Abstract
Nonalcoholic fatty liver disease (NAFLD) is a growing epidemic in the USA affecting ∼30% of the population. It has been closely linked to metabolic syndrome and type 2 diabetes, with strong implications for cardiovascular disease (CVD). This review focuses on the relationship between NAFLD and CVD and the proposed interactions interlinking these two diseases. This appraisal also discusses treatments targeting NAFLD in the context of CVD. NAFLD is a multisystem disease and ultimately the goals of therapy are to ameliorate CVD and prevent coronary artery disease morbidity and mortality.
Keywords: cardiovascular disease, metabolic syndrome, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis
Introduction
Nonalcoholic fatty liver disease (NAFLD) is defined as a spectrum of liver diseases ranging from simple steatosis to nonalcoholic steatohepatitis (NASH) leading to cirrhosis and risk for hepatocellular carcinoma (HCC). It is one of the most common causes of chronic liver disease in the USA and parallels the global epidemic of type 2 diabetes mellitus (T2DM) and metabolic syndrome (MetS) 1–4. The prevalence rates are as high as 20–30% in Europe 5,6 and 30–46% in the USA 7,8, and it is predicted to be the leading cause of liver transplantation by 2030 9. NAFLD was traditionally described as a hepatic manifestation of METs, but is increasingly being recognized as a multisystem disease 10–12, intricately involved in the pathogenesis of cardiovascular disease (CVD), T2DM, and chronic kidney disease (CKD), associated with significant morbidity and mortality 13–16. NAFLD has been associated with various subclinical markers of atherosclerosis such as increased carotid-intimal–medial thickness (IMT), increased arterial stiffness, and coronary artery calcification (reviewed here), independent of what we define as typical risk factors for CVD 17. CVD continues to be the leading cause of mortality in this patient population 18,19.
This review focuses on the relationship between NALFD and CVD, possible mechanisms linking the two, and implications for treatments.
Relationship between nonalcoholic fatty liver disease and cardiovascular disease
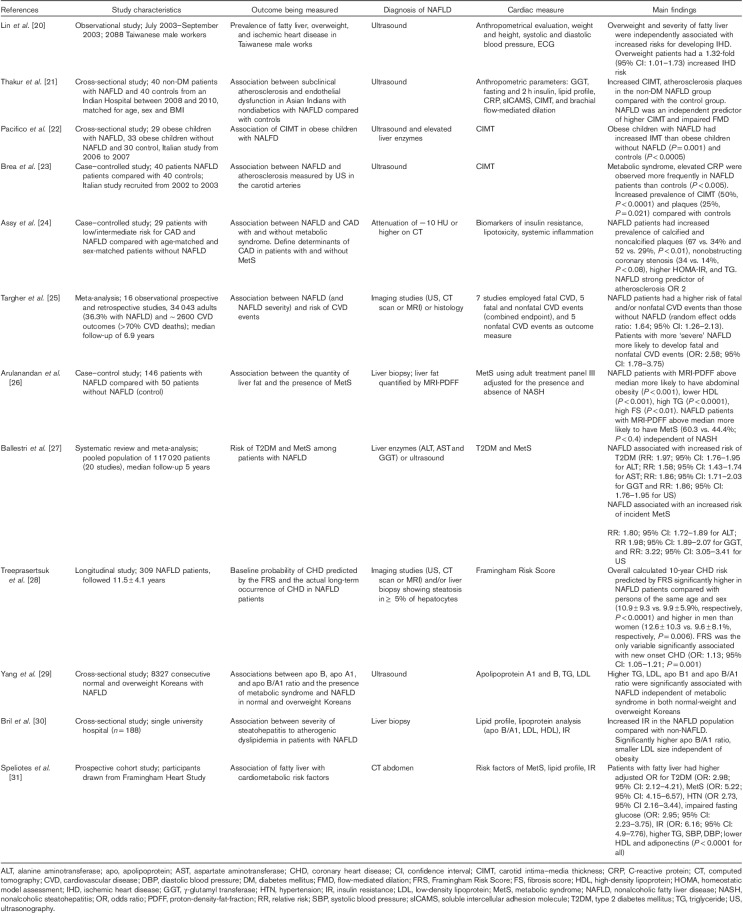
NAFLD is increasingly being recognized as an independent risk factor for CVD irrespective of the typical risk factors associated with MetS that is, hypertension, T2DM, dyslipidemia (specifically hypertriglyceridemia), and central obesity. Several retrospective and prospective population-based studies have effectively shown increased cardiovascular morbidity and mortality in patients with NAFLD (Table 1) 20.
Table 1.
Summary of studies examining the relationship between nonalcoholic fatty liver disease and the risk of cardiovascular disease
Carotid IMT is a known indicator for subclinical atherosclerosis, a predictor of myocardial infarction, and correlates with carotid atherosclerotic plaques 32,33. Thakur et al. 21 have shown that patients with NAFLD had higher average and maximum carotid intimal thickness than controls (0.6±0.12 and 0.684±0.16 mm, respectively, vs. 0.489±0.1 and 0.523±0.1 mm, respectively; P<0.05), and a higher prevalence of atherosclerotic plaques (20 vs. 5%, P<0.05), and this observation persisted even after adjusting for obesity, MetS, insulin resistance (IR), and lipid profile. Similar and striking findings have been reported by Pacifico et al. 22 in obese children. Obese children with NAFLD have a marked increase in carotid IMT compared with control healthy children. Furthermore, the carotid IMT was higher for obese children with NAFLD than obese children without liver involvement, but with similar BMI. Similarly, Brea et al. 23 reported that patients with NAFLD had increased carotid IMT, independent of the presence of MetS. Studies have also effectively shown that patients with NAFLD, even without MetS, have more vulnerable coronary soft plaques than healthy controls 24.
Several studies have shown that the severity of liver histology, specifically hepatic fibrosis, is associated with liver specific and all-cause mortality, with CVD being the leading cause of death in patients with NAFLD 19,34,35. Targher et al. 25 recent systematic review and meta-analysis of 16 observational prospective and retrospective studies with 34 043 adult individuals (36.3% with NAFLD) and ∼2600 CVD outcomes (>70% CVD deaths) over a median period of 6.9 years found that patients with NAFLD had a higher risk of fatal and/or nonfatal CVD events than those without NAFLD (odds ratio: 1.64; 95% confidence interval: 1.26–2.13). In addition, patients with more ‘severe’ NAFLD were also more likely to develop fatal and nonfatal CVD events (odds ratio: 2.58; 95% confidence interval: 1.78–3.75).
The degree of hepatic steatosis is also associated with worsening dyslipidemia and glucose control. From this, it can be postulated that the degree of severity of steatosis may dictate the risk of cardiovascular outcomes in patients with NAFLD. Arulanandan et al. 26 study reported that NAFLD patients with increased liver fat quantified by MRI and proton-density-fat-fraction were more likely to have abdominal obesity, decreased high-density lipoprotein levels, increased triglycerides (TGs), and increased fasting glucose levels (P<0.001). In addition, NAFLD patients with MRI-proton-density-fat-fraction above the median were more likely to have MetS (60.3 vs. 44.4%, P<0.04).
Although liver biopsy remains the gold standard for the diagnosis of NALFD, several noninvasive markers and serology-based tests have been used to assess the degree of hepatic steatosis and fibrosis. Liver enzymes [alanine aminotransferase (ALT), aspartate aminotransferase, and γ-glutamyl transferase] and noninvasive markers of fibrosis have been utilized to predict the risk of CVD and establish a relationship between NAFLD, T2DM and CVD. Ballestri et al. 27 meta-analysis and systematic review have reported this. NAFLD (defined by elevated liver enzymes and ultrasound) was associated with an almost two-fold increased risk of T2DM and MetS over a 5-year follow-up. Noninvasive markers, such as cytokeratin-18, nonalcoholic fatty liver disease fibrosis score (NFS), and transient elastography, have also been associated with increased liver-related mortality and CVD 16.
Mortality in patients with nonalcoholic fatty liver disease
Patients with NAFLD have an increased overall mortality compared with the general population. Several Third National Health and Nutrition Examination Survey-based studies 36–38 have evaluated the association between NAFLD and mortality and a recent meta-analysis has shown that NAFLD increased overall mortality by 57% mainly from liver-related and CVD causes and increased risk of T2DM by approximately two-fold 5.
To date, there are no specific scoring systems to assess CVD in patients with NAFLD. The Framingham Risk Score (FRS) has been used to assess the risk of CVD in patients with NAFLD on the basis of the close association of NAFLD and typical risk factors of CVD and MetS. The 10-year probability of CVD as calculated by the FRS in patients with NAFLD is high compared with an sex-matched and age-matched group 28. Dogan et al. 39 study and similar studies have taken this a step further to determine whether noninvasive markers of liver fibrosis in combination with FRS more accurately predict the risk of CVD in the NAFLD population. In their study, FRS was found to be higher in NAFLD patients than in controls (P<0.05). In addition, NFS was also higher in the intermediate/high probability coronary heart disease (CHD) risk group in NAFLD (P<0.05). The NFS cutoff point for identifying intermediate/high CHD risk in NAFLD patients was −2.1284, with a sensitivity and a specificity of 95.2 and 48.3%, respectively, with a predictive performance of 72% on the basis of the area under the curve value 39. There may be some value in utilizing FRS in combination with noninvasive markers of fibrosis in risk stratifying CHD in NAFLD patients.
Mechanisms linking nonalcoholic fatty liver disease and cardiovascular disease
NAFLD contributes toward atherosclerosis independent of typical risk factors for CVD, although the direct mechanisms of this relationship have not been completely defined. Proposed mechanisms include IR and a proinflammatory milieu, which alters the lipogenesis cycle and glucose metabolism promoting and facilitating arthrogenesis and hence CVD.
Insulin resistance
NAFLD has been associated with hepatic and adipose IR and decreased whole-body insulin sensitivity. Typical findings include an inability to control endogenous glucose. and hence increase susceptibility to developing diabetes, a known risk factor for CVD. Bonora et al. 40followed patients with T2DM and found that IR was independently predictive of coronary artery disease (CAD); a 1 U increase in the homeostatic model assessment index was associated with a 5.4% increased risk of CAD. In addition, NAFLD patients have a defect in insulin suppression of free fatty acids (FFAs), leading to increased delivery of FFA to the liver, inadequate FFA oxidation, and hence de-novo lipogenesis. It has been hypothesized that increased levels of FFA affect nitric oxide production and hence impair endothelial-dependent vasodilation that can lead to decreased oxygen transportation to myocardium, resulting in ischemia. Pilz et al. 41 have shown that FFA levels independently predict all-cause and cardiovascular mortality in patients with angiographic CAD.
Inflammation and oxidative stress
NAFLD is a proinflammatory state resulting in oxidative stress, and contributes toward endothelial dysfunction and IR. Increased oxidative stress has been reported in patients with NAFLD and CVD. Patients with NALFD have augmented production of reactive oxygen species (ROS) that leads to lipid peroxidation, resulting in inflammation and fibrogenesis through activation of stellate cells. Furthermore, ROS leads to diminished secretion of very low-density lipoprotein (LDL) and subsequent accumulation of fat in hepatocytes. Excess ROS production is also responsible for oxidation of LDL, which can promote transformation of macrophages to foam cells, which is considered to be the first step in the formation of an atherosclerotic lesion.
Hepatic steatosis produces proinflammatory cytokines including interleukin-6, interleukin-8, and tumor necrosis factor-α (TNF-α), which in turn triggers the production of acute-phase reactants, C-reactive protein (CRP) and fibrinogen, oxidative stress, and de-novo TNF-α pathways. Targher et al. 42 have shown markedly increased levels of plasma CRP, fibrinogen, von Willebrand factor, and plasminogen activator inhibitor-1 activity in healthy nonsmoking indiviudals with hepatic steatosis than in those without, even after controlling for other confounders such as age, BMI, blood pressure, IR, and TG levels. Similarly, proinflammatory cytokines are found in arthrogenic states. CRP is one of the most commonly used inflammatory markers for risk stratifying patients with CVD. CRP independently correlates with poor cardiovascular outcomes such as myocardial infarction, cerebrovascular accidents, and sudden cardiac death 43–46.
Adiponectin
Reduced levels of adiponectin have been observed in patients with NAFLD primarily driven by abundance of macrophage driven inflammation in adipose tissue 47,48. Adiponectin is a protein secreted by adipose tissue with antithrombotic properties and is considered to play a role in decreasing hepatic and systemic IR and attenuate hepatic inflammation and fibrosis; hence, decreased levels may represent another possible mechanism linking NAFLD and vascular disease.
Dyslipidemia
Lipid metabolism in patients with NAFLD is dysregulated. Typical findings include the following: increased levels of very LDL and small-density LDL (non-type A), increased levels of TG, a high apolipoprotein (apo) B/A1 ratio, and decreased levels of high-density lipoproteins, and that have been described and associated with increased CVD and found in patients with MetS 29,30. Defects have been found in multiple steps of lipid metabolism. One of the mechanisms of this imbalance is likely a result of upregulation of transcription factor sterol regulatory element binding protein-1c. Sterol regulatory element binding protein-1c inhibits FFA oxidation, but in addition to insulin, synergistically stimulates genes involved in de-novo lipogenesis, resulting in increased hepatocyte lipid content. 49–51. De-novo lipogenesis or FFA production is five-fold higher in patients with NAFLD compared with normal individuals and FFA also fails to increase postprandially. This is turn results in the production of the atherogenic lipid profile as described above. Dyslipidemia observed in patients with NAFLD is artherogenic likely because of differences in the subpopulations of LDL; for instance, there are increased levels of small-dense LDL, which is artherogenic, versus type A LDL 5,31,52,53. Small-density LDL particles enter the subendothelial space by passing easily through endothelial fenestrations, resulting in inflammation and plaque formation, leading to CAD.
Obstructive sleep apnea
Obstructive sleep apnea (OSA) is increasingly being recognized as a risk for NAFLD, already well established as a risk factor for CAD and cardiac arrhythmias. MetS and OSA are referred to in the literature as ‘Syndrome Z’ and the common denominator between these two is obesity. Almost 50% of NAFLD patients have symptoms of OSA 54. The mechanisms of this relationship are not well understood, but some proposed mechanisms include hypoxia leading to oxidative stress and generation of ROS, dysregulation of adipokines, specifically increased levels of leptin and decreased levels of ghrelin and adiponectin, and alterations in the hypothalamus–pituitary–adrenal axis that promote MetS. Patients with severe OSA were found to be more IR, with worse liver histology including necroinflammation, steatosis, and fibrosis, suggesting that the mechanism may be related to IR independent of BMI 55.
Postprandial hyperlipidemia
Postprandial hyperlipidemia is a risk factor for both NAFLD and CVD 56–58. Postprandial hyperlipidemia occurs as a result of dysregulated lipoprotein metabolism specifically from an increase in remnant lipoproteins 59. Swarbrick et al. 60 and Stanhope et al. 61 studies have shown increased de-novo lipogenesis as well as 24 h postprandial TGs including increased levels of apo B, LDL, oxidized LDL, remnant lipoprotein TG, and the apo B/apo A1 ratio (all biomarkers were increased for CVD) after consumption of fructose-sweetened beverages for 10 weeks. This observation may explain in part why some lean, overweight, and obese individuals with NAFLD may encounter CVD despite normal fasting lipid profiles or taking lipid-lowering medication.
Clinical implications and treatment
There is a strong association between NAFLD and CVD, with a common denominator being MetS. To date, there are no guidelines to aid clinicians on how to risk stratify patients with NAFLD to determine their risk of CVD. Wong et al. 62, similar to others, have shown that fatty liver disease is associated with CAD independent of other metabolic factors, but similar to other studies, the presence of fatty liver cannot predict cardiovascular mortality and morbidity in patients with established CAD.
Treatments targeted to improve NAFLD and hence improve CVD outcomes should be aimed at ameliorating the metabolic derangements, which are essentially all linked to IR. Once the diagnosis of NAFLD is made, a multidisciplinary approach should be adopted. Assessment of liver fibrosis should be performed with a combination of noninvasive markers of fibrosis and liver biopsy to determine the presence of NASH and other concomitant liver diseases. There should be a focus on healthy lifestyle modification, regular fitness or exercise, realistic weight loss goals, and close follow-up with a health coach and/or a dietician to understand and make better food choices including portion control, avoiding high-fructose corn syrup, artificial sweeteners, and high trans-saturated fats. Patients with NAFLD should be screened for impaired glucose tolerance and diabetes, dyslipidemia, and when appropriate OSA. Individuals should also be screened for metabolic causes of NAFLD. Finally, associated conditions should be treated aggressively and there should be appropriate referral to cardiology, endocrine, weight, and wellness centers and bariatric surgery as needed.
Lifestyle interventions
Weight loss has been shown to improve hepatic steatosis, inflammation, and furthermore improvement in the risk factors of MetS. Vilar-Gomez et al. 63. showed that after 52 weeks of lifestyle changes, 25% of the patient population achieved resolution of steatohepatitis, 47% showed a reduction in the nonalcoholic fatty liver disease activity score (NAS), and 19% showed regression of fibrosis. Furthermore, the degree of weight loss was associated independently with improvement in all NASH-related histologic parameters. 10% weight loss was associated with 90% resolution of NASH and 45% showed regression of fibrosis 63. The type of exercise regimen to achieve weight loss has been questioned and continues to be explored. Keating et al. 64 have shown that irrespective of the exercise regimen, improvement in liver and visceral fat can be achieved in overweight, sedentary, or inactive adults. In their study, 48 patients were randomized to one of four exercise groups for 8 weeks: a low-to-moderate intensity, high-volume aerobic exercise (LO : HI) group, a high-intensity, low-volume aerobic exercise (HI : LO) group, a low-to-moderate intensity, low-volume aerobic exercise (LO : LO), and a placebo group. Participants in the three exercise groups showed an average 18–29% reduction in liver fat during the 8-week period, with the greatest liver and visceral fat reduction found in participants in the HI : LO and LO : HI groups.
Diet
In general, hypercaloric diets, rich in trans-saturated fats and cholesterol, high-fructose corn syrup has been shown to increase visceral adiposity, and stimulate hepatic lipid accumulation and progression of disease. Current guidelines recommend a low-calorie diet with 30% energy deficit in individuals at risk for metabolic disease 65,66. Small studies have looked at the effects of a low-carbohydrate, ketogenic diet on NAFLD. Haufe et al. 67 studied the effects of reduced fat and carbohydrate diets on intrahepatic fat by liver spectroscopy. Decreased quantities of intrahepatic lipid content were found in the patients who completed the study, with the largest response in those with elevated fat content at baseline. De Luis et al. 68 have shown the beneficial effects of a low-carbohydrate and low-fat diet on IR measured by the homeostasis model assessment for insulin sensitivity and improvement in ALT. Several diets have been studied, with the most promising results described with a Mediterranean diet 69,70. Long-term trials testing the Mediterranean diet and similar diets need to be conducted. No specific dietary intervention has been proven to be effective, except calorie restriction. The focus should be on the importance of making sustainable changes versus taking drastic measures for weight loss.
As described earlier, individuals with NAFLD have depleted levels of polyunsaturated fatty acids (PUFAs), elevated TGs, and upregulation of lipogenesis. PUFAs are vital in the coordination of lipid oxygenation and downregulation of lipid synthesis. Spadaro et al. 71 randomized 40 patients to diet and PUFAs dosed at 2 g daily versus diet alone and followed patients for 6 months. At the end of the study period, patients in the diet and PUFA group showed a decrease in ALT, TG, TNF-α, and homeostatic model assessment, whereas no significant change was observed in the diet-alone group.
More recently, Argo et al. 72 and Dasarathy et al. 73 evaluated the effects of PUFAs on liver histology. Argo’s double-blind randomized placebo-controlled trail compared fish oil with placebo for 1 year with routine counseling on diet and exercise. Although they found a significant reduction in hepatic steatosis (by abdominal MRI), there was no reduction in NAS scores 72. Similar findings were observed in Dasarathy et al. 73 study; the difference was in the type of PUFAs administered (eicosapentaenoic acid and docasahexaenoic acid) and duration of the study (48 months). It remains unclear whether administration of PUFAs is beneficial; longer studies, higher doses, or specific populations may benefit from this intervention. The AASLD guidelines do not recommend the use of fish oil for the treatment of fatty liver disease unless hypertriglyceridemia is present.
Insulin resistance and diabetes
Biguanides (metformin) and thiazolidinediones are the two classes of insulin sensitizers studied in humans. Belfort et al. 74 randomized-controlled trial compared diet plus pioglitazone with diet plus placebo in 55 patients. The pioglitazone-treated group showed an improvement in ALT by 50%, steatosis by 54%, and insulin sensitivity by 48%. There was also amelioration of liver inflammation and ballooning necrosis, but not fibrosis. The PIVENS trial, a multicenter placebo-controlled trial that compared pioglitazone versus vitamin E versus placebo, in nondiabetic patients treated for 96 weeks showed similar results, with improvement of liver biochemistry, steatosis, and liver inflammation 75. Cusi et al. 76 recently conducted a single-center randomized double-blind placebo-controlled trial comparing a hypocaloric diet plus 45 mg of pioglitazone daily with a hypocaloric diet and placebo for 18 months, followed by 18 months of open-label pioglitazone. 58% of patients in the treatment group achieved an improvement in the NASH score by at least two points and 51% achieved resolution of NASH (P<0.001 for each). In addition, treatment with pioglitazone was associated with an improvement in the fibrosis score (P=0.039), hepatic TG content from 19 to 7% (P<0.001), and improved adipose tissue, hepatic, and muscle insulin sensitivity (P<0.001). These effects persisted at 36 months. There was no difference in adverse events between the two treatment groups, except that weight gain was greater in the pioglitazone-treated group 76. The positive effects of antidiabetic medications in improving CVD has also been shown in the cardiovascular literature) study. The Pioglitazone Effect on Regression of Intravascular Sonographic Coronary Obstruction Prospective Evaluation study 77 compared the effects of an insulin sensitizer, pioglitazone, with an insulin secretaogogue, glimeride, on the progression of coronary atherosclerosis in patients with T2DM. The percentage atheroma volume decreased with pioglitazone, 0.16%, and increased with glimeride, 0.73% (P=0.002). Similar beneficial effects were found with changes in HgA1c, high-density lipoprotein levels, and median TGs. Although no definitive recommendations can be made for pioglitazone, it can be used for selected patients with NASH and exercise caution should be exercised in patients with a history of congestive heart failure and women at risk of bone fractures.
Liraglutide, a glucagon-like peptide, has been studied in patients with NASH and T2DM. In the Armstrong et al. 78 multicenter double-blind randomized placebo-controlled study (LEAN trail), 40% of patients receiving liraglutide showed an improvement in histology of NASH, with fewer patients showing progression of fibrosis compared with placebo, 9%. Marso et al. 79 studied the effect of liraglutide on cardiovascular outcomes in the T2DM population with high cardiovascular risk (LEADER trial). A statistically significant all-cause mortality benefit and improvement in cardiovascular outcomes was observed in the liraglutide treatment group. Similarly, the effects of empagliflozin, a sodium-glucose transporter 2 inhibitor, have been studied in patients with T2DM on cardiovascular morbidity and mortality. In this phase 2 study, patients with T2DM were randomized to 10 or 25 mg of empagliflozin or placebo. The primary composite outcome (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) outcome occurred in 10.5% of the patients in the pooled empagliflozin group compared with 12.1% in the placebo group (P=0.04). In the empagliflozin group, there were significantly lower rates of death from cardiovascular causes [3.7 vs. 5.9% in the placebo group; 38% relative risk reduction (RRR)], hospitalization for heart failure (2.7 and 4.1%, respectively; 35% RRR), and death from any cause (5.7 and 8.3%, respectively; 32% RRR) 80. Both liraglutide and empagliflozin show promise in the treatment of T2DM and improvement of CVD, both risk factors for NAFLD.
Metformin is not recommended as a first-line agent in patients with NALFD because of lack of evidence to support improvement in liver histology 81. However, there are data to support that metformin may play a role in components of MetS. Metformin improves lipid metabolism, vascular smooth muscle, and cardiomyocyte intracellular calcium shuttling, affecting vasodilation, endothelial function, hypercoagulation, and platelet hyperactivity.
Antioxidants
Vitamin E in the PIVENS study 52 has effectively shown improvement in hepatic steatosis, inflammation, ballooning, and resolution of NASH in nondiabetic patients (36% compared with placebo, 21%). The concern with vitamin E is its long-term safety data on overall mortality, hemorrhagic stroke, and the association of prostate cancer in men older than 50 years of age. At present, vitamin E dosed at 800 IU daily can be used in nondiabetic patients with NASH.
There is a subset of patients who can benefit from vitamin E therapy in terms of reducing cardiovascular outcomes. The HOPE and ICARE studies investigated the role of vitamin E in reducing the cardiovascular events in patients with T2DM 82,83. The haptoglobin (Hp) protein has antioxidant activity and is associated with cardiovascular events in patients with T2DM. The Hp gene consists of two common alleles: 1 and 2. The Hp2 allele protein is inferior to the Hp1 allele protein in its antioxidant properties. A meta-analysis of these two studies showed a significant reduction in the composite endpoint (nonfatal myocardial infarction, stroke, and CVD death) in Hp2-2 individuals with vitamin E (P=0.006). Treatment of patients with the Hp2-2 genotype with vitamin E prevented one myocardial infarct, stroke, or cardiovascular death, with an estimated 3-year increase in life expectancy 84. Asgharpour et al. 85 recently reported that the presence of Hp2 is associated with a significant improvement in the NAS score, but in addition, improvements in ALT, AST, and cholesterol were observed. Further studies are needed to identify NAFLD patients who are likely to benefit from treatment with vitamin E to reduce cardiovascular risks and outcomes by the presence of Hp2 genotype.
Most interesting of all is a farsenoid X receptor agonist, obeticholic acid, with postulated multiple mechanisms of action including improvement in IR and hepatic steatosis, with possibly both antifibrotic and antioxidant activity 86. In the phase IIb FLINT trial 87, patients with noncirrhotic NASH were treated with 72 weeks of obeticholic acid, with improvement in NASH and fibrosis. Except for pruritis, treatment was well tolerated; long-term data on implications on the lipid profile and cardiovascular risk will be available soon.
Several RCTs have shown that ursodeoxycholic acid is ineffective in improving NASH 88,89.
Dyslipidemia
Statin therapy is recommended in patients with CAD or risk factors for CAD with a goal of LDL less than 100 mg/dl. There is no direct evidence to illustrate the beneficial effects of statin therapy on NASH; furthermore, statin therapy is generally underutilized in patients with liver disease because of concerns of safety and persistent elevated liver enzymes. Many studies have shown that statins are safe in patients with liver disease such as Lewis et al. 90 study, which reported the efficacy and safety of treating dyslipidemia in patients with chronic liver disease in their placebo-controlled double-blind multicenter study of high-dose pravastatin (80 mg/daily). In addition to improvement in LDL, there was a significant reduction in ALT and no adverse outcomes were observed.
In addition to an ameliorating lipid profile, statin therapy has been shown in in-vitro and in-vivo studies to inhibit and HCC. Several mechanisms have been described including induction of cell apoptosis 91 and inhibition of hepatic tumor cell growth 92. To date, there are no multicenter randomized-controlled trials assessing the chemopreventative effects of HCC in NAFLD. A single clinical trial supports the hypothesis that the use of statins might contribute toward survival in those with unresectable HCC. 91 patients with unresectable HCC were recruited and randomized to pravastatin versus placebo, with the pravastatin group showing an impressive 9-month longer survival 93. Other population-based cohort studies have shown a similar effect, but limited by study design, selection bias, and dose-dependent effects 94–96. On the basis of this, statin therapy should not be prescribed to patients with NAFLD for the sole purpose of chemoprevention of HCC, but indicated in those with a high risk of CHD as indicated by patient phenotype and associated cardiac equivalent risk factors.
As described earlier, omega-3 fatty acids should not be prescribed to all patients with NASH, except when indicated for hypertriglyceridemia.
Endoscopic and surgical therapies
The remarkable benefits of Roux-en-Y gastric bypass surgery on cardiovascular risk factors are well known from improvement in hypertension, dyslipidemia, and T2DM 97–101. There is also compelling evidence showing the beneficial effects of Roux-en-Y gastric bypass surgery on NASH. In the Lassailly et al. 102 prospective study, there was an improvement in NASH as early as 1 year after gastric bypass surgery in 85% of patients. Histological improvement was observed in ballooning and lobular inflammation and fibrosis was reduced in 33.8% of patients. There was also a statistically significant improvement in all parameters of the lipid profile, except LDL. Similar results have been reported by Taitano et al. 103, with resolution of NASH in 75% patients and improvement in fibrosis in 53% patients. Roux-en-Y gastric bypass can be considered in select patients with NASH with a careful assessment of the perioperative and postoperative risks and complications.
Endoscopic bariatric therapies are effective in the treatment of obesity and MetS, and small studies have shown its beneficial effects on NASH. A small study randomized patients with BMI of at least 27 kg/m2 and histology-proven NASH to diet and Orbera balloon and diet and Sham procedure for 6 months. Liver histology was assessed before and after treatment. The Orbera balloon placement group showed a significant reduction in BMI and histological features of NASH 104. Another study examined the liver fat content before and after placement of Orbera balloon, with a significantly higher decrease in liver fat, body fat composition, and liver biochemistries 105.
There is increasing interest in the use of endoscopic bariatric therapies beyond its primary objective of achieving weight loss, resolving MetS, and NASH. A small prospective pilot study assessed the effect of intragastric balloon therapy on left ventricular (LV) function and LV mass in morbidly obese patients at 6 months 106. BMI, LV mass index, and left volume index were significantly decreased (44±8 vs. 38±5, P<0.001, 112±21 vs. 93±17, P=0.001, and 20±6 vs. 14±5, P=0.02, respectively) following intragastric balloon placement. Similar results have been observed by Garza et al. 107 in their age-sex-BMI-controlled observational study of patients with obesity who underwent Roux-en-Y gastric bypass surgery and who had undergone echocardiography before and after surgery. Multivariate analysis showed a positive correlation between the change in body weight and ventricular septum thickness (R=0.33), posterior wall thickness (R=0.31), LV mass (R=0.38), right ventricular end-diastolic area (R=0.22), and estimated right ventricular systolic pressure (R=0.39), all with P values of less than 0.05 107.
Several endoscopic bariatric procedures such as intragastric balloons have been approved and in development for obesity. Prospective randomized studies need to be carried out before recommendations can be made for endoscopic bariatric surgery for the treatment of NASH exclusively.
Conclusion
Fatty liver disease is a growing pandemic with grave implications associated with cardiovascular and overall morbidity and mortality. The approach to the management of this patient population involves increased community and physician awareness, understanding that it is a multisystem disease requiring comprehensive collaborative efforts that are multidisciplinary. The focus of treatment should go beyond improvement in liver enzymes, but ameliorating diabetes, treating dyslipidemia and OSA to in turn preventing progression of liver disease and decreasing cardiovascular mortality.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kotronen A, Yki-Jarvinen H. Fatty liver: a novel component of metabolic syndrome. Arterioscler Thromb Vasc Biol 2008; 28:27–38. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and metabolic syndrome: an eleven-year follow-up study. Am J Gastroneterol 2009; 104:861–867. [DOI] [PubMed] [Google Scholar]
- 3.Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Liver Int 2009; 29:113–119. [DOI] [PubMed] [Google Scholar]
- 4.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of most common cause of chronic liver disease in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011; 9:524–530.e1. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R, Olliss S, et al. Presence and severity of nonalcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol 2012; 56:234–240. [DOI] [PubMed] [Google Scholar]
- 6.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology 2005; 42:44–52. [DOI] [PubMed] [Google Scholar]
- 7.Browning JD, Szczepaniak LS, Dobbins IR, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the Unites States: impact of ethnicity. Hepatology 2004; 40:1387–1395. [DOI] [PubMed] [Google Scholar]
- 8.Williams CD, Stengel J, Asike MI. Prevalence of nonalcoholic fatty liver disease and nonalcoholic seatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011; 140:124–131. [DOI] [PubMed] [Google Scholar]
- 9.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011; 141:1249–1253. [DOI] [PubMed] [Google Scholar]
- 10.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015; 62:S47–S64. [DOI] [PubMed] [Google Scholar]
- 11.Petta S, Valenti L, Bugianesi E, Targher G, Bellentani S, Bonino F. Special Interest Group on Personalised Hepatology of the Italian Association for the Study of the Liver (AISF); Special Interest Group on Personalised Hepatology of Italian Association for Study of Liver AISF. A ‘systems medicine’ approach to the study of nonalcoholic fatty liver disease. Dig Liver Dis 2016; 48:333–342.26698409 [Google Scholar]
- 12.MIkolasevic I, Milic S, Wensveen TT, Grgic I, Jakopcic I, Stimac D, et al. Nonalcoholic fatty liver disease – a multisystem disease? World J Gastroenterol 2016; 22:9488–9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013; 10:330–344. [DOI] [PubMed] [Google Scholar]
- 14.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of nonalcoholic fatty liver disease (NAFLD) and diagnostic accuracy on noninvasive tests for liver disease severity. Ann Med 2011; 43:617–649. [DOI] [PubMed] [Google Scholar]
- 15.Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis 2014; 64:638–652. [DOI] [PubMed] [Google Scholar]
- 16.Bang KB, Cho YK. Comorbidities and metabolic derangements of NAFLD. J Lifestyle Med 2015; 5:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oni EZT, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis 2013; 230:258–267. [DOI] [PubMed] [Google Scholar]
- 18.Adams LA, Lymp JF, St, Sauver J. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005; 129:113–121. [DOI] [PubMed] [Google Scholar]
- 19.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010; 363:1341–1350. [DOI] [PubMed] [Google Scholar]
- 20.Lin YC, Lo HM, Chen JD. Sonographic fatty liver, overweight and ischemic heart disease. World J Gastroenterol 2005; 11:4838–4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakur ML, Sharma S, Kumar A, Bhatt SP, Luthra K, Guleria R, et al. Nonalcoholic fatty liver disease is associated with subclinical atherosclerosis independent of obesity and metabolic syndrome in Asian Indians. Atherosclerosis 2012; 223:507–511. [DOI] [PubMed] [Google Scholar]
- 22.Pacifico L, Cantisani V, Ricci P, Osborn JF, Schiavo E, Ferrara E, et al. Nonalcoholic fatty liver disease and carotid atherosclerosis in children. Paediatr Res 2008; 63:423–427. [DOI] [PubMed] [Google Scholar]
- 23.Brea A, Mosquera D, Martin E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case–control study. Arterioscler Thromb Vasc Biol 2005; 5:1040–1050. [DOI] [PubMed] [Google Scholar]
- 24.Assy N, Djibre A, Farah R, Grosovski M, Marmor A. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology 2010; 254:393–400. [DOI] [PubMed] [Google Scholar]
- 25.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Nonalcoholic fatty liver disease and risk of cardiovascular disease: a meta-analysis. J Hepatol 2016; 65:589–600. [DOI] [PubMed] [Google Scholar]
- 26.Arulanandan A, Ang B, Bettencourt R, Hooker J, Behling C, Lin GY, et al. Association between quantity of liver fat and cardiovascular risk in patients with nonalcoholic fatty liver disease independent of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2015; 13:1513–20. e1. [DOI] [PubMed] [Google Scholar]
- 27.Ballestri S, Zona S, Targher G, Romagnoli D, Baldelli E, Nascimbeni F, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol 2016; 31:936–944. [DOI] [PubMed] [Google Scholar]
- 28.Treeprasertsuk S, Leverage S, Adams LA, Lindor KD, Sauver JS, Angulo P. The Framingham Risk Score and heart disease in nonalcoholic fatty liver disease. Liver Int 2012; 32:945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang MH, Sung J, Gwak GY. The associations between apolipoprotein B, A1 and the B/A1 ratio and nonalocoholic fatty liver disease in both normal- weight and overweight Korean population. J Clin Lipidol 2016; 10:289–298. [DOI] [PubMed] [Google Scholar]
- 30.Bril F, Sninsky JJ, Baca AM, Superko HR, Portillo Sanchez P, Biernacki D, et al. Hepatic steatosis and insulin resistance, but not steatohepatitis, promote atherogenic dyslipidemia in NAFLD. J Clin Endocrinol Metab 2016; 101:644–652. [DOI] [PubMed] [Google Scholar]
- 31.Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology 2010; 51:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cobble M, Bale B. Carotid intima–media thickness: knowledge and application to everyday practice. Postgrad Med 2010; 122:10–18. [DOI] [PubMed] [Google Scholar]
- 33.Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol 2014; 20:13306–13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol 2009; 7:234–238. [DOI] [PubMed] [Google Scholar]
- 35.Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow up. Hepatology 2010; 51:595–602. [DOI] [PubMed] [Google Scholar]
- 36.Dunne W, Xu R, Wingard DL, Rogers C, Angulo P, Younossi ZM, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol 2008; 103:2263–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol 2008; 49:608–612. [DOI] [PubMed] [Google Scholar]
- 38.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology 2009; 136:477–485. [DOI] [PubMed] [Google Scholar]
- 39.Dogan S, Celikbilek M, Yilmaz YK, Sarikaya S, Zararsiz G, Serin HI, et al. Association between liver fibrosis and coronary heart disease risk in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2015; 27:298–304. [DOI] [PubMed] [Google Scholar]
- 40.Bonora E, Formemtini G, Calcaterra F, Lombardi S, Marini F, Zenari L, et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care 2002; 25:1135–1141. [DOI] [PubMed] [Google Scholar]
- 41.Pilz S, Scharnagl H, Tiran B, Seelhorst U, Wllnitz B, Boehm BO, et al. Free fatty acids are independently associated with all-cause and cardiovascular mortality in subjects with coronary artery disease. J Clin Endocrinol Metab 2006; 91:2542–2547. [DOI] [PubMed] [Google Scholar]
- 42.Targher G, Bertolini L, Zoppini G, Zenari L, Falezza G. Increased plasma markers of inflammation and endothelial dysfunction and their association with microvascular complications in type1 diabetic patients without clinically manifest macroangiopathy. Diabet Med 2005; 22:999–1004. [DOI] [PubMed] [Google Scholar]
- 43.Ferreiros ER, Boissonnet CP, Pizarro R, Merletti PF, Corrado G, Cagide A, Bazzino OO. Independent prognostic value of elevated C-reactive protein in unstable angina. Circulation 1999; 100:1958–1963. [DOI] [PubMed] [Google Scholar]
- 44.Tomoda H, Aoki N. Prognostic value of C-reactive protein levels within six hours after the onset of acute myocardial infarction. Am Heart J 2000; 140:324–328. [DOI] [PubMed] [Google Scholar]
- 45.Tommasi S, Carluccio E, Bentivoglio M, Buccolieri M, Mariotti M, Politano M, Corea M. C-reactive protein as a marker for cardiac ischemic events in the year after a first, uncomplicated myocardial infarction. Am J Cardiol 1999; 83:1595–1599. [DOI] [PubMed] [Google Scholar]
- 46.Nikfardjam M, Müllner M, Schreiber W, Oschatz E, Exner M, Domanovits H, et al. The association between C-reactive protein on admission and mortality in patients with acute myocardial infarction. J Intern Med 2000; 247:341–345. [DOI] [PubMed] [Google Scholar]
- 47.Li G, Hu H, Shi W, Li Y, Liu L, Chen Y, et al. Elevated hematocrit in nonalcoholic fatty liver disease: a potential cause for the increased risk of cardiovascular disease? Clin Hemorheol Microcirc 2012; 51:59–68. [DOI] [PubMed] [Google Scholar]
- 48.Aygun C, Senturk O, Hulagu S, Uraz S, Celebi A, Konduk T, et al. Serum levels of hepatoprotective peptide adiponectin in non-alcoholic fatty liver disease. Eur J Gastroenterol Hepatol 2006; 18:175–180. [DOI] [PubMed] [Google Scholar]
- 49.Ahmed MH, Abu EO, Byrne CD. Non-alcoholic fatty liver disease (NAFLD): new challenge for general practitioners and important burden for health authorities? Prim Care Diabetes 2010; 4:129–137. [DOI] [PubMed] [Google Scholar]
- 50.Hacihamdioğlu B, Okutan V, Yozgat Y, Yildirim D, Kocaoğlu M, Lenk MK, Ozcan O. Abdominal obesity is an independent risk factor for increased carotid intima- media thickness in obese children. Turk J Pediatr 2011; 53:48–54. [PubMed] [Google Scholar]
- 51.Dogru T, Genc H, Tapan S, Ercin CN, Ors F, Aslan F, et al. Elevated asymmetric dimethylarginine in plasma: an early marker for endothelial dysfunction in non-alcoholic fatty liver disease? Diabetes Res Clin Pract 2012; 96:47–52. [DOI] [PubMed] [Google Scholar]
- 52.Nseir W, Shalata A, Marmor A, Assy N. Mechanisms linking nonalcoholic fatty liver disease with coronary artery disease. Dig Dis Sci 2011; 56:3439–3449. [DOI] [PubMed] [Google Scholar]
- 53.Jiang ZG, de Boer IH, Mackey RH, Jensen MK, Lai M, Lai M, et al. Associations of insulin resistance, inflammation and liver synthetic function with very low-density lipoprotein: the Cardiovascular Health Study. Metabolism 2016; 65:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh H, Pollock R, Uhanova J, Kryger M, Hawkins K, Minuk GY. Symptoms of obstructive sleep apnea in patients with nonalcoholic fatty liver disease. Dig Dis Sci 2005; 50:2338–2343. [DOI] [PubMed] [Google Scholar]
- 55.Tanné F, Gagnadoux F, Chazouillères O, Fleury B, Wendum D, Lasnier E, et al. Chronic liver injury during obstructive sleep apnea. Hepatology 2005; 41:1290–1296. [DOI] [PubMed] [Google Scholar]
- 56.Hessheimer AJ, Forner A, Varela M, Bruix J. Metabolic risk factors are a major comorbidity in patients with cirrhosis independent of the presence of hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2010; 22:1239–1244. [DOI] [PubMed] [Google Scholar]
- 57.Lee YJ, Shim JY, Moon BS, Shin YH, Jung DH, Lee JH, Lee HR. The relationship between arterial stiffness and nonalcoholic fatty liver disease. Dig Dis Sci 2012; 57:196–203. [DOI] [PubMed] [Google Scholar]
- 58.Ahmed MH, Barakat S, Almobarak AO. Nonalcoholic fatty liver disease and cardiovascular disease: has the time come for cardiologists to be hepatologists? J Obes 2012; 2012:483135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colak Y, Senates E, Yesil A, Yilmaz Y, Ozturk O, Doganay L, et al. Assessment of endothelial function in patients with nonalcoholic fatty liver disease. Endocrine 2013; 43:100–107. [DOI] [PubMed] [Google Scholar]
- 60.Swarbrick MM, Stanhope KL, Elliott SS, Graham JL, Krauss RM, Christiansen MP, et al. Consumption of fructose-sweetened beverages for 10 weeks increases postprandial triacylglycerol and apolipoprotein-B concentrations in overweight and obese women. Br J Nutr 2008; 100:947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, et al. Consuming fructose-sweetened, not glucose- sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009; 119:1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong VW, Wong GL, Yip GW, Limquiaco J, Chu WC, Chim AM, et al. Coronary artery disease and cardiovascular outcomes in patients with non-alcoholic fatty liver disease. Gut 2011; 60:1721–1727. [DOI] [PubMed] [Google Scholar]
- 63.Vilar- Gomez E, Martinez- Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015; 149:367–378. [DOI] [PubMed] [Google Scholar]
- 64.Keating SE, Hackett DA, Parker HM, O’Connor HT, Gerofi JA, Sainsbury A, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol 2015; 63:174–182. [DOI] [PubMed] [Google Scholar]
- 65.Rosenzweig JL, Ferrannini E, Grundy SM, Haffner SM, Heine RJ, Horton ES, Kawamori R. Primary prevention of cardiovascular disease and type 2 diabetes in patients at metabolic risk: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2008; 93:3671–3689. [DOI] [PubMed] [Google Scholar]
- 66.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014; 129 (Suppl 2):S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haufe S, Engeli S, Kast P, Böhnke J, Utz W, Haas V, et al. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology 2011; 53:1504–1514. [DOI] [PubMed] [Google Scholar]
- 68.De Luis DA, Aller R, Izaola O, Gonzalez Sagrado M, Conde R. Effect of two different hypocaloric diets in transaminases and insulin resistance in nonalcoholic fatty liver disease and obese patients. Nutr Hosp 2010; 25:730–735. [PubMed] [Google Scholar]
- 69.Trovato F, Catalano D, Martines G, Pace P, Trovato G. Mediterranean diet and non-alcoholic fatty liver disease: the need of extended and comprehensive interventions. Clin Nutr 2015; 34:86–88. [DOI] [PubMed] [Google Scholar]
- 70.Bozzetto L, Prinster A, Annuzzi G, Costagliola L, Mangione A, Vitelli A, et al. Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care 2012; 35:1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spadaro L, Magliocco O, Spampinato D, Piro S, Oliveri C, Aragona C, et al. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig Liver Dis 2008; 40:194–199. [DOI] [PubMed] [Google Scholar]
- 72.Argo CK, Patrie JT, Lackner C, Henry TD, de Lange EE, Weltman AL, et al. Effects of n-3 fish oil on metabolic and histological parameters in NASH: a double-blind, randomized, placebo-controlled trial. J Hepatol 2015; 62:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dasarathy S, Dasarathy J, Khiyami A, Yerian L, Hwakins C, Sargent R, McCullough AJ. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol 2015; 49:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006; 355:2297–2307. [DOI] [PubMed] [Google Scholar]
- 75.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010; 362:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-term Pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med 2016; 165:305–315. [DOI] [PubMed] [Google Scholar]
- 77.Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 2008; 299:1561–1573. [DOI] [PubMed] [Google Scholar]
- 78.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hul D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomized, placebo-controlled phase 2 study. Lancet 2016; 387:679–690. [DOI] [PubMed] [Google Scholar]
- 79.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcome in type 2 diabetes. N Engl J Med 2016; 375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 81.Chalsani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The Diagnosis and Management of Non-alcoholic Fatty Liver Disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol 2012; 107:811–826. [DOI] [PubMed] [Google Scholar]
- 82.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000; 342:154–160. [DOI] [PubMed] [Google Scholar]
- 83.Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, et al. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and haptoglobin 2-2 genotype: a prospective, double-blinded clinical trial. Arterioscler Thromb Vasc Biol 2008; 28:341–347. [DOI] [PubMed] [Google Scholar]
- 84.Blum S, Vardi M, Brown JB, Russell A, Milman U, Shapira C, et al. Vitamin E reduces cardiovascular disease in individuals with diabetes mellitus and the haptoglobin 2-2 genotype. Pharmacogenomics 2010; 11:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Asgharpour A, Cazanave SC, Yates KP, Vincent R, Kohli R, Hameed B, et al. Haptoglobin genotype 2 allele identifies adults and children with nonalcoholic steatohepatitis (NASH) who improve liver histology and cardiometabolic risk factors with vitamin E treatment. Hepatology 2015; 62:288A–291A. [Google Scholar]
- 86.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, et al. Efficacy and safety of the farsenoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013; 145:574–582. [DOI] [PubMed] [Google Scholar]
- 87.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomized, placebo-controlled trial. Lancet 2015; 385:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology 2004; 39:770–778. [DOI] [PubMed] [Google Scholar]
- 89.Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rössle M, Cordes HJ, et al. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology 2010; 52:472–479. [DOI] [PubMed] [Google Scholar]
- 90.Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology 2007; 46:1453–1463. [DOI] [PubMed] [Google Scholar]
- 91.Han KH, Rha SW, Kang HJ, Bae JW, Choi BJ, Choi SY, et al. Evaluation of short-term safety and efficacy or HMG-CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage. J Clin Lipidol 2012; 6:340–351. [DOI] [PubMed] [Google Scholar]
- 92.Paragh G, Kertai P, Kovacs P, Paragh G, Jr, Fülöp P, Foris G. HMG CoA reductase inhibitor fluvastatin arrests the development of implanted hepatocarcinoma in rats. Anticancer Res 2003; 23:3949–3954. [PubMed] [Google Scholar]
- 93.Kawata S, Yamasaki E, Nagase T, Inui Y, Ito N, Matsuda Y, et al. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br J Cancer 2011; 84:886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.El-Serag HB, Johnson ML, Hachem C, Morgana RO. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology 2009; 136:1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chiu HF, Ho SC, Chen CC, Yang CY. Statin use and the risk of liver cancer: a population-based case–control study. Am J Gastroenterol 2011; 106:894–898. [DOI] [PubMed] [Google Scholar]
- 96.Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol 2010; 30:623–630. [DOI] [PubMed] [Google Scholar]
- 97.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351:2683–2693. [DOI] [PubMed] [Google Scholar]
- 98.Adams TD, Pendleton RC, Strong MB, Kolotkin RL, Walker JM, Litwin SE, et al. Health outcomes of gastric bypass patients compared to nonsurgical, nonintervened severely obese. Obesity (Silver Spring) 2010; 18:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Batsis JA, Romero-Corral A, Collazo-Clavell ML, Sarr MG, Somers VK, Brekke L, Lopez-Jimenez F. Effect of weight loss on predicted cardiovascular risk: change in cardiac risk after bariatric surgery. Obesity (Silver Spring) 2007; 15:772–784. [DOI] [PubMed] [Google Scholar]
- 100.Busetto L, Sergi G, Enzi G, Segato G, De Marchi F, Foletto M, et al. Short-term effects of weight loss on the cardiovascular risk factors in morbidly obese patients. Obes Res 2004; 12:1256–1263. [DOI] [PubMed] [Google Scholar]
- 101.Torquati A, Wright K, Melvin W, Richards W. Effect of gastric bypass operation on Framingham and actual risk of cardiovascular events in class II to III obesity. J Am Coll Surg 2007; 204:776–782. [DOI] [PubMed] [Google Scholar]
- 102.Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology 2015; 149:379–388. [DOI] [PubMed] [Google Scholar]
- 103.Taitano AA, Markow M, Finan JE, Wheeler DE, Gonzalvo JP, Murr MM. Bariatric surgery improves histological features of nonalcoholic fatty liver disease and liver fibrosis. J Gastrointest Surg 2015; 19:429–436. [DOI] [PubMed] [Google Scholar]
- 104.Lee YM, Low HC, Lim LG, Dan YY, Aung MO, Cheng CL, et al. Intragastric balloon significantly improves nonalcoholic fatty liver disease activity score in obese patients with nonalcoholic steatohepatitis: a pilot study. Gastrointest Endosc 2012; 76:756–760. [DOI] [PubMed] [Google Scholar]
- 105.Folini L, Veronelli A, Benetti A, Pozzato C, Cappelletti M, Masci E, et al. Liver steatosis (LS) evaluated through chemical-shift magnetic resonance imaging liver enzymes in morbid obesity; effect of weight loss obtained with intragastric balloon gastric banding. Acta Diabetol 2014; 51:361–368. [DOI] [PubMed] [Google Scholar]
- 106.Koc F, Kavaoglu HA, Celik A, Altunkas F, Karayakali M, Ozbek K, et al. Effect of weight loss induced by intragastric balloon therapy on cardiac function in morbidly obese individuals: a pilot study. Med Princ Pract 2015; 24:432–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Garza CA, Pellikka PA, Somers VK, Sarr MG, Collazo-Clavell ML, Korenfeld Y, Lopez-Kimenez F. Structural and functional changes in left and right ventricles after major weight loss following bariatric surgery for morbid obesity. Am J Cardiol 2010; 105:550–556. [DOI] [PubMed] [Google Scholar]