Abstract
The opioid risk tool (ORT) is a commonly employed measure of risk of aberrant drug related behaviors (ADRB) in patients with chronic pain prescribed opioid therapy. In this study the discriminant predictive validity of the ORT was evaluated in a unique cohort of patients with chronic nonmalignant pain (CNMP) on long-term opioid therapy (LTOT) that displayed no evidence of developing an opioid use disorder (OUD) and a sample of patients with CNMP that developed an OUD after commencing opioid therapy. Results revealed that the original ORT was able to discriminate between patients with and without OUDs (OR=1.624; CI 95%: 1.539-1.715, p< 0.001). A weighted ORT eliminating the gender specific history of preadolescent sexual abuse item revealed comparable results (OR= 1.648; CI 95%: 1.539-1.742, p< 0.001). A revised unweighted ORT (ORT-OUD) removing the history of preadolescent sexual abuse item was notably superior in predicting the development of OUD in patients with CNMP on LTOT (OR= 3.085; CI 95%: 2.725-3.493, p< 0.001) with high specificity (0.851; CI 95%: 0.811-0.885), sensitivity (0.854; 95% CI: 0,799-0.898), positive (0.757; CI 95%: 0.709-0.799) and negative (0.914; CI 95%: 0.885-0.937) predictive values.
Perspective:
The revised ORT (ORT-OUD) is the first tool developed on a unique cohort to predict the risk of developing an OUD in patients with CNMP receiving opioid therapy, as opposed to ADRB that can reflect a number of other issues. The ORT-OUD has clinical utility in providing clinicians a simple, validated method to rapidly screen for the risk of developing OUD in patients on or being considered for opioid therapy.
Introduction
Both chronic pain, and the harms associated with prescription opioid abuse, including serious adverse events and fatalities, ae enormous public health problems. In parallel with a rise in long-term opioid use for chronic nonmalignant pain (CNMP), abuse of prescription opioids in the U.S. escalated more than 113% between 2004 and 2013.20 Safe and effective administration of opioid therapy is recognized as requiring initial and ongoing assessment of the risks related to prescription drug abuse and potential for developing an opioid use disorder (OUD).17 Recent clinical guidelines and professional society position papers for opioid prescribing recommend that prior to initiating opioid therapy in selected candidates, providers should screen patients to identify those at risk for developing an OUD.1,10,14,15,19,34 Efforts are underway to identify significant risk factors for the development of OUD, so that patient selection can be more informed, and the monitoring of those who receive these medications is appropriate to the level of risk severity. Accordingly, a number of validated risk assessment tools have been published to predict the likelihood that a specific patient will develop non-adherence behaviors with prescribed opioid therapy that could indicate possible misuse/abuse problems or an OUD.31 There are prescreening tools that were developed to assess risk in patients being considered for opioid therapy such as the Opioid Risk Tool32, Screener and Opioid Assessment for Patients with Pain3 , and the Diagnosis, Intractability, Risk, Efficacy.2 Other tools were designed to monitor for signs of non-adherence, misuse/abuse in patients currently prescribed opioids for example the Current Opioid Misuse Measure4
However, recent systematic reviews evaluating the predictive validity of these tools have revealed significant limitations.9,29 To date, the extant data are sparse, and the evidence on the accuracy of these tools in predicting abuse is relatively poor overall. For example, the sensitivity and specificity of the most commonly used tools (Opioid Risk Tool, Screener and Opioid Assessment for Patients with Pain-Revised, and Brief Risk Interview) ranges from 0.25 to 0.83 and 0.43 to 0.88 respectively, and the likelihood ratios show poor predictive accuracy.28 In addition, it has been argued that these tools were designed to assess the risk of developing aberrant drug-related behaviors (ADRB) (aberrant opioid use behaviors or opioid misuse), which are reported to be highly prevalent in pain populations (> 40%) and were not specifically developed or validated to predict the more serious outcome of OUD.5 To better guide patient selection for opioid therapy and promote the utilization of risk monitoring strategies and alternate therapies that improve treatment outcomes in patients at risk for developing OUD, tools with improved predictive validity are urgently needed.
Among the most promising screening tools presently available is the Opioid Risk Tool (ORT).32 Developed by Webster and Webster in 2005, this brief, clinician administered self-report tool was designed for use in primary care populations to assess the potential for opioid-related ADRB among adults who are candidates for commencing opioid therapy for CNMP. The 10 items assess family history of substance abuse; personal history of substance abuse; age (between 16-45 years); history of preadolescent sexual abuse (in women only); and presence of psychiatric disease (attention deficit disorder, obsessive compulsive disorder, bipolar disorder, schizophrenia and major depressive disorder). Each item is assigned a weight ranging from 5 to 1 and scoring is stratified by gender, with total scores summed to specify a low, moderate or high level of risk. Although intended to predict the development of opioid-related ADRB, the items characterize known risk factors for substance use disorder (SUD) in general (e.g., age, psychiatric co-morbidities and history of childhood trauma)12,33 and thus, is appropriate for a more extensive evaluation of its accuracy in predicting the development of OUD in patients with CNMP.
A large ongoing study assessing genotypic and phenotypic characteristics of OUD in patients with CNMP7 provided a unique opportunity to more extensively evaluate the ORT in terms of its discriminant predictive validity for OUD. In the parent study sample, approximately one-third of patients with CNMP recruited had developed a new OUD following the initiation of prescribed opioids. This permitted both the psychometric evaluation of the ORT in a large sample of chronic pain patients with and without OUDs, and initial exploration of whether evidence from this analysis would support the current weighting structure of the ORT, and yield data to guide the creation of a shorter, more robust tool for clinical use. Following factor analysis, the original versus revised tools were compared in terms of sensitivity and specificity, and positive and negative predictive values, using a split-half methodology that involved testing in half of the sample, with subsequent validation on the remaining half.
Materials and Methods
This is a secondary analysis of baseline data collected from 1178 patients who consented to participate in a cross sectional naturalistic study to create a phenotypic and genotypic profile of patients’ risk factors for the development of OUD when receiving long-term opioid therapy (LTOT) for CNMP.7 Control patients (patients with CNMP on LTOT displaying no evidence of an OUD) were recruited from multiple ambulatory pain and primary care practices and cases (patients with CNMP with no previous history of a substance use disorder who developed a OUD after commencing LTOT) were recruited from substance abuse treatment facilities in urban and surrounding suburban areas of the Northeast, Pacific Northwest, and Midwest. These sites included the Philadelphia region (University of Pennsylvania and suburbs), Seattle (University of Washington and suburbs), Salt Lake City (Lifetree Clinical Research and Pain Clinic and suburbs), and Boston (Brigham and Women's Hospital, Harvard Medical School). Patients entered the study between November 2012 and July 2018. The University of Pennsylvania, University of Washington, Lifetree and Harvard University Institutional Review Boards approved the protocol and patients provided written consent before participating.
Participants and Procedures
Subjects were patients with CNMP identified and recruited through referrals from practice physicians and staff, and those who consented for study eligibility using information from the electronic medical record (EMR) and self-report measures were screened for eligibility. Specifically, adult patients (age ≥18) were considered eligible if they were Caucasian and of European descent (i.e., 3 of 4 grandparents were of European origin) and the EMR indicated they had CNMP of musculoskeletal or neuropathic origin persisting ≥6 months in duration and had been receiving LTOT defined as receiving opioids consistently (monthly prescriptions) for 6 months or longer. The genotypic objectives of the parent study precluded the inclusion of patients of non-Caucasian races and ethnicities. Patients with pain syndromes due to cancer; gynecologic, abdominal, visceral, dental, trigeminal neuralgia, post-stroke syndrome, or migraine-related pain; neuropathic pain due to metabolic disease, or severe psychiatric conditions preventing the provision of informed consent or questionnaire completion, were excluded.
Classification of Patients with OUD and without OUD:
The EMR was reviewed for each participant from 6 months prior to the date of study consent. Patients were classified as not having an OUD (i.e., control cohort) if the information in the EMR indicated the following: patients were receiving LTOT as defined above; had no current or past history of SUD (except nicotine dependence) or evidence of ADRB (determined using an expert-derived checklist)6; and all urine drug screens in the previous six months were appropriate (presence of prescribed opioid and absence of non-prescribed opioid or illicit drug). To confirm that control subjects over time did not develop an OUD, EMR records were reviewed monthly for 12 months after completing baseline assessments and each control subject completed follow up comprehensive assessments at 6 and 12 months. The case cohort included patients with CNMP who had no previous history of SUD (except nicotine dependence) as defined by DSM-IV9 criteria prior to beginning LTOT; who currently endorsed DSM-IV criteria for “opioid dependence” (OUD) on both the MINI International Neuropsychiatric Interview30 and DSM-IV checklist, and were actively receiving treatment or had been in formal treatment for an OUD. The contact person at each substance abuse treatment facility corroborated that each enrolled case was receiving treatment for a prescription OUD. Enrolled patients were subsequently asked to provide baseline psychological and sociodemographic data, and were reimbursed for study participation.
Measures
Trained research assistants administered all assessments including the MINI and ORT over the phone. To ensure the fidelity of the assessment procedure, the research assistants underwent extensive training and role-play with senior research staff from our center and the principal investigator, are initially monitored and provided continuous feedback and supervision when conducting the phone assessments for a period of three months and subsequently periodically for quality assurance.
Opioid Risk Tool
The Opioid Risk Tool (ORT), is a 10-item instrument created to predict the risk of engaging in opioid-related ADRB among patients with chronic pain being considered for opioid therapy, and to classify the potential risk level as high, moderate, or low (Appendix 1).32 The items were developed based on factors in the empirical literature associated with the risk of substance abuse or considered relevant by the authors; the relative weighting of items was similarly based upon expert judgment. Items are weighed from 1 to 5 (i.e., history of prescription drug abuse=5; history of illegal drug abuse and family history of prescription drug abuse=4; history of alcohol abuse, preadolescent sexual abuse for women, and family history of illegal drug and alcohol abuse for men=3; attention deficit disorder, OCD, bipolar, schizophrenia=2; family history of illegal drug abuse in women=2; and family history of alcohol for women, age, and depression=1).
In its initial validation, the ORT significantly predicted ADRBs among patients with CNMP at one-year follow-up; more than 90% of patients who displayed ADRBs had been classified as "likely to abuse opioids." Furthermore, men (51%) were significantly more likely than women (34%) to display at least one aberrant behavior. Sex differences were not observed in the incidence of at least three aberrant behaviors (25% and 20%, respectively).32 Subsequent psychometric evaluations evaluating the tool’s predictive validity for later (6 – 12 months) ADRBs provided sensitivity values ranging from 0.20 (for high-risk scores) to 0.75 (combining medium- and high-risk scores),22-24,26,28 and specificity ranging from 0.54 to 0.88 (for low-risk scores).22-24,26 Combining both these characteristics, reported Area Under the Curve (AUC) values for the ORT ranged from .358 - .735.11,21,22 Each of these previous evaluations focused on the ability of the ORT to predict subsequent ADRBs, not the more severe outcome of development of OUD, a main goal of the current analysis.
MINI International Neuropsychiatric Interview 6.0
To rule out exclusionary psychiatric disorders and establish the primary study outcome (i.e., patients with versus without OUD), the MINI International Neuropsychiatric Interview 6.0 was administered to all patients. The MINI is a brief and well-validated, structured clinical interview that classifies one or more psychiatric disorders based on DSM-IV criteria. The interview establishes the diagnoses for major depressive disorder, mania, panic disorder, post-traumatic stress disorder, generalized anxiety disorder, psychotic symptoms and illicit drug and alcohol dependence (current and lifetime).30
Sociodemographics
Sociodemographic characteristics including age, sex, marital status, number in household, education level, employment status, disability status, and financial situation were obtained by patient interview.
Statistical Analysis and Validation Measures
Sociodemographic data were summarized (frequency and percent) within each category for discrete variables, and mean and standard deviations were calculated for continuous data. In this analysis, the primary outcome measure was the ORT total score used to distinguish between chronic pain patients with OUD (case cohort) versus patients without OUD (control cohort). Statistical analysis between patients with and without OUD for the sociodemographic data consisted of chi-square analysis and group t-tests. For all analysis, any p-value less than 0.05 was considered to be statistically significant.
The ORT contains 10 items for women and 9 items for men (assessment of the latter omits sexual abuse and the original validation of the ORT analyzed gender scores separately.32 Each ORT item is assigned a weight based on the probability that it predicts the potential for ADRB, and then summed to compute the total score. In the current study, factor analysis evaluated the underlying Eigen structure of the ORT, the number of principal components, and the cumulative percent explained by each component, first within the subset of women and then for the total sample (a distinct component was identified as having an Eigen value ≥ 1.00 using standard extraction methodology). To evaluate the predictive validity of the ORT’s weighting methodology, a binary variable was created (either 0 or 1) representing the unweighted item scores, and the factor analysis was repeated comparing the unweighted versus the weighted item scores. In addition, multivariate logistic regression models evaluated the weighted and unweighted scale total scores to evaluate the odds-ratio of correctly classifying patients with OUD versus without OUD.
Receiver Operating Characteristic curve (ROC) and AUC statistics, the standard error, p-values and the 95% confidence intervals were calculated using a randomly selected one-half of the sample to evaluate the cutoff scores and predictive validity of the revised ORT (validation sample); the sensitivity, specificity, positive predictive value (PPV) and negative predictive values (NPV) of the cutoff scores were evaluated with the remaining patients’ data (testing sample).
Results
Sociodemographic data and total ORT score for patients with (n=397) and without (n=781) OUDs are presented in Table 1. The overall sample was 67% women and on average 53 ± 12.7 years old. Almost half (48.5%) were married, and the majority (67.7%) had a college education. Of the minority who were employed (25.3%), 54% worked full-time, and 50.4% of those unemployed were disabled. As expected, when compared to patients without OUDs, patients with OUDs were more likely to be younger, men, unmarried, living with fewer household members, have lower education levels, lower socioeconomic status, receive disability benefits, and report higher ORT total scores (all p<0.001). Although the groups did not differ by employment status, patients with OUDs were less likely to be working full-time than those without OUD (p=0.013). Pain location by group is presented in Table 2. The majority of patients in both groups experienced low back pain. In Table 3 opioid type for controls (short acting, long acting and both short acting and long acting preparations revealed that that majority of controls were prescribed short acting preparations (70.3 percent) and the majority of cases received methadone (58.8 %).
Table 1:
Univariate analyses: Sociodemographics and total ORT score
| Variable | Category | Control | Cases | p-value | ||
|---|---|---|---|---|---|---|
| Count | % | Count | % | |||
| Gender | Men | 258 | 33.0 | 212 | 53.4 | <0.001 |
| Women | 523 | 67.0 | 185 | 46.6 | ||
|
Marital Status |
Married | 474 | 60.7 | 97 | 24.4 |
<0.001 |
| Separated | 26 | 3.3 | 31 | 7.8 | ||
| Divorced | 132 | 16.9 | 62 | 15.6 | ||
| Never Married | 116 | 14.9 | 195 | 49.1 | ||
| Widowed | 33 | 4.2 | 12 | 3.0 | ||
| How many people live with you? | Live alone | 128 | 16.4 | 73 | 18.5 | <0.001 |
| One other | 355 | 45.5 | 105 | 26.6 | ||
| More than one | 297 | 38.1 | 216 | 54.8 | ||
|
Education Level |
6th grade or less | 1 | 0.1 | 2 | 0.5 |
<0.001 |
| 7th to 12th grade | 42 | 5.4 | 73 | 18.4 | ||
| High School Graduate | 157 | 20.1 | 152 | 38.4 | ||
| Part College | 195 | 25.0 | 96 | 24.2 | ||
| 2-year Graduate | 108 | 13.8 | 25 | 6.3 | ||
| 4-year Graduate | 122 | 15.6 | 26 | 6.6 | ||
| Part Post-Graduate | 37 | 4.7 | 8 | 2.0 | ||
| Post-grad Graduate | 119 | 15.2 | 14 | 3.5 | ||
| Paid work | No | 581 | 74.6 | 299 | 75.5 | 0.730 |
| Yes | 198 | 25.4 | 97 | 24.5 | ||
| Hours worked | Full Time | 119 | 60.1 | 42 | 44.7 | 0.013 |
| Part Time | 79 | 39.6 | 52 | 55.3 | ||
| If not employed, why? | Retired | 110 | 19.3 | 8 | 2.7 | <0.001 |
| Laid off/Looking | 24 | 4.2 | 128 | 43.7 | ||
| Disabled | 437 | 76.5 | 157 | 53.6 | ||
| Disabled with benefits | No | 105 | 24.1 | 62 | 40.3 | <0.001 |
| Yes | 331 | 75.9 | 92 | 59.7 | ||
| Financial Situation | Cannot make ends | 147 | 19.0 | 199 | 50.6 | <0.001 |
| Have just enough | 359 | 46.4 | 159 | 40.5 | ||
| Comfortable | 268 | 34.6 | 35 | 8.9 | ||
| Continuous Variables | ||||||
| Variables | Control (n=781) | Cases (n= 397) | p-value | |||
| Mean | SD | Mean | SD | |||
| Age | 53.86 | 12.65 | 40.02 | 10.92 | <0.001 | |
| Total ORT Score | 2.38 | 2.61 | 12.73 | 5.73 | <0.001 | |
Table 2.
Pain location by group
| Pain Location (percent) | Controls | Cases |
|---|---|---|
| Back | 34.8 | 35.4 |
| Shoulder | 9.1 | 3.5 |
| Knee | 5.1 | 6.3 |
| Arm | 4.6 | 1.8 |
| Leg | 18.3 | 15.3 |
| Mixed | 28.1 | 37.7 |
Table 3.
Opioid Type by group
| Opioid Type (percent of population) | Controls |
|---|---|
| Short Acting | 70.3 |
| Long Acting | 20.7 |
| Short and Long acting | 8 |
| Average MEDD (Morphine equivalent daily dose) | 141.23 milligrams |
| Opioid Type (percent of Population) | Cases |
| Buprenorphine | 13.6 |
| Metdadone | 58.8 |
| Not listed or other | 27.6 |
Factor Analysis:
As noted, the ORT includes 10 items for women and 9 items for men (the item on history of sexual abuse is not weighted for men). Specifically, while both genders are asked about sexual abuse prior to adolescence, an affirmative response in men is not weighted and thus, not included in the total score. Although more women with OUD in the current sample endorsed preadolescent sexual abuse (13.0%) than women without OUD (6.3%, p=0.004), overall, very few patients endorsed this item (n=57, 4.8 %). Factor analysis for the subsample of women showed essentially no difference between models that included versus excluded the preadolescent sexual abuse item, suggesting it was a poor predictor of group membership, and therefore, we deleted it from subsequent analyses of validity, and combined the responses of men and women on a nine-item ORT.
The initial principal components analysis utilizing the weighted scores for the total sample revealed that three components had Eigen values > 1.00. These three extracted components explained 59.16% of the variance; the Eigen values for all components and the cumulative percent explained by each component are presented in Table 4.
Table 4:
Factor Analysis: Comparison of weighted and unweighted ORT factor structure for the total sample by the nine factor solution
| Component | Weighted ORT | Unweighted ORT (yes/no) | ||||
|---|---|---|---|---|---|---|
| Eigen Value |
Percent of variance |
Cumulative % |
Eigen Value |
Percent of variance |
Cumulative % |
|
| 1 | 3.047 | 33.859 | 33.859 | 3.012 | 33.464 | 33.464 |
| 2 | 1.236 | 13.729 | 47.588 | 1.343 | 14.921 | 48.385 |
| 3 | 1.041 | 11.569 | 59.157 | 1.002 | 11.136 | 59.521 |
| 4 | .850 | 9.446 | 68.603 | .871 | 9.674 | 69.195 |
| 5 | .776 | 8.617 | 77.221 | .771 | 8.571 | 77.766 |
| 6 | .729 | 8.104 | 85.324 | .704 | 7.822 | 85.588 |
| 7 | .625 | 6.949 | 92.274 | .630 | 7.005 | 92.594 |
| 8 | .511 | 5.682 | 97.956 | .482 | 5.355 | 97.948 |
| 9 | .184 | 2.044 | 100.000 | .185 | 2.052 | 100.000 |
The principal component extraction matrix in Table 5, revealed that six of the nine weighted ORT items loaded on the strongest factor explaining 33.86% of the variance. This included family history of substance abuse (except prescription drug abuse), personal history of substance abuse and age. Although family history of prescription drug abuse had a high loading score on the first factor (.439), it was the only item loading on the second factor (score of .640), accounting for 13.73% of the variance. Together, major depression (score of .701) and other psychiatric disorders (i.e., attention deficit disorder, obsessive compulsive disorder, bipolar disorder, and schizophrenia) (score of .485) loaded on a third factor (score of .692) which explained 11.57% of the variance.
Table 5:
Principal component extraction matrix
| Item | Weighted ORT | Unweighted ORT (Yes/No) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| Family History of Alcohol Abuse | .541 | .340 | −.339 | .532 | .486 | −.213 |
| Family History of Illegal Drugs | .585 | .543 | −.226 | .580 | .560 | −.247 |
| Family History of Prescription Drug Abuse | .439 | .640 | .023 | .454 | .552 | −.038 |
| Personal History of Alcohol Abuse | .506 | −.383 | −.254 | .490 | −.375 | −.265 |
| Personal History of Illegal Drug Abuse | .803 | −.374 | −.121 | .792 | −.403 | −.162 |
| Personal History of Prescription Drug Abuse | .813 | −.312 | −.088 | .802 | −.348 | −.126 |
| Age 16-45 years | .570 | −.126 | .247 | .565 | −.170 | .252 |
| Psych Disease: ADD, OCD, bipolar, schizophrenia | .471 | −.011 | .485 | .473 | −.046 | .524 |
| Psych Disease: Depression | .332 | .128 | .701 | .364 | .181 | .667 |
In an effort to simplify the ORT scoring, the principal components analysis was repeated using the binary variable we created for each item’s score (0,1). As noted in Tables 4 and 5, the results were notably similar to the prior analysis of the weighted items, with three components accounting for 59.52% of the variance and revealing the same factor structure. These findings suggest that the predictive validity of the ORT does not require weighting of items, but rather simple endorsement (yes/no) of each item can predict the risk for developing an OUD. Notably, the odds-ratio of predicting group membership (OUD versus non-OUD) was considerably higher using the unweighted versus weighted scores, in part due to the truncated scoring range of the latter (Table 6).
Table 6:
Mulitvariate logistic regression: Classification of patients with OUD versus without OUD as predicted by ORT total score with weighted and unweighted items
| Variable | Beta | p-value | OR | 95% Lower Bound |
95% Upper Bound |
|---|---|---|---|---|---|
| ORT Total Score (Weighted 10-items) | 0.485 | <0.001 | 1.624 | 1.539 | 1.715 |
| ORT Total Score (Weighted without Sexual Abuse item 9-item) | 0.500 | <0.001 | 1.648 | 1.559 | 1.742 |
| ORT Total Score (Unweighted) | 1.127 | <0.001 | 3.085 | 2.725 | 3.493 |
Sensitivity, Specificity, Positive and Negative Predictive Values:
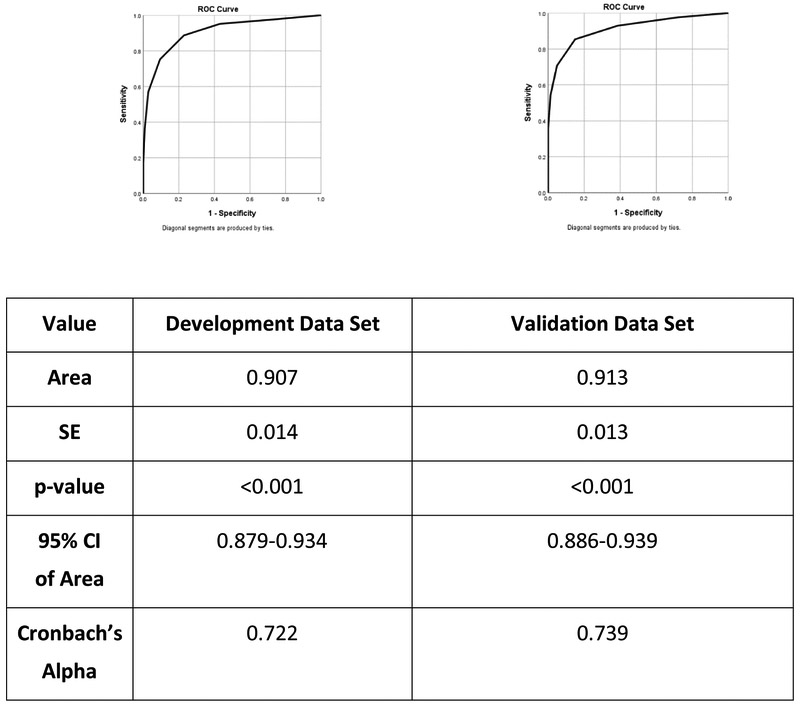
Lastly, the ROC of the nine-item unweighted ORT was calculated on a randomly selected one-half of the sample (validation subsample) and then tested for predictive validity on the remaining participants (testing subsample); statistical inspection of the composition of the two subsamples demonstrated equivalency with respect to percentages of patients with and without OUD (p=0.236). For the validation data set, the AUC was 0.907 (SE=0.014) and highly significant (p<0.001). A cut off score of 2.5 (scores 0, 1 or 2 indicating non-OUD, and scores ≥3 indicating patients with OUD) provided a sensitivity value of 0.887 and specificity value of 0.772. As presented in Table 7, this cut off score demonstrated an excellent ability to predict patient classification in the testing subsample, with sensitivity remaining high (85.4%) and improved specificity (85.1%). The PPV and NPV are similarly strong, with 75.7% of true positives cases and 91.4% of true negative cases accurately identified. The individual ROC curves for the validation and testing subsamples can be found in Figure 1.
Table 7:
Evaluation of sensitivity and specificity of varied cutoff scores (score 0-2 versus 3 or more)
| Patients with OUD |
Patients without OUD |
Total | ||
|---|---|---|---|---|
| Cut point on the 0-2 and 3+ sample | 3+ | 181 | 58 | 239 |
| 0-2 | 31 | 330 | 361 | |
| Total | 212 | 388 | 600 | |
| Value | Formula | Values | Result | 95% Confidence Interval |
| Sensitivity | A/(A+C) | 181/212 | 0.854 | 0.799-0.898 |
| Specificity | D/(D+B) | 330/388 | 0.851 | 0.811-0.885 |
| PPV | A/(A+B) | 181/239 | 0.757 | 0.709-0.799 |
| NPV | D/(C+D) | 330/361 | 0.914 | 0.885-0.937 |
Figure 1:
ROC curve on the Development and Validation data set.
Discussion
This study is the first to evaluate the sensitivity, specificity, and predictive value of the self-report ORT on a large cohort of patients with CNMP receiving LTOT and displaying no evidence of ADRB or meeting criteria for OUD, compared to a cohort of patients with CNMP with no previous history of a SUD who developed an OUD after commencing prescription opioid therapy. Both the item content and structure of the ORT in predicting OUD were evaluated. Overall, the results of this study support the validity of the original ORT to predict the risk of developing an OUD in patients with CNMP. However, additional psychometric analysis revealed that a revised 9-item unweighted version of ORT (ORT-OUD) was found to be superior to the original ORT in distinguishing patients with and without OUD. Principal component analysis suggested that in clinical practice ascertaining the patient’s personal and family history of SUD, age and concomitant psychiatric conditions, may be sufficient to determine general risk and need for further assessment of the potential of a patient developing an OUD when exposed to opioid therapy.
The main finding of this analysis suggests that the original ORT was able to discriminate between patients with and without OUDs to a great degree. The average ORT score for patients with and without OUD was 12.73 (SD=5.73) and 2.38 (SD=2.38), respectively.
A more granular evaluation of the ORTs structure and items, particularly the weighting of items and the item on preadolescent sexual abuse was conducted in an effort to simplify the ORT as a screener for OUD development. The item selection and item weighting of the ORT was based on clinical experience of the authors and the prevailing literature but had not been empirically based. The item on childhood sexual abuse weighted for women and not weighted for men was based on an article by Kendler and colleagues which found that women who reported a history of childhood sexual abuse were at increased risk for developing psychiatric disorders in adulthood including alcohol and drug abuse.27 The seminal ACE (adverse childhood events) study published in 1998 included both women and men and discovered that childhood exposure to a variety of adverse events including both abuse and household dysfunction increased the risk of developing adulthood health maladies including nicotine, alcohol and drug abuse for both genders.162 As noted in the results section, few women overall endorsed the sexual abuse question (4.8 %) and a factor analysis of the female subjects showed no statistically significant difference between models including or excluding the preadolescent sexual abuse item.
The weighting of items on the original ORT was not derived by psychometric testing principles, thus we evaluated a weighted full 10-item ORT (including the preadolescent sexual abuse question), a 9-item ORT that excluded the preadolescent question, and a 9-item unweighted scale (dichotomous yes or no response). A logistic regression revealed that the weighted 10-item and the weighted without sexual abuse 9-item scales were equally predictive of group membership (OR=1.624; 95% CI: 1.539-1.715, p <0.001 and OR=1.648; 95% CI: 1.559-1.742, p < 0.001, respectively). The unweighted 9-item scale, however, was superior to both the weighted 10 item and weighted 9 item scales (OR=3.085; 95% CI: 2.725-3.493, p < 0.001) (see Table 4). These findings indicate that a revised ORT with 9 unweighted items is more predictive of OUD classification and may have more clinical utility.
The principal components analysis on the 9-item weighted and unweighted versions revealed similar results of three key factors: 1) family history of alcohol and illicit drug abuse; personal history of alcohol, illicit and prescription drug abuse; 2) family history of prescription drug abuse; and 3) psychiatric disorders (OCD, ADHD, bipolar, schizophrenia) and depression. These results further support the use of the streamlined 9-item unweighted version of the ORT. Further, the grouping of the three components was intriguing. There is literature supporting that personal and family history of SUD and psychiatric co-morbidities are strong risk factors for developing a prescription OUD in patients with CNMP.18 It is unclear why family history of prescription drug abuse dropped out as a separate component. This could suggest a spurious finding or perhaps reflects a more specific risk factor for OUD in this patient population. However, family history of prescription drug abuse also weighed heavily in factor 1 suggesting that it was highly correlated with the other family history of substance abuse. This requires further exploration.
Further evaluation of the revised ORT-OUD support its use to predict the development of OUD in patients with CNMP on LTOT. Sensitivity, specificity and positive/negative predictive value of the 9-item unweighted ORT was validated on a randomly selected one-half of the sample and tested on the second half of the sample utilizing a 0-2 (non-OUD) and 3 + (OUD) cut off. Sensitivity (0.854) and specificity (0.851) were high as were the positive (.757) and negative (.914) predictive values.
The current standard of care regarding opioid therapy for chronic pain is to avoid or minimize opioids if possible, as first line therapeutic agents and rely on non-opioid pharmacotherapy (NSAIDs, antidepressants, anti-epileptic drugs, acetaminophen, etc) and non-pharmacologic interventions (physical therapy, cognitive behavioral therapy, acupuncture etc).14 In cases where patients have not responded to non-opioid therapies or lack access to forms of recommended pain management interventions (cognitive behavioral therapy, acupuncture, etc) and are functionally impaired, opioids may be indicated and efficacious in well-selected individuals. Patient selection when considering opioids relies on a number of strategies including querying the Prescription Drug Monitoring Program, urine drug testing and employing risk assessment tools. The ORT has been extensively utilized to assess the risk of ADRB in patients being considered for LTOT. The ORT, however, like all the risk assessment tools was not developed to predict the potential for a patient on opioid therapy to develop a true OUD, only ADRB or problematic drug taking behavior which could be a surrogate for OUD or a myriad of other maladaptive behaviors including general treatment non-adherence, misuse, abuse for non-euphoric effects such as self-treating medicating anxiety, depression or sleep disorders common in patients with CNMP.
There are cautionary limitations to this study. First, as the parent study was designed to discover genotypic characteristics of OUD in patients with CNMP, subjects were limited to Caucasians to increase the probability of discovering a genetic marker by reducing ethic variability. Our results, therefore, cannot be generalized to non-Caucasian populations, and require replication in other ethnic populations. A second limitation, which is inherent in all cross-sectional designs, is that we evaluated subjects at one point in time and did not assess the sequence of events leading to the development of an OUD which prevents us from inferring causality. Finally, being a self-administered tool, the ORT is subject to misreporting on the part of the patient, and there is evidence to suggest that when administered by a clinician25 or when self-administered but validated with the electronic health record,11 prediction of aberrant behaviors is improved. Not tested in the current evaluation of the tool, findings of predictive validity are limited to the context of self-administration, and may be improved under different administration conditions.
The ORT like all risk assessment tools is limited in scope to providing an assessment of risk of ADRB which may or may not be indicative of a patient having developed a true OUD but is useful in alerting a clinician to the possibility of misuse, abuse or OUD and prompting further investigation. Despite these limitations, these psychometric analyses represent a significant step forward in our ability to screen for risk in patients with CNMP who are candidates for LTOT. Beyond predicting risk for ADRB (which may reflect many issues other than OUD), we have demonstrated that the revised ORT (the ORT-OUD) (see Appendix 2) can actually detect risk for the more problematic and serious outcome of OUD. In addition, we have shown that a simpler and shorter version of the tool can predict OUD just as well, and was significantly better than the original 10-item weighted tool, with excellent sensitivity and specificity. In keeping with current calls for improved screening prior to opioid provision for patients with CNMP, the ORT-OUD provides a straightforward and validated method to quickly and effectively do so in the practice setting.
Supplementary Material
Highlights.
The opioid risk tool was evaluated on pain patients with and without an OUD
The ORT was able to discriminate between patients with and without OUDs (OR=1.624)
An ORT removing the preadolescent sexual abuse item had similar results (OR=1.648)
An unweighted ORT without the sexual abuse item was superior (OR= 3.085)
Acknowledgments
Disclosures: This work was supported by the National Institutes of Health [NIH/NIDA R01 DA032776-05] (Cheatle, O’Brien), The Patrick and Catherine Weldon Donaghue Medical Research Foundation, and The New York Community Trust (Dhingra) and the van Ameringen Endowed Chair (Compton). The authors have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Busse JW, Craigie S, Juurlink DN, Buckley DN, Wang L, Couban RJ, Agoritsas T, Akl EA, Carrasco-Labra A, Cooper L, Cull C, da Costa BR, Frank JW, Grant G, Iorio A, Persaud N, Stern S, Tugwell P, Vandvik PO, Guyatt GH: Guideline for opioid therapy and chronic noncancer pain. CMAJ 189:E659–E666, 2017. doi: 10.1503/cmaj.170363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belgrade M, Schamber C, Lindgren B. The DIRE score: predicting outcomes of opioid prescribing for chronic pain. J Pain. 7: 671–681. 2006. [DOI] [PubMed] [Google Scholar]
- 3.Butler S, Budman S et al. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain. 112: 65–75, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Butler S, Budman SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure. Pain 130: 144–156, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheatle MD, O'Brien CP: Opioid therapy in patients with chronic noncancer pain: diagnostic and clinical challenges. Adv Psychosom Med 30:61–91, 2011. doi: 10.1159/000324067 [DOI] [PubMed] [Google Scholar]
- 6.Cheatle MD, O'Brien CP, Mathai K, Hansen M, Grasso M, Yi P: Aberrant behaviors in a primary care-based cohort of patients with chronic pain identified as misusing prescription opioids. J Opioid Manag 9:315–24, 2013. doi: 10.5055/jom.2013.0174. [DOI] [PubMed] [Google Scholar]
- 7.Cheatle MD, O’Brien CP, Berrentini W, Turk D, Webster LR: Clinical and genetic characteristics of opioid addiction in chronic pain. NIH/NIDA R01 DA032776-05.
- 8.Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C: Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain 10:147–59, 2009. doi: 10.1016/j.jpain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, Donovan MI, Fishbain DA, Foley KM, Fudin J, Gilson AM, Kelter A, Mauskop A, O'Connor PG, Passik SD, Pasternak GW, Portenoy RK, Rich BA, Roberts RG, Todd KH, Miaskowski C: American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 10:113–30, 2009. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark MR, Hurley RW, Adams MCB: Re-assessing the validity of the Opioid Risk Tool in a tertiary academic pain management center population. Pain Med 19:1382–1395, 2018. doi: 10.1093/pm/pnx332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleland CM, Lanza ST, Vasilenko SA, Gwadz M: Syndemic Risk Classes and Substance Use Problems among Adults in High-Risk Urban Areas: A Latent Class Analysis. Front Public Health 5:237–249, 2017 10.3389/fpubh.2017.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC,American Psychiatric Association, 1994. [Google Scholar]
- 13.Dowell D, Haegerich TM, Chou R: CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm Rep 65(No. RR-1): 1–49, 2016. DOI: 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]
- 14.Federation of State Medical Boards. Guidelines for the Chronic Use of Opioid Analgesics. 2017. https://www.fsmb.org/globalassets/advocacy/policies/opioid_guidelines_as_adopted_april-2017_final.pdf [Google Scholar]
- 15.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS: Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14:245–58, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Fine P, Portenoy RK. Opioid analgesia. New York: McGraw Hill, 2004. [Google Scholar]
- 17.Hah JM, Sturgeon JA, Zocca J, Sharifzadeh Y, Mackey SC: Factors associated with prescription opioid misuse in a cross-sectional cohort of patients with chronic non-cancer pain. J Pain Res 10:979–987, 2017. doi: 10.2147/JPR.S131979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janke EA, Cheatle M, Keefe F, Dhingra L, Society of Behavioral Medicine Health Policy Committee: Society of Behavioral Medicine (SBM) position statement: improving access to psychosocial care for individuals with persistent pain: supporting the National Pain Strategy's call for interdisciplinary pain care. Transl Behav Med 8:305–308, 2018. [DOI] [PubMed] [Google Scholar]
- 19.Jones CM, Christensen A, Gladden RM: Increases in prescription opioid injection abuse among treatment admissions in the United States, 2004–2013. Drug Alcohol Depend 176:89–95, 2017. doi: 10.1016/j.drugalcdep.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones T, Lookatch S, Grant P, McIntyre J, Moore T: Further validation of an opioid risk assessment tool: The Brief Risk Interview. J Opioid Manage 10: 353–364, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Jones T, Lookatch S, Moore T: Validation of a new risk assessment tool: the Brief Risk Questionnaire. J Opioid Manage 11:171–183, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Jones T, Moore T, Levy JL, Daffron S, Browder JH, Allen L, Passik SD: A comparison of various risk screening methods in predicting discharge from opioid treatment. Clin J Pain 28:93–100, 2012. doi: 10.1097/AJP.0b013e318225da9e. [DOI] [PubMed] [Google Scholar]
- 23.Jones T, Moore T: Preliminary data on a new opioid risk assessment measure: The Brief Risk Interview. J Opioid Manage 9:19–27, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Jones T, Passik SD: A comparison of methods of administering the Opioid Risk Tool. J Opioid Manage 7: 347–351, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Jones T, Schmidt M, Moore T: Further Validation of an Opioid Risk Assessment Tool: The Brief Risk Questionnaire. Ann Psychiatry Ment Health 3: 1032–1039, 2015. [Google Scholar]
- 26.Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, Prescott CA: Childhood sexual abuse and adult psychiatric and substance use disorders in women: an epidemiological and cotwin control analysis. Arch Gen Psychiatry 57:953–9, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Moore TM, Jones T, Browder JH, Daffron S, Passik SD: A comparison of common screening methods for predicting aberrant drug-related behavior among patients receiving opioids for chronic pain management. Pain Med 10:1426–33, 2009. doi: 10.1111/j.1526-4637.2009.00743.x. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor AB, Turk DC, Dworkin RH, Katz NP, Colucci R, Haythornthwaite JA, Klein M, O'Brien C, Posner K, Rappaport BA, Reisfield G, Adams EH, Balster RL, Bigelow GE, Burke LB, Comer SD, Cone E, Cowan P, Denisco RA, Farrar JT, Foltin RW, Haddox JD, Hertz S, Jay GW, Junor R, Kopecky EA, Leiderman DB, McDermott MP, Palmer PP, Raja SN, Rauschkolb C, Rowbotham MC, Sampaio C, Setnik B, Smith SM, Sokolowska M, Stauffer JW, Walsh SL, Zacny JP: Abuse liability measures for use in analgesic clinical trials in patients with pain: IMMPACT recommendations. Pain 154:2324–34, 2013. doi: 10.1016/j.pain.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC: The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl 20:22–33, 1998. [PubMed] [Google Scholar]
- 30.Smith SM, Paillard F, McKeown A, Burke LB, Edwards RR, Katz NP, Papadopoulos EJ, Rappaport BA, Slagle A, Strain EC, Wasan AD, Turk DC, Dworkin RH: Instruments to Identify Prescription Medication Misuse, Abuse, and Related Events in Clinical Trials: An ACTTION Systematic Review. J Pain 16:389–411, 2015. doi: 10.1016/j.jpain.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Webster LR, Webster RM: Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med 6:432–442, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Webster LR: Risk Factors for Opioid-Use Disorder and Overdose. Anesth Analg 125:1741–1748, 2017. [DOI] [PubMed] [Google Scholar]
- 33.VA/DoD Evidence-Based Clinical Practice Guideline Work Group: VA/DoD Clinical practice guideline for opioid therapy for chronic pain. Department of Veterans Affairs, Department of Defense, Office of Quality and Performance. 2017 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.