Abstract
Sex differences in cardiovascular diseases can be classified as those which are specific to one sex and those that differ in incidence, prevalence, etiology, symptomatology, response to treatment, morbidity and mortality in one sex compared to the other. All sex differences in cardiovascular conditions have their basis in the combined expression of genetic and hormonal differences between women and men. This chapter addresses how advances in understanding basic mechanisms of hormone responses, imaging diagnostics, integration of genomics and proteomics have advanced diagnosis and improved outcomes for cardiovascular conditions, apart from those related to pregnancy, that are more prevalent in women. These conditions include occlusive and non-occlusive coronary artery disease, microvascular nonocclusive ischemic disease, spontaneous coronary artery dissection, diseases of the cardiac muscle including heart failure and takotsubo cardiomyopathy, and conditions related to neurovascular dysregulation including hot flashes and night sweats associated with menopause and effects of exogenous hormones on vascular function. Improvement in technologies allowing for noninvasive assessment of neuronally mediated vascular reactivity will further improve our understanding of the basic etiology of the neurovascular disorders. Consideration of sex, hormonal status, and pregnancy history in diagnosis and treatment protocols will improve prevention and outcomes of cardiovascular disease in women as they age.
Keywords: atrial fibrillation, cardiomyopathy, endothelial dysfunction, coronary microvascular dysfunction, 17β-estradiol, heart failure, hot flashes, ischemic nonobstructive coronary artery disease, INOCA, menopause, migraine, SCAD, spontaneous coronary artery dissection, takotsubo, vasomotor symptoms
A. Introduction
The fundamental origin of all sex differences in health and disease results from the presence of the sex chromosomes (XX for female and XY for males). Genes on these chromosomes influence expression of genes on the autosomes, as well as direct the development of the reproductive organs, and ultimately gonadal production of the sex steroids estrogen and testosterone.[1, 2] An important interaction between the sex chromosomes and sex hormones is through the gene for the androgen receptor located on the X chromosome. Variations in this gene will have greater effects in males who carry only one copy of the X chromosome. Females have two copies of the X chromosome but one is inactivated reducing the gene dosage effect, which also results in mosaic distribution of the genetic variants on each X in various tissues of the body. These differences in genes and hormones provide the basis for all sex differences in cardiovascular regulatory mechanisms in health and disease. It is essential when considering expression of various cardiovascular diseases to recall several points: 1) receptors for the sex steroids are present in most tissue of the body, but their relative expression may vary; 2) estrogen is a metabolic product of testosterone, thus both testosterone and estrogen are present in men and women but in different proportions; 3) concentrations of the sex steroids vary across the life span as evidence by increased production at puberty, increases during pregnancy, and decreases in women during the transition to menopause. The dramatic changes in hormone-mediated physiological changes in the cardiovascular (and other) systems of a woman’s body with pregnancy demonstrate the activational effects of the sex steroids; that is, reversible phenotypes expressed in the presence of the hormones but diminished in their absence. Specific aspects of cardiovascular physiology associated with pregnancy are discussed in a chapter by Fu. In this section, the focus will be on conditions of the coronary circulation, the heart and a group of conditions that require autonomic neurovascular regulation. A final section will discuss cardiovascular issues related to hormonal treatments in women.
B. The coronary circulation
Obstructive coronary artery disease:
Coronary artery disease (CAD) is the leading cause of death in women in the United States, affecting 6.6 million women a year with higher mortality rates than their male counterparts, from 1985 to 2011.[3] A recent reduction in these rates has been attributed to national and international efforts which focused on fostering understanding, awareness and application of evidence-based therapy to women. Women who present with obstructive CAD are generally older than their male counterparts, have more cardiovascular comorbidities and have a higher incidence of adverse cardiovascular outcomes including mortality following acute myocardial infarction (MI).[4] Occlusion of the coronary arteries is caused by disruption of plaque resulting from accumulation of infiltrating macrophages and low-density lipoproteins within the vessel wall. Formation of thrombus at the site of the disrupted plaque occludes the artery limiting blood flow to the myocardium resulting in MI. The pathophysiology of CAD and MI is distinctive in women, who are less likely than men to present with plaque rupture.[5] The reasons for this difference remain unclear.
Chest pain is the most prevalent symptom of acute MI in both sexes. However, women are more likely to present with atypical symptoms, including pain in the upper back and neck, fatigue, nausea and vomiting. These differences in symptom presentation, along with the longstanding notion that CAD and acute MI is uncommon in women accounts, in part, for delays in seeking medical advice, poor recognition of symptoms, under-diagnosis, and suboptimal treatment in women.[6–8] Women with MI also are less likely to undergo coronary angiograms when compared to men, despite that this test is the diagnostic and therapeutic gold standard. When obstructive lesions exist, revascularization is most often the treatment of choice in the clinical setting of both ST-elevation MI (STEMI), and high risk non-ST-elevation MI (NSTEMI).[9, 10] However, revascularization of the occluded artery may be more challenging women due to bleeding at the site of access, and small and more tortuous coronary arteries. Future studies should aim at improving the treatment of obstructive CAD in women.
Coronary microvascular dysfunction:
Approximately 30–50% of women who undergo coronary angiography for evaluation of chest pain do not have obstructive CAD.[11, 12] Ischemia in the setting of no obstructive coronary artery disease (INOCA) can be due to coronary microvascular dysfunction (CMD). Clinical suspicion for CMD should be high in patients that present with persistent chest pain, ischemic changes on noninvasive stress testing and no obstructive CAD by invasive or noninvasive diagnostic methods. Once thought to be a benign condition, CMD has been found to have a 2.5% annual rate of major adverse cardiovascular events (MACE) including stroke, heart failure or MI. [13, 14]
Risk factors for CMD are similar to traditional coronary risk factors for obstructive CAD such as smoking, hypertension, hyperlipidemia, and diabetes.[15] In addition, sex-specific risk factors have been identified such as autoimmune diseases [16], treatments for breast cancer with radiation to the chest wall area, as well as certain chemotherapy agents.[17, 18] The gold standard diagnostic test for CMD is an invasive coronary reactivity test (CRT) that uses vasoactive agents to functionally test the coronary microvasculature. During CRT, a Doppler guide wire is positioned in the proximal left anterior descending artery for sequential infusion of the vasoactive drugs adenosine, acetylcholine and nitroglycerin to assess microvascular and macrovascular (epicardial) endothelial and non-endothelial function. Coronary flow reserve (CFR) is the calculated ratio of peak flow velocity to baseline flow after adenosine has been administered. Data from the National Heart Lung and Blood-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study found that women with no obstructive CAD who have a low CFR to adenosine are at higher risk for major adverse cardiac events as compared to those with normal CFR.[14]
Noninvasive imaging of those with nonobstructive coronaries with cardiac magnetic resonance imaging (CMRI) can be used to evaluate the coronary microvascular at both stress (adenosine) and rest. The characteristic finding to diagnoses CMD by CMRI is a decreased perfusion pattern in the subendocardial region of the heart, which can also be seen in the affected coronary distribution of obstructed arteries. CMRI also has prognostic value in women with CMD.[19, 20] Cardiac positron emission tomography, and transthoracic Doppler echocardiography are other noninvasive techniques that measure CFR, but they do not replace diagnosis by intracoronary CRT.
As with obstructive CAD, treatment of CMD is focused on lifestyle modification and aggressive risk factor control. In addition to its diagnostic utility, CRT allows helps to guide treatment based on which abnormal pathway(s) (i.e. endothelium or smooth muscle) has been found. Medications to treat endothelial dysfunction include statins, angiotensin-converting enzyme inhibitors, and low dose aspirin. Medications to treatment nonendothelial dysfunction include beta-blockers, alpha-beta blockers, and nitrates.[21]
Spontaneous coronary artery dissection:
Spontaneous coronary artery dissection (SCAD) is a cause of acute coronary syndrome that affects women (81–92% of all cases) more than men. Women present with SCAD at an average age of between 42–53 years (range can be 17–71 years or older) and have minimal other cardiovascular risk factors.[22–27]
SCAD is caused by formation of a hematoma within the wall of one or more coronary artery(ies) leading to obstruction of coronary artery blood flow to the myocardium. A tear in the artery is not always observed either because it may be too small to detect or may have occurred before the formation of the hematoma. Associated conditions for SCAD include extracoronary vascular abnormalities (i.e. fibromuscular dysplasia, coronary tortuosity), pregnancy and the postpartum state, extreme emotion/stress/exercise, and connective tissue disease.
SCAD is diagnosed on coronary angiography and previously considered rare with reported prevalence of 0.07–1.1%.[28] However, recent technological advancements in the catheterization laboratory with the ability to visualize intramural hematoma of the coronary wall using intravascular imaging techniques such as intravascular ultrasound or optical coherence tomography has improved both awareness and diagnosis of this condition. Recent studies indicate that SCAD is the etiology of as many as 35% of myocardial infarctions among women <50 years of age, and that SCAD is the most common etiology of pregnancy associated MI.[26, 29]
Accurate diagnosis of SCAD is important as management strategies are different than those for coronary artery disease due to atherosclerosis. While atherosclerotic MI is best managed with revascularization, primarily with stenting, a similar approach for acute SCAD is associated with a high risk of complications.[30] SCAD can self-heal in the majority of conservatively managed patients. Therefore, as long as a patient has normal or near-normal distal coronary blood flow and is clinically stable, acute SCAD can be managed without revascularization.[30]
While patients with history of SCAD fare better survival rates that matched controls with atherosclerosis [23], there is a significant burden of major adverse cardiac events including recurrent SCAD, recurrent myocardial infarction, recurrent chest pain, and heart failure.[22–24] Precipitators of recurrent SCAD and underlying mechanisms of SCAD itself remain poorly understood requiring continued research into the causation, genetic predisposition, diagnostic and prognostic indicators, and treatment options for patients with recurrent SCAD with other comorbidities (e.g. migraine.)[31]
C. The heart
Atrial fibrillation:
Atrial fibrillation (AF) is due to chaotic atrial electrical activity with dysfunctional or nonexistent contraction of the atria; this can be accompanied with irregular and/or rapid ventricular contraction predisposing to heart failure. However, the most serious and immediate risk from AF is the association of stroke; one proposed mechanism is due to formation and dislodgement of thrombus in the poorly contractile and often dilated left atrial appendage.[32] This risk can be reduced with chronic anticoagulation; appropriate patients to treat can be selected using risk stratification techniques including the CHA2DS2VASc and HAS-BLED scores to predict risk of stroke and bleeding, respectively.[33] Of note, the female sex is considered an important risk factor for stroke associated with atrial fibrillation, and is accounted for on the CHA2DS2VASc tool.[34, 35] Despite many existing hypotheses, the exact pathophysiology as to why the female sex increases stroke risk in the setting of AF is not understood.
The proposed causes of AF are numerous. One hypothesis is that it may involve changes in function of the potassium channels on the atrial muscle leading to prolongation of the electrical refractory period. Variants in genes encoding subunits of the potassium channel, one of which is located on the X chromosome, may account for sex-differences in etiology, prevalence of and mortality from AF.[36, 37] Antiarrhythmic drugs that prolong the QT interval increase risk for torsade-de-pointes in women and was the reason some such drugs were removed from the market.[38] Estrogenic compounds modulate potassium currents in atrial tissue [39, 40], but the relationship between endogenous and exogenous estrogen on incidence of AF in women is not established.
Radiofrequency catheter ablation is effective in reducing AF in women. However, women are often older at the time of presentation, experience more bleeding events during procedures, and higher recurrence rates than men, which may be due to the older age and stage of disease when the procedures are performed.[41–43]
Heart failure:
HF, an important cause of morbidity and mortality [44], is caused by abnormal ventricular diastolic filling, reduced systolic ejection of blood, or a combination of both. It is a clinical diagnosis with a complex range of etiologies and mechanisms. Patients can present with symptoms such as dyspnea, fatigue, exercise intolerance, and fluid retention including edema and ascites.[45] Defining the prevalence of HF is challenging due to varying definitions and diagnosis methods. Regardless, over one million US hospitalizations in 2010 coded HF as the first discharge diagnosis, and 1 in 8 deaths mention HF on the death certificate.[44]
Left ventricular (LV) HF can be subcategorized into HF with ejection fraction (EF) preserved (HFpEF) and HF where EF is reduced (HFrEF). The severity of HF is stratified based on structural changes to the heart and severity of symptoms and response to treatment, or by severity of symptoms (Table 27.1 ).[45]
Table 27.1.
Classification of heart failure by structure and function
| ACCF AHA stages of heart failure | NYHA functional classification | |||
|---|---|---|---|---|
| A | At high risk for heart failure without structural heart disease or symptoms | None | ||
| B | Structural heart disease without heart failure signs or symptoms | I | No limitation of physical activity | Ordinary physical activity does not cause heart failure symptoms |
| C | Structural heart disease with prior or current heart failure symptoms | I | No limitation of physical activity | Ordinary physical activity does not cause heart failure symptoms |
| II | Slight limitation of physical activity | Comfortable at rest, but ordinary activity causes heart failure symptoms | ||
| III | Marked limitation of physical activity | Comfortable at rest, but less than ordinary activity causes heart failure symptoms | ||
| IV | Unable to cany on any physical activity without heart failure symptoms | Heart failure symptoms at rest | ||
| D | Refractory heart failure requiring specialized interventions | IV | Unable to cany on any physical activity without heart failure symptoms | Heart failure symptoms at rest |
Table adapted from Yancy et al. [49]
ACCF American College of Cardiology Foundation. AHA American Heart Association, NYHA New York Heart Association
In general, HFrEF refers to a patient whose LVEF is <40%. These patients may also have concurrent diastolic dysfunction with increased LV filling pressures. The diagnosis of HFpEF refers to a patient whose LVEF is >50%. In HFpEF, the diastolic relaxation of the LV is impaired.[45] On clinical evaluation, patients with HFpEF exhibit evidence of arterial and LV stiffness with chronically elevated LV filling pressures. At autopsy, patients with HFpEF have more cardiac hypertrophy, myocardial fibrosis, epicardial coronary atherosclerosis and coronary microvascular rarefaction than matched controls possibly accounting for the observed clinical findings.[46]
There is debate regarding the threshold for categorizing mid-range EF, and whether there is a need for sex-specific criterion for phenotyping HF. For example, patients with HF and LVEF of 41–49% are considered borderline HFpEF, because the clinical characteristics, response to treatment, and outcomes are similar to those who have HFpEF.[45] The risk of HFpEF is similar between men and women after adjustment for age and risk factors. [47] However, HFrEF is less frequent among women than men.[47] A recent study suggests women have more reverse remodeling with standard medical therapy than men (≥15% reduction in LV end-systolic volume index in those with HFrEF) resulting in fewer hospitalizations for HF, and fewer cardiovascular and all-cause mortality.[48]
Associated factors and predisposing conditions for HF include coronary artery disease (major etiology of HFrEF), diabetes, cigarette smoking, hypertension, vascular heart disease, hypertension, obesity and age. There are also less common causes of HF such as SCAD, myocarditis, infiltrative disease, substance abuse (e.g., alcohol, methamphetamines), chemotherapy induced (e.g., doxorubicin, trastuzumab), familial cardiomyopathy, which can be challenging to diagnose. Some patients have an unknown etiology of HF.[44, 45]
Current therapy known to benefit those with HFrEF include beta blockers and angiotensin converting enzyme inhibitors (ACEI) and aldosterone antagonists. However, depending on specific patient needs and clinical characteristics, additional therapies such as hydralazine-nitrates, ivabradine, implantable cardiac defibrillators/chronic resynchronization therapy, angiotensin receptor-neprilysin inhibitors, and advanced therapies including mechanical support and cardiac transplantation can be considered for patient management.[45, 47, 49]
Women have historically been underrepresented in clinical trials for the treatment of HFrEF, representing approximately a quarter of patients in studies examining the effects of beta-blockers on this condition.[50]. Women appear to benefit from most recommended goal-directed medical therapies in HFrEF, although the degree of benefit may differ slightly from men. In a meta-analysis of studies reporting the effects of beta-blockers that including 13 833 patients (24% women) from 11 trials, the benefits of beta-blockers remained significant in all ages categories, with no interaction according to sex.[50] In a cohort of 27,837 patients with congestive heart failure (53% women) who filled prescriptions for ACEI, women had improved survival, although to a lesser degree than men [HR 0.80 (0.76–0.85) for women vs. HR 0.71 (0.67–0.75) for men].[51] Although these findings remain to be confirmed in a randomized-controlled trial, a similar population study found that women diagnosed with heart failure appeared to have improved survival when they filled a prescription for angiotensin receptor blockers (ARB) as opposed to an ACEI, whereas men did not [HR 0.69 (0.59–0.80) for women vs. HR 1.10 (0.95 to 1.30) for men].[52]
The first left-ventricular assist devices (LVAD) developed for HF could only be used in patients with a body surface area >1.5m2, limiting their use in women with advanced HFrEF, who accounted for under a quarter of patients enrolled in early trials.[53–55] Despite current design-related limitations, women who received LVAD therapy while awaiting cardiac transplantation had greater risk of cerebrovascular complications than men but similar mortality rates to men (19% vs. 20%, p=0.89).[56, 57] Newer, smaller LVAD models have recently been developed and may allow more women with advanced HFrEF to benefit from this therapy.
Therapies for patients with HFpEF focus on reducing of volume overload and blood pressure, with a goal systolic blood pressure of less than 130 mmHg. [45, 49] HFpEF patients who have a lower heart rate at hospital dismissal are observed to have lower all-cause mortality.[58]
Takotsubo cardiomyopathy:
Also known as stress cardiomyopathy or broken heart syndrome, takostubo cardiomyopathy is as an acute onset cardiomyopathy first described in 1990.[59] Diagnostic criteria include characteristic wall motion abnormalities on left ventricular angiogram or cardiac echocardiogram extending beyond a single epicardial vascular distribution in the absence of atherosclerotic disease, most often akinesis of the apical and mid myocardial walls with hyperkinesis of the basal segments, resembling its namesake, a Japanese octopus or fish trap. Post-menopausal women account for 90% of diagnosed patients, and takotsubo cardiomyopathy is classically triggered by emotional stress or physical triggers, including anesthesia, infection and respiratory distress.[59] Potential pathophysiologic mechanisms include high catecholamine serum levels, leading to an overstimulation of β2-adrenoceptors and myocardial stunning, acute multivessel epicardial coronary artery spasm, and acute LV mid-chamber and/or outflow tract obstruction[60–63]
Clinical presentation for takotsubo cardiomyopathy is often indistinguishable from an acute MI, with over 75% of patients presenting with chest pain. Nearly all patients have ischemic ECG changes, and may present as STEMI. Serum troponin levels are elevated in 90% of cases, however peak levels are significantly lower than in patients with STEMI due to vessel occlusion. Brain type natriuretic protein (BNP) is elevated in the acute phase of the disease. Elderly patients and those with hypotension, ventricular arrhythmias, LVOTO, mitral regurgitation, and thrombus formation are at higher risk for complications including cardiogenic shock, which can occur in up to 25% of cases.[59] Acute ischemic stroke, occurring in up to 14% of patients, can also complicate the clinical course.[64]
Treatment of takotsubo cardiomyopathy includes cardiac monitoring, supportive measures (maintaining blood pressure, perfusion, hemodynamics, airway and fluid volume), as well as the initiation of goal-directed medical treatment for HF. It is important to identify whether there is concurrent left ventricular outflow tract obstruction (LVOTO) versus isolated ventricular systolic failure, which can be assessed by echocardiography, in order to delineate optimal treatment options and anticipated hemodynamic responses to therapies. Current expert consensus suggests 72-hour observation in the intensive cardiac care unit for higher risk patients.[59] In cases of cardiogenic shock specifically if LVOTO is present, the use of inotropic agents is discouraged to avoid adrenergic stimulation.
In conjunction with current HF treatment guidelines, beta-blockers and ACE inhibitors are recommended in the absence of contraindications such as acute cardiogenic shock. Beta-blockers have been considered to prevent detrimental myocardial response to sympathetic nervous system stimulation, however, in the in the largest reported takotsubo cohort which included 1750 patients, there was no association between the use of beta-blocking agents and improved survival.[65] In this same report, ACE inhibitors or angiotensin receptor antagonists were associated with significantly lower rates of death at 12 month follow-up (2% vs. 9%, p<0.001).[65] Cases of takotsubo cardiomyopathy demonstrate complete LV systolic function recovery at 3–6 months, and this characteristic is considered a diagnostic criterion by expert groups.[59] ECG changes and elevated BNP can persist up to a year following diagnosis. Takotsubo cardiomyopathy can recur in up to 22% of cases at 5 years, and one study reported that patients diagnosed with takotsubo are at similar 3-year mortality risk as patients who experience acute MI with evidence of occlusive atherosclerosis.[59] However, these findings remain to be confirmed, and future studies are needed to determine optimal medical therapy aimed at preventing complications, recurrence, and adverse cardiovascular events.
Peripartum cardiomyopathy:
Peripartum cardiomyopathy is a form of systolic HF that presents in the last month of pregnancy or in the first five months postpartum in the absence of another underlying etiology (such as acute MI). In some cases, chronic HF persists and may lead to cardiac transplantation. It affects about 1:1000 births worldwide, but the prevalence varies by geographic locations.[29, 66]
Although peripartum cardiomyopathy is the leading cause of maternal peripartum death, little is known regarding origin and underlying mechanisms of its progression. Preeclampsia and gestational diabetes may predispose women to peripartum cardiomyopathy, but the mediators connecting these conditions to the cardiomyopathy are incompletely defined. Some studies suggest that increased cleavage of prolactin by cathepsin D into the proapoptotic and antiangiogenic 16 kDa prolactin fragment may be involved.[67] Alternatively, other studies suggest an upregulation of soluble factors of placental origin (sFlt1, a soluble form of the vascular endothelial growth factor [VEGF] receptor) affects the coronary microcirculation.[67–69] Some women with peripartum cardiomyopathy bear mutations in titin, also known as connectin, which is a large sarcomeric protein involved in tethering the sarcomere to the cell wall.[70] Titin also binds a number of enzymes involved with maintaining high levels of ATP in regions of high energy demand in the sarcomere.[71] Whether any of these factors can be used as diagnostic, prognostic or treatment targets for the disease remains to be determined.
D. Autonomic neurovascular dysregulation
Vascular conditions associated with decreases in ovarian function:
With decreases or loss of ovarian function about 75% of women experience hot flashes and night sweats. [72] Three contemporary longitudinal studies identified 4 patterns of the vasomotor instability relative to their onset prior to menopause, the intensity of events and their duration relative to menopause (Figure 27.1). [73–75] These patterns are similar among women across the globe, suggesting physiological rather than cultural or environmental causes. Whether or not vasomotor instability places women at risk for various cardiovascular diseases is controversial. Part of the controversy is driven by the absence of reliable and systematic objective measurement of the hot flashes and night sweats. Most studies depend on self-report tools which may under (or over) estimate their number, frequency and severity. However, even with these unsophisticated reporting tools, the emerging data suggest that the severity of the hot flashes and night sweats may associate with reduced endothelium-dependent vasodilatation, hypertension, increased carotid intima-media thickness, coronary artery calcification and CMD.[76–80] The relationships between vasomotor irregularies and soluble markers associated with coagulation requires further study.[81–83] Resolving some of these controversies will require longitudinal studies that are designed to address the specific patterns of the hot flashes and night sweats with specific cardiovascular functions or events.
Figure 27.1.
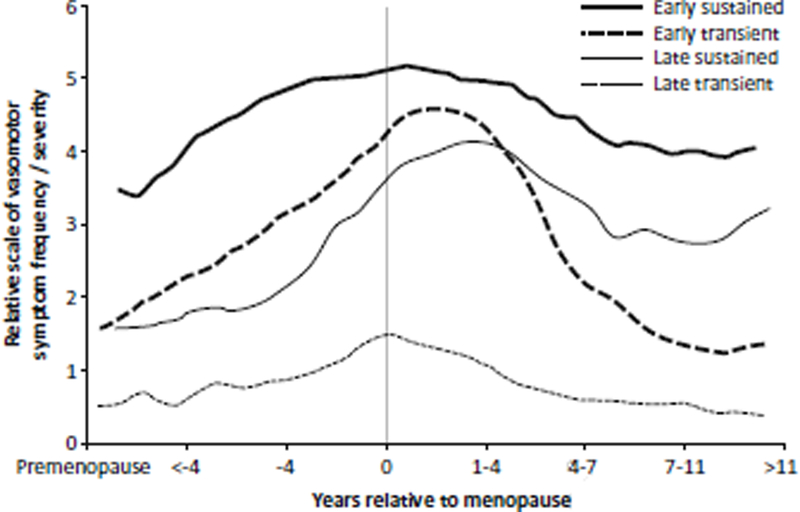
Composite representation of four patterns of vasomotor instability relative to their onset prior to menopause, the intensity of events and their duration relative to menopause reported in three contemporary studies. [75–77]
Hot flashes and night sweats involve the central thermoregulatory system in the hypothalamus, and are mediated through autonomic control of the peripheral vasculature and sweat glands. Therefore, the onset, frequency, intensity and duration of the symptoms may represent differences in underlying dysfunction in central and autonomic mechanisms and may involve other brain regions associated with sleep and mood.[84] Indeed, severe vasomotor symptoms may be related to obstructive sleep apnea [85], which is a risk factor for coronary artery calcification.[86]
The characterization of autonomic function associated with expression of hot flashes and night sweats has not been fully characterized. However, diminished parasympathetic tone may be involved as heart rate variability decreases at menopause [87], and sleep-related decreases in blood pressure are not observed in women who experience hot flashes with insomnia.[88]
Neurons of the hypothalamic-pituitary axis implicated in the development of hot flashes and night sweats are kisspeptin/neurokin B/dinorphin (KNDy) neurons.[89] Although menopausal hormone treatments (MHT) consisting of 17β estradiol, conjugated equine estrogen or progesterone (alone or in combination with the estrogen) reduce both hot flashes and night sweats in women, antagonists of the KNDy pathway offer a potential alternative option to treat these symptoms.[90]
In addition to menopausal hormone treatments [91], selective serotonin re-uptake inhibitors also are effective in reducing hot flashes in women. Stimulation of serotonergic receptors in the brain alters both sympathetic and parasympathetic nerve activity (see[92] for review). Estrogen modulates gene transcription of serotonin in the neuronal nucleus, augments activity of the serotonin uptake transporter, and increases the sensitivity of 5-HT1A receptors on post-synaptic neurons. Thus, declining levels of estrogen, as would occur at menopause, can decrease the activity of the serotonergic system. An interaction between the serotonergic and adrenergic systems [93] is suggested as both selective serotonin reuptake inhibitors (SSRIs) and clonidine, an alpha2-adrenergic receptor agonist which activates pre-synaptic inhibition in the brain, are effective in relieving vasomotor symptoms in some women.[94]
In addition to central autonomic control pathways mediating vasomotor symptoms, much remains to be learned about how peripheral pathways innervating the vascular smooth muscle (expression of adrenergic or cholinergic receptors), neurotransmitter synthesis, receptor coupling to vasodilatory pathways such as those mediated by cyclic guanylate cyclase [95–97] might differ among women who experience various patterns of hot flashes.
Although hot flashes and night sweats are typically referred to as menopausal symptoms, the fact that they can occur in men with androgen depletion [89] and have different patterns of expression in women suggests that the changes in hormonal milieu may unmask underlying autonomic and vascular dysregulation.
Migraine is another condition that may be unmasked by changes in the hormonal milieu. The hypothesis that changes in sex hormones, in particular estrogen, contribute to the etiology of migraine is supported by the observations that at puberty, migraines occur about 3 times in women than men, and that migraine is associated with estorgen levels during the menstrual cycle, with pregnancy and postpartum, and during perimenopause. While hormonally-related migraine is associated with abrupt declines in estrogen levels, as with menstrual-related migraine, postpartum and in perimenopause, it is unlikely that all migraine variants have a common hormonal etiology.(see [98–100]. Genetic variants considered in the etiology of migraine include those for the estrogen receptor alpha, receptors and neurotransmitters associated with adrenergic, GABAergic and nitrogeneric neurons, and enzymes associated with estrogen metabolism.[98] [101–103]. However, a systematic genomic studies of individuals within the various classifications of migraines may help to better understand comorbid conditions and provide insight into better treatment options.[104] Other emerging concepts for the etiology of migraine suggests an immunological component, mitochondrial dysregulation leading to oxidative stress, and iron deficiency. [105–108].
Although migraine was once considered a “essentially benign condition” [109], associations to stroke and SCAD remaining to be comprehensively examined with regard to mirgain with and without aura or relative to hormone levels, hormonal responsiveness and genomics.[31, 110, 111]
E. Exogenous hormones and cardiovascular disease
Exogenous hormones are used by women for contraception, replacement for primary and premature ovarian insufficiency and surgical oophorectomy, and treatment for vasomotor, mood, urogynecological symptoms and osteoporosis following menopause. Metabolism of sex steroid hormones is complex and genetic variants in any one of the numerous enzyme associated with synthesis, uptake and catabolism [112] of the hormones, as well as variation in the hormone receptors [113] will affect responses to exogenous products. The pharmacogenomics estrogen metabolism and response is an emerging area of investigation and greater understanding of genetic variants associated with ovarian function and hormone response will allow for individualizing treatments to maximize benefits and reduce the risk of adverse events with use of these products.
Hormonal Oral contraceptives:
Oral contraceptives to suppress ovulation modify endogenous production of sex steroids from a functioning ovary, and, thus, disrupt the hypothalamic-pituitary-ovary feedback regulatory pathway. Longitudinal randomized control trials evaluating adverse cardiovascular events and cardiovascular risk associated with oral hormone-based contraceptives are confounded by the fact that the composition of the products has changed over the years especially for the type of synthetic progestin allowing for longer cessation of ovulation and decreased frequency of menses (see Table 27.2 and Figure 27.2.) [114, 115] Evaluation of adverse cardiovascular events in older women who may have used various formulations of oral contraceptives is also confounded by their pregnancy history and perhaps use of menopausal hormone treatments. In spite of these limitations, there are some data to support that women who use oral contraceptives have decreased incidence of heart disease and stroke.[116, 117]
Table 27.2.
Progestins in oral contraceptives ranked according to risk for venous thrombosisa
| Oral contraceptive | Risk of thrombosis |
|---|---|
| First-generation <50 μg ethinyl estradiol with the progestins norctynodrel, norethisterone. and norcthisterone acetate | 6–12/10,000 women |
| Third-generation <50 μg ethinyl estradiol with the progestins desogcstrel or gestodene or norgestimate | 9–12/10.000 women |
| Fourth-generation <50 μg ethinyl estradiol with the progestins drospirenone. dienogest. or nomegcstrol acetate | 9–12/10.000 similar |
| Second-generation <50 μg ethinyl estradiol with the progestins norgestrel or levonorgestrel | 5–7/10.000 women |
| Progestin-only (norcthisterone. ethynodiol diacetate. levonorgestrel. desogestrel. lynestrenol) | 2–3/10.000 women |
Derived from Table 27.1 of (iialcraki ct al. [116] reprinted with permission
Figure 27.2.
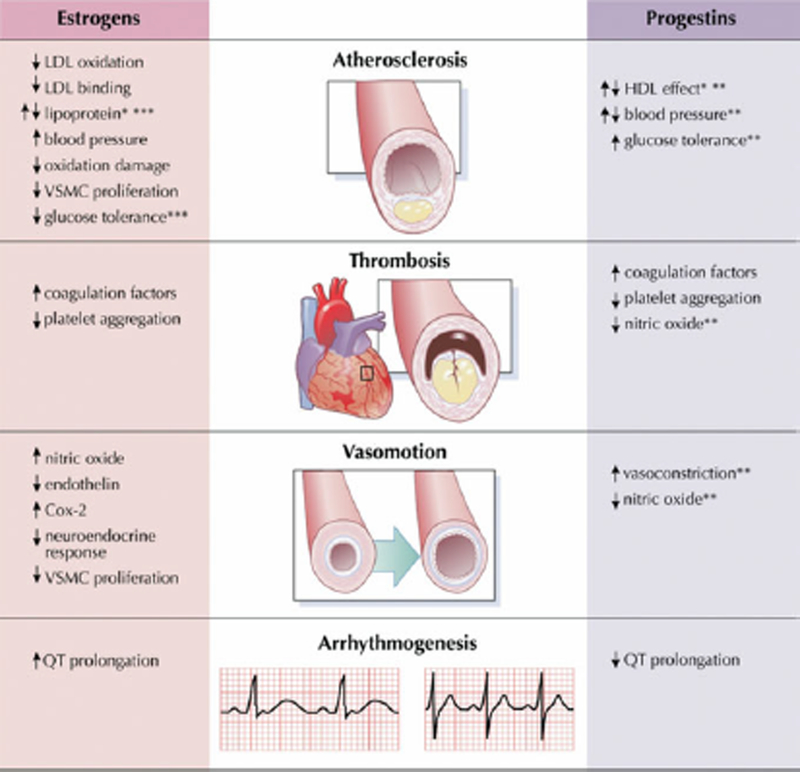
Impact of hormonal contraception on mechanisms of cardiovascular disease.
*Dependent on delivery route of estrogen; **dependent on type of progestin; ***dependent on the dose of estrogen. Cox-2 = cyclooxygenase-2; HDL = high-density lipoprotein; LDL= low density lipoprotein; VSMC = vascular smooth muscle cell. Reproduced with permission from reference [117].
Similar to all oral medications, oral hormonal products will enter the entero-hepatic circulation for first pass metabolism in liver. In addition to metabolism of the drugs in the liver, the hormones may stimulate production of proteins involved with coagulation and inflammation, and alter production of triglycerides and lipoproteins. These effects of the hormones on liver metabolism may be important for treating women with polycystic ovarian syndrome.[118] There is an increased risk of venous and pulmonary thrombosis with some products (Table 27.2), therefore, the use of oral contraceptives is not recommended for women with known thrombophilia.[114]
Hormone replacement for ovarian insufficiency and early oophorectormy:
Primary and premature ovarian insufficiencies are defined by loss of ovarian function before the age 40 years, and early oophorectomy is defined as surgical removal of the ovaries prior to 45 years of age. These conditions are characterized by increases in 18 comorbid conditions of premature aging [119–121], including incidence of and mortality from cardiovascular disease and stroke.[122–126] Cardiovascular mortality is reduced by treatment with estrogen at least until the age of natural menopause (Figure 27.3).[120, 122, 127]
Figure 27.3.
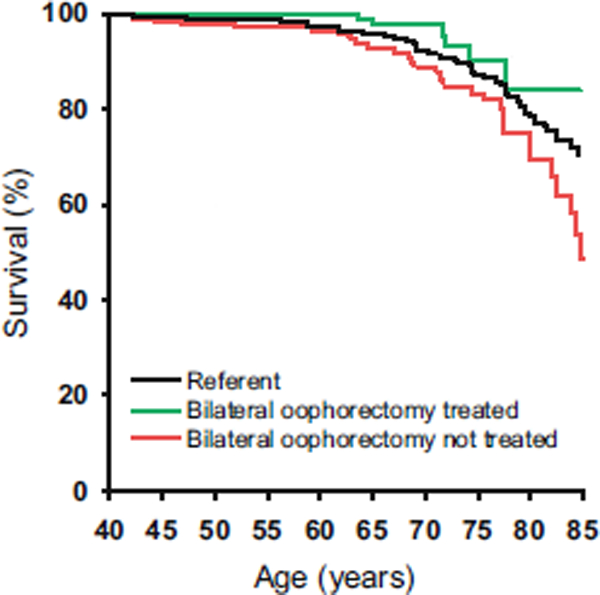
A population study of ccardiovascular mortality in women who underwent bilateral oophorectomy before age 45 years and were or were not treated with estrogen through age 45 years or longer. Reproduced with permission from reference [124].
Hormone treatment at menopause:
Until 2002, based on evidence from observational and epidemiological studies, it was accepted that use of hormones (in particular, estrogen) during menopause (MHT) reduced mortality due to cardiovascular disease. [128–134] The most frequently used formulation of MHT in those studies was conjugated equine estrogen, and most women initiated use of these products around the time of menopause. Cellular actions of 17β-estratiol encompass regulation of gene transcription, including regulation of nitric oxide synthase and angiotensin converting enzyme, and actions mediated through membrane receptors, ion channels, post-translational modification of enzymes, mitochondrial function and biochemical interactions with oxygen-derived free radicals (see Figure 27.2).[98] Collectively, these mechanistic actions are expected to promote vasodilatation, reduce adhesion of leukocytes to the vascular wall, reduce platelet activation, and thus slow development of atherosclerosis. In addition, oral estrogen products altered the serum lipid profile to reduce low-density lipoproteins and increase high density lipoproteins, an effect that was not observed in women using statins. [134, 135]
However, observational and epidemiological data may be biased as women self-select to use MHT and may represent a healthy subset of women (healthy user bias). Several prospective trials have been conducted to try to address the preventive actions of MHT (Table 27.3). [136]. The first large scale, prospective, randomized trial was the Women’s Health Initiative (WHI) that enrolled women between the ages of 50–79 years. The mean age for participants was 63 years, which was as many as 10 years past the age of menopause. Women with a uterus were randomized to placebo or a single dose of oral conjugated equine estrogen plus a continuous combined synthetic progestogen, medroxyprogesterone acetate; women without a uterus were randomized to placebo or oral conjugated equine estrogen alone. Outcomes for the study were adverse cardiovascular events, bone mineral density and breast cancer.[137] The WHI trial was stopped in 2002 because of an excess number of predetermined adverse events, including the cardiovascular outcomes of coronary heart disease, myocardial infarction, stroke and venous thromboembolism.[138] These results drastically altered clinical prescribing practice for MHT for menopausal symptoms and chronic diseases of aging such as osteoporosis, even though MHT was effective in reducing these conditions. However, subsequently over the course of about 15 years, several criticisms of the WHI have emerged that need to be considered. First, women in the WHI were many years past menopause, and, thus, the results cannot be generalized to women who would use MHT around the peri- and immediate time past menopause.[139, 140] Evidence from experimental animals indicates that the timing of initiation of hormonal treatments close to the time of oophorectomy or menopause (the timing hypothesis) is critical to prevent the initiation and progression of cardiovascular disease.[141] Indeed, subsequent sub-analysis of data from the WHI that stratified women by menopausal age at the time of treatment randomization, suggests that women who were within 5 years of menopause had reduced coronary artery calcification, fewer myocardial infarctions and lower all-cause mortality compared to those who were randomized after that time point.[142–144] In addition, the Danish Osteoporosis Prevention Study (DOPS), which began at the same time as the WHI, enrolled women between the ages of 45–58 years. Although these women were treated with different formulations of hormone products (triphasic estradiol and norethisterone acetate, a type of birth control pill with estrogen dose constant and variable progestin dose, or 2 mg estradiol a day if they had a hysterectomy), after 11 years of follow-up the number of myocardial infarctions, incidence of HF and death were reduced in the treated women compared to controls.[145]
Table 27.3.
Summary of variables for open-label and prospective trials of the use of menopausal hormone treatments and cardiovascular outcomes since the WHI
| Trial | Timing of treatment relative to the menopause |
Formulation | Participant characterization | Primary cardiovascular outcomcs |
Duration |
|---|---|---|---|---|---|
| DOPSa [146] | 3–24 months | Open label - | Women with and without a uterus 45–52 years of age with FSH >2 standard deviations ova premenopausal values | Death: hospitalisation for myocardial infarctions or heart failure | 11 years of intervention; 16 years of follow-up |
| Women with a uterus: triphasic estradiol and norcthisterone acetate (2 mg synthetic 17β-estradiol for 12 days, 2 mg 17β-estradiol plus 1 mg norethisterone acetate for 10 days, and 1 mg 17β-estradiol for 6 days) | Exclusion: history of bone disease (including non-traumatic vertebral fractures on radiography), uncontrolled chronic disease, previous or current cancer or thromboembolic disease, current or past treatment with glucocorticoids for more than 6 months, current or previous use of hormone replacement therapy within the past 3 months, and alcohol or drug dependency | ||||
| Women without a uterus: oral 17β-estradiol (2 mgAlay) | |||||
| EPAT [136] | Median 3.5 years | Oral micronized 17β-estradiol (1 mg/day) | Serum estradiol <20 pg/mL 45 years or older | Rate of change of carotid intima-medial thickness | 2 years |
| No preexisting cardiovascular disease; low-density lipoprotein cholesterol equal to or greater than 3.37 mmol/L (130 mg/ dL) | |||||
| KEEPS [153] | 6–36 months | Oral CEE <0.45 mg/day) or transdamai 17 |i-estradiol (50 (ig/day) each with oral progesterone <2(X) mg/day for 12 days in a 30-day cyclc) | Women with a uterus between ages of 42 and 58 years | Rate of change of carotid intima-medial thickncss: coronary arterial calcification | 4 years |
| Exclusion: history of cardiovascular disease or venous thrombosis: coronary calcification scores >50 Agatston units; body mass index >35 kg/m’: triglyceride >400 mg/dL: LDL cholesterol <190 mg/ dL; lipid4owcring medications: untrcatod hypertension (systolic >150 mmHg, diastolic >95 mmHg): diabetes: history of chronic diseases including canca: smoking more than 10 cigarettes/day | |||||
| ELITE [147] | <6 years or ≥ 10 years | Oral micronized 17β-estradiol (1 mg/day) plus vaginal progesterone (45 mg for 10 days in a 30-day cycle) for women with a uterus | Women with and without a uterus; median ages 55 and 65 years for the two groups, respectively | Rate of change of carotid intima-medial thickness; coronary arterial calcification | 6–7 years (median 5 years) |
| Exclusion: fasting plasma triglyceride level > 500 mg/dL; diabetes mdlitus or fasting serum blood glucose >140 mg/dL: scrum creatinine >2.0 mg/dL: uncontrolled hypertension (systolic/diastolic blood pressure > 16/110 mmHg); untreated thyroid disease; life-threatening disease with prognosis <5 years: history of deep vein thrombosis, pulmonary embolism: breast cancer |
Reproduced with permission from Miller and Harman [137]
“Abbreviations: DOPS Danish Osteoporosis Prevention Study, EPAT Estrogen in the Prevention of Atherosclerosis Trial, KEEPS Kronos Early Estrogen Prevention Study, ELITE Early versus Late Intervention Trial with Estradiol, CEE conjugated equine estrogen, LDL low-density lipoprotein cholesterol
The Early versus Late Intervention Trial (ELITE) directly tested the timing hypothesis. This study enrolled two age groups of women: an early group aged 55 years and a late group aged 65 years. After 5 years of randomization to oral 17β-estradiol, the progression of atherosclerotic plaque measured by intima-medial thickness of the carotid artery was lower in the early aged treated compared to early placebo group. However, the rate of progression of carotid plaque was similar between the treated and placebo in the late aged group.[146]
Another criticism of the WHI was that a conclusion based on single dose and formulation of conjugated equine estrogen was generalized to all hormonal products including those that contain the endogenous form of estrogen 17β-estradiol and to those that might be delivered transdermally.[140] Conjugated equine estrogen is a mixture of steroid metabolites with a ratio of estrone to 17β-estradiol that can range from 6–20:1[147]; sulfonated estrone and estradiol, equilin and other methylated products. The binding affinity of these different ligands for estrogen receptors vary and cannot be compared by dose equivalency with 17β-estradiol. In addition, oral products, which undergo metabolism in the liver before reaching the systemic circulation, will affect production of procoagulant and inflammatory proteins perhaps increasing risk for thrombosis. Indeed, in the Estrogen and Thromboembolism Risk (ESTHER) multicenter case-controlled study, the odd ratio for developing an idiopathic venous thromboembolism was about 4 times higher in women using oral compared to those using transdermal products. In addition, use of micronized progesterone also had a lower odds ratio for developing venous thromboembolism than use of synthetic progestogens.[148, 149] Risk of venous thromboembolism with use of MHT will also be affected by prothrombotic mutations.[150, 151].
The Kronos Early Estrogen Prevention Study (KEEPS) was designed to provide a direct comparison of oral conjugated equine estrogen (0.45 mg/day) and transdermal 17β estradiol (50μg/day) both with pulsed micronized progesterone compared to placebo on slowing progression of atherosclerosis.[152] Unlike women enrolled in the WHI, women in KEEPS were within three years of menopause and were at low risk for cardiovascular disease based on conventional risk factors of body mass index, serum lipid profile, insulin sensitivity, and blood pressure.[153] This phenotypic profile was similar to women in early observational studies and reflects a “healthy user” profile that with subsequent analysis of the WHI was one that demonstrated reduced cardiovascular risk with the treatments.[135, 154, 155] In KEEPS after 4 years of treatment, the rate of change of intima-media thickness in the carotid artery did not differ among groups.[156] However, there were no reports of venous thromboembolisms, and both formulations reduced vasomotor symptoms.[157] However, the products differed in regard to effectiveness in improving mood (oral conjugated equine estrogen being better than the transdermal 17β estradiol) and sexual function (17β estradiol was better than the oral conjugated equine estrogen).[158, 159] Variants in genes associated with innate immunity may have contributed to effects of the products on the cardiovascular outcomes measured in KEEPS.[160, 161]
In summary, evaluation of the effects of sex hormones on cardiovascular function in women need to consider the timing of the initiation of treatment, the type, dose and formulation of the product, the existing cardiovascular risk profile of the woman, and the potential pharmacogenomics effects of the hormones on the outcome of interest.
F. Conclusion
Sex differences in cardiovascular health and disease has origin in the presence of sex chromosomes and the production of sex steroids hormones that varies across the life span, is influenced by pregnancy, and by use of exogenous hormone products. Diagnosis and treatment of some cardiovascular diseases in women have been hampered by the lack of appreciation of symptom presentation in women, poor understanding of cellular mechanisms causing disease, and not treating women according to standard guidelines of care. However, much remains to be learned regarding identification of unique factors which may place women at risk for early development of cardiovascular diseases such as those that might be related to neurovascular autonomic dysregulation. In addition, research is needed to optimize diagnosis and treatment for conditions such as microvascular disease, SCAD and takostubo cardiomyopathies, and to develop prognostic indicators for women who might be at risk for recurrence of these conditions. Longitudinal studies are needed to determine effects of various types of hormonal treatments on cardiovascular function. Large scale clinical trials of new treatment options for cardiovascular conditions need to enroll women, and the results of such studies need to be analyzed by sex in order to inform individualized care that will lead to improved outcomes.
Contributor Information
Chrisandra Shufelt, Barbra Streisand Women’s Heart Center & Preventive and Rehabilitative Cardiac Center, Director, Women’s Hormone and Menopause Program, Associate Professor, Cedars-Sinai Medical Center, Cedars-Sinai Heart Institute, 8631 West Third Street, Suite 740 East, Los Angeles CA 90048.
Christine Pacheco, Constance A. Austin Fellow in Women’s Heart Health, Barbra Streisand Women’s Heart Center, Cedars-Sinai Heart Institute, 8631 West Third Street, Suite 740 East, Los Angeles CA 90048.
Marysia S. Tweet, Department of Cardiology, College of Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN 55905.
Virginia M. Miller, Director, Women’s Health Research Center, College of Medicine, Mayo Clinic, 200 First Street SW, Rochester, MN 55905.
References
- 1.Bellott DW, Hughes JF, Skaletsky H, Brown LG, Pyntikova T, Cho TJ, Koutseva N, Zaghlul S, Graves T, Rock S et al. : Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature 2014, 508(7497):494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold AP, Lusis AJ: Understanding the sexome: measuring and reporting sex differences in gene systems. Endocrinology 2012, 153(6):2551–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ et al. : Heart disease and stroke statistics−−2015 update: a report from the American Heart Association. Circulation 2015, 131(4):e29–322. [DOI] [PubMed] [Google Scholar]
- 4.Pancholy SB, Shantha GP, Patel T, Cheskin LJ: Sex differences in short-term and long-term all-cause mortality among patients with ST-segment elevation myocardial infarction treated by primary percutaneous intervention: a meta-analysis. JAMA Intern Med 2014, 174(11):1822–1830. [DOI] [PubMed] [Google Scholar]
- 5.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R: Update on acute coronary syndromes: the pathologists’ view. Eur Heart J 2013, 34(10):719–728. [DOI] [PubMed] [Google Scholar]
- 6.Roswell RO, Kunkes J, Chen AY, Chiswell K, Iqbal S, Roe MT, Bangalore S: Impact of Sex and Contact-to-Device Time on Clinical Outcomes in Acute ST-Segment Elevation Myocardial Infarction-Findings From the National Cardiovascular Data Registry. J Am Heart Assoc 2017, 6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi K, Shofer FS, Mills AM: Sex differences in STEMI activation for patients presenting to the ED 1939. Am J Emerg Med 2016, 34(10):1939–1943. [DOI] [PubMed] [Google Scholar]
- 8.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE et al. : Acute Myocardial Infarction in Women: A Scientific Statement From the American Heart Association. Circulation 2016, 133(9):916–947. [DOI] [PubMed] [Google Scholar]
- 9.O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr., Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA et al. : 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2013, 82(1):E1–27. [DOI] [PubMed] [Google Scholar]
- 10.Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE Jr., Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM et al. : 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012, 60(7):645–681. [DOI] [PubMed] [Google Scholar]
- 11.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ et al. : Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J 2001, 141(5):735–741. [DOI] [PubMed] [Google Scholar]
- 12.Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E: Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012, 33(6):734–744. [DOI] [PubMed] [Google Scholar]
- 13.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G et al. : Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004, 109(6):722–725. [DOI] [PubMed] [Google Scholar]
- 14.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN: Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010, 55(25):2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camici PG, Crea F: Coronary microvascular dysfunction. N Engl J Med 2007, 356(8):830–840. [DOI] [PubMed] [Google Scholar]
- 16.Ishimori ML, Martin R, Berman DS, Goykhman P, Shaw LJ, Shufelt C, Slomka PJ, Thomson LE, Schapira J, Yang Y et al. : Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc Imaging 2011, 4(1):27–33. [DOI] [PubMed] [Google Scholar]
- 17.Saiki H, Petersen IA, Scott CG, Bailey KR, Dunlay SM, Finley RR, Ruddy KJ, Yan E, Redfield MM: Risk of Heart Failure With Preserved Ejection Fraction in Older Women After Contemporary Radiotherapy for Breast Cancer. Circulation 2017, 135(15):1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandoo A, Kitas GD, Carmichael AR: Breast cancer therapy and cardiovascular risk: focus on trastuzumab. Vasc Health Risk Manag 2015, 11:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doyle M, Weinberg N, Pohost GM, Bairey Merz CN, Shaw LJ, Sopko G, Fuisz A, Rogers WJ, Walsh EG, Johnson BD et al. : Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women’s Ischemia Syndrome Evaluation) study. JACC Cardiovasc Imaging 2010, 3(10):1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freed BH, Narang A, Bhave NM, Czobor P, Mor-Avi V, Zaran ER, Turner KM, Cavanaugh KP, Chandra S, Tanaka SM et al. : Prognostic value of normal regadenoson stress perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2013, 15:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta PK, Bairey Merz CN: Treatment of angina in subjects with evidence of myocardial ischemia and no obstructive coroanry artery disease In: Braunwald’s Heart Disease. Edited by Bonow RO. Philadelphia: Elsevier; 2011. [Google Scholar]
- 22.Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, Gersh BJ, Khambatta S, Best PJ, Rihal CS et al. : Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012, 126(5):579–588. [DOI] [PubMed] [Google Scholar]
- 23.Tweet MS, Hayes SN, Codsi E, Gulati R, Rose CH, Best PJM: Spontaneous Coronary Artery Dissection Associated With Pregnancy. J Am Coll Cardiol 2017, 70(4):426–435. [DOI] [PubMed] [Google Scholar]
- 24.Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, Mancini GBJ: Spontaneous Coronary Artery Dissection: Clinical Outcomes and Risk of Recurrence. J Am Coll Cardiol 2017, 70(9):1148–1158. [DOI] [PubMed] [Google Scholar]
- 25.Lettieri C, Zavalloni D, Rossini R, Morici N, Ettori F, Leonzi O, Latib A, Ferlini M, Trabattoni D, Colombo P et al. : Management and Long-Term Prognosis of Spontaneous Coronary Artery Dissection. Am J Cardiol 2015, 116(1):66–73. [DOI] [PubMed] [Google Scholar]
- 26.Nakashima T, Noguchi T, Haruta S, Yamamoto Y, Oshima S, Nakao K, Taniguchi Y, Yamaguchi J, Tsuchihashi K, Seki A et al. : Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: A report from the Angina Pectoris-Myocardial Infarction Multicenter Investigators in Japan. Int J Cardiol 2016, 207:341–348. [DOI] [PubMed] [Google Scholar]
- 27.Rogowski S, Maeder MT, Weilenmann D, Haager PK, Ammann P, Rohner F, Joerg L, Rickli H: Spontaneous Coronary Artery Dissection: Angiographic Follow-Up and Long-Term Clinical Outcome in a Predominantly Medically Treated Population. Catheter Cardiovasc Interv 2017, 89(1):59–68. [DOI] [PubMed] [Google Scholar]
- 28.Tweet MS, Gulati R, Hayes SN: What Clinicians Should Know Alphabout Spontaneous Coronary Artery Dissection. Mayo Clinic proceedings 2015, 90(8):1125–1130. [DOI] [PubMed] [Google Scholar]
- 29.Elkayam U: Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol 2011, 58(7):659–670. [DOI] [PubMed] [Google Scholar]
- 30.Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS, Holmes DR Jr., Hayes SN, Gulati R: Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circulation Cardiovascular interventions 2014, 7(6):777–786. [DOI] [PubMed] [Google Scholar]
- 31.McGrath-Cadell L, McKenzie P, Emmanuel S, Muller DW, Graham RM, Holloway CJ: Outcomes of patients with spontaneous coronary artery dissection. Open Heart 2016, 3(2):e000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ko D, Rahman F, Schnabel RB, Yin X, Benjamin EJ, Christophersen IE: Atrial fibrillation in women: epidemiology, pathophysiology, presentation, and prognosis. Nat Rev Cardiol 2016, 13(6):321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr., Conti JB, Ellinor PT, Ezekowitz MD, Field ME et al. : 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130(23):2071–2104. [DOI] [PubMed] [Google Scholar]
- 34.Camm AJ, Savelieva I: Female gender as a risk factor for stroke associated with atrial fibrillation. Eur Heart J 2017, 38(19):1480–1484. [DOI] [PubMed] [Google Scholar]
- 35.Renoux C, Coulombe J, Suissa S: Revisiting sex differences in outcomes in non-valvular atrial fibrillation: a population-based cohort study. Eur Heart J 2017, 38(19):1473–1479. [DOI] [PubMed] [Google Scholar]
- 36.Abbott GW: KCNE4 and KCNE5: K(+) channel regulation and cardiac arrhythmogenesis. Gene 2016, 593(2):249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabeh MK, MacRae CA: The genetics of atrial fibrillation. Curr Opin Cardiol 2010, 25(3):186–191. [DOI] [PubMed] [Google Scholar]
- 38.Heinrich J: General Accounting Office, GAO-01–286R Drugs Withdrawn From Market. 2001. [Google Scholar]
- 39.Liu H, Jin MW, Xiang JZ, Huang Y, Sun HY, Chiu SW, Lau CP, Li GR: Raloxifene inhibits transient outward and ultra-rapid delayed rectifier potassium currents in human atrial myocytes. Eur J Pharmacol 2007, 563(1–3):61–68. [DOI] [PubMed] [Google Scholar]
- 40.Kocic I, Gruchala M, Petrusewicz J: Selective inhibition of pinacidil effects by estrogen in guinea pig heart. Int J Cardiol 2006, 110(1):22–26. [DOI] [PubMed] [Google Scholar]
- 41.Ko D, Rahman F, Martins MA, Hylek EM, Ellinor PT, Schnabel RB, Benjamin EJ, Christophersen IE: Atrial fibrillation in women: treatment. Nat Rev Cardiol 2017, 14(2):113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah SV, Kruse J, Andrei AC, Li Z, Malaisrie SC, Knight BP, Passman RS, McCarthy PM: Gender differences in outcomes after surgical ablation of atrial fibrillation. J Thorac Cardiovasc Surg 2016, 151(2):391–398 e392. [DOI] [PubMed] [Google Scholar]
- 43.Zylla MM, Brachmann J, Lewalter T, Hoffmann E, Kuck KH, Andresen D, Willems S, Eckardt L, Tebbenjohanns J, Spitzer SG et al. : Sex-related outcome of atrial fibrillation ablation: Insights from the German Ablation Registry. Heart Rhythm 2016, 13(9):1837–1844. [DOI] [PubMed] [Google Scholar]
- 44.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C et al. : Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL et al. : 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013, 62(16):e147–239. [DOI] [PubMed] [Google Scholar]
- 46.Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM: Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015, 131(6):550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM et al. : 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail 2017, 23(8):628–651. [DOI] [PubMed] [Google Scholar]
- 48.Lam PH, Dooley DJ, Deedwania P, Singh SN, Bhatt DL, Morgan CJ, Butler J, Mohammed SF, Wu WC, Panjrath G et al. : Heart Rate and Outcomes in Hospitalized Patients With Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2017, 70(15):1861–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunlay SM, Roger VL, Redfield MM: Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2017, 14(10):591–602. [DOI] [PubMed] [Google Scholar]
- 50.Kotecha D, Manzano L, Krum H, Rosano G, Holmes J, Altman DG, Collins PD, Packer M, Wikstrand J, Coats AJ et al. : Effect of age and sex on efficacy and tolerability of beta blockers in patients with heart failure with reduced ejection fraction: individual patient data meta-analysis. BMJ 2016, 353:i1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keyhan G, Chen SF, Pilote L: Angiotensin-converting enzyme inhibitors and survival in women and men with heart failure. Eur J Heart Fail 2007, 9(6–7):594–601. [DOI] [PubMed] [Google Scholar]
- 52.Hudson M, Rahme E, Behlouli H, Sheppard R, Pilote L: Sex differences in the effectiveness of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in patients with congestive heart failure--a population study. Eur J Heart Fail 2007, 9(6–7):602–609. [DOI] [PubMed] [Google Scholar]
- 53.Cook JL, Grady KL, Colvin M, Joseph SM, Brisco MA, Walsh MN, gen VADWG: Sex differences in the care of patients with advanced heart failure. Circ Cardiovasc Qual Outcomes 2015, 8(2 Suppl 1):S56–59. [DOI] [PubMed] [Google Scholar]
- 54.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG et al. : Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001, 345(20):1435–1443. [DOI] [PubMed] [Google Scholar]
- 55.Stevenson LW, Miller LW, Desvigne-Nickens P, Ascheim DD, Parides MK, Renlund DG, Oren RM, Krueger SK, Costanzo MR, Wann LS et al. : Left ventricular assist device as destination for patients undergoing intravenous inotropic therapy: a subset analysis from REMATCH (Randomized Evaluation of Mechanical Assistance in Treatment of Chronic Heart Failure). Circulation 2004, 110(8):975–981. [DOI] [PubMed] [Google Scholar]
- 56.Bogaev RC, Pamboukian SV, Moore SA, Chen L, John R, Boyle AJ, Sundareswaran KS, Farrar DJ, Frazier OH, HeartMate IICI: Comparison of outcomes in women versus men using a continuous-flow left ventricular assist device as a bridge to transplantation. J Heart Lung Transplant 2011, 30(5):515–522. [DOI] [PubMed] [Google Scholar]
- 57.Blumer V, Mendirichaga R, Hernandez GA, Zablah G, Chaparro SV: Sex-Specific Outcome Disparities in Patients Receiving Continuous-Flow Left Ventricular Assist Devices: A Systematic Review and Meta-analysis. ASAIO J 2017. [DOI] [PubMed] [Google Scholar]
- 58.Aimo A, Vergaro G, Castiglione V, Barison A, Pasanisi E, Petersen C, Chubuchny V, Giannoni A, Poletti R, Maffei S et al. : Effect of Sex on Reverse Remodeling in Chronic Systolic Heart Failure. JACC Heart Fail 2017, 5(10):735–742. [DOI] [PubMed] [Google Scholar]
- 59.Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi YJ et al. : Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2016, 18(1):8–27. [DOI] [PubMed] [Google Scholar]
- 60.Abraham J, Mudd JO, Kapur NK, Klein K, Champion HC, Wittstein IS: Stress cardiomyopathy after intravenous administration of catecholamines and beta-receptor agonists. J Am Coll Cardiol 2009, 53(15):1320–1325. [DOI] [PubMed] [Google Scholar]
- 61.Becker LC, Pepine CJ, Bonsall R, Cohen JD, Goldberg AD, Coghlan C, Stone PH, Forman S, Knatterud G, Sheps DS et al. : Left ventricular, peripheral vascular, and neurohumoral responses to mental stress in normal middle-aged men and women. Reference Group for the Psychophysiological Investigations of Myocardial Ischemia (PIMI) Study. Circulation 1996, 94(11):2768–2777. [DOI] [PubMed] [Google Scholar]
- 62.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC: Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005, 352(6):539–548. [DOI] [PubMed] [Google Scholar]
- 63.Merli E, Sutcliffe S, Gori M, Sutherland GG: Tako-Tsubo cardiomyopathy: new insights into the possible underlying pathophysiology. Eur J Echocardiogr 2006, 7(1):53–61. [DOI] [PubMed] [Google Scholar]
- 64.de Gregorio C, Grimaldi P, Lentini C: Left ventricular thrombus formation and cardioembolic complications in patients with Takotsubo-like syndrome: a systematic review. Int J Cardiol 2008, 131(1):18–24. [DOI] [PubMed] [Google Scholar]
- 65.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA et al. : Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med 2015, 373(10):929–938. [DOI] [PubMed] [Google Scholar]
- 66.Arany Z, Elkayam U: Peripartum Cardiomyopathy. Circulation 2016, 133(14):1397–1409. [DOI] [PubMed] [Google Scholar]
- 67.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C et al. : A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007, 128(3):589–600. [DOI] [PubMed] [Google Scholar]
- 68.Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, Hacker MR, Rhee JS, Mitchell J, Mahmood F et al. : Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012, 485(7398):333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Damp J, Givertz MM, Semigran M, Alharethi R, Ewald G, Felker GM, Bozkurt B, Boehmer J, Haythe J, Skopicki H et al. : Relaxin-2 and Soluble Flt1 Levels in Peripartum Cardiomyopathy: Results of the Multicenter IPAC Study. JACC Heart Fail 2016, 4(5):380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ware JS, Li J, Mazaika E, Yasso CM, DeSouza T, Cappola TP, Tsai EJ, Hilfiker-Kleiner D, Kamiya CA, Mazzarotto F et al. : Shared Genetic Predisposition in Peripartum and Dilated Cardiomyopathies. N Engl J Med 2016, 374(3):233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lange S, Auerbach D, McLoughlin P, Perriard E, Schafer BW, Perriard JC, Ehler E: Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. J Cell Sci 2002, 115(Pt 24):4925–4936. [DOI] [PubMed] [Google Scholar]
- 72.Freeman EW, Sherif K: Prevalence of hot flushes and night sweats around the world: a systematic review. Climacteric 2007, 10(3):197–214. [DOI] [PubMed] [Google Scholar]
- 73.Mishra GD, Kuh D: Health symptoms during midlife in relation to menopausal transition: British prospective cohort study. BMJ 2012, 344:e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tepper PG, Brooks MM, Randolph JF Jr., Crawford SL, El Khoudary SR, Gold EB, Lasley BL, Jones B, Joffe H, Hess R et al. : Characterizing the trajectories of vasomotor symptoms across the menopausal transition. Menopause 2016, 23(10):1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose SA, Gold EB, Hess R, Joffe H, Kravitz HM, Tepper PG et al. : Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med 2015, 175(4):531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang AJ, Sawaya GF, Vittinghoff E, Lin F, Grady D: Hot flushes, coronary heart disease, and hormone therapy in postmenopausal women. Menopause 2009, 16(4):639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jackson EA, El Khoudary SR, Crawford SL, Matthews K, Joffe H, Chae C, Thurston RC: Hot Flash Frequency and Blood Pressure: Data from the Study of Women’s Health Across the Nation. J Womens Health (Larchmt) 2016, 25(12):1204–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thurston RC, Sutton-Tyrell K, Everson-Rose SA, Hess R, Matthews KA: Hot Flashes and Subclinical Cardiovascular Disease. Findings From the Study of Women’s Health Across the Nation Heart Study. Circulation 2008. 118:1234–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muka T, Oliver-Williams C, Colpani V, Kunutsor S, Chowdhury S, Chowdhury R, Kavousi M, Franco OH: Association of Vasomotor and Other Menopausal Symptoms with Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis. PLoS One 2016, 11(6):e0157417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, Santen RJ: Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2015, 100(11):3975–4011. [DOI] [PubMed] [Google Scholar]
- 81.Svartberg J, von Muhlen D, Kritz-Silverstein D, Barrett-Connor E: Vasomotor symptoms and mortality: the Rancho Bernardo Study. Menopause 2009, 16(5):888–891. [DOI] [PubMed] [Google Scholar]
- 82.Allison MA, Manson JE, Aragaki A, Langer RD, Rossouw J, Curb D, Martin LW, Phillips L, Stefanick ML, Cochrane BB et al. : Vasomotor symptoms and coronary artery calcium in postmenopausal women. Menopause 2010, 17(6):1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Szmuilowicz ED, Manson JE, Rossouw JE, Howard BV, Margolis KL, Greep NC, Brzyski RG, Stefanick ML, O’Sullivan MJ, Wu C et al. : Vasomotor symptoms and cardiovascular events in postmenopausal women. Menopause 2011, 18(6):603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cintron D, Lipford M, Larrea-Mantilla L, Spencer-Bonilla G, Lloyd R, Gionfriddo MR, Gunjal S, Farrell AM, Miller VM, Murad MH: Efficacy of menopausal hormone therapy on sleep quality: systematic review and meta-analysis. Endocrine 2017, 55(3):702–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao C, Kapoor E, Lipford M, Miller V, Schroeder D, Mara K, Faubion S: Association of vasomotor symptoms and sleep apnea risk in midlife women. 2017, 25(4):in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kwon Y, Duprez DA, Jacobs DR, Nagayoshi M, McClelland RL, Shahar E, Budoff M, Redline S, Shea S, Carr JJ et al. : Obstructive sleep apnea and progression of coronary artery calcium: the multi-ethnic study of atherosclerosis study. J Am Heart Assoc 2014, 3(5):e001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mercuro G, Podda A, Pitzalis L, Zoncu S, Mascia M, Melis GB, Rosano GM: Evidence of a role of endogenous estrogen in the modulation of autonomic nervous system. Am J Cardiol 2000, 85(6):787–789, A789. [DOI] [PubMed] [Google Scholar]
- 88.de Zambotti M, Trinder J, Javitz H, Colrain IM, Baker FC: Altered nocturnal blood pressure profiles in women with insomnia disorder in the menopausal transition. Menopause 2017, 24(3):278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ: Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol 2013, 34(3):211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Nash Z, Doyle C, Papadopoulou DA, Bloom SR, Mohideen P et al. : Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2017, 389(10081):1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cintron D, Lahr BD, Bailey KR, Santoro N, Lloyd R, Manson JE, Neal-Perry G, Pal L, Taylor HS, Wharton W et al. : Effects of oral versus transdermal menopausal hormone treatments on self-reported sleep domains and their association with vasomotor symptoms in recently menopausal women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). Menopause 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCall RB, Clement ME: Role of serotonin1A and serotonin2 receptors in the central regulation of the cardiovascular system. PharmacolRev 1994, 46:231–243. [PubMed] [Google Scholar]
- 93.Deecher D, Andree TH, Sloan D, Schechter LE: From menarche to menopause: Exploring the underying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology 2008, 33:3–17. [DOI] [PubMed] [Google Scholar]
- 94.Santoro N: Symptoms of menopause: hot flushes. Clinical Obstetrics &Gynecology 2008, 51(3):539–548. [DOI] [PubMed] [Google Scholar]
- 95.Tuomikoski P, Ebert P, Groop PH, Haapalahti P, Hautamaki H, Ronnback M, Ylikorkala O, Mikkola TS: Evidence for a role of hot flushes in vascular function in recently postmenopausal women. Obstet Gynecol 2009, 113(4):902–908. [DOI] [PubMed] [Google Scholar]
- 96.Tuomikoski P, Ebert P, Groop PH, Haapalahti P, Hautamaki H, Ronnback M, Ylikorkala O, Mikkola TS: Effect of hot flushes on vascular function: a randomized controlled trial. Obstet Gynecol 2009, 114(4):777–785. [DOI] [PubMed] [Google Scholar]
- 97.Sassarini J, Fox H, Ferrell W, Sattar N, Lumsden MA: Vascular function and cardiovascular risk factors in women with severe flushing. Clin Endocrinol (Oxf) 2011, 74(1):97–103. [DOI] [PubMed] [Google Scholar]
- 98.Miller VM, Duckles SP: Vascular actions of estrogens: functional implications. PharmacolRev 2008, 60(2):210–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martin VT, Pavlovic J, Fanning KM, Buse DC, Reed ML, Lipton RB: Perimenopause and Menopause Are Associated With High Frequency Headache in Women With Migraine: Results of the American Migraine Prevalence and Prevention Study. Headache 2016, 56(2):292–305. [DOI] [PubMed] [Google Scholar]
- 100.Pavlovic JM, Allshouse AA, Santoro NF, Crawford SL, Thurston RC, Neal-Perry GS, Lipton RB, Derby CA: Sex hormones in women with and without migraine: Evidence of migraine-specific hormone profiles. Neurology 2016, 87(1):49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.An X, Fang J, Lin Q, Lu C, Ma Q, Qu H: New evidence for involvement of ESR1 gene in susceptibility to Chinese migraine. J Neurol 2017, 264(1):81–87. [DOI] [PubMed] [Google Scholar]
- 102.CoSkun S, Yucel Y, Cim A, Cengiz B, Oztuzcu S, Varol S, Ozdemir HH, Uzar E: Contribution of polymorphisms in ESR1, ESR2, FSHR, CYP19A1, SHBG, and NRIP1 genes to migraine susceptibility in Turkish population. J Genet 2016, 95(1):131–140. [DOI] [PubMed] [Google Scholar]
- 103.Sutherland HG, Champion M, Plays A, Stuart S, Haupt LM, Frith A, MacGregor EA, Griffiths LR: Investigation of polymorphisms in genes involved in estrogen metabolism in menstrual migraine. Gene 2017, 607:36–40. [DOI] [PubMed] [Google Scholar]
- 104.Hershey A, Horn P, Kabbouche M, O’Brien H, Powers S: Genomic expression patterns in menstrual-related migraine in adolescents. Headache 2012, 52(1):68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Loewendorf AI, Matynia A, Saribekyan H, Gross N, Csete M, Harrington M: Roads Less Traveled: Sexual Dimorphism and Mast Cell Contributions to Migraine Pathology. Front Immunol 2016, 7:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Borkum JM: Migraine Triggers and Oxidative Stress: A Narrative Review and Synthesis. Headache 2016, 56(1):12–35. [DOI] [PubMed] [Google Scholar]
- 107.Calhoun AH, Gill N: Presenting a New, Non-Hormonally Mediated Cyclic Headache in Women: End-Menstrual Migraine. Headache 2017, 57(1):17–20. [DOI] [PubMed] [Google Scholar]
- 108.Finsterer J, Zarrouk-Mahjoub S: Mitochondrial vasculopathy. World J Cardiol 2016, 8(5):333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bousser MG, Welch KMA: Relation between migraine and stroke. Lancet Neurol 2005, 4:533–542. [DOI] [PubMed] [Google Scholar]
- 110.Bushnell CD, Hurn P, Colton C, Miller VM, del Zoppo G, Elkind MSV, Stern BJ, Herrington D, Ford-Lynch G, Gorelick P et al. : Advancing the study of stroke in women. Summary and recommendations for future research from an NINDS-sponsored multidisciplinary working group. Stroke 2006, in press DOI: 10.1161/01.STR.0000236053.37695.15. [DOI] [PubMed] [Google Scholar]
- 111.Androulakis XM, Kodumuri N, Giamberardino LD, Rosamond WD, Gottesman RF, Yim E, Sen S: Ischemic stroke subtypes and migraine with visual aura in the ARIC study. Neurology 2016, 87(24):2527–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moyer AM, de Andrade M, Weinshilboum RM, Miller VM: Influence of SULT1A1 genetic variation on age at menopause, estrogen levels, and response to hormone therapy in recently postmenopausal white women. Menopause 2016, 23(8):863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sudhir K, Chou TM, Chatterjee K, Smith EP, Williams TC, Kane JP, Malloy MJ, Korach KS, Rubanyi GM: Premature coronary artery disease associated with a disruptive mutation in the estrogen receptor gene in a man. Circulation 1997, 96:3774–3777. [DOI] [PubMed] [Google Scholar]
- 114.Gialeraki A, Valsami S, Pittaras T, Panayiotakopoulos G, Politou M: Oral Contraceptives and HRT Risk of Thrombosis. Clin Appl Thromb Hemost 2016:1076029616683802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shufelt CL, Bairey Merz CN: Contraceptive hormone use and cardiovascular disease. J Am Coll Cardiol 2009, 53(3):221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Samson ME, Adams SA, Merchant AT, Maxwell WD, Zhang J, Bennett CL, Hebert JR: Cardiovascular disease incidence among females in South Carolina by type of oral contraceptives, 2000–2013: a retrospective cohort study. Arch Gynecol Obstet 2016, 294(5):991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dinger J, Do Minh T, Heinemann K: Impact of estrogen type on cardiovascular safety of combined oral contraceptives. Contraception 2016, 94(4):328–339. [DOI] [PubMed] [Google Scholar]
- 118.Dokras A: Noncontraceptive use of oral combined hormonal contraceptives in polycystic ovary syndrome-risks versus benefits. Fertil Steril 2016, 106(7):1572–1579. [DOI] [PubMed] [Google Scholar]
- 119.Shuster LT, Gostout BS, Grossardt BR, Rocca WA: Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int 2008, 14(3):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rocca WA, Gazzuola-Rocca L, Smith CY, Grossardt BR, Faubion SS, Shuster LT, Kirkland JL, Stewart EA, Miller VM: Accelerated Accumulation of Multimorbidity After Bilateral Oophorectomy: A Population-Based Cohort Study. Mayo Clin Proc 2016, 91(11):1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rocca WA, Gazzuola Rocca L, Smith CY, Grossardt BR, Faubion SS, Shuster LT, Kirkland JL, Stewart EA, Miller VM: Bilateral Oophorectomy and Accelerated Aging: Cause or Effect? J Gerontol A Biol Sci Med Sci 2017, 72(9):1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rivera CM, Grossardt BR, Rhodes DJ, Brown RD, Roger VL, Melton LJ, Rocca WA: Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009, 16(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Daan NM, Muka T, Koster MP, Roeters van Lennep JE, Lambalk CB, Laven JS, Fauser CG, Meun C, de Rijke YB, Boersma E et al. : Cardiovascular Risk in Women With Premature Ovarian Insufficiency Compared to Premenopausal Women at Middle Age. J Clin Endocrinol Metab 2016, 101(9):3306–3315. [DOI] [PubMed] [Google Scholar]
- 124.Roeters van Lennep JE, Heida KY, Bots ML, Hoek A, collaborators of the Dutch Multidisciplinary Guideline Development Group on Cardiovascular Risk Management after Reproductive D: Cardiovascular disease risk in women with premature ovarian insufficiency: A systematic review and meta-analysis. Eur J Prev Cardiol 2016, 23(2):178–186. [DOI] [PubMed] [Google Scholar]
- 125.Sullivan SD, Sarrel PM, Nelson LM: Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril 2016, 106(7):1588–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rocca WA, Grossardt BR, Miller VM, Shuster LT, Brown RD Jr., : Premature menopause or early menopause and risk of ischemic stroke. Menopause 2012, 19(3):272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rocca WA, Gazzuola Rocca L, Smith CY, Grossardt BR, Faubion SS, Shuster LT, Kirkland JL, Stewart EA, Miller VM: Bilateral Oophorectomy and Accelerated Aging: Cause or Effect? J Gerontol A Biol Sci Med Sci 2017, 72:1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Criqui MH, Suarez L, Barrett-Connor E, McPhillips J, Wingard DL, Garland C: Postmenopausal estrogen use and mortality. Results from a prospective study in a defined, homogeneous community. Am J Epidemiol 1988, 128:606–614. [DOI] [PubMed] [Google Scholar]
- 129.Falkeborn M, Persson I, Adami HO, Bergstrom R, Eaker E, Lithell H, Mohsen R, Naessen T: The risk of acute myocardial infarction after oestrogen and oestrogen-progestogen replacement. British journal of obstetrics and gynaecology 1992, 99(10):821–828. [DOI] [PubMed] [Google Scholar]
- 130.Henderson BE, Paganini-Hill A, Ross RK: Estrogen replacement therapy and protection from acute myocardial infarction. Am J Obstet Gynecol 1988, 159(2):312–317. [DOI] [PubMed] [Google Scholar]
- 131.Petitti DB, Perlman JA, Sidney S: Noncontraceptive estrogens and mortality: long-term follow-up of women in the Walnut Creek Study. Obstet Gynecol 1987, 70(3 Pt 1):289–293. [PubMed] [Google Scholar]
- 132.Stampfer MJ, Willett WC, Colditz GA, Rosner B, Speizer FE, Hennekens CH: A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med 1985, 313(17):1044–1049. [DOI] [PubMed] [Google Scholar]
- 133.Wolf PH, Madans JH, Finucane FF, Higgins M, Kleinman JC: Reduction of cardiovascular disease-related mortality among postmenopausal women who use hormones: Evidence from a national cohort. Am J Obstet Gynecol 1991, 164:489–494. [DOI] [PubMed] [Google Scholar]
- 134.Bush TL, Barrett-Connor E, Cowan LD, Criqui MH, Wallace RB, Suchindran CM, Tyroler HA, Rifkind BM: Cardiovascular mortality and noncontraceptive use of estrogen in women: Results from the Lipid Research Clinics Program Follow-up Study. Circulation 1987, 75:1102–1109. [DOI] [PubMed] [Google Scholar]
- 135.Hodis H, Mack W, Lobo R, Shoupe D, Sevanian A, Mahrer P, Selzer R, Liu C, Liu C, Azen S: Estrogen in the Prevention of Atherosclerosis. a randomized, double-blind, placebo-controlled trial. Annals of Internal Medicine 2001, 135(11):939–953. [DOI] [PubMed] [Google Scholar]
- 136.Miller VM, Harman SM: An update on hormone therapy in postmenopausal women: mini-review for the basic scientist. AmJPhysiol(Heart CircPhysiol42) 2017, 313(5):H1013–H1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anderson G, Cummings S, Freedman LS, Furberg C, Henderson M, Johnson SR, Kuller L, Manson J, Oberman A, Prentice RL et al. : Design of the Women’s Health Initiative Clinical Trial and Observational Study. Controlled Clin Trials 1998, 19(1):61–109. [DOI] [PubMed] [Google Scholar]
- 138.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC et al. : Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288(3):321–333. [DOI] [PubMed] [Google Scholar]
- 139.Langer RD, Simon JA, Pines A, Lobo RA, Hodis HN, Pickar JH, Archer DF, Sarrel PM, Utian WH: Menopausal hormone therapy for primary prevention: why the USPSTF is wrong. Menopause 2017, 24(10):1101–1112. [DOI] [PubMed] [Google Scholar]
- 140.Langer RD: The evidence base for HRT: what can we believe? Climacteric 2017, 20(2):91–96. [DOI] [PubMed] [Google Scholar]
- 141.Clarkson TB, Melendez GC, Appt SE: Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause 2013, 20(3):342–353. [DOI] [PubMed] [Google Scholar]
- 142.Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, Pettinger M, Heckbert SR, Greep N, Crawford S et al. : Conjugated equine estrogens and coronary heart disease. Arch Intern Med 2006, 166:357–365. [DOI] [PubMed] [Google Scholar]
- 143.Manson J, Allison M, Rossouw JE, Carr J, Langer R, Hsia J, Kuller L, Cochrane B, Hunt J, Ludlam S et al. : Estrogen Therapy and Coronary-Artery Calcification. N Engl J Med 2007, 356(25):2591–2602. [DOI] [PubMed] [Google Scholar]
- 144.Rossouw J, Prentice R, Manson J, Wu L, Barad D, Barnabei V, Ko M, LaCroix A, Margolis K, Stefanick M: Postmenopausal Hormone Therapy and Risk of Cardiovascular Disease by Age and Years Since Menopause. JAMA 2007, 297(13):1465. [DOI] [PubMed] [Google Scholar]
- 145.Schierbeck L, Rejnmark L, Tofteng C, Stilgren L, Eiken P, Mosekilde L, Kober L, Jensen J: Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomized trial. BMJ 2012, 345:e6409. [DOI] [PubMed] [Google Scholar]
- 146.Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N et al. : Vascular Effects of Early versus Late Postmenopausal Treatment with Estradiol. The New England journal of medicine 2016, 374(13):1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Faigle JW, Schenkel L: Pharmacokinetics of estrogens and progestogens, vol. 1 London: Chuyrchill Livingston; 1998. [Google Scholar]
- 148.Scarabin PY, Oger E, Plu-Bureau G: Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet 2003, 362:428–432. [DOI] [PubMed] [Google Scholar]
- 149.Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Levesque H, Trillot N, Barrellier MT, Wahl D, Emmerich J et al. : Estrogen and Thromboembolism Risk (ESTHER) Study Group. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation 2007, 115(7):840–845. [DOI] [PubMed] [Google Scholar]
- 150.Straczek C, Oger E, de Jonage-Canonico BY, Plu-Bureau G, Conard J, Meyer G, Alhenc-Gelas M, Levesque H, Trillot N, Barrellier M-T et al. : Prothrombotic mutations, hormone therapy, and venous thromboembolism among postmenopausal women. Impact of the route of estrogen administration. Circulation 2005, 112:3495–3500. [DOI] [PubMed] [Google Scholar]
- 151.de Andrade M, Armasu SM, McCauley BM, Petterson TM, Heit JA: Identification of Genetic Interaction with Risk Factors Using a Time-To-Event Model. Int J Environ Res Public Health 2017, 14(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N: KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric 2005, 8:3–12. [DOI] [PubMed] [Google Scholar]
- 153.Miller VM, Black DM, Brinton EA, Budoff MJ, Cedars MI, Hodis HN, Lobo RA, Manson JE, Merriam GR, Naftolin F et al. : Using basic science to design a clinical trial: baseline characteristics of women enrolled in the Kronos Early Estrogen Prevention Study (KEEPS). J Cardiovasc Transl Res 2009, 2(3):228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bray PF, Larson JC, Lacroix AZ, Manson J, Limacher MC, Rossouw JE, Lasser NL, Lawson WE, Stefanick ML, Langer RD et al. : Usefulness of baseline lipids and C-reactive protein in women receiving menopausal hormone therapy as predictors of treatment-related coronary events. Am J Cardiol 2008, 101(11):1599–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wild RA, Wu C, Curb JD, Martin LW, Phillips L, Stefanick M, Trevisan M, Manson JE: Coronary heart disease events in the Women’s Health Initiative hormone trials: effect modification by metabolic syndrome: A nested case-control study within the Women’s Health Initiative randomized clinical trials. Menopause 2013, 20:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, Hopkins PN, Lobo RA, Manson JEM, G R, Miller VM et al. : Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: A randomized trial. Ann Intern Med 2014, 161(4):249–260. [DOI] [PubMed] [Google Scholar]
- 157.Santoro N, Allshouse A, Neal-Perry G, Pal L, Lobo RA, Naftolin F, Black DM, Brinton EA, Budoff MJ, Cedars MI et al. : Longitudinal changes in menopausal symptoms comparing women randomized to low-dose oral conjugated estrogens or transdermal estradiol plus micronized progesterone versus placebo: the Kronos Early Estrogen Prevention Study. Menopause 2017, 24(3):238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, Brinton EA, Cedars MI, Lobo RA, Merriam GR et al. : Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS-Cognitive and Affective Study. PLoS medicine 2015, 12(6):e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Taylor HS, Tal A, Pal L, Li F, Black DM, Brinton EA, Budoff MJ, Cedars MI, Du W, Hodis HN et al. : Effects of Oral vs Transdermal Estrogen Therapy on Sexual Function in Early Postmenopause: Ancillary Study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med 2017, 177(10):1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Miller VM, Petterson TM, Jeavons EN, Lnu AS, Rider DN, Heit JA, Cunningham JM, Huggins GS, Hodis HN, Budoff MJ et al. : Genetic polymorphisms associated carotid artery intima-media thickness and coronary artery calcification in women of the Kronos Early Estrogen Prevention Study. Physiol Genomics 2013, 45(2):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Miller VM, Jenkins GD, Biernacka JM, Heit JA, Huggins GS, Hodis HN, Budoff MJ, Lobo RA, Taylor HS, Manson JE et al. : Pharmacogenomics of estrogens on changes in carotid artery intima-medial thickness and coronary arterial calcification: Kronos Early Estrogen Prevention Study. Physiol Genomics 2016, 48(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


