Abstract
Background
Progression from pre-diabetes to type 2 diabetes (T2D) and from T2D to insulin requirement proceeds at very heterogenous rates among patient populations, and the risk of developing different types of secondary complications is also different between patients. The diagnosis of pre-diabetes and T2D solely based on blood glucose measurements cannot capture this heterogeneity, thereby preventing proposition of therapeutic strategies adapted to individual needs and pathogenetic mechanisms. There is, thus, a need to identify novel means to stratify patient populations based on a molecular knowledge of the diverse underlying causes of the disease. Such knowledge would form the basis for a precision medicine approach to preventing and treating T2D according to the need of identified patient subgroups as well as allowing better follow up of pharmacological treatment.
Scope of review
Here, we review a systems biology approach that aims at identifying novel biomarkers for T2D susceptibility and identifying novel beta-cell and insulin target tissue genes that link the selected plasma biomarkers with insulin secretion and insulin action. This work was performed as part of two Innovative Medicine Initiative projects. The focus of the review will be on the use of preclinical models to find biomarker candidates for T2D prediction and novel regulators of beta-cell function. We will demonstrate that the study of mice with different genetic architecture and widely different adaptation to metabolic stress can be a powerful approach to identify biomarkers of T2D susceptibility in humans or for the identification of so far unrecognized genes controlling beta-cell function.
Major conclusions
The examples developed in this review will highlight the power of the systems biology approach, in particular as it allowed the discovery of dihydroceramide as a T2D biomarker candidate in mice and humans and the identification and characterization of novel regulators of beta-cell function.
Keywords: Type 2 diabetes, Biomarkers, Pancreatic islets, Beta-cells, Sphingolipids, Ceramides, Elongase, Insulin secretion
1. Introduction
A major challenge in the prevention and management of type 2 diabetes (T2D) is the poorly understood heterogeneity of the causes of the disease and of their secondary complications. It is well established that progression from a healthy state to pre-diabetes and to T2D differs in kinetics among individuals. For instance, deterioration of T2D, i.e., time to insulin requirement, proceeds with widely different rates between patients, as measured, for instance, by the rate of increase in HbA1C [1]. In addition, it is so far not possible to determine the risk of progression to, and deterioration of T2D of any individuals. Over recent years, genetic studies have led to the identification of several hundred genetic loci associated with increased susceptibility to T2D development [2] but taken globally, they only have a marginal role in predicting the development of T2D and are not sufficient for personalized prevention or therapeutic strategies [3], [4] although genetic studies have identified susceptibility loci for the development of secondary complications, such as diabetes kidney diseases [5]. Recent evidence has, nevertheless, been obtained that T2D patients can be stratified according to diabetes characteristics and risk of complications [6]. When six parameters were considered (age at diagnosis, body mass index (BMI), HbA1C, glutamic acid decarboxylase antibodies, homeostatic model assessment of insulin resistance (HOMA-IR) or of insulin secretion (HOMA-B)) cluster analysis revealed the possibility of identifying five T2D patient subgroups with markedly different combinations of diabetic characteristics and risk of developing kidney disease. This study thus supports the need to identify additional biomarkers to improve prediabetes and T2D stratification.
Established T2D is characterized by insulin resistance of liver, fat and muscle, and by insufficient insulin secretion by pancreatic beta-cells to counteract the insulin resistance of target tissues. A recurrent question, discussed now for decades, is whether T2D is initiated by primary defects in insulin sensitivity or in insulin secretion. This question now appears mundane. Indeed, it is clear now that glucose homeostasis is regulated by the balance between insulin secretion and insulin action [7] and that reduced insulin secretion leads to increased insulin sensitivity, and vice-versa, with the ultimate goal for the system to maintain normoglycemia. Viewed in this way, it appears obvious that any physiological defects, which can impair the insulin secretion/insulin action balance, can lead to T2D. From human genetic studies, in particular monogenic forms of diabetes [8], as well as from countless mouse models with tissue-specific inactivation of genes involved in differentiation, metabolic, or signaling pathways, it is recognized that prediabetes and T2D can originate from mutations in genes expressed exclusively in insulin target tissues, in beta-cells, but also in the hypothalamus or other brain areas [9]. The original dysfunctional cells then propagate metabolic alterations to other tissues, through metabolic or inflammatory signals, to eventually induce diabetic hyperglycemia. Therefore, it becomes obvious that the current diabetes therapies, which aim at increasing insulin secretion, at restoring insulin action, or at triggering renal glucose excretion, in most instances only address the symptoms rather than the causes of the disease. There is thus a need to get more mechanistic information about the diverse forms of T2D to improve patient's stratification and potentially deliver “the right treatment to the right patient at the right time”.
Over the recent years, as part of the European Innovative Medicine Initiative projects IMIDIA (https://www.imidia.org/) and RHAPSODY (https://imi-rhapsody.eu/), we applied different Systems Biology approaches to identify circulating biomarkers of pre-diabetes progression and of T2D deterioration. The overall idea is derived from a prediction made close to fifty years ago by Linus Pauling, who stated that “Information about the genetic nature of an individual human being, (…), could be obtained by the thorough quantitative analysis of body fluids. Moreover, the thorough quantitative analysis of body fluids might permit differential diagnosis of many diseases in a more effective way than is possible at the present time.” [10]. We thus postulated that biomarkers identified from extensive plasma metabolomic, lipidomic, and peptidomic analysis could help identify individuals at risk of developing T2D. Furthermore, we postulated that, combined with pancreatic islet and insulin target tissue transcriptomic as well as patient clinical information, such biomarkers could also inform us on defects in insulin secretion and insulin action. This combined information could then be used for better diagnostics, drug therapy, and treatment monitoring as well as for more refined clinical trials. These projects involve investigations in humans and the study of preclinical models and the translation of animal studies to human T2D. On the human side, several pre-diabetes and T2D diabetes cohorts have been harmonized to generate a federated database that can be interrogated and analyzed as a single large cohort, containing ∼50000 patients, with extensive clinical, genetic and plasma metabolomic data. This is complemented by the continuous development of a very large human islet biobank with extensive functional, genetic and transcriptomic data [11], [12]. This global resource provides exceptional capacity for novel discoveries in the pathogenesis of T2D and for patient stratification based on clinical, genetic and omics data.
The mouse studies were initiated with the goal of performing complementary studies on the pathogenesis of pre-diabetes and T2D. The major aims are to find plasma biomarkers predictive of i) the susceptibility to develop T2D, ii) of beta-cell dysfunction, iii) of insulin resistance in liver, adipose tissue or muscle. In addition, we aim at identifying whether these biomarkers are not only signatures of deregulated insulin secretion or action but also whether they can cause these defects and whether we can identify the tissues and metabolic pathways that produce them; such pathways could then become new therapeutic targets.
In the present review, we will first, illustrate the mouse studies that we have performed to identify novel biomarkers of diabetes susceptibility and validate them in humans [13] and, second, how this approach led us to uncover the role of a lipid modifying enzyme in protecting beta-cells against glucolipotoxicity [14], [15].
2. Heterogenous metabolic adaptation of inbred mice to a high fat, high sucrose diet
Mice fed a high fat, high sucrose (HFHS) diet develop insulin resistance and obesity, and progressively increase their fasting glycemia and insulinemia. These are characteristic parameters of pre-diabetes, which develop at different rates and extents in various inbred strains of mice [16], [17], [18], [19], [20], [21]. Here, we first characterized the adaptation to HFHS of six inbred strains of mice (C57Bl/6J, AKR/J, Balb/cJ, DBA/2J, 129S2/SvPas, A/J). We measured body weight, basal insulinemia, oral glucose tolerance tests and oral glucose-stimulated insulin secretion, fasting glycemia and intraperitoneal insulin tolerance tests, as well as pancreatic islet alpha and beta-cell areas. These phenotypic measurements were performed at day 2, 10, 30 and 90 after initiation of HFHS feeding of 8 week-old male mice; mice fed a regular chow for the same periods of time were used as controls. Pancreatic islets were prepared from mice from each strain, at each time point, and in the two feeding conditions; islet transcriptome was then characterized by RNA sequencing (RNASEq) analysis. Plasma from the same mice were also collected for quantitative measurements of glycerophospholipids, sphingolipids, glycerolipids, free cholesterol and cholesteryl esters totaling ∼135 lipid species. Figure 1 shows that these six strains of mice display marked differences in body weight gain, glucose tolerance, and basal and stimulated insulinemia. For instance, DBA/2J mice showed the fastest and highest body weight increase whereas C57Bl/6J mice showed only moderate body weight gain. HFHS diet induced a very strong glucose intolerance in Balb/cJ mice with no change in basal insulinemia, whereas DBA/2J mice displayed lower increase in glucose intolerance with a strong up-regulation of basal insulinemia. Thus, these different mouse strains show very different interactions between nutrition and genetic background in their susceptibility to develop glucose homeostasis dysregulations with diverse insulin secretion and insulin resistance defects. We therefore took advantage of this phenotypic diversity to: 1) search for plasma lipids that correlate with specific diabetic parameters across strains and conditions as an approach to identify biomarkers of diabetes susceptibility; 2) search for islet gene expression modules (groups of genes that behave in a coordinate manner) and specific genes that correlate with glycemia and insulin secretion to identify novel regulators of beta-cell function.
Figure 1.
Impact of HFD and age on metabolic parameters. Mice of the indicated strains were fed a regular chow (RC) or a high fat high sucrose diet (HFHS) for the indicated periods of time. They were then phenotyped as detailed in [14]. Boxplots show differences between HFHS (yellow) and RC (green) diet in the 6 mouse strains over time for (A) Body weight (g), (B) AUC glycemia measured during the glucose tolerance test (OGTT), (C) Basal insulinemia (ng/ml) measured at the start of the OGTT, and (D) Stimulated Insulinemia (ng/ml) measured at 15 min following glucose administration. Statistical significance between HFHS and RC at each time-point was measured using the two-sided Student's t-test and p-values were corrected for multiple comparisons using the Benjamini Hochberg FDR method. Statistically significant comparisons following FDR correction (FDR 0.05) are indicated by a double asterisk. Marginally significant comparisons (raw p-value 0.05) are indicated by a single asterisk. Figure reproduced from [14].
Highlight: Mice with different genetic architectures display highly divergent adaptation to the same metabolic stress. They are therefore useful models to investigate plasma biomarkers predictive of, and gene pathways causing susceptibility to, type 2 diabetes development.
3. Identification of dihydrocyeramides as diabetes susceptibility biomarkers
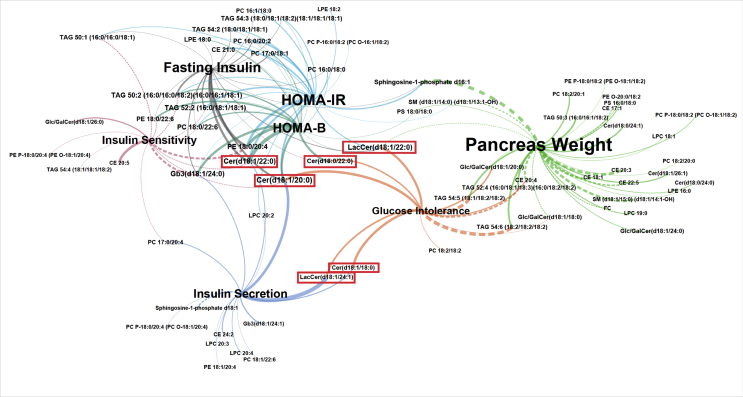
Quantitative plasma lipidomic data obtained from the mice described in Figure 1 revealed that the lipid profiles were influenced by diet but also by the mouse genetic background [13]. Correlation of lipids with phenotypic traits yielded the network shown in Figure 2. This analysis revealed interesting correlation of three ceramides (Cer(d18:1), one dihydroceramide (Cer(d18:0), and two lactosyl ceramides (LacCer(d18:1)) with glucose intolerance and insulin secretion. The evolution of the concentrations of these lipid species over time with HFHS feeding was dependent on the mouse genetic strains, with most plasma lipid concentrations usually increasing with time in all mouse strain. One exception was the Balb/cJ mice which showed the highest initial plasma ceramide (Cer(d18:1; 22:0)) concentration. As this mouse strain is the most sensitive to HFHS -induced diabetes (Figure 1), this suggests that the plasma level of this ceramide may indeed by associated with increased susceptibility to diabetes development. Overall, these mouse studies showed the highest correlation among the measured lipid species with pre-diabetes development in mice.
Figure 2.
Ceramides are correlated to glucose intolerance and insulin sensitivity in metabolically challenged mouse strains. Lipid-trait network showing plasma lipid correlations with five measured phenotypic traits. Correlations are represented as edges between lipid nodes and trait nodes. Only correlations with absolute value R > 0.4 are shown. Each trait node is depicted as a different color, and edges are colored according to the correlated trait. Edge width is proportional to correlation strength. Solid edge lines indicate positive correlations; dashed lines indicate negative correlations. Node label size is proportional to degree (total number of connections). Ceramide lipids that were chosen for further investigation based on their correlations to several mouse traits are boxed in red. Figure reproduced from [13].
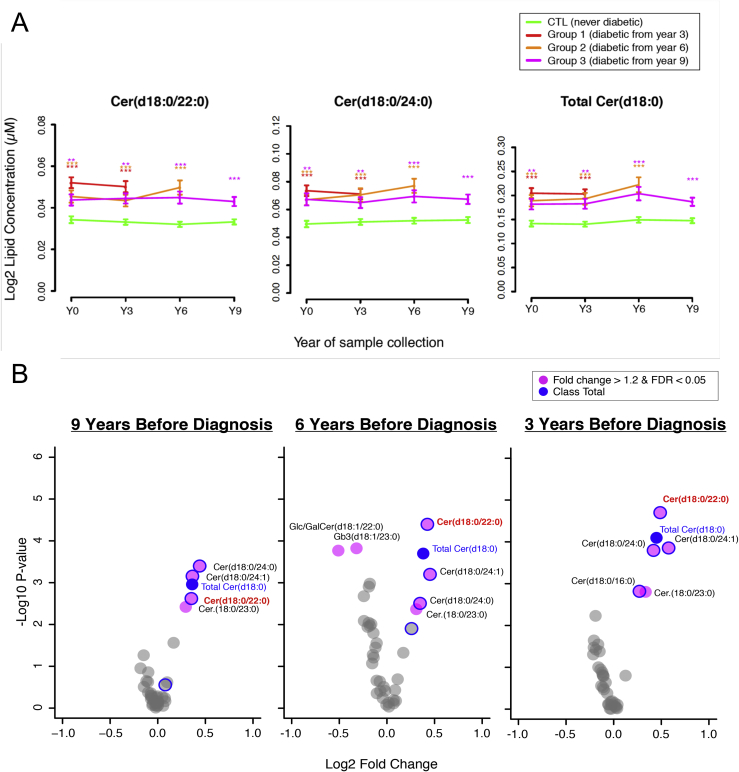
Testing the translatability of this observation to humans was made possible by the availability plasma samples collected from population-based human cohorts, which have been followed for several years and which have a large number of incident diabetes case. One cohort is DESIR [22], which included volunteers between 30 and 65 years who were healthy at the time of recruitment, and who have been followed on three occasions at 3 years intervals. Between 50 and 80 incident cases have been diagnosed at each of year 3, 6, and 9 after recruitment. The second replication cohort was CoLaus [23], a population-based cohort of approximately 6000 individuals followed for 5 years, at which time approximately 120 incident cases had been diagnosed. Plasma from an equivalent number of incident cases and individuals that remained diabetes-free for the period of observations were analyzed by targeted sphingolipid analysis. Figure 3 shows the results obtained with the DESIR cohort. All individuals who developed T2D at either year 3, 6 or 9, had levels of dihydroceramides that were significantly higher as those of the control individuals from the time of recruitment. Of note, these levels remained stable over time, suggesting that plasma dihydroceramides are not elevated secondary to glucose homeostasis deregulations but may precede them. The exact species that are significantly increased in prospective cases are shown in the volcano plots of Figure 3B. The same increase in plasma dihydroceramides was observed in prospective cases of the CoLaus study [13]. Statistical analysis of these data showed that these dihydroceramides are associated with increased risk of future diabetes, even when the data are corrected for age, sex, BMI or fasting glucose. Thus, dihydroceramides are potential biomarker candidates for diabetes susceptibility.
Figure 3.
Mean lipid concentration of dihydroceramides are significantly elevated at all time points in the DESIR Study. (A) Mean plasma concentrations of dihydroceramides plotted over time. The left two plots show the individual lipid species Cer(d18:0/22:0) and Cer(d18:0/24:0). The rightmost plot represents the class for total Cer(d18:0) species. The group means are consistently higher in diabetes cases as compared to control samples. x axis: time point of collection. y axis: mean lipid concentration in each of the groups. Error bars: SEM of the lipid concentration. Asterisks at each time point represent significance of the statistical test comparing cases to controls (age- and sex-corrected linear model): *adjusted p < 0.05, **adjusted p < 0.01, ***adjusted p < 0.001 (p values adjusted for multiple correction across 37 lipids by the Benjamini-Hochberg method). (B) Volcano plots of statistical tests comparing 37 lipid species in each group of diabetic subjects versus the control samples from the same sample collection period (linear model, containing factors for sex and age). The plots shown are from DESIR group of individuals tested 9, 6, and 3 years before diabetes diagnostic. Figure reproduced from [13].
Previous studies have linked increased circulating ceramide levels with various metabolic diseases, with a negative impact on beta-cells, adipose tissue, and heart [24], [25], [26], [27], [28], [29], [30]. Dihydroceramides, which are produced at the third step of the de novo ceramide synthesis pathway before being converted to ceramides by ceramide desaturases (Des1 or Des2) [27], have been considered as inactive sphingolipid species. However, more recently they have been shown to have potential negative impact on cellular viability by regulating autophagy, reactive oxygen production, cell proliferation and apoptosis [31], [32], [33], [34]. Mutations in Des1 leads to cellular accumulation of dihydroceramides and, in humans, cause hypomyelinating leukodystrophy [35]. Dihydroceramides have also been associated with reduced insulin sensitivity [36], obesity [37] and other metabolic diseases [37], [38], [39]. Clearly, more needs to be learned about the regulation of dihydroceramides, and whether their increased plasma concentrations lead to specific cellular alterations that may drive deregulated glucose homeostasis.
The above data also demonstrate that exploiting the genetic variability of different inbred strains of mice and their differential response to metabolic stress can be used for the identification of circulating biomarkers that are also relevant to predict disease susceptibility in humans. The interest of using animal models is the possibility to experimentally test whether the identified plasma biomarkers correlate with, and possibly cause, specific gene expression deregulations in pancreatic islets or in insulin target tissues. If this can be achieved, determining which tissue produces such biomarkers and which enzymatic step(s) is/are involved in generating them becomes possible. These enzymes could then form novel targets to prevent or treat diabetes.
Highlight: Increased plasma dihydroceramides have been identified in preclinical studies to be associated with prediabetes development. In humans, they have been found to be elevated compared to healthy individuals up to nine years before T2D diagnosis. Dihydroceramides are T2D susceptibility biomarkers.
4. Elovl2 as a novel regulator of glucose-stimulated insulin secretion
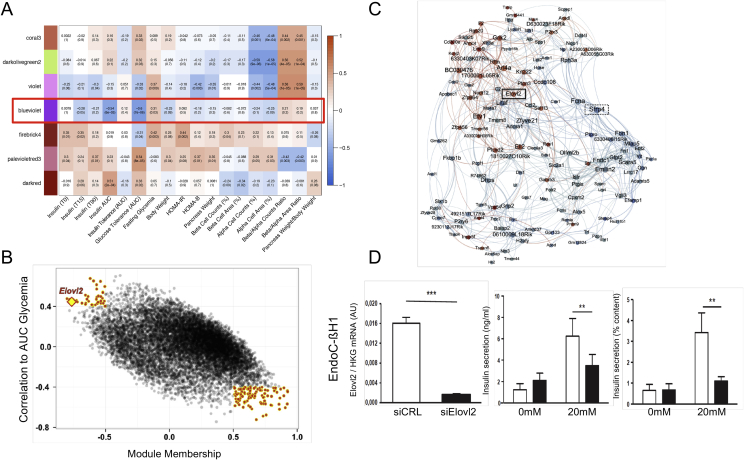
Pancreatic islets were isolated from the mouse strains and feeding conditions mentioned above (Figure 1) and their RNA was extracted for transcript profiling. Weighted correlation gene network analysis was then performed [40] to identify groups of islet genes (gene expression modules) that correlated with the measured phenotypes [14]. Figure 4A shows that gene modules were identified that showed various positive and negative correlations with the phenotypes. The gene modules are labeled with arbitrarily chosen color names. We focused our analysis on the blue-violet module, which shows a strong negative correlation with insulin secretion (insulin area under the curve (AUC)) and glucose intolerance (AUC glycemia in an oral glucose tolerance test). Figure 4B shows a scatter plot of all genes of the blue-violet module distributed according to their correlation with AUC glycemia and module membership (correlation to the module eigenegenes). Genes with the strongest correlations to both the module (Spearman's |R| ≥ 0.5) and to AUC glycemia (Spearman's |R| ≥ 0.4) were then used to generate a network of genes related to glucose tolerance (Figure 4C). Two prominent, highly connected genes in this network were Sfrp4, negatively correlated with AUC glycemia, and Elovl2 (elongase of very long chain fatty acids 2) which was positively correlated with glucose intolerance. Regarding insulin secretion in response to glucose, Sfrp4 was negatively correlated to the AUC insulinemia whereas Elovl2 was positively correlated. This finding is in line with previous findings showing that Sfrp4 is a negative regulator of insulin secretion [41]. Elovl2 is involved in the elongation of n-3 fatty acids and leads to increased production of docosahexaenoic acid (DHA) [42]. Silencing Elovl2 expression in mouse and human insulin cell lines markedly reduced glucose-stimulated insulin secretion (Figure 4D). Elovl2 also protected beta-cells against glucolipotoxicity-induced apoptosis and the loss of protection induced by Elovl2 silencing could be rescued by addition of DHA in rodent and human beta cells [15]. Interestingly, the inhibition of beta cell apoptosis by the Elovl2/DHA axis was associated with a decrease in ceramide content. This effect was linked to an increase in palmitate oxidation, as demonstrated by its attenuation by inhibition of carnitine palmitoyltransferase 1, the rate-limiting enzyme in fatty acid β-oxidation [15]. Finally, these results formally identified Elovl2 as a critical pro-survival enzyme for preventing beta cell death and dysfunction induced by glucolipotoxicity.
Figure 4.
A gene co-expression module correlated to insulin secretion and oral glucose intolerance. (A) Heat map showing correlations between module eigengenes and mouse phenotypic traits: darker colors indicate higher Spearman correlation. The red box indicates the correlations corresponding to the blue-violet module. (B) Scatter plot of AUC glycemia correlation against module membership (correlation to module) for all genes of the blue-violet module. Genes with the strongest correlations to both the module and to AUC glycemia are highlighted by red points. Elovl2 is indicated by a yellow diamond. (C) Network generated from the selected module genes. Node size is proportional to degree and node color indicates correlation to AUC glycemia (blue: negative correlation; red: positive correlation). Edges (connections) between nodes indicate correlation between genes (blue: negative; red: positive). Elovl2 and Sfrp4 are indicated in the network. (D) Effects of Elovl2 loss of function on glucose-stimulated insulin secretion in the human EndoC-βH1 cell line. Left: Elovl2 mRNA silencing; middle: insulin secretion expressing in ng/ml; right: insulin secretion as % of content. Values are mean (±SE) of three independent experiments. ***p < 0.001; **p < 0.01; *p < 0.05. Panels reproduced from [13].
5. Conclusions
The studies described above support the hypothesis that comparing the adaptation to metabolic stress, over several early time points, of multiple mouse lines with different genetic architectures is a powerful approach to identifying circulating biomarkers of diabetes susceptibility and genes whose deregulation may cause impaired insulin secretion or insulin action. In addition, we showed that this information can be translated to humans. This was shown for the dihydroceramides and their potential role as biomarkers for susceptibility to T2D. The role of Elovl2, which is required for the production of DHA, has now been well established, and silencing this gene in both mouse and human insulin cells leads to reduced glucose-stimulated insulin and increased susceptibility to glucolipotoxicity-induced apoptosis. Therefore, these animal studies are complementary to the study of human cohorts where clinical, genetic, and omics data can be combined to improve T2D patient stratification [6]. These complementary approaches have the potential to establish better treatment options for patient subgroups. An additional advantage of the mouse studies is the possibility to test whether the identified plasma biomarkers have not only predictive value on disease progression but also a have a direct impact on either beta-cell function or insulin action on liver, adipose tissue or muscle. If such direct effects of biomarkers on cellular function can be evidenced, then identification of the tissue and metabolic pathway that generate such biomarkers can provide new therapeutic targets. Furthermore, identification of specific metabolic, differentiation, or signaling pathways in beta-cells or insulin target tissues in mice, which when deregulated can lead to T2D, may be confirmed to be relevant to human diabetes using the wealth of clinical, genetic, and omics data available through the mentioned IMI2 project. This should lead to refinement of patient stratification according to risk of disease development but also to the underlying molecular pathogenic mechanisms. The hope is to develop improved precision medicine for T2D.
Acknowledgements
This project has received funding from the Innovative Medicines Initiative and Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 155005 (IMIDIA), No 115881 (RHAPSODY) and No 115797 (INNODIA). These Joint Undertakings received support from the European Union's FP7, Horizon 2020 research and innovation programs, EFPIA, JDRF and ‘The Leona M. and Harry B. Helmsley Charitable Trust'. RHAPSODY is also supported by the Swiss State Secretariat for Education’ Research and Innovation (SERI) under contract number 16.0097. B.T is supported by the Swiss National Science Foundation 310030_182496 and an ERC advanced grant (INTEGRATE). This project was also funding from the Agence Nationale de la Recherche and the Swiss National Science Foundation under a lead Agency program(ANR PRCI-15-CE14-0027-01 BetaDiamark).
Funding
This article is part of a supplement entitled ‘Biomarkers of Beta-Cell Health and Dysfunction: Towards Personalised Diabetes Care. Proceedings of the 20th Servier-IGIS Symposium’. The Symposium and the publication of the Supplement have been made possible by an unrestricted educational grant from Institut la Conférence Hippocrate – Servier Group.
Conflict of interest
None declared.
References
- 1.Donnelly L., Zhou K., Doney A., Jennison C., Franks P., Pearson E. Rates of glycaemic deterioration in a real-world population with type 2 diabetes. Diabetologia. 2018;61(3):607–615. doi: 10.1007/s00125-017-4519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahajan A., Taliun D., Thurner M., Robertson N.R., Torres J.M., Rayner N.W. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nature Genetics. 2018;50(11):1505–1513. doi: 10.1038/s41588-018-0241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grotz A.K., Gloyn A.L., Thomsen S.K. Prioritising causal genes at type 2 diabetes risk loci. Current Diabetes Report. 2017;17(9):76. doi: 10.1007/s11892-017-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kodama S., Fujihara K., Ishiguro H., Horikawa C., Ohara N., Yachi Y. Quantitative relationship between cumulative risk alleles based on genome-wide association studies and type 2 diabetes mellitus: a systematic review and meta-analysis. Journal of Epidemiology. 2018;28(1):3–18. doi: 10.2188/jea.JE20160151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li M., Pezzolesi M.G. Advances in understanding the genetic basis of diabetic kidney disease. Acta Diabetologia. 2018;55(11):1093–1104. doi: 10.1007/s00592-018-1193-0. [DOI] [PubMed] [Google Scholar]
- 6.Ahlqvist E., Storm P., Karajamaki A., Martinell M., Dorkhan M., Carlsson A. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinology. 2018;6(5):361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 7.Kahn S.E., Prigeon R.L., McCulloch D.K., Boyko E.J., Bergman R.N., Schwartz M.W. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42(11):1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 8.Bonnefond A., Froguel P. Rare and common genetic events in type 2 diabetes: what should biologists know? Cell Metabolism. 2015;21(3):357–368. doi: 10.1016/j.cmet.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 9.da Silva Xavier G., Hodson D.J. Mouse models of peripheral metabolic disease. Best Practice in Research and Clinical Endocrinology and Metabolism. 2018;32(3):299–315. doi: 10.1016/j.beem.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Pauling L., Robinson A.B., Teranishi R., Cary P. Quantitative analysis of urine vapor and breath by gas-liquid partition chromatography. Proceedings of the National Academy of Science United States of America. 1971;68(10):2374–2376. doi: 10.1073/pnas.68.10.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khamis A., Canouil M., Siddiq A., Crouch H., Falchi M., Bulow M.V. Laser capture microdissection of human pancreatic islets reveals novel eQTLs associated with type 2 diabetes. Molecular Metabolism. 2019;18(19):30130–30139. doi: 10.1016/j.molmet.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solimena M., Schulte A.M., Marselli L., Ehehalt F., Richter D., Kleeberg M. Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes. Diabetologia. 2018;61(3):641–657. doi: 10.1007/s00125-017-4500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wigger L., Cruciani-Guglielmacci C., Nicolas A., Denom J., Fernandez N., Fumeron F. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Reports. 2017;18(9):2269–2279. doi: 10.1016/j.celrep.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Cruciani-Guglielmacci C., Bellini L., Denom J., Oshima M., Fernandez N., Normandie-Levi P. Molecular phenotyping of multiple mouse strains under metabolic challenge uncovers a role for Elovl2 in glucose-induced insulin secretion. Molecular Metabolism. 2017;6(4):340–351. doi: 10.1016/j.molmet.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellini L., Campana M., Rouch C., Chacinska M., Bugliani M., Meneyrol K. Protective role of the ELOVL2/docosahexaenoic acid axis in glucolipotoxicity-induced apoptosis in rodent beta cells and human islets. Diabetologia. 2018;61(8):1780–1793. doi: 10.1007/s00125-018-4629-8. [DOI] [PubMed] [Google Scholar]
- 16.Surwit R.S., Feinglos M.N., Rodin J., Sutherland A., Petro A.E., Opara E.C. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44(5):645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 17.Surwit R.S., Kuhn C.M., Cochrane C., McCubbin J.A., Feinglos M.N. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988:371163–371167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 18.Andrikopoulos S., Massa C.M., Aston-Mourney K., Funkat A., Fam B.C., Hull R.L. Differential effect of inbred mouse strain (C57BL/6, DBA/2, 129T2) on insulin secretory function in response to a high fat diet. Journal of Endocrinology. 2005;187(1):45–53. doi: 10.1677/joe.1.06333. [DOI] [PubMed] [Google Scholar]
- 19.Burcelin R., Crivelli V., DaCosta A., Roy-Tirelli A., Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. American Journal of Physiology. 2002 doi: 10.1152/ajpendo.00332.2001. 282E834–E842. [DOI] [PubMed] [Google Scholar]
- 20.Watson P.M., Commins S.P., Beiler R.J., Hatcher H.C., Gettys T.W. Differential regulation of leptin expression and function in A/J vs. C57BL/6J mice during diet-induced obesity. American Journal of Physiology, Endocrinology and Metabolism. 2000;279(2):E356–E365. doi: 10.1152/ajpendo.2000.279.2.E356. [DOI] [PubMed] [Google Scholar]
- 21.Goren H.J., Kulkarni R.N., Kahn C.R. Glucose homeostasis and tissue transcript content of insulin signaling intermediates in four inbred strains of mice: C57BL/6, C57BLKS/6, DBA/2, and 129X1. Endocrinology. 2004;145(7):3307–3323. doi: 10.1210/en.2003-1400. [DOI] [PubMed] [Google Scholar]
- 22.Balkau B., Lange C., Fezeu L., Tichet J., de Lauzon-Guillain B., Czernichow S. Predicting diabetes: clinical, biological, and genetic approaches: data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Diabetes Care. 2008;31(10):2056–2061. doi: 10.2337/dc08-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firmann M., Mayor V., Vidal P.M., Bochud M., Pecoud A., Hayoz D. The CoLaus study: a population-based study to investigate the epidemiology and genetic determinants of cardiovascular risk factors and metabolic syndrome. BioMed Central Cardiovascular Disorders. 2008;8:6. doi: 10.1186/1471-2261-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergman B.C., Brozinick J.T., Strauss A., Bacon S., Kerege A., Bui H.H. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. American Journal of Physiology, Endocrinology and Metabolism. 2015;309(4):E398–E408. doi: 10.1152/ajpendo.00134.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bikman B.T., Summers S.A. Ceramides as modulators of cellular and whole-body metabolism. Journal of Clinical Investigation. 2011;121(11):4222–4230. doi: 10.1172/JCI57144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brozinick J.T., Hawkins E., Hoang Bui H., Kuo M.S., Tan B., Kievit P. Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet. International Journal of Obesity. 2013;37(8):1064–1070. doi: 10.1038/ijo.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaurasia B., Summers S.A. Ceramides - lipotoxic inducers of metabolic disorders. Trends in Endocrinology and Metabolism. 2015;26(10):538–550. doi: 10.1016/j.tem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Long S.D., Pekala P.H. Lipid mediators of insulin resistance: ceramide signalling down-regulates GLUT4 gene transcription in 3T3-L1 adipocytes. Biochememical Journal. 1996;31:9179–9184. doi: 10.1042/bj3190179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez X., Goldfine A.B., Holland W.L., Gordillo R., Scherer P.E. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. Journal of Pediatric Endocrinology & Metabolism. 2013;26(9–10):995–998. doi: 10.1515/jpem-2012-0407. [DOI] [PubMed] [Google Scholar]
- 30.Park T.S., Hu Y., Noh H.L., Drosatos K., Okajima K., Buchanan J. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. Journal of Lipid Research. 2008;49(10):2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Cuenca S., Barbarroja N., Vidal-Puig A. Dihydroceramide desaturase 1, the gatekeeper of ceramide induced lipotoxicity. Biochimica Biophysica Acta. 2015;1851(1):40–50. doi: 10.1016/j.bbalip.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 32.Siddique M.M., Li Y., Chaurasia B., Kaddai V.A., Summers S.A. Dihydroceramides: from bit players to lead actors. Journal of Biological Chemistry. 2015;290(25):15371–15379. doi: 10.1074/jbc.R115.653204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee A.Y., Lee J.W., Kim J.E., Mock H.J., Park S., Kim S. Dihydroceramide is a key metabolite that regulates autophagy and promotes fibrosis in hepatic steatosis model. Biochemical Biophysical Research Communications. 2017;494(3–4):460–469. doi: 10.1016/j.bbrc.2017.10.110. [DOI] [PubMed] [Google Scholar]
- 34.Barbarroja N., Rodriguez-Cuenca S., Nygren H., Camargo A., Pirraco A., Relat J. Increased dihydroceramide/ceramide ratio mediated by defective expression of degs1 impairs adipocyte differentiation and function. Diabetes. 2015;64(4):1180–1192. doi: 10.2337/db14-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pant D.C., Dorboz I., Schluter A., Fourcade S., Launay N., Joya J. Loss of the sphingolipid desaturase DEGS1 causes hypomyelinating leukodystrophy. Journal of Clinical Investigation. 2019;129(3):1240–1256. doi: 10.1172/JCI123959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mousa A., Naderpoor N., Mellett N., Wilson K., Plebanski M., Meikle P.J. Lipidomic profiling reveals early-stage metabolic dysfunction in overweight or obese humans. Biochimica Biophysica Acta Molecular Cell Biology of Lipids. 2019;1864(3):335–343. doi: 10.1016/j.bbalip.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Mamtani M., Meikle P.J., Kulkarni H., Weir J.M., Barlow C.K., Jowett J.B. Plasma dihydroceramide species associate with waist circumference in Mexican American families. Obesity (Silver Spring) 2014;22(3):950–956. doi: 10.1002/oby.20598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magaye R.R., Savira F., Hua Y., Kelly D.J., Reid C., Flynn B. The role of dihydrosphingolipids in disease. Cellular and Molecular Life Science. 2019;76(6):1107–1134. doi: 10.1007/s00018-018-2984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zuellig R.A., Hornemann T., Othman A., Hehl A.B., Bode H., Guntert T. Deoxysphingolipids, novel biomarkers for type 2 diabetes, are cytotoxic for insulin-producing cells. Diabetes. 2014;63(4):1326–1339. doi: 10.2337/db13-1042. [DOI] [PubMed] [Google Scholar]
- 40.Langfelder P., Horvath S. WGCNA: an R package for weighted correlation network analysis. BioMed Central Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahdi T., Hanzelmann S., Salehi A., Muhammed S.J., Reinbothe T.M., Tang Y. Secreted frizzled-related protein 4 reduces insulin secretion and is overexpressed in type 2 diabetes. Cell Metabolism. 2012;16(5):625–633. doi: 10.1016/j.cmet.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 42.Pauter A.M., Olsson P., Asadi A., Herslof B., Csikasz R.I., Zadravec D. Elovl2 ablation demonstrates that systemic DHA is endogenously produced and is essential for lipid homeostasis in mice. Journal of Lipid Research. 2014;55(4):718–728. doi: 10.1194/jlr.M046151. [DOI] [PMC free article] [PubMed] [Google Scholar]