Abstract
Hydrogels – water swollen cross-linked networks – have demonstrated considerable promise in tissue engineering and regenerative medicine applications. However, ambiguity over which rheological properties are needed to characterize these gels before crosslinking still exists. Most hydrogel research focuses on the performance of the hydrogel construct after implantation, but for clinical practice, and for related applications such as bioinks for 3D bioprinting, the behavior of the pre-gelled state is also critical. Therefore, the goal of this review is to emphasize the need for better rheological characterization of hydrogel precursor formulations, and standardized testing for surgical placement or 3D bioprinting. In particular, we consider engineering paste or putty precursor solutions (i.e., suspensions with a yield stress), and distinguish between these differences to ease the path to clinical translation. The connection between rheology and surgical application as well as how the use of paste and putty nomenclature can help to qualitatively identify material properties are explained. Quantitative rheological properties for defining materials as either pastes or putties are proposed to enable easier adoption to current methods. Specifically, the three-parameter Herschel-Bulkley model is proposed as a suitable model to correlate experimental data and provide a basis for meaningful comparison between different materials. This model combines a yield stress, the critical parameter distinguishing solutions from pastes (100–2000 Pa) and from putties (>2000 Pa), with power law fluid behavior once the yield stress is exceeded. Overall, successful implementation of paste or putty handling properties to the hydrogel precursor may minimize the surgeon-technology learning time and ultimately ease incorporation into current practice. Furthermore, improved understanding and reporting of rheological properties will lead to better theoretical explanations of how materials affect rheological performances, to better predict and design the next generation of biomaterials.
Keywords: 3D Printing, Bioprinting, Biomedical, Hydrogel, Herschel-Bulkley, Injectable, Paste, Putty, Syringeable
Graphical abstract
1. Introduction
Hydrogels are water-swellable networks held together by physical and/or covalent crosslinks. These networks can be tailored with varying degrees of control of the structural architectures from nano- to microscales and made from a wide variety of materials to suit particular applications.[1] Conventional hydrogel networks can be made from biological polymers (especially proteins and polysaccharides), synthetic polymers (e.g., poly(ethylene glycol)), or chemically modified (semi-synthetic) biopolymers such as cellulose ethers. Less conventional gels can be formed from inorganic colloids or even constructed from harvested tissues.[2–8] The molecular properties (e.g., hydrophilic/hydrophobic balance, net charge or charge distributions) and macroscopic physical properties (e.g., size) can be tuned based on the desired application.[9] Hydrophilicity and significant water content (typically in the 50–90% range) and a fixed physical geometry once the gel has formed are the defining features that fall under the broadest sense of the term ‘hydrogel’. The versatility of hydrogels has allowed them to be employed in a wide variety of applications, from consumer products such as diapers to various foods to common biomedical devices such as soft contact lenses.[10–18] Hydrogels have contributed to the rapid growth in publications for more complex biomedical engineering applications such as tissue engineering constructs and triggered drug delivery devices over the past 20 years.[19, 20] Most publications characterizing hydrogels focus on the performance of the gel after it has formed, with far less attention being paid to the behavior prior to gelation. For successful translation of hydrogels to surgical applications, the behavior of the pre-gelled solution is critical. In this review, we outline the rationale and methods for characterizing the hydrogel precursor for clinical translation.
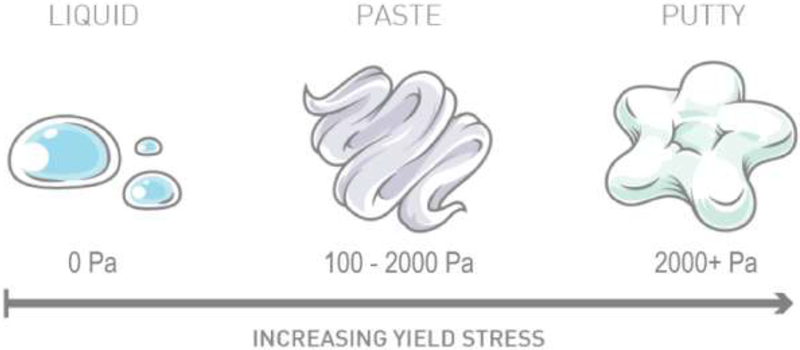
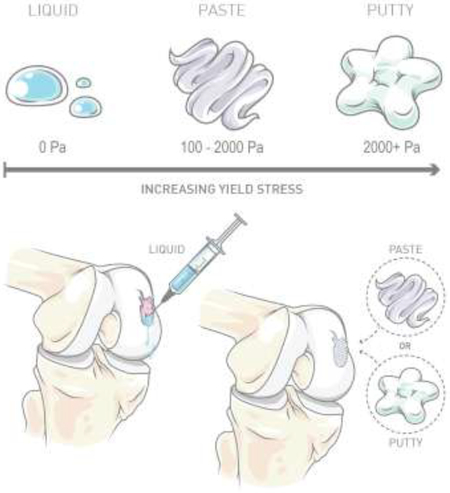
The use of hydrogels in regenerative medicine and biomedical applications has been rapidly increasing over the past 20 years, as the potential of hydrogels to treat complex medical issues has become widely recognized.[21] In situ forming hydrogels those that polymerize or set within the body -can be applied to the treatment of a wide range of complex medical issues such as those requiring filling of defects of various shapes and sizes. Hydrogels can be used to encapsulate and localize both living cells and materials such as hydroxyapatite (HAp) and demineralized bone matrix (DBM). HAp and DBM are commonly used materials in hydrogel formulations for treatment of bone defects.[16, 22] DBX® is a widely used commercially available bone replacement product that combines DBM and hyaluronic acid, the DBM concentration in these hydrogels modulates material pliability to achieve either paste or putty behavior for in situ placement.[16] In fluid mechanics, the yield stress is the stress required for a fluid to begin to flow (as opposed to solid mechanics, where the same ‘yield stress’ term refers to the point between elastic and plastic deformation), and quantifies where such a fluid lies on the spectrum of paste to putty behavior. Increasing the yield stress allows for different material consistencies as illustrated in Figure 1, in which materials with no yield stress exhibit traditional liquid behavior (i.e., flow upon exposure to any stress, no matter how small), while materials with increasing yield stress have paste or putty behavior. DBX® products, for example, vary the yield stress based on the application, employing high yield stress putties for craniofacial applications and lower yield stress pastes for filling mandibular resection defects.[23] The major difference between paste or putty being the route of application, where high yield stress materials can be easily molded and lower yield stress materials can easily flow for injection.
Fig. 1.
Illustration of increasing precursor yield stress to achieve different material consistencies. Precursor materials with no yield stress exhibit liquid properties, and materials with increasing yield stress exhibit paste and putty hydrogel precursor properties. The yield stress is the stress required for a material to begin to flow. Presence of a yield stress for the hydrogel precursor solution is beneficial for surgical placement and 3D bioprinting applications.
Although the material performance in vivo is the ultimate metric for translation, materials with handling properties similar to current products that are familiar to surgeons will have easier learning curves and will thus be easier to adopt into practice. To quantitatively assess these properties, we propose the use of rheology instruments to measure precursor solution characteristics such as yield stress, which have direct relevance to clinicians’ use of the materials. In practice, the three most relevant rheological parameters are the ease of injection (shear response), time for placement (recovery time), and retention of the hydrogel precursor solution at the defect site (yield stress). A review of the recent literature revealed few studies that have conducted hydrogel precursor rheology for one or more of the three previously mentioned properties of interest to clinicians (Table 1 and 2). Of those studies, approximately one in three characterized all rheologically dependent characteristics. Although previous studies commonly state the ability of the hydrogel precursor solution to be injectable or fill any irregularly-shaped defect, few publications reporting new hydrogels actually characterize the precursor solution. Various groups have reported their material to be injectable based only on the ability to pass through a syringe needle. Similarly, investigators have reported gel formation as determined by a simple tube inversion observation.[24, 25] Wang et al.[26] demonstrated that a mixture of oppositely charged nanospheres of anionic and cationic gelatins could be injected into a conical tube and inverted while maintaining the position and shape. Although these methods may qualitatively identify yield stress behavior and/or shear thinning properties, they do not provide quantitative or definitive information.
Table 1.
Summary of rheological properties of hydrogel precursor solutions. Dash marks (−) = data not provided.
| Reference | Hydrogel Type | Materials | Methods | Shear Response | Recovery Time | Yield Stress (Pa) |
|---|---|---|---|---|---|---|
| Abbadessa et al. [114] | Combinational Entanglement and Photoinitiated | Methacrylated chondroitin sulfate and PEG triblock copolymer | 20 mm cone and plate (1°), 27 μm gap | Thinning | < 10 s | 19.2 |
| Avery et al.[148] | Physical Charge | Gelatin and silicate nanoplatelets | 25 mm cone and plate (1°) | Thinning | <1 min | |
| Beck et al. [70] | Chemical Photoinitiated | Methacrylated hyaluronic acid and hyaluronic acid nanoparticles | 20 mm parallel plate | Thinning | ~18 to 160 | |
| Beck et al. [38] | Chemical Photoinitiated | Methacrylated hyaluronic acid and hyaluronic acid nanoparticles | 20 mm parallel plate, 500 μm gap | Thinning | <5 mins | ~200 to 700 |
| Beck et al.[2] | Chemical Photoinitiated | Methacrylated solubilized devitalized cartilage and devitalized cartilage microparticles | 20 mm parallel plate, 500 μm gap | Thinning | ~30 to 1500 | |
| Beck et al. [67] | Chemical Photoinitiated | Methacrylated hyaluronic acid and devitalized/decellulariz ed cartilage microparticles | 20 mm crosshatched parallel plate, 500 μm gap | Thinning | ~90 to 240 | |
| Dennis et al.[37] | Physical Colloidal Flocculation | Chondroitin sulfate or hyaluronic acid with hydroxyapatite nanoparticles | 20 mm parallel plate, 500 μm gap | Thinning | <5 mins | ~1 to 1400 |
| Dennis et al.[76] | Physical Colloidal Flocculation | Hyaluronic acid, hydroxyapatite nanoparticles, and micronized tissue ECM | 20 mm crosshatched parallel plate, 500 μm gap | Thinning | <5 mins | ~100 to 1000 |
| Dumas et al.[84] | Chemical Reaction | Lysine-derived polyurethane and bone particles | 25 mm parallel plate, 1000 μm gap | Thinning | 2.1 | |
| Fakhari et al. [68] | Physical Entanglement | Hyaluronic acid nanoparticles | 20 mm cone and plate (2°) | Thinning | ~500 to 2300 | |
| Gaharwar et al. [78] | Physical Charge | Gelatin and silicate nanoplatelets | Static Tests: 25 mm Parallel Plate, 500 μm gap Transient Tests: 25 mm Cone and Plate (1°) | Thinning | <10 s | 2 to 89 |
| Geisler et al.[64] | Physical Peptide Interactions | Peptide (VKVKVKVKVKV-NH2) | 25 mm parallel plate, 500 nm gap | Thinning | Immediate | |
| Glassman et al.[65] | Physical Peptide Interactions | Protein-polymer triblock copolymer | 25 mm sandblasted cone and plate (1°) | Thinning | ~9000 | |
| Hao et al.[77] | Physical Entanglement | Hydrophobically modified polyacrylamide and sodium oleate micelles | 40 mm cone and plate (1°) | <10 s | ||
| Li et al. [149] | Chemical Reaction | Glycol-chitosan and dibenzaldehyde-terminated polyethylene-glycol | 20 mm parallel plate | 40 to 84 min | ||
| Liu et al.[124] | Chemical Reaction | Catechol-modified PEG and Laponite | Immediate | |||
| Montalbano et al.[150] | Physical Entanglement | Collagen and strontium glass particles | 20 mm parallel plate, 500–1000 μm gap | Thinning | <100 s | 2.18 |
| Mouser et al.[112] | Combinational Entanglement and Photoinitiated | gelatin-methacryloyl and gellan gum | 20 mm cone and plate (1°) | Thinning | 0.13 to 48.2 | |
| Olsen et al.[79] | Physical Peptide Interactions | Telechelic proteins with coiled-coil endblocks and flexible polyelectrolyte midblocks | 25 mm cone and plate | Thinning | <10 s | ~1400 |
| Paxton et al.[111] | Physical Entanglement (poloxamer) and Charge (alginate) | Poloxamer 407, alginate, and alginate/gelatine | 25 mm parallel plate, 500 μm gap | Thinning | <200 s | 166 to 348 |
| Ribeiro et al.[98] | Physical Entanglement | Poloxamer 407 and PEG | 40 mm cone and plate (2°), 54 μm gap | Thinning | ~100 to 400 | |
| Rughani et al.[146] | Combinational Peptide Interactions and Photoinitiated | Self-assembling p-hairpin peptide incorporating non-natural sorbamide residues | 25 mm parallel plate, 500 μm gap | Thinning | <2 h | |
| Samaniuk et al.[48] | - | Mayonnaise | 25.4 mm diameter, 150 mm long vane rheometer | - | - | ~200 |
| - | Play-Doh | - | - | ~3000 | ||
| Townsend et al.[83] | Physical Colloidal Flocculation | Hyaluronic acid, hydroxyapatite nanoparticles, and micronized tissue ECM | 20 mm crosshatched parallel plate, 500 μm gap | Thinning | ~100 to 550 | |
| Tsaryk et al.[69] | Physical Entanglement | Collagen and hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres | 15 mm crosshatched parallel plate, 1000 μm gap | Thinning | ||
| Vulpe et al.[151] | Chemical Reaction | Collagen, hyaluronan, and sericin | Concentric cylinder geometry | Thinning | ||
| Wang et al.[26] | Physical Charge | Cationic and anionic gelatin nanospheres | 20 mm parallel plate, 500 μm gap | Thinning | <1 min | |
| Wilson et al.[108] | Physical Entanglement and Charge | Nanosilicates and kappa-cerrageenan | 40 mm parallel plate, 200 μm gap | Thinning | <5 s | 4.2 to 33 |
| Yavvari et al.[145] | Physical Charge | Catechol-modified chitosan and Fe(III) | 25 mm cone and plate (1°), 50 μm gap | Immediate |
Table 2.
Summary of rheological properties of hydrogel precursor solutions for groups presenting yield strain. Dash marks (−) = data not provided.
| Reference | Hydrogel Type | Materials | Methods | Shear Response | Recovery Time | Yield Strain (%) |
|---|---|---|---|---|---|---|
| Diba et al.[152] | Physical Colloidal Flocculation | Bisphosphonate-functionalized hyaluronan and bioactive glass particles | 8 mm parallel plate | Thinning | Immediate | ~160 to 260 |
| Gao et al.[81] | Physical Hydrophobic Interactions | Pyrene-tailored pyridinium and 2,4,7-trinitrofluorenone | 25 mm parallel plate | - | <180 s | 78 |
| Li et al. [137] | Physical Charge | Supramolecular gel containing charged micelles and laponite | 25 mm parallel plate | - | <10 s | 31.7 |
| Liu et al.[80] | Physical Hydrophobic Interactions | Dexamethasone phosphate, betamethasone phosphate, and hydrocortisone phosphate | 20 mm cone and plate | Thinning | <10 s | 9.8 |
| Lu et al.[66] | Physical Peptide Interactions | Peptide-PEG copolymer with dimerization and docking domain polypeptide | 20 mm cone and plate, 27 μm gap | Thinning | ~6 s | ~100 to 400 |
| Rodell et al.[153] | Physical Hydrophobic Interactions | Hyaluronic acid functionalized with guest (adamantine) and host (p-cyclodextrin) | 20 mm cone and plate (1°) | Thinning | <10 s | ~60 |
| Rodell et al.[154] | Combinational Hydrophobic Interactions and Chemical Reaction | Dual crosslinking hyaluronic acid functionalized with guest (adamantine) and host (p-cyclodextrin), and thiol/methacrylate groups | 20 mm cone and plate | Thinning | <3 s | 35 |
| Wang et al.[109] | Combinational Chemical Reaction and Photoinitiated | Hyaluronic acid modified with hydrazone crosslinking groups and with noroborene modified hyaluronic acid and pentaerythritol tetramercaptoacetate | 20 mm cone and plate | Thinning | <10 s | ~200 |
| Yu et al.[82] | Chemical Reaction | Chain-extended PEO-PPO-PEO multiblock copolymer | 40 mm cone and plate (1°) | - | Immediate | 130 |
Therefore, the purpose of this review article is to emphasize to biomaterials developers the importance of hydrogel precursor rheological characterization. Adoption of medical nomenclature can be leveraged as a translational advantage among hydrogel formulation developers, and the connection between rheological properties and surgical application requirements will be explained. In addition, the rheological characterization of bioinks for 3D bioprinting will be discussed providing a common framework for evaluating new bioinks. Hydrogel precursor rheology is also important for other impactful areas such as in vitro disease model systems, although the current review focuses on bioprinting for any application.[27–29] Finally, rheological modeling is proposed as the basis for comparison among hydrogel precursor groups, with additional considerations for physical, chemical, and combinational crosslinking approaches proposed to help move the community toward material design strategies to create precursor solutions with a target yield stress. In this review, we will show how implementing yield stress in hydrogel precursor solutions has a direct benefit for biomedical applications in terms of clinical translation.
2. Biomedical Hydrogel Perspective
2.1. Our Inspiration
More than 20 years ago, the concept of colloidal gels began to emerge as a potential material for regenerative medicine. At the time, pioneering work by Prof. Jennifer Lewis and others showed novel colloidal inks could be used for printing freestanding 3D structures.[30, 31] Such colloidal inks utilized weak interactions between a multitude of nanoparticles or between nanoparticles and polymers, which could be disrupted to allow the viscous ink to yield and flow before again ‘recovering’ bulk solid properties. We and others recognized these dynamic, viscoelastic colloidal inks offered promise as tissue fillers or as substrates for printing 3D scaffolds for regenerative medicine.[32, 33]
Our team began translating colloidal gels to applications in regenerative medicine based on biodegradable materials suitable for use as tissue fillers while still maintaining the desirable properties of colloidal inks. We rationalized that these dynamic, viscoelastic and biodegradable colloidal gels could facilitate placement of the material, retention at the site of administration, and recovery of elasticity after placement. Our first effort employed combinations of poly(lactic-co-glycolic acid) (PLGA) nanoparticles with opposite surface charges, which we demonstrated could support viable cells.[34] This work was followed by a study showing zero-order release and in vivo suitability for cranial defect repair.[35] Moving in a new direction, we then demonstrated that hyaluronic acid nanoparticles could form a dynamic, viscoelastic material believed to be the result of association of free surface chains, resulting in a colloidal gel viscosupplement that might improve upon the performance of traditional crosslinked or high molecular weight hyaluronic acid.[36] Having demonstrated some fundamental properties of colloidal gels, we then reported efforts to improve material performance by adding colloids such as hydroxyapatite,[37] or creating colloidal gels that could be chemically crosslinked after placement.[38] Today, we continue to explore the performance of colloidal gels in vivo as injectable bone or cartilage fillers, and we are refining material properties to create clinician-friendly materials that promote tissue regeneration.[39, 40]
The precursor properties of our materials are of critical importance to clinicians, perhaps even more so than the crosslinked solid mechanics, depending on the application. That is, it is important to have a material that is easy to place in the site and ensure that it will stay there, and that if new hydrogel precursor solutions have behaviors of other moldable materials that surgeons are used to implanting, they may be more likely to adopt the technology.
2.2. Translation of Technology to Surgeons
As previously discussed in the last section, it is important to keep in mind the needs of the clinician and the patient throughout the process of designing new hydrogel technologies. The transition from lab bench to clinic can be an arduous process, and a potentially significant product could be completely ignored if the surgeon must acquire new skills or buy new equipment. Wilson et al.[41] proposed that surgeons are attracted to new medical technologies that can be learned by passive observation rather than hands-on training sessions, and thus can be easily adopted into their practice without disruption. The gap between scientific knowledge and clinical application is well known, and with decreasing time available for some clinicians to spend on research, there are less comparative studies being conducted.[42] New methods are being employed to shorten the learning curve associated with new medical technologies and surgical procedures. The use of social media outlets and live streaming has provided the ability for surgeons to collaborate on difficult cases and provide coaching between surgical teams from different continents.[43, 44] Although steps are being taken to ease the translation of new research into the clinic, it is always going to be easier to utilize a surgeon’s current skill-set than to require training in a new technique.
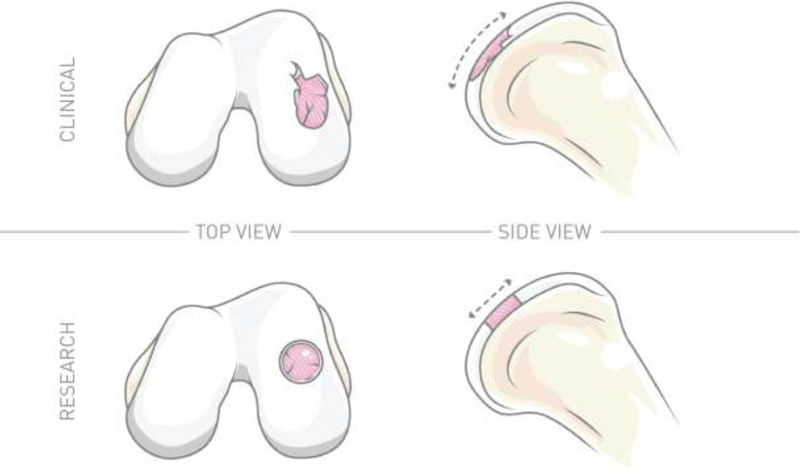
Lack of consideration of the method for the physical placement of hydrogels in an intended application directly ignores the needs of the surgeon. In the example of in situ hydrogels for cartilage regeneration, some groups have proposed the use of liquid hydrogel precursors with zero yield stress to fill defects before crosslinking.[45] The issue with this hydrogel precursor design is that it does not account for applications in which the defect may be angled, or in a hard to access area. In vivo studies generally employ ideal situations, such as a defect site that is perfectly cylindrical and applied to a single plane.[46] In contrast, real tissue defects are not perfectly cylindrical and uniplanar, as illustrated in Figure 2. In situ placement using a liquid hydrogel precursor solution with zero yield stress could become difficult as the material may leak after placement. In designing clinically translatable hydrogels, understanding the connection between rheological hydrogel precursor properties and the connection to surgical use is important for successful clinical translation.
Fig. 2.
Illustration depicting the difference between actual defects observed in patient populations (top) compared to well-defined, induced defects used for in vivo studies (bottom). Common differences between actual injuries and defects used for research include shape, size, and orientation, and emphasize the clinical requirement of a hydrogel precursor solution that can be carefully molded and contoured to fit the defect site prior to crosslinking.
2.3. Biomedical Hydrogel Perspective Summary
Over the past 10 years, we have evolved from using simple colloidal gels to more complex crosslinkable hydrogels for biomedical applications. A core philosophy of our team has focused on creating biomaterials capable of being translated from the lab to the clinic. A challenge of clinical translation is the poor adoption of new treatments by surgeons if the technique learning curve is too high. For the aforementioned reason, we have identified the handling properties of hydrogels, specifically the hydrogel precursor performance, as a crucial component of hydrogel design to allow future clinical translation.
3. Hydrogel Rheology Properties
3.1. Paste and Putty Nomenclature
As previously stated, paste and putty nomenclature has long been used in medical products to distinguish basic rheological properties.[16] Medical professionals and the general public already have a common connection to these words and their distinguishing characteristics. The paste and putty description is directly related to a material’s rheological properties for placement, specifically the material’s yield stress. A low yield stress material exhibits paste-like behavior, whereas a high yield stress material exhibits putty-like behavior.[34, 47] Because of the pre-existing connection to paste and putty nomenclature, the biomedical hydrogel community would be wise to adopt these words in describing rheological characteristics.
The distinction between paste and putty performance, although related to yield stress, remains qualitative and has yet to be successfully defined. In part, this review aims to propose relative ranges of yield stresses for both paste and putty solutions for improvement of material reporting. In determining the yield stress range for a paste-like material compared to a putty-like material, we can utilize known materials to help in creating the distinction between the two designations. Samaniuk et al.[48] previously published yield stress values for common household items that qualitatively exhibit either paste or putty behavior for reporting the design of a novel rheometer. For example, the yield stresses of mayonnaise and Play-Doh, approximately 200 and 3000 Pa, respectively, may be used to help define the ranges for paste and putty distinctions. Using the yield stress of known household items and previous publications from our own group, we propose the range of 100 to 2000 Pa be referred to as paste-like materials, whereas materials above 2000 Pa to be referred to as putty-like materials. By reporting material yield stress in this context, it is possible to better connect the hydrogel community to clinicians using a common set of nomenclature.
3.2. Paste and Putty Application
The choice between paste and putty behavior for hydrogel precursors remains application dependent, and no “one size fits all” approach exists. Examples of low yield stress, paste-like hydrogel precursors, and high yield stress, putty-like hydrogel precursors will be explored and how they can be beneficial to different applications. Nucleus pulposus regeneration and ophthalmology are two areas in which hydrogel use has been proposed.[49–52] The aforementioned applications generally propose precursor solutions to exhibit low to no yield stress for injection through a high-gauge needle; however, low yield stress, paste-like hydrogel precursor solutions may be ideal for such applications because they allow for injection through higher gauge needles while providing benefits tailored to the application desired.[53–55] High yield stress, putty-like hydrogel solutions are generally useful in applications where shape fitting is required, such as craniofacial bone regeneration.[56, 57] The higher yield stress precursor solutions and physical gels allow for better material shaping and directed tissue regeneration.
3.3. Herschel-Bulkley Model
Although paste and putty nomenclature qualitatively depicts low and high yield stress, the nomenclature alone does not provide enough information for an in-depth comparison. It is important for the hydrogel community to choose a standardized testing method that allows for a deeper look into the relationships between material properties and rheological performance. When reporting rheological properties of new materials, it is beneficial to fit experimental data to common, pre-existing rheological models for comparison. The Herschel-Bulkley (HB) model is an ideal candidate to fill this need for the hydrogel community. The HB model was first introduced by Winslow Herschel and Ronald Bulkley in 1926 and provides a simple and general model to explain the behavior of a non-Newtonian fluid.[58] The HB model equation shown in Equation 1 relates the fluids shear stress (τ), to the yield stress (τ 0), consistency index (k), shear rate (), and flow index (n).
| (Equation 1) |
The fluid exhibits solid properties when τ <τ0, and for τ > τ0, exhibits shear thinning when n<1 and shear thickening when n> 1. The HB model is an attractive option for the hydrogel community because it provides a general model that includes variables for both yield stress and shear response. Previous groups that have characterized hydrogel precursor solutions have reported that experimental results respect the HB model.[59–61] For reporting hydrogel precursor properties, quantification of yield stress and shear response adequately provides information for comparison of materials within the hydrogel community.
3.4. Rheological Methods
The assessment of rheological performance can be attained by various rheological testing systems. For in-depth background information regarding the mathematical theories of rheological testing, an introductory polymer rheology textbook by Osswald et al.[62] is recommended. Briefly, the two most commonly used testing fixtures, cone-plate and parallel-plate, will be discussed, detailing their individual uses and strengths/weaknesses. The cone-plate fixture allows for a wide variety of rheological tests such as yield stress, creep, recovery time, oscillation, and ramp tests.[62] The wide variety of rheological tests can be attributed to the design of the cone-plate fixture, where an upper cone of a small angle, generally between 1–4°, sandwiches the material being tested between a flat bottom plate. Due to the small angle of the cone, the shear rate and shear stress across the sample is nearly constant, allowing for accurate material measurements. The disadvantage of the cone-plate system is that it is limited to small particle sizes (≤ 10 μm), and thus cannot be used for suspensions of larger particles. Alternatively, the parallel-plate fixture, which sandwiches the material being tested between two flat plates, is not limited by particle size. Due to the design of the parallel plate fixture, materials with larger particle sizes (≥ 10 μm) can be tested. The disadvantage of the parallel-plate fixture is that the shear rate across the sample increases with radius, so the parameters obtained are average values over the range. while keeping in mind that these differences exist in comparing results between various studies. The cone-plate fixture is the recommended choice when performing a rheological test; however, in cases where the cone-plate fixture may not be used due to particle size, the parallel-plate fixture is recommended. The choice of testing apparatus, and the geometry of the probing fixture can influence the resulting data provided by a rheological test. Testing differences must be noted as sample measurements conducted by two separate studies using the same material and testing conditions, but different testing fixtures, can have varying results. It is necessary to report the choice of testing apparatus (e.g., cone-plate or parallel-plate), fixture geometry (e.g., 20 mm), and testing conditions, while keeping in mind that methodological differences exist in comparing results among various studies. The cone-plate fixture is the recommended choice when performing a rheological test; however, in cases where the cone-plate fixture may not be used due to particle size, the parallel-plate fixture is recommended.
3.5. Hydrogel Rheology Properties Summary
Clinicians and scientists using a common nomenclature is important for bridging the gap between the two fields. By incorporating the nomenclature, and defining the parameters, it is possible to better connect the two sides and eliminate the possibility of failed medical translation due to issues of poor surgeon adoption. In this section, we have defined the yield stress attributing to paste and putty nomenclature as the range of 100 to 2000 Pa, and materials above 2000 Pa, respectively. The Herschel-Bulkley model was proposed as the recommended model for reporting rheological parameters such as yield stress. In terms of methods for rheological application and choosing the best testing fixture, the cone-plate fixture is recommended overall but limited to materials with small particle sizes (≤ 10 μm), and the parallel-plate fixture is recommended for materials with larger particle sizes (≥ 10 μm). The recommended range for paste-like and putty-like materials, the use of the Herschel-Bulkley model, and employing the correct testing fixture will help to standardize the reporting of rheological parameters for consistency and cross-comparisons for bioprinting and for clinical translatability.
4. Surgical Context
4.1. Injectability/Syringeability and Shear Response
“Injectability” is a common buzzword among hydrogel publications to qualitatively communicate the ability of a material to flow through a syringe needle.[37, 63–66] Although prevalent, the use of this term is generally ambiguous with minimal quantitative information provided, as the injection pressure is strongly correlated with injection rate, needle gauge and length (i.e., the Hagen-Poiseuille equation). A similar term that has been used much less frequently in hydrogel publications is syringeability, which has become useful in our own publications to express the ability of the hydrogel precursor solution to flow through a syringe orifice rather than a needle.[2, 67] Both terms, injectability and syringeability, qualitatively express the ability to flow through a certain orifice size. In relation to the medical field, the performance of a material to easily flow from a needle or a syringe can dictate the delivery method and use by the surgeon. An injectable material (i.e., through a needle) could potentially have applications in laparoscopic surgery, whereas a syringeable material (i.e., through a syringe orifice) may require open surgery for placement.
The flow of a material in both situations is governed by the shear response exhibited when a force is applied as a function of flow rate. During extrusion, the material can undergo shear thinning or shear thickening. Shear thinning is where a material will exhibit a decreasing apparent viscosity with increasing rate of applied stress (i.e., shear rate), whereas shear thickening is where the material will exhibit an increasing apparent viscosity with increasing rate of applied stress. The apparent viscosity can also be time dependent, either decreasing with time (thixotropic) or increasing with time (rheopectic). For the injectable biomaterials of interest in this article, thixotropy could be desirable if it occurred over a short period of time, comparable or less than the time of injection or syringing. Hydrogel precursor solutions generally exhibit shear thinning behavior, which makes delivery from a syringe easier because as the shear rate increases in the needle, shear thinning will reduce the apparent viscosity and thus the relative resistance to flow (Table 1). Fakhari et al.[68] demonstrated shear thinning performance of colloidal gels comprised of hyaluronic acid nanoparticles by showing a decreasing viscosity with increasing shear rate. Similarly, Tsaryk et al.[69] indicated shear thinning performance of collagen/hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres by determining the load required to inject the material through a 16-gauge needle, showing a decreasing force required after the material began to flow. We previously fit experimental rheological data to the Herschel-Bulkley equation to determine the shear response, which allowed for a quantitative and comparable method for the degree of shear thinning.[37, 38, 70] The need for shear thinning physical hydrogels was previously identified by Guvendiren et al.[71] in biomedical applications; however, the benefit of shear thinning behavior can be extended to all hydrogel precursors. Shear thickening behavior has generally been avoided in biomedical applications due to issues with extruding, although desired in some industry based applications.[72] After injection or syringing, it is important for the material to regain the original properties prior to being injected or syringed. The time required for transition back to this starting time is referred to as the recovery time.
4.2. Placement and Recovery Time
Prior to injection or syringing, the bulk material is static and the microstructure is stable. After disruption, the material properties are in a state of disarray and take time to return to equilibrium. The time required for a material to return to the original equilibrium state is referred to as the recovery time, or the self-healing time.[71] The governing principle associated with the material recovery time is referred to as thixotropy, the reversible and time-dependent manifestation of viscosity-induced structural changes.[73–75] The recovery time of a syringed material is important in terms of initial placement. A material with a relatively slow recovery time could potentially have issues during placement as the material would initially be difficult to retain within the defect site. In terms of clinical usage, a fast recovery time after syringing is necessary for material manipulation or shaping, and especially for placement laparoscopically where it would be difficult to implement precautions for increased material retention. The recommended recovery time, and degree of material recovery for syringed or injected materials has yet to be defined and remains application dependent. We previously proposed the use of colloidal hydrogels comprised of hyaluronic acid, hydroxyapatite, and micronized native extracellular matrix as potential bone defect fillers.[76] The physical hydrogels presented were capable of nearly complete recovery of the storage modulus (G’) within 5 minutes after disruption (Table 1). Similarly, in a previous study, we reported hydrogels comprised of UV-crosslinking hyaluronic acid and hyaluronic acid nanoparticles, in which the precursor could recover their original storage modulus within 5 minutes.[38] Even faster recovery times on the magnitude of seconds have been reported.[64, 66, 77] Gaharwar et al.[78] demonstrated that an injectable nanocomposite hydrogel of gelatin and synthetic silicate nanoplatelets could be used for the treatment of internal hemorrhaging, as the hydrogel could recover the elastic gel strength in less than 10 seconds after disruption. In general, materials for biomedical applications should aim to have low recovery times to facilitate ease of use. After shearing and material placement, the material must remain in place, this retention is influenced by the yield stress of the material.
4.3. Retention and Yield Stress
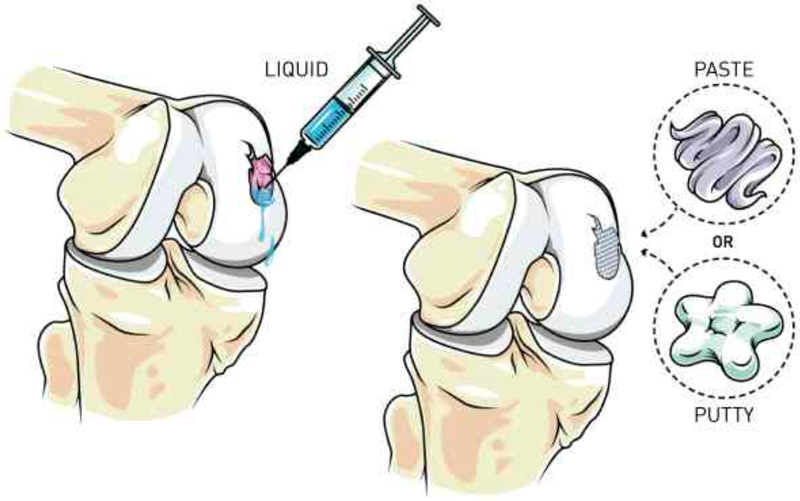
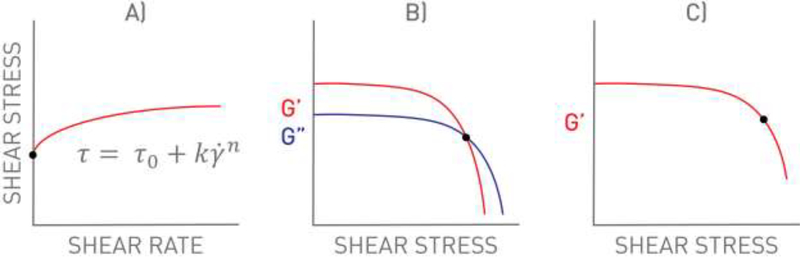
The stress required for a material to begin to deform under an applied shear stress is referred to as the yield stress, and it plays an important role in material performance. The yield stress is entirely separate from the apparent viscosity, and these two terms must not be confused. The yield stress contributes to both the syringeability/injectability of the material (i.e., initial force required to generate flow) and retention within the defect after placement (i.e., no movement in the absence of applied force). In contrast, the apparent viscosity relates to the force required to continue dispensing from the syringe after flow has commenced. Implementation of yield stress to the hydrogel precursor solution can positively aid in material retention within the defect site after surgical placement. Figure 3 illustrates the placement of a hydrogel precursor solution without a yield stress compared to a hydrogel precursor solution with a yield stress (i.e., exhibiting paste or putty rheological properties). Precursor solutions enabling a yield stress will experience better retention in the defect site, allow for shape-specific fitting, and will be easier for surgeons to handle.[70] Various methods are currently used to determine a material yield stress as depicted in Figure 4. The most common methods include 1) empirical model fitting (e.g., Herschel-Bulkley), 2) determining the shear stress at the crossover point of the storage and loss modulus (G’/G”), and 3) determining the shear stress related to a pre-determined deviation of storage modulus from linearity. We previously used a three-parameter fitting technique to the Herschel-Bulkley model to report the yield stress of UV-crosslinking hyaluronic acid hydrogels embedded with hyaluronic acid nanoparticles, and reported yield stress values in the range of ~18 to 160 Pa (Table 1).[70] Similarly, in another study, we used the Herschel-Bulkley model to determine the yield stress of hydroxyapatite colloidal gels with either hyaluronic acid or chondroitin sulfate, the resulting yield stress ranged from ~1 to 1400 Pa.[37] Alternatively, Olsen et al.[79] demonstrated that hydrogels comprised of self-assembling telechelic proteins had a yield stress of ~1400 Pa using the G’/G” crossover method, and could be injected from a syringe through a 22-gauge needle. Similarly, Liu et al.[80], Gao et al.[81], and Yu et al.[82] used the G’/G” crossover method to determine yield strains of 9.8, 78, and 130%, respectively. Yield strain, which is similar to yield stress, is the strain at which a material begins to flow (Table 2). The yield stress method is recommended over the use of yield strain as the yield stress allows for easier comparison amongst other studies. We previously used the storage modulus deviation method, choosing a storage modulus deviation of 10% from linearity, to determine the yield stress range of colloidal hydrogels comprised of hydroxyapatite and glycosaminoglycans from ~1 to 1400 Pa.[76] Gaharwar et al.[78] and Glassman et al.[65] similarly used the deviation from linearity method in determining the yield stress. As a general rule, yield stress values above 100 Pa are sufficient for material retention prior to secondary crosslinking.[2, 68, 78]
Fig. 3.
Illustration of how achieving paste or putty rheological properties in hydrogel precursor solutions can aid in material placement. (Left) Lack of material retention using an in situ liquid hydrogel precursor solution compared to (Right) precursor materials exhibiting desirable paste and putty behavior.
Fig. 4.
Graph illustrations of methods to determine the yield stress of a material, black dots correspond to the yield stress. A) Theoretical model fitting using the Herschel-Bulkley (HB) model as an example. B) Determining the shear stress at the crossover point of the storage and loss modulus (G’/G”). C) Determining the shear stress related to a pre-determined deviation of the storage modulus from linearity (i.e. 85% deviation). As a general rule, we recommend the HB model to calculate the yield stress (τ0).
Hydrogel precursor solutions can be created with a yield stress by several different available options. Perhaps the easiest route to employ a yield stress is the addition of micro-or nano-sized particles, the reduction in available space results in a thicker consistency, and we have previously used this method to create a hydrogel precursor solution with a yield stress.[83] Dumas et al.[84] developed an injectable self-setting lysine-derived polyurethane scaffold encapsulating allograft bone particles (180 μm) for bone restoration. Allograft bone particles were added to the precursor solution at a concentration of 45% (w/w), providing a modest yield stress of 2.1 Pa. An alternate route to employ a yield stress to a precursor solution is the use of oppositely charged materials, where electrostatic interactions increase precursor consistency. Gaharwar et al.[78] used oppositely charged gelatin and silicate nanoplatelets to create injectable self-assembling hydrogels for treatment of internal hemorrhaging. The oppositely-charged gelatin-silicate nanocomposite hydrogels provided a yield stress of 2 to 89 Pa, depending on nanoplatelet concentration. Another potential avenue for achieving a precursor yield stress is the use of fast-acting click chemistry to create materials that self-heal after shearing. For example, Yu et al.[82] proposed the use of a self-healing multi-block copolymer capable of quickly forming covalent bonds between acylhydrazines and aldehyde groups. Rapid covalent bond formation allowed for material healing after injection, creating a dynamic material with many potential applications to regenerative medicine.
4.4. Surgical Context Summary
The connection between rheological terminology and parameters observed in clinical practice is important for any researcher in developing the next generation of hydrogels intended for clinical adoption. In this section, we have outlined the connection between injectability/syringeability and shear response, material placement and recovery time, and material retention and yield stress to help bridge the gap between rheology and clinical relevance. Table 1 summarizes the rheological properties of published hydrogel precursor solutions. In general, we recommend that the design of in situ forming hydrogels for use in biomedical applications includes shear thinning properties that are application dependent for either injection through a needle or syringe orifice, incorporates an adequate recovery time after disruption to minimize any loss of material, and has a suitable yield stress (>100 Pa) to allow for material retention and shape fitting. Another area which can directly benefit from the same rheological characterization presented is 3D bioprinting.
5. 3D Bioprinting
5.1. Shape Fidelity
Beyond the benefit of paste and putty hydrogel precursor solutions for surgical use are direct applications to hydrogel bioinks in 3D printing. The aforementioned examples of hydrogel precursor performance of shear-thinning, rapid recovery time, and the presence of a yield stress for surgical placement are the same desirable parameters for development of bioinks for hydrogel 3D printing. The printing quality of 3D bioprinted structures generated from computer-aided modeling is referred to as the shape fidelity, which gauges how well the bioprinted architecture matches the original model.[85–89] The assessment of shape fidelity is becoming standard practice in bioprinting manuscripts; however, the shape fidelity evaluations are generally qualitative rather than quantitative, or non-existent.[90–94] A common semi-quantitative method of evaluating the shape fidelity of a bioink is image analysis, in which the printing quality of varying geometries is photographed and analyzed.[95–97] In a study by Ribeiro et al.[98] the shape fidelity of varying polymer blends was analyzed for hydrogels printed over varying gap distances and the amount of downward sag was quantified. In another study, Kyle et al.[99] used micro-computed tomography to image 3D-printed heart valves and compared the final product to the original 3D model, in which percent surface deviations were reported. In general, bioinks that are reported as having high shape fidelity do not employ fluid performance characterization (i.e., rheology). Due to the fact that important rheological considerations for 3D bioprinting have not been fully defined previously, the field heretofore has not had a standardized method for material evaluation that promotes quantitative material comparisons and characterization that defines high shape fidelity. To remedy this gap in the biomaterials field, the following sections propose three main rheological characteristics that influence shape fidelity to be the standardized characterization of bioinks in 3D printing.
5.2. Shear Performance
Similar to surgically-placed hydrogels, the shear performance of the material through a needle, nozzle, or tube is important for 3D bioprinting applications. Shear thinning allows for the decreasing proportional shear force required for flow allowing ease of extrusion through small orifice sizes (e.g., doubling the flow rate requires less than twice the force).[100, 101] Structural alignment of the printing solution after initiation of flow results in the shear thinning phenomenon.[102] In the pursuit of achieving great shape fidelity for 3D bioprinted materials, the shear thinning performance can be designed and leveraged for accomplishing high-resolution prints.[103] Ideal bioinks are those capable of passing through small orifice sizes to create narrow bioprinted lines while maintaining the printed architecture after extrusion. Clogging of the nozzle can be a potential issue during high resolution narrow line bioprinting if the material does not shear thin to an acceptable degree.[104] Ribeiro et al.[98] discussed the importance of shear thinning bioinks for the assessment of shape fidelity, including filament fusion testing to determine the minimum distance between filament lines for minimal line fusion. Beyond shear thinning bioinks for shape fidelity is the direct connection to cell viability for bioinks with encapsulated cells.[105] Shear thinning performance enables a decreasing proportional stress with increasing flow that results in less stress acting upon cells mixed into bioink solutions.[106] Holzl et al.[107] attributed the shear thinning performance as a contributing reason for high cell viability after 3D bioprinting. For shape fidelity or cell viability, the shear thinning performance can positively contribute to 3D bioprinting. Although shear thinning is an important consideration, the complementary connection between shear performance and recovery time for bioinks is necessary for achieving ideal shape fidelity.[98]
5.3. Recovery Time
As previously discussed, the shear thinning performance can be highly advantageous for print fidelity and protection of printed cells; however, the timely return to the original characteristics after extrusion is vital for high resolution bioprinting. The time required for the bioink to return to the original state after flow is referred to as the recovery time of the material.[108] Bioinks with relatively fast recovery times are ideal for bioprinting applications as the immediate return to equilibrium after dispensing may aid with the print fidelity and the homogenous incorporation of encapsulated cells.[109] Peak et al.[110] discussed the idea that the recovery time dictated the ease of cell incorporation, in which materials that recovered too quickly would be difficult to mix in cells and result in a heterogeneous cell distribution. Alternatively, too slow of a recovery time would potentially result in cell sedimentation, heterogeneous distribution, and poor shape retention. In contrast to the need for a fast bioink recovery time, Paxton et al.[111] discussed the notion that a bioink could instigate a crosslinking mechanism, such as chemical crosslinking agents, which could be used to crosslink the material between layers. Although there are potential avenues to accomplish bioprinting using slow recovery time bioinks, the printing fidelity will be greatly improved using a material that naturally recovers quickly. In general, recovery times that occur relatively quickly (i.e., >85% of G’ within 5–10 seconds) are recommended for bioprinting). After extrusion and recovery, the bioink must resist external forces such as the weight of stacking layers that would otherwise result in deformation and poor shape fidelity.
5.4. Yield Stress
In combination with shear thinning performance and a fast recovery time, bioinks that exhibit a yield stress will naturally resist deformation and maintain the printed structure, a major advantage for 3D bioprinting. Mouser et al.[112] attributed the printability of gelatin-methacryloyl and gellan gum hydrogels to the presence of a yield stress, which allowed for the creation of bioinks with appropriate shape fidelity. In another study, Malda et al.[113] discussed the positive benefits of bioinks exhibiting yield stress behavior for shape fidelity and preventing cell sedimentation. In addition, Malda et al. discussed high viscosity and yield stress and concluded that the yield stress was a more useful performance characteristic, as the presence of a yield stress could prevent deformation where high viscosity bioinks only delay eventual deformation. Although the presence of a yield stress is advantageous for bioprinting, the complementary synergy of shear thinning performance and an adequate recovery time is crucial for achieving high print fidelity. Abbadessa et al.[114] previously discussed the combination of these parameters for the printing of chondroitin sulfate-based hydrogels, concluding that the presence of these three characteristics (i.e., shear thinning, recovery time, and yield stress) still allowed for the tailoring of other characteristics such as porosity for cell encapsulation. As a general rule, yield stress values above 100 Pa are proposed for 3D bioprinting to achieve high print fidelity. Although bioinks with less than 100 Pa may be printable, the 100 Pa setpoint is proposed as a general rule to support layer stacking. Regardless of the hydrogel type, the yield stress can directly benefit 3D bioprinting for the reasons discussed above.
5.5. 3D Bioprinting Summary
The overlap in hydrogel precursor performance between 3D printing and surgical application further accentuates the need for a standardized set of parameters for hydrogel precursor rheological characterization. To standardize rheological testing, members of the field should report the yield stress (most important), shear performance (i.e., viscosity with increasing shear rate), and recovery time after shearing. The standardized characterization of the shear performance, recovery time, and yield stress for newly developed bioinks will allow for better comparisons between studies and development/adoption of new bioinks. Bioinks for 3D-printing applications that exhibit shear thinning behavior, fast recovery time (i.e., >85% of G’ within 5–10 seconds), and presence of a yield stress (>100 Pa) is adequate for 3D bioprinting applications. Implementing desired rheological performance in various hydrogel designs can be challenging and considerations for implementing these properties are proposed below.
6. Hydrogel Precursor Considerations
6.1. Chemically Crosslinked Hydrogels
Chemically crosslinked hydrogels are distinguished by the creation of covalent bonds between polymers to form an interconnected network.[21] A number of varying methods have been proposed to achieve the crosslinked network, the most common being photo-initiation, and enzymatic/reaction based technologies.[115–117] Various groups have proposed new and insightful ways for the development and application of chemical hydrogels for regenerative medicine.[117–119] Although the technologies developed have shown promise for the reported application, the intended delivery method and subsequent material mechanical characterization are sometimes unclear. In designing chemically crosslinked hydrogels for regenerative medicine, it is important to establish a delivery method suitable for the intended user that facilitates the ease of placement, and characterize these rheological properties.[120] Although some groups have proposed circumventing the need for in situ placement by crosslinking the hydrogels in Teflon molds, the pre-crosslinking method arguably diminishes the potential of this technology.[121] Although the end result of photo-initiated and reaction-based in situ hydrogels are similar in many ways, the considerations for designing their placement are distinct.
Photo-initiated hydrogels combine the positives of user-defined crosslinking initiation with the ability to form tunable covalent bonds to modulate crosslinking density.[115, 122] The downside of this method involves the need for an outside light source for initiation, generally requiring an open surgical site. The necessity of the open surgical site limits the available applications of photo-initiated hydrogels; however, an open surgical site offers many potential benefits for precursor solution placement. Photo-initiation allows for complete control over the precursor placement, enabling possible shaping and defect-fitting if the precursor exhibits the correct rheological properties. Earlier, we proposed the idea of a photo-initiated hydrogel that could allow for easier surgical placement in cartilage defects if the precursor solution exhibited a sufficient yield stress, enabling a surgeon to form-fit the material to the defect site before crosslinking.[2, 38, 67, 123] A potential limitation of photoinitiated hydrogels is the requirement for light penetration, thus opaque materials may encounter crosslinking issues due to limited light penetration. Reaction-based hydrogels, which are not limited by opacity issues like photoinitiated hydrogels are generally initiated by the mixing of two solutions to initiate crosslinking and subsequent hydrogel formation.[24] Liu et al.[124] used a catechol-modified poly(ethylene glycol) (PEG) and laponite to create a self-healing hydrogel, capable of reforming the bond between the catechol group and laponite. Similarly, Sato et al.[125] functionalized hyaluronic acid with catechol groups to create a self-healing hydrogel, highlighting the ability of catechol groups to operate in multiple methods. The ability of catechol-modified polymers to quickly recover after shearing is advantageous for biomedical applications.[126–128] The time-dependent nature of reaction-based hydrogels after crosslinking initiation can present challenges for placement if the crosslinking is too fast; however, a finely tuned system could circumvent the need for an initial material yield stress if the crosslinking initiated as placement occurred. The changing chemical structure of these hydrogels with time can be beneficial for medical translation, especially if the changing structure permits adequate rheological performance within a workable time frame.
Due to the need for achieving a well-mixed solution for reaction-based hydrogels, many studies have utilized low viscosity, zero yield stress precursor solutions.[129, 130] Although these hydrogels have shown great promise in promoting cell viability and proliferation, these hydrogels may encounter limitations in the intended surgical application. Employing a yield stress to the mixed precursor solution can greatly increase the available applications for reaction-based hydrogels.[63, 131] Special considerations need to be made for reaction-based hydrogels that employ a precursor yield stress. Issues arise in achieving a well-mixed solution, and an external mixing system such as a dual-syringe and mixing tip may be required. For both photo-initiated and enzymatic/reaction hydrogel precursor solutions, it is strongly recommended to characterize and report the yield stress, recovery time, shear response, and gelation time at physiological conditions.
6.2. Physically Crosslinked Hydrogels
Physical hydrogels are classified as hydrogels held together by polymer entanglements and/or secondary forces, and unlike chemical hydrogels, no formation of covalent binding occurs.[21] Some common methods for forming physical hydrogels are based on charge, colloidal flocculation, peptide interactions, and physical thermogelation.[25, 66, 132–136] Compared to chemically crosslinked hydrogels, physical hydrogels require specific rheological considerations as chemical binding does not occur post-placement. Physical hydrogels that exhibit a moderate yield stress, shear thinning behavior, and quick recovery time are ideal for biomedical applications.[37] Many research groups proposing the use of physical hydrogels for biomedical applications have characterized a wide variety of rheological properties.[68, 78] Li et al.[137] employed the use of a supramolecular hydrogel composed of charged micelles and laponite to create a self-healing material exhibiting fast recovery times. Supramolecular hydrogels have continued to gain support, highlighting their ability for fast recovery times after disruption.[138–143] An interesting material was proposed by Shao et al.[144] in which supramolecular hydrogels based on DNA self-assembly were created forming a hydrogel from complimentary base pairing. In another approach, Yavvari et al.[145] evaluated catechol-modified chitosan and Fe(III) self-assembling hydrogels, in which a naturally forming ligand interaction was formed between the catechol group and the iron. Catechol modified polymers and supramolecular hydrogels are two interesting approaches for forming robust materials exhibiting fast recovery times. For reference, Lu et al.[66] is a prime example of a physical hydrogel mechanical characterization study using rheology, providing information on shear response, gel recovery time, and yield stress.
Physical hydrogels based on charge, colloidal flocculation, and peptide interactions require the same rheological considerations. In designing physical hydrogels for biomedical applications, certain key rheological properties must be characterized and reported. Evaluation of yield stress, recovery-time, and shear response are strongly recommended when reporting new physical hydrogels to the community. Additionally, physical hydrogels for biomedical applications must be tested at body temperature as a proof of concept. Physical hydrogels utilizing thermogelation require the same considerations as the charge, colloidal, and peptide hydrogels; however, additional tests must be taken into consideration. Viscoelastic properties of thermally activated hydrogels should be mapped at varying temperature, and the time required for gelation at body temperature should be characterized.
6.3. Combinational Crosslinking Hydrogels
Combinational hydrogels are developed with the intention of utilizing both physical and chemical crosslinking principles in mind. Hydrogels have historically been separated as either chemically or physically crosslinked; however, there are potentially great benefits for hydrogel designs incorporating both physical and chemical crosslinking principles.[146] Lu et al.[4] proposed the use of a combinational crosslinking hydrogel utilizing both self-assembling peptides and secondary UV crosslinkable hyaluronic acid to form covalent bonds. Other groups have proposed the use of combinational hydrogels, such as polymer entanglements combined with UV crosslinking, and thermogelation combined with reaction based crosslinking.[146, 147] The rheological considerations for the combinational hydrogel precursors should incorporate the necessary experimental tests for both physically and chemically crosslinked hydrogels, as previously mentioned.
6.4. Crosslinking Hydrogels Summary
In this section, considerations for hydrogels incorporating chemical, physical, and combinational crosslinking mechanisms have been summarized. A general outline of rheological considerations and characteristics to report have been provided for each of the mentioned cases. Briefly, it is recommended to characterize and report the yield stress, recovery time, shear response, and gelation time at physiological conditions. The implementation of yield stress to precursor solutions can positively impact all hydrogel types and allow for an easier translation from the lab to clinic, while minimizing surgeon learning curve.
7. Conclusions
In the current review, we have summarized the need for hydrogel precursor rheological characterization and the advantage of implementing precursor solutions exhibiting yield stress for surgical placement and 3D bioprinting. This review has explored the gap in translation between hydrogel technology and clinical application and identified the rheology of the hydrogel precursor solution as an often overlooked, yet crucial, design consideration. Common pre-existing nomenclature of paste and putty behaviors were introduced for explaining low and high yield stress precursor properties, and the Herschel-Bulkley equation was proposed as the model to be used by the hydrogel community in reporting rheological properties for comparison. Basic rheological principles and their connection to the medical world and 3D bioprinting have been explored, providing examples of recent research in application-dependent hydrogel design. Considerations for hydrogels utilizing chemical, physical, and combinational crosslinking mechanisms were explored to better inform the community in designing new hydrogel materials for ease of implantation and surgeon adoption.
Hydrogels proposed for tissue engineering and regenerative medicine applications ultimately aspire to translate from the lab bench to the clinic. The design and intended placement of hydrogels may be tailored specifically with the surgeon in mind, minimizing the surgeon learning curve to ultimately improve translation. An emphasis within the hydrogel community on reporting rheological properties of hydrogel precursor solutions is the first step for meeting the clinical need. By utilizing the Herschel-Bulkley equation, the hydrogel community can easily compare rheological properties in a general and simplified manner that minimizes the need for an in-depth understanding of rheology. By better understanding the molecular level interactions and how they affect the performance of hydrogel precursor solutions, the community can begin to work toward theoretical explanations rather than relying on empirical correlations. By quantifying relationships rooted in fundamentals, future research will allow for better prediction and rational design of precursor solutions. Furthermore, the same rheological performance characteristics relevant to surgical placement are directly applicable for 3D bioprinting applications, an additional area that could benefit from standardized bioink evaluation for print fidelity. We recommend that members of the field report the the shear performance, recovery time, and yield stress for newly developed bioinks. We suggest a dialogue bringing together experts in both non-Newtonian fluids and hydrogels, to facilitate an understanding of how to characterize and design better biomaterials. This dialogue will help to bridge the gap in knowledge regarding non-Newtonian rheology and establish new collaborations across different fields.
Acknowledgments
The authors would like to recognize funding from the Stephenson Graduate Fellowship and thank Alysa Crum for development of graphical illustrations used in the manuscript. Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under Award Number R01 DE022472. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Nomenclature
- DBM
demineralized bone matrix
- HB
Herschel-Bulkley
- HAp
hydroxyapatite
- G’
storage modulus
- G”
loss modulus
- γ
shear strain
- k
consistency index
- n
flow index
- PEG
poly(ethylene glycole)
- PLGA
poly(lactic-co-glycolic acid)
- τ
shear stress
- τ0
yield stress
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm 2008;68:34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Beck EC, Barragan M, Tadros MH, Kiyotake EA, Acosta FM, Kieweg SL, et al. Chondroinductive Hydrogel Pastes Composed of Naturally Derived Devitalized Cartilage. Ann Biomed Eng 2016;44:1863–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ferreira JR, Padilla R, Urkasemsin G, Yoon K, Goeckner K, Hu WS, et al. Titanium-enriched hydroxyapatite-gelatin scaffolds with osteogenically differentiated progenitor cell aggregates for calvaria bone regeneration. Tissue Eng Part A 2013;19:1803–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lu HD, Soranno DE, Rodell CB, Kim IL, Burdick JA. Secondary photocrosslinking of injectable shear-thinning dock-and-lock hydrogels. Adv Healthc Mater 2013;2:1028–36. [DOI] [PubMed] [Google Scholar]
- [5].Visser J, Levett PA, te Moller NC, Besems J, Boere KW, van Rijen MH, et al. Crosslinkable hydrogels derived from cartilage, meniscus, and tendon tissue. Tissue Eng Part A 2015;21:1195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sridharan B, Sharma B, Detamore MS. A Road Map to Commercialization of Cartilage Therapy in the United States of America. Tissue Eng Part B Rev 2015;22:15–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sutherland AJ, Converse GL, Hopkins RA, Detamore MS. The bioactivity of cartilage extracellular matrix in articular cartilage regeneration. Adv Healthc Mater 2015;4:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brown TE, Marozas IA, Anseth KS. Amplified Photodegradation of Cell-Laden Hydrogels via an Addition-Fragmentation Chain Transfer Reaction. Adv Mater 2017;1605001/1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Peak CW, Wilker JJ, Schmidt G. A review on tough and sticky hydrogels. Colloid Polym Sci 2013;291:2031–47. [Google Scholar]
- [10].Rosiak JM, Yoshii F. Hydrogels and their medical applications. Nucl Instrum Methods Phys Res B 1999;151:56–64. [Google Scholar]
- [11].Peppas NA. Hydrogels and drug delivery. Curr Opin Colloid In 1997;2:531–7. [Google Scholar]
- [12].Shewan HM, Stokes JR. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. Journal Food Eng 2013;119:781–92. [Google Scholar]
- [13].Di Giuseppe E, Corbi F, Funiciello F, Massmeyer A, Santimano TN, Rosenau M, et al. Characterization of Carbopol® hydrogel rheology for experimental tectonics and geodynamics. Tectonophysics 2015;642:29–45. [Google Scholar]
- [14].Hu X, Hao L, Wang H, Yang X, Zhang G, Wang G, et al. Hydrogel Contact Lens for Extended Delivery of Ophthalmic Drugs. Int J Polym Sci 2011;2011: 814163/1–9. [Google Scholar]
- [15].Omidian H, Rocca JG, Park K. Advances in superporous hydrogels. J Control Release 2005;102:3–12. [DOI] [PubMed] [Google Scholar]
- [16].Gruskin E, Doll BA, Futrell FW, Schmitz JP, Hollinger JO. Demineralized bone matrix in bone repair: history and use. Adv Drug Deliv Rev 2012;64:1063–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Peppas N Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 2000;50:27–46. [DOI] [PubMed] [Google Scholar]
- [18].Murphy PS, Evans GR. Advances in wound healing: a review of current wound healing products. Plast Surg Int 2012;2012:190436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hunziker EB, Lippuner K, Keel MJ, Shintani N. An educational review of cartilage repair: precepts & practice--myths & misconceptions--progress & prospects. Osteoarthr Cartil 2015;23:334–50. [DOI] [PubMed] [Google Scholar]
- [20].Kretlow JD, Young S, Klouda L, Wong M, Mikos AG. Injectable biomaterials for regenerating complex craniofacial tissues. Adv Mater 2009;21:3368–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev 2012;64:18–23. [DOI] [PubMed] [Google Scholar]
- [22].D’Este M, Eglin D. Hydrogels in calcium phosphate moldable and injectable bone substitutes: Sticky excipients or advanced 3-D carriers? Acta Biomater 2013;9:5421–30. [DOI] [PubMed] [Google Scholar]
- [23].Bender SA, Rogalski JB, Mills MP, Arnold RM, Cochran DL, Mellonig JT. Evaluation of demineralized bone matrix paste and putty in periodontal intraosseous defects. J Periodontol 2005;76:768–77. [DOI] [PubMed] [Google Scholar]
- [24].Salgado CL, Sanchez EM, Zavaglia CA, Almeida AB, Granja PL. Injectable biodegradable polycaprolactone-sebacic acid gels for bone tissue engineering. Tissue Eng Part A 2012;18:137–46. [DOI] [PubMed] [Google Scholar]
- [25].Yu L, Ding J. Injectable hydrogels as unique biomedical materials. Chem Soc Rev 2008;37:1473–81. [DOI] [PubMed] [Google Scholar]
- [26].Wang H, Hansen MB, Lowik DW, van Hest JC, Li Y, Jansen JA, et al. Oppositely charged gelatin nanospheres as building blocks for injectable and biodegradable gels. Adv Mater 2011;23:H119–24. [DOI] [PubMed] [Google Scholar]
- [27].Knowlton S, Onal S, Yu CH, Zhao JJ, Tasoglu S. Bioprinting for cancer research. Trends Biotechnol 2015;33:504–13. [DOI] [PubMed] [Google Scholar]
- [28].Peng W, Unutmaz D, Ozbolat IT. Bioprinting towards Physiologically Relevant Tissue Models for Pharmaceutics. Trends Biotechnol 2016;34:722–32. [DOI] [PubMed] [Google Scholar]
- [29].Skardal A, Shupe T, Atala A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov Today 2016;21:1399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gratson G, Xu M, Lewis JA. Direct writing of three-dimensional webs. Nature 2004;428:386. [DOI] [PubMed] [Google Scholar]
- [31].Smay JE, Gratson GM, Shepherd RF, Cesarano J, Lewis JA. Directed Colloidal Assembly of 3D Periodic Structures. Adv Mater 2002;14:1279–83. [Google Scholar]
- [32].Arimura H, Ohya Y, Ouchi T, Yamada H. Preparation of a biodegradable matrix through polyion complex formation by mixing polylactide-based microspheres having oppositely charged surfaces. J Colloid Interface Sci 2004;270:299–303. [DOI] [PubMed] [Google Scholar]
- [33].Van Tomme SR, van Steenbergen MJ, De Smedt SC, van Nostrum CF, Hennink WE. Self-gelling hydrogels based on oppositely charged dextran microspheres. Biomaterials 2005;26:2129–35. [DOI] [PubMed] [Google Scholar]
- [34].Wang Q, Wang L, Detamore MS, Berkland C. Biodegradable Colloidal Gels as Moldable Tissue Engineering Scaffolds. Adv Mater 2008;20:236–9. [Google Scholar]
- [35].Wang Q, Wang J, Lu Q, Detamore MS, Berkland C. Injectable PLGA based colloidal gels for zero-order dexamethasone release in cranial defects. Biomaterials 2010;31:4980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fakhari A, Phan Q, Thakkar SV, Middaugh CR, Berkland C. Hyaluronic Acid Nanoparticles Titrate the Viscoelastic Properties of Viscosupplements. Langmuir 2013;29:5123–31. [DOI] [PubMed] [Google Scholar]
- [37].Dennis SC, Detamore MS, Kieweg SL, Berkland CJ. Mapping glycosaminoglycan-hydroxyapatite colloidal gels as potential tissue defect fillers. Langmuir 2014;30:3528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Beck EC, Lohman BL, Tabakh DB, Kieweg SL, Gehrke SH, Berkland CJ, et al. Enabling Surgical Placement of Hydrogels Through Achieving Paste-Like Rheological Behavior in Hydrogel Precursor Solutions. Ann Biomed Eng 2015;43:2569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Townsend JM, Zabel TA, Feng Y, Wang J, Andrews BT, Nudo RJ, et al. Effects of tissue processing on bioactivity of cartilage matrix-based hydrogels encapsulating osteoconductive particles. Biomed Mater 2018;13:034108/1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Townsend JM, Andrews BT, Feng Y, Wang J, Nudo RJ, Van Kampen E, et al. Superior calvarial bone regeneration using pentenoate-functionalized hyaluronic acid hydrogels with devitalized tendon particles. Acta Biomater 2018;71:148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wilson CB. Adoption of new surgical technology. BMJ 2006;332:112–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fernandez-Moure JS. Lost in Translation: The Gap in Scientific Advancements and Clinical Application. Front Bioeng Biotechnol 2016;4:43/1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ibrahim AM, Varban OA, Dimick JB. Novel Uses of Video to Accelerate the Surgical Learning Curve. J Laparoendosc Adv Surg Tech A 2016;26:240–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bruns NE, Irtan S, Rothenberg SS, Bogen EM, Kotobi H, Ponsky TA. Trans-Atlantic Telementoring with Pediatric Surgeons: Technical Considerations and Lessons Learned. J Laparoendosc Adv Surg Tech A 2016;26:75–8. [DOI] [PubMed] [Google Scholar]
- [45].Kwon JS, Yoon SM, Kwon DY, Kim DY, Tai GZ, Jin LM, et al. Injectable in situ-forming hydrogel for cartilage tissue engineering. Journal Mater Chem B 2013;1:3314–21. [DOI] [PubMed] [Google Scholar]
- [46].Spicer PP, Kretlow JD, Young S, Jansen JA, Kasper FK, Mikos AG. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protoc 2012;7:1918–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang Q, Jamal S, Detamore MS, Berkland C. PLGA-chitosan/PLGA-alginate nanoparticle blends as biodegradable colloidal gels for seeding human umbilical cord mesenchymal stem cells. J Biomed Mater Res A 2011;96:520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Samaniuk JR, Shay TW, Root TW, Klingenberg DJ, Scott CT. A novel rheometer design for yield stress fluids. AIChE Journal 2014;60:1523–8. [Google Scholar]
- [49].Priyadarshani P, Li Y, Yang S, Yao L. Injectable hydrogel provides growth-permissive environment for human nucleus pulposus cells. J Biomed Mater Res A 2016;104:419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ballios BG, Cooke MJ, Donaldson L, Coles BL, Morshead CM, van der Kooy D, et al. A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny following Transplantation. Stem Cell Reports 2015;4:1031–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Su WY, Chen YC, Lin FH. Injectable oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for nucleus pulposus regeneration. Acta Biomater 2010;6:3044–55. [DOI] [PubMed] [Google Scholar]
- [52].Vadala G, Russo F, Di Martino A, Denaro V. Intervertebral disc regeneration: from the degenerative cascade to molecular therapy and tissue engineering. J Tissue Eng Regen Med 2015;9:679–90. [DOI] [PubMed] [Google Scholar]
- [53].Xie B, Jin L, Luo Z, Yu J, Shi S, Zhang Z, et al. An injectable thermosensitive polymeric hydrogel for sustained release of Avastin(R) to treat posterior segment disease. Int J Pharm 2015;490:375–83. [DOI] [PubMed] [Google Scholar]
- [54].Zeng Y, Chen C, Liu W, Fu Q, Han Z, Li Y, et al. Injectable microcryogels reinforced alginate encapsulation of mesenchymal stromal cells for leak-proof delivery and alleviation of canine disc degeneration. Biomaterials 2015;59:53–65. [DOI] [PubMed] [Google Scholar]
- [55].Koreen IV, McClintic EA, Mott RT, Stanton C, Yeatts RP. Evisceration with Injectable Hydrogel Implant in a Rabbit Model. Ophthal Plast Reconstr Surg 2016;33:163–67. [DOI] [PubMed] [Google Scholar]
- [56].Suenaga H, Furukawa KS, Suzuki Y, Takato T, Ushida T. Bone regeneration in calvarial defects in a rat model by implantation of human bone marrow-derived mesenchymal stromal cell spheroids. J Mater Sci Mater Med 2015;26:254>/1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Oliveira SM, Barrias CC, Almeida IF, Costa PC, Ferreira MR, Bahia MF, et al. Injectability of a bone filler system based on hydroxyapatite microspheres and a vehicle with in situ gel-forming ability. J Biomed Mater Res B Appl Biomater 2008;87:49–58. [DOI] [PubMed] [Google Scholar]
- [58].Herschel WH, Bulkley R. Konsistenzmessungen von Gummi-Benzollösungen. Kolloid-Zeitschrift 1926;39:291–300. [Google Scholar]
- [59].Ahmad NH, Ahmed J, Hashim DM, Manap YA, Mustafa S. Oscillatory and steady shear rheology of gellan/dextran blends. J Food Sci Technol 2015;52:2902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Aktas S, Kalyon DM, Marín-Santibáñez BM, Pérez-González J. Shear viscosity and wall slip behavior of a viscoplastic hydrogel. Journal Rheol 2014;58:513–35. [Google Scholar]
- [61].Albu MG, Ghica MV, Popa L, Leca M, Trandafir V. Kinetics of in Vitro Release of Doxycycline Hyclate from Collagen Hydrogels. Rev Roum Chim 2009;54:373–79. [Google Scholar]
- [62].Osswald T, Rudolph N. Polymer Rheology -Fundamentals and Applications. Cincinnati: Hanser Publishers, 2015. . 221 pp. [Google Scholar]
- [63].Dumas JE, Zienkiewicz K, Tanner SA, Prieto EM, Bhattacharyya S, Guelcher SA. Synthesis and characterization of an injectable allograft bone/polymer composite bone void filler with tunable mechanical properties. Tissue Eng Part A 2010;16:2505–18. [DOI] [PubMed] [Google Scholar]
- [64].Geisler IM, Schneider JP. Evolution-Based Design of an Injectable Hydrogel. Adv Funct Mater 2012;22:529–37. [Google Scholar]
- [65].Glassman MJ, Chan J, Olsen BD. Reinforcement of Shear Thinning Protein Hydrogels by Responsive Block Copolymer Self-Assembly. Adv Funct Mater 2013;23:1182–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lu HD, Charati MB, Kim IL, Burdick JA. Injectable shear-thinning hydrogels engineered with a self-assembling Dock-and-Lock mechanism. Biomaterials 2012;33:2145–53. [DOI] [PubMed] [Google Scholar]
- [67].Beck EC, Barragan M, Libeer TB, Kieweg SL, Converse GL, Hopkins RA, et al. Chondroinduction from Naturally Derived Cartilage Matrix: A Comparison Between Devitalized and Decellularized Cartilage Encapsulated in Hydrogel Pastes. Tissue Eng Part A 2016;22:665–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Fakhari A, Phan Q, Berkland C. Hyaluronic acid colloidal gels as self-assembling elastic biomaterials. J Biomed Mater Res B Appl Biomater 2014;102:612–8. [DOI] [PubMed] [Google Scholar]
- [69].Tsaryk R, Gloria A, Russo T, Anspach L, De Santis R, Ghanaati S, et al. Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration. Acta Biomater 2015;20:10–21. [DOI] [PubMed] [Google Scholar]
- [70].Beck E, Berkland C, Gehrke S, Detamore M. Novel hyaluronic acid nanocomposite hydrogel for cartilage tissue engineering: utilizing yield stress for ease of implantation Summer Bioengineering Conference Sunriver, Oregon, USA: ASME; 2013. . p. 1–2. [Google Scholar]
- [71].Guvendiren M, Lu HD, Burdick JA. Shear-thinning hydrogels for biomedical applications. Soft Matter 2012;8:260–72. [Google Scholar]
- [72].Wagner NJ, Brady JF. Shear thickening in colloidal dispersions. Phys Today 2009;62:27–32. [Google Scholar]
- [73].Barbucci R, Leone G, Lamponi S. Thixotrophy property of hydrogels to evaluate the cell growing on the inside of the material bulk (Amber effect). J Biomed Mater Res B Appl Biomater 2006;76:33–40. [DOI] [PubMed] [Google Scholar]
- [74].Barnes HA. Thixotropy -A review. J Non-Newton Fluid 1997;70:1–33. [Google Scholar]
- [75].Lee CH, Moturi V, Lee Y. Thixotropic property in pharmaceutical formulations. J Control Release 2009;136:88–98. [DOI] [PubMed] [Google Scholar]
- [76].Dennis SC, Whitlow J, Detamore MS, Kieweg SL, Berkland CJ. Hyaluronic-Acid–Hydroxyapatite Colloidal Gels Combined with Micronized Native ECM as Potential Bone Defect Fillers. Langmuir 2017;33:206–18 [DOI] [PubMed] [Google Scholar]
- [77].Hao X, Liu H, Lu Z, Xie Y, Yang H. Endowing the conventional HMPAM hydrogel with pH-responsive and self-healing properties. J Mater Chem A Mater 2013;1:6920–7. [Google Scholar]
- [78].Gaharwar AK, Avery RK, Assmann A, Paul A, McKinley GH, Khademhosseini A, et al. Shear-thinning nanocomposite hydrogels for the treatment of hemorrhage. ACS Nano 2014;8:9833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Olsen BD, Kornfield JA, Tirrell DA. Yielding Behavior in Injectable Hydrogels from Telechelic Proteins. Macromolecules 2010;43:9094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Liu Q, Zhan C, Barhoumi A, Wang W, Santamaria C, McAlvin JB, et al. A Supramolecular Shear-Thinning Anti-Inflammatory Steroid Hydrogel. Adv Mater 2016;28:6680–6. [DOI] [PubMed] [Google Scholar]
- [81].Gao L, Gao Y, Lin Y, Ju Y, Yang S, Hu J. A Charge-Transfer-Induced Self-Healing Supramolecular Hydrogel. Chem Asian J 2016;11:3430–5. [DOI] [PubMed] [Google Scholar]
- [82].Yu H, Liu Y, Yang H, Peng K, Zhang X. An Injectable Self-Healing Hydrogel Based on Chain-Extended PEO-PPO-PEO Multiblock Copolymer. Macromol Rapid Commun 2016;37:1723–8. [DOI] [PubMed] [Google Scholar]
- [83].Townsend JM, Dennis SC, Whitlow J, Feng Y, Wang J, Andrews B, et al. Colloidal Gels with Extracellular Matrix Particles and Growth Factors for Bone Regeneration in Critical Size Rat Calvarial Defects. AAPS J 2017;19:703–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Dumas JE, BrownBaer PB, Prieto EM, Guda T, Hale RG, Wenke JC, et al. Injectable reactive biocomposites for bone healing in critical-size rabbit calvarial defects. Biomed Mater 2012;7:024112/1–14. [DOI] [PubMed] [Google Scholar]
- [85].Bertlein S, Brown G, Lim KS, Jungst T, Boeck T, Blunk T, et al. Thiol-Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Adv Mater 2017;29:1703404/1–6. [DOI] [PubMed] [Google Scholar]
- [86].Gao T, Gillispie GJ, Copus JS, Pr AK, Seol YJ, Atala A, et al. Optimization of gelatin-alginate composite bioink printability using rheological parameters: a systematic approach. Biofabrication 2018;10:034106/1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Stichler S, Bock T, Paxton N, Bertlein S, Levato R, Schill V, et al. Double printing of hyaluronic acid/poly(glycidol) hybrid hydrogels with poly(epsilon-caprolactone) for MSC chondrogenesis. Biofabrication 2017;9:044108/1–12. [DOI] [PubMed] [Google Scholar]
- [88].Chang CC, Boland ED, Williams SK, Hoying JB. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B Appl Biomater 2011;98:160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Chien KB, Makridakis E, Shah RN. Three-dimensional printing of soy protein scaffolds for tissue regeneration. Tissue Eng Part C Methods 2013;19:417–26. [DOI] [PubMed] [Google Scholar]
- [90].Sakai S, Ohi H, Hotta T, Kamei H, Taya M. Differentiation potential of human adipose stem cells bioprinted with hyaluronic acid/gelatin-based bioink through microextrusion and visible light-initiated crosslinking. Biopolymers 2018;109/1–8. [DOI] [PubMed] [Google Scholar]
- [91].Skardal A, Zhang J, McCoard L, Xu X, Oottamasathien S, Prestwich GD. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng Part A 2010;16:2675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wenz A, Borchers K, Tovar GEM, Kluger PJ. Bone matrix production in hydroxyapatite-modified hydrogels suitable for bone bioprinting. Biofabrication 2017;9:044103/1–14. [DOI] [PubMed] [Google Scholar]
- [93].Yin J, Yan M, Wang Y, Fu J, Suo H. 3D Bioprinting of Low-Concentration Cell-Laden Gelatin Methacrylate (GelMA) Bioinks with a Two-Step Cross-linking Strategy. ACS Appl Mater Interfaces 2018;10:6849–57. [DOI] [PubMed] [Google Scholar]
- [94].Zheng Z, Wu J, Liu M, Wang H, Li C, Rodriguez MJ, et al. 3D Bioprinting of Self-Standing Silk-Based Bioink. Adv Healthc Mater 2018;7:e1701026/1–12. [DOI] [PubMed] [Google Scholar]
- [95].Diamantides N, Wang L, Pruiksma T, Siemiatkoski J, Dugopolski C, Shortkroff S, et al. Correlating rheological properties and printability of collagen bioinks: the effects of riboflavin photocrosslinking and pH. Biofabrication 2017;9:034102/1–13. [DOI] [PubMed] [Google Scholar]
- [96].He Y, Yang F, Zhao H, Gao Q, Xia B, Fu J. Research on the printability of hydrogels in 3D bioprinting. Sci Rep 2016;6:29977/1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ouyang L, Yao R, Zhao Y, Sun W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016;8:035020/1–12. [DOI] [PubMed] [Google Scholar]
- [98].Ribeiro A, Blokzijl MM, Levato R, Visser CW, Castilho M, Hennink WE, et al. Assessing bioink shape fidelity to aid material development in 3D bioprinting. Biofabrication 2017;10:014102/1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kyle S, Jessop ZM, Al-Sabah A, Whitaker IS. ‘Printability’ of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art. Adv Healthc Mater 2017;6/1–16. [DOI] [PubMed] [Google Scholar]
- [100].Jammalamadaka U, Tappa K. Recent Advances in Biomaterials for 3D Printing and Tissue Engineering. J Funct Biomater 2018;9:22/1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bendtsen ST, Quinnell SP, Wei M. Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J Biomed Mater Res A 2017;105:1457–68. [DOI] [PubMed] [Google Scholar]
- [102].Critchley SE, Kelly DJ. Bioinks for bioprinting functional meniscus and articular cartilage. J 3D Print Med 2017;1:269–90. [Google Scholar]
- [103].Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014;32:773–85. [DOI] [PubMed] [Google Scholar]
- [104].Zhou M, Lee BH, Tan LP. A dual crosslinking strategy to tailor rheological properties of gelatin methacryloyl. Int J Bioprinting 2017;3:130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ooi HW, Mota C, Ten Cate AT, Calore A, Moroni L, Baker MB. Thiol-Ene Alginate Hydrogels as Versatile Bioinks for Bioprinting. Biomacromolecules 2018;19:3390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Panwar A, Tan LP. Current Status of Bioinks for Micro-Extrusion-Based 3D Bioprinting. Molecules 2016;21:685/1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hölzl K, Lin S, Tytgat L, Van Vlierberghe S, Gu L, Ovsianikov A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016;8:032002/1–19. [DOI] [PubMed] [Google Scholar]
- [108].Wilson SA, Cross LM, Peak CW, Gaharwar AK. Shear-Thinning and Thermo-Reversible Nanoengineered Inks for 3D Bioprinting. ACS Appl Mater Interfaces 2017;9:43449–58. [DOI] [PubMed] [Google Scholar]
- [109].Wang LL, Highley CB, Yeh YC, Galarraga JH, Uman S, Burdick JA. Three-dimensional extrusion bioprinting of single-and double-network hydrogels containing dynamic covalent crosslinks. J Biomed Mater Res A 2018;106:865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Peak CW, Stein J, Gold KA, Gaharwar AK. Nanoengineered Colloidal Inks for 3D Bioprinting. Langmuir 2018;34:917–25. [DOI] [PubMed] [Google Scholar]
- [111].Paxton N, Smolan W, Bock T, Melchels F, Groll J, Jungst T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017;9:044107/1–18. [DOI] [PubMed] [Google Scholar]
- [112].Mouser VH, Melchels FP, Visser J, Dhert WJ, Gawlitta D, Malda J. Yield stress determines bioprintability of hydrogels based on gelatin-methacryloyl and gellan gum for cartilage bioprinting. Biofabrication 2016;8:035003/1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Malda J, Visser J, Melchels FP, Jungst T, Hennink WE, Dhert WJ, et al. 25th anniversary article: Engineering hydrogels for biofabrication. Adv Mater 2013;25:5011–28. [DOI] [PubMed] [Google Scholar]
- [114].Abbadessa A, Blokzijl MM, Mouser VH, Marica P, Malda J, Hennink WE, et al. A thermo responsive and photo-polymerizable chondroitin sulfate-based hydrogel for 3D printing applications. Carbohydr Polym 2016;149:163–74. [DOI] [PubMed] [Google Scholar]
- [115].Francisco AT, Hwang PY, Jeong CG, Jing L, Chen J, Setton LA. Photocrosslinkable laminin-functionalized polyethylene glycol hydrogel for intervertebral disc regeneration. Acta Biomater 2014;10:1102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Li B, Wang L, Xu F, Gang X, Demirci U, Wei D, et al. Hydrosoluble, UV-crosslinkable and injectable chitosan for patterned cell-laden microgel and rapid transdermal curing hydrogel in vivo. Acta Biomater 2015;22:59–69. [DOI] [PubMed] [Google Scholar]
- [117].Chuang CH, Lin RZ, Tien HW, Chu YC, Li YC, Melero-Martin JM, et al. Enzymatic regulation of functional vascular networks using gelatin hydrogels. Acta Biomater 2015;19:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Duffy CV, David L, Crouzier T. Covalently-crosslinked mucin biopolymer hydrogels for sustained drug delivery. Acta Biomater 2015;20:51–9. [DOI] [PubMed] [Google Scholar]
- [119].Choi B, Kim S, Lin B, Li K, Bezouglaia O, Kim J, et al. Visible-light-initiated hydrogels preserving cartilage extracellular signaling for inducing chondrogenesis of mesenchymal stem cells. Acta Biomater 2015;12:30–41. [DOI] [PubMed] [Google Scholar]
- [120].Kumar D, Gerges I, Tamplenizza M, Lenardi C, Forsyth NR, Liu Y. Three-dimensional hypoxic culture of human mesenchymal stem cells encapsulated in a photocurable, biodegradable polymer hydrogel: a potential injectable cellular product for nucleus pulposus regeneration. Acta Biomater 2014;10:3463–74. [DOI] [PubMed] [Google Scholar]
- [121].Holloway JL, Ma H, Rai R, Hankenson KD, Burdick JA. Synergistic Effects of SDF-1alpha and BMP-2 Delivery from Proteolytically Degradable Hyaluronic Acid Hydrogels for Bone Repair. Macromol Biosci 2015;15:1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Levett PA, Melchels FP, Schrobback K, Hutmacher DW, Malda J, Klein TJ. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater 2014;10:214–23. [DOI] [PubMed] [Google Scholar]
- [123].Beck EC, Barragan M, Tadros MH, Gehrke SH, Detamore MS. Approaching the compressive modulus of articular cartilage with a decellularized cartilage-based hydrogel. Acta Biomater 2016;38:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Liu Y, Meng H, Qian Z, Fan N, Choi W, Zhao F, et al. A Moldable Nanocomposite Hydrogel Composed of a Mussel-Inspired Polymer and a Nanosilicate as a Fit-to-Shape Tissue Sealant. Angew Chem Int Ed Engl 2017;56:4224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Sato T, Aoyagi T, Ebara M, Auzély-Velty R. Catechol-modified hyaluronic acid: in situ-forming hydrogels by auto-oxidation of catechol or photo-oxidation using visible light. Polym Bull 2017;74:4069–85. [Google Scholar]
- [126].Li J, Zhang C, He W, Qiao H, Chen J, Wang K, et al. Coordination-driven assembly of catechol-modified chitosan for the kidney-specific delivery of salvianolic acid B to treat renal fibrosis. Biomater Sci 2017;6:179–88. [DOI] [PubMed] [Google Scholar]
- [127].Pinnaratip R, Meng H, Rajachar RM, Lee BP. Effect of incorporating clustered silica nanoparticles on the performance and biocompatibility of catechol-containing PEG-based bioadhesive. Biomed Mater 2018;13:025003/1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Zhang H, Zhao T, Newland B, Liu W, Wang W, Wang W. Catechol functionalized hyperbranched polymers as biomedical materials. Prog Polym Sci 2018;78:47–55. [Google Scholar]
- [129].Jin R, Teixeira LS, Dijkstra PJ, van Blitterswijk CA, Karperien M, Feijen J. Enzymatically-crosslinked injectable hydrogels based on biomimetic dextran-hyaluronic acid conjugates for cartilage tissue engineering. Biomaterials 2010;31:3103–13. [DOI] [PubMed] [Google Scholar]
- [130].Yuan L, Li B, Yang J, Ni Y, Teng Y, Guo L, et al. Effects of Composition and Mechanical Property of Injectable Collagen I/II Composite Hydrogels on Chondrocyte Behaviors. Tissue Eng Part A 2016;22:899–906. [DOI] [PubMed] [Google Scholar]
- [131].Ghosh K, Shu XZ, Mou R, Lombardi J, Prestwich GD, Rafailovich MH, et al. Rheological characterization of in situ cross-linkable hyaluronan hydrogels. Biomacromolecules 2005;6:2857–65. [DOI] [PubMed] [Google Scholar]
- [132].DeVolder RJ, Kong HJ. Three dimensionally flocculated proangiogenic microgels for neovascularization. Biomaterials 2010;31:6494–501. [DOI] [PubMed] [Google Scholar]
- [133].Ruel-Gariépy E, Shive M, Bichara A, Berrada M, Le Garrec D, Chenite A, et al. A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur J Pharm Biopharm 2004;57:53–63. [DOI] [PubMed] [Google Scholar]
- [134].Tadier S, Galea L, Charbonnier B, Baroud G, Bohner M. Phase and size separations occurring during the injection of model pastes composed of beta-tricalcium phosphate powder, glass beads and aqueous solutions. Acta Biomater 2014;10:2259–68. [DOI] [PubMed] [Google Scholar]
- [135].Kovtun A, Goeckelmann MJ, Niclas AA, Montufar EB, Ginebra MP, Planell JA, et al. In vivo performance of novel soybean/gelatin-based bioactive and injectable hydroxyapatite foams. Acta Biomater 2015;12:242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Zhu L, Yang J, Zhang J, Lei D, Xiao L, Cheng X, et al. In vitro and in vivo evaluation of a nanoparticulate bioceramic paste for dental pulp repair. Acta Biomater 2014;10:5156–68. [DOI] [PubMed] [Google Scholar]
- [137].Li Z, Hou Z, Fan H, Li H. Organic-Inorganic Hierarchical Self-Assembly into Robust Luminescent Supramolecular Hydrogel. Adv Funct Mater 2017;27:1604379/1–8. [Google Scholar]
- [138].Iglesias D, Melle-Franco M, Kurbasic M, Melchionna M, Abrami M, Grassi M, et al. Oxidized Nanocarbons-Tripeptide Supramolecular Hydrogels: Shape Matters! ACS Nano 2018;12:5530–38. [DOI] [PubMed] [Google Scholar]
- [139].Loebel C, Rodell CB, Chen MH, Burdick JA. Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat Protoc 2017;12:1521–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Lorson T, Jaksch S, Lubtow MM, Jungst T, Groll J, Luhmann T, et al. A Thermogelling Supramolecular Hydrogel with Sponge-Like Morphology as a Cytocompatible Bioink. Biomacromolecules 2017;18:2161–71. [DOI] [PubMed] [Google Scholar]
- [141].Mulvee M, Vasiljevic N, Mann S, Patil AJ. Construction of supramolecular hydrogels using photo-generated nitric oxide radicals. Soft Matter 2018;14:5950–4. [DOI] [PubMed] [Google Scholar]
- [142].Murphy R, Walsh DP, Hamilton CA, Cryan SA, In Het Panhuis M, Heise A. Degradable 3D-Printed Hydrogels Based on Star-Shaped Copolypeptides. Biomacromolecules 2018;19:2691–9. [DOI] [PubMed] [Google Scholar]
- [143].Xu W, Song Q, Xu JF, Serpe MJ, Zhang X. Supramolecular Hydrogels Fabricated from Supramonomers: A Novel Wound Dressing Material. ACS Appl Mater Interfaces 2017;9:11368–72. [DOI] [PubMed] [Google Scholar]
- [144].Shao Y, Jia H, Cao T, Liu D. Supramolecular Hydrogels Based on DNA Self-Assembly. Acc Chem Res 2017;50:659–68. [DOI] [PubMed] [Google Scholar]
- [145].Yavvari PS, Pal S, Kumar S, Kar A, Awasthi AK, Naaz A, et al. Injectable, Self-Healing Chimeric Catechol-Fe(III) Hydrogel for Localized Combination Cancer Therapy. ACS Biomater Sci Eng 2017;3:3404–13. [DOI] [PubMed] [Google Scholar]
- [146].Rughani RV, Branco MC, Pochan D, Schneider JP. De Novo Design of a Shear-Thin Recoverable Peptide-Based Hydrogel Capable of Intrafibrillar Photopolymerization. Macromolecules 2010;43:7924–30. [Google Scholar]
- [147].Ekenseair AK, Boere KW, Tzouanas SN, Vo TN, Kasper FK, Mikos AG. Structure-property evaluation of thermally and chemically gelling injectable hydrogels for tissue engineering. Biomacromolecules 2012;13:2821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Avery RK, Albadawi H, Akbari M, Zhang YS, Duggan MJ, Sahani DV, et al. An injectable shear-thinning biomaterial for endovascular embolization. Sci Transl Med 2016;8:365ra156/1–12. [DOI] [PubMed] [Google Scholar]
- [149].Li Y, Zhang Y, Shi F, Tao L, Wei Y, Wang X. Modulus-regulated 3D-cell proliferation in an injectable self-healing hydrogel. Colloids Surf B Biointerfaces 2017;149:168–73. [DOI] [PubMed] [Google Scholar]
- [150].Montalbano G, Fiorilli S, Caneschi A, Vitale-Brovarone C. Type I Collagen and Strontium-Containing Mesoporous Glass Particles as Hybrid Material for 3D Printing of Bone-Like Materials. Materials 2018;11:700/1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Vulpe R, Le Cerf D, Dulong V, Popa M, Peptu C, Verestiuc L, et al. Rheological study of in-situ crosslinkable hydrogels based on hyaluronanic acid, collagen and sericin. Mater Sci Eng C Mater Biol Appl 2016;69:388–97. [DOI] [PubMed] [Google Scholar]
- [152].Diba M, An J, Schmidt S, Hembury M, Ossipov D, Boccaccini AR, et al. Exploiting Bisphosphonate-Bioactive-Glass Interactions for the Development of Self-Healing and Bioactive Composite Hydrogels. Macromol Rapid Commun 2016;37:1952–9. [DOI] [PubMed] [Google Scholar]
- [153].Rodell CB, Kaminski AL, Burdick JA. Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules 2013;14:4125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Rodell CB, MacArthur JW, Dorsey SM, Wade RJ, Wang LL, Woo YJ, et al. Shear-Thinning Supramolecular Hydrogels with Secondary Autonomous Covalent Crosslinking to Modulate Viscoelastic Properties In Vivo. Adv Funct Mater 2015;25:636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]