Abstract
Background
Elucidation of the basic molecular mechanism of autophagy was a breakthrough in understanding various physiological events and pathogenesis of diverse diseases. In the fields of diabetes and metabolism, many cellular events associated with the development of disease or its treatment cannot be explained well without taking autophagy into account. While a grand picture of autophagy has been established, detailed aspects of autophagy, particularly that of selective autophagy responsible for homeostasis of specific organelles or metabolic intermediates, are still ambiguous and currently under intensive research.
Scope of review
Here, results from previous and current studies on the role of autophagy and its dysregulation in the physiology of metabolism and pathogenesis of diabetes are summarized, with an emphasis on the pancreatic β-cell autophagy. In addition to nonselective (bulk) autophagy, machinery and significance of selective autophagy such as mitophagy of pancreatic β-cells is discussed. Novel findings regarding autophagy types other than macroautophagy are also covered, since several types of autophagy or lysosomal degradation pathways other than macroautophagy coexist in pancreatic β-cells.
Major conclusion
Autophagy plays a critical role in cellular metabolism, homeostasis of the intracellular environment and function of organelles such as mitochondria and endoplasmic reticulum. Impaired autophagic activity due to aging, obesity or genetic predisposition could be a factor in the development of β-cell dysfunction and diabetes associated with lipid overload or human-type diabetes characterized by islet amyloid deposition. Modulation of autophagy of pancreatic β-cells is likely to be possible in the near future, which would be valuable in the treatment of diabetes associated with lipid overload or accumulation of islet amyloid.
Keywords: β-cells, Autophagy, Lysosome, Mitophagy, Islet-associated polypeptide (IAPP), Crinophagy
Highlights
-
•
Autophagy is critical for cellular metabolism, homeostasis and organelle function.
-
•
Impaired autophagic activity could predispose to β-cell dysfunction and diabetes.
-
•
Several types of autophagy coexist in pancreatic β-cells.
1. Introduction
Autophagy (meaning ‘self-eating’) is characterized by lysosomal degradation of cells' own material, which is in contrast to heterophagy or xenophagy, where degrading extracellular or foreign targets occurs. There are three major types of autophagy: macroautophagy, microautophagy and chaperone-mediated autophagy (CMA) [1]. In addition to the three major types, other types of autophagy have been reported such as crinophagy, starvation-induced nascent granule degradation (SINGD), Golgi membrane-associated degradation (GOMED) and vesicophagy, all of which are relevant to hormone-producing cells such as islet β-cells [2], [3], [4]. In addition, minor types of autophagy such as chaperone-assisted selective autophagy (CASA) and chaperone-assisted endosomal microautophagy (CAEMI) have been described, and more specialized types of autophagy are likely to be discovered. Among the many types of autophagy, macroautophagy is the most extensively studied and hereafter will be referred to as autophagy. Autophagy is a process of subcellular membrane rearrangement sequestering cytoplasm, proteins (or other cellular materials) and organelles, forming the autophagosome. Thus, autophagosome is characterized by organelles (or other cellular materials) surrounded by a double membrane, which is a morphological hallmark of autophagy identified by electron microscopy. Once the autophagosome is formed, it fuses with lysosome forming the autophagolysosome, and lysosomal enzymes digest the sequestered material inside the autophagolysosome [1], [5]. The main purpose of autophagy is to maintain intracellular nutritional homeostasis and achieve quality control of organelles and proteins.
While autophagy was originally considered nonselective regarding its targets (bulk autophagy), it is now clear that autophagy can target specific organelles or molecules (selective autophagy). Thus, each specific type of autophagy has its own name, e.g., mitophagy, endoplasmic reticulum (ER)-phagy, pexophagy, granulophagy, ribophagy, lipophagy, etc. Each type of selective autophagy has also its own function depending on the target organelle or molecule. Such diverse types of selective autophagy share a large part of the basic machinery of autophagy but also have specialized machinery and function of its own.
Because autophagy is crucial in the maintenance of cellular organelles such as mitochondria or ER that play a vital role in the survival or function of insulin-producing pancreatic β-cells and insulin sensitivity of insulin target tissues, dysregulation of autophagy would lead to dysfunction or death of β-cells or abnormal insulin sensitivity in insulin target tissues. Here, we summarize and discuss current data on the role of autophagy of pancreatic β-cells, focusing on that of dysregulated β-cell autophagy in the development of β-cell failure and diabetes.
2. Molecular machinery of autophagy
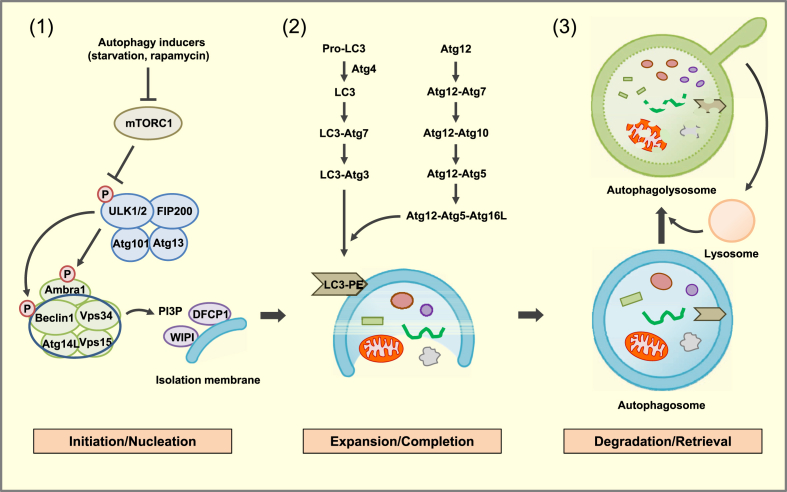
The autophagic process can be broadly divided into three stages: initiation/nucleation, expansion/completion and degradation/retrieval (Figure 1).
Figure 1.
Molecular pathways of autophagy. (1) Initiation/Nucleation: Autophagy inducers such as nutrient deprivation or rapamycin reduce mTORC1 phosphorylation, which liberates the ULK1/2 complex. The ULK1/2 complex increases activity of the Beclin1/Vps34 complex through phosphorylation of Ambra1 and Beclin 1. PI3P, produced by Vps34, recruits effector proteins such as DFCP1 and WIPI2 to promote formation of isolation membrane and to constitute the nucleation step. (2) Expansion/Completion: Expansion of autophagosome is mediated by two conjugated systems comprising Atg12-Atg5-Atg16L and LC3-Atg3 which lead to the formation of LC3-PE (LC3-II). (3) Degradation/Retrieval: After completion, mature autophagosome fuses with lysosome forming autophagolysosome, wherein the sequestered materials or organelles are degraded by lysosomal enzymes. After digestion of entrapped materials, nutrients become available and mTORC1 is reactivated. Proto-lysosomal tubules are then formed which mature into functional lysosome, constituting the retrieval process.
In the initiation/nucleation stage, the UNC51-like kinase 1 (ULK1) complex and the Bcl-2-interacting myosin-like coiled-coil protein (Beclin 1) complex play central roles. In a nutrient-replete condition, mTOR complex 1 (mTORC1) kinase is incorporated into the ULK1-Atg13-FIP200 complex and phosphorylates ULK1. Inhibition of mTORC1 by nutrient deprivation or rapamycin leads to its dissociation from the ULK complex. Liberated ULK1 phosphorylates mAtg13 and FIP200 initiating autophagy [1]. ULK1 also phosphorylates Ambra1, a Beclin 1-interacting protein, and makes Beclin 1 relocated to ER. Then, Beclin 1 forms a complex with Vps34, Vps15 and Atg14L. Vps34, a class III phosphatidylinositol 3-kinase (PI3K), produces phosphatidylinositol-3-phosphate (PI3P) and thereby recruits double FYVE-containing protein 1 (DFCP1), WIPI2 (a PI3P-binding effector protein) and Atg proteins, starting formation of isolation membrane and constituting a nucleation process (Figure 1).
The autophagosome expansion/completion consists of two ubiquitin-like pathways. The Atg system is critical for both of the two ubiquitin-like pathways. Here, Atg7 acts as an E1-like enzyme, and Atg10 and Atg3 act as E2-like enzymes. There is no E3-like enzyme; however, Atg12-Atg5-Atg16L1 acts as an E3-like enzyme complex [1]. Atg8 (also called microtubule-associated protein 1 light chain 3 [LC3]), a ubiquitin-like protein, is conjugated to Atg3 via Atg7 action, and Atg8-Atg3 intermediate is recruited to isolation membrane through the interaction between Atg3 and Atg12 in Atg12-Atg5-Atg16L1 complex bound to isolation membrane. Then, Atg8 is conjugated to its lipid target, phosphatidylethanolamine (PE) in the membrane, forming LC-II [1]. After processing, LC3-II is localized to both the outer and inner sides of autophagosome membrane. LC3-II on the outer side of the membrane is released to the cytosol. In mammals, there are seven LC3-related proteins including LC3A (two splice variants), LC3B, LC3C, GABARAP, GABARAPL1 and GABARAPL2. LC3B is the most extensively studied among them, however, each member of the LC3 family appears to have a specific nonredundant function.
After completion of autophagosome formation, the autophagosome fuses with the lysosome, and lysosomal enzymes induce hydrolysis of entrapped organelles and macromolecules. When nutrients become available and mTORC1 is reactivated, proto-lysosomal tubules are formed and ultimately mature into functional lysosome [6], completing the entire cycle of the autophagic process.
3. Machinery of selective autophagy
Selective autophagy targeting specific organelles or molecules has its own specialized autophagic machinery in addition to the common autophagic pathways shared by nonselective autophagy and most other types of autophagy. Selective autophagy targeting a specific organelle plays a critical role in the maintenance of the proper function of the respective organelle, without which organelle dysfunction ensues.
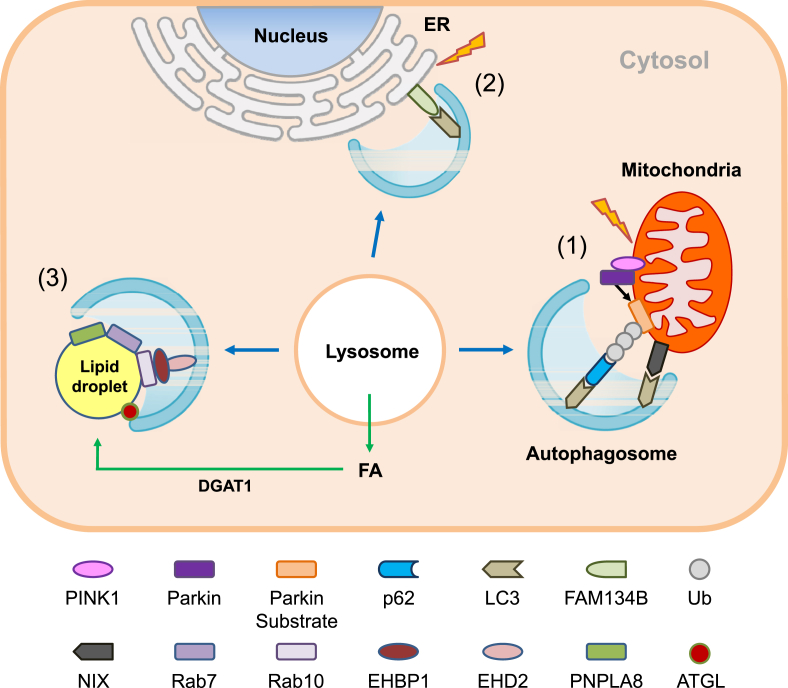
One of the foremost examples of organ-specific selective autophagy is mitophagy. Maintenance of mitochondrial integrity is especially critical for the survival of cells because mitochondria are particularly susceptible to damage from reactive oxygen species (ROS). Namely, mitochondria are the site of electron transfer and produce abundant oxygen radicals. Mitochondrial DNA, existing partly in a single stranded conformation, is not well protected by the nucleosome-like structures as is genomic DNA [7]. Thus, mitophagy serving to maintain mitochondrial function will be a critical element in the maintenance of energy balance and protection against oxidative stress. One of the most famous and important machineries of mitophagy is the PINK (PTEN-induced putative kinase)-Parkin pathway (Figure 2). PINK1 is a sensor of mitochondrial depolarization because PINK1 accumulates on the outer mitochondrial membrane (OMM) of depolarized mitochondria, while PINK1 is rapidly imported into the mitochondrial intermembrane space and degraded by presenilin-associated rhomboid-like protein (PARL) when mitochondrial potential is maintained [8]. PINK1 on the OMM of depolarized mitochondria recruits Parkin, an E3 ligase, which induces ubiquitination of several OMM proteins such as mitofusin, porin, Miro or VDAC and subsequently recruits autophagy receptors containing ubiquitin-binding domain and LC3-interacting region (LIR) domain such as p62, optineurin, NDP52 or NBR1 (Figure 2). In addition to the PINK-Parkin pathway, several types of receptor-mediated mitophagy have also been reported in which autophagic machinery is directly recruited to the mitochondrial target proteins that are localized to the MOM and harbor the LIR domain such as NIX, FUNDC1, BCL2L13, BNIP3 or FKBP8. Piecemeal-type basal mitophagy has recently been described which is Parkin- or LC3B-independent but p62-or LC3C-dependent [9].
Figure 2.
Machinery of selective autophagy. (1) Mitophagy: When mitochondrial potential is dissipated by mitochondrial stressors, PINK1 is stabilized on the outer membrane of mitochondria and recruits Parkin. Parkin, as an E3 ligase, ubiquitinates several substrates in mitochondria and induces mitophagy together with autophagy receptors including p62, optineurin, NDP52 and NBR1. In addition to Parkin-mediated ubiquitin-dependent mitophagy, Parkin-independent receptor-mediated autophagy and piecemeal-type mitophagy has also been described. (2) ER-phagy: An ER membrane protein, FAM134B, has been shown to mediate ER-phagy through the interaction of the LIR domain of FAM134B and LC3. In addition to FAM134B, Sec62 and RTN3 can also interact with LC3 through their LIR domains. FAM134B and Sec62 mediate ER-phagy of ER cistern, while RTN3 mediates that of ER tubule. (3) Lipophagy: Small GTPases such as Rab7 or Rab10 have been shown to mediate the formation of the ‘lipophagic synapse’ and autophagic degradation of LD. LC3 may also facilitate recruitment of ATGL to LD via the interaction with the LIR domain of ATGL, leading to lipolysis by cytosolic lipases. Autophagy may also contribute to the DGAT-1-dependent formation of LD by providing free fatty acids (FFAs) (green arrows), which may favor efficient oxidative degradation of FFAs through β-oxidation after transfer to mitochondria and reduce lipotoxicity of FFAs.
In addition to mitophagy, specific molecular pathways for other types of selective autophagy are being characterized. FAM134B has been identified as an ER-resident autophagy receptor in a screening for binding proteins for LC3B and its homologue (GATE16/GABARAPL2) (Figure 2). Furthermore, FAM134B knockout (KO) led to ER expansion, impaired ER turnover and susceptibility to ER stress, suggesting an important role of FAM134B in ER-phagy [10]. Follow-up studies identified other ER-resident autophagy receptors mediating ER phage including Sec62 and RTN3 [11], [12]. RTN3 appears to mediate the turnover of ER tubule rather than ER cistern [12].
In addition to authentic cellular organelles, several nutrients are targets of selective autophagy. A noteworthy example of such autophagic degradation of cellular nutrients is ‘lipophagy’ (Figure 2). In this process, triglycerides (TGs) stored in lipid droplets (LDs) are degraded by lysosomal lipase rather than by cytosolic lipases [13]. Regarding the molecular machinery of lipophagy, a role for Rab proteins, small GTPases mediating organelle trafficking, has been reported. Rab7, a regulator of the late endocytic pathway, is induced during starvation and is essential for the formation of a lipophagic synapse between LD, multivesicular body and lysosome, and finally LD breakdown in hepatocytes [14]. Rab10, which participates in insulin-mediated GLUT4 translocation, is recruited to LD during starvation and mediates LD breakdown together with EHBP1 and EHD2, Rab effectors [15]. In contrast to these results, Rab32 knockdown (KD) induces lipophagy through induction of adipocyte triglyceride lipase (ATGL), also called patatin-like phospholipase domain-containing protein 2 (PNPLA2) (Figure 2) [16].
ATGL, a major cytosolic TG hydrolase, interacts with LC3 through the LIR domain in brown adipocytes, which may be important for efficient localization of ATGL to LD and lipolysis [17]. Hormone-sensitive lipase (HSL), another important lipase, also has an LIR domain and interacts with LC3 in LD [17]. A role for other patatin-like phospholipase domain-containing protein (PNPLA) in lipophagy has also been reported. PNPLA8 is induced by statin or SREBP2, and leads to TG breakdown [18] (Figure 2).
In contrast to the degradation of lipid by lipophagy, LC3-mediated autophagy contributes to the formation of cytoplasmic LDs [19]. Starvation-induced autophagy replenishes free fatty acids (FFAs) to LDs, enabling FFA transfer to mitochondria via cytoplasmic lipase for mitochondrial β-oxidation [19] (Figure 2). Such a mechanism might be useful for efficient utilization of FFA during starvation while avoiding cellular toxicity by FFAs, and contribute to the paradoxically increased formation of LD during fasting. Diacylglycerol acyltransferase 1 (DGAT1) on ER has been shown to incorporate autophagy-liberated FFA to newly formed LD in the vicinity of mitochondria [20].
4. β-Cell autophagy in diabetes
As autophagy is critical in the maintenance of diverse organelles such as mitochondria and ER, autophagy plays an indispensable role in β-cell physiology. Indeed, autophagy-deficient β-cells show abnormal morphology and function of mitochondria and ER [21], [22], [23], while other organelles such as peroxisome or Golgi apparatus would be affected as well. In addition to the role of autophagy in β-cell physiology, dysregulated β-cell autophagy contributes to the development of diabetes. To study the role of dysregulated autophagy in the development of diabetes, we previously studied ER dysfunction in β-cell autophagy deficiency because ER distention is observed in autophagy-deficient β-cells [22] and ER stress plays a crucial role in β-cell failure associated with type 2 diabetes (T2D) [24], [25]. In autophagy-deficient β-cells, markedly depressed expression of almost all unfolded protein response (UPR) genes were noted using real-time RT-PCR, contrary to the expectation that UPR gene expression would be increased due to ER stress [23]. Because UPR is an adaptive response to ER stress in addition to its well-known role as an ER stress marker, inadequate UPR during ER stress could create a dangerous state [26]. Indeed, when Atg7 KO β-cells were treated with thapsigargin [27] or FFAs that can impose ER stress [28], more pronounced cell death occurred compared to autophagy-competent β-cells [23]. When primary islet cells from Atg7Δβ-cell mice were incubated with palmitic acid, the most abundant saturated FFA in vivo, cell death was again more pronounced compared to autophagy-competent type primary islet cells. These results suggest that autophagy is important for appropriate UPR in response to ER stress, although the molecular mechanism of inadequate UPR gene expression in autophagy-deficient β-cells is not clearly elucidated. In contrast to these results, the protein level of Epr57/GPR58, an ER protein that can be induced by cellular stresses, was increased in autophagy-deficient β-cells, while mRNA expression was not studied [29].
The significance of autophagy deficiency of β-cells in the development of diabetes in vivo was studied again focusing on ER stress. β-cell-specific Atg7-KO mice (Atg7Δβ-cell) that develop only mild impaired glucose intolerance (but not diabetes) were crossed to ob/ob mice, as obesity levies ER stress on β-cells [25]. As expected, UPR gene expression was increased in islets of ob/ob mice but such UPR gene induction was insufficient in islets of Atg7Δβ-cell-ob/ob mice [23], suggesting that the demand for UPR due to obesity is unmet in autophagy-deficient β-cells. Atg7Δβ-cell-ob/ob mice developed severe diabetes in conjunction with an increased number of apoptotic β-cells and decreased β-cell mass [23], which suggests that autophagy-deficient β-cells are susceptible to ER stress inflicted by obesity. This observation is consistent with a previous report that Atg7Δβ-cell mice are incapable of an adaptive increase in β-cell mass after high-fat diet (HFD) feeding [30]. Similarly, pancreatic ductal infusion of shAtg7 resulted in reduced β-cell mass, defective insulin release and increased apoptosis in mice fed a high-fat/high-glucose diet [31]. In addition to UPR genes, the expression of antioxidant genes such as SOD1, SOD2, Gpx1, Gpx2, HO-1 and catalase was downregulated in autophagy-deficient β-cells as revealed by real-time RT-PCR, which is consistent with increased ROS accumulation in autophagy-deficient β-cells and reversal of metabolic derangement of Atg7Δβ-cell mice by in vivo administration of N-acetyl-l-cysteine (NAC), an antioxidant [32].
These results are congruent with other papers showing the protective role of β-cell autophagy against ER stress caused by proinsulin misfolding, insulin secretory defects or cholesterol [33], [34], [35]. A recent paper reported a protective role of β-cell autophagy induced by C3 binding to ATG16L1 against apoptosis by palmitic acid or islet-associated polypeptide (IAPP) [36]. The protective effects of rosiglitazone, metformin or glucagon-like peptide 1 (GLP-1) receptor agonists against apoptosis of β-cells have also been attributed to autophagy [37], [38]. For instance, metformin, the first-line antidiabetic drug recommended by the ADA and EASD, appears to promote removal of accumulated autophagic vacuoles in β-cells by enhancing autophagy through AMPK activation [39], while it is not known whether the decreased accumulation of autophagic vacuole after metformin treatment is due to increased autophagic activity. In this regard, a recent paper demonstrated that phenformin, an analogue of metformin with a higher affinity for mitochondrial membranes, impaired autophagic activity through inhibition of mitochondrial complex I and phosphatidylserine decarboxylase (PISD) activity converting phosphatidylserine (PS) to mitochondrial PE [40]. Thus, it might be premature to conclude that AMPK activators such as metformin are autophagy activators in general. Exendin-4, a GLP-1 receptor agonist, has also been reported to ameliorate lysosomal dysfunction and defective autophagosome-lysosome fusion caused by tacrolimus [38].
Contrary to these beneficial effects of autophagy on β-cell survival, autophagy inhibition has been reported to reduce β-cell death due to Pdx1 KD or amino acid deprivation [41], suggesting possible occurrence of autophagic cell death. Impaired β-cell function and viability by rapamycin have also been attributed to upregulated autophagy associated with downregulation of insulin production and β-cell apoptosis [42]. On the functional aspect, a couple of papers reported that short-term KD of autophagy genes increased the content or release of insulin or proinsulin which is different from the results using autophagy KO β-cells and has been ascribed to reduced autophagic degradation of proinsulin or lysosomal lipid acting on insulin secretion [43], [44]. Inconsistencies between published data on the effect of autophagy on β-cell survival, death or function could be partly due to ambiguity concerning the definition or significance of autophagic cell death and methods or duration of genetic manipulation. Further studies would elucidate the molecular and cellular mechanisms of autophagic cell death and reveal its functional consequences.
5. β-Cell autophagy in human-type diabetes with islet amyloid
Most studies investigating the role of β-cell autophagy in diabetes have employed rodent models. However, human diabetes and rodent models of diabetes vary in several important aspects. One of the most striking differences is deposition of Congo Red-stained amyloid in pancreatic islets of ca. 90% of human T2D patients, which is never observed in islets of rodent diabetes [45]. Such a difference is due to the dissimilar amino acid sequence of IAPP also called amylin [45]. Amyloidogenic or aggregate-prone peptides such as human IAPP (hIAPP) are preferentially degraded by autophagy rather than by proteasomal degradation, which is different from soluble nonamyloidogenic peptides such as murine IAPP (mIAPP) that are well cleared by proteasomal degradation [46]. Thus, autophagy could be more relevant to human T2D characterized by islet amyloid accumulation than mouse T2D without islet amyloid.
The in vivo role of autophagy in hIAPP clearance was investigated employing transgenic mice expressing hIAPP in pancreatic β-cells (hIAPP+ mice) because mice do not express amyloidogenic IAPP. While hIAPP+ mice had only mild glucose intolerance, the same mice developed diabetes when they were crossed to β-cell-specific Atg7 KO mice, rendering them deficient in β-cell autophagy (hIAPP+Atg7Δβ-cell mice) [47], [48]. Furthermore, hIAPP oligomer could be clearly observed in islets of hIAPP+Atg7Δβ-cell mice but not in those of autophagy-competent hIAPP+Atg7F/F mice, suggesting efficient clearance of hIAPP oligomer when autophagy is intact. Islet amyloid stained with Thioflavin-S was also observed in hIAPP+Atg7Δβ-cell mice. Because hIAPP oligomer rather than islet amyloid is likely a dominant effector molecule in islet injury [45], accumulation of hIAPP oligomer in β-cells rather than that of islet amyloid likely led to the observed increase in apoptosis and fewer number of β-cells in hIAPP+Atg7Δβ-cell mice [47]. Consistent with these data, in vitro studies demonstrated islet cell death by transgenic hIAPP expression or hIAPP peptides when autophagy was inhibited [49], [50]. In addition to exogenous hIAPP, the effect of endogenous amyloidogenic IAPP in islet cell death has been tested in vitro. When monkey islet cells expressing amyloidogenic simian IAPP (sIAPP), similar to hIAPP, was incubated with bafilomycin A1 inhibiting lysosomal steps of autophagy, significant death of monkey islet cells was observed which was accompanied by accumulation of sIAPP oligomer. Significant cell death was not observed when rodent islet cells were treated in the same way [47]. The mechanism of β-cell death by hIAPP oligomer may entail ER stress [51], clogging of ER translocon [52], inflammasome activation [53] or membrane damage due to membrane pore formation leading to ion leakage [54], [55].
While autophagy insufficiency leads to accumulation of hIAPP oligomer and islet cell death, hIAPP oligomer conversely impairs autophagy as evidenced by accumulation of p62, a well-known autophagy substrate and autophagy receptor [47], [49]. Consistently, hIAPP overexpression leads to impaired mitophagy and unbalanced mitochondrial dynamics [56]. These results suggest that if hIAPP oligomer accumulation due to reduced autophagy reaches a certain level, it may further impair autophagic activity. This positive-feedback mechanism may lead to a profound decrease in autophagic activity and a large accumulation of hIAPP oligomer or islet amyloid, and then diabetes. Contrary to these results, increased autophagic activity by hIAPP peptide has also been reported [50]. These discrepancies could be attributable to the differences in experimental procedures, expression level of hIAPP, concentration of hIAPP peptides employed or the method of autophagy determination.
The role of hIAPP in diabetes has also been investigated employing an hIAPP knock-in mouse model instead of transgenic hIAPP expression models. In this model, the endogenous murine IAPP gene was replaced with the hIAPP gene employing a homologous recombination technology, which could be more physiological than transgenic hIAPP expression driven by an insulin promoter. When hIAPP knock-in mice were crossed to Rip-Cre:Atg7F/F mice to make them β-cell autophagy-deficient and fed HFD, they developed more severe metabolic changes and β-cell loss compared to control mice [50].
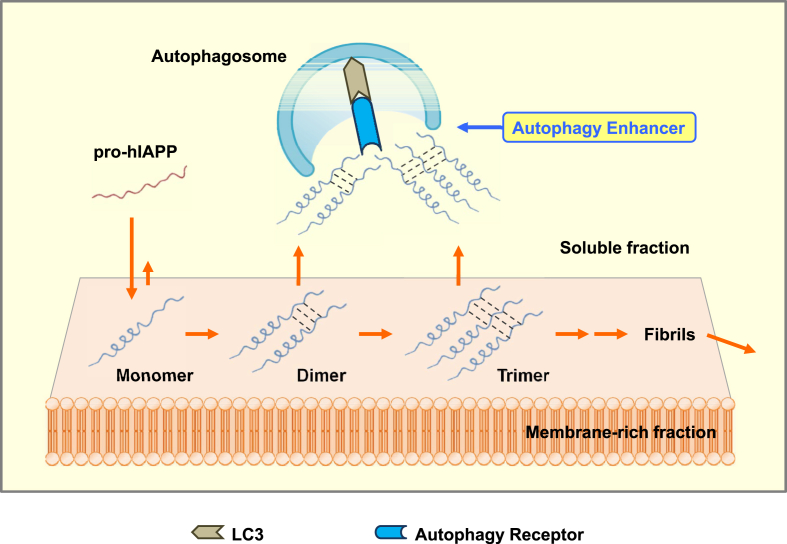
We have examined molecular species of hIAPP oligomer accumulating in autophagy-deficient β-cells by tagging an HA sequence to prepro-hIAPP which undergoes a similar maturation step to that of prepro-insulin by PC1/3 and PC2 [45]. When prepro-hIAPP was expressed, pro-hIAPP dimer was observed which was markedly increased by autophagy inhibition. When prepro-mIAPP was expressed, a pro-mIAPP dimer was not observed. Furthermore, pro-hIAPP dimer disappeared when three critical amino acid residues (one alanine residue at codon 58, and two serine residues at codon 61 and 62) were changed to prolines that were seen in prepro-mIAPP. Content of the pro-hIAPP dimer was higher in the membrane-rich fraction compared to the soluble cytosolic fraction. Furthermore, pro-hIAPP trimer was additionally observed in the membrane-rich fraction when autophagy was blocked [47], suggesting that pro-hIAPP dimerization or trimerization might be the initial step leading to the formation of hIAPP oligomer and amyloid. Thus, it was shown that pro-hIAPP dimer and trimer are molecular species targeted by autophagy in pancreatic β-cells, while high-n or fibrillary hIAPP oligomer could also be direct targets of autophagy (Figure 3).
Figure 3.
A model for autophagic clearance of pro-hIAPP oligomer. Pro-hIAPP dimer is formed in the membrane-rich fraction after binding of pro-hIAPP to the membrane fraction. Some pro-hIAPP dimers are translocated to the soluble fraction, while other pro-hIAPP dimers proceed to form pro-hIAPP timers. pro-hIAPP dimer and trimer are targets of autophagic clearance. high-n or fibrillary hIAPP oligomer could also be targets of autophagy. Autophagy enhancers may be able to expedite clearance of pro-hIAPP dimer, pro-trimer and hIAPP oligomer.
However, the mechanism of the maturation of hIAPP dimer or trimer to high-n or fibrillary hIAPP oligomer and then to hIAPP amyloid is far from clear and warrants future studies. Further work will be required to fully understand the biophysical process leading to the formation of hIAPP oligomer or amyloid and molecular details of autophagic clearance of hIAPP oligomer such as autophagic receptor(s) involved in the clearance of hIAPP oligomer, while p62 has been reported to be associated with hIAPP oligomer [48], [50].
6. Mitophagy and diabetes
While numerous previous papers have investigated the effect of autophagy on β-cell function in association with mitochondria, a relatively small number of papers examined the role of mitophagy per se on β-cell function. An earlier paper showed that mitochondrial fission followed by selective fusion and segregation of dysfunctional mitochondria could lead to mitophagy and removal of dysfunctional mitochondria in β-cells [57]. The role of authentic mediators of mitophagic machinery in systemic metabolism has also been studied. However, the metabolic effects of Parkin remain unclear despite several papers studying the role of Parkin in lipid metabolism or adipose tissue [58], [59]. One study assessed the role of Parkin in the mitophagy of pancreatic β-cells. Parkin KO mice showed further impaired glucose tolerance and reduced insulin release after treatment with streptozotocin and PFTα, a p53 inhibitor, compared to control mice [21], suggesting a protective role of Parkin-mediated mitophagy in stressed β-cells. A role of upstream regulators of Parkin-mediated mitophagy of β-cells has been reported. Clec16-RNF41-USP8 complex led to regulated mitophagy and normal insulin secretion, while disruption of the complex by stressors induced aberrant mitophagy and impaired β-cell function [60], [61].
The role of mitochondria movement in mitophagy of pancreatic β-cells has also been reported. Participation of Miro1, a mitochondrial outer membrane protein mediating the anchoring of kinesin-related protein 5 (KIF5) to mitochondria, in mitophagy of β-cells was reported [62].
Because lipids have a significant impact on autophagy, lipids are likely to affect mitophagy. Mitophagy has been shown to be suppressed by HFD feeding, as measured in the liver tissue of mice expressing a mitochondrial-targeted form of the fluorescent reporter Keima (mt-Keima), while the functional metabolic consequence of such decreased mitophagy was not investigated [63]. Changes of the mitophagy of pancreatic β-cells after HFD feeding have not been tested in those mice.
In addition to mitophagy, other types of selective autophagy such as ER-phage or lipophagy occur in pancreatic β-cells and play an important role in the pathophysiological events associated with glucose homeostasis or the development of diabetes. While changes of ER stress or lipid content in autophagy-deficient pancreatic β-cells in association with the development of diabetes have been investigated as discussed above, the occurrence of bona fide ER-phage or lipophagy in β-cells has not been clearly shown, partly because the lack of sensitive and specific tools to monitor such selective types of autophagy. Since several new markers of selective autophagy and novel autophagy receptors or adaptors associated with selective autophagy are becoming available, detailed investigation of selective autophagy related to β-cell function and diabetes will be possible, which will lead to a comprehensive knowledge of organelle function and dysfunction in the pathogenesis of diabetes and development of novel therapeutics based on such an innovative paradigm.
7. Other types of autophagy in pancratic β-cells
In addition to (macro)autophagy, other types of autophagy or lysosomal degradation pathways have been reported, including those acting specifically in pancreatic β-cells.
Crinophagy, a term coined by de Duve, is a phenomenon involving direct fusion of secretory granules into lysosomes, followed by subsequent degradation [64], and has been suggested to be an essential physiological mechanism of intracellular degradation of insulin [65]. Secretory granules containing mature insulin fuse with lysosomes, forming crinophagic bodies in pancreatic β-cells. This process can be precisely controlled by the concentration of glucose [66], indicating physiologic regulation of surplus insulin products.
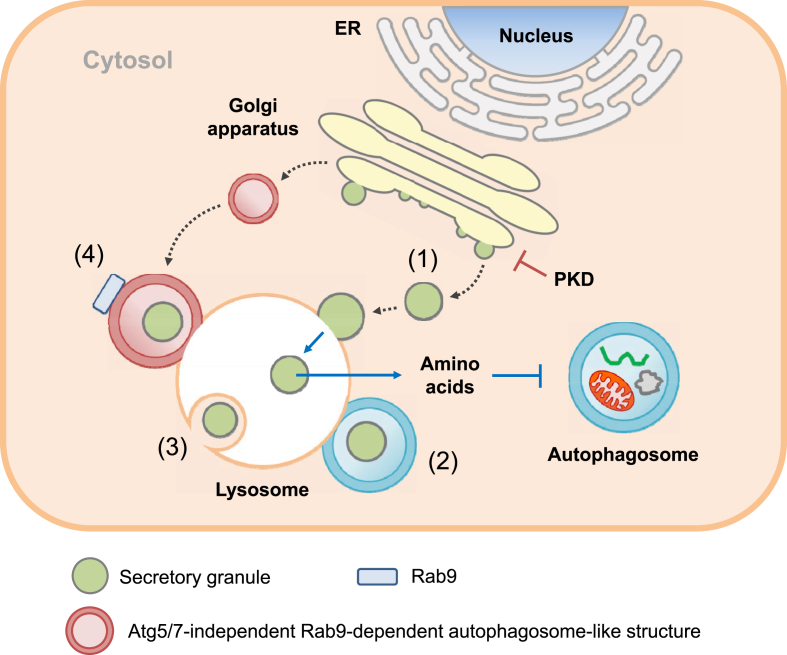
While nutrient deprivation induces autophagy in most types of cells, nutrient deprivation has been reported to inhibit autophagy in β-cells. In starvation, nascent insulin secretory granules were found to be degraded by lysosomes in a process called SINGD instead of autophagy, and nutrients released from SINGD inhibited autophagy. SINGD occurred in the vicinity of Golgi bodies, and could be inhibited by protein kinase D (PKD) which, in turn, is inhibited by p38δ [2] (Figure 4). SINGD and subsequent inhibition of autophagy could be a mechanism to reduce insulin release during starvation; however, the relationship between SIGND and crinophagy or GOMED (see below) needs to be elucidated. The role of SINGD in the development of diabetes also warrants further studies.
Figure 4.
Lysosomal autophagic degradation pathways other than macroautophagy in pancreatic β-cells. (1) SINGD: In starvation, PKD is inactivated at the Golgi area and nascent insulin secretory granules generated in Golgi apparatus directly fuse with lysosomes. Amino acids released from lysosomal degradation of insulin secretory granules inhibit autophagy through mTORC1. (2) Vesicophagy: Insulin granules are sequestered in autophagosome, and autophagosome containing insulin secretory granules as an autophagy cargo fuses with lysosome for degradation of insulin secretory granules. (3) Microautophagy: β-cell granules are engulfed by late endosome or multivesicular body in a manner similar to phagocytosis for degradation of the granule components. (4) GOMED: When the insulin secretory process from the Golgi apparatus is suppressed in autophagy-deficient β-cells, autophagosome-like double-membrane compartment is formed from Golgi membrane in an Atg5/7-independent but Rab9-dependent manner.
Metabolic effects of genetic hyperactivation of autophagy have been studied by transgenic expression of Becn1F121A, which inhibits Becn1 binding to Bcl-2 and makes Becn1 constitutive active [67]. HFD-fed Becn1F121A/F121A and Becn1F121A/+ mice showed improved insulin sensitivity due to ameliorated ER stress in insulin target tissues; however, the same mice developed aggravated glucose tolerance compared to nontransgenic mice due to reduced insulin release from β-cells [4]. Such a phenomenon was attributable to sequestration and degradation of insulin granules by hyperactive autophagy of β-cells, which has been termed ‘vesicophagy’, autophagic degradation of the insulin granule. Vesicophagy has been suggested to play a role in the inhibition of additional insulin release during fasting and could be distinct from crinophagy, direct fusion of insulin granule with lysosome (Figure 4) [4].
GOMED has been reported as another non-canonical, bulk degradation pathway that compensates for the absence of Atg5/Atg7-dependent autophagy in yeast and mammalian cells [3]. When Golgi-to-plasma membrane (PM) anterograde trafficking is disrupted, Golgi membranes are stacked and elongated, followed by the formation of autophagosome-like double-membrane compartments together with late endosomal membrane in a Rab9-dependent manner that envelop the cytoplasm and various organelles to compensate for autophagy. These structures then fuse with lysosomes to degrade cargo molecules. In β-cells, (pro)insulin granules are synthesized in the Golgi bodies and then secreted from cells in a way similar to Golgi-to-PM trafficking. When the insulin secretory process from the Golgi apparatus is suppressed, GOMED is activated in response to glucose depletion to alternatively degrade these unused (pro)insulin granules in Atg5-or Atg7-deficient β-cells. GOMED might be a crucial mechanism to regulate insulin secretion during starvation, especially in the condition of reduced protein degradation by conventional autophagy such as T2D or lipid overload [68]. If Atg5/Atg7-dependent autophagy is intact in β-cells, crinophagy or SINGD could be a major pathway for the degradation of unused (pro)insulin granules [3].
Microautophagy is another form of lysosomal degradation of insulin granule in pancreatic β-cells and is characterized by direct lysosomal engulfment of cytosolic content or granules in a phagocytic manner. Microautophagy is different from crinophagy showing fusion of β-cell granule membrane with lysosomal membrane (Figure 4). The molecular machinery of microautophagy involves delivery of cytosolic proteins to the late endosome or multivesicular body (MVB) in a manner dependent on the endosomal sorting complexes required for transport (ESCRT) system and hsc70 [69]. Microautophagy contributes to the degradation of intracellular insulin in Rab3A deficiency, however, it remains unclear whether microautophagy plays a significant role in the pathophysiology associated with β-cell function [70].
8. Autophagy deficiency in aging and lipid injury – implications of autophagy insufficiency as a cause of β-cell failure
As discussed above, β-cell autophagy is crucial in the physiology and appropriate insulin secretory function of β-cells, and its dysregulation leads to an impaired adaptive response to stresses and the development of diabetes associated with lipid overload or of human-type diabetes showing accumulation of hIAPP amyloid. Then, an important question is whether impaired autophagic activity can be indeed observed in the course of development of natural diabetes and contribute to β-cell failure, because autophagy impairment in the above experiments has been imposed mostly by genetic manipulation rather than by physiological processes. In this regard, an increase in autophagosome content has been observed in islets of T2D patients [39]. Because increased autophagosome content could be due to either enhanced autophagic activity or blockade of autophagy at the lysosomal step [5], such data do not prove that autophagic activity is reduced in islets of T2D patients. However, it is likely the case because metformin treatment reduced autophagosome content, probably through AMPK activation and autophagy activation, and expression of autophagy genes or lysosomal genes working at the later stage of autophagy were reduced in islets from patients from T2D patients [39]. Several papers reported depressed autophagy with aging. Expression of several autophagy genes including Beclin 1 or Atg5-Atg12 were reduced in several tissues of aged mice, accompanied by increased mTOR activity [71], [72]. A decreased number of LC3+ puncta was also noted in the kidney tissue of aged mice [73]. In addition to the changes of autophagosome number, a decreased autophagic flux was observed in stromal vascular fraction of adipose tissue from old mice [74]. Decreased autophagic activity in aging might be caused by reduced expression of motor proteins such as KIFC3 directing lysosomal movement to the perinuclear region for fusion with autophagosome rather than defective fusion activity itself [75]. In this regard, an observational study reported increased accumulation of hIAPP amyloid in islets of old nondiabetic subjects in a manner dependent on age [76]. Such data suggest the possibility that β-cell autophagic activity might decrease with aging because β-cell autophagy is a crucial element in the clearance of hIAPP oligomer or amyloid as discussed above, although several factors other than autophagic activity might also influence accumulation of hIAPP amyloid.
In addition to general autophagy, mitophagy has also been reported to decrease in the neurons of aged mice [63]. These results suggest that function of organelles such as mitochondria or ER can be compromised in aged animal or subjects due to insufficient rejuvenation of aged, senescent or damaged organelles, which can lead to impaired function of tissues or cells such as pancreatic β-cells. Thus, insufficiency of nonselective or selective autophagy due to aging might contribute to β-cell dysfunction in aging [77], although changes of β-cell function in aging could be different depending on the degree of aging, genetic predisposition, species or accompanying insulin resistance [77], [78]. In addition to autophagy, CMA has been reported to be reduced in aging [79]. Lysosomal activity includes (but is not restricted to) diverse types of autophagic activity, and also appears to be reduced in aging as shown by increased senescence-associated β-galactosidase activity measured at pH 6.0 in aged hippocampal neurons [80] and accumulation of lipofuscin, pigment granules comprising lipid-containing residues of lysosomal digestion [81] in diverse tissues in aging [82]. Lipofuscin can have deleterious effects on lysosomal function and autophagy [83], potentially inducing lysosomal dysfunction in aging.
Lipid overload or obesity also affects autophagic activity. Excessive lipids have been reported to inhibit fusion between autophagosome and lysosome [84]. In contrast to this result, increased autophagic flux by FFAs has also been reported [85], [86], which was attributed to JNK activation [86]. The influence of lipid overload or obesity on autophagic activity in vivo has also been investigated by employing GFP-LC3+ mice that express GFP-conjugated LC3 [87]. The number of GFP puncta representing the autophagosome was increased in pancreatic islets of GFP-LC3+-ob/ob mice compared to lean GFP-LC3+ mice [23]. GFP cleavage probably by lysosomal enzymes of autophagolysosome was also observed in islets of GFP-LC3+-ob/ob mice but not in islets of lean GFP-LC3+ mice [23]. These results suggest that autophagic activity is indeed increased in islets of ob/ob mice, which is consistent with a paper showing increased LC3 conversion in pancreatic islets of HFD-fed mice after chloroquine administration clamping lysosomal steps of autophagy [88]. However, the level of p62, a well-known substrate of autophagy was increased in islets of ob/ob mice, which is apparently incompatible with the increased autophagic activity [5]. Increased p62 accumulation has also been observed in islets of human T2D patients or db/db mice [89]. To address this conundrum, a proteolysis assay was conducted. Lysosomal proteolysis was reduced by FFAs [23], which is consistent with previous results showing decreased lysosomal enzyme activity and proteolytic activity by FFAs such as palmitic acid or oleic acid [90]. Downregulation of proteolysis by FFAs, despite an apparent increase in the autophagic activity, might be due to inhibition of autophagosome fusion with lysosome [84] or the sequestration of autophagic machinery to accumulated lipid in an attempt to remove excessive lipid [13]. In contrast to these results, oleic acid has been reported to increase autophagic activity of pancreatic β-cells through downregulation of the cAMP-Epac2 axis [91]. Such difference could be due to different methods of measuring autophagic flux (proteolysis vs. LC3 conversion).
The effect of lipid overload on autophagy in vivo may also depend on the duration of lipid overload, because a study reported increased and decreased autophagic flux after short-term and long-term HFD feeding, respectively [92]. The effect of HFD on mitophagy has also been studied. The paper that reported reduced mitophagic activity in neurons of aged mice also showed severely depressed mitophagy in the liver of mice fed HFD using a mitophagy reporter mouse model expressing mt-Keima [63], which is in line with the reduced autophagy in the liver of mice fed HFD [93]. Altogether, the relationship between lipids and nonselective autophagy or selective autophagy such as mitophagy or lipophagy is complex, and will be different depending on the types of cells or lipids employed, duration of lipid overload, the mode of autophagy studied (bulk vs. selective), experimental techniques or assay conditions. Further studies employing more refined technologies will be required to understand the mechanism and significance of selective autophagy, and the reciprocal interaction between lipid and autophagy in diverse physiological and pathological contexts.
Combined with the potential reduced β-cell autophagy in aging and reduced capability of autophagy-deficient β-cells to handle metabolic stress [23], these results suggest the possibility that autophagy insufficiency due to aging, lipid injury or genetic predisposition could contribute to β-cell failure, an indispensable component of the pathogenesis of diabetes.
9. Autophagy enhancer in diabetes
If autophagy insufficiency of β-cells is a cause of β-cell failure associated with the development of diabetes, autophagy enhancement by pharmacological measure or other methods could be a novel way to treat or prevent diabetes. Indeed, several anti-diabetic drugs such as metformin, rosiglitazone or GLP-1 receptor agonists have been reported to exert metabolic improvement partly by enhancing autophagic activity [37], [38], [94]. In addition to such currently available drugs, experimental drugs or chemical agents that can improve the metabolic profile of diabetic animals by enhancing autophagic activity have been described. For instance, trehalose has been administered to hIAPP+ mice expressing human-type amyloidogenic IAPP, because autophagy is important in the clearance of hIAPP oligomer or amyloid and trehalose increases autophagic activity in several mice or cell types [95]. When trehalose was administered to hIAPP+ mice on HFD, the glucose profile was significantly improved, and this was accompanied by increased islet autophagy, reduced hIAPP oligomer or amyloid accumulation in pancreatic islets and improved β-cell function [47]. Enhanced viability of hIAPP-expressing insulinoma or islet cells by rapamycin, amiodarone or trifluoperazine has also been reported in vitro which has been ascribed to autophagy [48], [96]. These results suggest that autophagy enhancers could be novel therapeutic agents for the treatment of human diabetes characterized by hIAPP oligomer or amyloid (Figure 3).
Several other novel or currently available chemicals have been shown to improve the metabolic profile of obese or diabetic mice, while their effects were mostly on insulin resistance rather than β-cell failure. An autophagy enhancer identified in a screening of phytochemical library (Rg2) improved insulin sensitivity and metabolic profile after in vivo administration to HFD-fed mice for 4 weeks [97]. Recent studies using a high-throughput screening for small molecule autophagy enhancers identified several novel agents that can enhance autophagic activity through activation of Tfeb [98], [99], a master regulator of autophagy gene expression and lysosomal biogenesis [100]. Such chemicals could improve the metabolic profile of ob/ob mice or HFD-fed mice by increasing insulin sensitivity, however, the effect of those chemicals on β-cell function has not been carefully addressed [98], [99]. Further studies of the effects of such autophagy enhancers on β-cell function, particularly in association with the hIAPP oligomer/amyloid clearing effect, could reveal therapeutic potential of such chemicals on human-type diabetes characterized by hIAPP oligomer/amyloid accumulation.
10. Conclusion and future directions
Changes of autophagy associated with diabetes and the pathophysiological role of such changes of autophagy have been extensively studied; however, a unanimous consensus has not been reached. The role of autophagy of pancreatic β-cells in diabetes has also been investigated in a number of studies. There seems to be a consensus regarding the homeostatic role of (macro)autophagy in pancreatic β-cells in that it preserves the function of organelles such as mitochondria or ER and regulates the appropriate insulin response to a glucose challenge. However, details of the role and regulation of autophagy in pancreatic β-cells are still elusive, particularly those of selective autophagy in pancreatic β-cells. Autophagy types other than macroautophagy including those that are specific to hormone secretory cells including crinophagy, SINGD, GOMED, vesicophagy and microautophagy have been described in pancreatic β-cells, and the molecular mechanism and pathophysiological roles of such autophagy types are far from clear. Another problem associated with the study of autophagy in pancreatic β-cells is paucity of physiologically relevant animal models simulating human-type diabetes with islet amyloid deposition. Thus, the results using animal models of diabetes as a tool for the study of autophagy in pancreatic β-cells may not accurately reflect real in vivo processes in human patients with diabetes.
Nonetheless, valuable new discoveries regarding the molecular machinery and regulation of diverse types of autophagy in pancreatic β-cells are being reported. The development of clinically applicable methods modulating autophagy of pancreatic β-cells for the treatment of human diabetes may be a feasible possibility in the near future.
FUNDING
This article is part of a supplement entitled ‘Biomarkers of Beta-Cell Health and Dysfunction: Towards Personalised Diabetes Care. Proceedings of the 20th Servier-IGIS Symposium’. The Symposium and the publication of the Supplement have been made possible by an unrestricted educational grant from Institut la Conférence Hippocrate – Servier Group.
Acknowledgments
Y-h. L., J. K., K. P. and M.-S. L. wrote the manuscript and drew the figures.
This study was supported by Global Research Laboratory Grant (NRF-2010-00347) and Bio & Medical Technology Development Program (2015M3A9B6073846 and 2017M3A9G7073521). M-S Lee is the recipient of grants from KHIDI (HI17C0913 and HR18C0012020018).
Conflict of interest
The authors declare no conflict of interest related to this article.
References
- 1.Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Goginashvili A., Zhang Z., Erbs E., Spiegelhalter C., Kessler P., Mihlan M. Insulin granules. Insulin secretory granules control autophagy in pancreatic β cells. Science. 2015;347:878–882. doi: 10.1126/science.aaa2628. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi H., Arakawa S., Kanaseki T., Miyatsuka T., Fujitani Y., Watada H. Golgi membrane-associated degradation pathway in yeast and mammals. The European Molecular Biology Organization Journal. 2016;35:1991–2007. doi: 10.15252/embj.201593191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto S., Kuramoto K., Wang N., Situ X., Priyadarshini M., Zhang W. Autophagy differentially regulates insulin production and insulin sensitivity. Cell Reports. 2018;23:3286–3299. doi: 10.1016/j.celrep.2018.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klionsky D.J., Abdelmohsen K., Abe A., Abedin M.J., Abeliovich H., Acevedo Arozena A. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu L., McPhee C.K., Zheng L., Mardones G.A., Rong Y., Peng J. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010;465:942–946. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohr V.A., Anson R.M. DNA damage, mutation and fine structure DNA repair in aging. Mutation Research. 1995;338:25–34. doi: 10.1016/0921-8734(95)00008-t. [DOI] [PubMed] [Google Scholar]
- 8.Pickrell A.M., Youle R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Guerroué F., Eck F., Jung J., Starzetz T., Mittelbronn M., Kaulich M. Autophagosomal content profiling reveals an LC3C-dependent piecemeal mitophagy pathway. Molecular Cell. 2017;68:786–796. doi: 10.1016/j.molcel.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Khaminets A., Heinrich T., Mari M., Grumati P., Huebner A.K., Akutsu M. Regulation of endoplasmic reticulum turnover by selective autophagy. Nature. 2015;522:354–358. doi: 10.1038/nature14498. [DOI] [PubMed] [Google Scholar]
- 11.Fumagalli F., Noack J., Bergmann T., Cebollero E., Pisoni G.B., Fasana E. Translocon component Sec62 acts in endoplasmic reticulum turnover during stress recovery. Nature Cell Biology. 2016;18:1173–1184. doi: 10.1038/ncb3423. [DOI] [PubMed] [Google Scholar]
- 12.Grumati P., Morozzi G., Hölper S., Mari M., Harwardt M.I., Yan R. Full length RTN3 regulates turnover of tubular endoplasmic reticulum via selective autophagy. eLife. 2017;6 doi: 10.7554/eLife.25555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeder B., Schulze R.J., Weller S.G., Sletten A.C., Casey C.A., McNiven M.A. The small GTPase Rab7 as a central regulator of hepatocellular lipophagy. Hepatology. 2015;61:1896–1907. doi: 10.1002/hep.27667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z., Schulze R.J., Weller S.G., Krueger E.W., Schott M.B., Zhang X. A novel Rab10-EHBP1-EHD2 complex essential for the autophagic engulfment of lipid droplets. Science Advances. 2016;2 doi: 10.1126/sciadv.1601470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q., Wang J., Wan Y., Chen D. Depletion of Rab32 decreases intracellular lipid accumulation and induces lipolysis through enhancing ATGL expression in hepatocytes. Biochemical and Biophysical Research Communications. 2016;471:492–496. doi: 10.1016/j.bbrc.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Lopez N., Garcia-Macia M., Sahu S., Athonvarangkul D., Liebling E., Merlo P. Autophagy in the CNS and periphery coordinate lipophagy and lipolysis in the brown adipose tissue and liver. Cell Metabolism. 2016;23:113–127. doi: 10.1016/j.cmet.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K.Y., Jang H.J., Yang Y.R., Park K.I., Seo J., Shin I.W. SREBP-2/PNPLA8 axis improves non-alcoholic fatty liver disease through activation of autophagy. Scientific Reports. 2016;6:35732. doi: 10.1038/srep35732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rambold A.S., Cohen S., Lippincott-Schwartz J. Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Developmental Cell. 2015;32:678–692. doi: 10.1016/j.devcel.2015.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen T.B., Louie S.M., Daniele J.R., Tran Q., Dillin A., Zoncu R. DGAT1-dependent lipid droplet biogenesis protects mitochondrial function during starvation-induced autophagy. Developmental Cell. 2017;42:9–21. doi: 10.1016/j.devcel.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoshino A., Ariyoshi M., Okawa Y., Kaimoto S., Uchihashi M., Fukai K. Inhibition of p53 preserves Parkin-mediated mitophagy and pancreatic β-cell function in diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3116–3121. doi: 10.1073/pnas.1318951111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung H.S., Chung K.W., Kim J.W., Kim J., Komatsu M., Tanaka K. Loss of autophagy diminishes pancreatic b-cell mass and function with resultant hyperglycemia. Cell Metabolism. 2008;8:318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Quan W., Hur K.Y., Lim Y., Oh S.H., Lee J.-C., Kim H.C. Autophagy deficiency in beta cells leads to compromised unfolded protein response and progression from obesity to diabetes in mice. Diabetologia. 2012;55:392–403. doi: 10.1007/s00125-011-2350-y. [DOI] [PubMed] [Google Scholar]
- 24.Back S.H., Scheuner D., Han J., Song B., Ribick M., Wang J. Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metabolism. 2009;10:13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheuner D., Vander Mierde D., Song B., Flamez D., Creemers J.W., Tsukamoto K. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nature Medicine. 2005;11:757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- 26.Merksamer P.I., Trusina A., Papa F.R. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell. 2008;135:933–947. doi: 10.1016/j.cell.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W.W., Alexander S.A., Cao X., Lee A.S. Transactivation of the grp78 promoter by Ca2+ depletion. Journal of Biological Chemistry. 1993;268:12003–12009. [PubMed] [Google Scholar]
- 28.Cunha D.A., Hekerman P., Ladrière L., Bazarra-Castro A., Ortis F., Wakeham M.C. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. Journal of Cell Science. 2008;121:2308–2318. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto E., Uchida T., Abe H., Taka H., Fujimura T., Komiya K. Increased expression of ERp57/GRP58 is protective against pancreatic beta cell death caused by autophagic failure. Biochemical and Biophysical Research Communications. 2014;453:19–24. doi: 10.1016/j.bbrc.2014.09.040. [DOI] [PubMed] [Google Scholar]
- 30.Ebato C., Uchida T., Arakawa M., Komatsu M., Ueno T., Komiya K. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metabolism. 2008;8:325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Sheng Q., Xiao X., Prasadan K., Chen C., Ming Y., Fusco J. Autophagy protects pancreatic beta cell mass and function in the setting of a high-fat and high-glucose diet. Scientific Reports. 2017;7:16348. doi: 10.1038/s41598-017-16485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J.J., Quijano C., Chen E., Liu H., Cao L., Fergusson M.M. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging. 2009:425–437. doi: 10.18632/aging.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachar-Wikstrom E., Wikstrom J.D., Ariav Y., Tirosh B., Kaiser N., Cerasi E. Stimulation of autophagy improves endoplasmic reticulum stress-induced diabetes. Diabetes. 2013;62:1227–1237. doi: 10.2337/db12-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartolome A., Guillen C., Benito M. Autophagy plays a protective role in endoplasmic reticulum stress-mediated pancreatic β cell death. Autophagy. 2012;8:1757–1768. doi: 10.4161/auto.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong F.J., Wu J.H., Sun S.Y., Zhou J.Q. The endoplasmic reticulum stress/autophagy pathway is involved in cholesterol-induced pancreatic β-cell injury. Scientific Reports. 2017;7:44746. doi: 10.1038/srep44746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King B.C., Kulak K., Krus U., Rosberg R., Golec E., Wozniak K. Complement component C3 Is highly expressed in human pancreatic islets and prevents β cell death via ATG16L1 interaction and autophagy regulation. Cell Metabolism. 2018;29:1–9. doi: 10.1016/j.cmet.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Y., Huang W., Wang J., Xu Z., He J., Lin X. Metformin plays a dual role in MIN6 pancreatic b cell function through AMPK-dependent autophagy. The International Journal of Biochemistry & Cell Biology. 2014;10:268–277. doi: 10.7150/ijbs.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim S.W., Jin L., Jin J., Yang C.W. Effect of exendin-4 on autophagy clearance in beta cell of rats with tacrolimus-induced diabetes mellitus. Scientific Reports. 2016;6:29921. doi: 10.1038/srep29921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masini M., Bugliani M., Lupi R., del Guerra S., Boggi U., Filipponi F. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia. 2009;52:1083–1086. doi: 10.1007/s00125-009-1347-2. [DOI] [PubMed] [Google Scholar]
- 40.Thomas H.E., Zhang Y., Stefely J.A., Veiga S.R., Thomas G., Kozma S.C. Mitochondrial complex I activity is required for maximal autophagy. Cell Reports. 2018;24:2404–2417. doi: 10.1016/j.celrep.2018.07.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujimoto K., Hanson P.T., Tran H., Ford E.L., Han Z., Johnson J.D. Autophagy regulates pancreatic beta cell death in response to Pdx1 deficiency and nutrient deprivation. Journal of Biological Chemistry. 2009;284:27664–27673. doi: 10.1074/jbc.M109.041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tenemura M., Ohmura Y., Deguchi T., Machida T., Tsukamoto R., Wada H. Rapamycin causes upregulation of autophagy and impairs islets function both in vitro and in vivo. Americal Journal of Transplantation. 2012;12:102–114. doi: 10.1111/j.1600-6143.2011.03771.x. [DOI] [PubMed] [Google Scholar]
- 43.Pearson G.L., Mellett N., Chu K.Y., Cantley J., Davenport A., Bourbon P. Lysosomal acid lipase and lipophagy are constitutive negative regulators of glucose-stimulated insulin secretion from pancreatic beta cells. Diabetologia. 2014;57:129–139. doi: 10.1007/s00125-013-3083-x. [DOI] [PubMed] [Google Scholar]
- 44.Riahi Y., Wikstrom J.D., Bachar-Wikstrom E., Polin N., Zucker H., Lee M.-S. Autophagy is a major regulator of beta cell insulin homeostasis. Diabetologia. 2016;59:1480–1491. doi: 10.1007/s00125-016-3868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westermark P., Andersson A., Westernark G.T. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiological Reviews. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 46.Rubinsztein D.C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 47.Kim J., Cheon H., Jeong Y.T., Quan Y., Kim K.H., Cho J.M. Amyloidogenic peptide oligomer accumulation in autophagy-deficient b-cells leads to diabetes. Journal of Clinical Investigation. 2014;125:3311–3324. doi: 10.1172/JCI69625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rivera J.F., Costes S., Gurlo T., Glabe C., Butler P.C. Autophagy defends pancreatic b-cells from human islet amyloid polypeptide-induced toxicity. Journal of Clinical Investigation. 2014;124:3489–3500. doi: 10.1172/JCI71981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rivera J.F., Curlo T., Daval M., Huang C.J., Matveyenko A.V., Butler P.C. Human-IAPP disrupts the autophagy/lysosomal pathway in pancreatic b-cells: protective role of p62-postivie cytoplasmic inclusions. Cell Death & Differentiation. 2011;18:415–426. doi: 10.1038/cdd.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shigihara N., Fukunaka A., Hara A., Komiya K., Honda A., Uchida T. Human IAPP-induced pancreatic beta-cell toxicity and its regulation by autophagy. Journal of Clinical Investigation. 2014;124:3634–3644. doi: 10.1172/JCI69866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gurlo T., Rivera J.F., Butler A.E., Cory M., Hoang J., Costes S. CHOP contributes to, but Is not the only mediator of, IAPP Induced β-cell apoptosis. Molecular Endocrinology. 2016;30:446–454. doi: 10.1210/me.2015-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kayatekin C., Amasino A., Gaglia G., Flannick J., Bonner J.M., Fanning S. Translocon declogger Ste24 protects against IAPP oligomer-induced proteotoxicity. Cell. 2018;173:62–73. doi: 10.1016/j.cell.2018.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masters S.L., Dunne A., Subramanian S.L., Hull R.L., Tannahill G.M., Sharp F.A. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1b in type 2 diabetes. Nature Immunology. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jayasinghe S.A., Langen R. Lipid membranes modulate the structure of islet amyloid polypeptide. Biochemistry. 2005;44:12113–12119. doi: 10.1021/bi050840w. [DOI] [PubMed] [Google Scholar]
- 55.Park K., Verchere C.B. Indentification of a heparin binding domain in the N-terminal cleavage site of pro-islet amyloid polypeptide. Journal of Biological Chemistry. 2001;276:16611–16616. doi: 10.1074/jbc.M008423200. [DOI] [PubMed] [Google Scholar]
- 56.Hernández M.G., Aguilar A.G., Burillo J., Oca R.G., Manca M.A., Novials A. Pancreatic β cells overexpressing hIAPP impaired mitophagy and unbalanced mitochondrial dynamics. Cell Death & Disease. 2018;9:481. doi: 10.1038/s41419-018-0533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Twig G., Elorza A., Molina A.J., Mohamed H., Wikstrom J.D., Walzer G. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. The European Molecular Biology Organization Journal. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim K.Y., Stevens M.V., Akter M.H., Rusk S.E., Huang R.J., Cohen A. Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. Journal of Clinical Investigation. 2011;121:3701–3712. doi: 10.1172/JCI44736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu X., Altshuler-Keylin S., Wang Q., Chen Y., Henrique Sponton C., Ikeda K. Mitophagy controls beige adipocyte maintenance through a Parkin-dependent and UCP1-independent mechanism. Science Signaling. 2018;11 doi: 10.1126/scisignal.aap8526. eaap8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pearson G., Chai B., Vozheiko T., Liu X., Kandarpa M., Piper R.C. Clec16a, Nrdp1, and USP8 form a ubiquitin-dependent tripartite complex that regulates β-cell mitophagy. Diabetes. 2018;67:265–277. doi: 10.2337/db17-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soleimanpour S.A., Ferrari A.M., Raum J.C., Groff D.N., Yang J., Kaufman B.A. Diabetes susceptibility genes Pdx1 and Clec16a function in a pathway regulating mitophagy in β-cells. Diabetes. 2015;64:3475–3484. doi: 10.2337/db15-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen L., Liu C., Gao J., Xie Z., Chan L.W.C., Keating D.J. Inhibition of Miro1 disturbs mitophagy and pancreatic β-cell function interfering insulin release via IRS-Akt-Foxo1 in diabetes. Oncotarget. 2017;8:90693–90705. doi: 10.18632/oncotarget.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun N., Yun J., Liu J., Malide D., Liu C., Rovira I.I. Measuring in vivo mitophagy. Molecular Cell. 2015;60:685–696. doi: 10.1016/j.molcel.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bainton D.F. The discovery of lysosome. Journal of Cell Biology. 1981;91:66s–76s. doi: 10.1083/jcb.91.3.66s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orci L., Ravazzola M., Amherdt M., Yanahira C., Yanihara N., Halban P. Insulin, not C-peptide, is present in crinophagic bodies of the pancreatic b-cell. Journal of Cell Biology. 1984;98:222–228. doi: 10.1083/jcb.98.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schnell A.H., Swenne I., Borg L.A. Lysosomes and pancreatic islet function. A quantitative estimation of crinophagy in the mouse pancreatic B-cell. Cell and Tissue Research. 1985;239:537–545. doi: 10.1007/BF00213820. [DOI] [PubMed] [Google Scholar]
- 67.Rocchi A., Yamamoto S., Ting T., Fan Y., Sadleir K., Wang Y. A Becn1 mutation mediates hyperactive autophagic sequestration of amyloid oligomers and improved cognition in Alzheimer's disease. Public Library of Science Genetics. 2017;13 doi: 10.1371/journal.pgen.1006962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lim Y.-M., Lim H.-J., Hur K.Y., Quan W., Lee H.-Y., Cheon H. Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nature Communications. 2014;5:4934. doi: 10.1038/ncomms5934. [DOI] [PubMed] [Google Scholar]
- 69.Sahu R., Kaushik S., Clement C.C., Cannizzo E.S., Scharf B., Follenzi A. Microautophagy of cytosolic proteins by late endosomes. Developmental Cell. 2011;20:131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marsh B.J., Soden C., Alarcon C., Wicksteed B.L., Yaekura K., Costin A.J. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine beta-cells. Molecular Endocrinology. 2007;21:2255–2269. doi: 10.1210/me.2007-0077. [DOI] [PubMed] [Google Scholar]
- 71.Caramés B., Taniguchi N., Otsuki S., Blanco F.J., Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis & Rheumatism. 2010;62:791–810. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ott C., König J., Höhn A., Jung T., Grune T. Macroautophagy is impaired in old murine brain tissue as well as in senescent human fibroblasts. Redox Biology. 2016;10:266–273. doi: 10.1016/j.redox.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernández Á., Sebti S., Wei Y., Zou Z., Shi M., McMillan K.L. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature. 2018;558:136–140. doi: 10.1038/s41586-018-0162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghosh A.K., Mau T., O'Brien M., Garg S., Yung R. Impaired autophagy activity is linked to elevated ER-stress and inflammation in aging adipose tissue. Aging. 2016;8:2525–2537. doi: 10.18632/aging.101083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bejarano E., Murray J.W., Wang X., Pampliega O., Yin D., Patel B. Defective recruitment of motor proteins to autophagic compartments contributes to autophagic failure in aging. Aging Cell. 2018;17 doi: 10.1111/acel.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Su Y., Misumi Y., Ueda M., Shono M., Takasi M., Guo J. The occurrence of islet amyloid polypeptide amyloidosis in Japanese subjects. Pancreas. 2012;41:971–973. doi: 10.1097/MPA.0b013e318249926a. [DOI] [PubMed] [Google Scholar]
- 77.Li L., Trifunovic A., Köhler M., Wang Y., Petrovic Berglund J., Illies C. Defects in β-cell Ca2+ dynamics in age-induced diabetes. Diabetes. 2014;63:4100–4114. doi: 10.2337/db13-1855. [DOI] [PubMed] [Google Scholar]
- 78.Ohn J.H., Kwak S.H., Cho Y.M., Lim S., Jang H.C., Park K.S. 10-year trajectory of β-cell function and insulin sensitivity in the development of type 2 diabetes: a community-based prospective cohort study. Lancet Diabetes-Endocrinology. 2016;4:27–34. doi: 10.1016/S2213-8587(15)00336-8. [DOI] [PubMed] [Google Scholar]
- 79.Kiffin R., Kaushik S., Zeng M., Bandyopadhyay U., Zhang C., Massey A.C. Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. Journal of Cell Science. 2007;120:782–791. doi: 10.1242/jcs.001073. [DOI] [PubMed] [Google Scholar]
- 80.Geng Y.Q., Guan J.T., Xu X.H., Fu Y.C. Senescence-associated beta-galactosidase activity expression in aging hippocampal neurons. Biochemical and Biophysical Research Communications. 2010;396:866–869. doi: 10.1016/j.bbrc.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 81.Terman A., Brunk U.T. Lipofuscin. The International Journal of Biochemistry & Cell Biology. 2004;36:1400–1404. doi: 10.1016/j.biocel.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 82.Hütter E., Skovbro M., Lener B., Prats C., Rabøl R., Dela F. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell. 2007;6:245–266. doi: 10.1111/j.1474-9726.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- 83.Bergmann M., Schütt F., Holz F.G., Kopitz J. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. FASEB Journal. 2004;18:562–564. doi: 10.1096/fj.03-0289fje. [DOI] [PubMed] [Google Scholar]
- 84.Koga H., Kaushik S., Cuervo A.M. Altered lipid content inhibits autophagic vesicular fusion. FASEB Journal. 2010;24:3052–3065. doi: 10.1096/fj.09-144519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi S.E., Lee S.M., Lee Y.J., Li L.J., Lee S.J., Lee J.H. Protective role of autophagy in palmitate-induced INS-1 beta cell death. Endocrinology. 2009;150:126–134. doi: 10.1210/en.2008-0483. [DOI] [PubMed] [Google Scholar]
- 86.Komiya K., Uchida T., Ueno T., Koike M., Abe H., Hirose T. Free fatty acids stimulates autophagy in pancreatic b-cells via JNK pathway. Biochemical and Biophysical Research Communications. 2010;401:561–567. doi: 10.1016/j.bbrc.2010.09.101. [DOI] [PubMed] [Google Scholar]
- 87.Hosokawa N., Hara Y., Mizushima N. Generation of cell lines with tetracycline-regulated autophagy and a role for autophagy in controlling cell size. FEBS Letters. 2006;580:2623–2629. doi: 10.1016/j.febslet.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 88.Chu K.Y., O'Reilly L., Ramm G., Biden T.J. High-fat diet increases autophagic flux in pancreatic beta cells in vivo and ex vivo in mice. Diabetologia. 2015;58:2074–2078. doi: 10.1007/s00125-015-3665-x. [DOI] [PubMed] [Google Scholar]
- 89.Abe H., Uchida T., Hara A., Mizukami H., Komiya K., Koike M. Exendin-4 improves β-cell function in autophagy-deficient β-cells. Endocrinology. 2013;154:4512–4524. doi: 10.1210/en.2013-1578. [DOI] [PubMed] [Google Scholar]
- 90.Las G., Serada S.B., Wilkstrom J.D., Twig G., Shirihai O.S. Fatty acids suppress autophagic turnover in b-cells. Journal of Biological Chemistry. 2011;286:42534–42544. doi: 10.1074/jbc.M111.242412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chu K.Y., O'Reilly L., Mellett N., Meikle P.J., Bartley C., Biden T.J. Oleate disrupts cAMP signaling, contributing to potent stimulation of pancreatic β-cell autophagy. Journal of Biological Chemistry. 2019;294:1218–1229. doi: 10.1074/jbc.RA118.004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Papáčková Z., Daňková H., Páleníčková E., Kazdová L., Cahová M. Effect of short- and long-term high-fat feeding on autophagy flux and lysosomal activity in rat liver. Physiological Reviews. 2012;61:S67–S76. doi: 10.33549/physiolres.932394. [DOI] [PubMed] [Google Scholar]
- 93.Yang L., Li P., Fu S., Calay E.S., Hotamisligil G.S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metabolism. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Z.-F., Li Y.-B., Han J.-Y., Yin J.-J., Wang Y., Zhu L.-B. Liraglutide prevents high glucose level induced insulinoma cells apoptosis by targeting autophagy. Chinese Medical Journal. 2013;126:937–941. [PubMed] [Google Scholar]
- 95.Sarkar S., Davies J.E., Huang Z., Tunnacliffe A., Rubinsztein D.C. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and a-synuclein. Journal of Biological Chemistry. 2007;282:5641–5652. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 96.Morita S., Sakagashira S., Shimajiiri Y., Eberhardt N.L., Kondo T., SKondo T. Autophagy protects against human islet amyloid polypeptide-associated apoptosis. Journal of Diabetes Investigation. 2011;2:48–55. doi: 10.1111/j.2040-1124.2010.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fan Y., Wang N., Rocchi A., Zhang W., Vassar R., Zhou Y. Identification of natural products with neuronal and metabolic benefits through autophagy induction. Autophagy. 2017;13:41–56. doi: 10.1080/15548627.2016.1240855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lim H., Lim Y.-M., Kim K.H., Jeon Y.E., Park K., Kim J. A novel autophagy enhancer as a therapeutic agent against metabolic syndrome and diabetes. Nature Communications. 2018;9:1438. doi: 10.1038/s41467-018-03939-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang C., Niederstrasser H., Douglas P.M., Lin R., Jaramillo J., Li Y. Small-molecule TFEB pathway agonists that ameliorate metabolic syndrome in mice and extend C. elegans lifespan. Nature Communications. 2017;8:2270. doi: 10.1038/s41467-017-02332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Settembre C., Di Malta C., Polito V.A., Arencibia M.G., Vetrini F., Erdin S. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332:1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]