Abstract
Polymerase chain reaction was used to determine the prevalence and correlates of human herpesvirus 8 (HHV8) in saliva, mouth, cervical, vaginal, plasma, and peripheral-blood mononuclear cell (PBMC) samples from 174 HHV8-sero-positive female prostitutes in Mombasa, Kenya. The prevalence of detection of HHV8 was 32% in saliva samples, 28% in mouth swabs, 4% in cervical swabs, 2.3% in vaginal swabs, 9% in plasma samples, and 18% in PBMC samples. Human immunodeficiency virus type 1 (HIV-1) seropositivity was associated with detection of HHV8 from any mucosal surface (odds ratio, 2.1 [95% confidence interval, 1.1–4.0]). In HIV-1–seropositive women, there was no association between detection of HHV8 and either CD4 count or HIV-1 viral load.
Human herpesvirus 8 (HHV8) has been implicated as a causative agent in Kaposi sarcoma (KS) [1]. Early epidemiologic studies suggested a sexual route of spread because of the high incidence of KS in HIV-1–seropositive homosexual males [2]. Reported risk factors for KS and HHV8 seropositivity in heterosexual men and women include age, HIV-1 seropositivity, number of sex partners, and history of sexually transmitted diseases (STDs), which suggests the possibility of heterosexual transmission [3, 4].
Viral-shedding studies of HHV8-seropositive men have demonstrated higher rates of shedding in saliva than in semen, urine, or stool, suggesting that saliva may be important in the horizontal spread of HHV8 [5–7]. The few studies of HHV8 shedding in women have been limited both by small sample sizes and by the inclusion of women with and without documented HHV8 seropositivity [8–10].
Seroprevalence rates of HHV8 are high (32%–66%) in African women, compared with those in women from developed countries [3, 4, 11, 12, 13]. To learn more about the epidemiology of HHV8 in women in Africa, we used polymerase chain reaction (PCR) methods to detect the presence of virus in saliva, mouth, cervical, vaginal, plasma, and peripheral-blood mononuclear cell (PBMC) samples from HHV8-seropositive female prostitutes in Mombasa, Kenya, and to evaluate risk factors for viral shedding.
Subjects, materials, and methods.
A prospective cohort study of HIV-1 and other STDs in female prostitutes in Mombasa, Kenya, has been ongoing since 1993, as described elsewhere [14]. In a survey of 736 participants in this cohort, we found the seroprevalence of HHV8 to be 44% [13]. Between September 2000 and September 2001, HHV8-seropositive women in this cohort were recruited for a cross-sectional study of detection of HHV8 in saliva, mouth, cervical, vaginal, plasma, and PBMC samples.
Women presenting to clinics for evaluation were offered serological testing for HIV-1 and HHV8. After written informed consent was obtained, 1 tube of blood was collected. Women who were HHV8 seropositive were offered enrollment in the cross-sectional study when they received their test results. A standardized questionnaire was administered that focused on recent sexual practices as well as on current symptoms of illness and contraceptive use. Blood samples were collected for detection of HHV8 in plasma and PBMCs and for determination of CD4 count in HIV-1–seropositive patients. Physical examination and collection of mucosal samples was then undertaken by 1 of 2 investigators (M.M.T. and W.H.). Patients demonstrating clinical or laboratory evidence of an STD received treatment according to guidelines of the Kenyan Ministry of Health. This study was approved by the Human Subjects Review Committee of the University of Washington and by the Human Subjects Review Committee of th University of Nairobi. All participants gave written consent.
Patients were asked to produce ~1 mL of saliva, which was collected in nonsterile spit cups. Approximately 0.8 mL of saliva was pipetted into 0.2 mL of 5 × proteinase K digestion buffer. Mouth swabs were collected by rotation of a dacron swab on both tonsils, on the buccal mucosa of both sides of the mouth, and on the center of the tongue. Cervical samples were collected by 3 complete rotations of a dacron swab in the cervical os. Vaginal samples were collected by wiping a dacron swab on lateral surfaces of the vaginal walls during an examination with a speculum. Dacron swabs were placed in cryovials containing 1 mL of proteinase K digestion buffer. Samples were stored on ice for up to 3 hours before being frozen at −70°C. Cryovials containing HHV8-shedding samples were shipped in liquid nitrogen to the Fred Hutchinson Cancer Research Center in Seattle, Washington, where they were stored at −70°C.
Serum or plasma samples were tested for the presence of HHV8 antibodies by ELISA, as described elsewhere [15]. Two enzyme immunoassays (Detect [Biochem ImmunoSystems] and Recombigen [Cambridge Biotech]) were used to detect and confirm the presence of antibodies to HIV-1. Screening for Neisseria gonorrhoeae, Haemophilus ducreyi, Trichomonas vaginalis, and Candida species, cervicitis, bacterial vaginosis, and syphilis was performed as described elsewhere [14].
A real-time quantitative PCR method was used to detect HHV8 viral segments [5]. The sensitivity of this assay is as few as 1–3 copies of HHV8 DNA in as much as 5 μg of cellular DNA. Swab samples from the mouth, cervix, and vagina were stored in 1 mL of proteinase K digestion buffer. DNA was extracted, by use of Qiagen columns, from 400 μL of the swab buffer, 1 million PBMCs, or 400 μL of saliva/buffer or plasma/buffer solution. DNA was eluted into 100 μL of 10 mmol/L Tris (pH 8.0); one-fifth of the DNA extracted was used for each PCR assay.
KS-1 and −2 primers and KS-NP probe (5′-ATGTGGTACACCAACAGCTGCTGCA-3′) were used to amplify and detect the 233 bp for the open-reading frame 26 region. KS-NP was labeled at the 5′ end with 6-carboxyfluorescin and at the 3′ end with 6-carboxytetramethylrhodamine (TAMRA) (Synthegen). PCR conditions were 50°C for 2 min and 95°C for 2 min, followed by 45 cycles of 95°C for 20 s and 60°C for 1 min. Each 50 μL of PCR mixture contained 830 nm of primers, 5 μL of 10× buffer II (Perkin Elmer), 10 mmol of MgCl2/L, 17.5 nmol of TaqStart antibody (Clontech)/L, 1.25 U of AmpliTaq (Perkin Elmer), 0.05 U of UNG (uracil-DNA-glycosylase), 5% of glycerol, and 60 nmol of 6-carboxy-x-rhodamin (ROX)/L. To ensure that negative results were not due to nonspecific inhibition of the PCR, each PCR reaction was spiked with 5000 copies of internal-control DNA (EXO), 30 nmol of primers (EXO186F and EXO314R)/L, and 50 nmol of probe (PiMP-242T)/L. The EXO DNA sequence is 5′-GCCTGGTGCCAAAAATTGCTTATCAATTGAACGGTCAATTGGAAGTGGCGGAAGAACAGCTATTGCAAACGCCATCGCACAATACCATAAACACACTTGTCTTAGGTTCACAAAAGAACAAATGAACGA-3′; the EXO186F and EXO314R sequences are 5′-GCCTGGTGCAAAAATTGCTT-3′and 5′-TCGTTCATTTGTTCTTTTGTGGAA-3′, respectively. PiMP-242T (5′-CAGCTATTGCAAACGCCATCGCAC-3′) is labeled at the 5′end with VIC and at the 3′end with TAMRA. All of the negative PCR results for the KS-1 and KS-2 primers required that the detection of EXO DNA be considered valid. One positive control with 5000 copies of HHV8 was coprocessed with the specimens, to verify DNA recovery. To monitor for possible false positives, specimens were processed in parallel with aliquots of HSB-2 cells, and each run contained PCR reactions without DNA input.
Statistical analysis was performed by SPSS software. Categorical variable comparisons were performed by Pearson χ2 test and Fisher’s exact test. Univariate and multivariate analyses of predictors of HHV8 shedding were computed by stepwise logistic regression. Comparison of differences for continuous variables was performed by Wilcoxon rank sum test and Kruskal-Wallis test.
Results.
One hundred seventy-four HHV8-seropositive women were enrolled in the cross-sectional study. The median age was 31 years, the median age at first sex was 17 years, and the median duration of prostitution was 3 years. Women re-ported a median of 2 sex acts per week and median condom use of 100%. Only 33% of women gave a history of deep kissing; 31% used hormonal contraception; 31% had bacterial vaginosis, 14% had vaginal candidiasis, 7% had trichomoniasis, 0.5% had syphilis, and 0.5% had gonorrhea; 50% (87/174) of the women were HIV-1 seropositive, with a median CD4 count of 343 cells/mm3, and 28% of this group (i.e., 14% of the women studied) had CD4 counts <200 cells/mm3. The median HIV-1 viral load was 65,600 copies/mL. None of these women had clinical evidence of KS.
HHV8 was detected by PCR in 40% of the women. HHV8 was detected more often from oral-cavity specimens than from genital-tract specimens (34% vs. 6%; P < .001). The prevalence of detection of HHV8 was 32% in saliva samples, 28% in mouth-swab samples, 4% in cervical-swab samples, 2.3% in vaginal-swab samples, 18% in PBMC samples, and 9% in plasma samples.
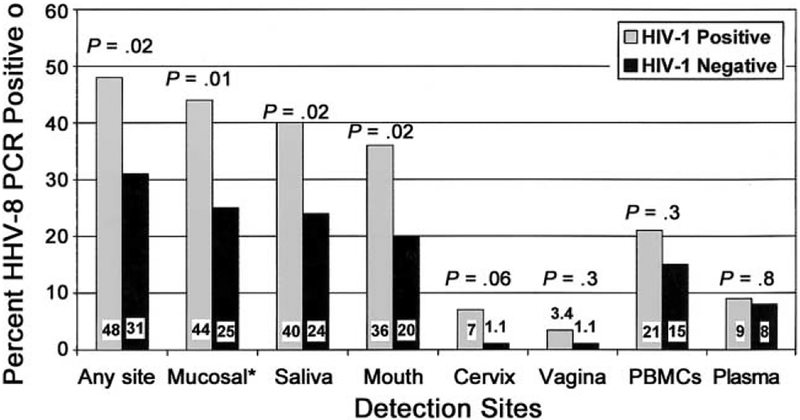
Viral shedding from mucosal sites (oral and genital) was associated with HIV-1 seropositivity (OR, 2.3 [95% confidence interval {CI}, 1.2–4.4]) and with older age at first sexual activity (P = .004) (table 1). Viral shedding in the oral cavity was detected in samples from 44% of the HIV-1–seropositive women and from 24% of the HIV-1–seronegative women (OR, 2.4 [95% CI, 1.3–4.7]) (figure 1). Virus was detected in genital samples from 10% of the HIV-1–seropositive women and from 2.3% of the HIV-1–seronegative women (OR, 4.9 [95% CI, 1.0–23.4]). The detection of virus in oral and genital sites in HIV-1–seropositive women was unrelated to CD4 count or HIV-1 viral load (table 1). In multivariate analysis, which included age at first sexual activity and HIV-1 serostatus, only HIV-1 seropositivity (OR, 2.1 [95% CI, 1.1–4.0]) remained significantly associated with HHV8 shedding from mucosal sites.
Table 1.
Univariate analysis of correlates of shedding of human herpesvirus 8 (HHV8) from mucosal (oral and genital) sites.
| Variablea | HHV8 shedding, median (range or %) | OR (95% CI) | P | |
|---|---|---|---|---|
| Present (N = 60) | Absent (N = 114) | |||
| Age | 32 years (18–46 years) | 31 years (19–19 years) | … | .9 |
| Education | 7.0 years (0–12 years) | 7.0 years (0–13 years) | … | .4 |
| Age at first sexual activity | 18 years (12–25 years) | 17 years (10–25 years) | … | .004 |
| Duration of prostitution | 4.0 years (0–30 years) | 3.0 years (0–20 years) | … | .6 |
| Frequency of sex acts | 2.0/week (1–20/week) | 2.0/week (0–12/week) | … | .4 |
| Frequency of condom use | 100% (0%–100%) | 67% (0%–100%) | … | .5 |
| Sex partners | 1.0/week (1–20/week) | 1.0/week (0–7/week) | … | .3 |
| History of deep kissing | 15 (25) | 41 (37) | 0.5 (0.3–1.1) | … |
| History of vaginal douching | 50 (83) | 92 (81) | 1.2 (0.5–2.7) | … |
| Oral-contraceptive use | 1 (2) | 11 (10) | 0.2 (0.02–1.3) | … |
| Depomedroxyprogesterone use | 18 (30) | 27 (24) | 1.4 (0.7–2.8) | … |
| Cigarette smoking | 8 (13) | 15 (13) | 1.0 (0.4–2.6) | … |
| Alcohol consumption >4 drinks/week | 35 (58) | 60 (53) | 1.3 (0.7–2.4) | … |
| Symptoms | ||||
| Fever | 14 (23) | 40 (35) | 0.6 (0.3–1.1) | … |
| Fatigue | 6 (17) | 14 (12) | 0.8 (0.3–2.1) | … |
| Sore throat | 4 (7) | 5 (4) | 1.5 (0.4–6.0) | … |
| Swollen glands | 3 (5) | 3 (3) | 1.9 (0.4–10) | … |
| Headache | 12 (20) | 31 (39) | 0.7 (0.3–1.5) | … |
| Muscle/joint aches | 7 (12) | 19 (17) | 0.7 (0.3–1.7) | … |
| Vaginal discharge | 9 (15) | 12 (11) | 1.5 (0.6–3.8) | … |
| Genital sores | 2 (3) | 5 (4) | 0.8 (0.1–1.0) | … |
| Vaginal itching | 13 (22) | 20 (18) | 1.3 (0.6–2.8) | … |
| Laboratory values | ||||
| Cervicitis | 2 (4) | 0 (0) | 1.0 (1.0–1.1) | … |
| Trichomoniasis | 9 (15) | 4 (4) | 4.9 (1.4–16.5) | … |
| Bacterial vaginosis | 15 (25) | 38 (34) | 0.7 (0.3–1.3) | … |
| Candida | 7 (12) | 17 (15) | 0.8 (0.3–1.9) | … |
| Gonorrhea | 1 (2) | 0 (0) | 1.0 (1.0–1.0) | … |
| RPR positive | 0 (0) | 1 (1) | 1.0 (1.0–1.0) | … |
| HIV-1-seropositive patients | 38 (62) | 49 (43) | 2.3 (1.2–4.4) | … |
| CD4 countb | 358 cells/mm3 (44–861 cells/mm3) | 343 cells/mm3 (30–1015 cells/mm3) | … | .9 |
| CD4 count <200 cells/cells/mm3 b | 11 (30) | 13 (27) | 1.2 (0.5–3.0) | … |
| HIV-1 viral loadb | 66,135 copies/mL (660–2,414,700 copies/mL) | 55,990 copies/mL (6–942,590 copies/mL) | … | .6 |
NOTE. Data are no. of women, unless otherwise indicated. CI, confidence interval; OR, odds ratio. RPR, rapid plasma reagin.
N = 174, except for history of deep kissing (N = 170) and cervicitis (N = 164).
Data are for HIV-1–seropositive patients only (N = 87).
Figure 1.
Prevalence of detection of HHV-8, by HIV-1 serostatus. *, “Mucosal” includes oral (saliva and mouth) and genital (cervical and vaginal) sites.
In contrast to the results from the analysis of shedding from mucosal sites, detection of HHV8 in PBMCs and plasma was unrelated to HIV-1 serostatus. HHV8 was detected in 21% and 15% of PBMC samples and in 9% and 8% of plasma samples from HIV-1–seropositive and HIV-1–seronegative women, respectively (figure 1). In HIV-1–seropositive women, there was no correlation between HHV8 shedding and either CD4 count or HIV-1 viral load.
The median log HHV8 viral copy number was 5.0/mL (range, 1.4–8.4) of sample/buffer solution in saliva samples, 3.8/swab (range, 1.2–7.5) in mouth samples, 3.2/swab (range, 1.1–4.2) in cervical samples, 1.5/swab (range, 1.2–4.3) in vaginal samples, and 5.1/1 million PBMCs (range, 2.9–5.8). No association between median viral copy number and HIV-1 serostatus was found, for any of the sample types. In none of the sites tested in HIV-1–seropositive women were HHV8 viral copy numbers associated with either CD4 count or HIV-1 viral load.
Discussion.
In this study of Kenyan female prostitutes, we found that HIV-1 seropositivity was associated with a 2.1-fold-increased odds of HHV8 shedding from mucosal (oral and genital) sites. Despite the presence of higher prevalence rates of detection of HHV8 in HIV-1–seropositive women, we did not find HHV8 viral copy numbers to be associated with HIV-1 infection, which suggests that factors other than HIV-1 infection affect HHV8 quantity at mucosal shedding sites.
In HIV-1–seropositive women, we found that neither HHV8 shedding from oral and genital sites nor HHV8 copy number was related to CD4 count and HIV-1 viral load. Other studies of HIV-1–seropositive men and women with and without KS have found that HHV8 shedding in the oral and genital tracts is unrelated to CD4 count [6, 10]. These results suggest that the mechanism of HHV8 shedding from mucosal sites that is stimulated by HIV-1 infection may be independent of cellular immunity as measured by CD4 count.
In contrast to our results with regard to HHV8 shedding from mucosal sites, we found that detection of HHV8 in PBMCs and plasma was unrelated to HIV-1 serostatus. In HIV-1–seropositive patients, neither detection of HHV8 nor the its quantity in either PBMCs or plasma was related to either CD4 count or HIV-1 viral load. Previous studies had found that the rates of detection of HHV8 in PBMCs in patients with KS were higher than those in patients without KS [8, 10], but data comparing HHV8-seropositive/KS-negative patients with and without HIV-1 are limited. The patients in the present study were fairly healthy (only 29% had CD4 counts <200 copies/mL, and none had KS), and it is possible that our results would have been different if we had studied a more severely immunocompromised group of women.
We found that the prevalence of HHV8 shedding was highest (34%) in the oral cavity and lowest (6%) in the genital tract. Oral-HHV8-shedding studies in men have found high prevalences of HHV8 shedding and high HHV8 copy numbers in saliva and in oral swabs [5–7]. Our findings contribute to the growing body of evidence that the oral cavity is an important site of HHV8 replication [5–6]. This in turn suggests that salivary exposure may be an important route of spread of HHV8.
In summary, our study is the largest of its kind published thus far and the first to compare HHV8 shedding from mucosal sites in HIV-1–seropositive and HIV-1–seronegative women. The high prevalence of HHV8 shedding in HIV-1–seropositive women suggests that this group may be more likely to transmit HHV8, compared with HIV-1–seronegative women. The higher prevalence of oral, compared with genital, shedding of HHV8 suggests that transmission through oral contact may be an important route of transfer of HHV8. Additional research, including HHV8-shedding studies that include a broad spectrum of HIV-1 disease stages, as well as prospective seroincidence studies that identify risk behaviors that predispose to HHV8 acquisition, is necessary to formulate recommendations regarding prevention of HHV8.
Acknowledgments
We are grateful for the participation of the women attending Ganjoni Clinic. We thank Esther Mutunga, Zubeda Basheikh, and Francis Kashonga, for their assistance with the recruitment of patients and the collection of samples; and Amina Abdalla, Khamis Mwinyikai, and Lizette Embuscado, for their analysis of samples.
Financial support: National Institutes of Health (grant CA-86795); International AIDS Research and Training Program, Fogarty International Center (grant D43-TW00007).
References
- 1.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994; 266:1865–9. [DOI] [PubMed] [Google Scholar]
- 2.Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med 1998; 338:948–54. [DOI] [PubMed] [Google Scholar]
- 3.Bestetti G, Renon G, Mauclere P, et al. High seroprevalence of human herpesvirus-8 in pregnant women and prostitutes from Cameroon. AIDS 1998; 12:541–3. [PubMed] [Google Scholar]
- 4.Eltom MA, Mbulaiteye SM, Dada AJ, Whitby D, Biggar RJ. Transmission of human herpesvirus 8 by sexual activity among adults in Lagos, Nigeria. AIDS 2002; 16:2473–8. [DOI] [PubMed] [Google Scholar]
- 5.Pauk J, Huang ML, Brodie SJ, et al. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med 2000; 343:1369–77. [DOI] [PubMed] [Google Scholar]
- 6.Koelle DM, Huang ML, Chandran B, Vieira J, Piepkorn M, Corey L. Frequent detection of Kaposi’s sarcoma–associated herpesvirus (human herpesvirus 8) DNA in saliva of human immunodeficiency virus–infected men: clinical and immunologic correlates. J Infect Dis 1997; 176:94–102. [DOI] [PubMed] [Google Scholar]
- 7.LaDuca JR, Love JL, Abbott LZ, Dube S, Freidman-Kien AE, Poiesz BJ. Detection of human herpesvirus 8 DNA sequences in tissues and bodily fluids. J Infect Dis 1998; 178:1610–15. [DOI] [PubMed] [Google Scholar]
- 8.Calabro ML, Fiore JR, Favero A, et al. Detection of human herpesvirus 8 in cervicovaginal secretions and seroprevalence in human immunodeficiency virus type 1–seropositive and –seronegative women. J Infect Dis 1999; 179:1534–7. [DOI] [PubMed] [Google Scholar]
- 9.Whitby D, Smith NA, Matthews S, et al. Human herpesvirus 8: seroepidemiology among women and detection in the genital tract of sero-positive women. J Infect Dis 1999; 179:234–6. [DOI] [PubMed] [Google Scholar]
- 10.Lampinen TM, Kulasingam S, Min J, et al. Detection of Kaposi’s sarcoma–associated herpesvirus in oral and genital secretions of Zimbab-wean women. J Infect Dis 2000; 181:1785–90. [DOI] [PubMed] [Google Scholar]
- 11.Kedes DH, Ganem D, Ameli N, Bacchetti P, Greenblatt R. The prevalence of serum antibody to human herpesvirus 8 (Kaposi sarcoma-associated herpesvirus) among HIV-seropositive and high-risk HIV-seronegative women. JAMA 1997; 277:478–81. [PubMed] [Google Scholar]
- 12.De The G, Bestetti G, van Beveren M, Gessain A. Prevalence of human herpesvirus 8 infection before the acquired immunodeficiency disease syndrome-related epidemic of Kaposi’s sarcoma in East Africa. J Nat Cancer Inst 1999; 91:1888–9. [DOI] [PubMed] [Google Scholar]
- 13.Lavreys L, Chohan B, Ashley R, et al. Human herpesvirus 8: sero-prevalence and correlates in prostitutes in Mombasa, Kenya. J Infect Dis 2003; 187:359–63. [DOI] [PubMed] [Google Scholar]
- 14.Martin HL, Nyange PM, Richardson BA, et al. Hormonal contracep tion, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis 1998; 178: 1053–9. [DOI] [PubMed] [Google Scholar]
- 15.Casper C, Krantz E, Taylor H, et al. Assessment of a combined testing strategy for detection of antibodies to human herpesvirus 8 (HHV-8) in persons with Kaposi’s sarcoma, persons with asymptomatic HHV-8 infection, and persons at low risk for HHV-8 infection. J Clin Microbiol 2002; 40:3822–25. [DOI] [PMC free article] [PubMed] [Google Scholar]