Abstract
Background
The extended and clinically silent progression of Type 1 diabetes (T1D) creates a challenge for clinical interventions and for understanding the mechanisms that underlie its pathogenesis. Over the course of the development of Type 1 diabetes, studies in animal models and of human tissues have identified adaptive changes in β cells that may affect their immunogenicity and susceptibility to killing. Loss of β cells has traditionally been identified by impairment in function but environmental factors may affect these measurements.
Scope of Review
In this review we will highlight features of β cell responses to cell death, particularly in the setting of inflammation, and focus on methods of detecting β cell death in vivo.
Major conclusions
We developed an assay to measure β cell death in vivo by detecting cell free DNA with epigenetic modifications of the INS gene that are found in β cells. This assay has robust technical performance and identifies killing in individuals at very high risk for disease, but its ability to identify β cell killing in at-risk relatives is limited by the short half-life of the cell free DNA and the need for repeated sampling over an extended course. We present results from the Diabetes Prevention Trial-1 using this assay. In addition, recent studies have identified cellular adaptations in some β cells that may avoid killing but impair metabolic function. Cells with these characteristics may aggravate the autoimmune response but also may represent a potentially recoverable source of functional β cells.
Keywords: Beta cells, Apoptosis, Death, Type 1 diabetes, Inflammation, Methylation
Abbreviations
- AEs
adverse events
- TPIAT
autologous islet transplantation following total pancreatectomy
- CTLA-4
cytotoxic T lymphocyte antigen 4
- DNMTs
DNA methyltransferases
- Fltp
Flattop
- qMSP
methylation specific PCR
- MMTT
mixed meal tolerance test
- NOD
non-obese diabetic
- OGTT
oral glucose tolerance test
- PPI
preproinsulin
- PD-1
programmed death-1
- PD-L1/PD-L2
programmed death-ligand 1 or 2
- ROS
reactive oxygen species
- SASP
senescence-associated secretory phenotype
- TSS
transcriptional start site
- T1D
Type 1 diabetes
- T2D
Type 2 diabetes
- TYK2
tyrosine kinase 2
1. Introduction
Type 1 diabetes (T1D) occurs as a result of the destruction of β cells that generally occurs over years [1], [2]. However, not all β cells are destroyed. In many patients with long-standing disease, residual β cell function may be identified by the presence of detectable C-peptide or proinsulin, but this limited function is insufficient to maintain metabolic control [3], [4]. As a result, exogenous insulin, diet, and exercise remain the cornerstones of therapy. Despite the improvements in delivery technologies, glucose monitoring, and pharmacokinetics of insulins, the majority of individuals with T1D, particularly adolescents, fail to achieve normal glucose levels or even meet the goals for therapy that have been identified by the American Diabetes Association [5]. Even at academic medical centers that can provide the most up to date technologies and support services, adolescents with T1D have average hemoglobin A1c levels (a measure of glucose control for which normal is <5.7%) above 9%: similar to the levels that had been reported in the standard management group in the Diabetes Control and Complications Trial [6], [7]. There has been progress in the management and prevention of secondary complications of the disease, but particularly for children who are diagnosed with T1D, there are ongoing risks of morbidity and loss of life-years [8], [9].
Studies over the past 3 decades have identified individuals who will ultimately develop disease on the basis of autoantibodies against β cell specific (e.g. insulin, ZnT8) and non-specific (e.g. GAD65) proteins [10], [11], [12], [13]. During that time, prior to diagnosis, immune cells interact with β cells and there is amplification and diversification of autoimmune responses (Stage 1) [14]. Autoimmunity is contained for years prior to the onset of β cell killing, but it is accelerated in the ∼2 years prior to diagnosis and heralded by impairment in provoked β cell responses to glucose (Stage 2) [15]. With the progression of β cell loss, metabolic control deteriorates and exogenous insulin is needed for survival (Stage 3).
It is challenging to identify individuals who will progress to T1D during Stage 1 or 2 of the disease – when autoantibodies are first found but frank hyperglycemia is not, particularly in the general population. Most individuals who develop T1D do not have a relative with T1D. The rate of positive autoantibodies, even among first-degree relatives of patients, is <6%. Accordingly, most immune therapies, which target the immune response, have been tested for their ability to stop the progression of autoimmune diabetes in individuals after clinical presentation. For example, anti-CD3 mAbs, teplizumab and otelixizumab, as well as anti-CD20 mAb, rituximab, CTLA4Ig, LFA3Ig, abatacept, and anti-thymocyte globulin have attenuated the decline in C-peptide responses during a mixed meal tolerance test (MMTT) over the first 2 years of disease [16], [17], [18], [19], [20], [21], [22], [23]. Some of these (e.g. anti-thymocyte globulin and anti-CD3 mAb have shown more robust effects in the first two years than others but none of these therapies have permanently prevented the metabolic decline even with continued or repeated treatment. The reasons for the ongoing decline are not understood.
In this review, we address two central issues involving the pathogenesis of the disease that are relevant to the design of therapies that may be used for prevention and even reversal of T1D. First, we discuss techniques to monitor β cell killing in vivo, which is silent but provides insights into the mechanisms of disease and is important to identify individuals in whom preventative therapies may be valuable. We present data of measurements of β cell death from the Diabetes Prevention Trial-1 (DPT-1). Second, we discuss experimental findings about the responses of β cell to immunologic assault. We have explored the hypothesis that, in response to inflammatory cells, β cells are modified that may affect their survival.
2. Mechanisms of beta cell death in autoimmune settings
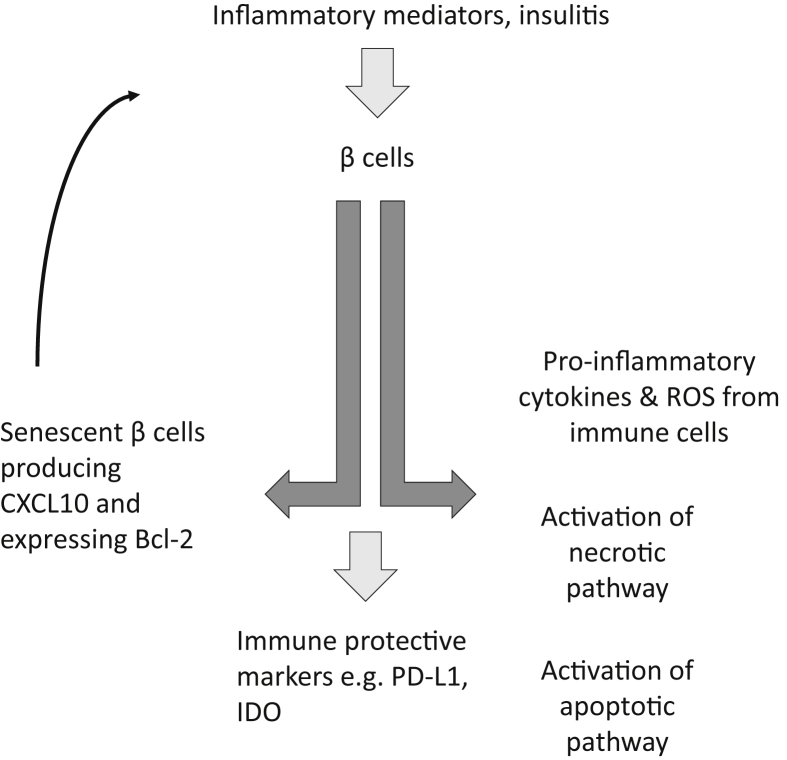
Several pathways are likely involved in β cell death in autoimmune diabetes. Whether there is a primary mechanism in vivo remains unclear [24], [25], [26]. Apoptotic pathways, both intrinsic and extrinsic have been described in β cells under immunologic stress. The intrinsic pathway, or mitochondrial pathway, is orchestrated by pro- and anti-apoptotic proteins of the Bcl superfamily, and the extrinsic pathway is comprised of TNF superfamily ligands, such as Fas-L and TNF-α, binding to their receptors, Fas and TNFR-1, respectively. Both pathways then converge by activating initiator and executioner caspases, which degrade crucial proteins and lead to apoptosis of β cells [27]. The importance of this mechanism in β cell death is highlighted by the expression of TNFR1 and Fas on β cells although other studies have suggested alternative (non-Fas mediated) mechanisms [24], [25], [28], [29]. However, the significance of Fas-mediated killing has been questioned since Thomas et al. found few Fas+ β cells shortly before the onset of diabetes in NOD mice. Nonetheless, Fas expression is found on human and murine β cells that are cultured with inflammatory cytokines and studies in vitro indicate that β cells can be killed by Fas-mediated cytotoxicity [29] (Figure 1).
Figure 1.
β cell death and survival. Recent studies of the fate of β cells during the progression of autoimmune diabetes in animal models and humans suggest that there may be alternate fates of cells that may lead to destruction, survival, or even acceleration of the immune response. Inflammatory mediators may induce changes in β cells that may lead to their demise through classical pathways such as Fas/FasL, cytolysis, or other mechanisms. However, there are also adaptive changes in β cells that may protect them from further destruction, such as increased expression of PD-L1 and IDO, or may also enhance the autoimmune response by the development of senenscent cells that can produce chemokines (e.g. CXCL10) that can recruit immune cells, or inflammatory cytokines (e.g. IL-6) that have direct toxic effects. Thus, there is a dynamic response to inflammatory stressors that may ultimately determine whether cells are killed by autoimmune responses or protected.
Necrosis and necroptosis have also been shown to play a role in mediating β cell death in T1D [30], but the consequences of these forms of cell death may be immunologically different: Whereas the programmed cell death via apoptosis evokes almost no inflammatory reaction, but both necrosis and necroptosis elicit potent inflammatory reactions [31], [32]. In addition to signaling downstream apoptosis, TNF-α/TNFR-1 interaction has been shown to also lead to downstream necroptosis. Necroptosis mediates programmed necrosis via production of reactive oxygen species (ROS) by modulating glutaminolysis, mitochondrial uncoupling, fragmentation of DNA and release of hydrolytic enzymes from lysosomes [27]. Calcium signaling appears to be an important component in the death pathway [33]. A pathway analysis of candidate genes expressed in human islets identified a central role for interferon (IFN)-regulated pathways and tyrosine kinase 2 (TYK2). Polymorphisms in the TYK2 gene were associated with a decreased risk of developing T1D. TYK2 inhibition prevented β cell apoptosis via the mitochondrial pathway of cell death suggesting that TYK2 regulates apoptotic and proinflammatory pathways in β cells and may also be involved in recruitment of T cells to the islets [34].The involvement of necrosis as a mechanism of β cell death is further suggested by the identification of an impaired vascular supply in pre-diabetic NOD islets, which could lead to ischemia and then necrosis [35]. Finally, conventional cell mediated toxicity leading to necrosis has been shown experimentally: Knight et al. showed that preproinsulin-specific CD8 T-cell clones recognizing either an HLA-A2 (A*0201) or HLA-A24 (A*2402)-restricted epitope (peptide of preproinsulin [PPI] (15–24), ALWGPDPAAA; or PPI (3–11), LWMRLLPLL) utilized conventional mediators of cytotoxicity [36].
3. Studies of β cell death in vivo
Methods that identify β cell death, the primary pathologic process in T1D, are indirect, and rely on β cell function or relative increased release of proinsulin. But these tests do not detect killing and may be affected by environmental factors. Immunologic markers such as autoantibodies (against GAD65, insulin, ICA512, or ZnT8) do not provide information about the extent or kinetics of disease progression. Progression of diabetes in individuals at-risk for and with T1D is variable [37]. Younger patients (<21 yrs) tend to have a more rapid fall in C-peptide than older patients but there is a large range of rate of fall. It has also been postulated that loss of β cells is cyclical but we do not have methods to identify when the process is occurring which makes intervention a challenge since some therapies (e.g. anti-CD3 mAbs) are best directed to an active immune response whereas others (e.g. antigen immunization) might not be effective when there are activated effector cells [38].
To detect β cell death, we developed a method to detect cell-free DNA that had epigenetic signatures of β cells. DNA is released into the circulation from dying cells [39], [40]. Because the epigenome is unique to each cell type, detection of DNA in the serum with specific epigenetic marks can identify the cell of origin. CpG sites are frequently unmethylated in cells that transcribe a gene but are often methylated in cells in which transcription does not occur. Following bisulfite treatment of the cell-free DNA in the serum, primers that are specific for methylated and unmethylated CpG sites can distinguish the methylation pattern. (Bisulfite-treated methyl-cytosines in CpG sites remain as cytosine but unmethylated sites are converted to uracil.) After analysis of the mouse methylome of Ins1 and Ins2 DNA in β cells we designed primers to detect, using a nested PCR reaction, unmethylated Ins1 DNA in mice and used it to study β cell death in mice that had been treated with streptozotocin and in NOD mice. In NOD mice, β cell death was detected prior to increased levels of glucose. Similar findings were reported by Hussieny et al. using methylation specific PCR (qMSP) in NOD mice [41]. Data from other groups confirmed that elevated levels of circulating unmethylated Ins DNA can be detected in serum prior to development of hyperglycemia in NOD mouse models [41], [42]. Similarly, we analyzed the human methylome and developed a nested PCR reaction to detect human INS DNA with epigenetic signatures of β cells and identified increased levels in a small proportion of individuals with new onset T1D.
We used this approach to evaluate β cell killing in patients with recent onset T1D participating in a clinical trial of teplizumab, and found that the anti-CD3 mAb decreased the levels of β cell killing as well as improved C-peptide responses to a mixed meal [43].
Because of the complex methods involved in the nested PCR reaction, we adapted the assay to use droplet digital PCR [40]. This method can quantify DNA without the use of standard curves [44], and it has recently been used as a tool for detecting mutations in circulating DNA [45], early detection of transplant rejection [46], copy number variation [47] residual HIV levels in patients [48]. The absolute quantification without reliance on an external standard, the capability to multiplex and the powerful sensitivity offered by ddPCR made it an ideal tool for our assay. The ddPCR reactions were designed as a single step PCR. The method involved in the ddPCR reaction entails a mixture of the PCR reaction with oil to generate up to 20,000 droplets, prior to the thermal cycling. The sample is randomly distributed into discrete partitions, such that each droplet behaves as an individual PCR reaction, which may contain from zero to five copies of the target gene. It is assumed, given the number of droplets generated, that the distribution of the template in the droplets adheres to a Poisson distribution. Therefore, the qualitative endpoint (positive/negative) of the reaction is converted into an absolute quantitation of the number of templates in the total PCR volume. This improves the sensitivity of finding rare gene targets significantly. The power of resolution of real-time PCR is limited to 5% of target concentration, while ddPCR can detect a target at 0.001% concentration [49] (i.e. 1 copy/μl). We developed primers that target a CpG sites at +396 and +399 from the transcriptional start site (TSS) in Exon 2 of the INS gene. The read out for the assay was initially determined as the ratio of the unmethylated copy number divided by the methylated copy number but the calculations were changed to the ratio of the unmethylated copy number divided by the unmethylated + methylated copy number. The assay performed well in two workshops with blinded duplicate samples. Using samples from autotransplants (see below), the mean CV of replicate samples ranged from 10.6 to 28.7%.
To evaluate the ability of the assay to identify β cell death and the kinetics of the INS DNA, we measured the levels of unmethylated INS DNA after autologous islet transplantation following total pancreatectomy (TPIAT) in serum and in the transplant material in 21 TPIAT recipients [50]. As expected, there was a decline in the ratio in the serum after pancreatectomy consistent with the pancreatic source of the unmethylated INS DNA. There were elevated ratios immediately after islet infusion in all recipients in the first 3 h, followed by a rapid decline in the ratio. It has been thought that there is a significant level of β cell death that occurred at the time when islets are infused into the portal vein during islet auto or allotransplantation. Interestingly, we found a high ratio and copy number of unmethylated INS DNA and methylated INS DNA in the cell-free supernatant of the islet product suggesting that much of the signal we had detected in the serum immediately after infusion originated from β cells that had died during the isolation process. Also, on the basis of the decline in unmethylated INS DNA after the first 3 h after infusion, we estimated that the t½ of the DNA was 2.2 h (95% confidence interval 1.7–2.9 h). There was variable elevation of the ratios after the initial spike. Between days 3 and 50, 52% patients had elevated ratios. After 90 days, 29% of patients had elevated ratios. Patients who had elevated ratios at day 90 had a high AUC for glucose during a MMTT suggesting that ongoing loss of β cells at day 90 may be associated with meal induced hyperglycemia.
We used the assay to study the at-risk individuals in the TrialNet Pathway to Prevention Study (TN-01) [51]. This study prospectively followed autoantibody+ relatives of patients with T1D. Some of these individuals (n = 10) developed T1D over the follow-up period of median = 1072 days (progressors) whereas others who were followed for a median = 1146 days did not (n = 10, non-progressors). In addition, we analyzed the levels of unmethylated INS DNA in participants from the TN-01 study who had been identified as “high-risk” (n = 30) because they had 2 + autoantibodies and dysglycemia on an OGTT test. Historical data suggested that approximately 75% of these individuals would develop T1D within 5 years.
Of those prospectively followed, 24.5% and14.2% of the ratios, measured at approximately 6 month intervals in the progressors and non-progressors were above the normal range. The average of the median ratios was modestly but significantly increased, compared to healthy control subjects but not compared to the non-progressors. Interestingly, in the progressors, an increase in the ratio was associated with a decline in the insulin secretory response during an OGTT, but not in the non-progressors. In the high-risk group the average ratios were increased in 22/27 subjects (3 samples were rejected due to technical issues) subjects and average ratio was higher compared to the progressor and non-progressor groups.
Since this initial study with participants in the relatively small cohort of TN-01 participants, we have analyzed the ratios in participants in the DPT-1 trial. This study, conducted in the 1990s, identified individuals at risk for T1D on the basis of a +ICA and diminished first phase insulin response during an IVGTT [52]. We analyzed the unmethylated INS/Total INS ratios in 97 participants who had serum collected approximately every 6 months (median of 4 samples/individual). We limited our analysis to participants who were in the placebo control group of the trial. Of these, 55 developed T1D.
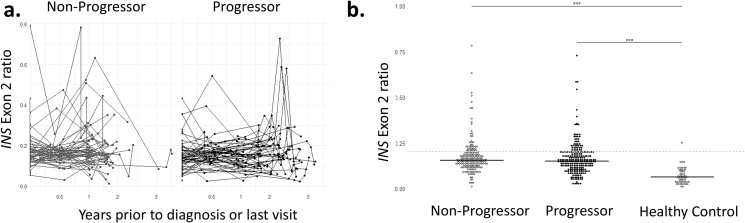
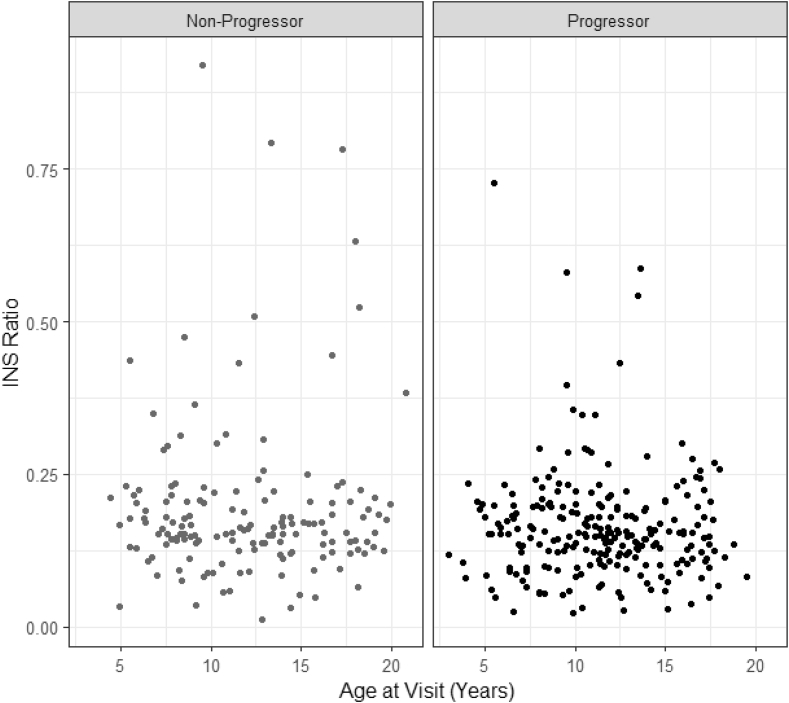
When all samples were compared, the ratios were increased in both those who progressed to T1D and those who did not compared to healthy control subjects (Figure 2A,B). Overall, the frequency of elevated ratios was 20% which is similar to the TN-01 cohort (19%). However, there was not a significant difference in the frequency of elevated levels between those who progressed (18%) and those who did not (24%) (Figure 3). We were unable to find a relationship between the ratio or change in the ratio and C-peptide responses during the OGTTs as we had done in the TN-01 analysis. In addition, there was not an association between age and the ratio (Figure 4).
Figure 2.
Ratios of unmethylated to total (unmethylated + methylated) INS DNA in the Diabetes Prevention Trial-1. The ratios of unmethylated:total INS DNA are shown over time in DPT-1 participants who did (progressors) or did not (non-progressors) develop T1D during the trial. (a) The time represents the years prior to the clinical diagnosis of T1D or the last study visit. Each line shows, with symbols, the measurements for an individual over time. (b) all of the data points from the progressors (n = 55) and non-progressors (n = 42) are shown. The levels were significantly higher in the progressors and non-progressors compared to healthy control subjects (***p < 0.001, ANOVA) but not to each other.
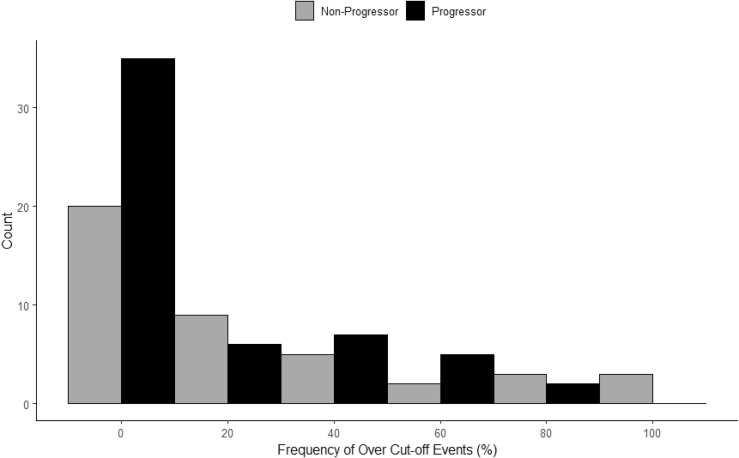
Figure 3.
Elevated unmethylated INS ratios in the DPT-1 trial. The number of elevated (>0.203) levels are shown for all of the data shown in Figure 1B for the progressors (n = 97) and non-progressors (n = 42). The threshold of 0.203 represents the 95th percentile of the ratios from healthy control subjects (n = 165).
Figure 4.
Relationship between age and the INS ratio in the DPT-1 in children. The relationships between the ratio of unmeth:total INS DNA is shown by age of the participants. The plot shows 504 individual observations (progressors = 310, non-progressors = 194).
Collectively, these studies suggest that β cell death is not readily identified when samples are collected every 6 months. This finding is not unexpected considering the short half-life of the INS DNA. Nonetheless, our data from the transplant and the high-risk groups suggest that the assay can identify β cell DNA in the serum when there is active killing. It is possible that in the individuals prospectively followed that the cytotoxicity is intermittent, absent, or below the level of detection with serum.
4. Other methods, other β cell genes
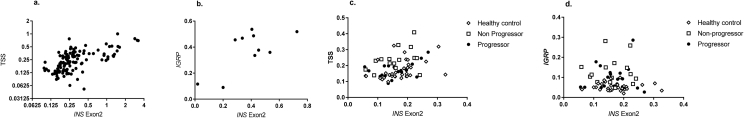
In addition to the CpG sites in Exon 2, we developed and tested a probe that identified unmethylated CpG sites in the INS promoter (−135 from the TSS) to detect unmethylated DNA and repeated the analysis of the TN-01 cohort. In islet autotransplant recipients we found a significant correlation between the ratios of unmethylated INS DNA at the TSS and in Exon 2 (r = 0.66, p < 0.0001) but a greater dynamic range of measurement with the probe measuring the CpG site in Exon 2 (Figure 5). Furthermore, when data using these two sites were compared in clinical samples (e.g. patients with new onset Type 1 diabetes or at risk), the correlation was not significant (r = 0.02). The most robust performance was using the probe that targeted Exon2 of the INS gene. Finally, we also identified CpG sites in Exon 1 of the IGRP gene that were unmethylated specifically in β cells. These assays had an average CV of <20% with internal testing of duplicate samples. There was agreement between measurements of the probe at the transcription start site and the original Exon 2 probe in clinical samples, and we also observed an agreement between the IGRP probe and the original Exon 2 probe in a subsets of autotransplant patients but the relationship in samples from patients with or at risk for T1D was less robust (Figure 5).
Figure 5.
Relationship between measures of unmethylated INS DNA. (a,b) a comparison of the unmethylated:total (unmethylated + methylated) INS DNA detected with (a) the Exon2 or the transcription start site (TSS) probe or the (b) Exon 2 and IGRP probe are shown in patients who received islet autotransplants. In (a) all of the samples (127 pairs) over the first 3 days are shown (r = 0.66, p < 0.0001, Pearson) and (b) a subset (10 pairs) of the samples (r = 0.69, p = 0.027, Pearson) (c) The relationship between the Exon 2 (r = 0.34, p = 0.005, Pearson)and TSS probe and (d) the Exon 2 and IGRP probe (r = −.01, p = ns) in a subset of samples from DPT-1 participants (n = 66 pairs).
High throughput sequencing identified a potential pitfall with the assumptions about epigenetic control of INS that has implications for the design of methods to identify β cell derived DNA. Neiman et al. reported that multiple islet cells share promoter hypomethylation which was not a determinant of protein expression. Rather than epigenetic modifications at the promotors, they found that cell-type-specific methylation in enhancers was responsible for differences in protein expression among the endocrine cells [53]. Based on this understanding Lehmann-Werman et al. described analysis of β cell derived INS DNA by identifying hypomethylation at multiple (6) CpG sites in the INS gene [54]. Their method has high specificity since the call of β cell DNA requires unmethylated CpGs at all six sites. Nonetheless, the specificity of the measurements was excellent. They were able to detect unmethylated CpG sites in the INS promoter DNA in cell free DNA from plasma of 11 recent T1D patients (2–16 wks after diagnosis). This methods was used in a setting of islet allotransplant and by using a new version of the above mentioned technique, based on next generation sequencing, Gala Lopez et al., reported that cell-free DNA-based estimation of β cell death 24 h after islet allotransplantation correlates with clinical outcome and could predict early engraftment [55]. The researchers studied cell-free DNA measurement after islet allotransplant in 37 subjects. They observed a peak in cell-free DNA 1 h after transplantation in 83% of the subjects, and like the observation in our islet autotransplant subjects, correlated this elevation to dead β cells carried over from the islet isolation process. Their study confirmed that the β cell death assay could have a predictive value in the islet allotransplant setting. They observed a second distinct peak at 24hrs post infusion in 21.6% of the patients which was associated with less favorable posttransplant outcomes, such as a higher 1-month insulin requirement, lower 1-month stimulated C-peptide levels and overall worse 3-month engraftment, by insulin independence scores.
5. Responses of β cells to immune assault and inflammatory mediators
Several reports have shown persistent β cell survival in individuals with long-standing T1D, which may be suggestive of beta cells that are capable of adapting and withstanding the autoimmune attack in T1D. However, it is not well understood how β cells may adapt and resist the immunological stressors of T1D. To follow the responses of β cells in vivo, we studied methylation marks in the Ins1 and Ins2 genes in the NOD model of T1D. We found that there was increased expression of DNA methyltransferases (DNMTs, particularly DNMT3a) during disease progression [56]. A strong association was seen between methylation of CpG sites in Ins1 exon 2 and Ins2 exon 1 and insulin gene expression. These epigenetic changes could be induced by cytokines that were expressed in β cells in the islets of NOD mice. We also found induction of DNMTs in human β cells that were exposed to inflammatory cytokines.
Further studies from our lab and others have identified transcriptomic and functional changes in the β cells in response to inflammation. We identified a subpopulation of β cells with features of “dedifferentiation” in NOD mice [57]. The cells were characterized by reduced insulin granularity and increased in direct relationship to infiltration of immune cells into the islets. There was reduced expression of Ins1 and Ins2 as well as transcription factors that are expressed in mature β cells such as MafA, Nkx6.1, and PDX-1. The cells did not represent simply stressed or dead β cells since the expression of genes associated with cellular stress responses such as Chop, Xbp1, Bip, Aft4, or Wfs1 or autophagy Atg5, LC3b, Becn1, Bnip3, and Gabarap, or cell apoptosis gene Casp3 were similar between mature β cells and the new subpopulation. Their functional responses (i.e. insulin secretion in response to glucose) was impaired but their ability to proliferate was enhanced. In addition, these cells acquired “stem-like” features reflected by increased expression of Neurog3, ALDH, and increased rates of proliferation relative to “normal” β cells that could be identified in the same islets. There was also increased expression of immune inhibitory ligands such as PD-L1 and Qa-2, but decreased expression of GAD and IGRP, antigens recognized by autoimmune T cells. They were resistant to killing by cytokines and inflammatory T cells and escaped killing when diabetes was induced in NOD mice with cyclophosphamide. We have postulated that these modified β cells may resist immunologic killing and may survive. Their functional properties are significantly impaired but they potentially are a recoverable source of β cells.
Recently Thompson and colleagues found a subset of senescent β cells developed during T1D in both humans and in NOD mice [58]. These β cells acquire a senescence-associated secretory phenotype (SASP), and are characterized but upregulated pro-survival mediator Bcl-2 and also have increased transcription of SASP factors such as IL-6, Mmp2, and Flnb and increased secretion of IL-6, Igfbp3, and Serpin1. A small molecule that inhibits Bcl-2, and ABT-199 cleared senescent β cells. Clearance of the senescent cells with ABT-199 reduced the rate of diabetes in NOD mice that were followed for 28 weeks. They suggest that senescent β cells may lead to enhanced immunologic destruction of β cells but the immune mechanisms remain undefined (Figure 1).
The secretion of pro-inflammatory cytokines is a peculiar feature of β cells. Several studies have implicated IL-6 and IL-1β as mediators of β cell death and in augmenting inflammatory cells but a clinical study with canakinumab failed to affect β cell decline [59], [60], [61], [62], [63], [64], [65]. An ongoing trial will test whether anti-IL-6 receptor (tocilizumab) will affect progression of new onset T1D. In contrast, other data have suggested that cytokines may also have a supportive role for example in preventing β cell apoptosis [66], [67]. Thus, the effects of cytokines are complicated – involving actions on β cells that may be different from immunologic effects. In a similar manner, inflammatory cytokines, such as IL-6 and IL-1β have been postulated to play a pathogenic role in Type 2 diabetes because of their direct effects on β cells and indirectly by effects on adipose and other cells [68].
6. Changes in β cells in other inflammatory conditions: checkpoint inhibitor diabetes
Checkpoint receptors, such as cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death-1 (PD-1), along with their respective ligands, CD80/CD86 and programmed death-ligand 1 or 2 (PD-L1/PD-L2), play an essential role in preventing unchecked T cell activation. Monoclonal antibodies blocking these receptors have shown efficacy in a growing number of cancers by enhancing immune responses towards tumor cells and increasing tumor cell destruction. However, the enhanced immunity induced by these therapeutic agents also results in immune-related adverse events (AEs) affecting various tissues, including pancreatic islets resulting in autoimmune diabetes [69], [70], [71], [72], [73], [74]. Preclinical studies have identified a role for immune checkpoint inhibitors in controlling β cell killing in mice with an autoimmune repertoire. For example blockade of B7.1 leads to rapid onset of diabetes in young NOD mice and blockade or genetic elimination of PD-1 leads to autoimmune diabetes on this background [75], [76], [77], [78]. Genetic loss of CTLA-4 leads to robust pancreatitis [79].
Checkpoint inhibitor-induced diabetes is a potentially life-threatening complication with an overall rate of 0.9% among patients receiving checkpoint inhibitors [71], [72], [80]. The presentation of checkpoint inhibitor-induced diabetes is often acute with pronounced increases in blood glucose and metabolic decompensation. The majority of patients present with diabetic ketoacidosis and have low or undetectable C-peptide at presentation indicating rapid and significant β cell death. The pathogenesis of checkpoint inhibitor-induced diabetes is not yet known but some clinical features are similar to T1D: many, although not all, patients are autoantibody positive. There is a predominance of the Class II MHC allele, HLA-DR4, in patients who develop this adverse event (AE) [71].
Although there are reports of some cases of autoimmune diabetes with anti-CTLA-4 alone, most of the cases to date have occurred with anti-PD-1 or anti-PD-L1 alone or in combination with anti-CTLA-4 pointing perhaps to central roles for PD-1 on T cells and PD-L1 in β cells for maintaining tolerance to β cells and preventing autoimmune diabetes [73], [81]. Recent studies point to increased expression of PD-L1 on mouse and human β cells in the setting of inflammation and with the development of autoimmune diabetes. Increased PD-L1 expression has been observed on β cells in NOD mice with infiltration of immune cells and progression of diabetes [82], including a population resistant to immunological attack [57]. Like murine β cells, human β cells induce expression of PD-L1 in response to inflammatory cytokines (IFNα and IFNγ) with known pathogenic roles in T1D, and is expressed in β cells of patients with T1D [82], [83]. These studies suggest that PD-L1 is induced on β cells in response to inflammation both in vitro and in vivo in human islets. It is not yet known how or whether checkpoint molecules impact β cell immunogenicity and susceptibility to cell death and their role among different β cell subsets. With the increasing use of checkpoint inhibitor therapy, the prevalence of autoimmune diabetes induced by these therapies is also increasing. Understanding mechanisms of β cell death in checkpoint inhibitor-induced diabetes could provide insights into mechanisms of β cell death in spontaneous T1D as well.
The basis for these changes in β cells is still under investigation but clinical observations may shed some light on the mechanisms. We found that a high proportion of individuals with this AE have biochemical evidence of exocrine pancreas inflammation: increased levels of lipase and/or amylase in 32% of patients with checkpoint inhibitor-induced diabetes suggesting that pancreatic exocrine inflammation may play a role in the development of diabetes in these patients. Interestingly, similar to our findings with checkpoint inhibitor induced diabetes, there have been changes in the exocrine pancreas in patients with T1D, such as reduced organ weight, identified through organ donor studies [84] and decreased pancreatic volumes by MRI during the first year of diagnosis [85]. Together these findings suggest that exocrine pancreatic inflammation may play a role in this form of autoimmune diabetes.
7. Conclusions
Studies of β cells during the progression of T1D have identified features of the tissue and immunologic responses that may be important in the design of therapies to prevent the disease. Detection of β cell-derived cell free DNA is technically feasible, specific, and reproducible in vivo but is limited by the short half-life of the β cell derived DNA of approximately 2 h. The findings from previous analysis of the participants in TN-01 who were followed prospectively, and the data from the DPT-1 study reported herein, and metabolic data from both studies, however, is informative about the disease progression. In contrast to a chronic destruction of β cells, these observations suggest that there are undetectable levels of killing in the years after autoantibodies are first identified but prior to glucose intolerance. When responses to oral glucose become impaired, in individuals who have at least 2 + autoantibodies (Stage 2) there is marked increase in β cell killing and progression to diabetes appears inevitable, but with individual variation in the kinetics. Therefore, use of drugs and agents for prevention that do not directly affect the effector immune response are best introduced in Stage 1. Once glucose tolerance is impaired, the effector immune response is well developed and agents that have broader effects on cellular immune responses are likely to be needed. These findings also suggest that unfortunately, repeated monitoring for β cell killing in individuals at risk is challenging since episodes or killing may be brief or the amount of DNA that is released by number of cells that are being killed at any one point is very small. Nonetheless, our data do show an increase in the total levels of unmethylated INS DNA in the at-risk population. The reasons for this finding are not clear at this time. It does not appear to be explained by age alone since the levels were elevated even with age matched control subjects. It is possible that there is increased turnover of β cells, with both generation of new cells and increased rates of cell death, that account for the findings, but further studies are needed to resolve this finding.
With improved technologies for study, is has become clear that β cells are heterogeneous and the subpopulations have distinguishing characteristics under normal conditions and in response to stressors. The studies from our lab and others also have shown that there is adaptation of β cells to the immunologic response. We have postulated that there are changes in β cells that may render them invisible or protected from immune killing. The loss of β cells in this manner is analogous to the dropout of β cells that has been described in the islets of mice with metabolic stressors and Type 2 diabetes. It is important to note that the source of “new” β cells remains unknown. They could reflect “dedifferentiation” of mature β cells in response to the immunologic and cellular stressors but it is also possible that they are derived from an alternate source. Many groups have shown how other cells, such as other endocrine, exocrine, ductal, or even gut cells can acquire characteristics of β cells. It is not clear, however, whether these cells are sufficient to recover metabolic control or whether, like those we have identified, the cells are markedly impaired in their functional capacity. Furthermore there is very little known about their immunogenicity.
Stressor responses are a common feature of scenarios in which β cell adaptive responses occur. Cytokines that may be produced by β cells themselves as well as in the islet environment can induce changes. The ability of β cells to produce these inflammatory mediators raises a question about their physiologic importance: Some work has suggested that both IL-1β and IL-6 are essential for normal β cell development and function. However, pharmacologic levels may be destructive rather than protective. β cells express receptors for IL-6 and IL-1β. Under physiologic conditions, turnover of β cells is relatively low but in the setting of cellular stressors it is aggravated. The responses of β cells themselves and other cells to clear the damaged cells may be overwhelmed in the setting of inflammation exposing neoantigens or invoking other cellular mechanisms that lead to β cell demise.
Funding
Supported by grants: R01DK057846, R01CA227473, R43DK116577, R21AI135562, R01DK120362, and T32DK007058 from the National Institutes of Health, and grants SRA-2014-158 and SRA-2014-142 from the Juvenile Diabetes Research Foundation.
This article is part of a supplement entitled ‘Biomarkers of Beta-Cell Health and Dysfunction: Towards Personalised Diabetes Care. Proceedings of the 20th Servier-IGIS Symposium’. The Symposium and the publication of the Supplement have been made possible by an unrestricted educational grant from Institut la Conférence Hippocrate – Servier Group.
Conflict of interest
S U–B has a patent application for an assay to measure β cell death using droplet digital PCR. KCH has a patent for measurement of β cell death with a PCR based assay to detect INS DNA with differential methylation patterns. ALP, NL, PC, MK, JR, GB have no conflicts to declare.
References
- 1.Bluestone J.A., Herold K., Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herold K., Vignali D.A., Cooke A., Bluestone J. Type 1 diabetes: translating mechanistic observations into effective clinical outcomes. Nature Reviews Immunology. 2013;13(4):243–256. doi: 10.1038/nri3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keenan H.A., Sun J.K., Levine J., Doria A., Aiello L.P., Eisenbarth G. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59:2846–2853. doi: 10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oram R.A., Jones A.G., Besser R.E., Knight B.A., Shields B.M., Brown R.J. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57:187–191. doi: 10.1007/s00125-013-3067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Professional practice committee for the standards of medical care in diabetes-2016. Diabetes Care. 2016;39(Suppl 1):S107–S108. doi: 10.2337/dc16-S018. [DOI] [PubMed] [Google Scholar]
- 6.Miller K.M., Foster N.C., Beck R.W., Bergenstal R.M., DuBose S.N., DiMeglio L.A. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38:971–978. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 7.McKnight J.A., Wild S.H., Lamb M.J., Cooper M.N., Jones T.W., Davis E.A. Glycaemic control of Type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabetic Medicine. 2015;32:1036–1050. doi: 10.1111/dme.12676. [DOI] [PubMed] [Google Scholar]
- 8.Livingstone S.J., Levin D., Looker H.C., Lindsay R.S., Wild S.H., Joss N. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. Journal of the American Medical Association. 2015;313:37–44. doi: 10.1001/jama.2014.16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rawshani A., Sattar N., Franzen S., Rawshani A., Hattersley A.T., Svensson A.M. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018;392:477–486. doi: 10.1016/S0140-6736(18)31506-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krischer J.P., Schatz D., Riley W.J., Spillar R.P., Silverstein J.H., Schwartz S. Insulin and islet cell autoantibodies as time-dependent covariates in the development of insulin-dependent diabetes: a prospective study in relatives. Journal of Clinical Endocrinology & Metabolism. 1993;77:743–749. doi: 10.1210/jcem.77.3.8370696. [DOI] [PubMed] [Google Scholar]
- 11.Orban T., Sosenko J.M., Cuthbertson D., Krischer J.P., Skyler J.S., Jackson R. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2009;32:2269–2274. doi: 10.2337/dc09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sosenko J.M., Palmer J.P., Greenbaum C.J., Mahon J., Cowie C., Krischer J.P. Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2006;29:643–649. doi: 10.2337/diacare.29.03.06.dc05-1006. [DOI] [PubMed] [Google Scholar]
- 13.Vehik K., Beam C.A., Mahon J.L., Schatz D.A., Haller M.J., Sosenko J.M. Development of autoantibodies in the TrialNet natural history study. Diabetes Care. 2011;34:1897–1901. doi: 10.2337/dc11-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Insel R.A., Dunne J.L., Atkinson M.A., Chiang J.L., Dabelea D., Gottlieb P.A. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964–1974. doi: 10.2337/dc15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrannini E., Mari A., Nofrate V., Sosenko J.M., Skyler J.S., DPT study group Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes. 2010;59:679–685. doi: 10.2337/db09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orban T., Bundy B., Becker D.J., Dimeglio L.A., Gitelman S.E., Goland R. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagopian W., Ferry R.J., Jr., Sherry N., Carlin D., Bonvini E., Johnson S. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protege trial. Diabetes. 2013;62:3901–3908. doi: 10.2337/db13-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herold K.C., Gitelman S.E., Ehlers M.R., Gottlieb P.A., Greenbaum C.J., Hagopian W. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62(11):3766–3774. doi: 10.2337/db13-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herold K.C., Gitelman S.E., Masharani U., Hagopian W., Bisikirska B., Donaldson D. A single course of anti-CD3 monoclonal antibody hOKT3{gamma}1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 Years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herold K.C., Hagopian W., Auger J.A., Poumian-Ruiz E., Taylor L., Donaldson D. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. New England Journal of Medicine. 2002;346:1692–1698. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 21.Sherry N., Hagopian W., Ludvigsson J., Jain S.M., Wahlen J., Ferry R.J., Jr. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378(9790):487–497. doi: 10.1016/S0140-6736(11)60931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigby M.R., Harris K.M., Pinckney A., DiMeglio L.A., Rendell M.S., Felner E.I. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. Journal of Clinical Investigation. 2015;125:3285–3296. doi: 10.1172/JCI81722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pescovitz M.D., Greenbaum C.J., Krause-Steinrauf H., Becker D.J., Gitelman S.E., Goland R. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. New England Journal of Medicine. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilcox N.S., Rui J., Hebrok M., Herold K.C. Life and death of beta cells in Type 1 diabetes: a comprehensive review. Journal of Autoimmunity. 2016;71:51–58. doi: 10.1016/j.jaut.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas H.E., Kay T.W. Beta cell destruction in the development of autoimmune diabetes in the non-obese diabetic (NOD) mouse. Diabetes Metabolism Research Review. 2000;16:251–261. doi: 10.1002/1520-7560(200007/08)16:4<251::aid-dmrr126>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 26.Thomas H.E., Kay T.W. How beta cells die in type 1 diabetes. Current Directions in Autoimmunity. 2001;4:144–170. doi: 10.1159/000060536. [DOI] [PubMed] [Google Scholar]
- 27.Rojas J., Bermudez V., Palmar J., Martinez M.S., Olivar L.C., Nava M. Pancreatic beta cell death: novel potential mechanisms in diabetes therapy. Journal of Diabetes Research. 2018;2018:9601801. doi: 10.1155/2018/9601801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darwiche R., Chong M.M., Santamaria P., Thomas H.E., Kay T.W. Fas is detectable on beta cells in accelerated, but not spontaneous, diabetes in nonobese diabetic mice. The Journal of Immunology. 2003;170:6292–6297. doi: 10.4049/jimmunol.170.12.6292. [DOI] [PubMed] [Google Scholar]
- 29.Thomas H.E., Darwiche R., Corbett J.A., Kay T.W. Evidence that beta cell death in the nonobese diabetic mouse is Fas independent. The Journal of Immunology. 1999;163:1562–1569. [PubMed] [Google Scholar]
- 30.Eizirik D.L., Darville M.I. beta-cell apoptosis and defense mechanisms: lessons from type 1 diabetes. Diabetes. 2001;50(Suppl 1):S64–S69. doi: 10.2337/diabetes.50.2007.s64. [DOI] [PubMed] [Google Scholar]
- 31.Eizirik D.L., Mandrup-Poulsen T. A choice of death--the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia. 2001;44:2115–2133. doi: 10.1007/s001250100021. [DOI] [PubMed] [Google Scholar]
- 32.Dhuriya Y.K., Sharma D. Necroptosis: a regulated inflammatory mode of cell death. Journal of Neuroinflammation. 2018;15:199. doi: 10.1186/s12974-018-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee M.S., Chang I., Kim S. Death effectors of beta-cell apoptosis in type 1 diabetes. Molecular Genetics and Metabolism. 2004;83:82–92. doi: 10.1016/j.ymgme.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Marroqui L., Dos Santos R.S., Floyel T., Grieco F.A., Santin I., Op de Beeck A. TYK2, a candidate gene for type 1 diabetes, modulates apoptosis and the innate immune response in human pancreatic beta-cells. Diabetes. 2015;64:3808–3817. doi: 10.2337/db15-0362. [DOI] [PubMed] [Google Scholar]
- 35.Akirav E.M., Baquero M.T., Opare-Addo L.W., Akirav M., Galvan E., Kushner J.A. Glucose and inflammation control islet vascular density and beta-cell function in NOD mice: control of islet vasculature and vascular endothelial growth factor by glucose. Diabetes. 2011;60:876–883. doi: 10.2337/db10-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knight R.R., Kronenberg D., Zhao M., Huang G.C., Eichmann M., Bulek A. Human beta-cell killing by autoreactive preproinsulin-specific CD8 T cells is predominantly granule-mediated with the potency dependent upon T-cell receptor avidity. Diabetes. 2013;62:205–213. doi: 10.2337/db12-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenbaum C.J., Beam C.A., Boulware D., Gitelman S.E., Gottlieb P.A., Herold K.C. Fall in C-peptide during first 2 Years from diagnosis: evidence of at least two distinct phases from composite TrialNet data. Diabetes. 2012 doi: 10.2337/db11-1538. db11-1538 [pii] 10.2337/db11-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatenoud L., Primo J., Bach J.F. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. The Journal of Immunology. 1997;158:2947–2954. [PubMed] [Google Scholar]
- 39.Akirav E.M., Lebastchi J., Galvan E.M., Henegariu O., Akirav M., Ablamunits V. Detection of beta cell death in diabetes using differentially methylated circulating DNA. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19018–19023. doi: 10.1073/pnas.1111008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Usmani-Brown S., Lebastchi J., Steck A.K., Beam C., Herold K.C., Ledizet M. Analysis of beta-cell death in type 1 diabetes by droplet digital PCR. Endocrinology. 2014;155:3694–3698. doi: 10.1210/en.2014-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Husseiny M.I., Kuroda A., Kaye A.N., Nair I., Kandeel F., Ferreri K. Development of a quantitative methylation-specific polymerase chain reaction method for monitoring beta cell death in type 1 diabetes. Public Library of Science one. 2012;7 doi: 10.1371/journal.pone.0047942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher M.M., Perez Chumbiauca C.N., Mather K.J., Mirmira R.G., Tersey S.A. Detection of islet beta-cell death in vivo by multiplex PCR analysis of differentially methylated DNA. Endocrinology. 2013;154:3476–3481. doi: 10.1210/en.2013-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lebastchi J., Deng S., Lebastchi A.H., Beshar I., Gitelman S., Willi S. Immune therapy and beta-cell death in type 1 diabetes. Diabetes. 2013;62:1676–1680. doi: 10.2337/db12-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinheiro L.B., Coleman V.A., Hindson C.M., Herrmann J., Hindson B.J., Bhat S. Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Analytical Chemistry. 2012;84:1003–1011. doi: 10.1021/ac202578x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taly V., Pekin D., Benhaim L., Kotsopoulos S.K., Le Corre D., Li X. Multiplex picodroplet digital PCR to detect KRAS mutations in circulating DNA from the plasma of colorectal cancer patients. Clinical Chemistry. 2013;59:1722–1731. doi: 10.1373/clinchem.2013.206359. [DOI] [PubMed] [Google Scholar]
- 46.Beck J., Bierau S., Balzer S., Andag R., Kanzow P., Schmitz J. Digital droplet PCR for rapid quantification of donor DNA in the circulation of transplant recipients as a potential universal biomarker of graft injury. Clinical Chemistry. 2013;59:1732–1741. doi: 10.1373/clinchem.2013.210328. [DOI] [PubMed] [Google Scholar]
- 47.Beck J., Hennecke S., Bornemann-Kolatzki K., Urnovitz H.B., Neumann S., Strobel P. Genome aberrations in canine mammary carcinomas and their detection in cell-free plasma DNA. Public Library of Science one. 2013;8:e75485. doi: 10.1371/journal.pone.0075485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strain M.C., Lada S.M., Luong T., Rought S.E., Gianella S., Terry V.H. Highly precise measurement of HIV DNA by droplet digital PCR. Public Library of Science one. 2013;8:e55943. doi: 10.1371/journal.pone.0055943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hindson B.J., Ness K.D., Masquelier D.A., Belgrader P., Heredia N.J., Makarewicz A.J. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Analytical Chemistry. 2011;83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellin M.D., Clark P., Usmani-Brown S., Dunn T.B., Beilman G.J., Chinnakotla S. Unmethylated insulin DNA is elevated after total pancreatectomy with islet autotransplantation: assessment of a novel beta cell marker. American Journal of Transplantation. 2017;17(4):1112–1118. doi: 10.1111/ajt.14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herold K.C., Usmani-Brown S., Ghazi T., Lebastchi J., Beam C.A., Bellin M.D. Beta Cell death and dysfunction during type 1 diabetes development in at-risk individuals. Journal of Clinical Investigation. 2015;125:1163–1173. doi: 10.1172/JCI78142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Effects of insulin in relatives of patients with type 1 diabetes mellitus. New England Journal of Medicine. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 53.Neiman D., Moss J., Hecht M., Magenheim J., Piyanzin S., Shapiro A.M.J. Islet cells share promoter hypomethylation independently of expression, but exhibit cell-type-specific methylation in enhancers. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:13525–13530. doi: 10.1073/pnas.1713736114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lehmann-Werman R., Neiman D., Zemmour H., Moss J., Magenheim J., Vaknin-Dembinsky A. Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E1826–E1834. doi: 10.1073/pnas.1519286113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gala-Lopez B.L., Neiman D., Kin T., O'Gorman D., Pepper A.R., Malcolm A.J. Beta cell death by cell-free DNA and outcome after clinical islet transplantation. Transplantation. 2018;102:978–985. doi: 10.1097/TP.0000000000002083. [DOI] [PubMed] [Google Scholar]
- 56.Rui J., Deng S., Lebastchi J., Clark P.L., Usmani-Brown S., Herold K.C. Methylation of insulin DNA in response to proinflammatory cytokines during the progression of autoimmune diabetes in NOD mice. Diabetologia. 2016;59(5):1021–1029. doi: 10.1007/s00125-016-3897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rui J., Deng S., Arazi A., Perdigoto A.L., Liu Z., Herold K.C. Beta cells that resist immunological attack develop during progression of autoimmune diabetes in NOD mice. Cell Metabolism. 2017;25(3):727–738. doi: 10.1016/j.cmet.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thompson P.J., Shah A., Ntranos V., Van Gool F., Atkinson M., Bhushan A. Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metabolism. 2019;29(5):1045–1060. doi: 10.1016/j.cmet.2019.01.021. e10. [DOI] [PubMed] [Google Scholar]
- 59.Campbell I.L., Cutri A., Wilson A., Harrison L.C. Evidence for IL-6 production by and effects on the pancreatic beta-cell. The Journal of Immunology. 1989;143:1188–1191. [PubMed] [Google Scholar]
- 60.DiCosmo B.F., Picarella D., Flavell R.A. Local production of human IL-6 promotes insulitis but retards the onset of insulin-dependent diabetes mellitus in non-obese diabetic mice. International Immunology. 1994;6:1829–1837. doi: 10.1093/intimm/6.12.1829. [DOI] [PubMed] [Google Scholar]
- 61.Hundhausen C., Roth A., Whalen E., Chen J., Schneider A., Long S.A. Enhanced T cell responses to IL-6 in type 1 diabetes are associated with early clinical disease and increased IL-6 receptor expression. Science Translational Medicine. 2016;8 doi: 10.1126/scitranslmed.aad9943. 356ra119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimura A., Kishimoto T. IL-6: regulator of Treg/Th17 balance. European Journal of Immunology. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 63.Moran A., Bundy B., Becker D.J., DiMeglio L.A., Gitelman S.E., Goland R. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet. 2013;381:1905–1915. doi: 10.1016/S0140-6736(13)60023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mandrup-Poulsen T., Spinas G.A., Prowse S.J., Hansen B.S., Jorgensen D.W., Bendtzen K. Islet cytotoxicity of interleukin 1. Influence of culture conditions and islet donor characteristics. Diabetes. 1987;36:641–647. doi: 10.2337/diab.36.5.641. [DOI] [PubMed] [Google Scholar]
- 65.Mandrup-Poulsen T., Zumsteg U., Reimers J., Pociot F., Morch L., Helqvist S. Involvement of interleukin 1 and interleukin 1 antagonist in pancreatic beta-cell destruction in insulin-dependent diabetes mellitus. Cytokine. 1993;5:185–191. doi: 10.1016/1043-4666(93)90003-n. [DOI] [PubMed] [Google Scholar]
- 66.Linnemann A.K., Blumer J., Marasco M.R., Battiola T.J., Umhoefer H.M., Han J.Y. Interleukin 6 protects pancreatic beta cells from apoptosis by stimulation of autophagy. The FASEB Journal. 2017;31:4140–4152. doi: 10.1096/fj.201700061RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kristiansen O.P., Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54(Suppl 2):S114–S124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- 68.Tsalamandris S., Antonopoulos A.S., Oikonomou E., Papamikroulis G.A., Vogiatzi G., Papaioannou S. The role of inflammation in diabetes: current concepts and future perspectives. European Cardiology. 2019;14:50–59. doi: 10.15420/ecr.2018.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Horvat T.Z., Adel N.G., Dang T.O., Momtaz P., Postow M.A., Callahan M.K. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. Journal of Clinical Oncology. 2015;33:3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barroso-Sousa R., Barry W.T., Garrido-Castro A.C., Hodi F.S., Min L., Krop I.E. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncology. 2018;4:173–182. doi: 10.1001/jamaoncol.2017.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stamatouli A.M., Quandt Z., Perdigoto A.L., Clark P.L., Kluger H., Weiss S.A. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 2018;67:1471–1480. doi: 10.2337/dbi18-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hughes J., Vudattu N., Sznol M., Gettinger S., Kluger H., Lupsa B. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38:e55–e57. doi: 10.2337/dc14-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clotman K., Janssens K., Specenier P., Weets I., De Block C.E.M. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. Journal of Clinical Endocrinology & Metabolism. 2018;103:3144–3154. doi: 10.1210/jc.2018-00728. [DOI] [PubMed] [Google Scholar]
- 74.Byun D.J., Wolchok J.D., Rosenberg L.M., Girotra M. Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nature Reviews Endocrinology. 2017;13:195–207. doi: 10.1038/nrendo.2016.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keir M.E., Liang S.C., Guleria I., Latchman Y.E., Qipo A., Albacker L.A. Tissue expression of PD-L1 mediates peripheral T cell tolerance. Journal of Experimental Medicine. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fife B.T., Guleria I., Gubbels Bupp M., Eagar T.N., Tang Q., Bour-Jordan H. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. Journal of Experimental Medicine. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fife B.T., Pauken K.E., Eagar T.N., Obu T., Wu J., Tang Q. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nature Immunology. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lenschow D.J., Ho S.C., Sattar H., Rhee L., Gray G., Nabavi N. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. Journal of Experimental Medicine. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tivol E.A., Borriello F., Schweitzer A.N., Lynch W.P., Bluestone J.A., Sharpe A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 80.Perdigoto A.L., Quandt Z., Anderson M., Herold K.C. Checkpoint inhibitor-induced insulin-dependent diabetes: an emerging syndrome. Lancet Diabetes Endocrinology. 2019;7:421–423. doi: 10.1016/S2213-8587(19)30072-5. [DOI] [PubMed] [Google Scholar]
- 81.Wright J.J., Salem J.E., Johnson D.B., Lebrun-Vignes B., Stamatouli A., Thomas J.W. Increased reporting of immune checkpoint inhibitor-associated diabetes. Diabetes Care. 2018;41:e150–e151. doi: 10.2337/dc18-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Osum K.C., Burrack A.L., Martinov T., Sahli N.L., Mitchell J.S., Tucker C.G. Interferon-gamma drives programmed death-ligand 1 expression on islet beta cells to limit T cell function during autoimmune diabetes. Scientific Reports. 2018;8:8295. doi: 10.1038/s41598-018-26471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Colli M.L., Hill J.L.E., Marroqui L., Chaffey J., Dos Santos R.S., Leete P. PDL1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-alpha and-gamma via IRF1 induction. EBioMedicine. 2018;36:367–375. doi: 10.1016/j.ebiom.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campbell-Thompson M.L., Kaddis J.S., Wasserfall C., Haller M.J., Pugliese A., Schatz D.A. The influence of type 1 diabetes on pancreatic weight. Diabetologia. 2016;59:217–221. doi: 10.1007/s00125-015-3752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Virostko J., Williams J., Hilmes M., Bowman C., Wright J.J., Du L. Pancreas volume declines during the first year after diagnosis of type 1 diabetes and exhibits altered diffusion at disease onset. Diabetes Care. 2019;42:248–257. doi: 10.2337/dc18-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]