Abstract
Background
The availability of human pancreatic islets with characteristics closely resembling those present in vivo is instrumental for ex vivo studies in diabetes research.
Scope of review
In this review we propose metabolically phenotyped surgical patients as a novel source of pancreatic tissue for islet research. Laser Capture Microdissection from snap frozen surgical specimens is a relatively simple, reproducible and scalable method to isolate islets of highest purity for many types of “omics” analyses. Fresh pancreatic tissue slices enable the functional characterization of living islet cells in situ through dynamic experiments. Access to complete medical history and laboratory values for each donor offers the opportunity of direct correlations with different “omics” data and detailed metabolic profiling prior to pancreas surgery. Peripheral blood samples complete the picture of each patient and represent a platform for pursuit of biomarkers with uniquely comprehensive background information in regard to the donor's islet cells.
Major conclusions
Living donors provide the scientific community with a steady and abundant supply of excellent material to study islets closest to their in situ environment, thus advancing our understanding of their physiology in health and diseases.
Keywords: Islets of Langerhans, Diabetes, Laser capture microdissection, Metabolically phenotyped living donor, Pancreatectomy, Biomarker
1. Introduction
Acquisition of knowledge about the insulin producing beta cells began with the discovery of pancreatic islets by Paul Langerhans in the late 19th century [1]. For almost a century the morphological and functional characterization of these highly specialized endocrine cells was able to almost exclusively rely on the application of histological methods to fixed pancreatic tissue sections of human or animal origin. Therefore, our early knowledge about beta cells and other islet cells in health and diseases, such as diabetes, was mostly restricted to static snapshots. The investigation of living islet cells was especially limited by the particular vulnerability of the pancreatic tissue to autolysis following the release of digestive enzymes by the surrounding exocrine cells. A leap forwards in our understanding of the physiology and pathophysiology of beta cells came in the second half of the 20th century with the development of protocols for the isolation of islets of Langerhans from the pancreas of animals and human cadavers or organ donors [2], [3], [4], [5]. In the decades to follow and to this day, enzymatically isolated islets have therefore been a most valuable in vitro model system to study in a dynamic fashion the function of islet cells and their dysfunctioning in diabetes [6].
With the remarkable progress of imaging and “omics” technologies, however, alternative approaches have emerged which allow the investigation of islet cells without their prior enucleation from the surrounding pancreatic tissue, i.e. in a more physiological context. In this short review we will briefly summarize how exploiting surgical specimens from metabolically phenotyped pancreatectomized patients as the source of pancreatic tissue for the study of islets in situ represents a paradigmatic shift for elucidating the cell biology and pathophysiology of beta cells.
2. Enzymatically isolated islets – the workhorse of research in diabetes
The first successful attempts to purify pancreatic endocrine cells were made by Hellerström, who in 1964 reported the isolation of islets using microdissection techniques [2]. A year later, Moskalewski published the use of collagenase digestion and mechanical disruption to “release” the islets from the surrounding tissue [3]. The islets would thereafter be picked by hand under a stereomicroscope. Even though this method yielded viable, insulin secreting islets, it was plagued by relatively high exocrine tissue contamination. This protocol was shortly afterwards optimized by Paul Lacey, who introduced additional steps of acinar tissue disruption prior to collagenase digestion and, most notably, sucrose gradient centrifugation. The fraction of islets of Langerhans in the final preparation was greatly increased, although still between 40 and 90% [4]. It took another 20 years of modifying and improving this initial protocol to finally succeed in making viable preparations of human islets with sufficient efficiency. Ricordi et al. first described the protocol and equipment setup that would enable, through an automatized process, the isolation of islets of Langerhans from an entire human pancreas [5]. This method greatly reduced enzymatic and mechanical damage done to the islets during the isolation procedure, but also facilitated an otherwise very labour-intensive process. It also reduced the amount of necessary tissue input to obtain a meaningful number of human islets, which also enabled their collection from organs derived from partial pancreatectomies [7]. Despite the various improvements to the protocol introduced by numerous researchers throughout the following decades, purity of the islet preparation remains one of the significant setbacks of this method of obtaining islets for diabetes research.
Due to the tendency of pancreatic tissue to undergo autolysis and suffer extensive damage ascribable to intrinsic digestive enzymes in a matter of hours after death, the source of organs for islet isolation are brain-dead, i.e. heart-beating, organ donors or, to a much lesser extent, subjects who have died following cardiocirculatory arrest. Frequently, medical and legal indications require these patients who have experienced a catastrophic brain injury to spend several days in the Intensive Care Unit (ICU) prior to organ donation. In order to improve severely aggravated homeostasis, aiming at preserving life, or ultimately to preserve organs for donation, these patients are subjected to extensive procedures and therapies in the days ante finem. Most receive some form of parenteral nutrition, continuous insulin infusions, as well as corticosteroid therapy. These therapeutic interventions affect endocrine axes, and in addition to the events that led to hospitalization in the ICU, perturb islet cells, with altered glucose homeostasis and metabolism. In addition to the metabolic stress induced by medical interventions, there are reports of islet inflammation and infiltration by macrophages in brain-dead rodents, while islets in situ harvested from brain dead human donors exhibit markers of beta cell stress [8], [9]. Further alterations can be induced by varying periods of warm and cold ischaemia that ensue during organ harvesting and by exposure of islets to physical and chemical damaging factors during the islet isolation procedure, which in essence represents a controlled form of induced pancreatitis and can elicit a wound response. Finally, isolated islets are usually cultured in an enriched medium for 24–48 hours after isolation before being subjected to analysis.
While the events prior to isolation contribute significantly to variability among islet preparations from donors to donors, the period of in vitro culture in a standardized milieu may mask some of the biologically relevant differences between islets coming from donors with different metabolic profiles. Furthermore, preparations of islets tend to vary substantially in purity, viability and functional capacity of the cells, which can affect downstream usage in various perturbation experiments. The availability of this precious resource for research has always posed a limitation, which has been further exacerbated in the past several years by rerouting of the islets to newly developed, more successful endocrine pancreas replacement therapies using human isolated islets. In order to maximise the usability of the islets that indeed are assigned to research, islets obtained from a single donor are divided into several portions and dispatched to the laboratories working with the particular islet isolation centre. The question of quality, viability and reproducibility of islet preparations after isolation and subsequent handling has been addressed in articles recently published in leading journals in the field such as Diabetologia and Diabetes [10], [11]. Identification of biological phenomena using this experimental model is hindered by limited clinical data available about the donors due to stringent legal requirements in regard to protection of personal information. In addition, restricted number of medical centres with the logistics and expertise in transplantation medicine and islet isolation limits the availability of isolated human islets.
3. Pancreatic tissue from living donors – a resource for the future of diabetes research
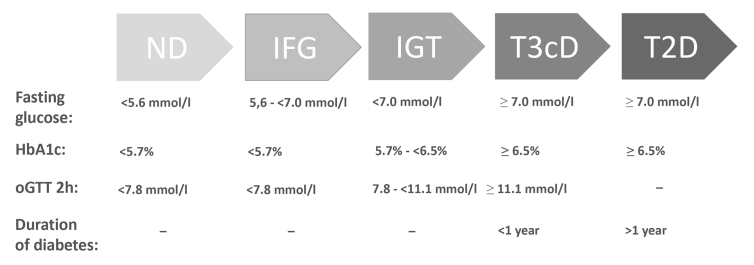
In order to overcome some of the limits described above, during the last decade we have resorted to surgical specimens of pancreatectomized patients as a complementary source of human islet tissue. Patients with varying indications for pancreatic surgery are recruited according to predefined criteria and metabolically profiled preoperatively (routine clinical biochemistry, oral glucose tolerance test (OGTT) including insulin, proinsulin, c-peptide and glucagon measurements). Despite its limitations in painting a complete metabolic picture of these living donors, the OGTT remains the gold standard clinical approach for assessing glucose tolerance and still offers very valuable information for downstream use [12]. This strategy in particular allows the recruitment not only of non-diabetic and subjects with overt type 2 diabetes but also of prediabetic subjects, hence opening up the possibility of cross sectional characterization of islets/beta cells in the full spectrum from normoglycaemia to type 2 diabetes (Fig. 1).
Figure. 1.
Criteria for stratification of surgical patients according to their preoperative glucose tolerance (adapted according to ADA Standards of Medical Care in Diabetes - 2018).
In the same procedure, additional peripheral blood samples are collected and promptly stored at −80 °C. The surgical specimen is at shortest notice delivered to a pathologist who excises healthy pancreatic tissue fragments, which are promptly snap frozen in liquid nitrogen thereby instantly stopping biological processes, and thereby minimizing artefacts in comparison to the perifused tissue in vivo. Owing to a streamlined routine process and privileged processing of research samples, total warm and cold ischaemia time is reduced to less than one hour. After freezing samples can be stored at −80 °C or lower temperatures for extended periods of time without risk of degradation and depletion of molecules relevant in contemporary research.
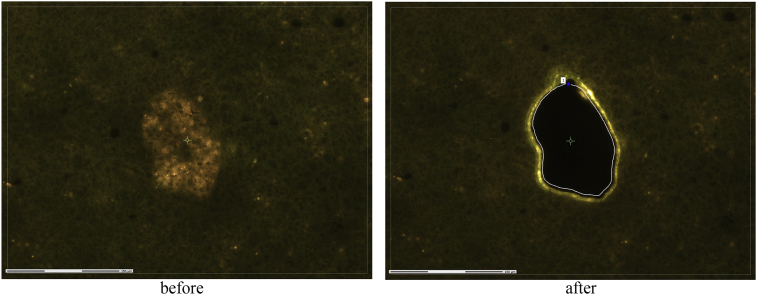
From the frozen pancreatic tissue fragments, serial sections are made in a cryostat and mounted on specialty slides. As part of the efforts in the IMI consortia IMIDIA and RHAPSODY we developed an improved protocol for retrieval of islets from frozen sections of pancreatic tissue using Laser Capture Microdissection (LCM) [13]. This technique, first introduced to the field of islet transcriptomic research by Lorella Marselli, Piero Marchetti and Gordon Weir, relies on the detection of lipofuscin autofluorescence in beta cells of human pancreatic specimens [14], [15]. Islets are then separated from the surrounding tissue by the cutting laser guided by a manually drawn path and catapulted from the slide into the collection cap by the catapulting laser (Fig. 2). Islet preparation obtained in this way is of highest purity and biomolecules are preserved virtually in the same state they had been prior to freezing of the samples very shortly after surgical removal. Tissue collected with this methodology is fully compatible with high throughput biology techniques such as DNA and RNA sequencing and microarrays [16].
Figure. 2.
Identification of islets of Langerhans using autofluorescence, snapshot of LCM isolation.
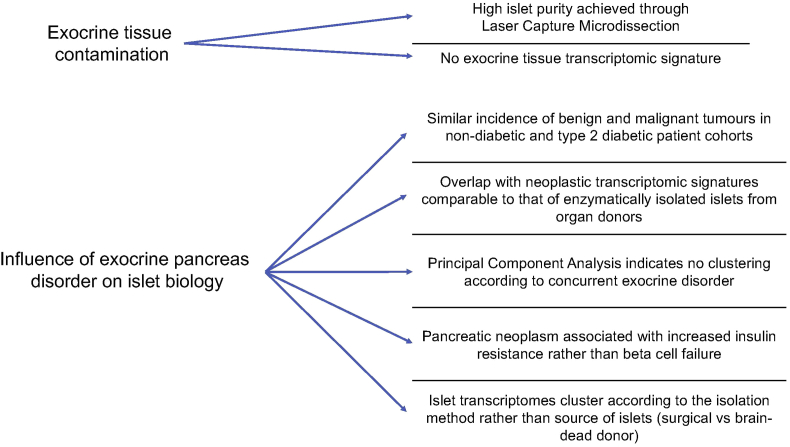
A concern about this approach pertains to the suitability of surgical specimens from subjects with pancreatic disorders, mainly of the exocrine tissue, for research on islets and diabetes pathophysiology. This question can be addressed on several levels schematically shown in Fig. 3. First, in the cohort of non-diabetic and type 2 diabetic surgical patients that we reported on recently, the prevalence of the most common disorders accounting for pancreatectomy, namely malignant and benign pancreatic tumours or chronic pancreatitis, was comparable [17]. Hence, the impact that these disorders might have on islet specificities should have been equivalent in the two groups. Key parameters such as age, gender and BMI of non-diabetic and type 2 diabetic surgical patients were overlapping with those of the corresponding groups among brain-dead organ donors whose islets had been retrieved post-mortem by enzymatic digestion. Principal component analysis indicated that the transcriptomes of islets isolated by LCM from snap frozen pancreatic tissue sections of several organ donors free from known pancreatic disorders clustered together with the transcriptome of LCM islets from pancreatectomized patients rather than with the transcriptome of enzymatically isolated from the same organ donors. Conversely, the transcriptomes of islets isolated enzymatically from surgical specimens clustered together with the transcriptomes of islets isolated enzymatically from organ donors rather than with the transcriptome of islets isolated by LCM from the same surgical patients. Hence, the phenotypic characteristics of the isolated islets are more driven by the method for their retrieval than by presence or absence of a concurrent disorder of the exocrine pancreas.
Figure. 3.
Considerations regarding potential pitfalls in the use of surgical specimens from pancreatectomized patients as a source of LCM islets for research on diabetes.
Transcriptomic analyses also revealed that islets recovered by LCM from surgical specimens were only minimally contaminated by pancreatic exocrine tissue, possibly less than those isolated enzymatically from the pancreas of organ donors. Most importantly LCM islets did not display transcriptomic signatures of neoplastic tissues, which is particularly of note considering that surgical indication for the majority of patients was some type of pancreatic neoplasm [17]. Likewise, pathological examination did not reveal infiltration of tumour cells in the islets of any of the surgical patients with pancreatic cancer. Nonetheless a strong association between recent onset diabetes and pancreatic cancer has been observed for several decades [18], [19]. However, the neoplastic processes in the pancreas do not seem to directly affect the biology of the islets, but rather impose a sudden rise in peripheral insulin resistance, leading in turn to impaired glucose tolerance and the clinical presentation of type 2 diabetes. The most likely cause for these alterations is cholestasis followed by post-hepatic hyperbilirubinemia and impaired liver function due to the compression exerted on the common bile duct by a tumour in the head of the pancreas. Indeed, cholestasis with insulin resistance and hyperglycaemia are uncommon among patients with cancers in the tail and body of the pancreas. Further support for this interpretation is the evidence of rapid improved glycaemic control in the majority of the patients who underwent resection of a tumour in the pancreas head, with amelioration of bile flow and liver parameters and despite the decreased total pancreas mass and insulin secretion [20], [21]. Remarkably, improved glycaemic control with reduced insulin resistance has been observed even in patients without cancer who underwent resection of the pancreas head in the context of pancreatoduodenectomy [22]. Additionally, islets isolated from rodents subjected to mechanical cholestasis in vivo did not exhibit impaired function in vitro [23]. The relationship between cholestasis and insulin resistance is further corroborated by evidence of metabolic like-syndrome and increased HOMA-IR in patients with intrahepatic cholestasis [24], [25]. Taken together, these considerations argue against glucose intolerance in patients with pancreatic cancer resulting from a remote effect of neoplastic cells on islets. On the contrary, it appears that also in subjects with pancreatic cancer decompensation of beta cell function occurs in the context of exacerbated insulin resistance, although the origin of the latter is mechanic rather than metabolic, which is the case for most other individuals with type 2 diabetes.
Another critical aspect to be considered whenever a new approach is introduced in scientific practice is its degree of reproducibility – a topic of particular relevance in the field of islet research. In the realm of the research activities of the German Center for Diabetes Research (DZD e.V.) and following our standard operating procedures for LCM of islets from surgical specimens, colleagues in Tübingen have been able to profile the islet transcriptome of an independent cohort of pancreatectomized patients who underwent surgery at their University Hospital. Despite the comparatively small number of patients, bioinformatic analyses of the transcriptomes obtained from these samples yielded remarkably similar results to those we had already observed in our cohort [26].
A further issue to be addressed by our approach is the limited availability of islets for research applications due to the limited number of organ donors as well as centres for pancreas transplantation and islet isolation in each microregion, further complicated by the special expertise and infrastructures required for such procedures. Despite being one of the most complex operations, pancreatectomy is on the other hand an elective surgical procedure that is performed on a routine basis at most university hospitals worldwide. In Germany, for instance, >10,000 pancreatectomies are carried out each year, with an average of >100 at each major hospital. Surgical pancreatic specimens from living donors could therefore represent a large and reliable local source of islets for research. The technique of LCM to obtain pure islets is, given the availability of a suitable microscopy system, not a limiting factor as it can be easily carried out with relatively little expertise. Snap frozen samples can alternatively be forwarded to interested investigators at remote locations with limited cost and time restrictions.
The reliance on surgical patients as donors of pancreatic tissue bypasses the stringent legal requirements regarding protection of personal data, that are in place for organ donors. This in practice means that, with appropriate consent and necessary deidentification procedures, personal and family history can be made readily available to researchers. Access to the patient before surgery offers the invaluable opportunity to collect preoperative blood samples and conduct necessary tests for full metabolic profiling. Hence, a comprehensive biobank can be established in each centre, containing not only the biological samples, but also complete clinical data and biochemical measurements needed for deep phenotypic characterisation of each donor.
The potential applications of surgical specimens for islet research have increased following the seminal work of the Speier lab, which pioneered protocols to conduct ex vivo dynamic imaging and physiological measurements in perifused pancreatic tissue slices [27]. This major advancement, in particular, tackles one of the critical limitations in the use of surgical specimens relative to enzymatically isolated islets, i.e. the possibility to analyse secretion of insulin and conceivably other islet hormones, all the while keeping the islets in situ without perturbing their anatomical and functional interactions [28].
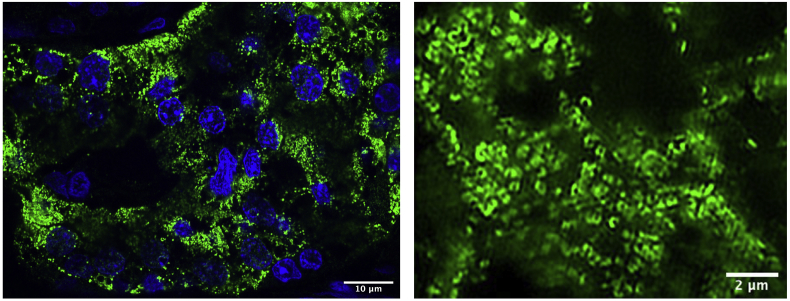
The issue of validation of data acquired using different high-throughput methods can be addressed through preservation of not only the snap frozen specimens from which the islets were retrieved by LCM but also additional formalin fixed paraffin embedded (FFPE) tissue samples. Snap frozen tissue samples can, with appropriate handling, be preserved for many years and still retain their integrity and serve as a source for new methods to be developed in the future. FFPE samples are inherently very durable and offer the possibility of revisions and validations using more traditional methods as well as novel microscopy techniques for decades after collection (Fig. 4). Access to histopathological reports completes the picture of the donor, enabling retrospective and prospective comprehensive investigations.
Figure. 4.
Left: Confocal image of a human islet from FFPE tissue; insulin immunofluorescence (in green), nuclear staining with DAPI (in blue). Right: Beta cell region in superresolution (Structured Illumination) from a FFPE human tissue; insulin immunofluorescence.
Finally, to complete the molecular and metabolic profile of living donors, peripheral blood samples are also routinely collected. These can, at the time of writing this review, be used for genomic profiling as well as plasma lipidomics, metabolomics and proteomics measurements. We believe that in the pursuit of circulating biomarkers predictive for beta cell failure, this resource is of utmost importance, especially given the possibility to integrate this data with those obtained from in situ islets of the same metabolically phenotyped donors.
4. Conclusions
Ever since their discovery and implication in diabetes, the cells of the islets of Langerhans have remained the focus of research for generations of scientists. Basic knowledge of structure and function of these cells has been established in static conditions using traditional histology techniques and methods and was a cornerstone for the many years of research that will follow. In the years to follow, owing to advances in the methodology for islet isolation and general developments in molecular biology, dynamic experiments and insight into the cell function in real time became possible. Despite the indicated disadvantages, the study of enzymatically isolated islets has provided a wealth of knowledge for the treatment and prevention of diabetes. The human model of primary islet cells remains therefore an asset to investigate their physiology and pathology [6].
In view of the background presented in this review, metabolically phenotyped surgical patients represent a novel and complementary source of pancreatic tissue and peripheral blood for diabetes research. The wealth of information and deep phenotyping of biological samples obtained from these donors are of particular interest, especially considering that this approach can be implemented in virtually any medical centre performing pancreatic surgery and with access to appropriately equipped laboratories.
Funding
Work in the Solimena lab is funded by the BMBF funded German Centre for Diabetes research (DZD e.V.). Additional funds for support of studies presented here came from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreements n° 155005 (IMIDIA) and n° 115881 (RHAPSODY), which include financial contributions from European Union's Seventh Framework Programme (FP7/2007–2013) and Horizon 2020 research and innovation programme and EFPIA. This work is also supported by the Swiss State Secretariat for Education Research and Innovation (SERI) under contract number 16.0097.
This article is part of a supplement entitled ‘Biomarkers of Beta-Cell Health and Dysfunction: Towards Personalised Diabetes Care. Proceedings of the 20th Servier-IGIS Symposium’. The Symposium and the publication of the Supplement have been made possible by an unrestricted educational grant from Institut la Conférence Hippocrate – Servier Group.
Acknowledgments
We thank members of the Solimena and Speier labs (PLID, TU Dresden) for discussion, our colleagues at the DZD- and HMGU-supported IDM at Univ. Tübingen and members of work packages 2B and 5 in IMIDIA and RHAPSODY, respectively. Special thanks go to Bernard Thorens for continued support.
Contributor Information
Marko Barovic, Email: marko.barovic@helmholtz-muenchen.de.
Marius Distler, Email: marius.distler@uniklinikum-dresden.de.
Eyke Schöniger, Email: eyke.schoeniger@helmholtz-muenchen.de.
Nicole Radisch, Email: nicole.radisch@uniklinikum-dresden.de.
Daniela Aust, Email: daniela.aust@uniklinikum-dresden.de.
Jürgen Weitz, Email: juergen.weitz@uniklinikum-dresden.de.
Mark Ibberson, Email: mark.ibberson@sib.swiss.
Anke M. Schulte, Email: anke.schulte@sanofi.com.
Michele Solimena, Email: michele.solimena@tu-dresden.de.
Conflict of interest
None.
References
- 1.Langerhans P. Friedrich-Wilhelms-Universität zu Berlin; 1869. Beiträge zur mikroskopischen Anatomie der Bauchspeicheldrüse : Inaugural-Dissertaton, zur Erlangung der Doctorwürde in der Medicin und Chirurgie vorgelegt der Medicinischen Facultät der Friedrich-Wilhelms-Universität zu Berlin. [Google Scholar]
- 2.Hellerström C. A method for the microdissection of intact pancreatic islets of mammals. Acta Endocrinologica. 1964;45:122–132. [PubMed] [Google Scholar]
- 3.Moskalewski S. Isolation and culture of the islets of Langerhans of the Guinea pig. General and Comparative Endocrinology. 1965;5(3):342–353. doi: 10.1016/0016-6480(65)90059-6. [DOI] [PubMed] [Google Scholar]
- 4.Lacy P.E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Ricordi C., Lacy P.E., Finke E.H., Olack B.J., Scharp D.W. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 6.Marchetti P., Suleiman M., Marselli L. Organ donor pancreases for the study of human islet cell histology and pathophysiology: a precious and valuable resource. Diabetologia. 2018;61(4):770–774. doi: 10.1007/s00125-018-4546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bötticher G., Sturm D., Ehehalt F., Knoch K.P., Kersting S., Grützmann R. Isolation of human islets from partially pancreatectomized patients. Journal of Visualized Experiments. 2011;(53):1–4. doi: 10.3791/2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebrahimi A., Jung M.H., Dreyfuss J.M., Pan H., Sgroi D., Bonner-Weir S. Evidence of stress in β cells obtained with laser capture microdissection from pancreases of brain dead donors. Islets. 2017;9(2):19–29. doi: 10.1080/19382014.2017.1283083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toyama H., Takada M., Suzuki Y., Kuroda Y. Activation of macrophage-associated molecules after brain death in islets. Cell Transplantation. 2003;12(1):27–32. doi: 10.3727/000000003783985205. [DOI] [PubMed] [Google Scholar]
- 10.Poitout V., Satin L.S., Kahn S.E., Stoffers D.A., Marchetti P., Gannon M. A call for improved reporting of human islet characteristics in research articles. Diabetes. 2019;68(2):209–211. doi: 10.2337/dbi18-0055. [DOI] [PubMed] [Google Scholar]
- 11.Hart N.J., Powers A.C. Use of human islets to understand islet biology and diabetes : progress, challenges and suggestions. Diabetologia. 2019;62(2):212–222. doi: 10.1007/s00125-018-4772-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babbar R., Heni M., Peter A., Hrabě de Angelis M., Häring H.-U., Fritsche A. Prediction of glucose tolerance without an oral glucose tolerance test. Frontiers in Endocrinology. 2018;9:82. doi: 10.3389/fendo.2018.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sturm D., Marselli L., Ehehalt F., Richter D., Distler M., Kersting S. Improved protocol for laser microdissection of human pancreatic islets from surgical specimens. Journal of Visualized Experiments. 2013;(71):2–7. doi: 10.3791/50231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marselli L., Sgroi D.C., Bonner-Weir S., Weir G.C. Laser capture microdissection of human pancreatic β-cells and RNA preparation for gene expression profiling. Methods in Molecular Biology. 2009;560:87–98. doi: 10.1007/978-1-59745-448-3_8. [DOI] [PubMed] [Google Scholar]
- 15.Cnop M., Hughes S.J., Igoillo-Esteve M., Hoppa M.B., Sayyed F., van de Laar L. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia. 2010;53(2):321–330. doi: 10.1007/s00125-009-1562-x. [DOI] [PubMed] [Google Scholar]
- 16.Khamis A., Canouil M., Siddiq A., Crouch H., Falchi M., von Bulow M. Laser capture microdissection of human pancreatic islets reveals novel eQTLs associated with type 2 diabetes. Molecular Metabolism. 2019;24:98–107. doi: 10.1016/j.molmet.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solimena M., Schulte A.M., Marselli L., Ehehalt F., Richter D., Kleeberg M. Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes. Diabetologia. 2018;61(3):641–657. doi: 10.1007/s00125-017-4500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gullo L., Pezzilli R., Morselli-Labate A.M., Italian pancreatic cancer study group Diabetes and the risk of pancreatic cancer. New England Journal of Medicine. 1994;331(2):81–84. doi: 10.1056/NEJM199407143310203. [DOI] [PubMed] [Google Scholar]
- 19.Permert J., Ihse I., Jorfeldt L., von Schenck H., Arnqvist H.J., Larsson J. Pancreatic cancer is associated with impaired glucose metabolism. The European Journal of Surgery Acta Chirurgica. 1993;159(2):101–107. [PubMed] [Google Scholar]
- 20.Ehehalt F., Sturm D., Rösler M., Distler M., Weitz J., Kersting S. Blood glucose homeostasis in the course of partial pancreatectomy – evidence for surgically reversible diabetes induced by cholestasis. Public Library of Science one. 2015;10(8):e0134140. doi: 10.1371/journal.pone.0134140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sohn S.Y., Lee E.K., Han S.-S., Lee Y.J., Hwangbo Y., Kang Y.H. Favorable glycemic response after pancreatoduodenectomy in both patients with pancreatic cancer and patients with non-pancreatic cancer. Medicine. 2018;97(18):e0590. doi: 10.1097/MD.0000000000010590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang M.J., Jung H.S., Jang J.-Y., Jung W., Chang J., Shin Y.C. Metabolic effect of pancreatoduodenectomy: resolution of diabetes mellitus after surgery. Pancreatology. 2016;16(2):272–277. doi: 10.1016/j.pan.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Holmberg J.T., Skoglund G., Ahrén B. Increased insulin secretion in response to glucose in isolated islets of Langerhans from bile duct-occluded rats. Pancreas. 1986;1(6):498–500. doi: 10.1097/00006676-198611000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Martineau M.G., Raker C., Dixon P.H., Chambers J., Machirori M., King N.M. The metabolic profile of intrahepatic cholestasis of pregnancy is associated with impaired glucose tolerance, dyslipidemia, and increased fetal growth. Diabetes Care. 2015;38(2):243–248. doi: 10.2337/dc14-2143. [DOI] [PubMed] [Google Scholar]
- 25.Shipovskaya A.A., Dudanova O.P. Intrahepatic cholestasis in nonalcoholic fatty liver disease. Terapevticheskii Arkhiv. 2018;90(2):69–74. doi: 10.26442/terarkh201890269-74. [DOI] [PubMed] [Google Scholar]
- 26.Gerst F., Jaghutriz B.A., Staiger H., Schulte A.M., Lorza-Gil E., Kaiser G. The expression of aldolase B in islets is negatively associated with insulin secretion in humans. Journal of Clinical Endocrinology & Metabolism. 2018;103(12):4373–4383. doi: 10.1210/jc.2018-00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marciniak A., Cohrs C.M., Tsata V., Chouinard J.A., Selck C., Stertmann J. Using pancreas tissue slices for in situ studies of islet of Langerhans and acinar cell biology. Nature Protocols. 2014;9(12):2809–2822. doi: 10.1038/nprot.2014.195. [DOI] [PubMed] [Google Scholar]
- 28.Cohrs C.M., Chen C., Jahn S.R., Stertmann J., Chmelova H., Weitz J. Vessel network architecture of adult human islets promotes distinct cell-cell interactions in situ and is altered after transplantation. Endocrinology. 2017;158(5):1373–1385. doi: 10.1210/en.2016-1184. [DOI] [PubMed] [Google Scholar]