Abstract:
Should we adopt the recently published American Cancer Society (ACS) recommendations to screen adults at 45 years of age? The main reasons for adopting the recommendation include the increase of colorectal cancer (CRC) in the young, especially late‐stage tumors. Screening at 45 years is also supported by predictive modeling, which the ACS employed using updated and improved models as compared to those previously used by the United States Preventive Services Task Force. Reasons against adopting include concerns with the models as well as diversion of scarce screening resources away from high‐risk populations. Readers are provided with opposing viewpoints regarding the ACS recommendation.
INTRODUCTION
While colorectal cancer (CRC) incidence and mortality rates in adults aged 50 years or older have steadily declined in recent years, rates for those younger than 50 years have increased [1, 2]. The decrease in incidence and mortality in older individuals is likely due in part to screening [3]. However, it is unclear why the rates have increased for younger individuals. Some experts have postulated that obesity and associated lifestyle risks such as diet may explain the increase [4]. In response, the American Cancer Society (ACS) has recently published recommendations to screen adults at 45 years [5]. Should we endorse and adopt these recommendations or wait for more evidence? We will provide readers with the opposing viewpoints regarding the ACS recommendation as well as a set of discussion points (Table 1) to have with their patients regarding this issue.
Table 1.
Talking points: short summary of talking points that physicians can use to discuss this recommendation with their patients and colleagues
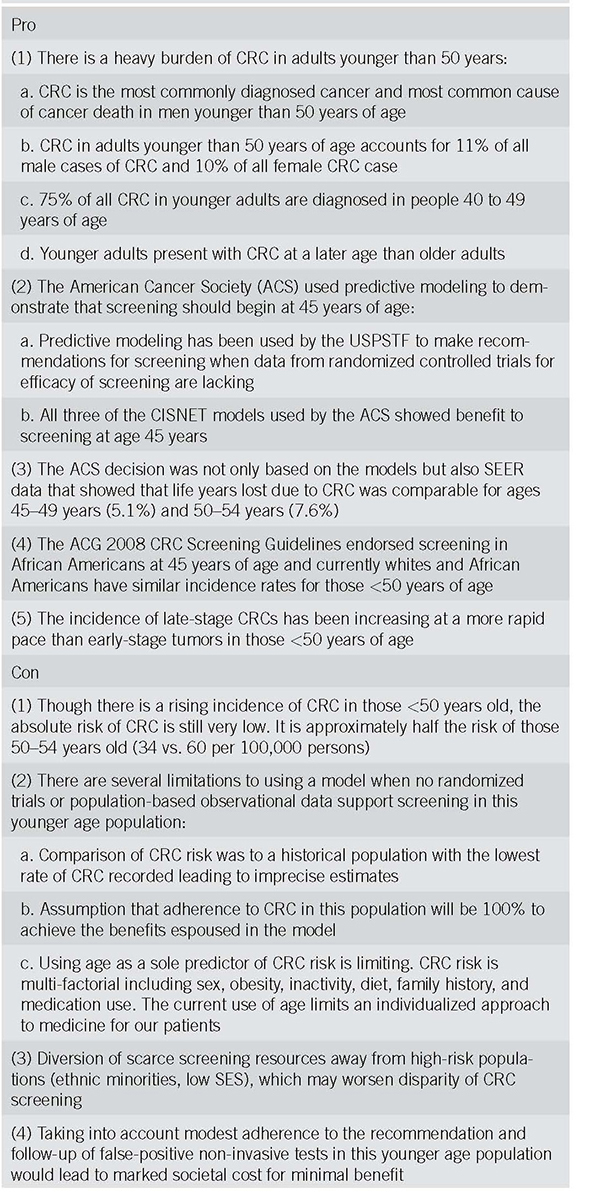
PRO
The ACS uses predictive modeling that has been updated and improved as compared to that utilized by the USPSTF
The ACS guidelines are based on sophisticated predictive modeling, which is an accepted method that has been used by the United States Preventive Services Task Force (USPSTF) to make screening recommendations for cancers such as lung and CRC when data from randomized controlled trials for efficacy of screening are lacking [6, 7]. Thus, predictive modeling is especially useful when examining whether to start CRC screening in younger adults, for which we have little data.
But if the USPSTF used predictive modeling and did not endorse CRC screening for adults <50 years of age [7], why should we accept the ACS recommendation? [5, 8]. It is important to note that the modeling approach used by the ACS [8] was different than that used for the USPSTF [7]. The USPSTF used three Cancer Intervention and Surveillance Modeling Network (CISNET) models: SimCRC, MIcrosimulation Screening Analysis (MISCAN), and CRC‐SPIN. All of the models were calibrated to CRC incidence from 1975 to 1979, a time period which predates CRC screening as well as the observed recent increase of CRC incidence in younger adults [7]. In addition, two (all but MISCAN) of the three CISNET models used by the USPSTF showed a benefit to starting screening at age 45 years, but they did not endorse the recommendation due to the lack of unanimous results for the models [7]. When two of the models (SimCRC and MISCAN) were modified and updated for the ACS analysis to reflect the recent increase in CRC incidence in contemporary birth cohorts, both models supported screening at age 45 years [1, 2, 4, 8]. Since the CRC‐SPIN, as noted above, had also previously supported earlier screening, ultimately all three models had unanimous findings. The ACS concluded that beginning CRC screening with a colonoscopy at 45 years of age, as opposed to 50 years, was associated with a higher benefit as manifested by an efficiency ratio, a measure of “burden to benefit” based on the ratio of the incremental number of colonoscopies divided by the incremental number of life years gained (LYG) compared with the nearest less effective efficient strategy [5].
ACS decision is based not only on modeling but also other recent important data
The ACS decision was not only based on modeling but also Surveillance, Epidemiology, and End Results (SEER) data that showed that life years lost due to CRC was comparable for ages 45‐49 years (5.1%) and 50‐54 years (7.6%) [5]. In addition, using data from SEER 9, the current incidence rate in adults aged 45 years during 2014‐2015 (28.0/100,000; 95% confidence interval: 24.5‐31.8/100,000) is comparable to that for individuals who were 50 years of age during the time period of 1992‐93 (32.0/100,000; 95% confidence interval: 27.5‐37.1/100,000), which was in the pre‐screening era (Rebecca Siegel MPH, personal communication, ACS) [5, 9, 10]. Thus, improvements in the modeling as well as recent empirical data explain why the ACS came to a different conclusion than the USPSTF.
Impediments to providing younger adults with CRC screening with colonoscopy are not significant
In recommending the earlier screening age, the ACS Guideline Development Group also considered some other important factors. They highlighted findings from a study that combined data from the 2012 Survey of Endoscopic Capacity with a modeling analysis and demonstrated that current US endoscopic capacity can absorb an additional 10.5 million exams [11]. In addition, younger adults have low rates of complications, especially when compared to older individuals [12]. Thus, screening younger adults would be expected to have negligible impact on morbidity and we have the capacity to provide younger adults with the benefit of CRC prevention using colonoscopy [13].
Young adults have a significant burden of CRC
CRC in those younger than 50 years accounts for 11% of all male cases of CRC and 10% of all female CRC cases [1, 4]. CRC is the most commonly diagnosed cancer and most common cause of cancer death in men younger than 50 years of age [5]. Based on SEER 9 data for 2015, 15% of rectal cancers were diagnosed in people younger than 50 years, half of whom (47%) were aged 45‐49 years [1, 10]. Furthermore, 75% of all CRC in younger adults are diagnosed in people 40‐49 years of age, thus screening the 45‐49 years group might have a great impact on younger adults with CRC [14, 15]. Adding to the high CRC burden in younger adults is the observation that they are more likely to present with a more advanced stage of CRC than older adults [16, 17]. In fact, the magnitude of the increase is largest for distant‐stage disease.
The rise in CRC in younger adults is not “artifactual”
One of the concerns raised about the rates has been if the increase is actually due to detection bias resulting from more endoscopic evaluation of younger adults [4, 18]. However, late‐stage CRCs are increasing in younger adults at a rate that is more rapid than early‐stage CRCs, a finding that would not be explained by increased uptake in colonoscopy [4, 19, 20]. In addition, although some data suggest that there is a delay in diagnosing CRC in younger individuals, one study has shown that this likely does not fully explain younger people having a more advanced disease [21]. Thus, screening this age group might have a great impact on reducing the large CRC burden in this age group.
Screening adults 45‐49 years will benefit the 50‐54‐year‐old group as well
Screening in the younger age group will also have a positive impact on individuals 50‐54 years of age who are also experiencing an increase in CRC incidence [4]. Furthermore, CRC mortality (not just incidence) is increasing in younger adults as well as those aged 50‐54 years [4]. Of course, the increase of CRC in the 50‐54 years age group reinforces the importance of compliance with CRC screening in this age group. Earlier screening will raise CRC screening awareness and enhance discussion in the 50‐54‐year‐old group, helping us to the “80 by 2018 goal” [22]. Screening younger adults would also yield benefits in the 50‐54‐year‐old group since the benefit of polypectomy for CRC prevention takes about 10 years for CRC prevention [13, 23]. This 10‐year time frame would support screening even in those 40 years of age.
ACG and other organizations already recommend screening in some younger patients
ACG (other organizations too such as the American College of Physicians and the Institute for Clinical Systems Improvement) [24,25,26] already recommend screening in select younger patients such as African Americans or those with family history of colorectal cancer or advanced adenomas. Currently, the CRC incidence in whites younger than 50 years is similar to African Americans for the same age range [5]. Thus, if ACG guidelines endorsed screening at 45 years of age based on similar rates for African Americans, they should support screening for all young adults.
CON
Though the prevention of colorectal cancer and associated deaths is a very desirable outcome, gastroenterologists and public health officials need to integrate the ACS recommendation [5] with other factors. These include the limitations of the model, societal cost for benefit, and the potential to divert scarce resources to a low absolute risk population with an unintentional effect of increasing disparity in populations that have been under screened in the past. Although the relative increase in CRC in this younger age (45‐49‐year‐old) population seems high (51%), the absolute incidence of the disease is still very low. CRC incidence in those 50 to 54 years of age is 60.2 cases per 100,000, while the rate in those 45 to 49 years of age is nearly half that value (34.0 per 100,000) [1, 2, 4, 5].
Problems with the model
There is no direct evidence concerning CRC screening in younger age population with dedicated observational studies or randomized trials in this age group. Thus, the ACS recommendation is based on a re‐analysis of the MISCAN‐Colon model after incorporating new incidence data [5] and may suffer from a birth cohort effect [8]. The rising incidence of CRC may be partly arti‐factual and due to the choice of an arbitrary reference population born in 1949 with a very low CRC rate. This assumption and age‐based sub‐group analysis led to imprecise estimates of cancer incidence rate ratios with wide confidence intervals that may impact the validity of the models in question. (1) The metric used in this model—efficiency ratio—is also not straightforward for clinicians or patients to comprehend and explain to others as compared to a more traditional metric of LYG. It also does not easily capture the cost‐effectiveness of alternative screening strategies. (2) The model also assumed a 100% rate of adherence to screening in the <49‐year‐old population. Real‐world data show that adherence rates are at most 60% in those above 50 years of age. (3) Finally, the greatest limitation of all such CRC screening guidelines to date is the reliance on age as a sole (or primary) risk factor. Though age is a strong risk factor in population‐based studies, its use in individual patients is not absolute. There are several integrated risk factors when seeing a patient in your clinic that correlates with CRC risk, including male sex, family history of CRC, obesity (body mass index), cigarette smoking, diet, and medication use (e.g., Nonsteroidal anti‐inflammatory drugs, aspirin, and hormone replacement therapies) [27]. At a time when “individualized medicine” is being touted as the new paradigm of care, the use of age as a sole risk factor to determine CRC screening is short sighted and belies the need to treat our patients individually. The Gail model uses a combination of age, gender, race, and reproductive information to determine breast cancer risk and determine need for chemoprevention or risk reduction surgery. It is well validated in a variety of ethnic populations and used extensively in breast cancer clinics [28]. In the gastrointestinal space, similar models (PREMM1,2,6) exist to determine the need for genetic testing in persons at risk of Lynch syndrome [29]. A similar approach could be taken to determine and guide decision making around colorectal cancer risk and screening.
Diverting resources away from high‐risk populations and increasing disparity in care
The National Colorectal Cancer Roundtable has set a goal for CRC screening uptake of 80% by 2018. Unfortunately, we are still performing far below this metric, with CRC screening at most reaching the low 60% rate in those 50‐74 years of age. Even more concerning is that there are several populations (including those with potentially a higher biological risk of CRC) that remain under screened. These include racial minorities including African Americans, recent immigrants, and those with limited financial means and limited access to broad health insurance [30]. In addition, colonoscopy follow‐up on those undergoing non‐invasive stool‐based testing for CRC is also very low, hovering below 60%. There is real concern that implementation of the ACS recommendations could divert important resources away from these populations at greatest risk of CRC (and where the actual benefit of CRC screening will be most apparent). This may only exacerbate the disparities in screening noted in these high‐risk populations. Hospitals and practices may also inadvertently direct scarce CRC screening resources (especially optical colonoscopy) to those with higher reimbursement commercial insurance—which is unlikely to be those at older age, lower socioeconomic status, or racial minority populations.
Heavy societal cost for minimal benefit
Even if the benefit of earlier screening is realized it will come with marked cost to society. The benefit of preventing a colorectal cancer diagnosis and need for surgical and systemic therapy can easily exceed $100,000 per person annually. However, the risk of false‐positive non‐invasive screening tests resulting in unnecessary colonoscopies, the use of surveillance colonoscopy in patients with non‐advanced adenomas and the potential for a healthy user effect in the 45‐49‐year‐old population will lead to substantially higher costs than predicted over time. This younger cohort of patients will add over 20 million persons to the list of currently eligible patients for CRC screening, and with the preference for endoscopic screening in the United States over non‐invasive testing, this raises the concern of diversion of existing scarce resources to a population with an absolute risk of CRC that is low.
Inability to formally study young‐onset CRC
Finally, our guidelines must be based on direct evidence whenever possible. The implementation of the ACS guideline ad hoc removes any possibility of studying this issue at a population level within a well‐designed clinical trial/study. The biology of cancers in those at younger age vs. older population may also be different and reflect the difference in location and stage of cancers that has been noted previously [31]. This raises the possibility that the biology of these tumors may make them less amenable to prevention or early detection through screening. Without formal studies this may further weaken the potential benefits of screening this population.
CONCLUSION
The prevention of CRC is a primary goal for all gastroenterologists. The rising incidence of CRC in those younger than 50 years of age has led to a new ACS recommendation to lower the screening age to 45 years. Such a recommendation has multiple nuances that could benefit patients and reduce CRC incidence/mortality but also divert resources from underserved population's potentially increasing disparity in CRC burden and the financial implications to society overall. How to best incorporate these recommendations into practice will rely on balancing the needs of the individual patient, society, and public health policy. Clinicians should continue to be vigilant in investigating any young person with gastrointestinal symptoms, especially in light of the rising incidence of CRC in this population and this commentary and associated ACS recommendation is not meant to alter the timely diagnostic work‐up of symptomatic patients [32]. Finally, these recommendations would also not apply to those with a family history of CRC. We hope the positives and negatives of this complex issue are articulated to the reader and provide a short summary of talking points that can guide this discussion between the clinician and patient.
CONFLICTS OF INTEREST
Guarantor of the article: Both authors serve as article guarantors.
Specific author contributions: Both authors wrote the editorial.
Financial Support: None to report.
Potential competing interests: None to report. Disclaimer: The contents of this work do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Correspondence: J.C.A. (email: Joseph.C.Anderson@dartmouth.edu) or N.J.S. (email: Samadder.jewel@mayo.edu)
Published online 1 November 2018
REFERENCES
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–93. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970-2014. JAMA. 2017;318:572–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welch HG, Robertson DJ. Colorectal cancer on the decline—why screening can't explain it all. N Engl J Med. 2016;374:1605–7. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250–81. [DOI] [PubMed] [Google Scholar]
- 6.Katki HA, Kovalchik SA, Berg CD, et al. Development and validation of risk models to select ever-smokers for CT lung cancer screening. JAMA. 2016;315:2300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterse EFP, Meester RGS, Siegel RL, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: Microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;124:2964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surveillance E, Program ER. (www.seer.cancer.gov) SEER* Stat Database: Mortality-All COD, Aggregated With State, Total US (1969-2010). 2015.
- 10.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 9 Regs Research Data with Delay-Adjustment, Malignant Only, Nov 2017 Sub (1975-2015) < Katrina/Rita Population Adjustment > - Linked to County Attributes—Total U.S., 1969-2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission.
- 11.Joseph DA, Meester RG, Zauber AG, et al. Colorectal cancer screening: estimated future colonoscopy need and current volume and capacity. Cancer. 2016;122:2479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880–6. [DOI] [PubMed] [Google Scholar]
- 13.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–81. [DOI] [PubMed] [Google Scholar]
- 14.Patel SG, Ahnen DJ. Colorectal cancer in the young. Curr Gastroenterol Rep. 2018;20:15. [DOI] [PubMed] [Google Scholar]
- 15.You YN, Xing Y, Feig BW, et al. Young-onset colorectal cancer: is it time to pay attention? Arch Intern Med. 2012;172:287–9. [DOI] [PubMed] [Google Scholar]
- 16.Dozois EJ, Boardman LA, Suwanthanma W, et al. Young-onset colorectal cancer in patients with no known genetic predisposition: can we increase early recognition and improve outcome? Medicine (Baltimore). 2008;87:259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teng A, Lee DY, Cai J, et al. Patterns and outcomes of colorectal cancer in adolescents and young adults. J Surg Res. 2016;205:19–27. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman DA, Williams JL, Holub JL, et al. Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc. 2014;80:133–43. [DOI] [PubMed] [Google Scholar]
- 19.Austin H, Henley SJ, King J, et al. Changes in colorectal cancer incidence rates in young and older adults in the United States: what does it tell us about screening. Cancer Causes Control. 2014;25:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomark Prev. 2009;18:1695–8. [DOI] [PubMed] [Google Scholar]
- 21.Chen FW, Sundaram V, Chew TA, et al. Advanced-stage colorectal cancer in persons younger than 50 years not associated with longer duration of symptoms or time to diagnosis. Clin Gastroenterol Hepatol. 2017;15:728–37 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlitz JJ, Oliphant AB, Greenwald DA, et al. The American College of Gastroenterology and the 80% by 2018 Colorectal Cancer Initiative: a multifaceted approach to maximize screening rates. Am J Gastroenterol. 2017;112:1360–2. [DOI] [PubMed] [Google Scholar]
- 23.Stryker SJ, Wolff BG, Culp CE, et al. Natural history of untreated colonic polyps. Gastroenterology. 1987;93:1009–13. [DOI] [PubMed] [Google Scholar]
- 24.Qaseem A, Denberg TD, Hopkins RH, Jr., et al. , Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156:378–86. [DOI] [PubMed] [Google Scholar]
- 25.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739–50. [DOI] [PubMed] [Google Scholar]
- 26.Institute for Clinical Systems Improvement. Health care guideline: colorectal cancer screening. 2010. www.icsi.org/colorectal_cancer_screening/colorectal_cancer_screening_5.html.
- 27.Hadjipetrou A, Anyfantakis D, Galanakis CG, et al. Colorectal cancer, screening and primary care: a mini literature review. World J Gastroenterol. 2017;23:6049–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86. [DOI] [PubMed] [Google Scholar]
- 29.Kastrinos F, Steyerberg EW, Balmana J, et al. Comparison of the clinical prediction model PREMM(1,2,6) and molecular testing for the systematic identification of Lynch syndrome in colorectal cancer. Gut. 2013;62:272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tammana VS, Laiyemo AO. Colorectal cancer disparities: issues, controversies and solutions. World J Gastroenterol. 2014;20:869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballester V, Rashtak S, Boardman L. Clinical and molecular features of young-onset colorectal cancer. World J Gastroenterol. 2016;22:1736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89:216–24. [DOI] [PubMed] [Google Scholar]


