Abstract
Background
Current therapeutic strategies for type 1 (T1DM) and type 2 diabetes mellitus (T2DM) rely on increasing or substituting endogenous insulin secretion in combination with lifestyle changes. β-cell regeneration, a process whereby new β-cells arise from progenitors, self-renewal or transdifferentiation, has the potential to become a viable route to insulin self-sufficiency. Current regeneration strategies capture many of the transcriptomic and protein features of native β-cells, generating cells capable of glucose-dependent insulin secretion in vitro and alleviation of hyperglycemia in vivo. However, whether novel β-cells display appreciable heterogeneity remains poorly understood, with potential consequences for long-term functional robustness.
Scope of review
The review brings together crucial discoveries in the β-cell regeneration field with state-of-the-art knowledge regarding β-cell heterogeneity. Aspects that might aid production of longer-lasting and more plastic regenerated β-cells are highlighted and discussed.
Major conclusions
Different β-cell regeneration approaches result in a similar outcome: glucose-sensitive, insulin-positive cells that mimic the native β-cell phenotype but which lack normal plasticity. The β-cell subpopulations identified to date expand our understanding of β-cell survival, proliferation and function, signposting the direction for future regeneration strategies. Therefore, regenerated β-cells should exhibit stimulus-dependent differences in gene and protein expression, as well as establish a functional network with different β-cells, all while coexisting with other cell types on a three-dimensional platform.
Keywords: Beta cell, Heterogeneity, Stem cell, Regenerative medicine, Diabetes
Highlights
-
•
Pancreatic β-cells are highly heterogeneous.
-
•
Heterogeneity helps maintain β-cell phenotype and function.
-
•
Heterogeneity is critical for islet plasticity in response to metabolic stress.
-
•
Regenerated β-cells share some molecular and cellular resemblance to native β-cells.
-
•
Heterogeneity in regenerated β-cells might increase robustness of islet-like structures.
1. Introduction
By 2045, it is predicted that 9.9% of the global adult population will live with type 1 (T1DM) and type 2 diabetes mellitus (T2DM) [1]. T1DM is generally associated with almost complete β-cell loss and dependency on insulin injections [2]. T2DM arises as a result of insufficient insulin secretion from pancreatic β-cells and/or impaired sensitivity of the liver, muscle and adipose tissue to insulin action [3]. Conventional therapeutic approaches rely on increasing insulin output, improving insulin sensitivity and during later stages of the disease, insulin replacement. While these treatments are effective when combined with diet and exercise, maintaining good glycemic control throughout the lifespan can be difficult. In patients who become non-responsive to therapy and/or struggle with hypoglycemia following insulin supplementation, islet transplantation is a therapy of choice, although limited donor supply and the need for immunosuppression [4], [5] make this a third-line option. Therefore, much recent research effort has been focused on β-cell regeneration, with the hope of producing a virtually unlimited supply of functional β-cells.
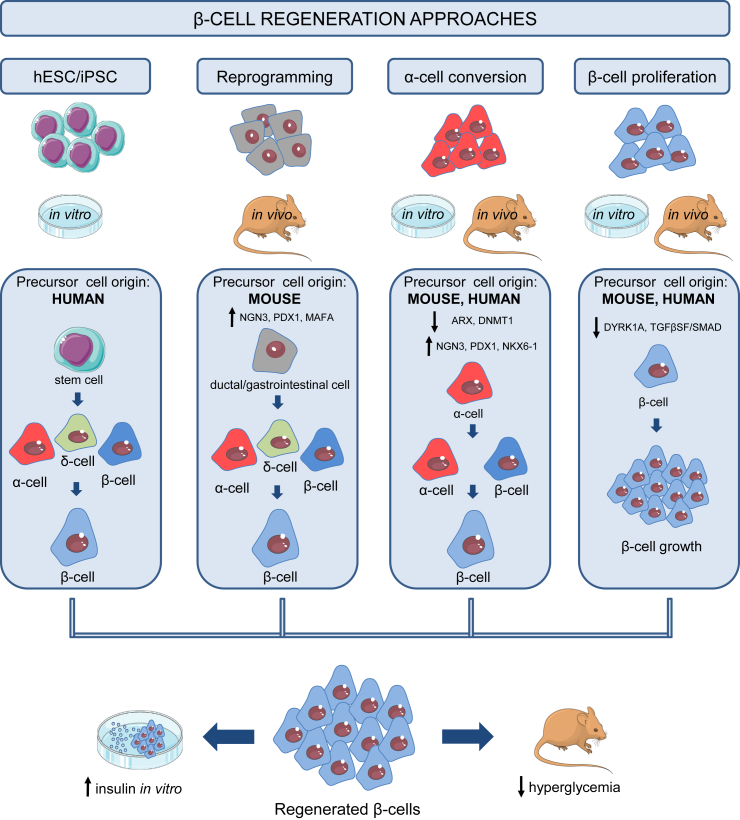
The manipulation of human embryonic stem cells (hESC) [6], [7] and induced pluripotent stem cells (iPSC) [8] has given rise to numerous studies describing generation of β-cells derived from pluripotent non-pancreatic progenitors. Newly-generated β-cells forming clusters were derived in vitro and, once transplanted into diabetic mice and rats, were able to mature, survive for a limited period of time, secrete insulin in response to glucose load and correct the hyperglycemia [9], [10], [11], [12], [13], [14], [15]. An alternative approach involves lineage reprogramming of non-endocrine cells in vivo (i.e. pancreatic exocrine, gastrointestinal), initiated by adenoviral- or TetO-driven polycistronic re-expression of the islet endocrine markers Ngn3, Pdx1 and Mafa [16], [17], [18], [19]. These studies described generation of insulin-positive cells exhibiting some β-cell morphological and functional traits. Although these β-like cells were able to manifest insulin secretion in vitro and alleviate hyperglycemia in diabetic mice, their phenotype remained relatively immature. A similar phenotype, albeit with improved β-cell maturity, was reported following FOXO1 inhibition in human gut organoids [20], highlighting this tissue as a promising pool of β-cell progenitors. Capitalizing on the islet plasticity concept, adult glucagon-secreting α-cells were used as a source of β-cells via transdifferentiation in vivo. The process could be initiated by inactivation of both Arx and Dnmt1 [21] or Arx alone [22], γ-aminobutiric acid (GABA) [23] or artemether treatment [24] (with caveats-see later), which lead to development of neo-β-cells with gene and protein expression patterns resembling normal β-cells in vivo. Very recent studies have shown that overexpression of PDX1, MAFA and NKX6-1 can also transform human adult α-cells into insulin-secreting cells able to form pseudoislets and with nearly normal insulin secretion in vitro [25], suggesting that transdifferentiation per se may be valuable route to replenishing βcells.
Current efforts are focused on increasing β-cell number, maturity, function and post-transplantation survival. The proportion of cells undergoing transformation to β-cells varies, depending on the experimental setting, but it does not include all cells targeted with the transformation/rederivation protocol. Once directed on a path towards β-cell identity, many insulin-positive cells stay polyhormonal, co-expressing glucagon together with insulin and often, somatostatin as well. Confirmation of β-cell phenotype depends mainly on measuring β-cell-specific gene and protein expression, without taking into account the subtle differences that exist among different β-cell subpopulations, as shown by studies of β-cell heterogeneity. In addition, a recent transcriptomic profiling study of emerging β-cells has highlighted the need to address the developmental intricacies that define regenerated cells throughout the differentiation protocol [26]. Furthermore, functioning of the new β-cells is often assessed only by the insulin response to glucose and/or other secretagogues, and the success in reducing hyperglycemia in vivo. Also, more work needs to be focused on increasing β-cell survival after transplantation and elucidating the underlying mechanisms. Finally, current protocols generate islet-like clusters, but the complex islet structure and the intercellular connectivity have yet to be recreated [27], [28], [29]. Addressing these limitations, together with translating the findings from mouse to human, might be the key to successful reconstruction of β-cells within novel islets.
2. Overview of β-cell regeneration efforts
All in vitro hESC- and iPSC-derived β-cell regeneration protocols were devised based on processes imitating normal pancreatic and islet development. Functional β-cells start developing from the embryonic definitive endoderm, undergoing changes taking them through formation of the primitive gut tube, posterior foregut and pancreatic endocrine progenitors, and finishing with specification of each individual islet cell type [30], [31]. For regenerative purposes, β-cell differentiation protocols are divided into four to seven stages, each driven by application of molecular stimuli and inhibitors able to turn on/off expression of stage-specific transcription factors until a β-like cell population is formed [9], [10], [11], [12], [13], [14], [15]. Novel β-like cells express key β-cell markers including PDX1, MAFA and NKX6-1 [9], [12], [19], while protein signatures confirm the presence of insulin and formation of insulin granules [9], [15], [16], [21], followed by aggregation of islet-like clusters [15], [17], [25]. Concentration-dependent responses to glucose are also present, with evidence of biphasic insulin secretion [12], [15], [25]. Subsequent transplantation of the β-cell clusters under the kidney capsule in diabetic mice leads to a gradual increase of circulating human C-peptide in response to glucose, as well as amelioration of hyperglycemia for upwards of a year [15], [19], [22]. Taken together, current approaches for generating novel human β-cells in vitro coupled with further maturation in vivo in mice show high resemblance to normal β-cell phenotype, although the functional quality lags (slightly) behind that of isolated human islets.
Lineage reprogramming of non-endocrine cell populations in vivo driven by re-expression or ectopic expression of Pdx1, Ngn3 and Mafa resulted in rapid development of insulin-positive cells in the pancreatic ducts [16], [18], the intestinal crypts [17] and the stomach epithelium [19]. Using adenoviral transduction and tetracycline induction strategies, these studies showed that the combination of the three transcription factors is essential in driving adult gastrointestinal extra-islet cells toward a β-cell lineage. Insulin-positive cells derived this way develop genetic and protein features of β-cells, lose expression of precursor markers and are able to form islet-resembling structures. As seen with other β-cell regeneration approaches, a significant improvement in blood glucose levels was achieved in diabetic mice in all cases. Importantly, similar reprogramming was achieved in bioengineered organoids and transplantation of these structures into diabetic mice also led to improved glucose tolerance [19].
Regeneration of β-cells can occur as a result of α-to β-conversion, as reported after β-cell loss [32], [33], [34]. Abrupt and near-total ablation of β-cells in mice initiated β-cell regeneration occurring not as a result of self-renewal, but instead due to downregulation of α-specific Arx and Dnmt1 and ensuing α-to β-transdifferentiation [21], [32]. Islets from T1DM donors also showed downregulation of their α-cell profile, with cells expressing less ARX and exhibiting insulin presence [21]. The new β-cells generated this way showed electrophysiological responses to glucose (when dispersed into single cells), with glucose-stimulated insulin secretion (GSIS) patterns resembling those of native β-cells. Similar features were described in α-cell-derived β-like cells arising due to Arx inactivation [22] and GABA administration [23] in mice with overt diabetes, with occurrence of repeated cycles of β-cell hyperplasia and significant improvements in glucose tolerance. In light of contradicting studies reporting that GABA or artemether have no effects on β-cell regeneration using different reporter models [35], [36], these data require careful consideration. Human α-to β-like conversion can also be driven by overexpression of PDX1, MAFA and NKX6-1 [25], enabling robust GSIS from the transformed α-cells.
Human studies employing high-throughput small molecule screening (HTS) [37] showed that small molecule inhibitors, such as harmine, are able to trigger β-cell proliferation in vitro and in vivo by inhibiting dual-specificity tyrosine-regulated kinase (DYRK) and transforming growth factor beta superfamily (TGFβSF) signalling [38]. The β-cells derived this way did not show dedifferentiation, but rather had abundant β-cell-specific gene and protein expression and showed satisfactory but non-optimal GSIS. Notably, the results were obtained from islets of both healthy and individuals with T2DM. Along similar lines, GABA was shown to trigger β-cell proliferation in rats, with the new β-cells possessing a distinct transcriptomic signature [39]. A summary of the approaches used for β-cell regeneration is shown in Figure 1.
Figure 1.
Summary of β-cell regeneration strategies. Novel β-cells can be derived through transdifferentiation of human pluripotent stem cells (hPSC) and induced pluripotent stem cells (iPSC), reprogramming of mature, non-endocrine (ductal and gastrointestinal) cell populations, α-to β-cell conversion and β-cell proliferation.
A consistent finding in all above-mentioned studies is that regenerated β-cells show hallmarks of their native counterparts and can restore normoglycemia in rodent models, but compromises exist in terms of electrical activity, metabolism, survival and insulin secretion. This might limit application in the clinical setting where regenerated β-cells will be expected to perform in patients spanning different ages, BMI, genetic backgrounds and genders [40].
3. Why might regenerated β-cells function relatively poorly?
Current regeneration protocols successfully replicate β-cell-specific gene and protein signatures [12], [15], [16], [19], [25], [38]. The majority of novel β-cells show expression of Pdx1, Mafa and Nkx6-1 and notable absence of exocrine or α-cell-specific transcription factors, such as Arx. These cells are monohormonal, positive for insulin and C-peptide, with insulin granules present throughout the cytoplasm. Expression of GLUT1/Glut2, Gck, Kir6.2 and Sur1 is detected, indicating development of glucose-sensing mechanisms. However, the dynamic range of glucose responsiveness and fine-tuning of insulin secretion is still inferior to that detected in normal (native) β-cells.
The performance of the novel cells is assessed by their insulin-secreting ability and Ca2+ responses in vitro, as well as by their glucose-lowering efficacy in diabetic mice. Static and dynamic GSIS in vitro from isolated differentiated cells and cell clusters showed dose-dependent insulin responses to rising concentration of glucose (11–20 mM glucose) and/or other stimuli (KCl, glibenclamide, Exendin-4, l-arginine) [15], [19]. Patterns of first and second phase insulin secretion were observed [15], [25], indicating functional insulin granule biogenesis and trafficking, although insulin levels failed to match those of isolated human islets. In addition, Ca2+ signaling traces from individual cells reflected those in mature human β-cells, but were delayed and with lower amplitude. Taken together, these findings are highly encouraging, but beg the question: if regenerated β-cells have the necessary stimulus-sensing and secretory machinery in place, what else is missing?
While impressive data exists on the single-cell transcriptomic landscape of developing/mature native β-cells [41], [42], [43] and hESC-derived pancreatic progenitors [44], the influence of the islet/tissue/in vivo environment remains poorly understood. Most work to date has shown that regenerated β-cells only reach full potential when engrafted in vivo [12], [15], [19], [22], underlining the importance of in situ mechanisms. Indeed, neural and vascular rewiring might ensure β-cell survival/further development, while intercellular connectivity based on gap junction communication might allow time-locked, coordinated and heightened responses to secretagogues. Furthermore, morphological and functional diversity, as shown by studies of β-cell heterogeneity (see below), could allow the new cells to adjust their insulin secretion and proliferative capacity according to metabolic demand (obesity and T2DM). In addition, the islet context might afford proper maturation and inter-lineage control of function. Finally, regenerated β-cells of human origin undergo further maturation and gain-of-function in mouse models, yet inter-species differences might exist and prove to be crucial barriers to full restoration of β-cell function.
3.1. Accounting for the influence of β-cell heterogeneity on insulin release
Extrapolating findings from single β-cells to the entire β-cell population is complicated by the existence of cellular and molecular heterogeneity. While β-cell identity is typified by the ability to secrete insulin, it has been apparent for almost 50 years that not all β-cells are the same. Thus, single, isolated β-cells respond differently to glucose stimulation [29], [45], which is reflected at the level of insulin secretion [46], [47], insulin content and granule morphology [48], [49]. In addition, differences in intraislet β-cell electrical activity and metabolism are also present [50], [51].
3.1.1. Differential gene and protein signatures
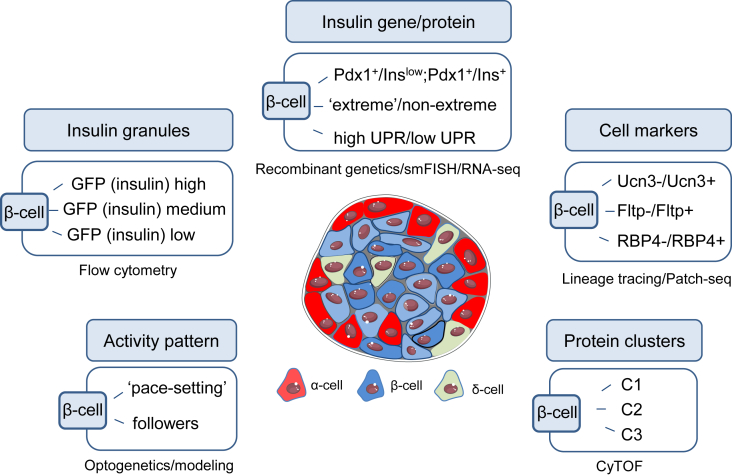
Studies in rodents and humans have identified β-cell subpopulations based on differential expression of specific gene and protein markers [52], [53], [54], [55], [56], insulin granularity [57], [58], and transcriptomic profile [42], [43]. Innovative promoter imaging showed that expression of Pdx1 and Ins is heterogeneous and reflects the maturity level and proliferative capacity of β-cells. A subgroup of β-cells in human and mouse islets, as well as MIN6 cells, were found to be Pdx1+/Ins+, and these cells actively secreted insulin, indicating maturity [56]. Another subgroup was represented by more immature Pdx1+/Inslow β-cells, which were highly proliferative [56]. The study also showed that immature Pdx1+/Inslow cells have the ability to become mature and secrete insulin without dividing, suggesting that immature cells are potentially a reservoir for more actively-secreting β-cells, should the demand arise [56]. Flow cytometry analyses stratified β-cells from MIP-GFP mice, which express green fluorescent protein (GFP) under the Ins1 promoter, into three different groups: cells with high, medium and low GFP brightness and granularity, indicative of differences in insulin gene expression [57]. Low GFP/low granularity β-cells with an immature profile were dominant in young mice, suggesting that age could be a factor imparting heterogeneity. Differences can also arise from the insulin mRNA expression and proinsulin content, as observed in ‘extreme’ β-cells, detected by single molecule fluorescence in situ hybridization (smFISH) in the intact pancreas [59]. The ‘extreme’ β-cells are a rare subgroup characterized by high insulin mRNA and proinsulin, normal Pdx1 expression, but low insulin, probably specialized in basal insulin secretion. The number of ‘extreme’ β-cells increased in insulin-resistant diabetic mice, implying a role in metabolic adaptation/failure.
RNA-sequencing studies have further revealed that some β-cell subpopulations are likely to be transient and dynamic rather than stable. Producing insulin is a demanding and highly oxidative process, rendering β-cells more prone to endoplasmic reticulum (ER) stress and activation of the unfolded protein response (UPR). Based on their UPR-coupled insulin gene expression, β-cells can exist in discrete states: this likely reflects transition from a state of active insulin secretion to a state of stress recovery under the UPR program in which insulin production is decreased [60]. Whether or not similar dynamics exist in neo-β-cells or islet-like structures is poorly understood, yet might be important to impart robustness and plasticity on the population. Moreover, strategies for regenerating β-cells, especially in T2DM patients, should take into consideration the possibility that these differences could be reintroduced in the newly-emerging cells under metabolic stress.
Next-generation sequencing and lineage-tracing revealed the presence of Urocortin-3 (Ucn3)-negative cells arising from α-cells at the islet periphery, but exhibiting traits of immature β-cells (so called “virgin” β-cells) [52]. The presence of these cells in mice and human neonatal islets is reassuring, as it indicates a highly-conserved process whose potential could be harnessed in autologous β-cell regeneration. The presence of a protein maintaining the islet 3D structure called Flattop (Fltp) classified β-cells as either Fltp+ or Fltp- cells [53]. Fltp+ cells bear most of the markers of mature β-cells, but have low proliferation potential, whereas Fltp- cells are immature and highly proliferative. Thus, confirmation of Fltp presence/absence in newly regenerated β-cells would be a useful initial indicator of heterogeneity. Single-cell mass cytometry has provided compelling data on the qualities of different β-cell subpopulations in human islets, based on their protein footprint [41]. It identified C1, C2 and C3 β-cell clusters, each associated differently with proliferation (more present in C2 and C3), maturity (higher insulin and PDX1 in C1), age and T2DM (characterized with a C2 decline). For a more complete list of β-cell molecular screening studies refer to reviews [61], [62], [63].
3.1.2. Functional diversity
The introduction of modeling [64], [65] to islet biology revealed the dynamics of β-cell intercellular networks and the characteristics of particular cells governing it. Optogenetic silencing during live Ca2+ imaging showed the existence of ‘hubs’–β-cells able to respond early to glucose stimuli and guide but not completely control the activity of the β-cell complement [50]. Hubs are relatively immature β-cells, with low Pdx1 and Nkx6-1 expression, but with highly-active mitochondria, prone to lipotoxic and pro-inflammatory attacks, in keeping with their low ER content [50]. Computational modelling of the electrical activity in mouse and human islets indicated species-specific differences in hub activity, with human hubs being potentially more susceptible to metabolic insults [66]. Intravital studies in zebrafish and mice have shown the presence of analogous cells, able to coordinate the activity of their neighbors [67]. Finally, using optogenetic excitation, different islet regions were shown to disproportionally regulate the function of the β-cell network [51]. It should be noted that such cell types are likely to overlap, since the techniques used describe subpopulations belonging to a distribution of cells spanning similar characteristics (e.g. maturity status, metabolism).
Recent Patch-seq studies have provided unprecedented insight into the molecular drivers of heterogeneity by directly correlating electrophysiological parameters of an individual β-cell with its transcriptome. Notably, the presence of retinol binding protein 4 clustered β-cells into RBP4+ and RBP4- subpopulations, the latter displaying improved electrophysiological parameters [68]. Together, these studies underline the importance of recreating the functional β-cell syncytium in neo-islets, built on a mixture of cells with desirable traits identified from single-cell screening and marker analyses. Figure 2 depicts selected β-cell subpopulations identified using different techniques.
Figure 2.
Overview of selected β-cell subpopulations. A combination of technologies and approaches identified β-cell subpopulations based on: differential expression of cell-specific markers, differences in their transcriptomic and protein blueprint and activity patterns. Abbreviations used: Pdx1, pancreatic and duodenal homeobox 1; Ins, insulin; GFP, green fluorescent protein; Ucn3, urocortin-3; Fltp, Flattop/Cfap126; RBP4, Retinol binding protein 4; C1/C2/C3, cell clusters with different protein signatures [41]; smFISH, single molecule fluorescent in situ hybridisation; RNA-seq, RNA sequencing; Patch-seq, patch sequencing; CyTOF, mass cytometry; UPR, unfolded protein response.
To summarize, molecular and functional heterogeneity contributes to subtle regulation of glucose homeostasis and appears to be important in ensuring survival, adaptability and resistance of β-cells to failure. Different responsiveness and thresholds allow β-cells to fine-tune responses to changing stimuli and ultimately, might prolong function and ensure survival of newly regenerated β-cells. This is an important feature that regenerated β-cells currently lack and which could contribute to limited function and survival. It should therefore not be ignored but integrated into regenerated β-cells.
3.2. Islets as the 3D scaffold promoting β-cell heterogeneity, survival and function
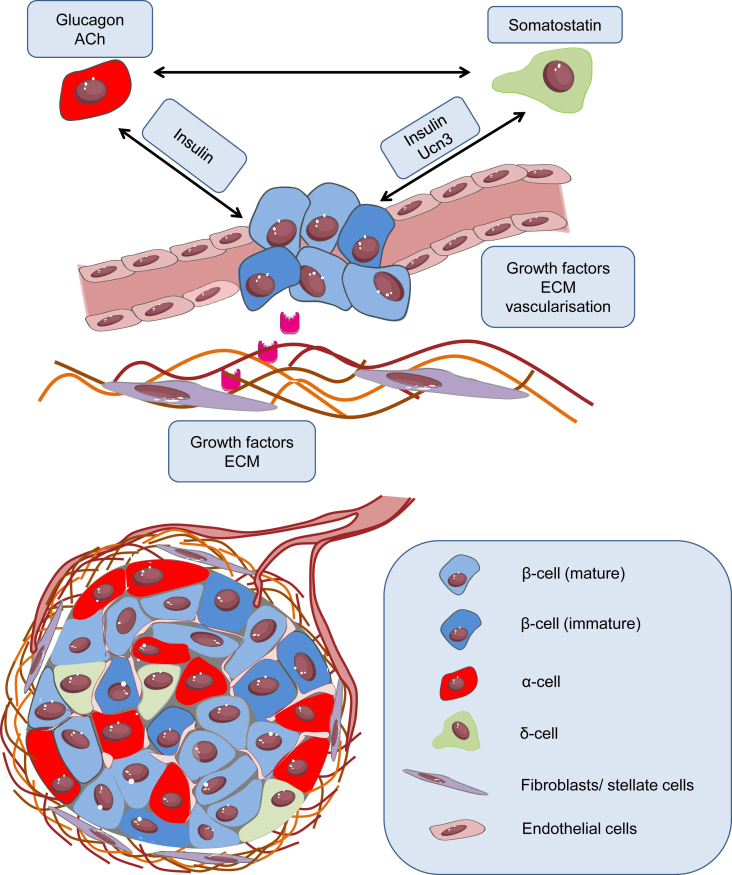
Islet architecture is a dynamic niche, which undergoes constant remodeling. Individual β-cells are thus exposed to a different microenvironment over time, altering their function [69]. In mouse islets, regional differences in membrane potential and electrical coupling have been observed between β-cells in the core and those at the periphery, indicating the presence of functional heterogeneity [69], [70], [71], [72]. Additionally, heterogeneity in insulin secretion capacity has been observed depending on location and density of blood vessels [73], [74], and β-cells target the release of insulin granules toward the vasculature [75]. Indeed, islets are highly-vascularized structures, with blood flow and vascularization representing important adaptive components in response to demand [76], [77]. Furthermore, species differences exist in the proportion of different endocrine cell types, as well as their localization relative to one another. Mouse islets contain ∼80% β-cells, with α-cells arranged at the periphery, probably reflecting the inner→outer blood flow pattern [78] and orientation of intercellular feedback loops [79]. By contrast, human islets contain ∼50% β-cells, with a tertiary folding step encouraging heterotypic cell contacts with α-cells [80], [81]. Evidently, regenerated β-cells are likely to benefit from the specific endocrine arrangement, as well as from the association with other islet components, including the extracellular matrix (ECM) and the vascular network (Figure 3).
Figure 3.
Tweaking regenerated β-cell function. Integration of islet components is essential to maintain β-cell heterogeneity and survival of the regenerated cells. α and δ-cells monitor β-cells and prevent hypersecretion through glucagon, acetylcholine and somatostatin. Stromal cells and the ECM may promote revascularisation and mediate cell-cell communications. Abbreviations used: Ach, Acetylcholine; Ucn3, urocortin-3; ECM, extracellular matrix.
3.2.1. α-cells
Glucagon is an important counter-regulatory hormone, which prevents potentially dangerous drops in blood glucose induced by insulin. At low glucose concentration, α-cells secrete glucagon, which stimulates hepatic gluconeogenesis and release of glucose into the circulation [82]. Species diferences exist, with data indicating that glucagon paradoxically promotes insulin secretion in humans, most likely via direct effects on β-cells, as well as via somatostatin release from δ-cells [83], [84], [85]. Although α-cells normally reduce secretion as glucose levels reach 5 mM, glucagon secretion has been observed even at 20 mM glucose [86], [87]. A rise in glucose may stimulate glucagon release by activating ER Ca2+ stores, priming β-cells to respond to impending hyperglycemia [83], [84], [88]. In human islets, glucagon receptor activation in β-cells is required to amplify insulin secretion [84]. Furthermore, parasympathetic nerve innervation is more sporadic in humans compared to rodents, with acetylcholine derived from α-cells rather than from neurons, as in mice [89]. At basal glucose, acetylcholine is released and subsequently stimulates insulin secretion, thereby preparing β-cells for a rise in glucose [89], [90]. However, acetylcholine can also indirectly inhibit insulin secretion through activation of somatostatin release [90]. From this, it can be envisaged that, rather than inhibiting regenerated β-cell function, inclusion of α-cells with the correct phenotype might facilitate proper responses to hypoglycemia and amplify insulin release.
3.2.2. δ-cells
Another “master inhibitor” hormone is δ-cell-derived somatostatin, which downregulates both α- and β-cell activity. Comprising only ∼5% of mouse and human islets, δ-cells nonetheless make large contributions to glucagon and insulin secretion. Somatostatin secretion ramps up in response to glucose stimulation, during which time it inhibits glucagon and exerts tonic influence over insulin release [83]. Somatostatin release is stimulated by a paracrine mechanism involving UCN3 co-secreted with insulin from β-cells [91]. Notably, somatostatin secretion is pulsatile, trailing insulin peaks by ∼30 s, probably reflecting the kinetics of UCN3 [91], [92]. Although suppression of insulin at high glucose seems counterintuitive, δ-cell→β-cell interactions represent an intrinsic “buffer” to prevent insulin hypersecretion and rebound hypoglycemia [83]. δ-cell morphology in mice is neuron-like with long, protruding arms for increased cell contacts, while in humans δ-cells tend to be more interspersed, suggesting a more intimate somatostatinergic regulation of α- and β-cell function [83]. As such, δ-cells are not only critical for glucose counter-regulation, but might also be important for restraining activity of regenerated β-cells during increased demand, alleviating UPR-initiated stress.
3.2.3. Stromal cells
The islets are home to resident stromal cells such as stellate cells, fibroblasts and endothelial cells. These supportive cells secrete growth factors and ECM-regulating proteins, contributing to extracellular signaling. Endothelial cells promote angiogenesis and their role in regulating islet function and survival has been well documented [93]. During development, the vasculature is instructive for β-cell and ultimately islet specification [94], [95]. Resident endothelial cells are also required for angiogenesis in newly-formed islets, as well as for connecting the islet to the host endothelium following transplantation [93], [96], [97]. The secretome of endothelial cells includes fibroblast growth factor-2 (FGF-2) and hepatocyte growth factor (HGF), which have been shown to induce β-cell proliferation (HGF) and improve insulin secretion (FGF-2) [98], [99], [100]. Further demonstrating the importance of stromal cells during transplantation are studies in islets co-cultured with mesenchymal stromal cells (MSCs), fibroblasts or endothelial cells, which show increased insulin secretion and improved survival [101], [102], [103], [104], [105]. Factors secreted by MSCs include C3a, annexin A1, and stromal cell-derived factor (SDF-1), which protect against cytokine-induced apoptosis after transplantation, increase graft survival and improve blood glucose levels in diabetic mice [102]. It will be worth revisiting such studies to determine whether stromal-β-cell interactions improve maturation/stress status, heterogeneity or both.
3.2.4. The ECM
The ECM determines tissue structure but importantly, also modulates gene expression and cell behavior. The regulatory effects of the ECM vary as its composition changes. Remodeling throughout development and tissue repair [106] might therefore influence β-cell function. ECM proteins are involved in the regulation of β-cell proliferation, islet mass, survival and insulin secretion [107], [108], [109], [110], [111]. Islets and β-cells cultured on an ECM survive and function better compared to those in conventional culture plates [107], [108], [109], [110], [111]. Previous evidence has shown that endothelial cell-derived collagen IV potentiates insulin secretion through β-cell α1β1 integrins [112]. Mice with β-cell-specific β1 integrin knockout exhibit a decrease in β-cell mass and proliferation [113], suggesting that establishment of islet–matrix interactions is essential for β-cell development. In addition, the matrix might be important in regulating β-cell heterogeneity: expression of α6β1 integrin varies in β-cells, reflecting heterogeneity in their spreading behavior on matrix cultures [108]. Matricellular components have been shown to exert specific regulatory effects on islet adhesion, survival and function [114]. Human islets cultured in collagen I and IV have increased insulin, GLUT2 and GCK gene expression, whereas fibronectin is more important for insulin release [114]. Matricellular structure further protects islets by influencing immune activation, islet infiltration with immune cells and ensuing β-cell destruction [115], [116]. Restoring islet-ECM interactions might therefore provide an additional mechanism to preserve tissue architecture and support the function of regenerated β-cells.
4. Avenues to restoring function in regenerated β-cells
Heterogeneity is not the only mechanism that dictates viability and function of synthetic β-cells, but it could provide a more solid foundation for introducing plasticity and robustness into islet-like structures. Moreover, missing β-cell subpopulations should be re-established according to the timeline during which they appear in the islet during development.
4.1. Balancing β-cell heterogeneity
Establishing robust glucose responsiveness and insulin secretion is the major goal when producing regenerated β-cells. Recreating a degree of heterogeneity is likely to facilitate this by allowing newly-formed cells to be more dynamic and robust when present in islet-like structures. Indeed, heterogeneous populations might impart improved recovery from ER stress by allowing β-cells to temporarily opt out of insulin secretion should UPR occur. This will become an increasingly important concept as regenerated β-cells are produced with ever higher insulin-secretory capacity. Moreover, metabolic stress expected to occur naturally during transplant-recipient ageing, is likely to be better compensated if grafts contain immature cells capable of undergoing proliferation. This might boost insulin secretion across the population without driving individual cells into hypersecretion and ER stress. Subpopulations with highly secretory characteristics could also be harnessed, but caution should be extended to avoid exhaustion and eventual failure. In this regard, a balance of different β-cell subpopulations spanning immature → mature is likely to be more important to support insulin secretion while maintaining functional robustness.
How can such heterogeneity be achieved in regenerated β-cells? One potential way is to integrate β-cells with different levels of maturity, proliferative potential and secretory/metabolic capacity at time points relative to when they first appear in the islet. This is likely to be challenging, however, since native β-cells tend to lose these heterogeneous characteristics once dissociated, meaning that regenerated β-cells may never attain them in the first place. A possible solution is to mimic the 3D islet architecture by integration of islet cells that maintain β-cells, such as α-cells, δ-cells, stromal cells as well as the extracellular matrix (ECM) (see below).
4.2. Balancing islet architecture
Establishing the islet architectural components will be important to mimic the islet niche that might contribute to maintenance of heterogeneity and function of regenerated β-cells (Figure 3). Glucose homeostasis does not solely depend on β-cells, but rather on the overall balance between insulin and other counter-regulatory hormones. For example, α- and δ-cells are essential to monitor β-cell secretion, ultimately preventing hypersecretion and stress while allowing replenishment of insulin and avoiding hypoglycemia. On the other hand, stromal cells and the extracellular matrix are essential to create a microenvironment supportive of growth while maintaining tissue architecture. Several studies have shown that islet function declines in vitro, which may be due to the loss of β-cell–β-cell interactions, indirect interactions with other cell types, and inappropriate extracellular signaling [69], [107], [117]. Therefore, promotion of cell–cell interactions and cell–matrix interactions will be essential to establish functional heterogeneity and therapeutic use of regenerated β-cells. Recent studies using decellularized pancreatic scaffolds show the potential of such approaches to increase maturation and function of hESC-derived β-cells [118]. Lastly, next generation tools, such as CRISPR genome editing, could be used to impart specific characteristics onto β-cells [119] before their inclusion in the islet.
5. Summary - going forward
Replenishing β-cells after their numbers have been decimated (in the case of T1DM) or once their function has deteriorated beyond remission (occurring in T2DM) is still a revolutionary concept. A number of impressive studies describe novel β-cells with adequate β-cell phenotype, glucose-sensing and insulin-secretory properties, and with the potential to form islet-like structures. However, their proliferative potential, maturity, age/time/demand-dependent distribution, as well as diversity of function remain inferior to those of native β-cells. These differences might reflect the regeneration protocol used, the limited plasticity of the newly-derived β-cells, or the method by which regenerated cells are encapsulated and transplanted into the body. Recent studies elucidating β-cell heterogeneity have helped identify some of the potential shortcomings of current β-cell regeneration efforts, while promising feasible solutions. The existence of discrete β-cell subpopulations with morphological differences has shown that driving expression of β-cell-specific markers is only the first step; differential expression conferring levels of maturity, proliferation or other characteristics should follow next. Furthermore, recreating the signaling identity of the whole β-cell population would correct the single-cell activity patterns in regenerated β-cell clusters, potentially rendering them more resistant to metabolic insults. Finally, research focusing on the non-β-cell islet tissue has underlined the importance of the islet context in generating/maintaining functional β-cell populations. Embedding ‘synthesized’ β-cells in a 3D microenvironment with other endocrine cell lines, on a vascular and neural scaffold, is the last level of complexity to achieve. Although impairment of β-cell survival and function drives the onset of T2DM, it eventually becomes a disease encompassing a failure of the islet as a whole.
Future multidisciplinary and collaborative approaches are necessary for meeting the above-mentioned objectives. Validation of existing regeneration protocols, supported by current technologies detecting different β-cell subpopulations, offers a template for the regeneration of more robust islets.
Funding
This article is part of a supplement entitled ‘Biomarkers of Beta-Cell Health and Dysfunction: Towards Personalised Diabetes Care. Proceedings of the 20th Servier-IGIS Symposium’. The Symposium and the publication of the Supplement have been made possible by an unrestricted educational grant from Institut la Conférence Hippocrate – Servier Group.
Acknowledgements
D.J.H. was supported by a Diabetes UK R.D. Lawrence (12/0004431) Fellowship, a Wellcome Trust Institutional Support Award, and MRC (MR/N00275X/1 and MR/S025618/1) and Diabetes UK (17/0005681) Project Grants. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Starting Grant 715884 to D.J.H.). We thank Dr Julia Ast for critical reading of the manuscript. We apologize to the many researchers whose work we could not cite due to space limitations.
Conflict of interest
None declared.
References
- 1.Cho N.H., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone J.A., Herold K., Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeFronzo R.A., Ferrannini E., Groop L., Henry R.R., Herman W.H., Holst J.J. Type 2 diabetes mellitus. Nature Reviews Disease Primers. 2015;1:15019. doi: 10.1038/nrdp.2015.19. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro A.M., Lakey J.R., Ryan E.A., Korbutt G.S., Toth E., Warnock G.L. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. New England Journal of Medicine. 2000;343(4):230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 5.Bellin M.D., Barton F.B., Heitman A., Harmon J.V., Kandaswamy R., Balamurugan A.N. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. American Journal of Transplantation. 2012;12(6):1576–1583. doi: 10.1111/j.1600-6143.2011.03977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 7.Rosler E.S., Fisk G.J., Ares X., Irving J., Miura T., Rao M.S. Long-term culture of human embryonic stem cells in feeder-free conditions. Developmental Dynamics. 2004;229(2):259–274. doi: 10.1002/dvdy.10430. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.D'Amour K.A., Bang A.G., Eliazer S., Kelly O.G., Agulnick A.D., Smart N.G. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nature Biotechnology. 2006;24(11):1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 10.Kroon E., Martinson L.A., Kadoya K., Bang A.G., Kelly O.G., Eliazer S. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nature Biotechnology. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 11.Rezania A., Bruin J.E., Riedel M.J., Mojibian M., Asadi A., Xu J. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rezania A., Bruin J.E., Arora P., Rubin A., Batushansky I., Asadi A. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nature Biotechnology. 2014;32(11):1121–1133. doi: 10.1038/nbt.3033. [DOI] [PubMed] [Google Scholar]
- 13.Pagliuca F.W., Millman J.R., Gurtler M., Segel M., Van Dervort A., Ryu J.H. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159(2):428–439. doi: 10.1016/j.cell.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millman J.R., Xie C., Van Dervort A., Gurtler M., Pagliuca F.W., Melton D.A. Generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nature Communications. 2016;7:11463. doi: 10.1038/ncomms11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velazco-Cruz L., Song J., Maxwell K.G., Goedegebuure M.M., Augsornworawat P., Hogrebe N.J. Acquisition of dynamic function in human stem cell-derived beta cells. Stem Cell Reports. 2019;12(2):351–365. doi: 10.1016/j.stemcr.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Q., Brown J., Kanarek A., Rajagopal J., Melton D.A. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y.J., Finkbeiner S.R., Weinblatt D., Emmett M.J., Tameire F., Yousefi M. De novo formation of insulin-producing “neo-beta cell islets” from intestinal crypts. Cell Reports. 2014;6(6):1046–1058. doi: 10.1016/j.celrep.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada T., Cavelti-Weder C., Caballero F., Lysy P.A., Guo L., Sharma A. Reprogramming mouse cells with a pancreatic duct phenotype to insulin-producing beta-like cells. Endocrinology. 2015;156(6):2029–2038. doi: 10.1210/en.2014-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ariyachet C., Tovaglieri A., Xiang G., Lu J., Shah M.S., Richmond C.A. Reprogrammed stomach tissue as a renewable source of functional beta cells for blood glucose regulation. Cell Stem Cell. 2016;18(3):410–421. doi: 10.1016/j.stem.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouchi R., Foo K.S., Hua H., Tsuchiya K., Ohmura Y., Sandoval P.R. FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nature Communications. 2014;5:4242. doi: 10.1038/ncomms5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakravarthy H., Gu X., Enge M., Dai X., Wang Y., Damond N. Converting adult pancreatic islet alpha cells into beta cells by targeting both Dnmt1 and Arx. Cell Metabolism. 2017;25(3):622–634. doi: 10.1016/j.cmet.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Courtney M., Gjernes E., Druelle N., Ravaud C., Vieira A., Ben-Othman N. The inactivation of Arx in pancreatic alpha-cells triggers their neogenesis and conversion into functional beta-like cells. Public Library of Science Genetics. 2013;9(10) doi: 10.1371/journal.pgen.1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Othman N., Vieira A., Courtney M., Record F., Gjernes E., Avolio F. Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell. 2017;168(1–2):73–85 e11. doi: 10.1016/j.cell.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Li J., Casteels T., Frogne T., Ingvorsen C., Honore C., Courtney M. Artemisinins target GABAA receptor signaling and impair alpha cell identity. Cell. 2017;168(1–2):86–100. doi: 10.1016/j.cell.2016.11.010. e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuyama K., Chera S., van Gurp L., Oropeza D., Ghila L., Damond N. Diabetes relief in mice by glucose-sensing insulin-secreting human alpha-cells. Nature. 2019;567(7746):43–48. doi: 10.1038/s41586-019-0942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veres A., Faust A.L., Bushnell H.L., Engquist E.N., Kenty J.H.-R., Harb G. Charting cellular identity during human in vitro β-cell differentiation. Nature. 2019;569(7756):368–373. doi: 10.1038/s41586-019-1168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodson D.J., Mitchell R.K., Bellomo E.A., Sun G., Vinet L., Meda P. Lipotoxicity disrupts incretin-regulated human beta cell connectivity. Journal of Clinical Investigation. 2013;123(10):4182–4194. doi: 10.1172/JCI68459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stozer A., Gosak M., Dolensek J., Perc M., Marhl M., Rupnik M.S. Functional connectivity in islets of Langerhans from mouse pancreas tissue slices. Public Library of Science Computational Biology. 2013;9(2) doi: 10.1371/journal.pcbi.1002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benninger R.K., Piston D.W. Cellular communication and heterogeneity in pancreatic islet insulin secretion dynamics. Trends in Endocrinology and Metabolism. 2014;25(8):399–406. doi: 10.1016/j.tem.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Q., Melton D.A. Pancreas regeneration. Nature. 2018;557(7705):351–358. doi: 10.1038/s41586-018-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baeyens L., Lemper M., Staels W., De Groef S., De Leu N., Heremans Y. (Re)generating human beta cells: status, pitfalls, and perspectives. Physiological Reviews. 2018;98(3):1143–1167. doi: 10.1152/physrev.00034.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorel F., Nepote V., Avril I., Kohno K., Desgraz R., Chera S. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464(7292):1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonner-Weir S., Baxter L.A., Schuppin G.T., Smith F.E. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42(12):1715–1720. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 34.Xu X., D'Hoker J., Stange G., Bonne S., De Leu N., Xiao X. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Ackermann A.M., Moss N.G., Kaestner K.H. GABA and artesunate do not induce pancreatic alpha-to-beta cell transdifferentiation in vivo. Cell Metabolism. 2018;28(5):787–792. doi: 10.1016/j.cmet.2018.07.002. e783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Meulen T., Lee S., Noordeloos E., Donaldson C.J., Adams M.W., Noguchi G.M. Artemether does not turn alpha cells into beta cells. Cell Metabolism. 2018;27(1):218–225. doi: 10.1016/j.cmet.2017.10.002. e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang P., Alvarez-Perez J.C., Felsenfeld D.P., Liu H., Sivendran S., Bender A. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nature Medicine. 2015;21(4):383–388. doi: 10.1038/nm.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang P., Karakose E., Liu H., Swartz E., Ackeifi C., Zlatanic V. Combined inhibition of DYRK1A, SMAD, and trithorax pathways synergizes to induce robust replication in adult human beta cells. Cell Metabolism. 2019;29(3):638–652. doi: 10.1016/j.cmet.2018.12.005. e635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Untereiner A., Abdo S., Bhattacharjee A., Gohil H., Pourasgari F., Ibeh N. GABA promotes beta-cell proliferation, but does not overcome impaired glucose homeostasis associated with diet-induced obesity. FASEB Journal. 2019;33(3):3968–3984. doi: 10.1096/fj.201801397R. [DOI] [PubMed] [Google Scholar]
- 40.Saber N., Bruin J.E., O'Dwyer S., Schuster H., Rezania A., Kieffer T.J. Sex differences in maturation of human embryonic stem cell-derived beta cells in mice. Endocrinology. 2018;159(4):1827–1841. doi: 10.1210/en.2018-00048. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y.J., Golson M.L., Schug J., Traum D., Liu C., Vivek K. Single-Cell mass cytometry analysis of the human endocrine pancreas. Cell Metabolism. 2016;24(4):616–626. doi: 10.1016/j.cmet.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Segerstolpe A., Palasantza A., Eliasson P., Andersson E.M., Andreasson A.C., Sun X. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metabolism. 2016;24(4):593–607. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xin Y., Kim J., Okamoto H., Ni M., Wei Y., Adler C. RNA sequencing of single human islet cells reveals type 2 diabetes genes. Cell Metabolism. 2016;24(4):608–615. doi: 10.1016/j.cmet.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Krentz N.A.J., Lee M.Y.Y., Xu E.E., Sproul S.L.J., Maslova A., Sasaki S. Single-cell transcriptome profiling of mouse and hESC-derived pancreatic progenitors. Stem Cell Reports. 2018;11(6):1551–1564. doi: 10.1016/j.stemcr.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pipeleers D.G. Heterogeneity in pancreatic beta-cell population. Diabetes. 1992;41(7):777–781. doi: 10.2337/diab.41.7.777. [DOI] [PubMed] [Google Scholar]
- 46.Hiriart M., Ramirez-Medeles M.C. Functional subpopulations of individual pancreatic B-cells in culture. Endocrinology. 1991;128(6):3193–3198. doi: 10.1210/endo-128-6-3193. [DOI] [PubMed] [Google Scholar]
- 47.Navarro-Tableros V., Sanchez-Soto M.C., Garcia S., Hiriart M. Autocrine regulation of single pancreatic beta-cell survival. Diabetes. 2004;53(8):2018–2023. doi: 10.2337/diabetes.53.8.2018. [DOI] [PubMed] [Google Scholar]
- 48.Van Schravendijk C.F., Kiekens R., Pipeleers D.G. Pancreatic beta cell heterogeneity in glucose-induced insulin secretion. Journal of Biological Chemistry. 1992;267(30):21344–21348. [PubMed] [Google Scholar]
- 49.Kiekens R., In 't Veld P., Mahler T., Schuit F., Van De Winkel M., Pipeleers D. Differences in glucose recognition by individual rat pancreatic B cells are associated with intercellular differences in glucose-induced biosynthetic activity. Journal of Clinical Investigation. 1992;89(1):117–125. doi: 10.1172/JCI115551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnston N.R., Mitchell R.K., Haythorne E., Pessoa M.P., Semplici F., Ferrer J. Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metabolism. 2016;24(3):389–401. doi: 10.1016/j.cmet.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westacott M.J., Ludin N.W.F., Benninger R.K.P. Spatially organized beta-cell subpopulations control electrical dynamics across islets of Langerhans. Biophysical Journal. 2017;113(5):1093–1108. doi: 10.1016/j.bpj.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Meulen T., Mawla A.M., DiGruccio M.R., Adams M.W., Nies V., Dolleman S. Virgin beta cells persist throughout life at a neogenic niche within pancreatic islets. Cell Metabolism. 2017;25(4):911–926. doi: 10.1016/j.cmet.2017.03.017. e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bader E., Migliorini A., Gegg M., Moruzzi N., Gerdes J., Roscioni S.S. Identification of proliferative and mature beta-cells in the islets of Langerhans. Nature. 2016;535(7612):430–434. doi: 10.1038/nature18624. [DOI] [PubMed] [Google Scholar]
- 54.Karaca M., Castel J., Tourrel-Cuzin C., Brun M., Geant A., Dubois M. Exploring functional beta-cell heterogeneity in vivo using PSA-NCAM as a specific marker. Public Library of Science one. 2009;4(5):e5555. doi: 10.1371/journal.pone.0005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dorrell C., Schug J., Canaday P.S., Russ H.A., Tarlow B.D., Grompe M.T. Human islets contain four distinct subtypes of beta cells. Nature Communications. 2016;7:11756. doi: 10.1038/ncomms11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szabat M., Luciani D.S., Piret J.M., Johnson J.D. Maturation of adult beta-cells revealed using a Pdx1/insulin dual-reporter lentivirus. Endocrinology. 2009;150(4):1627–1635. doi: 10.1210/en.2008-1224. [DOI] [PubMed] [Google Scholar]
- 57.Katsuta H., Aguayo-Mazzucato C., Katsuta R., Akashi T., Hollister-Lock J., Sharma A.J. Subpopulations of GFP-marked mouse pancreatic beta-cells differ in size, granularity, and insulin secretion. Endocrinology. 2012;153(11):5180–5187. doi: 10.1210/en.2012-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aguayo-Mazzucato C., van Haaren M., Mruk M., Lee T.B., Jr., Crawford C., Hollister-Lock J. Beta cell aging markers have heterogeneous distribution and are induced by insulin resistance. Cell Metabolism. 2017;25(4):898–910. doi: 10.1016/j.cmet.2017.03.015. e895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farack L., Golan M., Egozi A., Dezorella N., Bahar Halpern K., Ben-Moshe S. Transcriptional heterogeneity of beta cells in the intact pancreas. Developmental Cell. 2019;48(1):115–125. doi: 10.1016/j.devcel.2018.11.001. e114. [DOI] [PubMed] [Google Scholar]
- 60.Xin Y., Dominguez Gutierrez G., Okamoto H., Kim J., Lee A.H., Adler C. Pseudotime ordering of single human beta-cells reveals states of insulin production and unfolded protein response. Diabetes. 2018;67(9):1783–1794. doi: 10.2337/db18-0365. [DOI] [PubMed] [Google Scholar]
- 61.Carrano A.C., Mulas F., Zeng C., Sander M. Interrogating islets in health and disease with single-cell technologies. Molecular Metabolism. 2017;6(9):991–1001. doi: 10.1016/j.molmet.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Avrahami D., Klochendler A., Dor Y., Glaser B. Beta cell heterogeneity: an evolving concept. Diabetologia. 2017;60(8):1363–1369. doi: 10.1007/s00125-017-4326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y.J., Kaestner K.H. Single-cell RNA-seq of the pancreatic islets--a promise not yet fulfilled? Cell Metabolism. 2019;29(3):539–544. doi: 10.1016/j.cmet.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dolensek J., Spelic D., Klemen M.S., Zalik B., Gosak M., Rupnik M.S. Membrane potential and calcium dynamics in beta cells from mouse pancreas tissue slices: theory, experimentation, and analysis. Sensors (Basel) 2015;15(11):27393–27419. doi: 10.3390/s151127393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gosak M., Stozer A., Markovic R., Dolensek J., Marhl M., Rupnik M.S. The relationship between node degree and dissipation rate in networks of diffusively coupled oscillators and its significance for pancreatic beta cells. Chaos. 2015;25(7):073115. doi: 10.1063/1.4926673. [DOI] [PubMed] [Google Scholar]
- 66.Lei C.L., Kellard J.A., Hara M., Johnson J.D., Rodriguez B., Briant L.J.B. Beta-cell hubs maintain Ca(2+) oscillations in human and mouse islet simulations. Islets. 2018;10(4):151–167. doi: 10.1080/19382014.2018.1493316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salem V., Delgadillo Silva L., Suba K., Georgiadou E., Neda Mousavy S., Akhtar N. Leader β cells coordinate Ca2+ dynamics across pancreatic islets in vivo. Nature Metabolism. 2019;1:615–629. doi: 10.1038/s42255-019-0075-2. [DOI] [PubMed] [Google Scholar]
- 68.Camunas-Soler J., Dai X., Hang Y., Bautista A., Lyon J., Suzuki K. bioRxiv; 2019. Pancreas patch-seq links physiologic dysfunction in diabetes to single-cell transcriptomic phenotypes. [Pre-print] [DOI] [Google Scholar]
- 69.Roscioni S.S., Migliorini A., Gegg M., Lickert H. Impact of islet architecture on beta-cell heterogeneity, plasticity and function. Nature Reviews Endocrinology. 2016;12(12):695–709. doi: 10.1038/nrendo.2016.147. [DOI] [PubMed] [Google Scholar]
- 70.Dean P., Matthews E. Electrical activity in pancreatic islet cells. Nature. 1968;219(5152):389. doi: 10.1038/219389a0. [DOI] [PubMed] [Google Scholar]
- 71.Meda P., Orci L., Atwater I., Bangham A., Rojas E., Gonçalves A. The topography of electrical synchrony among β-cells in the mouse islet of langerhans. Quarterly Journal of Experimental Physiology. 1984;69(4):716–735. [PubMed] [Google Scholar]
- 72.Meda P., Chanson M., Pepper M., Giordano E., Bosco D., Traub O. In vivo modulation of connexin 43 gene expression and junctional coupling of pancreatic B-cells. Experimental Cell Research. 1991;192(2):469–480. doi: 10.1016/0014-4827(91)90066-4. [DOI] [PubMed] [Google Scholar]
- 73.Olsson R., Carlsson P.-O. A low-oxygenated subpopulation of pancreatic islets constitutes a functional reserve of endocrine cells. Diabetes. 2011;60(8):2068–2075. doi: 10.2337/db09-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ullsten S., Lau J., Carlsson P.O. Vascular heterogeneity between native rat pancreatic islets is responsible for differences in survival and revascularisation post transplantation. Diabetologia. 2015;58(1):132–139. doi: 10.1007/s00125-014-3385-7. [DOI] [PubMed] [Google Scholar]
- 75.Low J.T., Zavortink M., Mitchell J.M., Gan W.J., Do O.H., Schwiening C.J. Insulin secretion from beta cells in intact mouse islets is targeted towards the vasculature. Diabetologia. 2014;57(8):1655–1663. doi: 10.1007/s00125-014-3252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Michau A., Hodson D.J., Fontanaud P., Guillou A., Espinosa-Carrasco G., Molino F. Metabolism regulates exposure of pancreatic islets to circulating molecules in vivo. Diabetes. 2015;65(2):463–475. doi: 10.2337/db15-1168. [DOI] [PubMed] [Google Scholar]
- 77.Nyman L.R., Ford E., Powers A.C., Piston D.W. Glucose-dependent blood flow dynamics in murine pancreatic islets in vivo. American Journal of Physiology Endocrinology and Metabolism. 2010;298(4):E807–E814. doi: 10.1152/ajpendo.00715.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nyman L.R., Wells K.S., Head W.S., McCaughey M., Ford E., Brissova M. Real-time, multidimensional in vivo imaging used to investigate blood flow in mouse pancreatic islets. Journal of Clinical Investigation. 2008;118(11):3790–3797. doi: 10.1172/JCI36209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brereton M.F., Vergari E., Zhang Q., Clark A. Alpha-, Delta- and PP-cells: are they the architectural cornerstones of islet structure and co-ordination? Journal of Histochemistry and Cytochemistry. 2015;63(8):575–591. doi: 10.1369/0022155415583535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bosco D., Armanet M., Morel P., Niclauss N., Sgroi A., Muller Y.D. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59(5):1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cabrera O., Berman D.M., Kenyon N.S., Ricordi C., Berggren P.O., Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Band G.C., Jones C.T. Functional activation by glucagon of glucose 6-phosphatase and gluconeogenesis in the perfused liver of the fetal Guinea pig. FEBS Letters. 1980;119(1):190–194. doi: 10.1016/0014-5793(80)81028-3. [DOI] [PubMed] [Google Scholar]
- 83.Huising M.O., van der Meulen T., Huang J.L., Pourhosseinzadeh M.S., Noguchi G.M. The difference delta-cells make in glucose control. Physiology (Bethesda) 2018;33(6):403–411. doi: 10.1152/physiol.00029.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodriguez-Diaz R., Molano R.D., Weitz J.R., Abdulreda M.H., Berman D.M., Leibiger B. Paracrine interactions within the pancreatic islet determine the glycemic set point. Cell Metabolism. 2018;27(3):549–558. doi: 10.1016/j.cmet.2018.01.015. e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Samols E., Marri G., Marks V. Promotion of insulin secretion by glucagon. The Lancet. 1965;286(7409):415–416. doi: 10.1016/s0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- 86.MacDonald P.E., De Marinis Y.Z., Ramracheya R., Salehi A., Ma X., Johnson P.R. A KATP channel-dependent pathway within α cells regulates glucagon release from both rodent and human islets of Langerhans. Public Library of Science Biology. 2007;5(6):e143. doi: 10.1371/journal.pbio.0050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salehi A., Vieira E., Gylfe E. Paradoxical stimulation of glucagon secretion by high glucose concentrations. Diabetes. 2006;55(8):2318–2323. doi: 10.2337/db06-0080. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y.-J., Vieira E., Gylfe E. A store-operated mechanism determines the activity of the electrically excitable glucagon-secreting pancreatic α-cell. Cell Calcium. 2004;35(4):357–365. doi: 10.1016/j.ceca.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Rodriguez-Diaz R., Dando R., Jacques-Silva M.C., Fachado A., Molina J., Abdulreda M.H. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nature Medicine. 2011;17(7):888–892. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molina J., Rodriguez-Diaz R., Fachado A., Jacques-Silva M.C., Berggren P.-O., Caicedo A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes. 2014;63(8):2714–2726. doi: 10.2337/db13-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van der Meulen T., Donaldson C.J., Cáceres E., Hunter A.E., Cowing-Zitron C., Pound L.D. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nature Medicine. 2015;21:769–776. doi: 10.1038/nm.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hellman B., Salehi A., Grapengiesser E., Gylfe E. Isolated mouse islets respond to glucose with an initial peak of glucagon release followed by pulses of insulin and somatostatin in antisynchrony with glucagon. Biochemical and Biophysical Research Communications. 2012;417(4):1219–1223. doi: 10.1016/j.bbrc.2011.12.113. [DOI] [PubMed] [Google Scholar]
- 93.Narayanan S., Loganathan G., Dhanasekaran M., Tucker W., Patel A., Subhashree V. Intra-islet endothelial cell and β-cell crosstalk: implication for islet cell transplantation. World Journal of Transplantation. 2017;7(2):117. doi: 10.5500/wjt.v7.i2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cleaver O., Dor Y. Vascular instruction of pancreas development. Development. 2012;139(16):2833–2843. doi: 10.1242/dev.065953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Magenheim J., Ilovich O., Lazarus A., Klochendler A., Ziv O., Werman R. Blood vessels restrain pancreas branching, differentiation and growth. Development. 2011;138(21):4743–4752. doi: 10.1242/dev.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pepper A.R., Gala-Lopez B., Ziff O., Shapiro A.M. Revascularization of transplanted pancreatic islets and role of the transplantation site. Clinical and Developmental Immunology. 2013;2013:352315. doi: 10.1155/2013/352315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Del Toro-Arreola A., Robles-Murillo A.K., Daneri-Navarro A., Rivas-Carrillo J.D. The role of endothelial cells on islet function and revascularization after islet transplantation. Organogenesis. 2016;12(1):28–32. doi: 10.1080/15476278.2016.1165378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alvarez-Perez J.C., Ernst S., Demirci C., Casinelli G.P., Mellado-Gil J.M.D., Rausell-Palamos F. Hepatocyte growth factor/c-Met signaling is required for β-cell regeneration. Diabetes. 2014;63(1):216–223. doi: 10.2337/db13-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Demirci C., Ernst S., Alvarez-Perez J.C., Rosa T., Valle S., Shridhar V. Loss of HGF/c-Met signaling in pancreatic beta-cells leads to incomplete maternal beta-cell adaptation and gestational diabetes mellitus. Diabetes. 2012;61(5):1143–1152. doi: 10.2337/db11-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rivas-Carrillo J.D., Navarro-Alvarez N., Soto-Gutierrez A., Okitsu T., Chen Y., Tabata Y. Amelioration of diabetes in mice after single-donor islet transplantation using the controlled release of gelatinized FGF-2. Cell Transplantation. 2006;15(10):939–944. doi: 10.3727/000000006783981323. [DOI] [PubMed] [Google Scholar]
- 101.Arzouni A.A., Vargas-Seymour A., Rackham C.L., Dhadda P., Huang G.C., Choudhary P. Mesenchymal stromal cells improve human islet function through released products and extracellular matrix. Clinical Science. 2017;131(23):2835–2845. doi: 10.1042/CS20171251. [DOI] [PubMed] [Google Scholar]
- 102.Rackham C.L., Amisten S., Persaud S.J., King A.J.F., Jones P.M. Mesenchymal stromal cell secretory factors induce sustained improvements in islet function pre- and post-transplantation. Cytotherapy. 2018;20(12):1427–1436. doi: 10.1016/j.jcyt.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 103.Rackham C.L., Dhadda P.K., Le Lay A.M., King A.J., Jones P.M. Preculturing islets with adipose-derived mesenchymal stromal cells Is an effective strategy for improving transplantation efficiency at the clinically preferred intraportal site. Cell Medicine. 2014;7(1):37–47. doi: 10.3727/215517914X680047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jalili R.B., Moeen Rezakhanlou A., Hosseini-Tabatabaei A., Ao Z., Warnock G.L., Ghahary A. Fibroblast populated collagen matrix promotes islet survival and reduces the number of islets required for diabetes reversal. Journal of Cellular Physiology. 2011;226(7):1813–1819. doi: 10.1002/jcp.22515. [DOI] [PubMed] [Google Scholar]
- 105.Pan X., Xue W., Li Y., Feng X., Tian X., Ding C. Islet graft survival and function: concomitant culture and transplantation with vascular endothelial cells in diabetic rats. Transplantation. 2011;92(11):1208–1214. doi: 10.1097/TP.0b013e3182356ca7. [DOI] [PubMed] [Google Scholar]
- 106.Stendahl J.C., Kaufman D.B., Stupp S.I. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplantation. 2009;18(1):1–12. doi: 10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang R., Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. Journal of Endocrinology. 1999;163(2):181–190. doi: 10.1677/joe.0.1630181. [DOI] [PubMed] [Google Scholar]
- 108.Bosco D., Meda P., Halban P.A., Rouiller D.G. Importance of cell-matrix interactions in rat islet beta-cell secretion in vitro: role of alpha6beta1 integrin. Diabetes. 2000;49(2):233–243. doi: 10.2337/diabetes.49.2.233. [DOI] [PubMed] [Google Scholar]
- 109.Beattie G.M., Montgomery A.M., Lopez A.D., Hao E., Perez B., Just M.L. A novel approach to increase human islet cell mass while preserving β-cell function. Diabetes. 2002;51(12):3435–3439. doi: 10.2337/diabetes.51.12.3435. [DOI] [PubMed] [Google Scholar]
- 110.Nagata N., Gu Y., Hori H., Balamurugan A., Touma M., Kawakami Y. Evaluation of insulin secretion of isolated rat islets cultured in extracellular matrix. Cell Transplantation. 2001;10:447–451. [PubMed] [Google Scholar]
- 111.Zhang Y., Jalili R.B., Warnock G.L., Ao Z., Marzban L., Ghahary A. Three-dimensional scaffolds reduce islet amyloid formation and enhance survival and function of cultured human islets. American Journal Of Pathology. 2012;181(4):1296–1305. doi: 10.1016/j.ajpath.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 112.Kaido T., Yebra M., Cirulli V., Montgomery A.M. Regulation of human beta-cell adhesion, motility, and insulin secretion by collagen IV and its receptor alpha1beta1. Journal of Biological Chemistry. 2004;279(51):53762–53769. doi: 10.1074/jbc.M411202200. [DOI] [PubMed] [Google Scholar]
- 113.Diaferia G.R., Jimenez-Caliani A.J., Ranjitkar P., Yang W., Hardiman G., Rhodes C.J. beta1 integrin is a crucial regulator of pancreatic beta-cell expansion. Development. 2013;140(16):3360–3372. doi: 10.1242/dev.098533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Daoud J., Petropavlovskaia M., Rosenberg L., Tabrizian M. The effect of extracellular matrix components on the preservation of human islet function in vitro. Biomaterials. 2010;31(7):1676–1682. doi: 10.1016/j.biomaterials.2009.11.057. [DOI] [PubMed] [Google Scholar]
- 115.Irving-Rodgers H.F., Ziolkowski A.F., Parish C.R., Sado Y., Ninomiya Y., Simeonovic C.J. Molecular composition of the peri-islet basement membrane in NOD mice: a barrier against destructive insulitis. Diabetologia. 2008;51(9):1680–1688. doi: 10.1007/s00125-008-1085-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bogdani M., Korpos E., Simeonovic C.J., Parish C.R., Sorokin L., Wight T.N. Extracellular matrix components in the pathogenesis of type 1 diabetes. Current Diabetes Reports. 2014;14(12):552. doi: 10.1007/s11892-014-0552-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ahn Y.B., Xu G., Marselli L., Toschi E., Sharma A., Bonner-Weir S. Changes in gene expression in beta cells after islet isolation and transplantation using laser-capture microdissection. Diabetologia. 2007;50(2):334–342. doi: 10.1007/s00125-006-0536-5. [DOI] [PubMed] [Google Scholar]
- 118.Sackett S.D., Tremmel D.M., Ma F., Feeney A.K., Maguire R.M., Brown M.E. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Scientific Reports. 2018;8(1):10452. doi: 10.1038/s41598-018-28857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Balboa D., Saarimaki-Vire J., Otonkoski T. Concise review: human pluripotent stem cells for the modeling of pancreatic beta-cell pathology. Stem Cells. 2019;37(1):33–41. doi: 10.1002/stem.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]