Abstract
Object.
Pallidal deep brain stimulation (DBS) is a treatment option for those with early-onset dystonia. However, there are limited data on long-term outcome and treatment complications. The authors report on the short- and long-term effects of pallidal DBS in a cohort of patients with early-onset dystonia.
Methods.
Fourteen consecutive pediatric patients with early-onset dystonia were systematically evaluated and treated. The duration of follow-up ranged from 16 to 84 months.
Results.
There were no immediate postoperative complications. At last follow-up, 12 of the 14 patients displayed a significant decline in the Burke-Fahn-Marsden Dystonia Rating Scale motor subscale score, with an average decrease of 62% ± 8.4%. The most common hardware complication was lead fracture (14.3%).
Conclusions.
These data provide further evidence that DBS is a safe and effective treatment for those with early-onset dystonia.
Keywords: early-onset dystonia, deep brain stimulation, functional neurosurgery
DEEP brain stimulation (DBS) of the globus pallidus interna (GPi) has become a therapeutic option for patients with early-onset dystonias that do not respond to medical management.7,10,15,20 A variety of case series and prospective trials have been reported focusing on the short-term effects of GPi DBS in dystonia.2,5,14,16 In addition, some case series provide data on the long-term effects of DBS, up to 8 years in a single case of primary generalized dystonia and up to 10 years in a case of DYT11 dystonia.3,9,10 Although these papers have provided further evidence regarding the efficacy of pallidal DBS for the treatment of dystonia, many questions regarding optimal patient selection and, in particular, effective programming parameters remain. We report on a consecutive case series of pediatric patients with focal, segmental, or generalized dystonia, and their genotypes, clinical outcomes, and stimulation parameters, to contribute to the understanding of which patients experience the greatest benefit from DBS and how to program their systems most effectively while conserving battery life.
Methods
We reviewed the clinical records of 14 consecutive patients with dystonia who were implanted with bilateral pallidal DBS by the same neurosurgical team (E.N.E. and A.C.D.) and followed by the same neurological movement disorders team (M.T.P., L.R.P., M.T.H., and N.S.). Prior to surgery and at every subsequent visit, dystonia severity was determined using the motor subscale of the Burke-Fahn-Marsden Dystonia Rating Scale (BFMDRS). For all patients, clinical outcome data were available for at least 16 months after surgery. The mean length of follow-up was 50.8 months (median 52.5 months, range 16–84 months). The local institutional review board of Partners HealthCare approved the study.
Patient Selection
Diagnosis and confirmation that the patients had early-onset focal, segmental, or generalized dystonia was determined according to published criteria by a neurologist trained in movement disorders (N.S.).6 Age of onset was confirmed by review of medical history and/or available medical records. The patients underwent DBS if their symptoms were not well controlled with pharmacological management. In addition, patient 14 underwent 1 treatment with electromyography-guided botulinum toxin injections that did not produce significant benefit. Patient 14 and the patient’s parents elected to proceed with DBS. All patients underwent MRI of the brain prior to surgery. Twelve patients were screened for the DYT1 mutation. The remaining 2 cases were not screened, as they were homozygous for the PANK2 mutation. Patients 1, 4, and 6 were evaluated after clinical genetic testing for DYT6 was available and also underwent screening for its presence. Nine patients underwent neuropsychological testing to ensure that there were no underlying undiagnosed cognitive difficulties or untreated psychiatric illnesses. Six patients did not undergo neuropsychological testing: those who had DYT1 dystonia (Cases 7–9), the PANK2 mutation (Cases 10–11), and 1 patient with developmental delay of unknown origin (Case 12).
Neurosurgical Procedure
Each patient underwent bilateral frame-based MRI and microelectrode-guided stereotactic implantation of DBS leads into the GPi. The patients were lightly sedated but alert throughout the surgical procedure. Briefly, quad-ripolar electrodes (3387, Medtronic Neurological) were placed using standard stereotactic techniques and intra-operative microelectrode recordings. Coordinates were calculated using stereotactic CT obtained on the day of surgery, fused with preoperative thin-slice MR images. The location of the GPi was confirmed using intraoperative microelectrode recordings (Alpha Omega). The permanent electrodes were tested using macrostimulation to ensure there were no side effects. Each patient received either MRI or CT postoperatively to verify correct lead placement in the GPi and exclude complications. The patients recovered in-house and were typically discharged after 1 or 2 days. Two weeks later, each patient returned for implantation of either 1 Kinetra or 2 Soletra implantable pulse generators (IPGs; Medtronic Neurological). In some patients, the IPGs were implanted as the same time as the intracranial electrodes to avoid a second surgical procedure. The choice of whether to implant 1 Kinetra or 2 Soletra IPGs was made on a case-by-case basis, depending on the age and body habitus of the patient. This procedure was conducted with the patient under general anesthesia, and the patient was discharged on the same day as the surgery.
Deep Brain Stimulation Programming
Deep brain stimulation programming was conducted in a standardized fashion. Initial programming began 3–5 weeks after implantation of the intracranial leads. At the first programming session, each contact was analyzed in a monopolar configuration to determine tolerance to increasing voltage (to a maximum of 4.0 V or to a lesser voltage if intolerable effects occurred). The second, third, and fourth programming sessions occurred 4, 8, and 12 weeks after the initial session, respectively. At subsequent sessions, adjustments were made to improve clinical symptoms and/or reduce adverse events; these adjustments typically consisted of a gradual increase in voltage. After 4 consecutive monthly programming sessions, patients were transitioned to returning after 3 months for 2 additional visits, at which time DBS system integrity was verified and further changes in voltage, pulse width, or frequency were made, if warranted based on symptoms. Afterward, patients were evaluated once every 6 months for routine follow-up consisting of a neurological examination, analysis of DBS system integrity, and a check of IPG capacity to identify those who were in need of IPG replacement.
Statistical Analysis
The Wilcoxon signed-rank test for matched pairs was used to compare BFMDRS motor subscale scores at baseline and at 6 months and last follow-up evaluation. A Pearson correlation coefficient was calculated to analyze the relationship between clinical outcome and demographic features. A p value < 0.05 was considered statistically significant.
Results
Clinical Characteristics
Clinical characteristics of those who underwent DBS are summarized in Table 1. Eleven patients had generalized dystonia, including 3 with the DYT1 mutation and 2 with the PANK2 mutation. Three remaining patients had focal dystonia. The mean disease duration was 65.4 ± 11.7 months (5.4 years), with a range of 12–168 months (1–14 years). The mean age at surgery was 14.6 ± 1 years (range 9–25 years).
TABLE 1:
Clinical features of case series patients*
| Case No. | Sex | Age at Onset (yrs) | Age at Surgery (yrs) | Symptom Duration Before DBS (mos) | Dystonia Distribution | DYT1 | DYT6 | PANK2 |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 0.5 | 9 | 102 | primary generalized | − | − | ND |
| 2 | F | 4 | 13 | 108 | primary generalized | − | NA | ND |
| 3 | M | 10 | 17 | 84 | primary generalized | − | NA | ND |
| 4 | M | 7 | 11 | 48 | primary generalized | − | − | ND |
| 5 | M | 8 | 15 | 84 | primary generalized | − | NA | ND |
| 6 | F | 12 | 25 | 168 | primary generalized | − | − | ND |
| 7 | M | 11 | 12 | 21 | primary generalized | + | ND | ND |
| 8 | M | 14 | 17 | 36 | primary generalized | + | ND | ND |
| 9 | M | 9 | 12 | 36 | primary generalized | + | ND | ND |
| 10 | M | 9 | 17 | 96 | secondary generalized | ND | ND | + |
| 11 | M | 9 | 15 | 60 | secondary generalized | ND | ND | + |
| 12 | M | 12 | 13 | 12 | primary focal w/global developmental delay | − | NA | ND |
| 13 | M | 12 | 15 | 36 | primary focal | − | NA | ND |
| 14 | F | 11 | 13 | 24 | primary focal | − | ND | ND |
− = negative; + = positive; NA = testing not available at the time of clinical evaluation; ND = testing not determined.
Twelve patients (Cases 1–9, 12–14) had normal MRI of the brain. Patients 10 and 11, who were homozygous for the PANK2 mutation, demonstrated the characteristic “eye of the tiger” sign in the globus pallidus bilaterally on MRI.8 The short-term effect (up to 12 months) of DBS in patients 10 and 11 was previously reported.19
Surgical Complications
A total of 28 leads were implanted in the initial surgical procedures. There were no intracranial hemorrhages. Five patients (36%) experienced 7 hardware-related adverse events. Four of the leads fractured, in 2 patients, giving a lead fracture rate of 14.3%. One lead fracture occurred after head trauma and no inciting cause could be identified for the others.
Two patients (Cases 1 and 6) developed infections at the site of an IPG pocket. Patient 5 underwent unilateral explantation of the IPG, lead extensions, and intracranial lead, and treatment with antibiotics. The remaining lead was programmed and provided control of symptoms to the contralateral side. Patient 6 developed drainage from the infraclavicular IPG pocket following its insertion. The patient was treated with antibiotics and during this time, underwent programming with a good result (44% decline in motor score). Seven months after IPG insertion, the patient exhibited drainage from the right scalp incision and all hardware was removed. Within 48 hours of hardware removal, symptoms returned, resulting in difficulty walking. Three months after battery removal, the patient displayed a complete recurrence of generalized dystonia, with a BFMDRS motor subscale score of 41. The patient is scheduled for reimplantation.
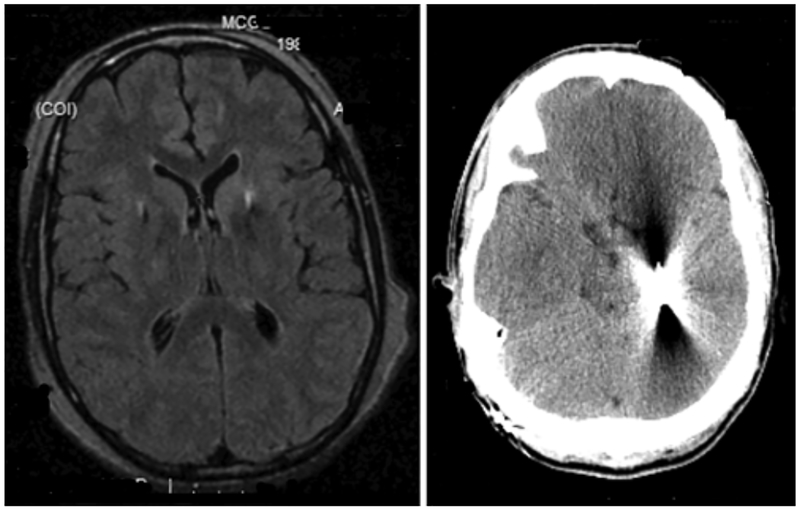
Patient 10, with a PANK2 mutation, developed complications 7 years after his initial surgery. Immediately after the initial surgery, MRI showed appropriate placement of intracranial electrodes in the GPi bilaterally. His BFMDRS motor subscale score declined by 13%, and he remained stable, with medication and stimulator adjustments, until he began having complex partial seizures 7 years later. An extended ambulatory electroencephalogram showed interictal and ictal focal epileptiform abnormalities in bilateral temporal lobes, with some suggestion of earlier involvement in the right anterior to midtemporal leads. A CT scan showed lead migration, with the left lead terminating in the left temporal lobe (Fig. 1). Impedance testing of the left-sided system triggered a complex partial seizure similar to a previously witnessed event. The stimulator was turned off and he did not experience further seizures. The hardware was then removed and replaced.
Fig. 1.
Case 10. Radiographic images of electrode location. Left: Axial MR image depicting GPi location of both DBS leads immediately after the original surgery. Right: Axial CT scan depicting lead migration in the left hemisphere
BFMDRS Motor Subscale Score
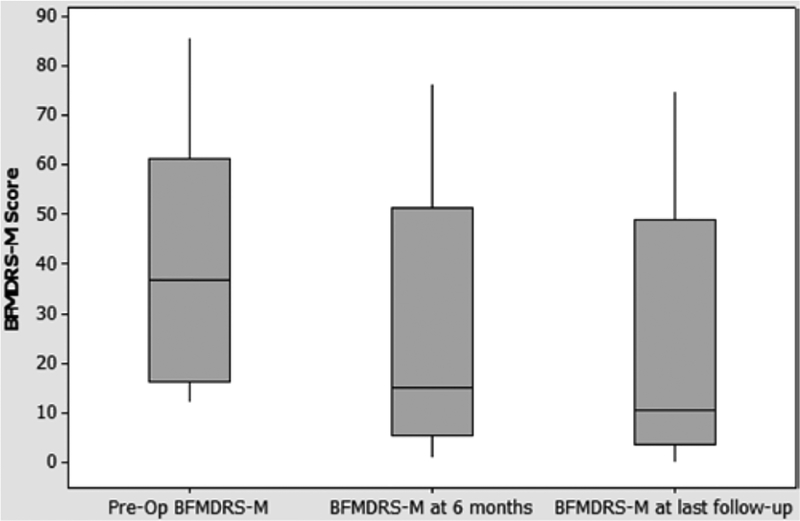
At 6 months after DBS, a point at which others have reported an improvement in cervical dystonia, BFMDRS motor subscale scores were available for 10 patients (Table 2).12,17 Of those 10 patients, 8 displayed a reduction in BFMDRS motor subscale scores. When all 10 were analyzed together, the average decline in BFMDRS motor subscale score was 38% ± 12.0% (Fig. 2). A Wilcoxon signed-rank test revealed that the decline was statistically significant (z = 2.37, p = 0.0089). At last follow-up, 12 of the 14 patients displayed a decline in BFMDRS motor subscale score, with an average decrease of 62% ± 8.4% (Fig. 2). A Wilcoxon signed-rank test revealed that this decline was also statistically significant (z = 2.72, p = 0.0033). Cases 3 and 11 did not display any reduction in BFMDRS motor subscale score at last follow-up.
TABLE 2:
Summary of the BFMDRS motor subscale scores preoperatively, at 6 months, and at the last follow-up evaluation
| BFMDRS Motor Subscale Score | |||||
|---|---|---|---|---|---|
| Case No. | Preop | 6 Mos | % Decline | At Last Follow-Up (Mos) | % Decline |
| 1 | 72 | NA | NA | 18 (29) | 75 |
| 2 | 73 | 48 | 34 | 49 (65) | 33 |
| 3 | 58 | 61.5 | none | 61.5 (20) | none |
| 4 | 17.5 | 16 | 9 | 2 (56) | 89 |
| 5 | 37 | 2 | 95 | 2 (56) | 95 |
| 6 | 42 | 35 | 17 | 23.5 (16) | 44 |
| 7 | 37 | 11 | 70 | 4 (80) | 9 8 |
| 8 | 30 3 |
1 | 97 | 0 (37) | 0 0 |
| 9 | 40 4 |
NA | NA | 13 (39) | 67 |
| 10 | 86 8 |
76.5 | 11 | 75 (84) | 13 |
| 11 | 30 | NA | NA | 49 (84) | none |
| 12 | 13 | 14 | none | 8 (49) | 38 |
| 13 | 12 | 6.5 | 46 | 4 (64) | 67 |
| 14 | 12 | NA | NA | 8 (32) | 33 |
Fig. 2.
Boxplot of BFMDRS motor subscale (-M) score of entire cohort at 6 months after DBS and at last follow-up. The line in the middle of each box represents the median value.
In our patients who did respond to stimulation, there was no consistent correlation of disease duration and outcome (n = 14, Pearson correlation = −0.273). Excluding patients with focal dystonia from the analysis, the correlation of disease duration and DBS outcome in our patients was still not consistent (n = 11, Pearson correlation = −0.403).
Dystonia Distribution
Of the 11 patients with generalized dystonia (Cases 1–11), 8 (Cases 1, 4, 5, 7–11) showed a reduction in the number of affected body regions at last follow-up. Patients 2 and 6 exhibited a decline in overall dystonia severity, but still displayed dystonia in the same body regions after DBS (Table 3).
TABLE 3:
Summary of dystonia distribution before and after DBS surgery at last follow-up
| Case No. | Preop Distribution | Postop Distribution |
|---|---|---|
| 1 | neck, trunk, rt & lt arm, rt & lt leg | trunk, lt arm |
| 2 | mouth, speech/swallowing, neck, rt & lt arm, trunk, rt & lt leg | same as preop |
| 3 | mouth, speech/swallowing, neck, trunk, rt & lt arm, rt & lt leg | same as preop |
| 4 | neck, lt arm, rt & lt leg | speech/swallowing, trunk |
| 5 | trunk, rt arm, rt & lt leg | rt & lt leg |
| 6 | neck, trunk, rt & lt arm, rt leg | same as preop |
| 7 | neck, trunk, rt & lt arm, rt & lt leg | trunk, lt arm, rt leg |
| 8 | neck, trunk, rt arm, rt & lt leg | none |
| 9 | neck, trunk, lt arm, rt & lt leg | rt leg |
| 10 | mouth, speech/swallowing, neck, trunk, rt & lt arm, rt & lt leg | mouth, speech/swallowing, trunk, rt & lt arm, rt & lt leg |
| 11 | rt & lt arm, rt & lt leg | rt & lt arm, rt leg |
| 12 | trunk | same as preop |
| 13 | trunk | same as preop |
| 14 | lt leg | same as preop |
Programming Parameters
Programming parameters at last follow-up varied between patients (Table 4). Twelve patients were on monopolar settings (Cases 1–5 and 7–13), 1 patient was on bipolar settings (Case 14), and 1 patient had all hardware explanted due to an infection (Case 6).
TABLE 4:
Implantable pulse generator parameters at last follow-up
| Case No. | Rt Hemisphere | Lt Hemisphere | ||||||
|---|---|---|---|---|---|---|---|---|
| Contact | Amp (V) | Pulse Width (ms) | Frequency (Hz) | Contact | Amp (V) | Pulse Width (ms) | Frequency (Hz) | |
| 1 | explanted | C+ 2− | 2.2 | 90 | 145 | |||
| 2 | C+ 5− 6− | 2.5 | 120 | 140 | C+ 3− | 3.4 | 120 | 140 |
| 3 | C+ 6− | 2.2 | 90 | 130 | C+ 2− | 2.2 | 90 | 130 |
| 4 | C+ 7− | 3.0 | 90 | 130 | C+ 1− | 3.3 | 90 | 130 |
| 5 | C+ 6− | 2.8 | 90 | 130 | C+ 2− | 2.9 | 90 | 130 |
| 6 | explanted | explanted | ||||||
| 7 | C+ 5− | 3.6 | 150 | 60 | C+ 1− 2− | 3.6 | 150 | 60 |
| 8 | C+ 5− | 3.2 | 120 | 60 | C+ 1− | 3.5 | 120 | 60 |
| 9 | C+ 6− 7− | 3.1 | 120 | 60 | C+ 3− | 3.6 | 120 | 60 |
| 10 | C+ 6− 5− | 3.3 | 180 | 185 | C+ 1− 2− | 2.8 | 180 | 185 |
| 11 | C+ 6− 5− | 4.0 | 210 | 160 | C+ 1− 2− 3− | 3.8 | 120 | 160 |
| 12 | C+ 6− 7− | 3.0 | 90 | 130 | C+ 1− 2− | 2.4 | 120 | 130 |
| 13 | C+ 6− 5− | 2.8 | 150 | 130 | C+ 1− 2− | 3.0 | 150 | 130 |
| 14 | 0+ 6− 7− | 3.5 | 90 | 130 | off | off | off | off |
Discussion
In our series of 14 consecutive patients with medically refractory pediatric-onset dystonia treated with pallidal DBS, a reduction in BFMDRS motor subscale scores was noted at 6 months in 8 of the 10 patients for whom 6-month follow-up data were available. At last follow-up, which ranged from 16 to 84 months after initial surgery, 12 patients showed a reduction in BFMDRS motor subscale score. In the remaining 2 patients, 1 had an early-onset generalized dystonia of uncertain etiology (Case 3) and 1 had generalized dystonia due to the PANK2 mutation (Case 11). For Case 3, the unknown underlying cause of the dystonia may have made his symptoms refractory to improvement with GPi stimulation. For Case 11, the effects of the underlying PANK2 mutation may have counteracted any effect of DBS. However, given the small sample size and unique underlying pathophysiology in each of these patients, no clear conclusion can be drawn.
Programming parameters, established based on patient response, varied. The majority of patients were on monopolar settings. In a monopolar configuration, the single active intracranial contact is established as the negative pole (cathode) and the IPG case serves as the positive pole (anode). Thus, a monopolar setting creates a wide electric field in which stimulation spreads equally in all directions. In a bipolar configuration, 1 active intracranial lead serves as the cathode and a second active intracranial lead serves as the anode. Thus, a bipolar setting results in less current spread and a more narrow area of stimulation.
The most common complication was lead fracture; the next most common was infection. This rate of hardware complication is consistent with previous reports.10,21 Regarding the patient with delayed lead migration resulting in partial seizures during stimulation (but none with the stimulator turned off), a literature review yields only cases of immediate postoperative seizures (typically in the first 24–48 hours, up to 7 days).4 In a case series of 161 patients who underwent DBS for movement disorders, seizures were associated with identifiable brain abnormalities on imaging such as hemorrhage, edema, or ischemia in 86%.18 In that series, 86% of seizures occurred within 48 hours postoperatively, and 100% occurred within 1 week. While lead migration has been reported in the literature, this appears to be the first case of delayed onset of complex partial seizures due to lead migration.1,13 The discrepancy between left lead migration and electroencephalography evidence of earlier seizure activity in the right temporal leads may be related to difficulty in interpretation due to significant myogenic artifact and the unclear onset of the ictus, or to rapid bilateral spread of seizure activity.
Previous reports have suggested that response to pallidal DBS is improved in younger patients and those with shorter disease duration. Specifically, Isaias et al. in 2011 showed that patients with a younger age at surgery (< 27 years) and shorter disease duration (≤ 17 years) displayed 87% improvement in BFMDRS motor subscale score at 1 year compared with 63% improvement in patients with older age at surgery (≥ 27 years) and longer disease duration (> 17 years).10 In the series reported in this paper, no such correlations were identified. However, this series represents a heterogeneous patient population and may lack statistical power to identify a relationship between age at surgery and disease duration with outcome. Our sample size was also too small to accurately determine a relationship of DYT1 status with DBS outcome.
Conclusions
Our case series provides added evidence that treatment with DBS is an effective and safe long-term treatment for cases of early-onset dystonia that do not respond to medical management. The most common complication was lead fracture. Programming parameters varied considerably between patients, although the majority both tolerated and responded well to monopolar configurations. Multicenter prospective trials, focusing on patient selection and the establishment of an optimal, systematic programming protocol, are needed to help improve patient outcome.
Disclosure
This study was funded by a grant (no. P50NS037409) to Dr. Sharma from the National Institute of Neurological Disorders and Stroke, NIH.
Abbreviations:
- BFMDRS
Burke-Fahn-Marsden Dystonia Rating Scale
- DBS
deep brain stimulation
- GPi
globus pallidus interna
- IPG
implantable pulse generator
References
- 1.Boviatsis EJ, Stavrinou LC, Themistocleous M, Kouyialis AT, Sakas DE: Surgical and hardware complications of deep brain stimulation. A seven-year experience and review of the literature. Acta Neurochir (Wien) 152:2053–2062, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Cersosimo MG, Raina GB, Piedimonte F, Antico J, Graff P, Micheli FE: Pallidal surgery for the treatment of primary gen eralized dystonia: long-term follow-up. Clin Neurol Neurosurg 110:145–150, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Cif L, Vasques X, Gonzalez V, Ravel P, Biolsi B, Collod-Beroud G, et al. : Long-term follow-up of DYT1 dystonia patients treated by deep brain stimulation: an open-label study. Mov Dis ord 25:289–299, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Coley E, Farhadi R, Lewis S, Whittle IR: The incidence of seizures following deep brain stimulating electrode implantation for movement disorders, pain and psychiatric conditions. Br J Neurosurg 23:179–183, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Egidi M, Franzini A, Marras C, Cavallo M, Mondani M, Lavano A, et al. : A survey of Italian cases of dystonia treated by deep brain stimulation. J Neurosurg Sci 51:153–158, 2007 [PubMed] [Google Scholar]
- 6.Fahn S, Bressman SB, Marsden CD: Classification of dystonia. Adv Neurol 78:1–10, 1998 [PubMed] [Google Scholar]
- 7.Ghosh PS, Machado AG, Deogaonkar M, Ghosh D: Deep brain stimulation in children with dystonia: experience from a tertiary care center. Pediatr Neurosurg 48:146–151, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Gregory A, Polster BJ, Hayflick SJ: Clinical and genetic delineation of neurodegeneration with brain iron accumulation. J Med Genet 46:73–80, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruber D, Kühn AA, Schoenecker T, Kivi A, Trottenberg T, Hoffmann KT, et al. : Pallidal and thalamic deep brain stimulation in myoclonus-dystonia. Mov Disord 25:1733–1743, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Isaias IU, Alterman RL, Tagliati M: Deep brain stimulation for primary generalized dystonia: long-term outcomes. Arch Neurol 66:465–470, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Isaias IU, Volkmann J, Kupsch A, Burgunder JM, Ostrem JL, Alterman RL, et al. : Factors predicting protracted improvement after pallidal DBS for primary dystonia: the role of age and disease duration. J Neurol 258:1469–1476, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Jeong SG, Lee MK, Kang JY, Jun SM, Lee WH, Ghang CG: Pallidal deep brain stimulation in primary cervical dystonia with phasic type: clinical outcome and postoperative course. J Korean Neurosurg Soc 46:346–350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenney C, Simpson R, Hunter C, Ondo W, Almaguer M, Davidson A, et al. : Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J Neurosurg 106:621–625, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Krause M, Fogel W, Kloss M, Rasche D, Volkmann J, Tronnier V: Pallidal stimulation for dystonia. Neurosurgery 55:1361–1370, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Kupsch A, Benecke R, Müller J, Trottenberg T, Schneider GH, Poewe W, et al. : Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 355:1978–1990, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Loher TJ, Capelle HH, Kaelin-Lang A, Weber S, Weigel R, Burgunder JM, et al. : Deep brain stimulation for dystonia: outcome at long-term follow-up. J Neurol 255:881–884, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Ostrem JL, Racine CA, Glass GA, Grace JK, Volz MM, Heath SL, et al. : Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology 76:870–878, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Pouratian N, Reames DL, Frysinger R, Elias WJ: Comprehensive analysis of risk factors for seizures after deep brain stimulation surgery. Clinical article. J Neurosurg 115:310–315, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Timmermann L, Pauls KAM, Wieland K, Jech R, Kurlemann G, Sharma N, et al. : Dystonia in neurodegeneration with brain iron accumulation: outcome of bilateral pallidal stimulation. Brain 133:701–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, et al. : Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 352:459–467, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Yianni J, Nandi D, Shad A, Bain P, Gregory R, Aziz T: Increased risk of lead fracture and migration in dystonia compared with other movement disorders following deep brain stim ulation. J Clin Neurosci 11:243–245, 2004 [DOI] [PubMed] [Google Scholar]