Abstract
Sickle cell disease (SCD) pain associates with cold temperature and touch. Patients and murine models with SCD have baseline thermal and mechanical pain. In SCD mice, the baseline hypersensitivity is exacerbated by experimental vaso-occlusive crises. We hypothesized that SCD patients will similarly experience increased hypersensitivity to thermal and mechanical stimuli during acute painful events compared to baseline health. We conducted a prospective study of 24 SCD patients ages 7–19 yrs. Patients underwent quantitative sensory testing to thermal (cold/heat) and mechanical stimuli on the thenar eminence of the non-dominant hand (glabrous skin) and the lateral dorsum of the foot (hairy skin) during baseline health and within 48 hrs of hospitalization for acute pain. Primary outcomes were changes in: 1) Cold Pain Threshold (°C), 2) Heat Pain Threshold (°C) and 3) Mechanical Pain Threshold (g). Median age was 10.5 (IQR 9–14.8) yrs, 67% were female and 92% were on hydroxyurea. SCD patients had increased cold pain sensitivity in the hand during hospitalization compared to baseline [25.2°C (IQR 18.4–27.5°C) vs. 21.3°C (IQR 4.9–26.2°C); p=0.011] and increased mechanical pain sensitivity in the foot during hospitalization [0.32g (IQR 0.09–1.1g) vs. 1.7g (IQR 0.4–8.3g); p=0.003]. There were no differences in heat pain sensitivity. The increased cold (p=0.02) and mechanical (p=0.0016) pain sensitivity during hospitalization persisted after adjusting for age, gender, hydroxyurea use, opioid consumption and numeric pain score. Thus, cold and mechanical pain is significantly worse during an acute SCD painful event as compared to baseline health in patients with SCD.
Keywords: sickle cell disease, acute pain, cold pain threshold, mechanical pain threshold
Summary
Quantitative sensory testing in patients with sickle cell disease prospectively shows increased cold and mechanical hypersensitivity during acute painful events as compared to baseline health.
Introduction
Severe, debilitating pain is the most common complication of sickle cell disease (SCD), an inherited hemoglobinopathy affecting ~100,000 people in the US and over 3 million worldwide.[16; 70] Epidemiological studies reveal that SCD pain frequency and intensity in adults are associated with cold temperatures, increased wind speed, increased barometric pressure and skin touch.[66; 73; 82]. Further, heightened sensitivity to cold temperatures is also a major complaint of SCD patients and can be a pain trigger.[3; 46; 72; 75; 83] The biological causes of these associations are not yet known. SCD pain has clinical features of neuropathic pain as adolescents and adults with SCD describe their pain as “cold”, “hot” and “shooting” and approximately 40% score positive on neuropathic pain screening questionnaires.[12; 35; 93] The precipitating environmental factors and the array of pain descriptors suggest that SCD patients have hypersensitivity to evoked tactile stimuli, a characteristic of several pain types including neuropathic pain, inflammatory pain, peripheral neuropathies and centralized pain disorders.[7; 47; 94; 97]
SCD patients in baseline health have evidence of nervous system sensitization. Compared to race-matched healthy controls, adolescents and adults with SCD during their baseline state of health have hypersensitivity to cold and heat stimuli assessed using quantitative sensory testing (QST).[13–15] QST is a psychophysical testing modality used to interrogate human pain processing through the application of thermal (cold, heat) and mechanical stimuli. These data in adolescents and adults are corroborated by similar findings in SCD murine models where mice display marked behavioral hypersensitivity to mechanical (tactile), cold and heat stimuli at baseline.[44; 54; 89] The baseline mechanical and cold hypersensitivity in mice is exacerbated by hypoxia-reoxygenation, an experimental method used to induce acute vaso-occlusion.[18; 44] To date, published QST studies in adolescents and adults have assessed patients during baseline health[15; 20; 35; 68], and in children, after a painful event has occurred.[6] It is not known if exacerbations in thermal or mechanical pain occur during episodes of acute painful events in SCD patients. Therefore, the primary objective of this study was to quantify differences in thermal and mechanical pain thresholds in the same child or adolescent with SCD during baseline health and during an acute painful event. We hypothesized that children ages 7–19 years with SCD experience increased sensitivity to thermal (cold, heat) and mechanical stimuli during acute painful events as compared to their baseline health.
Methods
Study Setting and Subjects
A prospective cohort study was conducted between 2014 and 2017. Inclusion criteria were: 1) Age 7–19 years[61] and 2) Diagnosis of SCD (all genotypes). Exclusion Criteria were: 1) Other chronic disease resulting in pain phenotype or neurological manifestation, 2) History of overt or silent stroke based on known clinical history and available neuroimaging, 3) Currently receiving chronic red blood cell transfusions and 4) Pregnancy.
Subject recruitment occurred by three mechanisms: 1) Pre-consenting during routine SCD clinic visits; desire to participate was affirmed if subject was hospitalized for pain[17], 2) Within 48 hours of pain admission and 3) By phone contact from our previously established database of subjects that consented to be contacted for future pain studies. Patients returned for baseline testing at a time when they had not received opioids for the prior 24 hours (baseline health cohort) and were evaluated when admitted for treatment of an acute painful event (acute pain cohort). All patients were evaluated at both time points. To enhance recruitment and the acquisition of paired data, the order of testing could vary. Thus, some patients had baseline assessments performed first and some had acute pain assessments first. Standard of care for an acute painful event, at the discretion of the treating physician, was given to all patients and included opioids and intravenous fluids. No patients in the study were on chronic opioid therapy at home.
The Children’s Hospital of Wisconsin Institutional Review Board granted approval for the study. Informed written consent was obtained from the parent or legal guardian and informed written assent was obtained from the child when age appropriate. Stipends were given for participation and transportation to the study site was provided for baseline testing. Data were analyzed by co-authors (Pan and Nugent) and all authors had access to the data.
Primary Outcomes
The primary outcomes were the change in the following thresholds between the disease states of baseline health and acute pain: 1) Cold pain threshold (°C), 2) Heat pain threshold (°C) and 3) Mechanical pain threshold (g). All outcomes were assessed using QST in the same patient during baseline health and during admission for an acute painful event. Per our prior work, baseline state of health was defined as the absence of an acute SCD painful event severe enough to require intravenous opioids within 2 weeks prior to testing[14; 15] and with no opioids consumed in past 24 hours.
Variables of interest
Age, gender, hydroxyurea use, opioid consumption and clinical pain score were examined as potential variables of interest that could affect our primary outcome. Opioid consumption was defined in two ways: 1) total oral morphine equivalents (OME) consumed from presentation in ED through time of QST and 2) total OME in the immediate one hour prior to QST. Older age has been associated with decreased cold and heat pain thresholds (heightened sensitivity)[32–34] and females have been shown to have higher pain sensitivity than males.[38; 69; 74] Hydroxyurea lessens SCD severity and has been shown to decrease the pain frequency in patients and therefore, we accounted for hydroxyurea consumption.[25; 90] Opioids have the potential to induce hypersensitivity and pain with chronic administration, and therefore, we accounted for opioid consumption.[27]
Study Procedure
Quantitative Sensory Testing (QST), a psychophysical evaluation of the somatosensory system[59], was conducted at both study timepoints (baseline and acute pain). The standard protocol for QST from our previous work was used for this study.[14; 15] QST utilizes the application of physical stimuli (mechanical, heat and cold) to activate peripheral sensory receptors that subsequently generate pain signals in the pain pathways of the central nervous system. QST can evaluate sensory loss (hyposensitivity) or sensory gain (hypersensitivity) to the applied stimuli.[4; 5] QST has been used in children and adults with SCD[6; 15; 20; 35; 68], a variety of pain conditions such as migraine and headaches, recurrent abdominal pain, rheumatoid pain[45; 98; 99] and is often utilized as an outcome in pain clinical trials.[19; 84; 85]
QST was conducted by one of two research personnel for the duration of the study. In order to minimize experimenter bias, the principal investigator did not conduct testing. The site of QST included the thenar eminence (glabrous skin) of the non-dominant hand and the lateral dorsum (hairy skin) of the foot; both hand and foot sites were randomized to right/left using a table of random numbers. These testing sites were chosen to be consistent with our prior work and have been used as reference sites in other QST studies.[14; 15; 61] Room temperature was kept constant between 68–72°C and subjects acclimated to this temperature for 10–15 minutes before testing began. Scripted instructions were read to each subject to ensure the testing methodology was standardized. QST was completed in the following order: (1) mechanical pain threshold, (2) cold pain threshold and (3) heat pain threshold. This testing order was chosen since cold/heat pain thresholds tested before mechanical pain thresholds could influence the outcome of mechanical thresholds.[43] Data support that the conduct of QST in this manner is reproducible.[43; 67]
The primary outcomes were: 1) Cold Pain Threshold (median of 3 measures), 2) Heat Pain Threshold (median of 3 measures) and 3) Mechanical Pain Threshold (median of 5 measures). The thermal and mechanical thresholds were determined utilizing the “method of limits”.[61]
Thermal (cold, heat) testing
Cold and heat stimulation was performed with a Thermal Sensory Analyzer (TSA-II; Medoc; Israel)[1], an FDA approved computer assisted QST device. This device was used in our prior SCD work and has been used in QST studies conducted by many other groups in other childhood and adult diseases (i.e., chronic regional pain syndrome, migraines, juvenile rheumatoid arthritis and normal healthy controls).[45; 61; 80; 99] This device delivers cold or warm stimuli through a computer-driven thermode attached to the skin. The baseline temperature was 32°C (average normal skin temperature in patients) and the stimulus range was 0–50°C. Cold and heat pain thresholds were determined utilizing the “method of limits” where participants were directed to push a button when the sensation of pain was perceived.[61] These thresholds were chosen based on murine studies and other prior QST studies in humans.[61; 99] Cold and heat pain thresholds. The probe temperature decreased/increased linearly from baseline temperature (32°C) at 1.5 °C/sec. When the stimulus was painful, a button was pressed, and this was defined as the cold pain or heat pain threshold. The temperature change was then halted and the thermode temperature was reset to baseline at a rate of 10°C/sec. This procedure was repeated three times with an interstimulus interval of 10 sec. The final cold or heat pain threshold was determined by calculating the median of the three threshold temperatures (°C).[10; 61; 76; 80]
Mechanical testing
Mechanical testing was performed using a standardized set of graded von Frey monofilaments that have an applied force upon bending that ranges from 0.026 grams (g) to 110 g.[51] Similar monofilaments have been used in our prior work and other QST studies.[10; 14; 15; 51] Mechanical pain threshold. A subject’s hand and foot were placed behind a screen, so he/she could not see the filament applied to the skin. Starting with the lowest intensity, von Frey filaments were applied with a 1–2 second contact time. The “method of limits”[61] was used, where the subject was asked to reply “yes” if the stimulus was “painful.” Once this threshold was identified, the operator started again with the lowest von Frey monofilament to assess another threshold. Five threshold determinations (subject answered “yes”) were done and the final threshold was the median of the varying forces (g) of these five series.[10; 76]
Assessment of sedation and pain level
Pain scores were assessed at both testing time points (baseline and acute pain) using the Numeric Rating Scale.[86] To ensure the patient was able to complete the testing protocol during inpatient admission, sedation scores were obtained prior to testing. The Pasero Opioid Induced Sedation Scale[65] was used (S = Sleep, easy to arouse, 1 = Awake and alert, 2 = Slightly drowsy, easily aroused, 3 = Frequently drowsy, arousable, drifts off to sleep during conversation, 4 = Somnolent, minimal or no response to physical stimulation). If the sedation score was greater than 2 and the subject could not give a pain score, testing was not performed, and the subject’s sedation level and pain score were reassessed every 2 hours until the sedation score was 2 or less.
Collection of clinical data
To characterize the study population, age, gender, hydroxyurea use, hemoglobin genotype and baseline laboratory data (hemoglobin, reticulocyte count, white blood cell count and hemoglobin F) were collected. Total oral morphine equivalents[77] consumed in the emergency department and inpatient unit from arrival until completion of inpatient QST were collected (i.e., opioid consumption). All data were extracted from the medical record. Patients completed a questionnaire that elicited responses to weather-related pain triggers and included the following questions: 1) “Do temperature changes from warm to cold trigger your pain?” and 2) “Do temperature changes from cold to warm trigger your pain?”
Data Analysis
Patients’ characteristics were summarized as median with interquartile range (IQR) for continuous data and n (%) for categorical data. Either appropriate transformations were employed to meet parametric assumptions, or a non-parametric method was used. Medians of outcomes were compared between baseline health and acute painful events using Wilcoxon signed-rank test since the data were non-normally distributed. Additionally, repeated measures analysis was done with an unstructured within-subject variance-covariance matrix. The impact of testing order (baseline before acute pain vs. acute pain before baseline) on thresholds was investigated by including order in the model. The impact of genotype [HbSS/HbSO-Arab (most severe) and HbSC/HbSB+-thal (least severe)] was examined similarly. Age, gender, hydroxyurea use, opioid consumption and numeric pain score at the time of testing, were included as covariates. Opioid consumption was defined in two ways: 1) total opioids consumed from ED arrival to QST and 2) total opioids consumed in the one hour prior to QST. Log or square transformed data were used to ensure fit. A McNemar’s exact test was used to determine whether there was a proportional difference in responses to questions regarding weather pain triggers. A p-value of <0.05, with no correction for multiple testing, was considered significant. SPSS version 24 (IBM Software, Chicago, IL, USA) and SAS 9.4 (SAS Institute, Cary, NC) were used to analyze the data.
Results
Study Population
A total of 24 SCD patients completed QST during baseline health and inpatient admission for an acute painful event. Median age at enrollment was 10.5 (IQR 9.0–14.8) yrs, 67% were female and 92% were on hydroxyurea. The remainder of the patient characteristics upon study entry are summarized in Table 1. Median interval between testing timepoints was 15.8 (IQR 5.1–22.1) months. The median Pasero opioid-induced sedation scale at the time of inpatient testing was 1 (IQR 1–1); only two patients had a score of two. This suggests that nearly all patients were awake and alert and only 2 were slightly drowsy, but easily aroused. Patients were tested a median of 25 (IQR 19–35) hours from hospital admission. The median pain score at the time of baseline testing was 0 and at the time of inpatient testing was 6 (IQR 4–7.75), suggesting patients were experiencing ongoing pain at the time of testing. A median of 115.2 (IQR 54.6–164.4) mg of oral morphine equivalents (OME) were consumed from treatment in the emergency department through the time of inpatient testing. A median of 0.3 (IQR 0–4.7) mg of OME were consumed in the one hour prior to inpatient testing.
Table 1.
Patients’ characteristics at study entry (n=24)
| Variable | n (%), median (IQR) |
|---|---|
| Age (years) | 10.5 (9–14.8) |
| Gender: Female | 16 (67) |
| Genotype | |
| HbSS | 15 (63) |
| HbSC | 7 (29) |
| HbSβ+thal | 1 (4) |
| HbSOArab | 1 (4) |
| Hydroxyurea use | 22 (92) |
| Labs | |
| Hb (g/dL) | 9.8 (8.9−11.1) |
| Retic (%) | 6.1 (3.3−11.4) |
| WBC (K/uL) | 7.7 (5.4−10.5) |
| HbF (%) | 14.1 (3.1−23.0) |
| Opioids consumed from ED treatment to inpatient testing (oral morphine equivalents, mg) |
115.2 (54.6–164.4) |
| Opioids consumed in one hour prior to inpatient testing (oral morphine equivalents, mg) |
0.3 (0–4.7) |
| Time between admission and inpatient testing (hours) | 25 (19–35) |
SCD patients have increased cold pain sensitivity in the hand during acute painful events
Figure 1 displays the change in median cold pain thresholds between baseline health and acute painful events in the thenar eminence (glabrous skin) of the hand and the lateral dorsum (hairy skin) of the foot. As displayed, the threshold temperature for cold pain in the hand was higher (i.e., warmer; pain felt closer to 32°C) during an acute painful event as compared to baseline health [25.2°C (IQR 18.4–27.5°C) vs. 21.3°C (IQR 4.9–26.2°C); p=0.011]. This finding reflects increased cold pain sensitivity during acute painful events as compared to baseline health. There were no significant changes in cold sensitivity in the foot during an acute painful event compared to baseline health. The sickle cell disease genotype had no effect on the outcome (p>0.39). Further, the order of testing (baseline or acute pain first or second) had no effect on the outcome (p>0.91).
Figure 1.
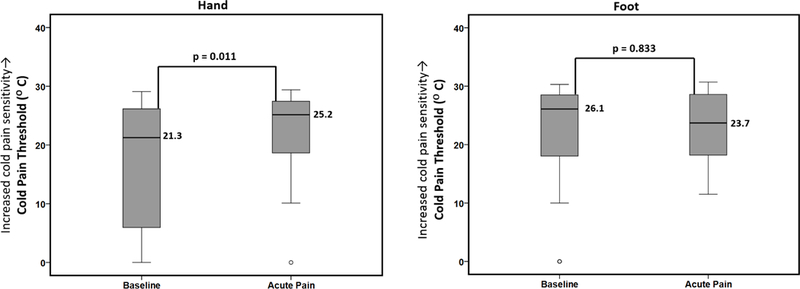
Differences in cold pain thresholds between baseline health and acute painful events. Boxplots displaying differences in median cold pain thresholds between SCD patients at baseline health and during acute pain (n=24, measured at both timepoints) analyzed using Wilcoxon signed-rank test. There was a significant increase in the median cold pain threshold in the hand (i.e., pain felt closer to 32°C) during acute pain as compared to baseline health reflecting increased cold pain sensitivity during acute pain. There were no measured differences in the foot. Note: open circles represent outliers.
SCD patients do not display differences in heat pain sensitivity during acute painful events
Figure 2 displays the change in median heat pain thresholds between baseline health and acute painful events in the hand and foot. As displayed, there were no significant changes in the heat pain thresholds between baseline health and acute painful events in either the hand or the foot.
Figure 2.
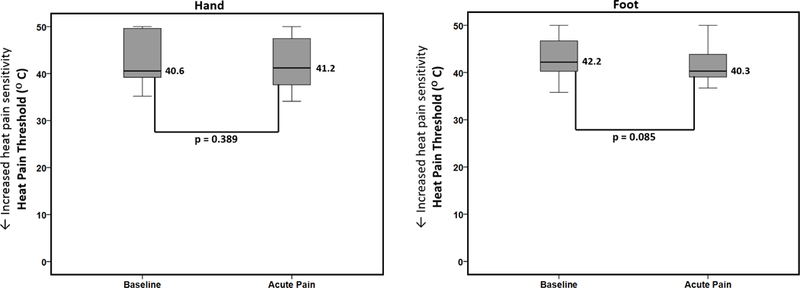
Differences in heat pain thresholds between baseline health and acute painful events. Boxplots displaying differences in median heat pain thresholds between SCD patients at baseline health and during acute pain (n=24, measured at both timepoints) analyzed using Wilcoxon signed-rank test. There were no differences in heat pain sensitivity between the two timepoints in the hand or the foot.
SCD patients have increased mechanical pain sensitivity in the foot during acute painful events
Figure 3 displays the change in median mechanical pain thresholds between baseline health and acute painful events in the hand and foot. There was a significant decrease in the mechanical pain threshold in the foot during acute painful events as compared to baseline health reflecting an increase in mechanical pain sensitivity during acute pain [0.32g (IQR 0.09–1.1) vs. 1.7g (IQR 0.4–8.3); p=0.003]. There was also a decrease in mechanical pain thresholds in the hand during acute painful events as compared to baseline health that approached significance in the direction of our hypothesis [0.4g (IQR 0.15–17.0) vs. 3.3g (IQR 0.32–34.0); p=0.051]. The decrease in the mechanical pain thresholds indicates that patients have increased mechanical pain sensitivity during acute painful events as compared to their own baseline health. The sickle cell disease genotype had no effect on the outcome (p>0.99). Further, the order of testing had no effect on the outcome (p>0.17).
Figure 3.
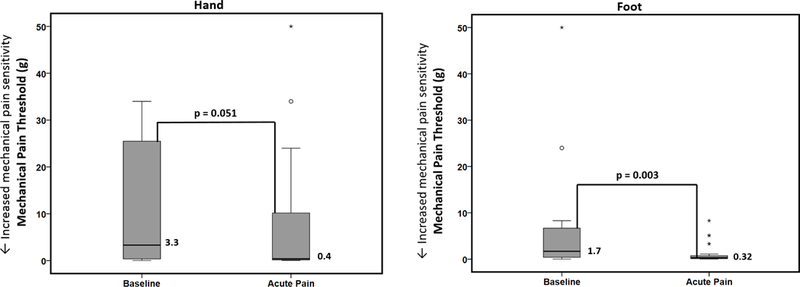
Differences in mechanical pain thresholds between baseline health and acute painful events. Boxplots displaying differences in median mechanical pain thresholds between SCD patients at baseline health and during acute pain (n=24, measured at both timepoints) analyzed using Wilcoxon signed-rank test. There was a significant decrease in the mechanical pain threshold in the foot during acute pain as compared to baseline health reflecting an increase in mechanical pain sensitivity during acute pain. There was also a decrease in mechanical pain thresholds in the hand during acute pain as compared to baseline health that approached (but did not reach) significance in the direction of our hypothesis. Note: open circles and asterisks represent outliers.
Age, gender, hydroxyurea use, opioid consumption and clinical pain score do not impact the change in cold and mechanical pain thresholds between baseline health and acute painful events
We evaluated the impact of age, gender, hydroxyurea use, opioid consumption and clinical pain score on the pain thresholds that significantly changed between baseline and acute painful events. Specifically, we determined whether these covariates affect the change in cold pain thresholds (hand) and mechanical pain thresholds (foot) between the two disease states (baseline and acute pain). Age, gender, hydroxyurea use, opioid consumption and clinical pain score had no significant impact on the change in cold and mechanical pain outcomes (Tables 2, 3, 4 and 5). Disease state, which represents the change between baseline health and acute painful events, with baseline health as the reference in the model, remained significant for cold and mechanical pain after adjusting for the other covariates.
Table 2.
Effect of time, gender, age, hydroxyurea use, total opioid consumption and clinical pain score on cold pain threshold of hand
| Variable | Estimate | SE | P value |
|---|---|---|---|
| Time (Acute pain vs Baseline) | 137.05 | 53.62 | 0.02 |
| Gender (Female vs Male) | 171.27 | 114.73 | 0.15 |
| Age | 32.08 | 16.27 | 0.06 |
| Hydroxyurea (Yes vs No) | −267.93 | 172.67 | 0.14 |
| Opioid consumption (total) Clinical Pain Score |
−0.75 −33.64 |
0.59 22.26 |
0.22 0.15 |
Data were square transformed to ensure fit
SE: standard error
Table 3.
Effect of time, gender, age, hydroxyurea use and opioid consumption one-hour prior and clinical pain score on cold pain threshold of hand
| Variable | Estimate | SE | P value |
|---|---|---|---|
| Time (Acute Pain vs Baseline) | 137.05 | 53.62 | 0.02 |
| Gender (Female vs Male) | 155.48 | 117.32 | 0.20 |
| Age | 25.49 | 16.27 | 0.13 |
| Hydroxyurea (Yes vs No) | −293.38 | 180.07 | 0.12 |
| Opioid consumption (one-hour prior) | −9.20 | 14.91 | 0.54 |
| Clinical Pain Score | −19.43 | 25.72 | 0.46 |
Data were square transformed to ensure fit
SE: standard error
Table 4.
Effect of time, gender, age, hydroxyurea use and total opioid consumption and clinical pain score on mechanical pain threshold of foot
| Variable | Estimate | SE | P value |
|---|---|---|---|
| Time (Acute pain vs Baseline) | −0.82 | 0.22 | 0.0017 |
| Gender (Female vs Male) | −0.04 | 0.40 | 0.92 |
| Age | 0.03 | 0.05 | 0.54 |
| Hydroxyurea (Yes vs No) | −0.59 | 0.54 | 0.29 |
| Opioid consumption (total) Clinical Pain Score |
0.001 −0.08 |
0.002 0.08 |
0.57 0.32 |
Data were log transformed to ensure fit
SE: standard error
Table 5.
Effect of time, gender, age, hydroxyurea use and opioid consumption one-hour prior and clinical pain score on mechanical pain threshold of foot
| Variable | Estimate | SE | P value |
|---|---|---|---|
| Time (Acute pain vs Baseline) | −0.82 | 0.22 | 0.0017 |
| Gender (Female vs Male) | 0.01 | 0.40 | 0.98 |
| Age | 0.04 | 0.05 | 0.45 |
| Hydroxyurea (Yes vs No) | −0.52 | 0.55 | 0.36 |
| Opioid consumption (one-hour prior) | −0.003 | 0.05 | 0.95 |
| Clinical Pain Score | −0.09 | 0.09 | 0.31 |
Data were log transformed to ensure fit
SE: standard error
Patients report that environmental temperature changes from warm to cold trigger pain
More patients report that temperature changes from warm to cold trigger their pain (Yes: 63%) as compared to temperature changes from cold to warm (Yes: 26%) (63% vs. 26%; p=0.039). These data are consistent with hypersensitivity to cold but not warmth in SCD.
Discussion
Using QST, we report that youth with SCD display enhanced sensitivity to cold and mechanical stimuli during acute painful events as compared to their baseline health. This enhanced sensitivity persisted after controlling for age, gender, hydroxyurea use, opioid consumption and clinical pain. Our data are strengthened by the paired study design where each patient served as their own control, thereby reducing variability.
Epidemiological studies indicate higher pain frequency and intensity are associated colder temperatures.[66, 73, 82] We previously showed that SCD patients have higher cold pain sensitivity compared to healthy African American controls during baseline health.[15] Similarly, SCD mice display significantly greater sensitivity to cold stimuli at baseline health compared to age-matched controls, and the cold hypersensitivity worsens with age.[89; 96] Furthermore, the baseline cold hypersensitivity in SCD mice is exacerbated by hypoxia-reoxygenation, an experimental method that induces RBC sickling and vaso-occlusion and that mimics the acute pain state in SCD patients.[18; 44; 54; 96] Data presented here corroborate murine SCD findings by showing that patients have worse cold pain sensitivity during acute painful events compared to their baseline health. Our findings of increased mechanical sensitivity during acute painful events also corroborate patients’ pain descriptions and data from neuropathic pain screening tools that show increased sensitivity to touch or hyperalgesia during vaso-occlusive events.[12; 35; 93] The murine model of SCD has also shown higher baseline mechanical sensitivity than age matched controls[24; 44; 54], and the hypersensitivity is exacerbated in SCD mice during hypoxia-reoxygenation induced acute crises.[18; 44; 79; 91; 96] Thus, our data present new evidence that patients with SCD may have biologic changes that account for the previously described epidemiological, patient-reported and animal studies of cold and mechanical hypersensitivity.[62; 73; 82; 92]
Enhanced cold and mechanical pain sensitivity during acute painful events could be mediated by peripheral nervous system abnormalities. Using in vivo and ex vivo recordings, peripheral C-fibers from SCD mice are shown to have more sensitive cold thresholds.[89; 96] C- and Aδ fibers from SCD mice also show enhanced responsiveness to mechanical stimuli.[39; 44; 89] Candidate cold channels in sensory neurons include Transient Receptor Potential Melastatin 8 (TRPM8)[8; 48; 60; 88] and Transient Receptor Potential Ankyrin 1 (TRPA1).[49; 87] However, there were no changes in mRNA levels or functional responses to agonists for TRPM8 or TRPA1 in dorsal root ganglion sensory neurons from SCD mice.[96] Other putative cold receptors such as KCNK2, KCNQ2[2; 56] and TASK-3 potassium channel[58; 64] could be explored in SCD. The mechanical sensitization of sensory neurons in SCD mice is mediated in part by Transient Receptor Potential Vanilloid 1 (TRPV1).[44] Recent data suggest the chemokine (c-c motif) ligand 2 (CCL2) and it’s receptor CCR2 are upstream of both mechanical and cold hypersensitivity in SCD mice.[79] Furthermore, pharmacologic inhibition of peripheral anandamide hydrolysis decreased mechanical hypersensitivity in SCD[89] via a cannabinoid receptor type 1 (CB1) but not CB2 mechanism. Thus, increasing peripheral endocannabinoids could be a novel SCD pain therapy. Other mechanosensitive ion channels such as Piezo2[28; 71] may contribute to enhanced mechanical sensitivity in SCD patients during acute pain and require investigation. Since increased pain sensitivity during acute painful events in patients closely resembles the exacerbation of mechanical sensitivity following hypoxia in SCD mice, the translational mouse model could be used to determine whether altered peripheral mechanisms might serve as novel therapeutic targets for acute SCD pain.
Central nervous system (CNS) mechanisms could also mediate the enhanced cold and mechanical pain during hospitalization. Little is known about CNS pain mechanisms in SCD. Descending inhibitory pain mechanisms and cortical processing could be altered in SCD and contribute to exacerbation of cold and mechanical hypersensitivity. Pharmacologic activation of mu-opioid receptors and inhibition of chemokine receptor-5 (CCR-5) attenuated responses of spinal cord neurons to noxious cold and mechanical stimulation of the skin.[23] Further, spinal cord neurons from SCD mice have greater ongoing spontaneous activity, larger receptive fields and greater discharge rates to the same force of mechanical stimulation of skin.[24] Ca2+/calmodulin-dependent protein kinase II (CaMKIIα) has also been studied in SCD mice. CaMKIIα is expressed in the dorsal horn of the spinal cord and some primary sensory neurons. CaMKIIα, activated by intracellular calcium signaling, is implicated in neuroplasticity and its activation is associated with chronic pain states.[26; 29; 36; 95] CaMKIIα inhibition was shown to improve ongoing spontaneous mechanical pain and transiently reverse evoked mechanical sensitivity in SCD mice.[41] A Phase I study of trifluoperazine, a CaMKIIα inhibitor, has shown some analgesic effects for SCD pain with neuropathic features.[63] Alternatively, Protein kinase C delta (PKCdelta) is shown to be increased in spinal cord GABAnergic inhibitory neurons of SCD mice and mechanical hyperalgesia was diminished with PKCdelta inhibition.[42] Functional magnetic resonance imaging (fMRI) shows SCD mice displaying mechanical hyperalgesia have decreased BOLD changes in the thalamus following hypoxia-reoxygenation.[91] Collectively, these data implicate spinal cord and/or brain involvement in the enhanced mechanical sensitivity associated with SCD pain. Notably, with exception of the fMRI study, most experiments were conducted in SCD mice during baseline state, not after hypoxia-reoxygenation. Similar or different mechanisms could contribute to the enhanced mechanical sensitization experienced during acute SCD pain versus baseline sensitization. Thus, our work supports the need for additional investigations after hypoxia-reoxygenation in animal models. Alternatively, vascular changes or effects of ischemia on chronic red blood cell sickling may contribute to pain sensitization. For example, blood vessels are innervated by sensory afferent neurons and cold temperatures could stimulate vasoconstriction thereby contributing to pain exacerbation.[57; 78]
Our current study found no changes in heat pain thresholds between baseline and acute pain. Our prior work compared SCD patients at baseline to healthy African American controls and showed SCD patients experience heat hyperalgesia.[15] It is possible that heat hyperalgesia in SCD patients is static. Patients utilize heat for analgesia; thus lack of increased heat hyperalgesia during acute pain may be consistent with patient experience. SCD mice show behavioral evidence of heat hyperalgesia compared to controls during baseline state.[44; 54] The impact of hypoxia-reoxygenation induced vaso-occlusion on heat hyperalgesia reveals mixed results. Post-hypoxia, some data show increased heat hyperalgesia[18] while other data show no change.[44]
Patients with SCD experienced cold hypersensitivity in glabrous skin of the hand but not in hairy skin of the foot. There is greater epidermal nerve fiber density in the hands compared to feet, and a recent study indicates hands are twice as sensitive to thermal stimuli as feet. However, hairy skin is more sensitive than glabrous skin to temperature.[37; 52] The difference may be due to glabrous versus hairy skin. Psychophysical studies show cold pain intensity rises faster in glabrous compared to hairy skin and spatial summation of cold thresholds is greater in glabrous skin.[30; 31; 40] Perception of gentle and noxious cold temperatures is medicated largely by Aδ and C fibers[9; 21; 22; 55; 81] but a detailed study of cold receptors innervating glabrous versus hairy skin has not been reported.[30] Peripheral factors that may underlie the differences include location, depth or density of noxious cold-responsive receptors, or how close the stimulus is to superficial veins innervated by cold-sensitive nociceptors.[30] Further, cold pain perception is likely a function of both the central and peripheral nervous systems, as well as CNS integration of different thermosensitive afferent populations that respond to the stimulus.[30] Differences between hand and foot mechanical pain thresholds were less pronounced than cold. Mechanical pain sensitivity of the foot significantly increased during acute pain and a trend toward increased mechanical pain sensitivity in the hand (p=0.05) was observed.
Limitations
Limitations to our study exist. Opioids can cause sedation; however, we used the Pasero Opioid Induced Sedation Scale[65] prior to QST and deferred testing if patients were sedated. Self-completion of patient-reported measures during acute pain while receiving opioids has been demonstrated.[11; 50; 53] We cannot definitively determine threshold changes were independent of opioids. Withholding opioids would be unethical in any SCD acute pain study. We attempted to address this issue by accounting for quantity of opioids received in two ways as outlined above. Our data show the quantity of opioids received had no impact on the magnitude of threshold change between baseline and acute pain, suggesting opioids were not driving threshold changes. Since no published data exist regarding pain thresholds during SCD acute painful events, this is the first step in determining the effect of acute pain on these outcomes. Blinding QST testers as to whether subjects were at baseline or acute pain was not possible. QST testers were not blinded to the hypothesis which could have introduced bias. However, the same tester did not always conduct both baseline and hospitalized assessments on a given subject which could minimize bias. Further, thermal thresholds were computer driven and all thresholds were patient-reported (not tester determined), which further decreases bias. Since all thresholds did not show differences between baseline and acute pain, the data further support bias was likely not driving the findings. Finally, interim data analyses were not performed so QST testers did not know data trends mid-study. The sample size was relatively small; however, the paired study design increases the robustness of our findings. The median interval between testing timepoints was 15.8 (IQR 5.1–22.1) months. To enhance recruitment and acquisition of paired data, we allowed for a 2-year interval between assessments. We found age did not impact threshold changes and longitudinal pain threshold data within a SCD patient are currently absent. This is a focus of our current work and could inform this question. We did not measure pain thresholds at the active pain site for two reasons. First, we felt it was unacceptable to do QST at the active pain site. Second, we were interested in generalized versus localized pain sensitivity (i.e., active pain site). Testing at the pain site could reflect only the sensitivity of the location affected. Other potential confounders may exist and the ability to account for these in multivariate analyses may be limited due to sample size.
Conclusions
SCD patients experience worse cold and mechanical hypersensitivity during hospitalization for acute painful events, however, no changes in heat sensitivity. Investigation into the etiology of these cold and mechanical sensitivity changes with a focus on peripheral, ascending, descending and cortical pain pathways could lead to identification of therapeutic targets that could be modulated for treatment or prevention of SCD pain.
Acknowledgments
We acknowledge all the patients and families who participated in the study. We also acknowledge the staff in the Pediatric Translational Research Unit at Children’s Hospital of Wisconsin. This study was supported in part by funding from NIH 5 K23 HL114636–05 (AB), American Society of Hematology (AB) and the Midwest Athletes Against Childhood Cancer and Blood Disorders Fund (AB).
Conflict of interest statement
The authors declare no financial arrangements that represent a conflict of interest.
This work was presented in abstract form as a poster at the American Society of Hematology meeting in December 2017.
The authors declare no conflicts of interest
References
- [1].Medoc TSA-II NeuroSensory Analyzer, Vol. 2011. pp. http://www.medoc-web.com/medoc_en_tsaII_analyzer.aspx.
- [2].Abd-Elsayed AA, Ikeda R, Jia Z, Ling J, Zuo X, Li M, Gu JG. KCNQ channels in nociceptive cold-sensing trigeminal ganglion neurons as therapeutic targets for treating orofacial cold hyperalgesia. Molecular pain 2015;11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Amjad H, Bannerman RM, Judisch JM. Letter: Sickling pain and season. Br Med J 1974;2(5909):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Arendt-Nielsen L, Curatolo M, Drewes A. Human experimental pain models in drug development: translational pain research. Curr Opin Investig Drugs 2007;8(1):41–53. [PubMed] [Google Scholar]
- [5].Backonja MM, Walk D, Edwards RR, Sehgal N, Moeller-Bertram T, Wasan A, Irving G, Argoff C, Wallace M. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain 2009;25(7):641–647. [DOI] [PubMed] [Google Scholar]
- [6].Bakshi N, Lukombo I, Belfer I, Krishnamurti L. Quantitative sensory testing is feasible and is well-tolerated in patients with sickle cell disease following a vaso-occlusive episode. J Pain Res 2018;11:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009;139(2):267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007;448(7150):204–208. [DOI] [PubMed] [Google Scholar]
- [9].Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol 1969;32(6):1025–1043. [DOI] [PubMed] [Google Scholar]
- [10].Blankenburg M, Boekens H, Hechler T, Maier C, Krumova E, Scherens A, Magerl W, Aksu F, Zernikow B. Reference values for quantitative sensory testing in children and adolescents: developmental and gender differences of somatosensory perception. Pain 2010;149(1):76–88. [DOI] [PubMed] [Google Scholar]
- [11].Brandow AM, Brousseau DC, Pajewski NM, Panepinto JA. Vaso-occlusive painful events in sickle cell disease: impact on child well-being. Pediatr Blood Cancer 2010;54(1):92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer 2014;61(3):512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brandow AM, Farley RA, Panepinto JA. Early insights into the neurobiology of pain in sickle cell disease: A systematic review of the literature. Pediatr Blood Cancer 2015;62(9):1501–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brandow AM, Panepinto JA. Clinical Interpretation of Quantitative Sensory Testing as a Measure of Pain Sensitivity in Patients With Sickle Cell Disease. J Pediatr Hematol Oncol 2016;38(4):288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brandow AM, Stucky CL, Hillery CA, Hoffmann RG, Panepinto JA. Patients with sickle cell disease have increased sensitivity to cold and heat. Am J Hematol 2013;88(1):37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brousseau DC, Panepinto JA, Nimmer M, Hoffmann RG. The number of people with sickle-cell disease in the United States: national and state estimates. Am J Hematol 2010;85(1):77–78. [DOI] [PubMed] [Google Scholar]
- [17].Brousseau DC, Scott JP, Badaki-Makun O, Darbari DS, Chumpitazi CE, Airewele GE, Ellison AM, Smith-Whitley K, Mahajan P, Sarnaik SA, Casper TC, Cook LJ, Dean JM, Leonard J, Hulbert ML, Powell EC, Liem RI, Hickey R, Krishnamurti L, Hillery CA, Nimmer M, Panepinto JA, Pediatric Emergency Care Applied Research N. A multicenter randomized controlled trial of intravenous magnesium for sickle cell pain crisis in children. Blood 2015;126(14):1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cain DM, Vang D, Simone DA, Hebbel RP, Gupta K. Mouse models for studying pain in sickle disease: effects of strain, age, and acuteness. Br J Haematol 2012;156(4):535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Campbell BK, Fillingim RB, Lee S, Brao R, Price DD, Neubert JK. Effects of High-Dose Capsaicin on TMD Subjects: A Randomized Clinical Study. JDR Clin Trans Res 2017;2(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Campbell CM, Moscou-Jackson G, Carroll CP, Kiley K, Haywood Jr C, Lanzkron S, Hand M, Edwards RR, Haythornthwaite JA. An Evaluation of Central Sensitization in Patients With Sickle Cell Disease. J Pain 2016;17(5):617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Campero M, Serra J, Bostock H, Ochoa JL. Slowly conducting afferents activated by innocuous low temperature in human skin. The Journal of physiology 2001;535(Pt 3):855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Campero M, Serra J, Ochoa JL. C-polymodal nociceptors activated by noxious low temperature in human skin. The Journal of physiology 1996;497 ( Pt 2):565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cataldo G, Lunzer MM, Olson JK, Akgun E, Belcher JD, Vercellotti GM, Portoghese PS, Simone DA. The bivalent ligand MCC22 potently attenuates nociception in a murine model of sickle cell disease. Pain 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cataldo G, Rajput S, Gupta K, Simone DA. Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease. Pain 2015;156(4):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 1995;332(20):1317–1322. [DOI] [PubMed] [Google Scholar]
- [26].Chen Y, Luo F, Yang C, Kirkmire CM, Wang ZJ. Acute inhibition of Ca2+/calmodulin-dependent protein kinase II reverses experimental neuropathic pain in mice. The Journal of pharmacology and experimental therapeutics 2009;330(2):650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain 2008;24(6):479–496. [DOI] [PubMed] [Google Scholar]
- [28].Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010;330(6000):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Crown ED, Gwak YS, Ye Z, Yu Tan H, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Calcium/calmodulin dependent kinase II contributes to persistent central neuropathic pain following spinal cord injury. Pain 2012;153(3):710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Davis KD. Cold-induced pain and prickle in the glabrous and hairy skin. Pain 1998;75(1):47–57. [DOI] [PubMed] [Google Scholar]
- [31].Defrin R, Petrini L, Arendt-Nielsen L. Spatial summation of thermal sensations depends on skin type and skin sensitivity. Exp Brain Res 2009;198(1):29–36. [DOI] [PubMed] [Google Scholar]
- [32].Edwards RR, Fillingim RB. Age-associated differences in responses to noxious stimuli. J Gerontol A Biol Sci Med Sci 2001;56(3):M180–185. [DOI] [PubMed] [Google Scholar]
- [33].Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: clinical relevance in healthy older and younger adults. J Pain 2001;2(6):307–317. [DOI] [PubMed] [Google Scholar]
- [34].Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain 2003;101(1–2):155–165. [DOI] [PubMed] [Google Scholar]
- [35].Ezenwa MO, Molokie RE, Wang ZJ, Yao Y, Suarez ML, Pullum C, Schlaeger JM, Fillingim RB, Wilkie DJ. Safety and Utility of Quantitative Sensory Testing among Adults with Sickle Cell Disease: Indicators of Neuropathic Pain? Pain practice : the official journal of World Institute of Pain 2016;16(3):282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ferrari LF, Bogen O, Levine JD. Role of nociceptor alphaCaMKII in transition from acute to chronic pain (hyperalgesic priming) in male and female rats. J Neurosci 2013;33(27):11002–11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Filingeri D, Zhang H, Arens EA. Thermosensory micromapping of warm and cold sensitivity across glabrous and hairy skin of male and female hands and feet. J Appl Physiol (1985) 2018. [DOI] [PubMed] [Google Scholar]
- [38].Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL, 3rd. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10(5):447–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Garrison SR, Kramer AA, Gerges NZ, Hillery CA, Stucky CL. Sickle cell mice exhibit mechanical allodynia and enhanced responsiveness in light touch cutaneous mechanoreceptors. Molecular pain 2012;8:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Harrison JL, Davis KD. Cold-evoked pain varies with skin type and cooling rate: a psychophysical study in humans. Pain 1999;83(2):123–135. [DOI] [PubMed] [Google Scholar]
- [41].He Y, Chen Y, Tian X, Yang C, Lu J, Xiao C, DeSimone J, Wilkie DJ, Molokie RE, Wang ZJ. CaMKIIalpha underlies spontaneous and evoked pain behaviors in Berkeley sickle cell transgenic mice. Pain 2016;157(12):2798–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].He Y, Wilkie DJ, Nazari J, Wang R, Messing RO, DeSimone J, Molokie RE, Wang ZJ. PKCdelta-targeted intervention relieves chronic pain in a murine sickle cell disease model. J Clin Invest 2016;126(8):3053–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Heldestad V, Linder J, Sellersjo L, Nordh E. Reproducibility and influence of test modality order on thermal perception and thermal pain thresholds in quantitative sensory testing. Clin Neurophysiol 2010;121(11):1878–1885. [DOI] [PubMed] [Google Scholar]
- [44].Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, Wandersee NJ, Stucky CL. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood 2011;118(12):3376–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hogeweg JA, Kuis W, Huygen AC, de Jong-de vos van Steenwijk C, Bernards AT, Oostendorp RA, Helders PJ. The pain threshold in juvenile chronic arthritis. Br J Rheumatol 1995;34(1):61–67. [DOI] [PubMed] [Google Scholar]
- [46].Ibrahim AS. Relationship between meteorological changes and occurrence of painful sickle cell crises in Kuwait. Trans R Soc Trop Med Hyg 1980;74(2):159–161. [DOI] [PubMed] [Google Scholar]
- [47].Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 2014;13(9):924–935. [DOI] [PubMed] [Google Scholar]
- [48].Jordt SE, Ehrlich BE. TRP channels in disease. Subcell Biochem 2007;45:253–271. [DOI] [PubMed] [Google Scholar]
- [49].Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A 2009;106(4):1273–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kawamata M, Ishitani K, Ishikawa K, Sasaki H, Ota K, Omote K, Namiki A. Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer pain. Pain 1996;64(3):597–602. [DOI] [PubMed] [Google Scholar]
- [51].Keizer D, van Wijhe M, Post WJ, Uges DR, Wierda JM. Assessment of the clinical relevance of quantitative sensory testing with Von Frey monofilaments in patients with allodynia and neuropathic pain. A pilot study. Eur J Anaesthesiol 2007;24(8):658–663. [DOI] [PubMed] [Google Scholar]
- [52].Kennedy WRW-CG, Polydefkis M and McArthur JC. Pathology and Quantitation of Cutaneous Innervation Peripheral Neuropathy (Fourth Edition). Philadelphia, Pennsylvania: Elsevier Saunders, 2005. pp. 869–895. [Google Scholar]
- [53].Klepstad P, Borchgrevink PC, Kaasa S. Effects on cancer patients’ health-related quality of life after the start of morphine therapy. J Pain Symptom Manage 2000;20(1):19–26. [DOI] [PubMed] [Google Scholar]
- [54].Kohli DR, Li Y, Khasabov SG, Gupta P, Kehl LJ, Ericson ME, Nguyen J, Gupta V, Hebbel RP, Simone DA, Gupta K. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood 2010;116(3):456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].LaMotte RH, Thalhammer JG. Response properties of high-threshold cutaneous cold receptors in the primate. Brain Res 1982;244(2):279–287. [DOI] [PubMed] [Google Scholar]
- [56].Ling J, Erol F, Gu JG. Role of KCNQ2 channels in orofacial cold sensitivity: KCNQ2 upregulation in trigeminal ganglion neurons after infraorbital nerve chronic constrictive injury. Neurosci Lett 2018;664:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Link AS, Kuris A, Edvinsson L. Treatment of migraine attacks based on the interaction with the trigemino-cerebrovascular system. J Headache Pain 2008;9(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lolignier S, Gkika D, Andersson D, Leipold E, Vetter I, Viana F, Noel J, Busserolles J. New Insight in Cold Pain: Role of Ion Channels, Modulation, and Clinical Perspectives. J Neurosci 2016;36(45):11435–11439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].McGrath PA, Brown SC. Quantitative Sensory Testing in children: practical considerations for research and clinical practice. Pain 2006;123(1–2):1–2. [DOI] [PubMed] [Google Scholar]
- [60].McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002;416(6876):52–58. [DOI] [PubMed] [Google Scholar]
- [61].Meier PM, Berde CB, DiCanzio J, Zurakowski D, Sethna NF. Quantitative assessment of cutaneous thermal and vibration sensation and thermal pain detection thresholds in healthy children and adolescents. Muscle Nerve 2001;24(10):1339–1345. [DOI] [PubMed] [Google Scholar]
- [62].Molokie RE, Wang ZJ, Wilkie DJ. Presence of neuropathic pain as an underlying mechanism for pain associated with cold weather in patients with sickle cell disease. Medical hypotheses 2011;77(4):491–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Molokie RE, Wilkie DJ, Wittert H, Suarez ML, Yao Y, Zhao Z, He Y, Wang ZJ. Mechanism-driven phase I translational study of trifluoperazine in adults with sickle cell disease. Eur J Pharmacol 2014;723:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Morenilla-Palao C, Luis E, Fernandez-Pena C, Quintero E, Weaver JL, Bayliss DA, Viana F. Ion channel profile of TRPM8 cold receptors reveals a role of TASK-3 potassium channels in thermosensation. Cell Rep 2014;8(5):1571–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nisbet AT, Mooney-Cotter F. Comparison of selected sedation scales for reporting opioid-induced sedation assessment. Pain Manag Nurs 2009;10(3):154–164. [DOI] [PubMed] [Google Scholar]
- [66].Nolan VG, Zhang Y, Lash T, Sebastiani P, Steinberg MH. Association between wind speed and the occurrence of sickle cell acute painful episodes: results of a case-crossover study. Br J Haematol 2008;143(3):433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Nothnagel H, Puta C, Lehmann T, Baumbach P, Menard MB, Gabriel B, Gabriel HHW, Weiss T, Musial F. How stable are quantitative sensory testing measurements over time? Report on 10-week reliability and agreement of results in healthy volunteers. J Pain Res 2017;10:2067–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].O’Leary JD, Crawford MW, Odame I, Shorten GD, McGrath PA. Thermal pain and sensory processing in children with sickle cell disease. Clin J Pain 2014;30(3):244–250. [DOI] [PubMed] [Google Scholar]
- [69].Paller CJ, Campbell CM, Edwards RR, Dobs AS. Sex-based differences in pain perception and treatment. Pain Med 2009;10(2):289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, Temperley WH, Williams TN, Weatherall DJ, Hay SI. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet 2013;381(9861):142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, Patapoutian A. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 2014;516(7529):121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Redwood AM, Williams EM, Desal P, Serjeant GR. Climate and painful crisis of sickle-cell disease in Jamaica. Br Med J 1976;1(6001):66–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Resar LM, Oski FA. Cold water exposure and vaso-occlusive crises in sickle cell anemia. J Pediatr 1991;118(3):407–409. [DOI] [PubMed] [Google Scholar]
- [74].Riley JL 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain 1998;74(2–3):181–187. [DOI] [PubMed] [Google Scholar]
- [75].Rogovik AL, Persaud J, Friedman JN, Kirby MA, Goldman RD. Pediatric vasoocclusive crisis and weather conditions. J Emerg Med 2011;41(5):559–565. [DOI] [PubMed] [Google Scholar]
- [76].Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 2006;123(3):231–243. [DOI] [PubMed] [Google Scholar]
- [77].Ruddock B Chronic pain: switching to an alternate opioid analgesic. CPJ/RPC 2005;138:44–45. [Google Scholar]
- [78].Ruocco I, Cuello AC, Parent A, Ribeiro-da-Silva A. Skin blood vessels are simultaneously innervated by sensory, sympathetic, and parasympathetic fibers. J Comp Neurol 2002;448(4):323–336. [DOI] [PubMed] [Google Scholar]
- [79].Sadler KE, Zappia KJ, O’Hara CL, Langer SN, Weyer AD, Hillery CA, Stucky CL. Chemokine (c-c motif) receptor 2 mediates mechanical and cold hypersensitivity in sickle cell disease mice. Pain 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Sethna NF, Meier PM, Zurakowski D, Berde CB. Cutaneous sensory abnormalities in children and adolescents with complex regional pain syndromes. Pain 2007;131(1–2):153–161. [DOI] [PubMed] [Google Scholar]
- [81].Simone DA, Kajander KC. Responses of cutaneous A-fiber nociceptors to noxious cold. J Neurophysiol 1997;77(4):2049–2060. [DOI] [PubMed] [Google Scholar]
- [82].Smith WR, Bauserman RL, Ballas SK, McCarthy WF, Steinberg MH, Swerdlow PS, Waclawiw MA, Barton BA, Multicenter Study of Hydroxyurea in Sickle Cell A, Smith WR, Bauserman RL, Ballas SK, McCarthy WF, Steinberg MH, Swerdlow PS, Waclawiw MA, Barton BA. Climatic and geographic temporal patterns of pain in the Multicenter Study of Hydroxyurea. Pain 2009;146(1–2):91–98. [DOI] [PubMed] [Google Scholar]
- [83].Smith WR, Coyne P, Smith VS, Mercier B. Temperature changes, temperature extremes, and their relationship to emergency department visits and hospitalizations for sickle cell crisis. Pain Manag Nurs 2003;4(3):106–111. [DOI] [PubMed] [Google Scholar]
- [84].Soin A, Bock G, Giordano A, Patel C, Drachman D. A Randomized, Double-Blind Study of the Effects of a Sustained Release Formulation of Sodium Nitrite (SR-nitrite) on Patients with Diabetic Neuropathy. Pain physician 2018;21(2):179–190. [PubMed] [Google Scholar]
- [85].Sorensen J, Bengtsson A, Ahlner J, Henriksson KG, Ekselius L, Bengtsson M. Fibromyalgia--are there different mechanisms in the processing of pain? A double blind crossover comparison of analgesic drugs. J Rheumatol 1997;24(8):1615–1621. [PubMed] [Google Scholar]
- [86].Stinson JN, Kavanagh T, Yamada J, Gill N, Stevens B. Systematic review of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain 2006;125(1–2):143–157. [DOI] [PubMed] [Google Scholar]
- [87].Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003;112(6):819–829. [DOI] [PubMed] [Google Scholar]
- [88].Stucky CL, Dubin AE, Jeske NA, Malin SA, McKemy DD, Story GM. Roles of transient receptor potential channels in pain. Brain Res Rev 2009;60(1):2–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Uhelski ML, Gupta K, Simone DA. Sensitization of C-fiber nociceptors in mice with sickle cell disease is decreased by local inhibition of anandamide hydrolysis. Pain 2017;158(9):1711–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wang WC, Ware RE, Miller ST, Iyer RV, Casella JF, Minniti CP, Rana S, Thornburg CD, Rogers ZR, Kalpatthi RV, Barredo JC, Brown RC, Sarnaik SA, Howard TH, Wynn LW, Kutlar A, Armstrong FD, Files BA, Goldsmith JC, Waclawiw MA, Huang X, Thompson BW. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet 2011;377(9778):1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wang Y, Wang X, Chen W, Gupta K, Zhu XH. Functional MRI BOLD response in sickle mice with hyperalgesia. Blood Cells Mol Dis 2017;65:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang ZJ, Molokie RE, Wilkie DJ. Does cold hypersensitivity increase with age in sickle cell disease? Pain 2014;155(12):2439–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wilkie DJ, Molokie R, Boyd-Seal D, Suarez ML, Kim YO, Zong S, Wittert H, Zhao Z, Saunthararajah Y, Wang ZJ. Patient-reported outcomes: descriptors of nociceptive and neuropathic pain and barriers to effective pain management in adult outpatients with sickle cell disease. J Natl Med Assoc 2010;102(1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152(3 Suppl):S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yu H, Pan B, Weyer A, Wu HE, Meng J, Fischer G, Vilceanu D, Light AR, Stucky C, Rice FL, Hudmon A, Hogan Q. CaMKII Controls Whether Touch Is Painful. J Neurosci 2015;35(42):14086–14102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zappia KJ, Garrison SR, Hillery CA, Stucky CL. Cold hypersensitivity increases with age in mice with sickle cell disease. Pain 2014;155(12):2476–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Zimmermann M Pathobiology of neuropathic pain. Eur J Pharmacol 2001;429(1–3):23–37. [DOI] [PubMed] [Google Scholar]
- [98].Zohsel K, Hohmeister J, Flor H, Hermann C. Somatic pain sensitivity in children with recurrent abdominal pain. Am J Gastroenterol 2008;103(6):1517–1523. [DOI] [PubMed] [Google Scholar]
- [99].Zohsel K, Hohmeister J, Oelkers-Ax R, Flor H, Hermann C. Quantitative sensory testing in children with migraine: preliminary evidence for enhanced sensitivity to painful stimuli especially in girls. Pain 2006;123(1–2):10–18. [DOI] [PubMed] [Google Scholar]


