Structured abstract:
Background:
Heart failure (HF) is associated with an increased risk for sudden cardiac death (SCD). Persons living with HIV (PHIV) are at heightened risk of HF. The study aim was to determine the incidence of SCD among PHIV with HF, who were hospitalized for HF, and the risk factors associated with it.
Methods:
This was a retrospective study of 2,578 patients hospitalized with HF from a single academic center, of whom 344 were PHIV. The outcome of interest was SCD. Sub-group analyses were performed by strata of viral load (VL), and LVEF <35%, 35–49%, >50%).
Results:
Of 2,578 patients with HF, 2,149 (86%) did not have an ICD; of these, there were 344 PHIV and 1,805 uninfected controls. Among PHIV with HF, 313 (91%) were prescribed ART and 64% were virally suppressed. There were 191 SCD’s over a median follow-up period of 19 months. Compared to controls, PHIV had a 3-fold increase in SCD [(21 vs. 6.4%), adjusted OR=3.0, CI (1.78—4.24)]. Among PHIV, cocaine use, lower LVEF, absence of beta-blocker prescription, and VL were SCD predictors. The SCD rate among PHIV with an undetectable VL was similar to the rate among HIV-uninfected individuals. Similar findings were observed by LVEF strata. Among PHIV with HF without a conventional indication for an ICD, the rate of SCD was 10% per year.
Conclusions:
PHIV hospitalized with HF are at a markedly increased risk for SCD. SCD risk was increased in patients with a lower LVEF, lower CD4 count, and a higher VL.
Keywords: Heart failure, Human immunodeficiency virus, Sudden cardiac death
Introduction
Sudden cardiac death (SCD) risk is increased among patients with heart failure (HF) (1). Among selected patients with HF, insertion of an implantable cardioverter defibrillator (ICD) for primary prevention of SCD is indicated (2). However, conventional indications for primary prevention of SCD among patients with HF are imperfect or have limitations, including: 1. Some patients, especially those with a non-ischemic cardiomyopathy, who meet left ventricular ejection fraction (LVEF) criteria for an ICD may not derive benefit (3), and 2. Most SCD cases occur among those with HF with a LVEF above the conventional cut-off (4). Therefore, an improved characterization of populations at risk for SCD may be of help.
PHIV may be one such vulnerable population as PHIV are at increased risk of HF (5) and among PHIV without HF, the risk for SCD is increased (6). There are no data on the risk of SCD among PHIV with HF and there are significant epidemiological and pathophysiological data to support our hypothesis that, as compared to uninfected controls with HF, the risk of SCD is increased among PHIV with HF (1,6,7). Specifically, key risk factors for SCD among broad populations are increased in HIV; for example, rates of concomitant coronary artery disease (CAD), diabetes, smoking, and illicit drug use are increased in HIV (7,8). Additionally, pathophysiologic changes in the myocardium associated with SCD are also increased in HIV (9,10). For example, PHIV without HF have an increase in both the presence and the extent of myocardial fibrosis, which is an established electrophysiological mediator for SCD (9-11). Therefore, the aim of this study was to analyze the incidence of SCD among PHIV with HF and the risk factors associated with it.
Methods
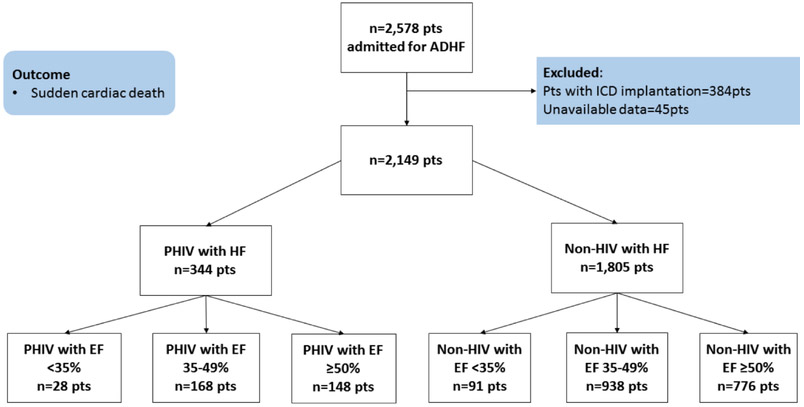
After obtaining Institutional Board Review (IRB) approval, we created a prospective observational registry of all patients admitted to a US tertiary care hospital (Bronx-Lebanon Hospital Center of Icahn School of Medicine at Mount Sinai, Bronx, New York) in 2011 with a primary diagnosis of acute decompensated HF. Individuals who had an ICD or who received an ICD during follow up were excluded (n=358, Figure 1). An acute decompensated HF admission was identified by HF ICD-9 codes at the index HF hospitalization discharge (428.21, 428.31, 428.23, 428.33, 428.41 and 428.43) and confirmed after individual electronic health record (EHR) review using established criteria (12). HIV infection was determined using HIV ICD-9 codes (13,14). The use of these ICD-9 codes for identification of HIV- infected individuals has previously been shown to be 99% sensitive and 89% specific (15). The diagnosis was further confirmed using individual chart review. Similarly, clinically relevant variables were also confirmed through manual review of each of the individual EHR. The information on the diagnosis of CAD (which included myocardial infarction, angina and other ischemic heart disease) was determined by ICD-9 codes (13,14,16) and was confirmed using EHR review, demonstrating high levels of agreement with expert chart review.
Figure 1. Consort diagram.
Consort diagram for the study. ADHF = Acute decompensated heart failure. Pts = Patients, CV = Cardiovascular, PHIV = Persons living with Human Immunodeficiency Virus. LVEF = left ventricular ejection fraction. HF= Heart failure, EF= Ejection fraction.
Outcomes
The follow-up period began on the date of discharge from the first HF hospitalization in 2011. Our primary outcome was the occurrence of SCD. Death was initially identified through the Social Security Death Index (SSDI) and cause of death was determined by physician-adjudicated individual EHR review. Sudden cardiac death was defined using the standardized definition as death within 1 hour of onset of symptoms if witnessed or within 24 hours of being observed alive and asymptomatic if unwitnessed or unexpected out of hospital death (6). The adjudications for SCD were made by two individual reviewers. The inter-observer agreement was determined using kappa between the two physicians, using 50 random patients)). The disagreements/variability was resolved by consensus by an additional reviewer. After complete chart review of these patients, 191 deaths were qualified as SCD. Deaths occurring in a hospice setting, with documented drug overdose, with a positive urine toxicology at death, in patients with ESRD One hemodialysis, in patients with metastatic cancer, among those with a CD4 count <50 cell/mm3 or those with another hospitalization within one month of death were not considered as SCDs. The adjudication was blinded to HIV status.
Covariates
Data on traditional HF risk factors as well as SCD, LVEF, ECG variables, and medication use were collected at discharge from the index HF hospitalization by EHR review. Details on HIV-specific parameters (CD4, VL) were recorded from those available closest to the time of discharge from the index HF hospitalization.
Statistical analysis
Continuous variables are presented as mean and SD or median (IQR) as appropriate based on normality, and categorical variables are presented as percentages. Continuous data were compared with the use of unpaired Student t tests or Wilcoxon rank-sum tests, as appropriate. Categorical data were compared using the chi-square or the Fisher exact test. Survival curves were plotted using Kaplan Meier curves. Univariate and multivariate analyses (Cox proportional hazard regression adjusting for confounding factors (such as age, beta blocker use, CD4 count, cocaine use, and history of CAD), ECG parameters and left ventricular ejection fraction were performed to determine the association between covariates and the occurrence of SCD. In another model, we included patients with and without an ICD as well. Sub-group analyses were performed by CD4 count (<200, >200 cells/mm3), viral load (VL) (<200 - suppressed, >200 copies/mL - non-suppressed), and established strata of LVEF (<35%, 35-49%, >50%). Statistical significance was defined using a two-tailed p-value <0.05. Statistical analyses were performed using SPSS software version 24.
Results
Demographics and baseline characteristics
Among 2,149 individuals hospitalized with HF without an ICD at a single US academic hospital in 2011, there were 344 living with HIV. Among PHIV, 91% (313/344) were prescribed ART, and the median duration of ART prescription was 9 years (IQR 4-16 years). The mean CD4 cell count was 336 cells/mm3, 55% (189/344) had a CD4 cell count of >200 cell/mm3, and 64% (221/344) were virologically suppressed. As per Table 1, among PHIV and HF, the presence of CAD and pulmonary artery systolic pressure (PASP) were higher (47±9.2 vs. 40±9.0 mmHg, p<0.001), as was cocaine use (34 vs. 19%, p<0.001), and co-infection with hepatitis C virus (HCV, 13% vs. 7%, p<0.001) compare to the non-HIV group with HF. Groups were also separated according to strata of LVEF (Figure 1). The characteristics of the cohort stratified based on LVEF are shown in Supplemental Tables 1, 2 and 3. The prevalence of cocaine use stratified by LVEF is shown in supplemental table 4
Table 1.
Baseline Characteristics PHIV vs. non-HIV
| PHIV (n=344) |
Non-HIV (n=1805) |
p-value | |
|---|---|---|---|
| Females | 178 (52%) | 876 (49%) | 0.275 |
| Age (yrs, mean±SD) | 60±9.7 | 60±9.4 | 0.994 |
| Race | |||
| Hispanic | 132 (38%) | 756 (42%) | |
| African American | 139 (40%) | 632 (35%) | 0.167 |
| Others | 73 (21%) | 414 (23%) | |
| Cardiovascular risk factors | |||
| Diabetes | 134 (39%) | 628 (34%) | 0.139 |
| Hypertension | 221 (64%) | 1120 (62%) | 0.441 |
| Hyperlipidemia | 153 (44%) | 723 (39%) | 0.126 |
| Smoking | 162 (47%) | 821 (44%) | 0.583 |
| LVEF (%, mean±SD) | 47±12.0 | 48±12.4 | 0.168 |
| PASP (mmHg, mean±SD) | 47±9.2 | 40±9.0 | <0.001 |
| SBP (mmHg) | 141±27.5 | 139±27.1 | 0.211 |
| DBP (mmHg) | 78±18.3 | 79±18.0 | 0.346 |
| HR (bpm) | 85±21.2 | 83±21.5 | 0.113 |
| QRS duration (ms) | 116±25.3 | 114±25.6 | 0.184 |
| QTc duration (ms) | 435±30.4 | 432±30.7 | 0.100 |
| Serum creatinine (mg/dL) | 1.37±1.0 | 1.31±1.1 | 0.347 |
| BMI (kg/m2, mean±SD) | 27±5.8 | 34±5.9 | <0.001 |
| Sleep apnea | 101 (29%) | 478 (26%) | 0.270 |
| CAD | 162 (46%) | 581 (32%) | <0.001 |
| Cocaine use | 116 (34%) | 344 (19%) | <0.001 |
| Mode of cocaine administration | |||
| Intranasal | 33 (28%) | 107 (31%) | |
| Smoking | 45 (39%) | 151 (44%) | 0.263 |
| Intravenous | 38 (33%) | 86 (25%) | |
| HCV | 45 (13%) | 126 (7%) | <0.001 |
| HBV | 26 (8%) | 119 (6%) | 0.513 |
| HIV parameters | |||
| CD4 count cells/mm3(mean±SD) | 336±244 | ||
| VL <200 copies/mL | 221 (64%) | ||
| ART prescription | 313 (91%) | ||
| Duration of ART prescription (yrs), median (IQR) | 9 (4–16) | ||
| Duration of HIV (yrs), median (IQR) | 9 (4–16) | ||
| Cardiovascular Medications | |||
| Beta blocker | 293 (85%) | 1573 (87%) | 0.321 |
| ACE/ARB | 286 (83%) | 1544 (86%) | 0.251 |
| Spironolactone | 40 (12%) | 187 (10%) | 0.483 |
| Furosemide | 263 (77%) | 1444 (80%) | 0.136 |
BMI= body mass index, LVEF = left ventricular ejection fraction, PASP= pulmonary artery systolic pressure, CAD= coronary artery disease, ICD= implantable cardioverter defibrillator, HCV- hepatitis C virus infection, HBV= hepatitis B virus infection, ACE I= angiotensin converting enzyme inhibitor, ARB= angiotensin receptor blocker, ART = antiretroviral therapy.VL= viral load.
Outcomes
There were 191 SCD’s (8.8%) during a median follow-up duration of 19 months (IQR 3-24).
Entire cohort:
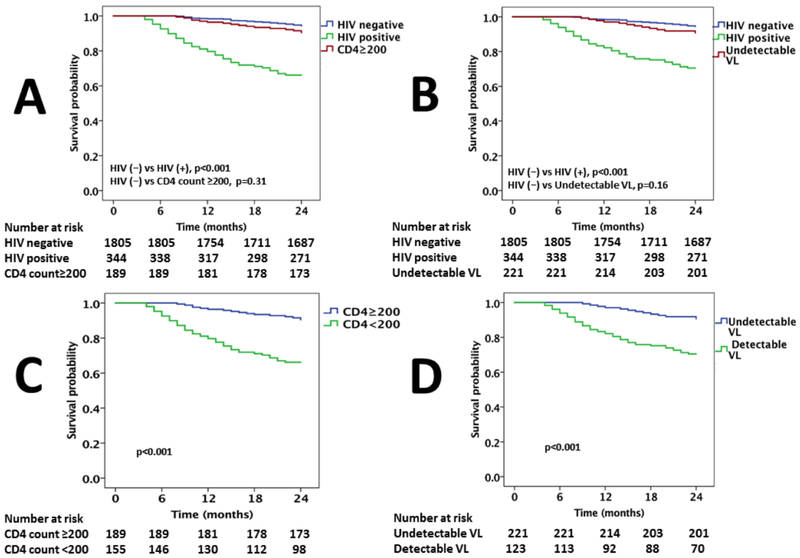
The rate of SCD was higher among PHIV vs. non-HIV infected individuals (21 vs. 6.4%, p<0.001; Figure 2A & 2B) [adjusted OR=3.0, CI = (1.78—4.24)]. Among PHIV, those with a CD4 count of <200 cells/mm3 had a higher SCD rate as compared to those with a CD4 count >200 cells /mm3 (37 vs. 8.4%, p< 0.001; Figure 2C). Similarly, among PHIV those with detectable VL had a higher SCD rate as compared with those with a suppressed VL (43 vs. 9%, p<0.001; Figure 2D). The SCD rate among PHIV with HF with a CD4 count >200 cells/mm3 was higher among PHIV but did not differ significantly from the rate among non-HIV infected individuals (8.4 vs. 6.4%, p=0.31; Figure 2A). Similarly, the SCD rate among PHIV with HF with an undetectable VL was higher but not significantly increased as compared to the rate of SCD among HIV-uninfected individuals with HF (9 vs. 6.4%, p=0.16; Figure 2B). Among PHIV, factors associated with SCD on univariate analysis included traditional CV risk factors (such as history of CAD, cocaine use, a low LVEF), lower rates of beta blocker prescription, QRS width, QTc duration, and HIV parameters (including low CD4 count and non-suppressed VL; Table 2). In a multivariable model, after adjusting for confounding factors, CAD was the strongest predictor of SCD followed by lower CD4 count (or non-suppressed VL), cocaine use, non-prescription of beta blockers, a low LVEF, wider QRS, and an increased QTc duration (Table 2, Supplemental Tables 5 & 6). In a multivariate model, including all PHIV with HF with and without and ICD, the same factors as well as presence of an ICD device remained independent predictors of SCD/SCD equivalents (ICD discharge for ventricular arrythmnias). (Supplemental Table 7). In another multivatiate model, including the entire cohort (PHIV with HF plus HIV-uninfected with HF), same factors as well as presence of HIV disease remained independent predictors of SCD/SCD equivalents (ICD discharge for VT/VF) (Supplemental Table 8). Similar results were noted after matching a similar profile of cocaine use, poor medication adherence (i.e. as represented by poor adherence to HF guideline based medical therapy) among PLHIV with non-infected patients after exclusion of patients with ICD and without exclusion of patients with ICD (Supplemental Tables 9 & 10).
Figure 2. Kaplan Meier survival curves.
Kaplan Meier survival curves comparing sudden cardiac death among (A) PHIV and uninfected controls and PHIV with a CD4 count >200 cells/mm3, (B) PHIV and uninfected controls and PHIV with an undetectable VL, (C) PHIV with a CD4 >200 cells/mm3 vs. patients with CD4 count <200 cells/mm3, (D) PHIV with an undetectable VL with those with a detectable VL.
Table 2:
Analysis for association and predictors of SCD among PHIV with HF
| Univariate analysis | † Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio |
95% CI | p-value | Hazard ratio |
95% CI | p-value | |||
| Lower | Upper | Lower | Upper | |||||
| Gender | 1.223 | 0.821 | 1.843 | 0.28 | ||||
| Age | 1.027 | 0.965 | 1.086 | 0.22 | ||||
| BMI | 0.937 | 0.856 | 1.018 | 0.14 | ||||
| Diabetes | 1.453 | 0.827 | 2.639 | 0.27 | ||||
| Hypertension | 1.077 | 0.652 | 1.745 | 0.83 | ||||
| Hyperlipidemia | 1.242 | 0.854 | 1.866 | 0.16 | ||||
| Smoking | 1.162 | 0.825 | 1.724 | 0.37 | ||||
| H/o CAD | 1.231 | 1.127 | 1.311 | <0.001* | 1.772 | 1.264 | 2.368 | <0.001 |
| Cocaine | 1.372 | 1.185 | 1.516 | <0.001* | 1.571 | 1.223 | 2.113 | 0.002 |
| LVEF | 0.956 | 0.929 | 0.983 | 0.009* | 0.803 | 0.727 | 0.985 | 0.017 |
| PASP | 1.035 | 0.986 | 1.164 | 0.09 | ||||
| SBP | 1.122 | 0.632 | 1.868 | 0.53 | ||||
| DBP | 1.138 | 0.588 | 1.726 | 0.50 | ||||
| HR | 1.151 | 0.863 | 1.611 | 0.47 | ||||
| QRS duration | 1.654 | 1.136 | 2.842 | <0.001* | 1.433 | 1.175 | 2.151 | 0.005 |
| QTc duration | 1.132 | 1.008 | 1.648 | 0.009* | ||||
| SA | 1.020 | 0.814 | 1.253 | 0.97 | ||||
| Viral load | 1.211 | 1.123 | 1.492 | <0.001* | ||||
| CD4 count | 0.983 | 0.969 | 0.991 | <0.001* | 0.907 | 0.861 | 0.997 | 0.004 |
| ART duration | 1.011 | 0.933 | 1.245 | 0.83 | ||||
| Beta blocker | 0.754 | 0.622 | 0.944 | <0.001* | 0.535 | 0.341 | 0.773 | 0.002 |
| ACE I/ARB | 0.966 | 0.842 | 1.224 | 0.73 | ||||
| Spironolactone | 1.114 | 0.883 | 1.463 | 0.81 | ||||
| Furosemide | 1.231 | 0.655 | 2.453 | 0.56 | ||||
p<0.05,
CAD= coronary artery disease, SA= sleep apnea, CAD= coronary artery disease, ICD= implantable cardioverter defibrillator, ACE I= angiotensin converting enzyme inhibitor, ARB= angiotensin receptor blocker, LVEF = left ventricular ejection fraction, PASP= pulmonary artery systolic pressure, BMI= body mass index.
Cox proportional hazard regression for multivariate analysis for primary outcome (sudden cardiac death) among PHIV with HF adjusting for confounding factors (such as (age, beta blocker use, CD4 count, cocaine use, and history of coronary artery disease), ECG parameters and left ventricular ejection fraction.
PHIV with HF with an LVEF >50%:
The SCD rate was higher among PHIV with a preserved LVEF as compared to HIV-uninfected individuals with a preserved LVEF (18 vs. 5.4%, p<0.001). Among PHIV with an LVEF >50%, those with a CD4 count of <200 cells/mm3 had a higher SCD rate as compared to those with a CD4 count of >200 cells/mm3 (30 vs. 8.6 %, p<0.001). Similarly, among PHIV those with detectable VL had a higher SCD rate as compared with those with suppressed VL (37 vs. 9%, p<0.001). The SCD rate among PHIV with a preserved LVEF with a CD4 count of >200 cells/mm3 or suppressed VL was higher but did not differ significantly from the rate among HIV-uninfected individuals with a preserved LVEF (8.6 vs. 5.4%, p=0.23; 9 vs. 5%, p=0.14, respectively). Factors associated with SCD among PHIV with HF were similar to the entire cohort (Supplemental Tables 11 and 12).
PHIV with HF with an LVEF from 35-49%:
Parallel findings were noted among PHIV hospitalized with HF with LVEF 35-49%. Specifically, the SCD rate was higher among PHIV vs. non-HIV-infected individuals with the same range of LVEF (20 vs. 6.5%, p<0.001). Among PHIV, those with a CD4 count of <200 cells/mm3 had a higher SCD rate as compared to those with a CD4 count of >200 cells/mm3 (32 vs. 10%, p<0.001). Similarly, among PHIV those with detectable VL had a higher SCD rate as compared with those with suppressed VL (39 vs. 10%, p<0.001). The SCD rate among PHIV with HF with a CD4 count of >200 cells /mm3 and an undetectable VL was higher but did not differ significantly from the rate among non-HIV-infected individuals with HF (10 vs. 6.5%, p=0.26; 10 vs. 6.5%, p=0.21, respectively). Factors associated with SCD among PHIV with HF were similar to the entire cohort (Supplemental Tables 13 & 14).
PHIV with an LVEF <35%:
There were 28 patients with HIV, HF and an LVEF <35%. These patients did not have an ICD because they refused ICD implantation (n=17), were in the process of optimization of HF medical therapy prior to ICD implantation (n=9), or had NYHA class IV HF refractory to medical treatment (n=2). Among the individuals with LVEF <35%, PHIV had a higher SCD rate compared to the uninfected controls (42 vs. 14%, p=0.001). All PHIV with LVEF <35% who had SCD also had a CD4 count of <200 cells/mm3 and a VL >200 copies/ml. During follow-up, the incidence of SCD among PHIV with a CD4 count of <200 cells /mm3 or a detectable VL without an ICD and LVEF<35% approached 86% and 80% respectively.
SCD incidence and sex difference:
Among women in the entire cohort (HIV and non-HIV infected women; n=1,054), the SCD rate was higher among women with HIV as compared to HIV-uninfected women (18 vs. 5%, p<0.001). A trend toward decreased SCD was noted among women with HIV with HF compared to men with HIV (18 vs. 25%, p=0.13) (Supplemental Figure 1A. Similar partially protective effect of CD4 count and VL on SCD were noted among women with HIV(Supplemental Figure 2).
SCD incidence in African Americans:
The SCD rate was higher among African Americans living with HIV compared to non-African Americans living with HIV (27 vs. 18%, p=0.02) (Supplemental Figure 1B. Similar effects of a partially protective effect of CD4 count and VL were noted on SCD (Supplemental Figure 3).
Discussion
In this study, we present data on characteristics and predictors of SCD among PHIV with HF. This analysis highlights the following key and novel findings: 1. As compared to the uninfected controls with HF, the rate of SCD was markedly higher among PHIV with HF. 2. Improved HIV disease control measures (higher CD4 count and undetectable VL) were associated with lower, but not background, rates of SCD among PHIV. 3. CAD, cocaine use, absence of beta-blockers, a low LVEF, and ECG parameters including QRS width and QTc duration were predictors of SCD, 4. These statements held true when we stratified the cohort based on LVEF (LVEF >50%, LVEF 35-49% and LVEF <35%), some of whom would not be considered for an ICD and 5. These statements were also true when applied to women and African-Americans. To our knowledge, this is the first study to evaluate SCD among PHIV with HF and identifies a cohort at markedly increased risk of adverse events.
Our results expand upon previous findings of Tseng et al., reporting SCD rates 4.5-fold higher among PHIV compared to the general population (6). In that study, the records of 2,860 consecutive patients from a HIV clinic in San Francisco, CA were examined. Viral suppression rates were lower (21% had an undetectable VL) in that study and of 230 deaths occurring over a median 3.7 years of follow-up, 30 (13%) met criteria for SCD. Consistent with our findings, SCD was related to the presence of CAD, a known cardiomyopathy, or clinical heart failure. Incident HF is increased in HIV and PHIV are living longer. Due to this combination, the numbers of patients with HIV and HF are increasing. Our study found that PHIV with HF have a 3-fold increased relative risk, however the overall risk is much higher. Specifically, in the study of Tseng and colleagues, the rate of SCD was about 3.5% per year, while in our study of PHIV with HF, the rate of SCD was 13% per year in the entire cohort and 10% per year among those with an LVEF of >35%. These data suggest the need for additional research into whether patients with HIV and HF would benefit from, at a minimum, close surveillance for a malignant arrhythmia.
The mechanisms involved in the increased risk of SCD in our cohort were not clear. However, based on previous data, we hypothesize that this increase may partially relate to an increase in myocardial fibrosis (11). Specifically, robust data have shown an increase in myocardial fibrosis by MRI is associated with an increased risk for SCD (9) and data have shown that among PHIV without HF, there is an increase in both the presence and extent of myocardial fibrosis by MRI (10) and recent pathological data, have shown an increase in fibrosis among PHIV without HF with SCD (17).
An additional key finding of our study was that measures of HIV disease control (low CD4 count and high VL) were associated with an increased rate of SCD among patients with HF. Specifically, PHIV hospitalized with HF with a CD4 count of <200 cells/mm3 had higher rates of SCD compared with those with a CD4 count of >200 cells/mm3. Parallel findings were noted when stratified by detectable and undetectable VL. Several studies suggest that lower CD4 and higher VL are associated with increased CV disease risk (14,18). In a study involving 2,860 consecutive patients in a public HIV clinic, Moyers et al. found that LV systolic and diastolic dysfunction predicted SCD patients among patients with HIV who were predominately free of HF. This effect was greater in those with a delectable VL (18). In our study, we have evaluated SCD in PHIV with HF who had additionally been hospitalized with decompensated HF. In the SMART study, individuals with HIV randomized to intermittent ART, who had a lower CD4, had increased rates of ischemic CV events compared to individuals with HIV randomized to continuous ART (19). The SMART study suggested that improved virologic control is associated with a lower rate of ischemic CV events. In an observational cohort of PHIV with HF who are at a markedly increased risk of non-ischemic CV events, we found that effective ART (with immunologic rebound and suppressed VL) protected PHIV against adverse outcomes associated with HF such as SCD. The mechanisms involved in potential protection of higher CD4 and viral suppression against SCD are incompletely understood.
Two other findings are notable. Both cocaine use and a lower prescription of beta-blockers were associated with SCD. The exact mechanisms involved in the association between cocaine use and SCD are unclear. Possibilities include the link between cocaine use and the heightened sympathetic tone resulting from catecholamine re-uptake inhibition as well as an increase in the sensitivity of adrenergic nerve endings to norepinephrine (20). Cocaine use can lead to cardiac injury and focal myocardial fibrosis leading to an increased risk of arrhythmias (20). Additionally, cardio-selective beta-blockers are not recommended among active cocaine users (21). This is mainly because of the concern that the use of cardio-selective beta-blockers can lead to vasoconstrictive or hypertensive complications due to unopposed α-stimulation in patients on beta-blockers, especially during acute intoxication and chest pain crises (22). However, recent data suggest that beta-blockers may be safe in patients admitted for cocaine induced chest pain (23). In this study, we found that those PHIV with HF who were not on a beta-blocker were at an increased risk of SCD. Therefore, additional studies should include both a focus on whether continued beta-blockade is beneficial among PHIV, and, potentially, uninfected controls with HF who have previously or still use cocaine.
There are limitations to this study. We do not have autopsy data for these presumed primary cardiac deaths. Hence all cases of SCD in this study were presumed and not confirmed due to lack of autopsy reports. This limitation was highlighted in two recent abstracts where, even among patients meeting WHO criteria for SCD, almost 50% of the sudden cardiac deaths were determined via pathological examination to be likely non-arrhythmic (24,25) and that the event may be related to a drug overdose (17). In this retrospective cohort study we do not have data on pill counts or prescription refills and adherence to treatment and diet could not be assessed. Finally, approximately 6% of deaths occurred either at home or in a different hospital; therefore, the cause of death in those patients was not available, however, there were no differences in the proportion of patients with missing mortality data between the PHIV group and non-HIV infected group.
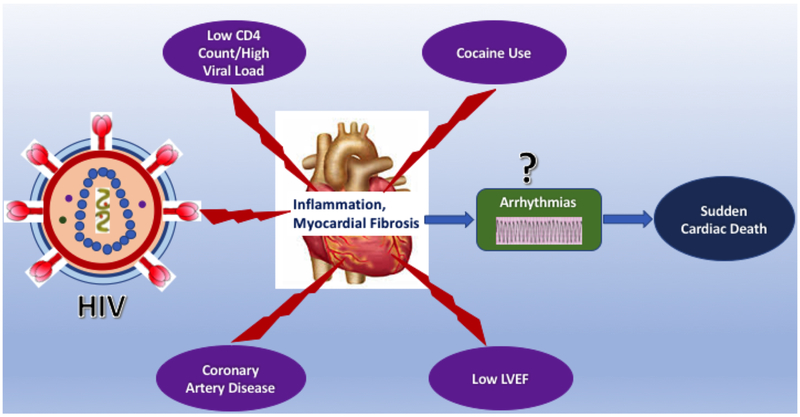
In conclusion, PHIV with HF are at a heightened relative and absolute risk for SCD. The risk of SCD was present across all strata of LVEF and was related to the use of cocaine, lower use of beta-blockers, lower CD4, and non-suppressed VL (Central illustration). This study advances our understanding of SCD among PHIV with HF, re-emphasizing the already recognized importance of disease control among PHIV regardless of LVEF. Moreover, the present study highlights the fact that among PHIV, a clear majority of the patients with HF who do not fall in the standard guideline-criteria for prophylactic ICD placement for primary prevention may need close supervision for arrhythmia risk, continued counselling regarding prevention of risk, and additional emphasis on the potential protective role of beta-blockers. Additionally, the heightened risk of SCD among PHIV with HF should act as a basis for consideration of strategies such as additional surveillance, temporizing strategies such as a LifeVest (26) where transient modifiable factors exist, or subcutaneous ICD implantation in those in whom the infectious risk is still considered prohibitive (27).
Central illustration: Central illustration:
This figure illustrates that Patients living with HIV and heart failure are at a heightened relative and absolute risk for SCD. This figure illustrates that among these patients, a lower CD4 count, high viral load, coronary artery disease, cocaine use and lower left ventricular ejection fraction were independently associated with SCD.
Supplementary Material
Clinical prespectives:
Competency in medical knowledge:
PHIV with HF are at a heightened relative and absolute risk for SCD. The risk of SCD is present across all strata of LVEF and is associated with use of cocaine, lower use of beta-blockers, lower CD4, and a non-suppressed viral load.
Translational outlook:
Additional studies in this cohort are needed to determine the pathophysiological mechanisms responsible for the heightened risk for SCD as well as preventative strategies such as additional surveillance, LifeVest where transient modifiable factors exist or subcutaneous ICD implantation in those in whom the infection risk is still considered prohibitive.
Acknowledgments
Funding: This work was supported by National Institutes of Health / National Heart, Lung, Blood Institute [5T32HL076136 to R.A.], [1R01HL137562 - 01A1to M.Z. and T.N], [1R01HL132786 - 01A1 to V.T.], [1R01HL130539-01A1, and K24HL113128-06 to T.N.], A. Curtis Greer, JD, Pamela Kohlberg and the Kohlberg Foundation, an American Heart Association Fellow to Faculty Award [12FTF12060588 to T.N.], Eunice Kennedy Shriver National Institute for Child Health and Human Development [K08HD094638 to A.N.], National Institutes of Health/ Harvard Center for AIDS Research [P30- AI060354 to A.N. M.Z and T.N.], International Maternal Pediatric AIDS Clinical Trials Network Early Investigator Award [UM1AI068632 to A.N.], and the Nutrition Obesity Research Center at Harvard [P30-DK040561 to M.Z.].
Abbreviations:
- PHIV
Persons living with Human immunodeficiency virus
- SCD
Sudden cardiac death
- VL
Viral load
- HFrEF
Heart failure with reduced ejection fraction
- HFpEF
Heart failure with preserved ejection fraction
- HFbEF
Heart failure with borderline ejection fraction
Footnotes
Disclosures: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vaduganathan M, Patel RB, Michel A et al. Mode of Death in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol 2017;69:556–569. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khatib SM, Stevenson WG, Ackerman MJ et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2017. [DOI] [PubMed] [Google Scholar]
- 3.Kober L, Thune JJ, Nielsen JC et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med 2016;375:1221–30. [DOI] [PubMed] [Google Scholar]
- 4.Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation 2012;125:620–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butt AA, Chang CC, Kuller L et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med 2011;171:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tseng ZH, Secemsky EA, Dowdy D et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol 2012;59:1891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvi RM, Neilan AM, Tariq N et al. Incidence, predictors, and outcomes of implantable cardioverter-defibrillator discharge among people living with HIV. J Am Heart Assoc 2018;7:e009857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvi RM, Neilan AM, Tariq N et al. Protease Inhibitors and Cardiovascular Outcomes in Patients With HIV and Heart Failure. J Am Coll Cardiol 2018;72:518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neilan TG, Farhad H, Mayrhofer T et al. Late gadolinium enhancement among survivors of sudden cardiac arrest. JACC Cardiovasc Imaging 2015;8:414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holloway CJ, Ntusi N, Suttie J et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation 2013;128:814–22. [DOI] [PubMed] [Google Scholar]
- 11.Neilan TG, Coelho-Filho OR, Danik SB et al. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc Imaging 2013;6:944–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hicks KA, Tcheng JE, Bozkurt B et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J Am Coll Cardiol 2015;66:403–69. [DOI] [PubMed] [Google Scholar]
- 13.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009;51:268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr 2010;55:615–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke; a journal of cerebral circulation 2002;33:2465–70. [DOI] [PubMed] [Google Scholar]
- 16.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One 2014;9:e92286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng ZH ME, Bedigian A, Wong JK, VIttinghoff E, Connolly A, Olgin JE, Hsue P. HIV Post SCD: Rates of autopsy-defined sudden arrhythmic death and cardiac fibrosis are >60% higher in persons with HIV. ACC; 2019. March 15–18; New Orleans, LA. Abstract Number 1028–03. [Google Scholar]
- 18.Moyers BS, Secemsky EA, Vittinghoff E et al. Effect of left ventricular dysfunction and viral load on risk of sudden cardiac death in patients with human immunodeficiency virus. Am J Cardiol 2014;113:1260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips AN, Carr A, Neuhaus J et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther 2008;13:177–87. [DOI] [PubMed] [Google Scholar]
- 20.Havakuk O, Rezkalla SH, Kloner RA. The Cardiovascular Effects of Cocaine. J Am Coll Cardiol 2017;70:101–113. [DOI] [PubMed] [Google Scholar]
- 21.McCord J, Jneid H, Hollander JE et al. Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation 2008;117:1897–907. [DOI] [PubMed] [Google Scholar]
- 22.Lange RA, Cigarroa RG, Flores ED et al. Potentiation of cocaine-induced coronary vasoconstriction by beta-adrenergic blockade. Ann Intern Med 1990;112:897–903. [DOI] [PubMed] [Google Scholar]
- 23.Rangel C, Shu RG, Lazar LD, Vittinghoff E, Hsue PY, Marcus GM. Beta-blockers for chest pain associated with recent cocaine use. Arch Intern Med 2010;170:874–9. [DOI] [PubMed] [Google Scholar]
- 24.Freiberg M DM, Kundu S, Mumpuni A, Epstein E, Bedigian A, Justice AC, Vittinghoff E, Tseng ZH. . Sudden cardiac death among HIV-infected and uninfected veterans [Abstract], Annual Conference on Retroviruses and Opportunistic Infections; 2019. March 4–7; Seattle, WA. Abstract Number 32. [Google Scholar]
- 25.Tseng ZH ME, VIttinghoff E, Bedigian A, Wong JK, Ursell P, Connolly A, Olgin J, Hsue P. HIV Post SCD Study: 80% higher rate of autopsy-defined sudden arrhythmic death in HIV. Annual Conference on Retroviruses and Opportunistic Infections; 2019. March 4–7; Seattle, WA. Abstract Number 33. [Google Scholar]
- 26.Klein HU, Goldenberg I, Moss AJ. Risk stratification for implantable cardioverter defibrillator therapy: the role of the wearable cardioverter-defibrillator. Eur Heart J 2013;34:2230–42. [DOI] [PubMed] [Google Scholar]
- 27.Brouwer TF, Yilmaz D, Lindeboom R et al. Long-Term Clinical Outcomes of Subcutaneous Versus Transvenous Implantable Defibrillator Therapy. J Am Coll Cardiol 2016;68:2047–2055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.