Abstract
Background
Myriad challenges to the proper folding and structural maturation of secretory pathway client proteins in the endoplasmic reticulum (ER) — a condition referred to as “ER stress” — activate intracellular signaling pathways termed the unfolded protein response (UPR).
Scope of review
Through executing transcriptional and translational programs the UPR restores homeostasis in those cells experiencing manageable levels of ER stress. But the UPR also actively triggers cell degeneration and apoptosis in those cells that are encountering ER stress levels that exceed irremediable thresholds. Thus, UPR outputs are “double-edged”. In pancreatic islet β-cells, numerous genetic mutations affecting the balance between these opposing UPR functions cause diabetes mellitus in both rodents and humans, amply demonstrating the principle that the UPR is critical for the proper functioning and survival of the cell.
Major conclusions
Specifically, we have found that the UPR master regulator IRE1α kinase/endoribonuclease (RNase) triggers apoptosis, β-cell degeneration, and diabetes, when ER stress reaches critical levels. Based on these mechanistic findings, we find that novel small molecule compounds that inhibit IRE1α during such “terminal” UPR signaling can spare ER stressed β-cells from death, perhaps affording future opportunities to test new drug candidates for disease modification in patients suffering from diabetes.
Keywords: Endoplasmic reticulum stress, Unfolded protein response, Diabetes mellitus, Kinase, Endoribonuclease, Apoptosis, Small molecule kinase inhibitor
1. Introduction
One third of all eukaryotic proteins are secreted to the cell exterior or inserted into membranes. Biogenesis of these secretory pathway client proteins begins in the endoplasmic reticulum (ER) through the concerted actions of an ER-resident protein-folding machinery comprising chaperones, glycosylating enzymes, and oxido-reductases [1]. But if demand on the ER protein-folding machinery exceeds its capacity, unfolded client secretory proteins start to accumulate within the organelle. Such “ER stress” can occur from simply having to make too much of any given secretory protein; thus, professional secretory cells — such as insulin-producing pancreatic islet β-cells — are especially at risk because they typically work at the limits of their secretory capability. Under ER stress, secretory proteins accumulate in unfolded/immature forms to activate intracellular signaling pathways called the unfolded protein response (UPR). Through adaptive (‘A’-UPR) outputs, transcription of genes encoding ER-resident protein-folding enzymes and ER-associated degradation (ERAD) components is augmented while translation of secretory proteins is temporarily reduced [2], [3], [4], [5], [6], [7].
But if the cell still continues to experience ER stress at levels that cannot be remedied, the UPR instead maladaptively “overshoots”, causing cell cycle arrest, dedifferentiation, senescence, and sterile inflammation, ultimately culminating in apoptosis; the spectrum of these destructive outputs is termed the terminal UPR (‘T’-UPR) [3]. Apoptosis of irremediably stressed cells is an extreme, yet definitive, quality control strategy that protects multicellular organisms from exposure to immature and damaged secretory proteins. At the cost of losing some cells, multicellular organisms may benefit temporarily from T-UPR-induced apoptosis. However, many human cell degenerative diseases occur when too many cells die through this process and are not replaced by proliferation. For instance, diabetes mellitus develops once the functioning mass of an individual's insulin-producing β-cells is reduced to below a critical threshold; thus, this may be an archetype of the overall T-UPR induced concept for other diseases.
Despite having evolved a large and robust ER, pancreatic β-cells have an innate fragility and are susceptible to secretory pathway overload because they are mandated to synthesize and secrete insulin in the large and requisite amounts needed to buffer blood glucose fluxes [8] — more than 1 million molecules of insulin produced/β-cell/minute (i.e., working near limits of their protein synthetic capacity). Under elevated insulin demand, protein folding and structural maturation in the β-cell ER becomes exhausted as the homeostatic ‘A’-UPR morphs into a destructive ‘T’-UPR [9], [10].
As detailed later in this review, rare gene mutations in both β-cell secretory cargo (i.e., proinsulin variants), and in UPR gene products cause UPR dysregulation and β-cell degeneration/apoptosis, leading to diabetes in rodent models (and in some human diabetes syndromes); furthermore, peripheral insulin resistance has also been linked to premature β-cell death and diabetes through UPR dysregulation [8], [11], [12], [13], [14]. For instance, type 2 diabetes (T2D) occurs under obese/overweight conditions that force β-cells to overproduce insulin in order to counter peripheral insulin resistance. Chronic UPR activation is evident in islets of patients with T2D and those at risk before overt disease appears [15]. Similarly, we, and others, have found that ‘A’- to ‘T’-UPR conversions occur in murine models of type 1 diabetes (T1D), a disease initiated by autoimmune T-cell-mediated attack against β-cells [13], [16], [17]. Specifically, stereotypic ‘A’- to ‘T’-UPR conversions occur in β-cells of the T1D model, non-obese diabetic (NOD) mouse, after innate/adaptive immune infiltration (insulitis) starts, but before diabetes ensues [17]. While ‘A’-UPR homeostatic outputs wane, ‘T’-UPR–destructive outcomes in the immune-targeted β-cells cause β-cell functional mass to decline, further increasing stress in remaining β-cells [13].
Thus in T1D, the β-cell may become complicit in its own demise (i.e., through suicide — not just homicide) under unrelenting stress caused by immune attack [18]. A unifying theory of β-cell failure posits that (as in the rare, monogenetic syndromes) UPR dysregulation contributes to β-cell failure in the etiology and pathogenesis of the common (polygenic) syndromes of diabetes (types 1 and 2; Figure 1). However, our knowledge of these events and translation of these experimental studies into human diabetes contexts is still very much in the preliminary stages. In this review we will highlight salient features of the early secretory pathway and the general case for UPR involvement in β-cell failure, as well as focus attention on an emerging kinase target for small molecule modulation of the UPR as potential therapies for β-cell sparing during ER stress.
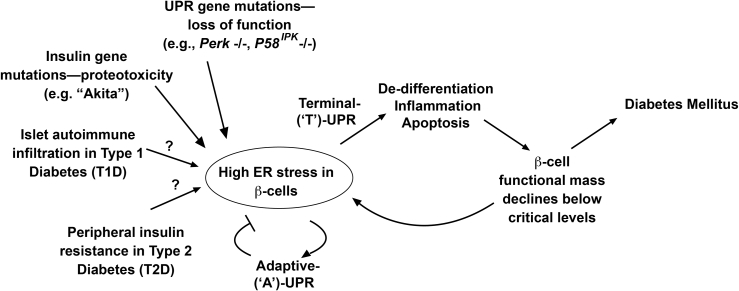
Figure 1.
‘A’- to ‘T’-UPR conversions may promote β-cell death in diabetes. During ER stress, adaptive UPR outputs maintain cellular homeostasis. If ER stress remains unmitigated, terminal UPR signaling drives destructive outputs culminating in β-cell apoptosis. Declining β-cell mass elevates workload/ER stress in remaining β-cells in vicious cycles promoting diabetes. These principles may be a unifying feature in monogenetic and polygenetic forms of diabetes (both rare and common). In various UPR gene mutations, loss of function of critical UPR components (e.g., PERK) dysregulates the pathway leading to infantile diabetes. Gain-of-function proteotoxicity accompanies mutant proinsulin expression in the MIDY syndrome caused by the (C96Y) Akita variant. In T1D, islet immune cell infiltration elevates ER stress in β-cells. Peripheral insulin resistance elevates β-cell overwork in T2D. It is conceivable that similar events that occur in the murine models drive β-cell degeneration in human T1D and T2D.
2. Endoplasmic reticulum (ER) stress and unfolded protein response (UPR) signaling
In all eukaryotic cells, soluble and transmembrane proteins destined for many intra-cellular organelles, the plasma membrane, or the extracellular space, initially fold to their native shapes and structurally mature within the ER [19], [20]. These protein-folding and maturation reactions are catalyzed by ER-resident molecular chaperones, glycosylating enzymes, oxido-reductases, and other protein-modifying activities. Molecular chaperones hydrolyze ATP to sequentially bind and release nascent secretory proteins, thereby reducing off-pathway aggregation during folding of secretory protein clients. Glycosylating enzymes add and trim sugar moieties from client proteins. ER oxido-reductases generate and sustain an oxidizing environment within the ER that facilitates formation of disulfide bonds in maturing client proteins [21], [22]. Overall, these early secretory pathway processes are energy-intensive and enzymatically mediated — the ER-resident activities that regulate these processes constitute a “protein-folding machinery” that maximizes the probability that secretory pathway client proteins, such as proinsulin, become properly folded, modified, and sometimes assembled into multi-protein complexes before they exit the ER towards the Golgi and distal organelles.
It is however the case that a substantial fraction of secretory proteins normally fails to fold and mature properly within the ER. Such proteins are extracted from the ER to the cytosol, (poly)ubiquitylated, and degraded by the 26S proteasome (a process referred to as ER-associated degradation or ERAD) [5], [23], [24]. Misfolded secretory proteins are also removed through autophagy [25], [26]. These stringent quality control processes notwithstanding, cells frequently encounter acute and/or chronic environmental challenges during which protein-folding demand in the ER overwhelms protein-folding capacity, causing these protein quality control systems to break down. Myriad challenges, both physiological and pathological, can provoke such ER stress. For instance, ischemia depletes cellular energy stores due to nutrient and oxygen deprivation, which in turn compromises the energy-intensive processes of protein modification and folding in the ER [27]. While overproduction of secretory proteins can generate ER stress, this is especially true for mutant forms of secretory proteins that are particularly difficult to fold — see the example of “Akita” insulin later.
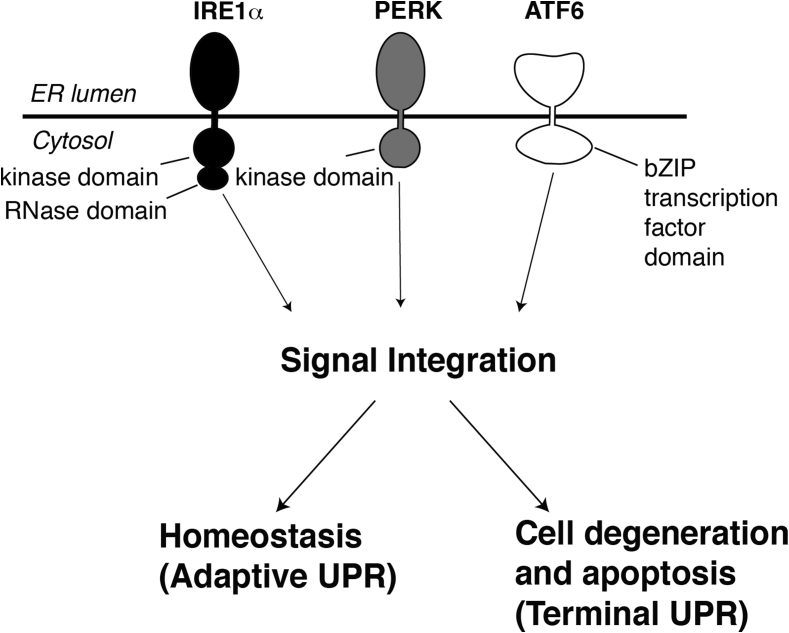
Cells are alerted to the presence of unfolded proteins within the ER by three ER trans-membrane signaling proteins named IRE1α, PERK, and ATF6 (Figure 2). These “first responders” become activated through direct and/or indirect binding of unfolded proteins within the ER [28], [29], [30], [31], [32]. Combinatorial signals from IRE1α, PERK, and ATF6 initially trigger transcriptional programs that upregulate genes encoding many of the aforementioned ER-resident protein-folding machines. Specifically, ATF6 is a latent transcription factor that gets cleaved in the plane of ER membrane during ER stress and translocates to the nucleus to upregulate numerous genes that encode ER protein folding activities. IRE1α and PERK are both serine/threonine kinases whose outputs also lead to production of specific transcription factors — ATF4 in the case of PERK and XBP1 in the case of IRE1α that trans-activate genes necessary for augmenting ER secretory function. Thus, by increasing the complement of ER protein-folding and quality control machinery [2], the UPR increases the cell's capacity to maintain healthy protein secretion in the face of manageable levels of ER stress. Through PERK activation the UPR also imposes a transient translational block (at the level of translation initiation) during ER stress, thereby concentrating available resources to aid the maturation of existing proteins before new ones arrive into the ER. If these adaptive UPR outputs are successful, the decline in levels of unfolded proteins causes UPR signaling to wane as homeostasis is restored
Figure 2.
The three first responders of the UPR are diagrammed. Upon activation under ER stress, three sensors, IRE1α, PERK, and ATF6 send intracellular signals that allow the cell to either adapt or commit apoptosis.
However, if ER stress still remains high and/or chronic despite these attempts, signal integration from these three UPR sensor/effectors causes the pathway to switch its output from promoting adaptation to instead promoting cell destruction, ultimately culminating in apoptosis (Figure 2). Cells also appear to transition under high ER stress through intermediate dysfunctional or de-differentiated states before committing to apoptosis. Apoptosis during irremediable ER stress may perhaps be the most stringent and definitive strategy for exerting protein quality control since culling highly stressed cells will ensure that they will not produce potentially dysfunctional secretory products. As proteins of the secretory pathway include cell surface receptors and polypeptide hormones, which mediate crucial signaling roles, the cell needs to ensure that these protein products are pristine and will not tolerate faulty versions. Thus, at the cost of culling irreversibly-stressed cells, multi-cellular organisms may thus benefit from employing UPR-induced apoptosis as a stringent and definitive protein quality control mechanism. However, if UPR-induced apoptosis becomes too vigorous and unchecked, organisms will suffer organ failure due to an insufficient mass of functioning cells. This principle is on vivid display in many different examples of pancreatic islet β-cell degeneration during ER stress-induced development of diabetes mellitus.
3. Irremediable ER stress promotes apoptosis of β-cells to cause diabetes
β-cells contain highly developed ERs since their raison d'être is to produce insulin continuously during their lives [8]. Insulin biogenesis requires a complex series of molecular biosynthetic events that initiate in the ER [33]. After translation, insulin precursor, pre-proinsulin, is translocated into the ER lumen of β-cells, where its signal sequence is removed, generating proinsulin. ER-resident oxido-reductases catalyze formation of three intramolecular disulfides in proinsulin to help it fold to its native shape. This oxidative folding event is critical for proper structural maturation and subsequent trafficking of proinsulin to the Golgi. In the “Akita” mouse mutant, which expresses the proinsulin variant gene, Ins2 (C96Y), an unpaired cysteine leads to futile cycling of the oxidative folding process; Akita insulin is therefore arrested by the protein quality control system in the β-cell ER where it acts as a continuous irritant to hyper-activate the UPR [34], [35], [36], [37]. Diabetes in the Akita mouse is not directly caused by reduction of mature insulin levels from the C96Y mutation. Instead, Akita insulin causes a toxic “gain-of-function” phenotype, leading to diabetes early in life; hence it is classified as a ‘MIDY’ syndrome — “mutant insulin-gene induced diabetes of youth” [36]. By accumulating in the ER as a conformationally-altered immature species that also generates reactive oxygen species (ROS) due to futile oxidative folding, Akita insulin acts as a “proteotoxin” that chronically activates the UPR [34]. Continuous UPR activation exhausts homeostatic ‘A’-UPR outputs, causing β-cells to enter the ‘T’-UPR-driven apoptotic pathway and leading to early diabetes in the mice [38], [39], [40]. Interestingly, rare diabetes-causing Akita-like insulin mutations have also been described in humans [41].
Loss-of-function mutations in the UPR also have deleterious effects in β-cells. In one of the more interesting examples, homozygous deletion in mice of the PERK gene encoding the UPR sensor Perk — an ER transmembrane kinase that when activated by ER unfolded proteins phosphorylates the eIF-2α translation initiation factor on the Ser51 residue to temporarily shut off translation — causes massive and rapid β-cell apoptosis leading to early diabetes [42], [43]. Without Perk, β-cells cannot attenuate translation to match ER protein folding capacity, and thereby suffer deposition of ER unfolded proteins, leading to apoptosis [42]. Furthermore, as will be detailed below, the Perk −/− genotype causes compensatory hyper-activation of the IRE1α arm, to drive the T-UPR in professional secretory cells. A rare human diabetic syndrome caused by PERK gene mutations (called Wolcott-Rallison syndrome) is a phenocopy of the mouse knockout. Further, a homozygous genetic knock-in of an unphosphorylatable eIF-2α (Ser51Ala) variant (the substrate of Perk) develops a severe neonatal wasting syndrome [44], [45], and an insulin-resistant T2D-like syndrome in the heterozygote [45]. Finally, another diabetic syndrome follows homozygous loss of p58IPK [46], [47], [48], a co-chaperone needed to inhibit Perk. In the absence of p58IPK, a continued translational block through Perk signals a frustrated UPR cycle, leading to UPR signaling dysregulation.
Together, these experimental genetic syndromes are rare textbook examples of ER stress deterministically causing β-cell death leading to diabetes. But extending these concepts into human pathophysiology, we may be able to learn whether common human diabetes syndromes, such as types 1 and 2 diabetes (and gestational diabetes) proceed, at least in part, from similar mechanisms. For example, T2D is a heterogeneous syndrome initiating with peripheral insulin resistance, leading eventually to β-cell failure and death. Measuring ER stress in β-cells of living humans is currently not feasible, as the pancreatic islet β-cell mass and insulin secretory function during life cannot be studied simultaneously. However, pancreatic autopsy series from T2D patients show a reduction in β-cell mass [49], with relics of activated UPR apoptotic markers. In addition, human type 2 diabetic islets contain protein aggregates in the form of amyloid [50]. Islet amyloid is composed of a 37-residue amyloidogenic polypeptide called islet amyloid polypeptide (IAPP). IAPP spontaneously forms ER membrane-damaging sheets of amyloid [51]. Therefore, IAPP could be another ER stress link promoting β-cell death. IAPP-mediated β-cell death likely induces ER stress through activation of the pro-apoptotic transcription factor Chop, and Chop has been reported to be detected in islets of human diabetics at the time of autopsy [15].
These studies inform a unified model for the development of T2D in humans. In obese states, an acquired state of peripheral insulin resistance causes β-cells to overwork by overproducing insulin. This chronic protein overproduction should elevate β-cell ER stress, provoking the UPR whose outputs initially counter the stress. Over time, in the absence of relief from the stress signal, β-cells may start to die through UPR-mediated apoptosis. Once β-cell death commences, the remaining β-cells will become even more overworked as they attempt to make up for the insulin deficit, therefore experiencing further ER stress. This β-cell “burn out” may become a vicious cycle, until apoptosis of sufficient numbers of β-cells locks in the T2D state [52], [53].
These aforementioned mechanisms may also be operative in type 1 diabetes (T1D), a disease that initiates from autoimmune attack by T-cells against β-cells. In addition, during gestation, increasing insulin requirements require an approximate doubling of the β-cell mass in humans, which we propose may expose the β-cells of pregnant mothers to increased ER stress. In some individuals (perhaps those who are already at risk for T2D), this could increase risk for gestational diabetes (GDM) [54]. Indeed it is well appreciated that GDM, which often resolves after pregnancy, puts affected mothers at risk for T2D later in life, perhaps by reducing β-cell mass.
4. ER Kinase/RNase IRE1α controls entry into the T-UPR
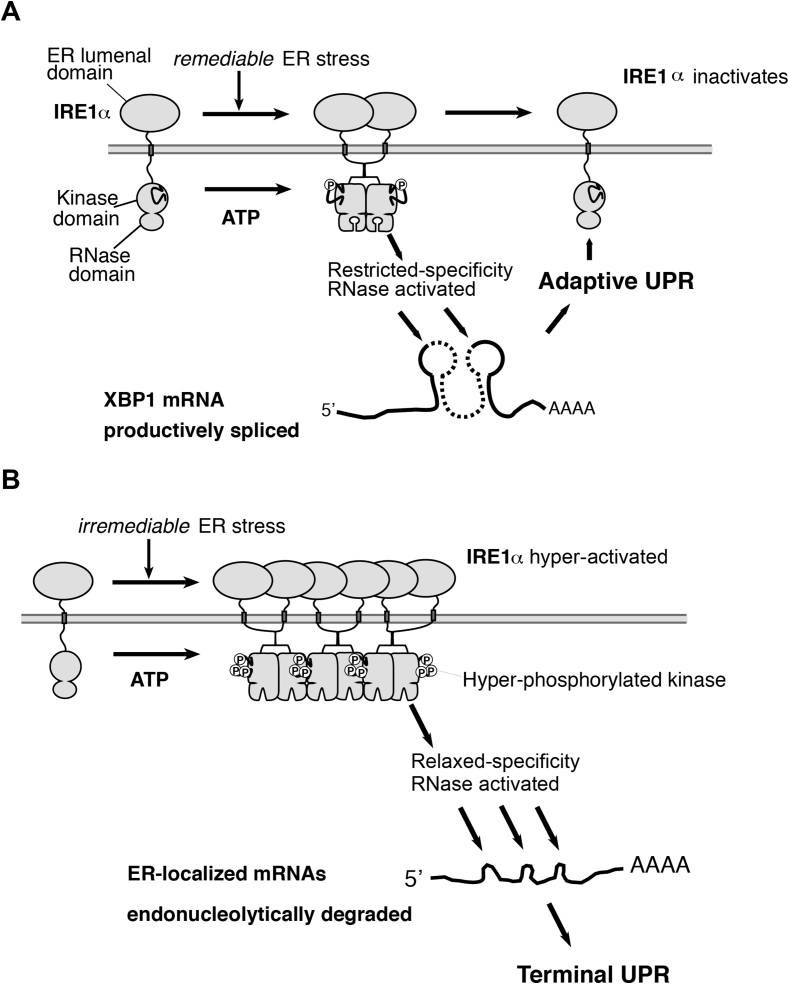
How does the UPR promote binary cell fate outcomes? We found that IRE1α — a bifunctional kinase/endoribonuclease (RNase) — is a critical UPR life-death switch that employs its kinase auto-phosphorylation levels as a “rheostat” to determine cell fate [55], [56]. Remediable ER stress causes low-level kinase auto-phosphorylation and dimerization that restricts IRE1α′s RNase activity to a single adaptive task: excising an intron in XBP1 mRNA; re-ligation of spliced XBP1 mRNA shifts its open reading frame, and its translation produces the homeostatic transcription factor XBP1s (s = spliced) [57], [58], which upregulates genes encoding ER protein-folding and quality control components [59]. Sustained, high-level kinase autophosphorylation, however, causes higher-order IRE1α oligomerization. Under these conditions, IRE1α′s RNase relaxes its specificity and degrades hundreds of mRNAs at the ER membrane that encode co-translationally translocated secretory proteins [55], [56], [60]. Depletion of cargo-encoding transcripts may initially be protective because this event reduces ER protein-folding burden; but eventually the wholescale endonucleolytic mRNA decay depletes transcripts encoding the very structural and enzymatic components of the ER protein-folding machinery itself — i.e., ER-resident molecular chaperones, oxido-reductases, and glycosylating enzymes. Consequently, ER protein-folding function actively deteriorates. Thus, hyperactive IRE1α RNase induces a T-UPR that eclipses ‘A’-UPR XBP1s outputs over time, causing loss of differentiated β-cell identity (e.g., depletion of insulin mRNAs), sterile inflammation, and apoptosis (Figure 3).
Figure 3.
IRE1α acts as a homeostatic-apoptotic switch in response to ER stress. (A) Homeostatic XBP1 splicing progresses to apoptotic mRNA decay if ER stress is unresolved, (B).
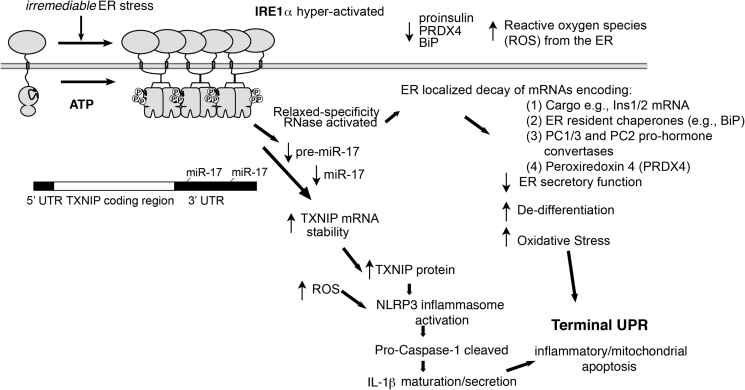
Furthermore, hyperactivated IRE1α RNase may promote multiple, interlinked, downstream destructive outcomes specific to the β-cell during high/chronic ER stress through this regulated endonucleolytic decay of RNAs–Figure 4. For instance, as wholescale mRNA decay proceeds, loss of differentiated cell identity ensues (decreases in insulin levels, proinsulin-modifying prohormone PC1/3, PC2 convertases, etc.). Other ER-localized mRNAs encoding enzymatic factors that dispose of reactive oxygen species (ROS) such as PRDX4 also decline under IRE1α hyperactivation, perhaps causing oxidative stress. Also, hyperactive IRE1α RNase — in competition with DICER — causes decay of select micro RNAs (miRs) at the pre-miR level. Notably repressive miR-17 targets the T-UPR mediator, TXNIP mRNA (a naturally short-lived mRNA with two 3′UTR miR-17 seed sequences). Depletion of miR-17 causes rapid and robust increases in pro-oxidant TXNIP protein, which then activates the NLRP3 inflammasome, leading to pro-caspase 1 cleavage and IL-1β maturation/secretion in islets. Therefore, genetic removal of Txnip is β-cell sparing in the Akita mouse, [17], [39]. Finally, miR-17 depletion also derepresses p21 expression, cyclin-dependent kinase inhibitor 1, through a post-transcriptional mechanism similar to that of TXNIP — through two miR-17 seed sequences in its 3′UTR (Figure 4).
Figure 4.
Through IRE1α RNase hyperactivation — and ER-localized mRNA and miR decay — ER stress can secondarily morph to cause oxidative stress (loss of PRDX4), loss of differentiated cell identity (decreases in insulin mRNA and protein), inflammation (TXNIP induction), proliferation blocks, and pyroptotic/apoptotic cell death. See text for details.
5. ‘KIRAs’ reset endogenous IRE1α′s execution point to separate the A-UPR from the T-UPR
Through mechanistic and chemical-biology studies, we learned that the IRE1α-induced A-UPR could be entirely uncoupled from its T-UPR by enforcing intermediate activation states (sufficient for splicing XBP1 mRNA, but insufficient for causing endonucleolytic decay) with select small molecule kinase inhibitors. IRE1α′s RNase activation is normally dependent on kinase autophosphorylation [61], but we discovered that an allosteric relationship between these two domains exists, which allows nucleotides (ADP and ATP) that stabilize an active ATP-binding site conformation (called type I) to directly activate the RNase, while bypassing autophosphorylation [62], [63], [64].
Based on this knowledge, we hypothesized that a different class of kinase inhibitors (called type II) should stabilize an inactive ATP-binding site conformation of endogenous IRE1α and inhibit its RNase activity by breaking high-order oligomerization, to consequently reduce both kinase and RNase activities in tandem and thereby also enforce an intermediate activation state. We predicted that these “type II” kinase inhibitors should stabilize the inactive ATP-binding site conformation of IRE1α by stabilizing its Helix-αC-out conformation. This should cause back-to-back clashes between IRE1α protomers, shutting down RNase activity [65]. We named these small molecules ‘KIRA's — the acronym stands for kinase-inhibiting RNase-attenuators — and developed robust biochemical assays to discover them [66].
One early-stage KIRA, KIRA6, attenuates endogenous IRE1α in vivo, to generally promote cell survival under ER stress in diverse models of premature cell degeneration. For example, provided intravitreally, KIRA6 preserved photoreceptor functional viability in rat models of ER stress-induced retinal degeneration, specifically in a model that expresses P23H rhodopsin (a folding mutant), which causes autosomal dominant retinitis pigmentosa [56]. Systemically, KIRA6 preserved β-cells, increased insulin content, and reduced hyperglycemia in Akita mice (mimicking effects of TXNIP gene deletion) [56]. Biochemical, cellular, and in vivo data showed that a wide “therapeutic window” exists between the ‘A’- and ‘T’-UPR because endonucleolytic decay of extra-XBP1 target mRNAs such as the mRNAs encoding secretory target cargo, i.e., proinsulin (Ins1 or Ins2) can be quenched with lower KIRA6 concentrations (∼nM IC50) than those needed to inhibit XBP1 mRNA cleavage (μM IC50), owing to the fact that XBP1 mRNA is a much more efficient substrate for IRE1α RNAse than the hundreds of other ER-localized mRNA substrates. In other words, because lower-order species suffice for XBP1 mRNA splicing, sub-complete IRE1α inhibition with KIRAs selectively attenuates the T-UPR while preserving the A-UPR — with an approximate three log10 IC50 difference separating the two outcomes.
In general, rheostatic control over A-versus T-UPR IRE1α outputs proceeds through changes in IRE1α′s homo-oligomerization levels. However, it has also been learned that other proteins also bind IRE1α at the ER membrane to regulate its oligomeric state and outputs. Various factors in other signaling pathways interact with IRE1α, including protein-tyrosine phosphatase 1B, BAX inhibitor-1, ASK1, RACK1, and nonmuscle myosin IIB, and tune IRE1α-outputs to affect cell fate [67], [68], [69], [70], [71]. For example, the ABL-family of tyrosine kinases, consisting of two paralogs, c-Abl and Arg [72] are also integral components of this “UPRosome” that drives IRE1α hyperactivity at the ER. While oncogenic BCR-Abl is the intended target of Gleevec (imatinib) for CML, c-Abl and Arg kinases are also inhibited by this drug. Based on successful efficacy in the NOD using Gleevec, repurposed Gleevec has been tested clinically for treatment of new-onset T1D in adult human patients. However, in spite of imatinib's efficacy in the NOD, little was known about the relative contributions of the efficacy of this drug on the autoimmune system versus β-cells themselves. c-Abl kinase is induced and activated during ER stress in β-cells of NOD mice after immune infiltration [17]. c-Abl activation relocalizes it from the cytosol to the ER membrane to rheostatically hyperactivate IRE1α, causing T-UPR induction and β-cell apoptosis. Imatinib reverses T-UPR endpoints by sequestering c-Abl in the cytosol and preventing it from binding IRE1α. Thus, FDA-approved tyrosine kinase inhibitors (TKIs) may be repurposed to truncate the T-UPR based on rationally understanding their underlying mechanism of action.
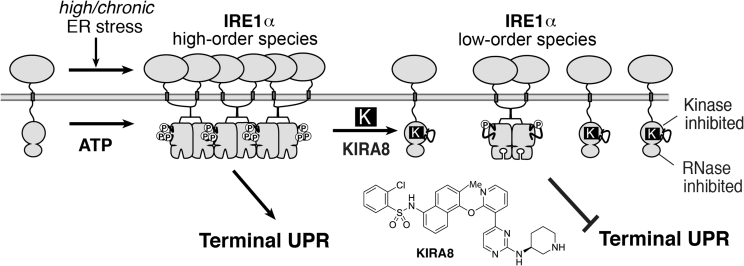
A significant advance in the UPR field to directly target IRE1α occurred with the development of a highly potent (sub-nanomolar) kinase inhibitor of IRE1α called compound 18 by Amgen [73]. This molecule came out of a medicinal chemistry analoging campaign to optimize Tie2 kinase inhibitors, but unexpectedly produced a near mono-selective inhibitor of IRE1α. Compound 18 was further tested for its ability to reduce survival of multiple cancer cell lines by Amgen (and abandoned when it could not). Using an XBP1 RNA mini-substrate [65] we confirmed that resynthesized compound 18 has all the properties of a KIRA (hence renamed KIRA8). KIRA8 breaks IRE1α oligomerization in vitro (Figure 5), and is highly selective against a panel of >300 kinases, including Abl (demonstrating even negligible activity against IRE1α′s closely-related gut-expressed paralog, IRE1β) [17]Favorable pharmacokinetic properties of KIRA8 guided daily intraperitoneal (i.p.) dosing in pre-diabetic NODs, which within one showed decreased TXNIP mRNA, and rescued proinsulin- and (ER chaperone) BiP-encoding mRNAs in islets. By 4-weeks, first phase insulin responses to an i.p. glucose load were preserved in the KIRA8 group, concomitant with 3-fold-enhanced insulin-staining mass in pancreata. Finally, even after overt diabetes is manifested, KIRA8 led to >90% diabetes reversal within three weeks [17]
Figure 5.
KIRA8 dose-dependently breaks IRE1α homo-oligomers and allosterically reduces RNAse hyperactivation. Because lower-order species suffice for adaptive XBP1 mRNA splicing, sub-complete IRE1α inhibition with KIRAs selectively attenuates the T-UPR under high/chronic ER stress.
In toto, the aforementioned positive efficacy results in the NOD may therefore offer a translational opportunity in humans to study and understand the mechanistic and physiological consequences of modulating the ABL-IRE1α axis — from either the Abl (with TKIs such as imatinib or nilotinib) or the IRE1α end (with evolving KIRA molecules) — for healthy secretion. For instance, we predict that either imatinib or KIRA8 will cause in human β-cells, either ex-vivo or transplanted into immune-deficient mice: (1) blunting of human insulin mRNA endonucleolytic decay; (2) stabilization of IRE1α′s RNase substrate, (pre)miR-17, (3) consequently attenuate TXNIP, the NLRP3 inflammasome, and IL-1β; and (4) enhance proinsulin to insulin processing (with increased human C-peptide detectable) as pro-hormone convertases and ER-resident chaperones are preserved, thereby increasing GSIS. Because of these intermediary outcomes, (5) we may expect that as with murine β-cells the functioning mass of human β-cells should be preserved. Finally, we predict that these β-cell-sparing effects should be general — i.e., without regard to the upstream stress that initiates disease — and extend to obesity-related diabetes (in addition to autoimmune-induced diabetes and MIDY models).
From a development standpoint, the next challenges facing the pharmaceutical industry in advancing KIRAs for human testing is to improve their drug-likeness. At the time of the writing of this review, current KIRAs lack oral bioavailability and are poorly soluble (both liabilities should be straightforward to correct by medicinal chemistry analoging efforts). Further testing of KIRA candidates in numerous in vivo murine diabetes models and in human islets reconstituted into immune-deficient mice for dose optimization should further inform pharmacokinetic/pharmacodynamic (A-vs. T-UPR markers) and relate these PK/PD studies back to chronic efficacy (durability) studies as was done for imatinib. Furthermore, while the accumulating experience with in vivo use of KIRA8 has not shown adverse effects in rodents to date, de-risking this class of inhibitors will require formal chronic toxicity studies with dose-escalation in animals — especially given that KIRAs monomerize IRE1α, it will be necessary to define the dimensions of an in vivo therapeutic window. Given the evolving interest in the pharmaceutical industry for drugging the UPR through IRE1α it is conceivable that clinical candidates for testing KIRAs for a systemic UPR-driven cell-degenerative indication (not necessarily confined to diabetes) in humans may be available for an investigational new drug application (IND) to the United States Food and Drug Administration (FDA) in the next few years.
In conclusion, the long path of basic discovery and study of an intracellular stress signaling pathway — the UPR — has led to potential targets, understanding of a mechanism of action and mode of intervention, and new chemical development through which the goal of β-cell sparing in diabetes may be tested in the future. We predict that the next few years will provide interesting insights and advances into these topics.
Funding
This article is part of a supplement entitled ‘Biomarkers of Beta-Cell Health and Dysfunction: Towards Personalised Diabetes Care. Proceedings of the 20th Servier-IGIS Symposium’. The Symposium and the publication of the Supplement have been made possible by an unrestricted educational grant from Institut la Conférence Hippocrate – Servier Group.
Conflict of interest
F.R.P. is a founder and equity holder of OptiKira, LLC, a UPR-focused ophthalmology biotech company. R.G. and K. C.-N. have no conflicts of interest.
References
- 1.van Anken E., Braakman I. Versatility of the endoplasmic reticulum protein folding factory. Critical Reviews in Biochemistry and Molecular Biology. 2005;40:191–228. doi: 10.1080/10409230591008161. [DOI] [PubMed] [Google Scholar]
- 2.Travers K.J., Patil C.K., Wodicka L., Lockhart D.J., Weissman J.S., Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 3.Zhang K., Kaufman R.J. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102–S109. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
- 4.Harding H.P., Calfon M., Urano F., Novoa I., Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annual Review of Cell and Developmental Biology. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 5.Meusser B., Hirsch C., Jarosch E., Sommer T. ERAD: the long road to destruction. Nature Cell Biology. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- 6.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 7.Brodsky J.L., McCracken A.A. ER protein quality control and proteasome-mediated protein degradation. Seminars in Cell & Developmental Biology. 1999;10:507–513. doi: 10.1006/scdb.1999.0321. [DOI] [PubMed] [Google Scholar]
- 8.Scheuner D., Kaufman R.J. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocrine Reviews. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merksamer P.I., Papa F.R. The UPR and cell fate at a glance. Journal of Cell Science. 2010;123:1003–1006. doi: 10.1242/jcs.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oakes S.A., Papa F.R. The role of endoplasmic reticulum stress in human pathology. Annual Review of Pathology: Mechanisms of Disease. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papa F.R. Endoplasmic reticulum stress, pancreatic beta-cell degeneration, and diabetes. Cold Spring Harbor Perspectives Medicine. 2012;2 doi: 10.1101/cshperspect.a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozcan U., Cao Q., Yilmaz E., Lee A.H., Iwakoshi N.N., Ozdelen E. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 13.Tersey S.A., Nishiki Y., Templin A.T., Cabrera S.M., Stull N.D., Colvin S.C. Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61:818–827. doi: 10.2337/db11-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eizirik D.L., Cardozo A.K., Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocrine Reviews. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 15.Huang C.J., Lin C.Y., Haataja L., Gurlo T., Butler A.E., Rizza R.A. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. doi: 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- 16.Engin F., Yermalovich A., Nguyen T., Hummasti S., Fu W., Eizirik D.L. Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Science Translational Medicine. 2013;5:211ra156. doi: 10.1126/scitranslmed.3006534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita S., Villalta S.A., Feldman H.C., Register A.C., Rosenthal W., Hoffmann-Petersen I.T. Targeting ABL-IRE1alpha signaling spares ER-stressed pancreatic beta cells to reverse autoimmune diabetes. Cell Metabolism. 2017;25:883–897. doi: 10.1016/j.cmet.2017.03.018. e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottazzo G.F. Lawrence lecture. Death of a beta cell: homicide or suicide? Diabetic Medicine. 1986;3:119–130. doi: 10.1111/j.1464-5491.1986.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 19.Gaut J.R., Hendershot L.M. The modification and assembly of proteins in the endoplasmic reticulum. Current Opinion in Cell Biology. 1993;5:589–595. doi: 10.1016/0955-0674(93)90127-c. [DOI] [PubMed] [Google Scholar]
- 20.Gething M.-J., Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 21.Tu B.P., Weissman J.S. Oxidative protein folding in eukaryotes: mechanisms and consequences. The Journal of Cell Biology. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sevier C.S., Kaiser C.A. Formation and transfer of disulphide bonds in living cells. Nature Reviews Molecular Cell Biology. 2002;3:836–847. doi: 10.1038/nrm954. [DOI] [PubMed] [Google Scholar]
- 23.McCracken A.A., Brodsky J.L. Evolving questions and paradigm shifts in endoplasmic-reticulum-associated degradation (ERAD) BioEssays. 2003;25:868–877. doi: 10.1002/bies.10320. [DOI] [PubMed] [Google Scholar]
- 24.Smith M.H., Ploegh H.L., Weissman J.S. Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science. 2011;334:1086–1090. doi: 10.1126/science.1209235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yorimitsu T., Klionsky D.J. Eating the endoplasmic reticulum: quality control by autophagy. Trends in Cell Biology. 2007;17:279–285. doi: 10.1016/j.tcb.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Kaniuk N.A., Kiraly M., Bates H., Vranic M., Volchuk A., Brumell J.H. Ubiquitinated-protein aggregates form in pancreatic beta-cells during diabetes-induced oxidative stress and are regulated by autophagy. Diabetes. 2007;56:930–939. doi: 10.2337/db06-1160. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman R.J. Orchestrating the unfolded protein response in health and disease. Journal of Clinical Investigation. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner B.M., Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333:1891–1894. doi: 10.1126/science.1209126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Credle J.J., Finer-Moore J.S., Papa F.R., Stroud R.M., Walter P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18773–18784. doi: 10.1073/pnas.0509487102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J., Liu C.Y., Back S.H., Clark R.L., Peisach D., Xu Z. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14343–14348. doi: 10.1073/pnas.0606480103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertolotti A., Zhang Y., Hendershot L.M., Harding H.P., Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nature Cell Biology. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 32.Carrara M., Prischi F., Nowak P.R., Kopp M.C., Ali M.M. Noncanonical binding of BiP ATPase domain to Ire1 and Perk is dissociated by unfolded protein CH1 to initiate ER stress signaling. Elife. 2015;4:e03522. doi: 10.7554/eLife.03522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steiner D.F. New aspects of proinsulin physiology and pathophysiology. Journal of Pediatric Endocrinology & Metabolism. 2000;13:229–239. doi: 10.1515/jpem.2000.13.3.229. [DOI] [PubMed] [Google Scholar]
- 34.Ron D. Proteotoxicity in the endoplasmic reticulum: lessons from the Akita diabetic mouse. Journal of Clinical Investigation. 2002;109:443–445. doi: 10.1172/JCI15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izumi T., Yokota-Hashimoto H., Zhao S., Wang J., Halban P.A., Takeuchi T. Dominant negative pathogenesis by mutant proinsulin in the Akita diabetic mouse. Diabetes. 2003;52:409–416. doi: 10.2337/diabetes.52.2.409. [DOI] [PubMed] [Google Scholar]
- 36.Wang J., Takeuchi T., Tanaka S., Kubo S.K., Kayo T., Lu D. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. Journal of Clinical Investigation. 1999;103:27–37. doi: 10.1172/JCI4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haataja L., Manickam N., Soliman A., Tsai B., Liu M., Arvan P. Disulfide mispairing during proinsulin folding in the endoplasmic reticulum. Diabetes. 2016;65:1050–1060. doi: 10.2337/db15-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oyadomari S., Koizumi A., Takeda K., Gotoh T., Akira S., Araki E. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. Journal of Clinical Investigation. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerner A.G., Upton J.P., Praveen P.V., Ghosh R., Nakagawa Y., Igbaria A. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metabolism. 2012;16:250–264. doi: 10.1016/j.cmet.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oslowski C.M., Hara T., O'Sullivan-Murphy B., Kanekura K., Lu S., Hara M. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metabolism. 2012;16:265–273. doi: 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoy J., Edghill E.L., Flanagan S.E., Ye H., Paz V.P., Pluzhnikov A. Insulin gene mutations as a cause of permanent neonatal diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15040–15044. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harding H.P., Zhang Y., Bertolotti A., Zeng H., Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Molecular Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 43.Delepine M., Nicolino M., Barrett T., Golamaully M., Lathrop G.M., Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nature Genetics. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 44.Scheuner D., Song B., McEwen E., Liu C., Laybutt R., Gillespie P. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Molecular Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 45.Scheuner D., Vander Mierde D., Song B., Flamez D., Creemers J.W., Tsukamoto Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nature Medicine. 2005;11:757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- 46.Laybutt D.R., Preston A.M., Akerfeldt M.C., Kench J.G., Busch A.K., Biankin A.V. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 47.Oyadomari S., Yun C., Fisher E.A., Kreglinger N., Kreibich G., Oyadomari M. Cotranslocational degradation protects the stressed endoplasmic reticulum from protein overload. Cell. 2006;126:727–739. doi: 10.1016/j.cell.2006.06.051. [DOI] [PubMed] [Google Scholar]
- 48.Ladiges W.C., Knoblaugh S.E., Morton J.F., Korth M.J., Sopher B.L., Baskin C.R. Pancreatic beta-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes. 2005;54:1074–1081. doi: 10.2337/diabetes.54.4.1074. [DOI] [PubMed] [Google Scholar]
- 49.Yoon K.H., Ko S.H., Cho J.H., Lee J.M., Ahn Y.B., Song K.H. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. Journal of Clinical Endocrinology & Metabolism. 2003;88:2300–2308. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]
- 50.Westermark P., Johnson K.H., O'Brien T.D., Betsholtz C. Islet amyloid polypeptide--a novel controversy in diabetes research. Diabetologia. 1992;35:297–303. doi: 10.1007/BF00401195. [DOI] [PubMed] [Google Scholar]
- 51.Sawaya M.R., Sambashivan S., Nelson R., Ivanova M.I., Sievers S.A., Apostol M.I. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature. 2007;447:453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- 52.Weir G.C., Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(Suppl 3):S16–S21. doi: 10.2337/diabetes.53.suppl_3.s16. [DOI] [PubMed] [Google Scholar]
- 53.Stefan Y., Orci L., Malaisse-Lagae F., Perrelet A., Patel Y., Unger R.H. Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. Diabetes. 1982;31:694–700. doi: 10.2337/diab.31.8.694. [DOI] [PubMed] [Google Scholar]
- 54.Buchanan T.A., Xiang A.H. Gestational diabetes mellitus. Journal of Clinical Investigation. 2005;115:485–491. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han D., Lerner A.G., Vande Walle L., Upton J.P., Xu W., Hagen A. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghosh R., Wang L., Wang E.S., Perera B.G., Igbaria A., Morita S. Allosteric inhibition of the IRE1alpha RNase preserves cell viability and function during endoplasmic reticulum stress. Cell. 2014;158:534–548. doi: 10.1016/j.cell.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calfon M., Zeng H., Urano F., Till J.H., Hubbard S.R., Harding H.P. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida H., Matsui T., Yamamoto A., Okada T., Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 59.Lee A.H., Iwakoshi N.N., Glimcher L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Molecular and Cellular Biology. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hollien J., Lin J.H., Li H., Stevens N., Walter P., Weissman J.S. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. The Journal of Cell Biology. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tirasophon W., Welihinda A.A., Kaufman R.J. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes & Development. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papa F.R., Zhang C., Shokat K., Walter P. Bypassing a kinase activity with an ATP-competitive drug. Science. 2003;302:1533–1537. doi: 10.1126/science.1090031. [DOI] [PubMed] [Google Scholar]
- 63.Han D., Upton J.P., Hagen A., Callahan J., Oakes S.A., Papa F.R. A kinase inhibitor activates the IRE1alpha RNase to confer cytoprotection against ER stress. Biochemical and Biophysical Research Communications. 2008;365:777–783. doi: 10.1016/j.bbrc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 64.Korennykh A.V., Egea P.F., Korostelev A.A., Finer-Moore J., Stroud R.M., Zhang C. Cofactor-mediated conformational control in the bifunctional kinase/RNase Ire1. BioMed Central Biology. 2011;9:48. doi: 10.1186/1741-7007-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L., Perera B.G., Hari S.B., Bhhatarai B., Backes B.J., Seeliger M.A. Divergent allosteric control of the IRE1alpha endoribonuclease using kinase inhibitors. Nature Chemical Biology. 2012;8:982–989. doi: 10.1038/nchembio.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feldman H.C., Tong M., Wang L., Meza-Acevedo R., Gobillot T.A., Lebedev I. Structural and functional analysis of the allosteric inhibition of IRE1alpha with ATP-competitive ligands. ACS Chemical Biology. 2016;11:2195–2205. doi: 10.1021/acschembio.5b00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qiu Y., Mao T., Zhang Y., Shao M., You J., Ding Q. A crucial role for RACK1 in the regulation of glucose-stimulated IRE1alpha activation in pancreatic beta cells. Science Signaling. 2010;3:ra7. doi: 10.1126/scisignal.2000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishitoh H., Matsuzawa A., Tobiume K., Saegusa K., Takeda K., Inoue K. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes & Development. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lisbona F., Rojas-Rivera D., Thielen P., Zamorano S., Todd D., Martinon F. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Molecular Cell. 2009;33:679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He Y., Beatty A., Han X., Ji Y., Ma X., Adelstein R.S. Nonmuscle myosin IIB links cytoskeleton to IRE1alpha signaling during ER stress. Developmental Cell. 2012;23:1141–1152. doi: 10.1016/j.devcel.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gu F., Nguyen D.T., Stuible M., Dube N., Tremblay M.L., Chevet E. Protein-tyrosine phosphatase 1B potentiates IRE1 signaling during endoplasmic reticulum stress. Journal of Biological Chemistry. 2004;279:49689–49693. doi: 10.1074/jbc.C400261200. [DOI] [PubMed] [Google Scholar]
- 72.Hantschel O. Structure, regulation, signaling, and targeting of abl kinases in cancer. Genes Cancer. 2012;3:436–446. doi: 10.1177/1947601912458584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harrington P.E., Biswas K., Malwitz D., Tasker A.S., Mohr C., Andrews K.L. Unfolded protein response in cancer: IRE1alpha inhibition by selective kinase ligands does not impair tumor cell viability. ACS Medicinal Chemistry Letters. 2015;6:68–72. doi: 10.1021/ml500315b. [DOI] [PMC free article] [PubMed] [Google Scholar]