Abstract
Background
Metabolic diseases represent a wide category of alterations affecting metabolism. These pathologies are notably marked by inflammation that implicates the immune system. Mucosal Associated Invariant (MAI)T cells are immune cells expressing a semi-invariant TCR able to recognize bacterial and fungal vitamin B metabolites. MAIT cells can promote inflammation and are present in many organs central to metabolism, suggesting a role in the etiopathology of these diseases.
Scope of the review
Here, we will review what is known of the involvement of MAIT cells in metabolic pathologies in humans and mice.
Major conclusions
MAIT cells are severely affected, overactivated with a frequency reduction and a phenotype shift from protective to deleterious. Therefore, they might be a novel target to treat, in particular, pancreas and liver metabolic diseases.
Keywords: Mucosal associated invariant T cells, Metabolic disease, Liver, Pancreas, Diabetes, Microbiota
1. Introduction
Metabolism is defined as a list of chemical reactions taking place in a living organism. In the human body, any abnormal metabolic processes can lead to a broad spectrum of pathologies called metabolic diseases. They can either be congenital with an inherited enzyme deficiency or acquired through disease or failure of an important metabolic organ [1]. Amongst others, affected organs include endocrine glands, liver, pancreas, kidneys, and the cardiovascular system. Their malfunction has consequences on human health ranging from mild to life-threatening.
Since the end of the 20th century, worldwide prevalence of these pathologies has considerably increased, especially for acquired metabolic diseases affecting liver and pancreas. One of the most striking examples is diabetes mellitus, whose frequency has been quadrupled since 1980 and which now affects more than 8.5% of the human population [2]. This increase has been linked with the mean Body Mass Index (BMI) augmentation worldwide. Indeed, more than 13% of men and 21% of women are now obese worldwide, and this proportion is expected to keep rising [3].
In recent years, it has become clear that both inflammatory processes and immune system involvement are central in initiation, development and pathogenesis of several metabolic diseases.
In particular, autoimmunity and chronic inflammation in metabolic organs are the main drivers of metabolic syndromes, of liver diseases and of diabetes mellitus [4].
Amongst all immune actors involved in these processes, Mucosal Associated Invariant-T cells (MAIT cells) are of particular interest. As their name suggests, they are mainly found in mucosal tissues and lamina propria, at the interfaces with environment; and they circulate in blood and lymph [5], [6], [7]. These cells bear characteristics of the innate immune system, such as their effector phenotype after development, or their presence in mucosal tissues [8], [9]. Yet, they also develop properties that are specific to the adaptive immune system. This includes semi-variant T-Cell Receptor (TCR) expression [10], [11]. These cells act on the immune system memory [10], [11], [12]. Therefore, they are classified as innate-like lymphocytes. MAIT cells are mainly involved in early antimicrobial immunity, as first suggested by Le Bourhis et al. [13].
Mr1−/− mice deficient in MAIT cells were notably found to be much more sensitive to several bacterial and fungal infections, including Mycobacteria, Escherichia coli, Klebsellia pneumoniae, Salmonella typhimurium, Francisella tularensis and Candida albicans [14]. MAIT cells are also activated during virus-associated infections, mainly through inflammatory contexts [15].
Interestingly, these cells have been reported to be involved in several chronic inflammatory and autoimmune pathologies, notably in inflammatory bowel diseases [16]. Therefore, MAIT cells are now thought to play an important role in the development of several other metabolic diseases, associated with gut microbiome alteration, since they closely interact with gut.
This review will thus describe MAIT cells characteristics, what is known about their involvement in metabolic disease pathogenesis and development both in humans and mouse models. We will also report the interactions between microbiota, MAIT cells, and metabolic disease initiation.
2. Characteristics of MAIT cells
In 1993, Porcelli et al. described specific double-negative (DN) lymphocytes present in the blood. These cells express TCRαβ, enriched in some conserved recombinations on the α chain, especially Vα7.2-Jα33 with two variable amino acids at the junction. More variability was found in the TCR β chain, yet with a marked enrichment of the Vβ2, 8, 11 and 13 germline families [17].
These cells were further analyzed by Tilloy and collaborators, who confirmed the presence of this invariant TCR, Vα7.2-Jα33 (hAV7S2AJ33) in humans and Vα19-Jα33 (mAV19AJ33) in mice, and the bias towards the Vβ2 and Vβ13 segments in humans and Vβ8 and Vβ6 in mice. They also demonstrated that these cells were selected by an unknown ligand requesting β2 microglobulin, but not the transporter associated with antigen processing (TAP) [18].
Limited TCR variability of these cells suggested that they may recognize a reduced family of antigens on a non-classical MHC molecule [17]. Ten years later, it was confirmed that these cells recognize antigens presented by the major histocompatibility complex related class I-like molecule (MR1), expressed notably on B cells [5].
In addition, these cells are mostly localized in the lamina propria of the gut. Therefore they were named mucosal-associated invariant T cells (MAIT) [5].
Later, MAIT cells were also reported in several tissues, including lungs, intestines, colon, liver, pancreas, female genital mucosa, spleen and lymph nodes both in humans and in mice [19], [20], [21], [22], [23]. They represent approximately 6% of all CD3+ T-cells in human blood, compared to a much lower value (0.1%) in wild-type C57BL-6 mice. Their presence varies between 0.05% and 3.5% of total T-cells in tissues in mice [22].
Development of MR1 tetramers has allowed a better knowledge on MAIT cells TCR diversity, now considered as a semi-invariant TCR. Indeed, this TCRα chain is composed of the Vα7.2/19-Jα33 segments but several combinations with Jα10, 20, 17 and 12 were reported. Moreover, TCRβ also display variations and can be composed of Vβ20, Vβ6-4 and Vβ4-2 and several other Vβ segments at lower frequency. These combinations and the localization of MAIT cells are related, as for example jejunal MAIT cells in majority are Jα33+ cells [10].
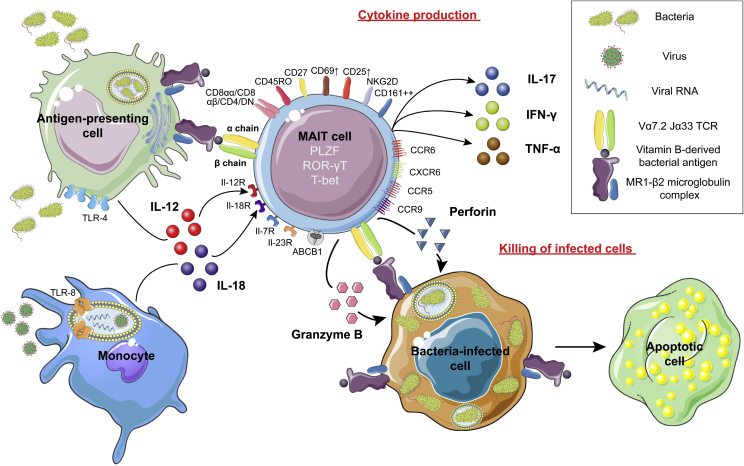
MAIT cells can express CD4 or CD8 coreceptors, although at a lower rate compared to conventional T cells. Of note, both CD8αα+ and CD8αβ+ MAIT cells have been described [18]. Surface markers of MAIT cells classify them as memory and effector immune cells, including CD45RO, CD95hi, CD27, CD26hi, CD44hi, CD62Llo, CD69lo, CD103 (Integrin αE) (Figure 1).
Figure 1.
MAIT cells activation and function in immunity. MAIT cells are innate lymphoid cells, which express a Vα7.2/19 Jα33 semi-invariant TCR, several tissue-homing cytokine receptors, and several markers that classify them as effector (CD44, CD27) and memory (CD45RO) immune cells. MAIT cells are able to recognize pathogens directly through recognition of riboflavin-derived antigens presented by MR1. They are also activated indirectly by IL-12 and IL-18 in diverse inflammatory contexts. In response, they secrete Th1 and Th17 cytokines, and acquire the ability to kill bacteria-infected cells with granzyme B and perforin. This killing action is restricted by the engagement of the TCR with MR1 exposing riboflavin-derived antigen.
MAIT cells express several NK receptors such as NKG2D, NKP30 and CD161lo-hi (NK1.1) (high in humans). They also harbor multiple cytokine receptors, including CD127 (IL-7Rα), CD218 (IL-18R-α), IL-12Rβ2, IL-23R and the CXCR6, CCR5, CCR6 and CCR9 chemokine receptors, as well as the multidrug resistance transporter ABCB1 [20], [22], [24], [25]. They are characterized by PLZF transcription factor (Promyelocytic Leukemia Zinc Finger, ZBTB16) expression as well as RORγT or Tbet (Figure 1) [22].
These cells develop from common lymphoid progenitors in three main developmental stages based on their cell-surface phenotype. MAIT cell development is similar both in human and mice thymus and competes with NKT cell development [9]. Stage 1 developing MAIT cells are small, immature-like T cells, lack CD161 and CD27 surface markers (CD24+ CD44- in mice) and are mostly either double positive (DP) or CD4+ CD8- thymocytes. Stage 2 developing MAIT cells are larger CD161- CD27+ cells (CD24- CD44- in mice) and mostly CD4+ CD8- cells. Next, their development is regulated by miRNA. This leads to the beginning of PLZF expression. This is required for the next stages of maturation [22].
Stage 3 developing MAIT cells start to migrate outside thymus and present a close phenotype compared to mature MAIT cells. Most of them either do not express CD4 nor CD8 (double negative, DN) or CD8+ CD4-; CD161+ CD27lo cells (CD24- CD44+ in mice). Stage 3 developing MAIT cells also start to highly express either RORγT or Tbet transcription factors (or both in mice), suggesting functional maturity [9]. This development requires presentation of MR1 to the MAIT cells by DP thymocytes and B cells after stage 2 [5], [26]. After thymus exit, MAIT cells expand by engaging MR1 complexes, despite the fact that they rapidly lose Ki-67 expression [20].
As soon as their maturation is underway, MAIT cells are able to recognize bacterial and fungal antigens loaded on MR1 complex. These antigens belong to vitamin B metabolites from riboflavin (B2) and folic acid (B9) pathways, although only riboflavin-derived antigens are able to activate MAIT cells [27]. MAIT cells can also in inflammatory contexts be activated independently of a TCR-MR1 interaction, both by IL-12 and by IL-18 which are produced by monocytes activated by TLR-4 or TLR-8 activation (Figure 1) [28].
Once activated, MAIT cells upregulate CD25 and CD69 expression [25]. Then, they can produce several cytokines, notably IFN-γ, TNF-α and IL-17. This is consistent with a major mixed Th1/Th17 secretion pattern, as early as stage 3 development [20], [22], [25]. IL-10, IL-4, IL-13 and GM-CSF secretion were reported, although Th2 cytokine secretion by MAIT cells is still controversial [22], [25].
In addition to their cytokine production, MAIT cells have a cytotoxic ability. Resting MAIT cells produce granzyme A and K. Once activated, they can destroy bacterially-infected cells through degranulation of granzyme B (GzB) and perforin, after engagement of their TCR on a MR1 complex loaded with a recognized antigen (Figure 1) [29], [30].
MAIT cells are therefore a specific population of innate-like lymphoid cells involved in early immunity against pathogens in peripheral tissue, where, when activated, will produce pro-inflammatory cytokines and can lyse infected cells. Alteration of these cells may therefore be critical in the pathophysiology of several inflammatory metabolic diseases; we will now review their involvement in these pathologies.
3. MAIT cells in liver diseases
Liver dysfunction is one of the major causes of metabolic diseases, due to the liver critical role of this organ in body metabolism.
Metabolic diseases affecting the liver are extremely diverse and include inflammatory and autoimmune diseases, amongst others, alcoholic (ALD) and non-alcoholic fatty (NALFD) liver disease, non-alcoholic steatohepatitis (NASH), and terminal cirrhosis. Pathophysiology of these diseases is extremely diverse, but they share an important immune cell activation, fibrosis, and in some diseases, steatosis.
In healthy individuals, liver is characterized by a tolerogenic and sterile environment. However, it also acts as a second barrier against antigens coming from the gut through the portal vein. This prevents their spread through blood to other parts of the body [31]. This function is supported by the extensive immune cell population present in liver. This includes Kupffer cells, dendritic cells, Natural-Killer (NK) cells, innate Natural-Killer T cells and also MAIT cells [23].
Indeed, MAIT cells can represent almost 20–50% of all T cells in human liver in humans, compared to 0.6% in wild-type C57BL-6 mice [23]. They reside mainly around bile ducts in portal tracts and in the liver parenchyma [32]. The liver produces constitutively CXCL16 and CCL20, which are bound to CXCR6 and CCR6 respectively and attracts MAIT cells. MAIT cells also express integrin αEβ7, LFA1 and VLA-4, which recruits them to the biliary epithelium [32]. Even in a tolerogenic environment, MAIT cells are constitutively activated, with a high expression of both CD25 and CD69, as well as HLA-DR and CD38. This mechanism is believed to be an “alerted status” to react against pathogens that have crossed gut immune barrier. They are also very sensitive to IL-12 and IL-18 mediated activation produced notably by Kupffer cells and monocytes in the course of viral infection (Figure 1). MAIT cells can also be activated by IL-7 produced by hepatocytes and are the major producers of IL-17 present in the liver, which in turn activates multiple other immune cells [33].
MAIT cells are therefore probably key modulators of inflammation in the liver [23]. Thus, they might play both a protective role against bacterial infections in a normal liver, but might be detrimental, with over-inflammation, in abnormal livers.
During late stages of NAFLD, MAIT cells accumulate predominantly around hepatocytes overloaded with lipid deposits [34]. In a disease course, tissue-homing chemokine and cytokine receptor expression is not modified on liver MAIT cells [32]. However, during inflammation liver cells produce more CCL20, CXCL9 and CXL10 chemokines, secretion of the latter being stimulated by IFN-γ. On the other hand, MAIT cells in the blood upregulate CCR5 and CXCR3 expression, which bind CCL20 for the first one and CXCL9 and CXCL10 for the second. This promotes MAIT cell migration to liver [32].
However, MAIT cell frequency in liver as in blood is reduced, with an inverse correlation between disease severity and MAIT cell reduction [35]. Taken together, these results suggests a recruitment of MAIT cells from blood to liver during liver inflammation, until the pool of these cells is exhausted [32].
Moreover, chronic hyper-activation and exhaustion of MAIT cells have been reported in several metabolic liver diseases. Indeed, MAIT cells present increased CD69, CD25, HLA-DR, CTLA-4 and PD-1 expression compared to healthy patients [35]. Kupffer cells also express more MR1 molecules with increased levels of free fatty acids (FFA). They can further activate MAIT cells in NASH, as well as hepatic myofibroblasts in cirrhosis [34], [36]. Therefore, there might be a direct correlation between total fat quantity in the liver and MAIT cell activation [34].
During liver disease, activated MAIT cells produce high quantities of IFN-γ, TNF-α and IL-17 (although at lower levels) and GzB amount. Thus, this further promotes inflammation and tissue damage, except in alcohol-related liver diseases where their antibacterial activity is severely reduced [32], [37]. MAIT cells also produce TNF-α, which stimulates IL-6 and IL-8 proinflammatory cytokines released by macrophages and hepatic myofibroblasts [36].
During chronic liver disease, MAIT cells also produce IL-17 and thus activate primary hepatic stellate cells which cause fibrosis [35], [36]. Indeed, MAIT cells accumulate in liver fibrosis septa and promote myofibroblasts mitogenesis through direct contact. Moreover, MAIT cell-deficient mice possess less fibrosis in liver, while conversely Vα19TCRTg mice possessing ten times the normal quantity of MAIT cells shown increased fibrosis [36]. However, another study in Mr1−/− mice found that MAIT cells may protect against inflammation during NASH by inducing a polarization of monocytes toward M2 macrophages, which promote tolerance and tissue repair (demonstrated in vitro) [34].
MAIT cells have a protective role against bacterial infection in liver, but their high number and effector functions can also promote and cause severe live damages both in sterile- and infectious-originated metabolic conditions of the liver.
4. MAIT cells in diabetic and cardiovascular disorders
4.1. MAIT cells in type 1 diabetes
Type I diabetes (T1D) is an autoimmune metabolic disease caused by a lack of production of insulin due to the destruction of β pancreatic cells by the immune system [38].
In a comprehensive study, Rouxel et al. showed recently that MAIT cells frequency and numbers are reduced in children with recent onset diabetes, compared to children who have established diabetes or healthy children [39]. At the onset of T1D, blood MAIT cells express less CCR6, suggesting an increased former tissue recruitment. They express higher levels of exhaustion markers (CD25 and PD-1), as well as reduced levels of anti-apoptotic BCL-2, suggesting a reduced resistance to apoptosis.
After in-vitro stimulation with PMA-ionomycin blood MAIT cells from children with recent onset T1D produce less IFN-γ and more TNF-α, GzB and IL-17 compared to established T1D and control children. They are also less activated by direct TCR stimulation.
Similar results were also observed in animal models of T1D, notably NOD mice. Here, MAIT cells are activated and are producing higher levels of cytokines in the pancreatic lymph nodes (pLNs), as well as in the pancreatic islets and the ileum, compared to control C57BL/6 mice. MAIT cell frequency in those organs increases with the progression of insulitis in NOD mice.
Interestingly, on both surface markers and molecule production, blood MAIT cells from established T1D have an intermediate phenotype between children at the onset of T1D and control children, suggesting a partial return to homeostasis of MAIT cells after insulin treatment.
Moreover, pancreatic MAIT cells might be directly involved in the pathophysiology of T1D and the destruction of β pancreatic cells. Indeed, GzB+ MAIT cell frequency is positively correlated with level of glycated hemoglobin in the youngest children, suggesting that MAIT cells are more activated in the context of an acute T1D development. Moreover, MAIT cells produce IFN-γ and TNF, which induce expression of MR1 complex on β pancreatic cell lines. It has been shown by co-culture experiments that MAIT cells are able to directly kill β pancreatic cells in vitro through degranulation [39]. However, another study found in humans no significant higher infiltration of MAIT cells in pancreatic islets at the onset of T1D, questioning their role in the direct pathogenesis of T1D [40].
On the other hand, gut mucosa MAIT cells might be protective against diabetes [39]. Gut mucosa alterations increase with progression of T1D and blood MAIT cell diminution in NOD mice. Moreover, Mr1−/− NOD mice develop diabetes faster than control NOD mice. Specific CD8+ T cells against β pancreatic cells are present in higher frequency in pancreatic islets in Mr1−/− NOD mice. Activated DCs are also present in higher frequency in pLNs, and their expression of MHCII and costimulatory molecules CD80/86 is also increased.
Of note, bacterial DNA was found in higher quantity in the pLNs of mice lacking MAIT cells.
Therefore, Rouxel et al. proposed that gut leakiness due to MAIT cell deregulation in the mucosa may increase the translocation of bacterial elements to the pancreas, which could increase local auto-immune responses in T1D progression [39].
We will review further the links between MAIT cells, microbiota, and disease in the next section.
4.2. MAIT cells in type 2 diabetes, obesity and cardiovascular disease
Metabolic syndrome, type 2 diabetes and cardiovascular disease are a wide range of closely-related pathologies characterized notably by insulin resistance, high glucose levels in blood, dyslipidemia and overweight.
MAIT cells are highly affected in T2D and obesity. Circulating MAIT cells levels are strongly reduced in T2D patients as well as in severely obese patients, compared to healthy subjects. MAIT cells are even undetectable in some morbidly obese patients (less than 0.04% of total T cells, compared to 6% in healthy controls). Moreover, MAIT cell frequency is negatively correlated with the BMI and insulin sensitivity.
Blood MAIT cells display a major activated pro-inflammatory Th17 phenotype, producing after stimulation increased levels of GzB, IL-17 and TNF-α in T2D, and only IL-17 in obese patients, compared to healthy controls [41], [42].
Moreover, MAIT cells in inflamed adipose tissue in obese patients also display a higher production of IL-17 compared to healthy controls [41], [42]. Conversely, they produce less regulatory IL-10 cytokine both in blood and adipose tissue [42]. In obese patients, Ki-67+ MAIT cells frequency is increased in adipose tissue and they present a reduced expression of anti-apoptotic BCL-2 [41].
Overweight may induce therefore a higher recruitment of MAIT cells to inflamed adipose tissue from the blood. With the local inflammatory context, MAIT cells are overactivated, have a higher replication rate and present an exhausted phenotype (increased expression of CD69). They are therefore more susceptible to cell death, possibly through glucotoxicity [41], [42]. This is supported by the fact that high level of glucose stimulates MAIT cell apoptosis in vitro [43].
Furthermore, bariatric surgery, which reduces weight, is associated with an increase in circulating MAIT frequency as well as a reduction in their cytokine production [41].
On the other hand, little is known about the influence of MAIT cells in cardiovascular diseases. These pathologies are often associated with T2D and obesity, however not systematically. A recent study by Touch and colleagues showed that blood MAIT cell numbers are reduced in coronary artery disease (CAD). This inversely correlates with left heart ventricular enlargement and a higher brain natriuretic peptide (BNP) serum concentration. Both are characteristic of heart failure [43].
In all these metabolic diseases, MAIT cell frequency is therefore altered in a similar way (Figure 2). One common link is also that all organs affected could be in contact with bacterial antigens coming from the gut microbiota. As MAIT cells main supposed function is to guard against bacterial and fungal pathogens, several links between MAIT cells, microbiota and metabolic diseases have emerged, that we will now discuss.
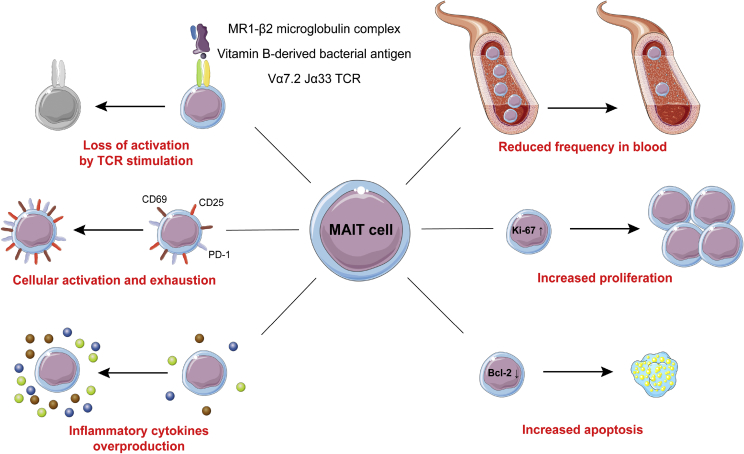
Figure 2.
MAIT cells phenotype in metabolic diseases. Blood MAIT cells frequency is reduced in metabolic diseases, which could reflect their recruitment to inflamed and dysfunctional tissues. MAIT cells express a chronically overactivated phenotype and at the same time, are refractory to TCR activation. They display an increase in proliferation as well as signs of cellular exhaustion (CD25, CD69, PD-1) and abnormal apoptosis (reduction of BCL-2). Moreover, they produce abnormal levels of Th1, Th17 and cytotoxic factors. Taken together, this indicates major MAIT cell alterations, including loss of antibacterial function.
5. MAIT cells and microbiota in the pathophysiology of metabolic diseases
MAIT cells and gut microbiota are in interaction: they are absent in peripheral tissues from germ-free mice, suggesting that microbiota is essential for their development and expansion [5]. Initially, they were thought to be activated only by bacterial and fungi riboflavin, however now we know that their activation can occur in multiple different situations.
The pathogenic role of MAIT cells has been directly observed in inflammatory bowel diseases where a breach of the gut epithelial barrier is associated with a MAIT pathogenic phenotype extremely similar to what is observed in metabolic diseases (Figure 2) [44].
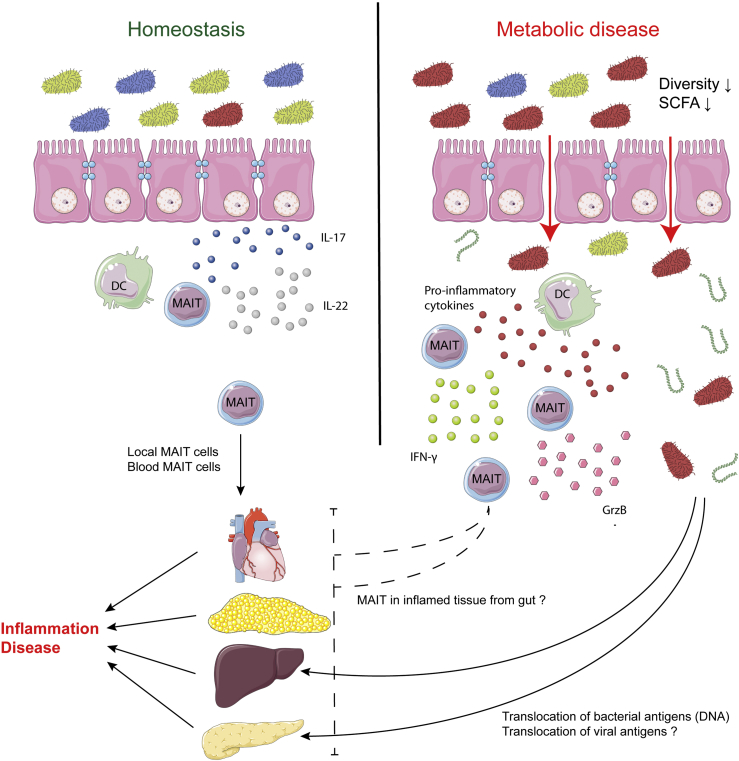
Rouxel et al. suggested in a model that gut MAIT cells are protective against metabolic diseases and produce IL-17 and IL-22 in the ileum, reinforcing the intestinal barrier function. As diabetes progresses and MAIT cells are depleted, this protection fades and the barrier becomes compromised [39]. Expression of occludin and mucin2 in the gut is reduced confirming the increased permeability along with a higher immune infiltration suggesting loss of local tolerance. Gülden et al. further developed this model by proposing that at homeostasis DCs present bacterial metabolites to MAIT cells and promote IL-17 and IL-22 secretion. However, in dysbiosis, MAIT cells switch to GrzB production and increase release of IFN-γ and TNF, promoting inflammation (Figure 3) [45].
Figure 3.
Suspected roles of the microbiota in the activation of MAIT cells and the onset of metabolic disease. At homeostasis, gut MAIT cells may promote the immune barrier function by producing IL-17 and IL-22. However, in dysbiosis situations observed in several metabolic diseases, gut barrier is compromised and MAIT cells in gut mucosa become pathogenic after activation by cytokines produced by DCs. This increases inflammation, as local and blood MAIT cells do in the affected organs. Adapted from [39], [45].
Meanwhile, higher bacterial 16S DNA has been detected in the pLNs in diabetes in Mr1−/− NOD mice by Rouxel et al. They suggested that this DNA could trigger DC activation as well as resident immune cells including pancreatic MAIT cells, which would promote more severe insulitis and destruction of β pancreatic cells in T1D [39]. Therefore, protective or pathogenic roles of MAIT cells in metabolic diseases seem to highly depend on their localization.
One remaining question is to assess whether MAIT cells are altered by changes in the microbiota, or if microbiota is altered by the chronic gut inflammation in those diseases. A recent comprehensive study by Vatanen et al. found that microbiota of T1D children was very low in bacteria producing Short Chain Fatty Acids (SCFA) [46]. These SCFA are known to promote general immune tolerance and to reduce inflammation [47]. Same results were also described in patients suffering from T2D and liver pathologies [48], [49].
In ALD, a higher bacteria presence in both liver and blood is observed, indicating that the firewall role of liver is failing. While causes of dysbiosis are unknown in the majority of metabolic diseases, in ALD chronic ethanol exposure is toxic for SCFA-producing bacteria [48].
A dysbiosis followed by a reduction in SCFA production from the gut might reduce the tolerogenic environment and favor MAIT cell dysfunction (Figure 3). This hypothesis is reinforced by the fact that in T1D transferring microbiota from mice resistant to T1D delays or even prevent T1D in sensitive mice [50].
On the other hand, gut virome is far less in the spotlight. As MAIT cells are able to be indirectly activated by viruses, initial MAIT activation may be potentially due to viral infection [28]. This may notably shed potential new light on the hypothesis of an enteroviral origin of T1D [51], [52].
6. Conclusion
All metabolic diseases in which MAIT cells are altered are characterized by innate immune system activation, detrimental inflammation and pro-inflammatory cytokine production in affected organs: pancreas, adipose tissue, gut, and liver [41], [52], [53], [54]. It is likely that local MAIT cells are activated by this inflammatory context and amplify it by pro-inflammatory cytokine production, increasing all inflammation-mediated damage to organs and tissues. On the other hand, the failure of anti-bacterial MAIT cell function at microbiota–gut and gut–liver interfaces may be responsible for bacterial metabolites and pathogen-associated molecular patterns (PAMP) penetration in metabolic organs, also amplifying local inflammation [39], [45], [48], [55]. Finally, MAIT cells may be furtherly pathogenic by direct cytotoxicity against cells presenting bacterial metabolites and PAMP in affected tissues [39]. Recruitment of activated, pathogenic MAIT cells to inflamed organs leads to their exhaustion both in numbers and in phenotype, aggravating their loss of anti-bacterial function at microbiota–host interfaces [35], [39], [41], [42], [43].
Therefore, MAIT cells are likely to be an actor in the event chains leading to inflammation and metabolic diseases, but they might even be the initiator with failure of their guardian role at microbiota/host interfaces. Indeed, microbiota alteration is shared as well between all these metabolic diseases [46], [49], [55], [56], [57]. It is possible that changes in microbiota reduce the activation of MAIT cells in the gut and deplete them, weakening gut integrity, or that gut inflammation induces microbiota changes affecting MAIT cells, weakening the gut and thus inducing even more inflammation.
MAIT cells are emerging as important players in the initiation of several metabolic diseases affecting various organs, consistently with their confirmed role in several other inflammatory and auto-immune pathologies. However, several other immune cell types are affected in metabolic diseases which are multifactorial, and their interplay has to be taken into account to understand these pathologies.
Their close relationship with the gut microbiota confirms their interest as a target to treat these diseases efficiently. Exploring the consequences of microbiota manipulation on MAIT cells, such as fecal treatments, might help to improve further their efficiency and reduce the frequency of metabolic diseases.
FUNDING
This article is part of a supplement entitled ‘Biomarkers of Beta-Cell Health and Dysfunction: Towards Personalised Diabetes Care. Proceedings of the 20th Servier-IGIS Symposium’. The Symposium and the publication of the Supplement have been made possible by an unrestricted educational grant from Institut la Conférence Hippocrate – Servier Group.
Acknowledgment
We are grateful to Renaud Mahieux and Carole Passone for critical reading of this review. This work was supported by grants from INSERM, CNRS, Laboratoire d’Excellence consortium Inflamex ANR-11-IDEX-0005-02 and the Fondation pour la Recherche Médicale (FRM grant number DEQ20140329520 to A.L.), EFSD/JDRF/Lilly and EFSD/Lilly to A.L., Fondation Francophone pour la Recherche sur le Diabète to A.L., Agence National de la Recherche (Provide and Diab1MAIT to A.L.) and Servier Medical Art.
Conflict of interest
None declared.
References
- 1.Medical subject heading. National Health Institute; 2018. https://meshb.nlm.nih.gov/record/ui?ui=D0086599. (Accessed 24 November 2018) [Google Scholar]
- 2.World Health Organization . World Health Organization; 2016. Global report on diabetes. [Google Scholar]
- 3.NCD Risk Factor Collaboration (NCD-RisC) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387(10026):1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 5.Treiner E., Duban L., Bahram S., Radosavljevic M., Wanner V., Tilloy F. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422(6928):164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 6.Martin E., Treiner E., Duban L., Guerri L., Laude H., Toly C. Stepwise development of MAIT cells in mouse and human. Public Library of Science Biology. 2009;7(3):e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voillet V., Buggert M., Slichter C.K., Berkson J.D., Mair F., Addison M.M. Human MAIT cells exit peripheral tissues and recirculate via lymph in steady state conditions. Journal of clinical investigation insight. 2018;3(7):e98487. doi: 10.1172/jci.insight.98487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gold M.C., Eid T., Smyk-Pearson S., Eberling Y., Swarbrick G.M., Langley S.M. Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal Immunology. 2013;6(1):35–44. doi: 10.1038/mi.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koay H.-F., Gherardin N.A., Enders A., Loh L., Mackay L.K., Almeida C.F. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nature Immunology. 2016;17(11):1300–1311. doi: 10.1038/ni.3565. [DOI] [PubMed] [Google Scholar]
- 10.Reantragoon R., Corbett A.J., Sakala I.G., Gherardin N.A., Furness J.B., Chen Z. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. The Journal of Experimental Medicine. 2013;210(11):2305–2320. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lepore M., Kalinichenko A., Kalinicenko A., Colone A., Paleja B., Singhal A. Parallel T-cell cloning and deep sequencing of human MAIT cells reveal stable oligoclonal TCRβ repertoire. Nature Communications. 2014;5:3866. doi: 10.1038/ncomms4866. [DOI] [PubMed] [Google Scholar]
- 12.Gold M.C., McLaren J.E., Reistetter J.A., Smyk-Pearson S., Ladell K., Swarbrick G.M. MR1-restricted MAIT cells display ligand discrimination and pathogen selectivity through distinct T cell receptor usage. The Journal of Experimental Medicine. 2014;211(8):1601–1610. doi: 10.1084/jem.20140507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Bourhis L., Martin E., Pιguillet I., Guihot A., Froux N., Corι M. Antimicrobial activity of mucosal-associated invariant T cells. Nature Immunology. 2010;11(8):701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 14.Napier R.J., Adams E.J., Gold M.C., Lewinsohn D.M. The role of mucosal associated invariant T cells in antimicrobial immunity. Frontiers in Immunology. 2015;6:344. doi: 10.3389/fimmu.2015.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Wilgenburg B., Scherwitzl I., Hutchinson E.C., Leng T., Kurioka A., Kulicke C. MAIT cells are activated during human viral infections. Nature Communications. 2016;7:11653. doi: 10.1038/ncomms11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouxel O., Lehuen A. Mucosal-associated invariant T cells in autoimmune and immune-mediated diseases. Immunology and Cell Biology. 2018;96(6):618–629. doi: 10.1111/imcb.12011. [DOI] [PubMed] [Google Scholar]
- 17.Porcelli S., Yockey C.E., Brenner M.B., Balk S.P. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. The Journal of Experimental Medicine. 1993;178(1):1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilloy F., Treiner E., Park S.H., Garcia C., Lemonnier F., de la Salle H. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. The Journal of Experimental Medicine. 1999;189(12):1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jo J., Tan A.T., Ussher J.E., Sandalova E., Tang X.-Z., Tan-Garcia A. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. Public Library of Science Pathogens. 2014;10(6):e1004210. doi: 10.1371/journal.ppat.1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dusseaux M., Martin E., Serriari N., Pιguillet I., Premel V., Louis D. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117(4):1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 21.Gold M.C., Cerri S., Smyk-Pearson S., Cansler M.E., Vogt T.M., Delepine J. Human mucosal associated invariant T cells detect bacterially infected cells. Public Library of Science Biology. 2010;8(6) doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahimpour A., Koay H.F., Enders A., Clanchy R., Eckle S.B.G., Meehan B. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. The Journal of Experimental Medicine. 2015;212(7):1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurioka A., Walker L.J., Klenerman P., Willberg C.B. MAIT cells: new guardians of the liver. Clinical and Translational Immunology. 2016;5(8):e98. doi: 10.1038/cti.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma P.K., Wong E.B., Napier R.J., Bishai W.R., Ndung’u T., Kasprowicz V.O. High expression of CD26 accurately identifies human bacteria-reactive MR1-restricted MAIT cells. Immunology. 2015;145(3):443–453. doi: 10.1111/imm.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franciszkiewicz K., Salou M., Legoux F., Zhou Q., Cui Y., Bessoles S. MHC class I-related molecule, MR1, and mucosal-associated invariant T cells. Immunological Reviews. 2016;272(1):120–138. doi: 10.1111/imr.12423. [DOI] [PubMed] [Google Scholar]
- 26.Seach N., Guerri L., Le Bourhis L., Mburu Y., Cui Y., Bessoles S. Double-positive thymocytes select mucosal-associated invariant T cells. Journal of Immunology (Baltimore, Md.: 1950) 2013;191(12):6002–6009. doi: 10.4049/jimmunol.1301212. [DOI] [PubMed] [Google Scholar]
- 27.Kjer-Nielsen L., Patel O., Corbett A.J., Le Nours J., Meehan B., Liu L. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491(7426):717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 28.Ussher J.E., Bilton M., Attwod E., Shadwell J., Richardson R., de Lara C. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. European Journal of Immunology. 2014;44(1):195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurioka A., Ussher J.E., Cosgrove C., Clough C., Fergusson J.R., Smith K. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunology. 2015;8(2):429–440. doi: 10.1038/mi.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Bourhis L., Dusseaux M., Bohineust A., Bessoles S., Martin E., Premel V. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. Public Library of Science Pathogens. 2013;9(10) doi: 10.1371/journal.ppat.1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balmer M.L., Slack E., de Gottardi A., Lawson M.A.E., Hapfelmeier S., Miele L. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Science Translational Medicine. 2014;6(237):237ra66. doi: 10.1126/scitranslmed.3008618. [DOI] [PubMed] [Google Scholar]
- 32.Jeffery H.C., van Wilgenburg B., Kurioka A., Parekh K., Stirling K., Roberts S. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. Journal of Hepatology. 2016;64(5):1118–1127. doi: 10.1016/j.jhep.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang X.-Z., Jo J., Tan A.T., Sandalova E., Chia A., Tan K.C. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. Journal of Immunology. 2013;190(7):3142–3152. doi: 10.4049/jimmunol.1203218. [DOI] [PubMed] [Google Scholar]
- 34.Li Y., Huang B., Jiang X., Chen W., Zhang J., Wei Y. Mucosal-associated invariant T cells improve nonalcoholic fatty liver disease through regulating macrophage polarization. Frontiers in Immunology. 2018;9:1994. doi: 10.3389/fimmu.2018.01994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Böttcher K., Rombouts K., Saffioti F., Roccarina D., Rosselli M., Hall A. MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology. 2018;68(1):172–186. doi: 10.1002/hep.29782. [DOI] [PubMed] [Google Scholar]
- 36.Hegde P., Weiss E., Paradis V., Wan J., Mabire M., Sukriti S. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nature Communications. 2018;9(1):2146. doi: 10.1038/s41467-018-04450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riva A., Patel V., Kurioka A., Jeffery H.C., Wright G., Tarff S. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut. 2018;67(5):918–930. doi: 10.1136/gutjnl-2017-314458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katsarou A., Gudbjörnsdottir S., Rawshani A., Dabelea D., Bonifacio E., Anderson B.J. Type 1 diabetes mellitus. Nature Reviews Disease Primers. 2017;3:17016. doi: 10.1038/nrdp.2017.16. [DOI] [PubMed] [Google Scholar]
- 39.Rouxel O., Da Silva J., Beaudoin L., Nel I., Tard C., Cagninacci L. Cytotoxic and regulatory roles of mucosal-associated invariant T cells in type 1 diabetes. Nature Immunology. 2017;18(12):1321–1331. doi: 10.1038/ni.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuric E., Krogvold L., Hanssen K.F., Dahl-Jørgensen K., Skog O., Korsgren O. No evidence for presence of mucosal-associated invariant T cells in the insulitic lesions in patients recently diagnosed with type 1 diabetes. The American Journal of Pathology. 2018;188(8):1744–1748. doi: 10.1016/j.ajpath.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Magalhaes I., Pingris K., Poitou C., Bessoles S., Venteclef N., Kiaf B. Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. The Journal of Clinical Investigation. 2015;125(4):1752–1762. doi: 10.1172/JCI78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carolan E., Tobin L.M., Mangan B.A., Corrigan M., Gaoatswe G., Byrne G. Altered distribution and increased IL-17 production by mucosal-associated invariant T cells in adult and childhood obesity. Journal of Immunology. 2015;194(12):5775–5780. doi: 10.4049/jimmunol.1402945. [DOI] [PubMed] [Google Scholar]
- 43.Touch S., Assmann K.E., Aron-Wisnewsky J., Marquet F., Rouault C., Fradet M. Mucosal-associated invariant T (MAIT) cells are depleted and prone to apoptosis in cardiometabolic disorders. Federation of American Societies for Experimental Biology journal. 2018;32(9) doi: 10.1096/fj.201800052RR. [DOI] [PubMed] [Google Scholar]
- 44.Serriari N.-E., Eoche M., Lamotte L., Lion J., Fumery M., Marcelo P. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clinical and Experimental Immunology. 2014;176(2):266–274. doi: 10.1111/cei.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gülden E., Palm N., Herold K.C. MAIT cells: a link between gut integrity and type 1 diabetes. Cell Metabolism. 2017;26(6):813–815. doi: 10.1016/j.cmet.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Vatanen T., Franzosa E.A., Schwager R., Tripathi S., Arthur T.D., Vehik K. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562(7728):589–594. doi: 10.1038/s41586-018-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corrêa-Oliveira R., Fachi J.L., Vieira A., Sato F.T., Vinolo M.A.R. Regulation of immune cell function by short-chain fatty acids. Clinical and Translational Immunology. 2016;5(4):e73. doi: 10.1038/cti.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartmann P., Seebauer C.T., Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcoholism Clinical and Experimental Research. 2015;39(5):763–775. doi: 10.1111/acer.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 50.Peng J., Narasimhan S., Marchesi J.R., Benson A., Wong F.S., Wen L. Long term effect of gut microbiota transfer on diabetes development. Journal of Autoimmunity. 2014;53:85–94. doi: 10.1016/j.jaut.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dotta F., Censini S., van Halteren A.G.S., Marselli L., Masini M., Dionisi S. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(12):5115–5120. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehuen A., Diana J., Zaccone P., Cooke A. Immune cell crosstalk in type 1 diabetes. Nature Reviews Immunology. 2010;10(7):501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 53.Tai N., Wong F.S., Wen L. The role of the innate immune system in destruction of pancreatic beta cells in NOD mice and humans with type I diabetes. Journal of Autoimmunity. 2016;71:26–34. doi: 10.1016/j.jaut.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diana J., Simoni Y., Furio L., Beaudoin L., Agerberth B., Barrat F. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nature Medicine. 2013;19(1):65–73. doi: 10.1038/nm.3042. [DOI] [PubMed] [Google Scholar]
- 55.Brandl K., Schnabl B. Intestinal microbiota and nonalcoholic steatohepatitis. Current Opinion in Gastroenterology. 2017;33(3):128–133. doi: 10.1097/MOG.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kostic A.D., Gevers D., Siljander H., Vatanen T., Hyötyläinen T., Hämäläinen A.-M. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host and Microbe. 2015;17(2):260–273. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boursier J., Mueller O., Barret M., Machado M., Fizanne L., Araujo-Perez F. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63(3):764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]