in Fig. 1(a). Since the growth curves obtained from the optical turbidity of cell cultures in the presence of Li+ and Mn2+ ions show minimal impact on B. subtilis as predicted (Fig. S4†), we focused mainly on the impact of Ni2+ and Co2+ ions in this study. In the presence of given amounts of Ni2+ and Co2+, we evaluated bacterial respiration, shown in Fig. 3(a). The oxygen uptake curves reveal that the impact on bacterial respiration of Co2+ is less significant than that of Ni2+. In the presence of 0.096 mg L−1 Co2+, the respirometry profile of B. subtilis showed a delayed lag-phase with recovery beyond 30 hours. However, Ni2+ significantly suppressed the respiration with minimal recovery. Remarkably, the O2 uptake curve of B. subtilis with Ni2+ in growth medium completely overlapped with that in the presence of 5 mg L−1 NMC. This result suggests that the Ni ion released from NMC is the major contributor to the hindered respiration of B. subtilis by NMC. The combined impact of Ni2+ and Co2+ ions on bacterial respiration also traces that indicated by the respiration curves in NMC and Ni2+ ion-containing media (plotted separately for clarity in Fig. S5†).
Fig. 3.
Response of B. subtilis cultures to NMC and selected ions dissolved from NMC monitored by oxygen uptake (a) and optical density (b) in minimal growth medium (black) or media containing0.279 mg L−1 Ni2+ (green), 0.096 mg L−1 Co2+ (blue), a combination of Ni2+ and Co2+ (purple) and 5.0 mg L−1 NMC (brown). Chloride salts were used for all the ions. Error bars represent the standard error of means from at least three replicates.
Optical turbidity measurements of B. subtilis cultures in the presence of Ni2+ and Co2+ ions, respectively, have also both resulted in reduced growth, as shown in Fig. 3(b). Due to the optical interference by the NMC colloidal suspension mentioned previously, no direct comparisons of growth curves were made between that in the presence of ions and that of the NMC suspension (i.e. no brown trace in Fig. 3(b)). However, comparing the growth curves of Ni2+ and Co2+-containing media, we observe that Co2+ ions affect growth more severely than Ni2+ ions, opposite to that observed in respirometry measurements. The results in Fig. 3 demonstrate the subtle differences in these assays. When we examine the effect of both Ni2+ and Co2+ ions, the growth curves fall in between those from the two individual ion-containing media, with no statistical differences in OD readings at the end of a 24 hour growth period.
Overall, material characterization and ion dissolution studies indicate the incongruent dissolution to produce Li, Ni and Co ions in B. subtilis growth medium, similar to that previously reported.8,28 However, medium constituents (lactate in the case of S. oneidensis media vs. dextrose in B. subtilis media) dictate that the dissolved ions mainly stay in their free ion form, which impacts B. subtilis growth and respiration continuously.
B. subtilis DNA damage by NMC
Previous studies have suggested that metal toxicity in bacterial species could be through the displacement of essential metals from their binding sites or through interactions with nucleic acids and proteins by altering their conformations.18 In addition, because cobalt is in a 3+ oxidation state in NMC, dissolution leads to ions in valence states that can induce oxidation of water or OH−, producing reactive oxygen species such as hydroxyl radicals.8 A solution ROS assay using a fluorescence probe also showed the formation of ROS species from NMC nanosheet suspensions.16 Therefore, we hypothesize that solution suspensions of NMC may induce oxidative stress and DNA damage in B. subtilis as a mechanism of toxicity.
Bacterial DNA damage induced by the presence of the NMC material was investigated with single cell gel electrophoresis, also known as the comet assay. The comet assay, a term that originated from the comet-like tails of the fragmented DNA in single cells, is a highly sensitive tool that provides direct visualization of lesions in DNA breakage and allows for quantification by comparing the damaged DNA tail lengths.29,30 Its application in genotoxicity studies of nanomaterials, primarily in mammalian cells, has been demonstrated recently.31,32 The method has recently been adapted to bacterial cells,25,33,34 yielding DNA tails less resemblant of comets but more of bolts of lightning. Yet its application in understanding genotoxicity induced by nanomaterials in bacterial species is rare.35 In our study, DNA tail lengths from individual NMC-exposed cells were compared to those of unexposed cells. Sample fluorescence microscopy images are shown in Fig. S6,† where the longer the DNA tails, the more severe the DNA damage.
Fig. 4(a) shows the results of DNA tail length comparisons from cells exposed to 0 and 5 mg L−1 NMC suspensions, as well as to the corresponding amounts of dissolved Ni2+ and Co2+ ions after 48 hours of growth. Control samples resulted in an average tail length of 5.3 ± 0.4 μm with many intact cells in fluorescence images. In contrast, 5 mg L−1 NMC-exposed B. subtilis cells had average tail lengths of 18.2 ± 0.8 μm. Statistical analysis reveals that compared to the control group, NMC exposed populations had significant DNA damage (denoted by letters “a” and “b”).
Fig. 4.
DNA comet tail length analysis from the comet assay showing DNA damage in B. subtilis when exposed to 5 mg L−1 of NMC (brown),0.279 mg L−1 Ni2+ (green), 0.096 mg L−1 Co2+ (blue), and both Ni2+ and Co2+ (purple) in a box-and-whisker plot (a) and histograms (b). Data sets with different letters (a–c) differ statistically (p < 0.05).
The results from the respirometry and growth curves in Fig. 3 suggest that Ni2+ and Co2+ ions are key contributors to NMC toxicity to B. subtilis. The comet assay results in Fig. 4 reveal that the extent of DNA damage induced by the combination of Ni2+ and Co2+ ions recapitulates that of NMC. In comparison, cells from Ni2+ and Co2+ ion-containing media resulted in average DNA tail lengths of 10 ± 1 μm and 11.3 ±0.4 μm, respectively. The two groups yielded no statistical difference with an unpaired t-test (both denoted by letter “c”). We observe that the DNA damage was more heterogeneous at the single cell level with Ni2+ ions, compared to that from Co2+ ions, as demonstrated by the standard deviations of tail lengths. In addition, Ni2+ and Co2+ ions individually, although they both induced DNA damage when compared to the control group, resulted in less DNA damage than those from 5 mg L−1 NMC (denoted by letters “a” and “c”). These results reveal that the amount of Ni2+ and Co2+ ions dissolved from 5 mg L−1 NMC caused significant DNA damage to B. subtilis, yet the extent of the damage is less than that with NMC nanoparticles. Remarkably, however, when both ions are present, as shown in the purple bar in Fig. 4(a), the resulting average of DNA tail lengths is 19 ± 1 μm, statistically identical to that of the 5 mg L−1 NMC (denoted by letter “b”). This comparison is also shown more clearly when replotted in a histogram (Fig. 4(b)).
Ni2+ has been linked to genotoxicity to both mammalian and bacterial cells by inducing oxidative DNA damage and interfering with DNA repair mechanisms in the presence of additional DNA damaging agents.36 A molecular-level genotoxicity study in E. coli has found that Ni(II) complexed to the tripeptide Gly–Gly–His in combination with hydrogen peroxide induced mutations in vitro on single-stranded DNA typical for damage by oxygen free radicals.37 Co2+ has also been reported to be mutagenic in certain bacterial strains.38 Our finding from DNA damage analysis reveals an important mechanism of toxicity by NMC to B. subtilis. Although ROS generation leading to DNA damage is often considered as a mode of nanotoxicity to bacteria,39 only a few studies have examined DNA damage directly.35,40,41 In contrast to studies that focus on intracellular ROS species, which are often transient, the comet assay probes an end-point of resulting DNA damage with high sensitivity at the single cell level.
Mn-Enriched NMC
An earlier study has reported that the Ni2+ and Co2+ ions released from nanoscale NMC materials have been the main contributors to NMC toxicity toward a model Gram-negative bacterium, S. oneidensis.8 In an effort to remediate the adverse biological impact of NMC, several NMC materials with reduced Ni and Co contents were synthesized, and their biological impact was tested against S. oneidensis.16 In that work, increasing the Mn fraction (with concurrent reduction in Ni and Co content) decreased the amount of Ni and Co ions released into solution, and this reduction mitigated the impact on S. oneidensis respiration. Therefore, we are interested in examining the toxicity of these Mn-enriched NMC nanomaterials on a Gram-positive model, B. subtilis, and in evaluating if the redesign is a viable approach across multiple biological species. We note that although the Mn-enriched NMC material studied here has not been tested in commercial batteries, a variety of NMC compositions are commercially available,7,11 which suggests the variabilities in this class of materials that have comparable performance. Two Mn-enriched NMC materials were evaluated in this study, Li0.61Ni0.23Mn0.55Co0.22O2 (55% Mn) and Li0.52Ni0.14Mn0.72Co0.14O2 (72% Mn). These materials were compared to NMC-111 (33% Mn) in terms of their biological impact.
Guided by the hypothesis that the released ions, especially Ni2+ and Co2+, in growth media are the key contributors to toxicity to B. subtilis, we analyzed the ionic species present in minimal media with 5 mg L−1 Mn-enriched NMC nanosheets after 48 hours by ICP-OES. Fig. 5 shows a comparison of dissolved ions from the three NMC variants for each of the four metals. Distinctively, Li, Ni, and Co ions present in the media decreased as the Mn fraction increased from 33% to 72% in the material lattice, while the corresponding amounts of Mn ion increased. This trend echoes that observed in the S. oneidensis growth medium.16
Fig. 5.
Ionic concentrations of species in B. subtilis minimal growth media dissolved from NMC materials containing varying amounts of Mn: 33% (light blue), 56% (medium blue), and 70% (dark blue) quantified by ICP-MS.
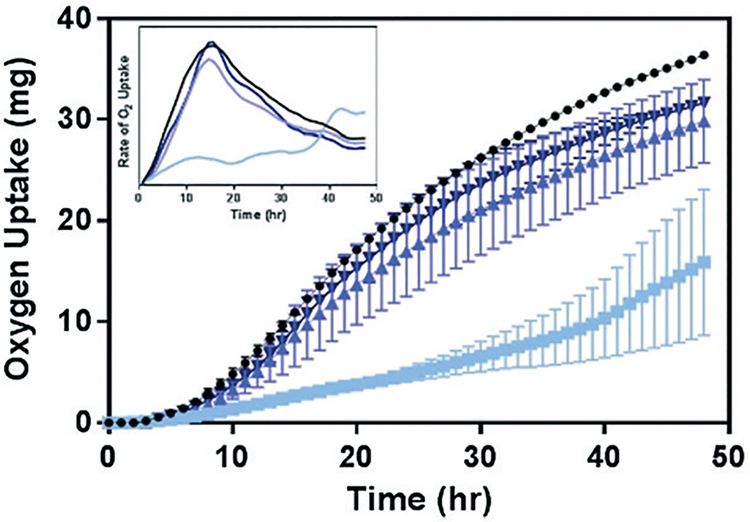
Given the impact of Ni2+ and Co2+ on the growth and respiration of B. subtilis (Fig. 3), we hypothesize that the decrease in their concentrations in media with Mn-enriched NMC variants would lead to reduced toxicity of these materials to B. subtilis. The biological impact of the Mn-enriched NMC to B. subtilis was assessed again with respirometry and compared to that of NMC-111 (33% Mn). Fig. 6 shows the oxygen uptake over a 48 hour period by B. subtilis cultures in pressure-regulated vessels with 5 mg L−1 of each NMC variants. We observe that, while 33% Mn severely suppressed the oxygen uptake by bacterial cultures, the cellular respiration profiles from cultures incubated with either 55% or 72% Mn NMC variants strongly resemble that of the control culture. The final levels of oxygen uptake at 48 hours follow the trend 33% Mn < 55% Mn ≤ 72% Mn < no NMC, with no statistical significance between 55% and 72% Mn using a t-test. We also note that the rates of oxygen uptake by cultures incubated with NMC were nearly identical among control samples and those with either 55% or 72% Mn NMC, as shown in the first derivative plot of the respirometry curves in the inset. These results support the hypothesis that Mn-enrichment in NMC that resulted in lower nickel and cobalt fractions in the material lattice reduced Ni2+ and Co2+ ion release in aqueous suspension, and hence successfully mitigated the toxicity to B. subtilis.
Fig. 6.
Oxygen uptake of cellular respiration by B. subtilis upon exposure to NMC materials containing various amounts of Mn: 33% (light blue), 56% (medium blue), and 70% (dark blue). Inset: The first derivative of the respirometry curves to indicate the rate of oxygen uptake.
Additional biological impact of Mn-enrichment on B. subtilis
Previous studies on the impact of metal ions on bacterial species have revealed that Mn2+ is an essential component in the growth environment of B. subtilis, affecting cellular activities and pathways of various processes, such as mobility, endospore and biofilm formation, and antibiotic production.42,43 Therefore, to thoroughly evaluate any additional unintended impact of NMC redesign, we examine B. subtilis sporulation and surfactin production activities as biological indicators.
Sporulation is an adaptive response to environmental changes for certain bacterial species, such as those in the genera of Bacillus.44–46 In the sporulation process, vegetative cells undergo asymmetric division, where chromosomes are copied and protected by a special cortex. Endospores are usually formed under harsh environmental conditions, such as heat, radiation, or nutrient depletion. The mechanism allows cells to re-germinate with the conserved genetic information when the growth environment is restored.44,47 Mn2+ has been identified as an essential mineral for endospore formation48 by controlling the proper functions of phosphoglycerate phosphomutase (PGA-mutase), an enzyme needed for optimal sporulation in growth medium.49,50
We examined bacterial cultures after 48 hour incubation in growth media containing Mn-enriched NMC nanomaterials using differential staining to identify spores versus vegetative cells under a bright field optical microscope. Malachite green, retained by the spore coat, stains the spores green, while Gram stain using safranin O stains the vegetative cells red. As shown in Fig. 7, image analysis reveals that there is an increasing trend from 33% to 70% Mn-enriched NMC samples in the spore-to-cell ratios. Cultures exposed to growth media containing NMC with 70% Mn resulted in the most endospores per view, yet the large majority of cells are still in the vegetative state. The spore-to-cell ratio from these images shows a significant difference from that from the 33% Mn samples. The results confirm that the increased Mn in NMC nanosheets which led to increased Mn2+ concentrations in media indeed induces sporulation in B. subtilis.
Fig. 7.
Spore-to-cell ratio from microscopy image analysis of cells exposed to 5 mg L−1 of Mn-enriched varieties of NMC.
Surfactin is one of the well-known biosurfactant lipopeptides secreted by B. subtilis and has potent antimicrobial activities.51 Certain metal cations, especially manganese and iron, have been identified to spur its production and have been applied in industrial bioreactors to induce the bio-synthesis of surfactin.52 We hypothesize that the redesigned Mn-enriched NMC may also induce surfactin production in B. subtilis cultures. Therefore, surfactin secretion was compared by growing B. subtilis cultures in 0.072 mg L−1 Mn2+ containing medium, corresponding to the amount of Mn2+ dissolved from 5 mg L−1 of the 72% Mn-enriched NMC to avoid potential interference of NMC nanosheets in the LCMS analysis. A feature was detected at m/z 1036.7,51 corresponding to a C15 isoform of surfactin, which was then compared to unexposed cultures. Fig. 8 shows that Mn doping induces 8.5 times more surfactin production from B. subtilis. Surfactin is used as a signalling molecule, which can induce biofilm formation,53 and has a moderate ability to chelate metal ions.54 Therefore, surfactin secretion on its own has been suggested as a defense mechanism against an increase in metal concentrations, especially in Mn2+, as observed here.
Fig. 8.
Surfactin production from B. subtilis cultures grown in media with and without 0.072 mg L−1 Mn2+ (unpaired t-test, p < 0.0001).
The sporulation and surfactin production studies highlight the ecological sensitivity to material compositions. In this case, the observed impacts are in agreement with those predicted from ion dissolution studies. These observations also suggest the need for evaluating the environmental impact of nanomaterials with mixed cultures over longer periods of time to examine the long-term impact of nanomaterials on microbial communities.
Conclusions
In this study, we expand our understanding of the biological impact of an emerging class of nanomaterials as potential environmental stressors, complex metal oxides, NMC, to a Gram-positive model bacterium, B. subtilis. Although the environmental impact, especially on microbial communities, of nanomaterials composed of simple metal oxides has been more extensively studied,21 these new materials pose more complex challenges where multiple factors in determining environmental impacts need to be considered. Together with our earlier publications in this area, the current study demonstrates that the incongruent dissolution behavior of the material, as well as solution constituents, is an important factor when dissolved ions are major contributors to the biological impact. In addition, this is our first step towards a mechanistic understanding of genotoxicity induced by complex metal oxides where transition metal oxidative states may pose additional oxidative stress in an organism as a means for DNA damage. This preliminary study calls for a more in-depth investigation of the mechanism of intracellular ROS generation and chemical evidence of DNA damage. Lastly, our effort in material redesign, which has successfully reduced toxicity to another model bacterium, S. oneidensis, has been shown to also be effective in mitigating toxicity to B. subtilis. Yet, additional biological consequences, such as increased sporulation and antibiotic secretion, have also been observed. Although thorough material redesign also needs to take functionality into consideration, this proof-of-concept effort demonstrates the importance of a fundamental understanding of biological impacts from all aspects of material redesign.
Supplementary Material
Environmental significance.
Nanoscale complex metal oxides are a class of emerging nanomaterials widely used in energy storage and catalysis applications. Although these materials are produced in increasing quantities, there are few incentives or infrastructure for recycling currently, which poses questions for their environmental impact upon disposal at the end of their life cycle. This work examines the biological impact of a class of lithium intercalating battery cathode materials to a model environmentally beneficial bacterium in terms of bacterial growth, metabolic respiration, spore formation, and antibiotic production, as well as DNA damage. This initial attempt at probing the genotoxicity of these nanomaterials guides us towards an understanding of their toxicity mechanism at a molecular level and provides insights into material redesign.
Acknowledgements
This work was supported by the National Science Foundation under the Center for Sustainable Nanotechnology (CSN) (CHE-1503408). The CSN is part of the Centers for Chemical Innovation Program. S. L. M. acknowledges the NIH Chemistry−Biology Interface Training Grant 5T32GM008700–18. T. P. acknowledges the Lindstrom Research Fund through Augsburg University. The authors gratefully acknowledge Dr. Michael P. Schwartz for helpful discussion.
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c8en00995c
Conflicts of interest
There are no conflicts to declare.
References
- 1.Patel P, Improving the Lithium-Ion Battery, ACS Cent. Sci,2015, 1(4), 161–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etacheri V, Marom R, Elazari R, Salitra G and Aurbach D, Challenges in the Development of Advanced Li-Ion Batteries: A Review, Energy Environ. Sci, 2011, 4(9), 3243. [Google Scholar]
- 3.Goodenough JB and Park K-S, The Li-Ion Rechargeable Battery: A Perspective, J. Am. Chem. Soc, 2013, 135(4), 1167–1176. [DOI] [PubMed] [Google Scholar]
- 4.Goodenough JB and Kim Y, Challenges for Rechargeable Li Batteries, Chem. Mater, 2010, 22(3), 587–603. [Google Scholar]
- 5.Wang Y and Cao G, Developments in Nanostructured Cathode Materials for High-Performance Lithium-Ion Batteries, Adv. Mater, 2008, 20(12), 2251–2269. [Google Scholar]
- 6.Meng J, Niu C, Liu X, Liu Z, Chen H, Wang X, Li J, Chen W, Guo X and Mai L, Interface-Modulated Approach toward Multilevel Metal Oxide Nanotubes for Lithium-Ion Batteries and Oxygen Reduction Reaction, Nano Res, 2016, 9(8), 2445–2457. [Google Scholar]
- 7.Andre D, Kim S-J, Lamp P, Lux SF, Maglia F, Paschos O and Stiaszny B, Future Generations of Cathode Materials: An Automotive Industry Perspective, J. Mater. Chem. A, 2015, 3(13), 6709–6732. [Google Scholar]
- 8.Hang MN, Gunsolus IL, Wayland H, Melby ES, Mensch AC, Hurley KR, Pedersen JA, Haynes CL and Hamers RJ, Impact of Nanoscale Lithium Nickel Manganese Cobalt Oxide (NMC) on the Bacterium Shewanella Oneidensis MR-1, Chem. Mater, 2016, 28(4), 1092–1100. [Google Scholar]
- 9.Sun Y, Liu N and Cui Y, Promises and Challenges of Nanomaterials for Lithium-Based Rechargeable Batteries, Nat. Energy, 2016, 1(7), 16071. [Google Scholar]
- 10.Martha SK, Sclar H, Szmuk Framowitz Z, Kovacheva D,Saliyski N, Gofer Y, Sharon P, Golik E, Markovsky B and Aurbach D, A Comparative Study of Electrodes Comprising Nanometric and Submicron Particles of LiNi0.50Mn0.50O2, LiNi0.33Mn0.33Co0.33O2, and LiNi0.40Mn0.40Co0.20O2 Layered Compounds, J. Power Sources, 2009, 189(1), 248–255. [Google Scholar]
- 11.Olivetti EA, Ceder G, Gaustad GG and Fu X, Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals, Joule, 2017, 1(2), 229–243. [Google Scholar]
- 12.Dewulf J, der Vorst G, Denturck K, Van Langenhove H, Ghyoot W, Tytgat J and Vandeputte K, Recycling Rechargeable Lithium Ion Batteries: Critical Analysis of Natural Resource Savings, Resour., Conserv. Recycl, 2010, 54(4), 229–234. [Google Scholar]
- 13.Notter DA, Gauch M, Widmer R, Wäger P, Stamp A, Zah R and Althaus H-J, Contribution of Li-Ion Batteries to the Environmental Impact of Electric Vehicles, Environ. Sci. Technol, 2010, 44(17), 6550–6556. [DOI] [PubMed] [Google Scholar]
- 14.Larcher D and Tarascon J-M, Towards Greener and More Sustainable Batteries for Electrical Energy Storage, Nat. Chem, 2014, 7(1), 19–29. [DOI] [PubMed] [Google Scholar]
- 15.Doğangün M, Hang MN, Troiano JM, McGeachy AC,Melby ES, Pedersen JA, Hamers RJ and Geiger FM, Alteration of Membrane Compositional Asymmetry by LiCoO2 Nanosheets, ACS Nano, 2015, 9(9), 8755–8765. [DOI] [PubMed] [Google Scholar]
- 16.Gunsolus IL, Hang MN, Hudson-Smith NV, Buchman JT, Bennett JW, Conroy D, Mason SE, Hamers RJ and Haynes CL, Influence of Nickel Manganese Cobalt Oxide Nanoparticle Composition on Toxicity toward Shewanella Oneidensis MR-1: Redesigning for Reduced Biological Impact, Environ. Sci.: Nano, 2017, 4(3), 636–646. [Google Scholar]
- 17.Bozich J, Hang M, Hamers R and Klaper R, Core Chemistry Influences the Toxicity of Multicomponent Metal Oxide Nanomaterials, Lithium Nickel Manganese Cobalt Oxide, and Lithium Cobalt Oxide to Daphnia Magna: Battery Material Core Chemistry Influences Aquatic Toxicology, Environ. Toxicol. Chem, 2017, 36(9), 2493–2502. [DOI] [PubMed] [Google Scholar]
- 18.Bruins MR, Kapil S and Oehme FW, Microbial Resistance to Metals in the Environment, Ecotoxicol. Environ. Saf, 2000, 45(3), 198–207. [DOI] [PubMed] [Google Scholar]
- 19.Malik A, Metal Bioremediation through Growing Cells, Environ. Int, 2004, 30(2), 261–278. [DOI] [PubMed] [Google Scholar]
- 20.Valentine NB, Bolton H, Kingsley MT, Drake GR, Balkwill DL and Plymale AE, Biosorption of Cadmium, Cobalt, Nickel, and Strontium by aBacillus Simplex Strain Isolated from the Vadose Zone, J. Ind. Microbiol, 1996, 16(3), 189–196. [Google Scholar]
- 21.McKee MS and Filser J, Impacts of Metal-Based Engineered Nanomaterials on Soil Communities, Environ. Sci.: Nano, 2016, 3(3), 506–533. [Google Scholar]
- 22.Okubo M, Hosono E, Kim J, Enomoto M, Kojima N, Kudo T, Zhou H and Honma I, Nanosize Effect on High-Rate Li-Ion Intercalation in LiCoO2 Electrode, J. Am. Chem. Soc, 2007, 129(23), 7444–7452. [DOI] [PubMed] [Google Scholar]
- 23.Lu Z, MacNeil DD and Dahn JR, Layered Li[Ni[Sub x] Co[Sub 1−2x]Mn[Sub x]]O[Sub 2] Cathode Materials for Lithium-Ion Batteries, Electrochem. Solid-State Lett, 2001, 4(12), A200. [Google Scholar]
- 24.Feng ZV, Gunsolus IL, Qiu TA, Hurley KR, Nyberg LH, Frew H, Johnson KP, Vartanian AM, Jacob LM and Lohse SE, et al. , Impacts of Gold Nanoparticle Charge and Ligand Type on Surface Binding and Toxicity to Gram-Negative and Gram-Positive Bacteria, Chem. Sci, 2015, 6(9), 5186–5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solanky D and Haydel SE, Adaptation of the Neutral Bacterial Comet Assay to Assess Antimicrobial-Mediated DNA Double-Strand Breaks in Escherichia Coli, J. Microbiol. Methods, 2012, 91(2), 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olive PL and Banáth JP, The Comet Assay: A Method to Measure DNA Damage in Individual Cells, Nat. Protoc, 2006, 1(1), 23–29. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds J, Moyes R and Breakwell DP, Differential Staining of Bacteria: Endospore Stain, in Current Protocols in Microbiology, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2009, vol. Appendix 3, p. Appendix 3J. [DOI] [PubMed] [Google Scholar]
- 28.Bennett JW, Jones D, Huang X, Hamers RJ and Mason SE, Dissolution of Complex Metal Oxides from First-Principles and Thermodynamics: Cation Removal from the(001) Surface of Li(Ni 1/3 Mn 1/3 Co 1/3)O2, Environ. Sci. Technol, 2018, 52(10), 5792–5802. [DOI] [PubMed] [Google Scholar]
- 29.Azqueta A and Collins AR, The Essential Comet Assay: A Comprehensive Guide to Measuring DNA Damage and Repair, Arch. Toxicol, 2013, 87(6), 949–968. [DOI] [PubMed] [Google Scholar]
- 30.Liao W, McNutt MA and Zhu W-G, The Comet Assay: A Sensitive Method for Detecting DNA Damage in Individual Cells, Methods, 2009, 48(1), 46–53. [DOI] [PubMed] [Google Scholar]
- 31.Armand L, Tarantini A, Beal D, Biola-Clier M, Bobyk L, Sorieul S, Pernet-Gallay K, Marie-Desvergne C, Lynch I and Herlin-Boime N, et al. Long-Term Exposure of A549 Cells to Titanium Dioxide Nanoparticles Induces DNA Damage and Sensitizes Cells towards Genotoxic Agents, Nanotoxicology, 2016, 10(7), 913–923. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson HL, The Comet Assay in Nanotoxicology Research, Anal. Bioanal. Chem, 2010, 398(2), 651–666. [DOI] [PubMed] [Google Scholar]
- 33.Singh N, Manshian B, Jenkins GJS, Griffiths SM, Williams PM, Maffeis TGG, Wright CJ and Doak SH, NanoGenotoxicology: The DNA Damaging Potential of Engineered Nanomaterials, Biomaterials, 2009, 30(23–24), 3891–3914. [DOI] [PubMed] [Google Scholar]
- 34.El-Sonbaty SM and El-Hadedy DE, Combined Effect of Cadmium, Lead, and UV Rays on Bacillus Cereus Using Comet Assay and Oxidative Stress Parameters, Environ. Sci. Pollut. Res, 2015, 22(5), 3400–3407. [DOI] [PubMed] [Google Scholar]
- 35.Anas A, Jiya J, Rameez MJ, Anand PB, Anantharaman MR and Nair S, Sequential Interactions of Silver-Silica Nanocomposite (Ag-SiO NC) with Cell Wall, Metabolism and Genetic Stability of Pseudomonas Aeruginosa, a Multiple Antibiotic-Resistant Bacterium, Lett. Appl. Micro-biol, 2013, 56(1), 57–62. [DOI] [PubMed] [Google Scholar]
- 36.Hartwig A, Current Aspects in Metal Genotoxicity, BioMetals, 1995, 8(1), 3–11. [DOI] [PubMed] [Google Scholar]
- 37.Tkeshelashvili LK, Reid TM, Mi-Bride TJ and Loe LA, Nickel Induces a Signature Mutation for Oxygen Free Radical Damage1, 1993, vol. 53. [PubMed] [Google Scholar]
- 38.Pagano DA and Zeiger E, Conditions for Detecting the Mutagenicity of Divalent Metals In Salmonella Typhimurium, Environ. Mol. Mutagen, 1992, 19(2), 139–146. [DOI] [PubMed] [Google Scholar]
- 39.Suresh AK, Pelletier DA and Doktycz MJ, Relating Nanomaterial Properties and Microbial Toxicity, Nanoscale, 2013, 5(2), 463. [DOI] [PubMed] [Google Scholar]
- 40.Hwang ET, Lee JH, Chae YJ, Kim YS, Kim BC, Sang B-I and Gu MB, Analysis of the Toxic Mode of Action of Silver Nanoparticles Using Stress-Specific Bioluminescent Bacteria, Small, 2008, 4(6), 746–750. [DOI] [PubMed] [Google Scholar]
- 41.Guo Z, Xue J, Liu T, Song X, Shen Y and Wu H, Antibacterial Mechanisms of Silica/Polydopamine/Silver Nanoparticles against Gram Positive and Gram Negative Bacteria, Micro Nano Lett, 2014, 9(3), 210–214. [Google Scholar]
- 42.Mhatre E, Troszok A, Gallegos-Monterrosa R, Lindstädt S,Hölscher T, Kuipers OP and Kovács ÁT, The Impact of Manganese on Biofilm Development of Bacillus Subtilis, Microbiology, 2016, 162(8), 1468–1478. [DOI] [PubMed] [Google Scholar]
- 43.Stöckel S, Meisel S, Böhme R, Elschner M, Rösch P and Popp J, Effect of Supplementary Manganese on the Sporulation of textitBacillus Endospores Analysed by Raman Spectroscopy: Raman Study of the Effect of Manganese on the Sporulation of Bacillus Endospores, J. Raman Spectrosc, 2009, 40(11), 1469–1477. [Google Scholar]
- 44.Stephenson K and Lewis R, Molecular Insights into the Initiation of Sporulation in Gram-Positive Bacteria: New Technologies for an Old Phenomenon, FEMS Microbiol. Rev, 2005, 29(2), 281–301. [DOI] [PubMed] [Google Scholar]
- 45.Nicholson WL, Roles of Bacillus Endospores in the Environment, Cell. Mol. Life Sci, 2002, 59(3), 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y, Wu H-J, Lin L, Zhu Q, Borriss R and Gao X-W, A Plasmid-Born Rap-Phr System Regulates Surfactin Production, Sporulation and Genetic Competence in the Heterologous Host, Bacillus Subtilis OKB105, Appl. Microbiol. Biotechnol, 2015, 99(17), 7241–7252. [DOI] [PubMed] [Google Scholar]
- 47.McKenney PT, Driks A and Eichenberger P, The Bacillus Subtilis Endospore: Assembly and Functions of the Multilayered Coat, Nat. Rev. Microbiol, 2012, 11(1), 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Charney J, Fisher WP and Hegarty CP, Manganese as an Essential Element for Sporulation in the Genus Bacillus,J. Bacteriol, 1951, 62(2), 145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasantha N and Freese E, The Role of Manganese in Growth and Sporulation of Bacillus Subtilis, J. Gen. Microbiol, 1979, 112(2), 329–336. [DOI] [PubMed] [Google Scholar]
- 50.Hachisuka Y, Imura H, Nakata A and Hattori E, Sporulation Promoting Factor in Vegetative Cells of Bacillus Subtilis, Microbiol. Immunol, 1983, 27(12), 1005–1019. [DOI] [PubMed] [Google Scholar]
- 51.Vater J, Kablitz B, Wilde C, Franke P, Mehta N and Cameotra SS, Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry of Lipopeptide Biosurfactants in Whole Cells and Culture Filtrates of Bacillus Subtilis C-1 Isolated from Petroleum Sludge, Appl. Environ. Microbiol, 2002, 68(12), 6210–6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooper DG, Macdonald CR, Duff SJ and Kosaric N, Enhanced Production of Surfactin from Bacillus Subtilis by Continuous Product Removal and Metal Cation Additions, Appl. Environ. Microbiol, 1981, 42(3), 408–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cawoy H, Mariutto M, Henry G, Fisher C, Vasilyeva N, Thonart P, Dommes J and Ongena M, Plant Defense Stimulation by Natural Isolates of Bacillus Depends on Efficient Surfactin Production, Mol. Plant-Microbe Interact, 2014, 27(2), 87–100. [DOI] [PubMed] [Google Scholar]
- 54.Mulligan CN, Yong RN and Gibbs BF, Heavy Metal Removal from Sediments by Biosurfactants, J. Hazard. Mater, 2001, 85(1–2), 111–125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.